Abstract
The importance of (micro)vascular contributions to cognitive impairment and dementia (VCID) in aging cannot be overemphasized, and the pathogenesis and prevention of age-related cerebromicrovascular pathologies are a subject of intensive research. In particular, aging impairs the increase in cerebral blood flow triggered by neural activation (termed neurovascular coupling or functional hyperemia), a critical mechanism that matches oxygen and nutrient delivery with the increased demands in active brain regions. From epidemiological, clinical and experimental studies the picture emerges of a complex functional impairment of cerebral microvessels and astrocytes, which likely contribute to neurovascular dysfunction and cognitive decline in aging and in age-related neurodegenerative diseases. This overview discusses age-related alterations in neurovascular coupling responses responsible for impaired functional hyperemia. The mechanisms and consequences of astrocyte dysfunction (including potential alteration of astrocytic endfeet calcium signaling, dysregulation of eicosanoid gliotransmitters and astrocyte energetics) and functional impairment of the microvascular endothelium are explored. Age-related mechanisms (cellular oxidative stress, senescence, circulating IGF-1 deficiency) impairing the function of cells of the neurovascular unit are discussed and the evidence for the causal role of neurovascular uncoupling in cognitive decline is critically examined.
Keywords: geroscience, senescence, vascular aging, microcirculation, cerebral circulation, cerebrovascular, functional hyperemia, neurovascular coupling, VCID, VCI
Introduction
The brain is the most metabolically active organ in the human body. While it only accounts for 2% of the body mass, it consumes 20–25% of the body’s total energy requirements. Constant provision of nutrients to the brain is crucial to its health and function since brain energy stores are scarce. Therefore the brain must rely on the circulation for continuous supply of nutrients as well as oxygen, and for effective washout of metabolic waste products. Cerebromicrovascular health is essential for the maintenance of adequate brain perfusion and thus the preservation of normal cerebral function.
Energy demand of the brain varies both spatially and temporally with changes in neuronal activity, which require prompt CBF adjustments in a highly regulated fashion to maintain cellular homeostasis and function (Enager et al. 2009; Mathiesen et al. 1998). This is accomplished through a process termed neurovascular coupling (or “functional hyperemia”; Figure 1), which is orchestrated by an inter-cellular signaling network comprised of neurons and astrocytes, as well as smooth muscle cells and endothelial cells of cerebral microvessels (Chen et al. 2014; Petzold and Murthy 2011; Stobart et al. 2013; Wells et al. 2015). Compelling evidence obtained both in elderly patients and rodent models shows that aging significantly impairs neurovascular coupling responses (Balbi et al. 2015; Fabiani et al. 2013; Park et al. 2007; Sorond et al. 2013; Tong et al. 2012; Toth et al. 2014; Zaletel et al. 2005).
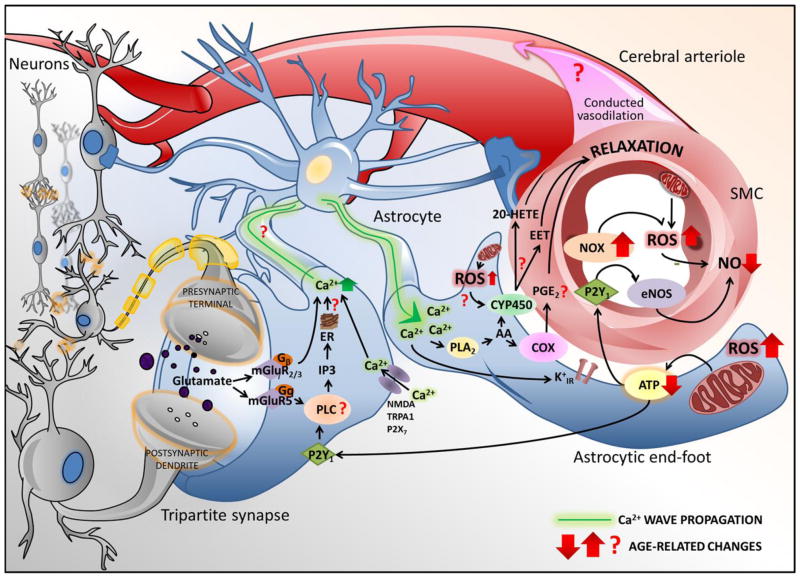
Figure 1. Aging impairs neurovascular coupling responses: synergistic roles of astrocyte dysfunction and endothelial impairment.
