Highlights
-
•
Greater amygdala reactivity to angry faces predicted youth antisocial behavior.
-
•
Callous-unemotional traits were not related to amygdala reactivity.
-
•
Findings were similar across sex, ethnicity, and pubertal stage.
Keywords: Antisocial behavior, Callous-unemotional traits, Amygdala, Threat, fMRI
Abstract
Recent neuroimaging studies have suggested divergent relationships between antisocial behavior (AB) and callous-unemotional (CU) traits and amygdala reactivity to fearful and angry facial expressions in adolescents. However, little work has examined if these findings extend to dimensional measures of behavior in ethnically diverse, non-clinical samples, or if participant sex, ethnicity, pubertal stage, and age moderate associations. We examined links between amygdala reactivity and dimensions of AB and CU traits in 220 Hispanic and non-Hispanic Caucasian adolescents (age 11–15; 49.5% female; 38.2% Hispanic), half of whom had a family history for depression and thus were at relatively elevated risk for late starting, emotionally dysregulated AB. We found that AB was significantly related to increased right amygdala reactivity to angry facial expressions independent of sex, ethnicity, pubertal stage, age, and familial risk status for depression. CU traits were not related to fear- or anger-related amygdala reactivity. The present study further demonstrates that AB is related to increased amygdala reactivity to interpersonal threat cues in adolescents, and that this relationship generalizes across sex, ethnicity, pubertal stage, age, and familial risk status for depression.
1. Introduction
Antisocial behavior (AB) includes behaviors such as rule breaking, lying, and aggression. AB is a major public health concern because of its high prevalence and the negative impact of AB on perpetrators, victims, and their families (Foster and Jones, 2005, Nock et al., 2006, Odgers et al., 2007). Recent research has suggested that youth with AB are a heterogeneous group (Frick and Viding, 2009), which may undermine intervention success. Thus, research that examines the divergent etiologies of specific dimensions within AB has the potential to inform more effective, personalized treatments (Frick and Nigg, 2012, Kahn et al., 2012, Moffitt et al., 2008).
1.1. Divergent pathways of amygdala reactivity
One recent approach to parsing AB into etiologically distinct subtypes is to measure the presence of callous-unemotional (CU) traits. CU traits were added as a subtyping specifier to the antisocial behavior diagnosis of Conduct Disorder in the latest version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013), titled “with limited prosocial behavior.” These traits are characterized by low empathy, lack of remorse, and shallow interpersonal affect (Frick and White, 2008, Viding et al., 2012). AB in the presence of CU traits is more highly heritable (Viding et al., 2005), and research is beginning to suggest that AB and CU traits may have divergent relationships with neural reactivity, particularly in the amygdala (Hyde et al., 2013).
The amygdala has been implicated in cognitive and affective processes believed to underlie behavioral deficits characteristic of youth AB, such as poor fear conditioning and impaired emotional regulation, potentially via two divergent pathways (Blair et al., 2014, Hyde et al., 2013). In one pathway, in those high on CU traits, relatively decreased amygdala reactivity to signals of interpersonal distress may prevent the processing of interpersonal distress cues, and disrupt important conditioning early in life that contributes to the development of empathy (Blair et al., 2014). In a second pathway, in those low on CU traits, but high on AB, relatively increased amygdala reactivity to cues of social threat may result in over-reactivity to threat and lead to emotional dysregulation, manifesting behaviorally as increased reactive aggression and AB (Hyde et al., 2013, Viding et al., 2012). Consistent with a dual pathway model, antisocial adolescents with high levels of CU traits (AB+/CU + ) demonstrate relatively decreased amygdala reactivity to signals of interpersonal distress such as fearful facial expressions with directed eye gaze (Jones et al., 2009, Lozier et al., 2014, Marsh et al., 2008, Viding et al., 2012). In contrast, antisocial adolescents low on CU traits (AB+/CU-) exhibit relatively increased reactivity of the amygdala to negative emotional stimuli, particularly interpersonal signals of threat, such as angry facial expressions with directed eye gaze (Herpertz et al., 2008, Sebastian et al., 2012, Sebastian et al., 2014, Viding et al., 2012).
Though a growing literature supports a dual pathway model for AB+/CU+ versus AB+/CU- adolescents, the majority of these studies have focused on small case-control samples of those extreme on AB and CU traits. Thus, beyond a few exceptions (e.g., Viding et al., 2014), studies of adolescents have been unable to separate the relative contribution of amygdala reactivity to AB versus CU traits. As evidence continues to accumulate emphasizing the dimensional nature of AB and CU traits (Blonigen et al., 2006, Krueger et al., 2007), research is needed to test these relationships across the distribution of AB and CU traits, particularly in samples with non-clinical levels of AB. Although recent studies have begun to examine AB dimensionally in healthy and at-risk adults (e.g., Carré et al., 2013, Hyde et al., 2014), it is important to examine this question in early adolescence at the cusp of the emergence of serious AB and other psychopathology. Thus, the primary goal of our study was to examine the relationships between AB, CU traits, and amygdala reactivity to fearful and angry facial expressions in a relatively large sample of teens with variability in AB and CU traits but without diagnosable levels of AB.