Shown is a schematic illustration of putative aging-induced alterations in glio-vascular coupling mechanisms, which may contribute to impaired functional hyperemia and thereby promote cognitive decline in the elderly. A complex interaction between neurons, astrocytes, microvascular smooth muscle and endothelial cells ensures adequate cerebral blood flow at all times. Neurotransmitters (e.g. glutamate) released from active excitatory synapses elicits elevations of [Ca2+]i in astrocytes via G protein–coupled receptors, whereas P2X and NMDA receptors contribute to channel-mediated increases in [Ca2+]i, initiating the propagation of a calcium waves through the astrocyte’s processes to the soma and to its microvascular end-feet wrapped around the smooth muscle cells. The surge in astrocyte end-feet [Ca2+]i promotes ATP release and activates PLA2 to release arachidonic acid (AA), which lead to the CYP450- and cyclooxygenase (COX)-mediated production of vasodilator eicosanoids (epoxyeicosatrienoic acids [EETs] and prostaglandins, respectively). Astrocyte-derived ATP promotes endothelial release of vasodilator NO via activation of P2Y1 receptors and also contributes to the propagation of the signal(Chen et al. 2014; Toth et al. 2015b). The aforementioned mechanisms together elicit smooth muscle relaxation in cerebral microvessels leading to localized hyperemia. The model predicts that the effects of aging on the neurovascular unit are manifest at multiple levels. Arrows indicate known age-related changes, whereas question marks highlight predicted aging effects yet to be verified experimentally. Of particular importance is the role of age-related oxidative stress as increased ROS production by NOX oxidases(Park et al. 2007) and mitochondrial sources can potentially affect both endothelial NO-mediated dilation and may play a critical role in dysregulation of eicosanoid synthesis. The model highlights the importance of new research investigating age-related alterations in astrocyte calcium signaling pathways. The schematic does not include the potential effects of aging on neuronal release of vasodilator mediators and/or alterations in astrocytic regulation of pericyte function and capillary dilation.
In this review, the effects of aging on key cellular and molecular mechanisms involved in neurovascular coupling are considered in terms of potential mechanisms involved in astrocyte dysfunction and microvascular impairment and their potential role in age-related cognitive decline.
Role of oxidative stress and endothelial dysfunction in age-related neurovascular un-coupling
The age-related mechanisms that contribute to neurovascular un-coupling are likely multifaceted (Figure 1). Considering the available evidence, the picture emerges that age-related dysfunction of microvascular endothelial cells contributes significantly to neurovascular un-coupling. Several lines of evidence suggest that oxidative stress and consequential endothelial dysfunction have a critical role in age-related cerebromicrovascular impairment and neurovascular uncoupling (Park et al. 2007; Toth et al. 2014). There is strong evidence that endothelial NO production contributes to neurovascular coupling (Toth et al. 2015b) and that in aging increased levels O2.− decreases bioavailability of endothelium-derived NO by forming peroxynitrite (Csiszar et al. 2002; Park et al. 2007; Toth et al. 2014). Importantly, neurovascular uncoupling in aging is reversible by interventions that improve endothelial function and cerebromicrovascular reactivity (Park et al. 2007; Toth et al. 2014). For example, previous studies showed that acute inhibition of NADPH oxidases (Park et al. 2007) and/or mitochondria-derived production of reactive oxygen species (ROS)(Toth et al. 2014) in aged mice is able to significantly improve microvascular endothelial function and improve neurovascular coupling responses. The pathophysiology of age-related endothelial dysfunction has been extensively studied in the peripheral circulation and a number of therapeutic interventions (ranging from dietary and lifestyle interventions to anti-inflammatory and anti-oxidant treatments) with endothelial protective effects have been identified (reviewed in (Ungvari et al. 2010)). These interventions can likely be adapted for improvement of the endothelial component of neurovascular coupling in aging and, consequently, for protection of higher brain function in the elderly. Further studies are needed to determine how these treatment approaches affect age-related functional alterations of astrocytes (see below) and/or interneurons(Jessen et al. 2015), which also contribute to neurovascular dysfunction.
Role of astrocyte dysfunction in age-related neurovascular uncoupling
Astrocytes are ideally positioned between neurons and cerebral microvessels to translate information on the activity level and energy demands of neurons to the vascular cells. Numerous studies conducted primarily in brain slices has built a model of neurovascular coupling where it is thought that astrocytes first respond to elevations in extracellular glutamate released from synapses during neuronal activation. Glutamate activates group 1 metabotropic glutamate receptors on the astrocytes leading to elevation of intracellular calcium via IP3 signaling (Zonta et al. 2003). The astrocyte endfeet calcium signals trigger multiple parallel acting pathways that lead to the release of vasoactive mediators that modulate the tone of adjacent vascular smooth muscle cells. Activation of calcium sensitive phospholipase A2 releases arachidonic acid, which is converted by cyclooxygenases into prostaglandins such as PGE2 or PGI2, and by epoxygenases to epoxyeicosatrienoic acids (EETs). These eicosanoid gliotransmitters relax vascular smooth muscle cells via mechanisms that involve EP receptor activation, BKCa channels and/or TRPV4 channel opening (Fernandes et al. 2008; Nilius et al. 2003). Arachidonic acid, especially under pathological conditions, also can be converted into 20-hydroxyeicosatetraenoic acid (20-HETE), which elicits vasoconstriction counteracting the dilatory stimuli mediated by NO, EETs and prostaglandins.