1.2. Moderators of the links between AB, CU traits, and amygdala reactivity
Beyond this first goal, recent work has highlighted that several factors may moderate the relationship between AB and amygdala reactivity to interpersonal threat and distress, at least in adults. For example, in a recent study of young men, we found that participant race moderated the associations between AB, CU traits, and amygdala reactivity (Hyde et al., 2016). This study demonstrated that amygdala reactivity was related to AB in response to fearful facial expressions but only in African Americans (versus European Americans). Though these results were intriguing, the study focused exclusively on African and European American young men, and thus could not examine the extent to which sex or ethnicity (i.e., Hispanic versus non-Hispanic) may also moderate these relationships. Moreover, these recent studies have often focused on adults (e.g., Carré et al., 2013, Hyde et al., 2014) with little attention to how age or stage of puberty may also moderate these pathways, which is particularly important during adolescence. Rapid pubertal development occurs in early adolescence and results in hormonal changes that affect neural correlates of social and affective processing (Crone and Dahl, 2012), which likely result in changes in amygdala reactivity to emotional facial expressions (Hare et al., 2008). Thus, a second goal of the current study was to examine the ways in which the relationship between amygdala reactivity to emotional facial expressions and AB and CU traits may vary based on participant sex, ethnicity, pubertal timing, and age.
Finally, previous studies have focused on relationships between AB, CU traits and amygdala reactivity in adolescents with extreme, early-starting, diagnosable levels of AB. Yet, in contrast to this extreme group, many adolescents will show subclinical and/or late onset AB during adolescence (up to 25% of teens show late onset AB; Moffitt, 1993, Moffitt et al., 2002). Many of these subclinical and “late starting” adolescents will be at risk for AB because of comorbid diagnoses associated with emotional dysregulation (e.g., depression). Two factors may contribute to subclinical and later onset of AB: First, adolescence is a period in which teens are learning to control their emotions and are more impulsive (Hinshaw, 2003) and second, internalizing disorders rapidly increase in prevalence during adolescence, which can lead to AB as a result of underlying premorbid or comorbid internalizing symptoms (e.g., depression, anxiety; Hinshaw et al., 1993). However, little research has examined neural pathways to subclinical AB in adolescents at higher risk for depression, even though these youths comprise a substantial portion of the population. To address this significant gap in the literature, in the present study we also sought to examine our aims in a unique sample for this research: adolescents without diagnosable levels of AB, but with enrichment for familial risk for depression that could lead to the emergence of later AB.
1.3. Present study
The goal of the present study was to investigate relationships between amygdala reactivity and dimensional measures of AB and CU traits in a sample of adolescents with nonclinical levels of AB at the start of adolescence. We examined reactivity to fearful and angry facial expressions compared to a non-face control condition to evaluate the differential contribution of reactivity to interpersonal distress or threat, respectively. Consistent with prominent theories of AB and CU traits (Blair et al., 2014), we expected that AB would be related to increased amygdala reactivity to angry facial expressions, whereas CU traits would be uniquely related to decreased amygdala reactivity to fearful facial expressions. Finally, given previous research suggesting differential neural correlates for AB between men and women (Carré et al., 2013), as well as across race/ethnicity (Hyde et al., 2016), and research suggesting that pubertal onset may influence amygdala reactivity (Crone and Dahl, 2012), we examined these relationships in an early adolescent sample containing substantial proportions of Hispanic participants and girls during early to middle adolescence to analyze potential moderation of findings based on sex, ethnicity, pubertal timing, and age. Though sex, ethnicity and puberty based analyses were exploratory, we expected that relationships between AB, CU traits, and amygdala reactivity would be strongest in male participants and Caucasians in early stages of puberty, given that prior research in this area has focused on Caucasian boys during early adolescence (Jones et al., 2009, Sebastian et al., 2014, Viding et al., 2012). Finally, though we focused on the amygdala globally, given the different roles in fear learning, potential implications for the development of later psychopathy (Moul et al., 2012), as well as the different afferent and efferent connections of the centro-medial (CM) versus the basolateral (BL) regions (Amunts et al., 2005), we also conducted exploratory analyses within the CM versus BL sub-regions. We examined these questions in a large sample of psychiatrically healthy participants, in a sample that was enriched with adolescents with familial risk for depression (50% of the sample) and thus potentially at risk for later comorbid and emotionally dysregulated late onset AB.
2. Methods
2.1. Participants
Participants were recruited as part of the Teen Alcohol Outcomes Study (TAOS), designed to examine the association between the development of depression and alcohol use disorders by recruiting adolescents at high and low familial risk for depression. Adolescents at high-risk had a first- and a second-degree relative with a history of major depression, whereas those at low-risk did not have any first- and a limited number of second-degree relatives with a history of depression. Sampling and recruitment procedures for the TAOS have been described previously (Bogdan et al., 2012, Swartz et al., 2015, White et al., 2012a). Participants, who were age 11–15 years, provided assent, and parents provided written informed consent following procedures approved by the Institutional Review Board at the University of Texas Health Sciences Center at San Antonio. Participants had to be free of psychiatric diagnoses, including externalizing disorders, at the baseline clinical assessment using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), with the exception that an anxiety disorder diagnosis was permitted in the group with a positive family history of depression. Participants were also excluded if they met criteria for a substance use disorder or reported any binge drinking at baseline (based on National Institute on Alcohol Abuse and Alcoholism guidelines) and/or met lifetime criteria for conduct disorder or attention deficit disorder. Thus, by excluding adolescents with diagnoses of CD and other externalizing disorders, the participants in this sample did not present with clinically significant levels of self-reported AB at the time of entry. The final sample consisted of 220 participants (49.5% female), with a mean age of 13.42 (+/− 0.96) (Table 1). Of these participants, 116 also had data on AB and CU traits, as these measures were added to the study after data collection began. However as noted below, using missing data procedures, we were able to include all 220 participants in the current study. In the final sample, based on parent-reported ethnicity, 61.8% of participants were Caucasian, while 38.2% were Hispanic. A small number of the participants were siblings (18 pairs in total), which was adjusted for in the statistical model.
Table 1.
Sample Characteristics.
| Variable | n | (%) | Mean | SD | Range |
|---|---|---|---|---|---|
| Female (coded as 0) | 109 | 49.5 | |||
| Non-Hispanic (coded as 0) | 136 | 61.8 | |||
| Puberty > Tanner Stage III | 126 | 57.3 | |||
| Low Familial Risk for Depression | 108 | 49.1 | |||
| Age | 13.42 | 0.96 | 11–15 | ||
| Pubertal Development | 3.41 | 0.89 | 1–5 | ||
| Antisocial Behavior | 5.44 | 5.28 | 0–24 | ||
| Callous-Unemotional Traits | 2.12 | 1.61 | 0–6 |
2.2. Amygdala reactivity paradigm
In the study, we adopted a widely-used paradigm known to robustly elicit amygdala reactivity (Carré et al., 2012, Hariri et al., 2002). In this task, participants complete four blocks of a perceptual face-processing task in which they view a trio of faces (expressing either anger or fear) and select one of two faces (displayed on the bottom) identical to the target stimulus (displayed on top). Each block consists of six images derived from a standard set of facial affect pictures (Ekman and Friesen, 1976). Faces are balanced for sex and target affect. Each of the six face trios is presented for 4 s with a variable inter-stimulus interval of 2–6 s, for a total block length of 48 s. Interleaved between these blocks, participants complete five blocks of a sensorimotor control task during which they view a trio of geometric shapes (circles, horizontal ellipses and vertical ellipses) and select one of two shapes (displayed on the bottom) identical to the target shape (displayed on top). Each sensorimotor task consists of six different shape trios. Each of the six different shape trios is presented for 4 s with a fixed inter-stimulus interval of 2 s, for a total block length of 36 s. The total paradigm length is 390 s. Reaction times and accuracy were recorded throughout (Table 2).
Table 2.
Relationships Between Antisocial Behavior, Callous-Unemotional Traits, and Amygdala Reactivity.
| Antisocial Behavior |
Callous-Unemotional Traits |
|||||
|---|---|---|---|---|---|---|
| B | SD | ß | B | SD | ß | |
| Callous-Unemotional Traits | 0.86 | 0.60 | 0.10 | |||
| Amygdala Reactivity to Fearful Facial Expressions | ||||||
| Left Amygdala | −0.00 | 0.01 | −0.05 | −0.02 | 0.02 | −0.09 |
| Right Amygdala | 0.00 | 0.01 | 0.07 | 0.00 | 0.02 | 0.01 |
| Amygdala Reactivity to Angry Facial Expressions | ||||||
| Left Amygdala | 0.00 | 0.01 | 0.04 | −0.02 | 0.02 | −0.11 |
| Right Amygdala | .01* | 0.01 | 0.19 | 0.02 | 0.02 | 0.08 |
Note: †p < 0.10, *p < 0.05; **p < 0.01; ***p < 0.001. Relationships between callous-unemotional traits, antisocial behavior, and all amygdala contrasts were estimated simultaneously. Sex, ethnicity, pubertal maturity, age, familial risk for depression, and internalizing symptoms were included as covariates.
2.3. Analysis of fMRI data
Blood oxygen level dependent (BOLD) fMRI data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). BOLD parameter estimates were extracted from functional clusters that were activated within the left or right amygdala at p < 0.05, family- wise error corrected. In our exploratory analyses on distinct functional subregions within the amygdala, we constructed separate ventral (i.e., basolateral complex; BL region) and dorsal (i.e., central nucleus and substantia inominata; CM region) amygdala ROIs as previously described (Amunts et al., 2005, Hyde et al., 2016).