Our current understanding is that the balance between generation of vasodilator and vasoconstrictor eicosanoid gliotransmitters is determined by the preceding arteriolar tone (Blanco et al. 2008), tissue O2, lactate and adenosine levels (Gordon et al. 2008) and the bioavailability of NO (Metea and Newman 2006), among other factors. However, the exact role of astrocytes in functional hyperemia in vivo is now questioned due to difficulty of observing correlative astrocyte Ca2+ signals during sensory evoked blood flow increases (Bonder and McCarthy 2014; Nizar et al. 2013), and because IP3R2 knockout eliminates evoked astrocyte Ca2+ elevations yet does not affect functional hyperemia (Takata et al. 2013). Others though, have been able to detect very fast Ca2+ transients that precede the onset of vasodilation (Lind et al. 2013; Otsu et al. 2015), a signal that likely do not reflect the involvement of Ca2+ stores. This creates the possibilities that this ultrafast signal is important for neurovascular coupling, however, its causative role has not yet been demonstrated. Alternatively, others have proposed that astrocytes may instead be involved in blood flow control that is temporally much slower than functional hyperemia, where astrocytes may act to set baseline blood flow to the brain in a tonic, steady-state manner (Kur and Newman 2014; Rosenegger et al. 2015). If true, the same astrocyte cell pathways listed above could still be involved, relying instead on smaller, slower changes to the free Ca2+ concentration in the cytosol to promote the release vasodilators or vasoconstrictors, instead of large transients mediated by IP3R2. For example, one such ‘tonic’ pathway has indicated the role of PLA2 and COX1 mediating a tonic vasodilation in the neocortex via the constant release of prostaglandins (Rosenegger et al. 2015). New brain cell-derived pathways involved in arteriole tone control are interesting in light of the fact that the brain has a tremendous resting blood supply, which decreases with age (Amin-Hanjani et al. 2015). Thus, these pathways may be important therapeutic targets for the elderly or to ward off dementia.
Despite the importance of astrocyte-derived eicosanoid gliotransmitters in regulation of cerebral blood flow whether fast or slow, age-related alterations in astrocytic release of arachidonic acid metabolites are not well understood (Keleshian et al. 2013). Further studies are evidently needed to elucidate age-related alterations in endfeet Ca2+ signaling pathways (Mathiesen et al. 2013). New experimental preps and tools that are now being applied to the study of the neurovascular unit in young animals, will be key to making observations in the aged brain. For example, two-photon Ca2+ and vascular imaging has been an important technique for elucidating cellular mechanisms of cerebral blood flow control in brain slices and in vivo. The latest in vivo techniques allow observations of micro-vessel diameter changes and cell-type specific Ca2+ changes in either fully awake and behaving animals (Tran and Gordon 2015a; Tran and Gordon 2015b) or lightly sedated animals (Bonder and McCarthy 2014). By using cell-type specific promoters (like GLAST for astrocytes and TEK for endothelium) and new bright genetically encoded Ca2+ probes, astrocyte endfeet or vascular endothelial cell Ca2+ dynamics can be interrogated (Figure 2). This of course can be coupled with a variety of pharmacological or molecular approaches to understand the cell pathways involved or affected in old animals. By training animals for head-restraint, realistic Ca2+ and diameter measurements can be made in the absence of anesthesia. This is critical to avoid the side-effect that anesthetic have on brain activity and blood flow responses (reviewed in (Tran and Gordon 2015a; Tran and Gordon 2015b)). These technologies should be able to be implemented in aged mice and rats, as long as healthy cranial windows (Drew et al. 2010) can be achieved and the animal can tolerate head-restraint.