2.3.1. Preprocessing and quality control procedures
Functional images were slice-timing corrected and then realigned to the first volume in the time series. Images were then normalized into standardized Montreal Neurological Institute space and smoothed with a 6 mm FWHM Gaussian filter. The Artifact Detection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) was used to identify images with excessive movement (>2 mm or degrees relative to the previous timeframe) or spiking artifacts (global mean intensity >4 standard deviations from the time series). Participants with >5% of functional images flagged for motion or artifact using ART were excluded from further analyses. For the remaining participants, ART generated a regressor for volumes with high motion or artifact to control for these volumes in analyses.
2.3.2. Exclusion of fMRI data for quality control
Of the 331 participants who underwent the scan, 99 were excluded due to either problems with their fMRI data, or diagnoses of major depressive disorder (MDD) or anxiety (see Supplemental Table 1). Additionally, 12 participants that identified their ethnicity as “Other” were excluded from analyses that compared relationships between Caucasian and Hispanic participants.
2.3.3. Extraction of contrast values
As in prior research, contrast values were extracted from functional clusters within anatomically defined (via the Automated Anatomical Labeling Atlas) amygdala regions of interest (ROIs) exhibiting significant main effects of task (i.e., fearful facial expressions > shapes) at p < 0.05 family-wise error corrected across the volumes of the ROIs. The procedure for extraction of contrast values was identical to that performed in Swartz et al. (2015). BOLD parameter estimates were extracted for the following contrasts: fearful facial expressions > shapes and angry facial expressions > shapes. All participants had >98% BOLD signal coverage in these amygdala ROIs.
2.4. Self-report measures
2.4.1. Pubertal development
Pubertal development was assessed via mean scores derived from two development items from the Tanner scales (Marshall and Tanner, 1969, Marshall and Tanner, 1970). A dichotomous variable was created to distinguish participants with less pubertal development (i.e., below or at Tanner Stage III; mean score ≤ 3, N = 93), from those who were more developed (i.e., above Tanner Stage III; mean score >3, N = 126).
2.4.2. Antisocial behavior
Youth AB was assessed using a sum score from the 62-item Self-Report of Delinquency Questionnaire that assesses frequency of aggressive and delinquent behavior, substance use, and related offenses during the prior year (SRD; Elliott et al., 1985).
2.4.3. Callous-unemotional traits
CU traits were measured through self-report using a sum of five items from the CU factor from the Antisocial Process Screening Device that assesses callousness, lack of empathy, and shallow affect (e.g., “concerned about the feelings of others”) on a 3-point rating scale (APSD; Frick et al., 2000). Consistent with our past work with this measure (Hyde et al., 2015), one item (“hides feelings from others”) did not have a significant loading (ß = −0.09) during a confirmatory factor analysis using WLSMV estimation in Mplus 7.2 (Mplus; Muthén and Muthén, 2014), and thus was excluded from the scale.
2.5. Data analytic strategy
After extracting mean parameter estimates of amygdala reactivity in SPM8, all subsequent analyses were performed in Mplus 7.2. Maximum likelihood with robust standard errors (MLR) estimation was used in all analyses to account for potential non-normality in the behavioral data and to accommodate the missing behavioral data. Simulation studies have shown that full-information maximum likelihood estimation provides unbiased estimates even with up to 50% missing data when data is missing at random (Schafer and Graham, 2002) and thus we included all 220 participants to maximize available data for these individuals. Youth with neuroimaging, but without behavioral data, did not differ in age or familial risk for depression, but displayed trend-level differences in sex and ethnicity, which were thus included as covariates in all analyses (except those examining these variables as moderators). There were no significant differences between groups in other potentially relevant measures (e.g., measures of anxiety and trauma which were not the focus of this study). As maximum likelihood estimation utilizes the information in the entire covariance matrix, we included all covariates and broader measures of anxiety and trauma, to assure that using missing data procedures would result in robust estimates. We therefore also controlled for concurrent internalizing symptoms in all analyses. To account for siblings in the model (18 pairs), we nested individuals within family using the TYPE = COMPLEX command (Muthén and Muthén, 2014).
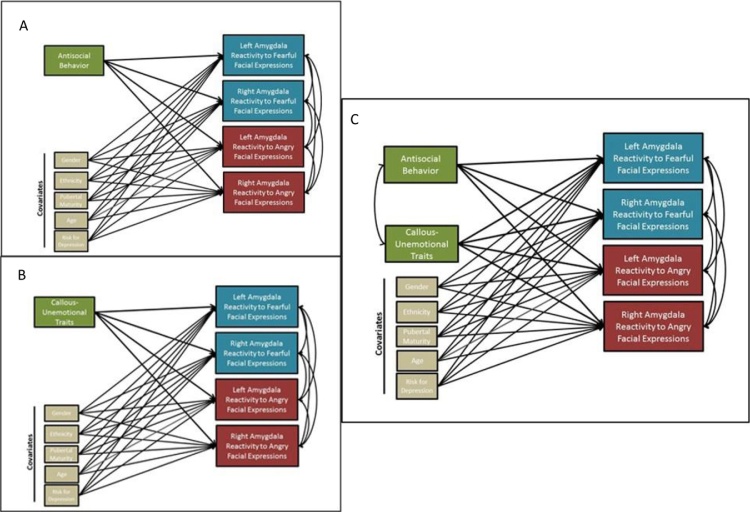
To test our hypotheses, we ran a series of path models to determine associations between AB and CU traits and amygdala reactivity (Fig. 1). First, individual models including covariates (sex, ethnicity, pubertal timing, age, familial risk for depression, and internalizing symptoms), were run that included all amygdala contrasts as outcomes and either AB or CU traits as the independent variable (which leverages a single path model to decrease multiple comparison concerns). Next, we ran identical models that included both AB and CU traits as independent variables to determine if relationships remained when accounting for concurrent AB and CU traits (and to examine for any potential suppression effects; e.g., Hyde et al., 2016, Lozier et al., 2014, Sebastian et al., 2012). The results of the first set of analyses were identical to the results found in our models that controlled for overlap of AB and CU traits, and as we found no evidence of suppression, nor that controlling for the overlap of AB and CU traits affected estimates substantially, we only report the full model with both AB and CU traits included simultaneously. To examine sex, ethnicity, pubertal timing, and age (mean split) as moderators, we ran multi-group models for each amygdala region. Each moderator was analyzed in a separate model. Though not our primary focus, due to half the sample being at high familial risk for depression, we also examined familial risk status as a potential moderator. In all multi-group models, parameters were first constrained to be equal across groups (e.g., boys and girls). Model fit was then compared to the same model when parameters were not constrained to be equal using the Satorra-Bentler scaled χ2 difference test, as is appropriate when using MLR estimation in Mplus 7.2 (Satorra, 2000). Thus, moderation was tested by examining whether the path models fit equally well across groups. To ensure that missing data did not influence the results, we also re-ran all analyses (e.g., zero-order, path models, moderator analyses) when only including participants with both neuroimaging and behavioral data (i.e., list-wise deletion), and the pattern of results was identical (N = 116; see Supplemental Fig. 1). Finally, in an exploratory set of analyses, we re-ran initial models examining AB and CU traits as predictors of the CM region and BLA region contrasts separately.
Fig. 1.
Summary Of Data Analytic Steps In Aim 1. In Step 1 (Panels A and B) separate models were run for antisocial behavior (AB) versus callous-unemotional (CU) traits that included all covariates and all amygdala contrasts. In Step 2 (Panel C) a model was run that included both AB and CU traits, as well as all covariates and all amygdala contrasts.
3. Results
3.1. Task effects
As reported in prior research conducted with the TAOS sample, the emotional face-matching task reliably elicited activation in the whole anatomically-defined amygdala as well as in the BLA and CM sub-regions (Bogdan et al., 2012, Swartz et al., 2015, White et al., 2012a). Whole-brain main effects are also summarized in the supplemental materials (see Supplemental Table 2 and Swartz et al., 2015 for more details).
3.2. Antisocial behavior, callous-unemotional traits, and amygdala reactivity
3.2.1. Path models
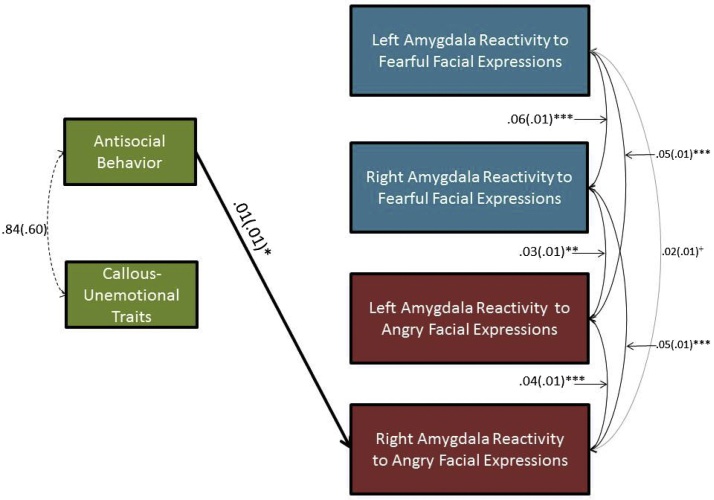
Zero-order correlations are reported in Supplemental Table 3. Consistent with our hypothesis that AB would be associated with increased amygdala reactivity to threat, after accounting for covariates, concurrent CU traits, and all amygdala contrasts, AB was related to increased right amygdala reactivity (B = 0.01, SD = 0.01, ß = 0.20, p < 0.01) to angry facial expressions (Fig. 2, Fig. 3). Unexpectedly, CU traits were not significantly related to amygdala reactivity to either fearful or angry facial expressions. Thus, AB was uniquely related to relatively increased right amygdala reactivity to angry facial expressions, whereas CU traits were not significantly related to amygdala reactivity.