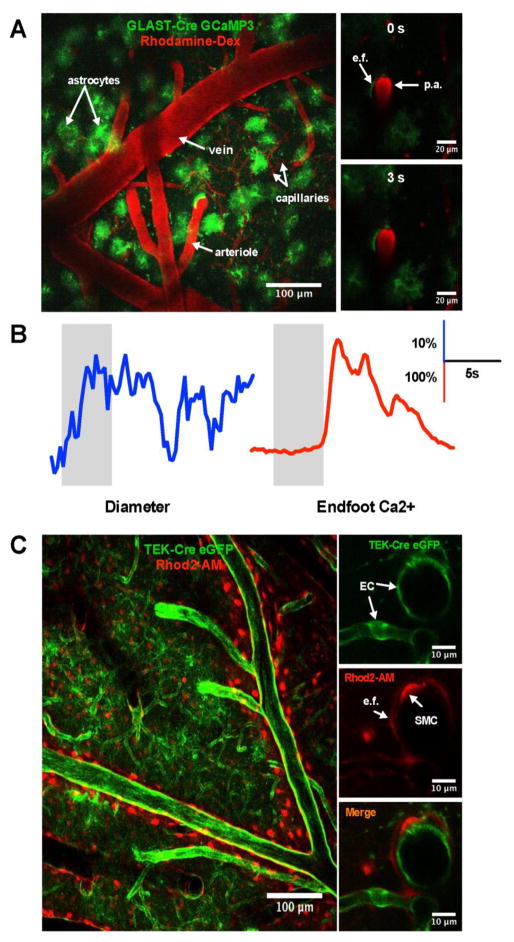
Figure 2. Imaging of neurovascular coupling events using two-photon microscopy.
A. Wide-field two-photon max intensity Z-stack of the barrel cortex from a GLAST-Cre GCaMP3 mouse with GCaMP3 expressed in the astrocytes (green) and vascular network labeled with Rhodamine B-dextran (red). Images of a penetrating arteriole (p.a.) enwrapped by an endfoot (e.f.) prior to whiskers stimulation (0 second, top and at 3 seconds, bottom). B. Representative traces of arteriole diameter (left) and endfoot Ca2+ (right) in response to whiskers stimulation (grey bars). C. Wide-field two-photon max intensity Z-stack of the barrel cortex from TEK-Cre ArchT eGFP mouse with ArchT eGFP expressed in the endothelial cells (EC) (green) and astrocytes labeled with Rhod2-AM (red). Images of a cross section of a penetrating arteriole and a capillary with EC expressing ArchT eGFP (top) with, astrocytic endfoot and smooth muscle cell (SMC) labeled with Rhod2-AM (middle) and merge image (bottom).
Another highly important signaling molecule by which astrocytes communicate both with each other and with vascular cells is ATP and its metabolites, adenosine and ADP (Toth et al. 2015b; Wells et al. 2015). When ATP is released from astrocytes in response to neuronal activation it contributes to microvascular dilation by triggering the production of endothelial NO (Toth et al. 2015b). Further, astrocyte-derived ATP can be also hydrolyzed to adenosine, which can relax vascular smooth muscle cells by acting on A2A and A2B receptors. Notably, a recently described mechanism in the retina involves the release of ATP acting through P2X1 receptors to mediate a tonic vasoconstriction to microvessels (Kur and Newman 2014). Here, Muller glial cells (the astrocyte equivalent in the retina) set steady-state vessel tone and thus this mechanism appears independent of phasic, neurovascular coupling. ATP is directly linked to astrocyte metabolism. In aging, cellular energy metabolism and ATP production are altered in most cell types studied, yet, surprisingly little is known about age-related alterations in purinergic gliovascular coupling mechanisms and the contribution of age-related astrocyte dysfunction to impaired endothelial-dependent vasomotor responses.
The cellular senescence theory of aging posits that progressive acquisition of senescent phenotypes by multiple cell types is a major driving force in aging. In addition to loss of cell proliferation, senescent cells are characterized by altered metabolism, functional impairment, acquisition of a pro-inflammatory senescence-associated secretory phenotype (a major contributor to “inflamm-aging”), activation of DNA-damage response pathways, altered secretion of extracellular matrix constituents and enzymes involved in degradation of the extracellular matrix and alterations of cell morphology and cytoarchitecture. Recent studies demonstrate that astrocytes also acquire a senescent phenotype in human aging, as indicated by the up-regulation of the senescence-specific molecular marker p16INK4a (Bhat et al. 2012). Although several pathways involved in functional hyperemia may be affected in senescent astrocytes, the role of astrocyte senescence in neurovascular uncoupling remains unexplored. Future investigations should also provide answers to a number of additional important questions about the role of astrocyte dysfunction in neurovascular uncoupling in aging. What is the functional role of mitochondrial/cellular oxidative stress in age-related astrocyte dysfunction? How does aging affect the expression and activity of K+ channels or mGluRs involved in gliovascular coupling, as well as the action and metabolism of glutamate? How does aging affect astrocyte-pericyte communication? Treatments that target astrocyte metabolism in order to prevent/reverse gliovascular dysregulation in aging also need to be tested.
Role of age-related IGF-1 deficiency in neurovascular dysfunction
Humans and experimental animals exhibit progressive age-related decline in circulating IGF-1 levels. There is overwhelming evidence that age-related IGF-1 deficiency significantly contributes to cardiovascular aging, promoting cerebromicrovascular alterations associated with old age (Sonntag et al. 2013; Ungvari and Csiszar 2012). Each cell type involved in neurovascular coupling (i.e. neurons, astrocytes, endothelial cells) are known targets of IGF-1 (Pardo et al. 2016; Sonntag et al. 2013) and there is good reason to believe that circulating IGF-1 deficiency is an important contributing factor to neurovascular dysfunction in aging. In endothelial cells IGF-1 was shown to regulate ROS production, mitochondrial oxidative stress, NO bioavailability and antioxidant response pathways (Bailey-Downs et al. 2012; Csiszar et al. 2008). Importantly, we recently found that in mouse models of circulating IGF-1 deficiency, neurovascular coupling is impaired, in part, due to endothelial dysfunction (Toth et al. 2015a). In addition, IGF-1 deficiency also promotes astrocyte dysfunction, impairs production of astrocyte-derived EETs and increases production of 20-HETE (Toth et al. 2015a). Further studies are warranted to test the effect of IGF-1 deficiency on astrocytic calcium signaling mechanisms, to elucidate the link between IGF-1 deficiency and impaired functional hyperemia in older individuals, as well as to assess the effects of IGF-1 treatment on neurovascular coupling responses in aged experimental animals.
Neurovascular un-coupling in Alzheimer’s disease
There is growing evidence for microvascular pathophysiological alterations having a causal role both in the development of Alzheimer’s disease (AD) and AD-related cognitive decline (reviewed in (Snyder et al. 2015a)). Importantly, AD patients exhibit significant impairment of neurovascular coupling responses (Hock et al. 1997; Rombouts et al. 2000). In mouse models of AD, neurovascular coupling is also impaired (Shin et al. 2007) due to enhanced oxidative stress (Nicolakakis et al. 2008). The experimental data suggest that up-regulation of NADPH-derived ROS production and/or mitochondrial oxidative stress may contribute to neurovascular uncoupling in AD (Park et al. 2005; Park et al. 2008). Importantly, there is increasing evidence that treatments that improve neurovascular coupling responses are associated with improved cognitive function in mice with AD pathologies (Tong et al. 2012). Future studies are needed to translate these findings to the clinical setting.
Pathophysiological consequences of age-related neurovascular uncoupling
In elderly patients, inadequate blood flow augmentation during neuronal activation likely results in a mismatch between supply and demand of oxygen and metabolic substrates in functioning cerebral tissue(Jessen et al. 2015). This homeostatic imbalance is likely causally related to compromised neuronal and cerebral function (Fabiani et al. 2013; Sorond et al. 2008; Stefanova et al. 2013; Topcuoglu et al. 2009; Zaletel et al. 2005). Importantly, age-related deficiencies in neurovascular coupling responses have been linked to impaired higher cognitive function (Sorond et al. 2013) and gait abnormalities (Sorond et al. 2011). Studies conducted in rodent models of aging have substantiated these findings showing that neurovascular uncoupling associates with cognitive decline. Experimental studies support a causal link between neurovascular uncoupling and cognitive decline. Importantly, pharmacologically-induced neurovascular coupling was shown result in impairment of spatial and recognition memory, mimicking the aging phenotype (Tarantini et al. 2015). Studies showing that treatment of aged mice with the naturally occurring dietary polyphenol resveratrol rescues neurovascular coupling responses also provide important proof-of-concept (Toth et al. 2014), as resveratrol treatment improved cognitive function in aged rodents (Liu et al. 2012; Oomen et al. 2009; Zhao et al. 2013). Recently we found that treatment with the mitochondria-targeted antioxidative peptide SS-31 also significantly improves neurovascular coupling responses in aged mice, which is associated with significantly improved spatial working memory and motor skill learning (Tarantini, Csiszar and Ungvari, 2016, manuscript in preparation). Recently, to recognize the contribution of cerebromicrovascular mechanisms to age-related cognitive decline the phrase “vascular contributions to cognitive impairment and dementia (VCID)” was introduced (Corriveau et al. 2016; Gorelick et al. 2011; Snyder et al. 2015b). The concept of VCID implies that a spectrum of age-related vascular and microvascular pathologies (including cerebral microhemorrhages, microinfarcts, blood brain barrier disruption and leukoaraiosis) contribute to cognitive impairment in elderly patients. We posit that neurovascular uncoupling superimposed on these vascular pathologies is likely to significantly exacerbate cognitive decline. Hypertensive vasculopathies and amyloid pathologies associated with Alzheimer models are associated with neurovascular dysfunction (Iadecola 2013; Iadecola and Davisson 2008; Iadecola et al. 2009; Nicolakakis et al. 2008; Tong et al. 2012). On the basis of the aforementioned findings the neurovascular unit should be considered as a target for therapeutic intervention.