Fig. 2.
Antisocial Behavior (AB) Was Related To Increased Right Amygdala Reactivity Specifically To Angry Facial Expressions In A Sample Of 220 Adolescents. +p < 0.10, *p < 0.05; **p < 0.01; *** p < 0.001. N = 220. Only relationships that were significant (black) or trend-level (grey) are depicted. Sex, ethnicity, pubertal maturity, age, familial risk for depression, and internalizing symptoms were included as covariates.
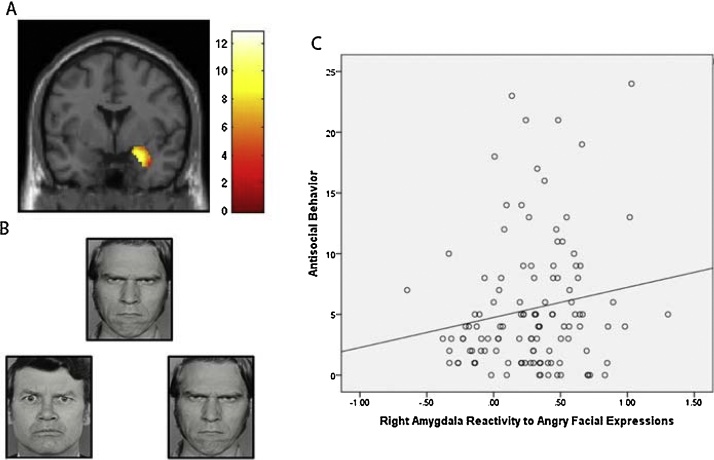
Fig. 3.
Increased Right Amygdala Reactivity To Angry Facial Expressions Was Related To Increased Antisocial Behavior (AB). Panel A: Functional cluster for right amygdala reactivity to angry facial expressions > shapes. The threshold is set at p < 0.05 family-wise error corrected for the right amygdala region of interest. The color bar is showing T-values. Panel B: Example of facial expression affect matching stimuli used in the amygdala reactivity paradigm. Panel C: Zero-order relationship between AB and increased right amygdala reactivity to angry facial expressions in the 116 adolescents who had both neuroimaging and behavioral data.
3.2.2. AB × CU moderation
Our findings diverged from previous reports, as we did not find any significant associations between CU traits and amygdala reactivity. Thus, we also examined AB x CU traits interactions to determine if CU traits are only significantly associated with amygdala reactivity at high levels of AB. We tested both a continuous interaction for AB x CU traits and dichotomous interactions comparing participants with high versus low levels of AB (mean split, low = ≤5.44, N = 79; high = > 5.44, N = 38), as well as high versus low levels of CU traits (mean split, low = ≤2.12, N = 79; high = > 2.12, N = 50). However, we did not find any significant interactions and thus no evidence that CU traits were related to amygdala reactivity at higher (though still subclinical) levels of AB.
3.2.3. Amygdala subregions
In exploratory analyses of amygdala subregional reactivity, we found relationships between AB and amygdala reactivity to angry facial expressions were present within the CM, which is consistent with the two past studies in adults that examined amygdala subregional anatomy (Carré et al., 2013, Hyde et al., 2016). However, this relationship was also present in the BL region. Specifically, AB was related to increased left CM reactivity to angry facial expressions (Supplemental Fig. 2) and increased right BL reactivity to angry facial expressions (B = 0.01, SD = 0.02, ß = 0.14, p = 0.049). Consistent with whole amygdala findings, CU traits were not significantly related to either CM or BL amygdala reactivity.
3.3. Differences in relationships according to sex, ethnicity, pubertal timing and familial risk for depression
To test for moderation by sex, ethnicity, pubertal timing, and age, as well as familial risk for depression, we first started with an omnibus test where we compared a model in which all paths were constrained to be equivalent (e.g., across sex) with one in which they could vary. Examining separate models for each moderator, we found that models did not differ statistically when parameters were fixed across each moderator, indicating that all models generally fit equally across sex, ethnicity, pubertal timing, age and risk status, (i.e., no evidence of moderation of these models by any of these moderators). Using an approach more sensitive to smaller moderation effects, we also fixed and freed each individual path to determine if specific paths differed according to sex, ethnicity, pubertal timing, age, or familial risk for depression. As with the omnibus tests, these individual paths did not differ across levels of any of the moderators (i.e., the relationships were the same regardless of sex, ethnicity, pubertal timing, age, or familial risk for depression). Finally, to confirm that these moderators did not also act in tandem, we conducted exploratory analyses to test for two-way interactions between our main covariates of interest: sex, ethnicity, and pubertal timing (e.g., sex × ethnicity). Though two of these interactions were significant, after applying a Bonferroni correction to address the exploratory nature of these analyses, none of the interactions remained significant (see Supplemental materials). Thus, our findings appeared to generalize across sex, ethnicity, pubertal development, and age. Moreover, familial risk for depression also did not appear to moderate these pathways.