Gait dysfunction in the elderly population is a major cause of functional impairment, contributes to falls and predicts increased risk of institutionalization and mortality. Although senile gait disorders are likely multifactorial, there is emerging evidence that an association exists between neurovascular coupling and gait coordination (Sorond et al. 2010; Sorond et al. 2011). Further research is warranted to explore the mechanistic link between neurovascular uncoupling and gait dysfunction using animal models and to determine whether interventions, which rescue functional hyperemia are also effective in improving gait in aging.
Age-related dysregulation of neurovascular coupling responses also has relevance for altered hemodynamic effects associated with cortical spreading depolarizations (CSDs). CSDs are intense self-propagating waves of depolarization in the cortex that trigger rapid vasoconstriction, followed by a hyperemic response and then a long-lasting post-spreading depolarization oligemia. CSDs often occur after intracerebral hemorrhage, ischemic stroke, subarachnoid hemorrhage or traumatic brain injury and are thought to exacerbate ischemic neuronal damage worsening the clinical outcome (Farkas and Bari 2014; Hartings et al. 2016). There is strong evidence that aging-induced alterations in neurovascular coupling pathways exacerbate the deleterious functional consequences of CSDs (Farkas and Bari 2014; Menyhart et al. 2015). Future studies should determine how novel pharmacological interventions that rescue neurovascular coupling in aging impact vasomotor responses associated with CSD and functional recovery after neuronal injury.
Perspectives
In recent years there have been major conceptual shifts in our understanding of mechanisms and consequences of age-related impairment of neurovascular coupling responses. These developments not only provide an increased understanding of the cerebromicrovascular aging process, they also offer opportunities to develop novel approaches for preventive and therapeutic interventions for age-related cognitive impairment. Future studies should provide answers to a number of critical questions about neurovascular uncoupling and its role in VCID. How aging alters calcium signaling processes and eicosanoid metabolism in astrocytes? What is the role of age-related mitochondrial oxidative stress and mitochondrial dysfunction? What is the functional role of cellular senescence in neurovascular aging? Under what circumstances does neurovascular uncoupling contribute to the pathogenesis of VCID? In that regard studies focusing on the synergistic effects of multiple comorbidities and aging (e.g. the complex effects of hypertension and obesity in geriatric patients) will be highly informative. Future studies should also explore the link between age-related neurovascular dysfunction and its role in diffuse white matter disease. Further, pathways involved in neurovascular coupling can be potentially modulated by pharmacological treatments used in the elderly. For instance, there are studies extant showing that commonly used, non-steroidal anti-inflammatory drugs (NSAIDs) significantly attenuate neurovascular coupling responses in humans (Bruhn et al. 2001; Szabo et al. 2014). Potential adverse neurovascular effects of these medications should be considered when used in older patients. A critical area for future research will be to develop clinically relevant therapeutic interventions that improve function of the neurovascular unit and to determine whether these treatments can prevent and/or reverse cognitive decline associated with aging and age-related pathologies. Finally, comorbidity is common in elderly persons. Previous studies show that multiple comorbid conditions prevalent in the elderly (e.g. hypertension (Girouard et al. 2007), obesity (Tucsek et al. 2014)) promote neurovascular uncoupling, exacerbating the effects of aging (Tucsek et al. 2014). Additional research efforts are needed to test the efficacy of pharmacological interventions that rescue neurovascular coupling in aging under comorbid conditions.
Highlights.
Normal neurovascular coupling is critical for cognitive health.
Clinical and experimental evidence shows that aging impairs functional hyperemia.
ROS and IGF-1 deficiency contribute to neurovascular uncoupling in aging.
Acknowledgments
This work was supported by grants from the American Heart Association (to ST, AC and ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; 3P30AG050911-02S1), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC), the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to ZU; P30 AG028718), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU), the Reynolds Foundation (to ZU and AC), the Canadian Institutes of Health Research (GG), Canada Research Chair (GG) and Alberta Innovates Health Solutions (CT). Portions of this work were presented and published in thesis form in fulfillment of the requirements for the PhD for Stefano Tarantini from the University of Oklahoma Health Sciences Center.
Footnotes
Disclosure: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin-Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT. Effect of age and vascular anatomy on blood flow in major cerebral vessels. J Cereb Blood Flow Metab. 2015;35:312–318. doi: 10.1038/jcbfm.2014.203. http://dx.doi.org/10.1038/jcbfm.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi M, Ghosh M, Longden TA, Jativa Vega M, Gesierich B, Hellal F, Lourbopoulos A, Nelson MT, Plesnila N. Dysfunction of mouse cerebral arteries during early aging. J Cereb Blood Flow Metab. 2015;35:1445–1453. doi: 10.1038/jcbfm.2015.107. http://dx.doi.org/10.1038/jcbfm.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. http://dx.doi.org/10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–2863. doi: 10.1152/ajpheart.91451.2007. http://dx.doi.org/10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci. 2014;34:13139–13150. doi: 10.1523/JNEUROSCI.2591-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn H, Fransson P, Frahm J. Modulation of cerebral blood oxygenation by indomethacin: MRI at rest and functional brain activation. J Magn Reson Imaging. 2001;13:325–334. doi: 10.1002/jmri.1047. http://dx.doi.org/10.1002/jmri.1047. [DOI] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. http://dx.doi.org/10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol. 2016;36:281–288. doi: 10.1007/s10571-016-0334-7. http://dx.doi.org/10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–1894. doi: 10.1152/ajpheart.412.2008. http://dx.doi.org/412.2008 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Blinder P, Davalos D, Akassoglou K, Tsai PS, Kleinfeld D. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 2010;7:981–984. doi: 10.1038/nmeth.1530. http://dx.doi.org/10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. http://dx.doi.org/10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.04.113. http://dx.doi.org/S1053-8119(13)00480-1 [pii] [DOI] [PMC free article] [PubMed]
- Farkas E, Bari F. Spreading depolarization in the ischemic brain: does aging have an impact? J Gerontol A Biol Sci Med Sci. 2014;69:1363–1370. doi: 10.1093/gerona/glu066. http://dx.doi.org/10.1093/gerona/glu066. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. The Journal of cell biology. 2008;181:143–155. doi: 10.1083/jcb.200712058. http://dx.doi.org/10.1083/jcb.200712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. http://dx.doi.org/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. http://dx.doi.org/10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. http://dx.doi.org/STR.0b013e3182299496 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Shuttleworth CW, Kirov SA, Ayata C, Hinzman JM, Foreman B, Andrew RD, Boutelle MG, Brennan KC, Carlson AP, Dahlem MA, Drenckhahn C, Dohmen C, Fabricius M, Farkas E, Feuerstein D, Graf R, Helbok R, Lauritzen M, Major S, Oliveira-Ferreira AI, Richter F, Rosenthal ES, Sakowitz OW, Sanchez-Porras R, Santos E, Scholl M, Strong AJ, Urbach A, Westover MB, Winkler MK, Witte OW, Woitzik J, Dreier JP. The continuum of spreading depolarizations in acute cortical lesion development: Examining Leao’s legacy. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16654495. http://dx.doi.org/0271678X16654495 [pii] [DOI] [PMC free article] [PubMed]
- Hock C, Villringer K, Muller-Spahn F, Wenzel R, Heekeren H, Schuh-Hofer S, Hofmann M, Minoshima S, Schwaiger M, Dirnagl U, Villringer A. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer’s disease monitored by means of near-infrared spectroscopy (NIRS)--correlation with simultaneous rCBF-PET measurements. Brain Res. 1997;755:293–303. doi: 10.1016/s0006-8993(97)00122-4. http://dx.doi.org/S0006-8993(97)00122-4 [pii] [DOI] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. http://dx.doi.org/10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. http://dx.doi.org/10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–44. doi: 10.1161/STROKEAHA.108.533638. http://dx.doi.org/10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen SB, Mathiesen C, Lind BL, Lauritzen M. Interneuron Deficit Associates Attenuated Network Synchronization to Mismatch of Energy Supply and Demand in Aging Mouse Brains. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv261. http://dx.doi.org/bhv261. [DOI] [PubMed]
- Keleshian VL, Modi HR, Rapoport SI, Rao JS. Aging is associated with altered inflammatory, arachidonic acid cascade, and synaptic markers, influenced by epigenetic modifications, in the human frontal cortex. J Neurochem. 2013;125:63–73. doi: 10.1111/jnc.12153. http://dx.doi.org/10.1111/jnc.12153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kur J, Newman EA. Purinergic control of vascular tone in the retina. J Physiol. 2014;592:491–504. doi: 10.1113/jphysiol.2013.267294. http://dx.doi.org/10.1113/jphysiol.2013.267294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci U S A. 2013;110:E4678–4687. doi: 10.1073/pnas.1310065110. http://dx.doi.org/10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GS, Zhang ZS, Yang B, He W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life sciences. 2012;91:872–877. doi: 10.1016/j.lfs.2012.08.033. http://dx.doi.org/10.1016/j.lfs.2012.08.033. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Brazhe A, Thomsen K, Lauritzen M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J Cereb Blood Flow Metab. 2013;33:161–169. doi: 10.1038/jcbfm.2012.175. http://dx.doi.org/10.1038/jcbfm.