4. Discussion
The present study identified a positive relationship between a dimensional measure of AB, but not CU traits, and amygdala reactivity to angry, but not fearful, facial expressions in an ethnically diverse non-clinical sample of adolescents. The moderating effects of familial risk for depression, ethnicity, sex, pubertal status, and age were further assessed. Analyses revealed that AB was related to increased amygdala reactivity specifically to angry facial expressions, whereas CU traits were not related to amygdala reactivity to fearful or angry facial expressions. In addition, we found that relationships between AB and CU traits and amygdala reactivity to threat and distress generally did not vary across sex, ethnicity, pubertal development, age, or familial risk for depression in a relatively large sample with power to detect these potential differences. These results are important as they demonstrate relationships between AB and increased amygdala reactivity to interpersonal threat cues in a larger sample than past studies of adolescents. Moreover, by testing these relationships dimensionally, the results demonstrate that the relationship between AB and amygdala reactivity to angry faces is present even at normative, subclinical levels of AB.
4.1. Increased AB was associated with increased amygdala reactivity to angry facial expressions
Consistent with theory (Blair et al., 2014), AB was related to relatively increased amygdala reactivity specifically to angry facial expressions. This relationship remained significant while controlling for concurrent CU traits, concurrent internalizing symptoms, and additionally in the smaller sample using list-wise deletion. Our findings thus support the notion that over-reactivity to interpersonal threat, a characteristic behavioral phenotype of AB (Dodge, 1993), could be the result of increased amygdala reactivity to threat-related cues for those high on AB (Blair et al., 2014, Viding et al., 2012). Importantly, these results were specific to angry facial expressions, paralleling studies in adults with impulsive aggression (Coccaro et al., 2007) and emphasizing that these neural differences are specific to interpersonal threat, and not distress, at least at this age and in this sample. In contrast, most prior research in adolescents with AB has identified abnormalities specifically related to processing fearful facial expressions (Lozier et al., 2014, Marsh et al., 2008, Viding et al., 2014). Few studies have actively compared the relationship between AB and amygdala reactivity to angry versus fearful facial expressions, particularly while controlling for concurrent CU traits (though see Fairchild et al., 2014, Passamonti et al., 2010). Moreover, participants were excluded from the current sample if they were diagnosed with an externalizing disorder such as conduct disorder, which contrasts with prior studies that have focused on extreme levels of AB. Additionally, in our analyses we controlled for concurrent internalizing symptoms, demonstrating that amygdala reactivity was specifically associated with AB, even in this sample, half of whom were at higher familial risk for depression. Thus, the use of a dimensional measure of AB in the current sample of adolescents at familial risk for depression demonstrates that these relationships are present across the range of AB and not only present in those with clinically high levels of AB.
4.2. CU traits were not associated with amygdala reactivity to angry or fearful facial expressions
In contrast, we found that CU traits were not generally associated with amygdala reactivity to fearful or angry facial expressions. This result marks a relatively large non-replication of previous findings (Jones et al., 2009, Lozier et al., 2014, Marsh et al., 2008, Viding et al., 2014, White et al., 2012b), though in a very different type of sample and using a slightly different fMRI paradigm. This null finding is consistent with two previous studies that found no relationship between CU traits and amygdala reactivity, in a study of adolescent boys with early and late starting AB (Passamonti et al., 2010) and a study of adolescent girls with conduct disorder (Fairchild et al., 2014). Our finding is also somewhat consistent with our recent work in low-income community adults that also found no relationship between CU traits and amygdala reactivity using the same fMRI task (Hyde et al., 2016). Importantly, the current study represents the first investigation of these relationships in an adolescent sample without clinically diagnosable levels of AB. Thus, while there was a considerable range of scores for both CU traits (0–6 on a scale up to 10) and AB (0–24 on a scale up to 124) in this sample, the mean scores (CU traits = 2.12; SD = 1.61; AB = 5.44; SD = 5.28) were lower than in previously studied samples of clinical populations assessing children with diagnosable AB disorders (Jones et al., 2009, Viding et al., 2014, White et al., 2012b) and our own recent study of adults, described as “at risk” for externalizing disorders and using the same measures (e.g., CU traits = 7.58; SD = 1.70; AB = 62.87; SD = 7.76; though note that this sample was of 20 year old men; Hyde et al., 2016). In contrast, here we examined these relationships in relatively healthy individuals, half of whom had familial risk for internalizing disorders. Thus, it could be that relationships between CU traits and amygdala reactivity to interpersonal distress only emerge when also in the presence of diagnosable levels of AB or might only predict future, longitudinal increases in AB or CU traits. Future studies with a very wide range of AB and CU traits could address this issue and longitudinal studies are needed to examine whether amygdala reactivity to interpersonal threat and distress can predict AB and CU traits over time or even their emergence in the future.