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512(Pt 2):555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menyhart A, Makra P, Szepes BE, Toth OM, Hertelendy P, Bari F, Farkas E. High incidence of adverse cerebral blood flow responses to spreading depolarization in the aged ischemic rat brain. Neurobiol Aging. 2015;36:3269–3277. doi: 10.1016/j.neurobiolaging.2015.08.014. http://dx.doi.org/10.1016/j.neurobiolaging.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. http://dx.doi.org/26/11/2862 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Watanabe H, Vriens J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch. 2003;446:298–303. doi: 10.1007/s00424-003-1028-9. http://dx.doi.org/10.1007/s00424-003-1028-9. [DOI] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzic S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. http://dx.doi.org/10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci. 2015;18:210–218. doi: 10.1038/nn.3906. http://dx.doi.org/10.1038/nn.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Uriarte M, Console GM, Reggiani PC, Outeiro TF, Morel GR, Goya RG. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci. 2016;44:2120–2128. doi: 10.1111/ejn.13278. http://dx.doi.org/10.1111/ejn.13278. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. http://dx.doi.org/10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. http://dx.doi.org/10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. http://dx.doi.org/10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Rosenegger DG, Tran CH, Wamsteeker Cusulin JI, Gordon GR. Tonic Local Brain Blood Flow Control by Astrocytes Independent of Phasic Neurovascular Coupling. J Neurosci. 2015;35:13463–13474. doi: 10.1523/JNEUROSCI.1780-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.1780-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130:2310–2319. doi: 10.1093/brain/awm156. http://dx.doi.org/awm156 [pii] [DOI] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015a;11:710–717. doi: 10.1016/j.jalz.2014.10.008. http://dx.doi.org/10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015b;11:710–717. doi: 10.1016/j.jalz.2014.10.008. http://dx.doi.org/S1552-5260(14)02862-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. http://dx.doi.org/10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Galica A, Serrador JM, Kiely DK, Iloputaife I, Cupples LA, Lipsitz LA. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology. 2010;74:1627–1633. doi: 10.1212/WNL.0b013e3181df0982. http://dx.doi.org/10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a351aa. http://dx.doi.org/WNL.0b013e3182a351aa [pii] [DOI] [PMC free article] [PubMed]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell’Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. http://dx.doi.org/10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex. 2008;44:179–184. doi: 10.1016/j.cortex.2006.01.003. http://dx.doi.org/10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging. 2013;34:2277–2286. doi: 10.1016/j.neurobiolaging.2013.03.031. http://dx.doi.org/10.1016/j.neurobiolaging.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2013;110:3149–3154. doi: 10.1073/pnas.1215929110. http://dx.doi.org/10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo K, Rosengarten B, Juhasz T, Lako E, Csiba L, Olah L. Effect of non-steroid anti-inflammatory drugs on neurovascular coupling in humans. J Neurol Sci. 2014;336:227–231. doi: 10.1016/j.jns.2013.10.048. http://dx.doi.org/10.1016/j.jns.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One. 2013;8:e66525. doi: 10.1371/journal.pone.0066525. http://dx.doi.org/10.1371/journal.pone.0066525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–4715. doi: 10.1523/JNEUROSCI.0169-12.2012. http://dx.doi.org/10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett. 2009;452:17–22. doi: 10.1016/j.neulet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015a;14:1034–1044. doi: 10.1111/acel.12372. http://dx.doi.org/10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015b;309:H1837–1845. doi: 10.1152/ajpheart.00463.2015. http://dx.doi.org/10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. http://dx.doi.org/ajpheart.00744.2013 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CH, Gordon GR. Acute two-photon imaging of the neurovascular unit in the cortex of active mice. Front Cell Neurosci. 2015a;9:11. doi: 10.3389/fncel.2015.00011. http://dx.doi.org/10.3389/fncel.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CH, Gordon GR. Astrocyte and microvascular imaging in awake animals using two-photon microscopy. Microcirculation. 2015b;22:219–227. doi: 10.1111/micc.12188. http://dx.doi.org/10.1111/micc.12188. [DOI] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. http://dx.doi.org/10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. http://dx.doi.org/10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. http://dx.doi.org/glq113 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JA, Christie IN, Hosford PS, Huckstepp RT, Angelova PR, Vihko P, Cork SC, Abramov AY, Teschemacher AG, Kasparov S, Lythgoe MF, Gourine AV. A Critical Role for Purinergic Signalling in the Mechanisms Underlying Generation of BOLD fMRI Responses. J Neurosci. 2015;35:5284–5292. doi: 10.1523/JNEUROSCI.3787-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.3787-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. http://dx.doi.org/893 [pii] [PubMed] [Google Scholar]
- Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435:597–602. doi: 10.1016/j.bbrc.2013.05.025. http://dx.doi.org/10.1016/j.bbrc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. http://dx.doi.org/10.1038/nn980. [DOI] [PubMed] [Google Scholar]