Beyond sample composition, the current study differed from prior research on CU traits specifically in the task used to elicit amygdala reactivity. A majority of previous studies have examined associations between amygdala reactivity and CU traits during an implicit emotional processing task, in which participants were asked to identify the gender of a single actor’s face expressing an emotion (Jones et al., 2009, Lozier et al., 2014, Marsh et al., 2008, Passamonti et al., 2010, White et al., 2012b). In the current study, participants were instead instructed to match one face on the top to an identical face on the bottom. This somewhat more complex task may require greater attention to the faces or parts of the faces that may drive implicit emotion responding (i.e., the eyes). Importantly, another recent study that utilized this same matching task similarly did not find relationships between CU traits and amygdala reactivity (Hyde et al., 2016). These variations in task demand may be important when considering research showing that attention to the eye region during emotional face processing may moderate amygdala effects (Han et al., 2011), such that under-reactivity to emotional faces may stem from less time spent looking at the eyes (Dadds et al., 2012). Thus, if the identity matching of our task (versus gender identification) elicits greater attention to the eyes, this small variation may explain discrepant findings relating CU traits to amygdala reactivity to fearful facial expressions.
Finally, recent studies suggest that the level of attentional load required by imaging tasks may explain differences in findings for amygdala reactivity in relation to CU traits (e.g., Baskin-Sommers et al., 2011). Indeed, White et al. (2012b) found that CU traits were associated with amygdala reactivity, but only under low attention load; that is, when participants were not required by the task to attend to the fearful facial expressions. Thus, our findings may reflect that, rather than specific amygdala deficits (i.e., bottom up under-reactivity to affective cues), CU traits may only be related to amygdala reactivity under low attentional demands and/or when tasks require less attention to the eye and the small variations in task demands (i.e., gender identification of a single face versus matching the identity of two faces) may lead to divergent relationships between CU traits and amygdala reactivity to emotional facial expressions.
4.3. Relationships were similar across sex, ethnicity, pubertal timing, and familial risk for depression
Finally, to our knowledge this is the first study to investigate moderation by sex, ethnicity, pubertal timing, and familial risk for depression between AB and amygdala reactivity. Most previous studies have focused on boys and those identified as non-Hispanic Caucasian and several studies have suggested that “classic” findings regarding AB and CU traits or psychopathy do not replicate in minority groups (e.g., Baskin-Sommers et al., 2011, Lorenz and Newman, 2002). Interestingly, we did not find any moderation effects. In contrast, a recent paper from our group found that race strongly moderated relationships between AB and amygdala reactivity, with associations only significant in African-American participants (Hyde et al., 2016). However, this previous study used an urban sample of young adult men at high sociodemographic risk for externalizing disorders that may have had increased exposure to a higher rate of a broad range of acute and chronic stressors that could differ from the stressors experienced by the present mixed sex sample of adolescents. Thus, these two somewhat conflicting reports suggest further research is needed to disentangle whether differential neural correlates of AB stem from race (versus ethnicity) specifically or the context that participants live and grow up in (Raine, 2002).
Additionally, we found that relationships between amygdala reactivity and AB were similar between adolescents with and without familial risk for depression. These findings suggest that increased familial risk for depression does not alter the relationship between amygdala reactivity and AB, at least prior to the emergence of significant depressive symptoms. That is, these results could suggest that the neural etiology of AB may be similar across those with and without familiar risk for depression, at least during the premorbid period before depression onset. Future research comparing these pathways to neural correlates of late-onset AB in young adulthood with comorbid internalizing is the next step in understanding the ways in which comorbid AB and internalizing disorders develop. However, a clear contribution of the present data are that they show that early in this process, familial risk for depression does not appear to affect amygdala reactivity-AB pathways. Overall, these results, in a relatively large neuroimaging study, suggest that neural pathways to AB in early adolescence may be similar across sex, ethnicity, pubertal timing, and familial risk for depression.
4.4. Limitations
Despite the strengths of this study, including a relatively large and ethnically diverse sample of Hispanic and non-Hispanic Caucasian adolescents, dimensional assessments of AB and CU traits, ability to examine the effect of familial risk for depression on these pathways, and a well-established task for eliciting amygdala reactivity, there are limitations worth noting. First, the validity of self-reported measures of CU traits has been debated in the field, given that deceit and grandiosity are inherent to the construct of CU traits (Lilienfeld, 1994). Thus, it may be that we found few significant relationships with CU traits because of the error inherent in self-reports of CU traits. Moreover, our measure of CU traits was limited, as it only contained 5 items, in contrast to other studies that have begun to use more comprehensive and extensive measures of CU traits, such as the 24-item Inventory of Callous-Unemotional Traits (Frick, 2004). It could be that AB was more strongly related to amygdala reactivity in the present study because our measure of AB was psychometrically stronger. Studies that incorporate parent- and teacher-report or clinical interview of CU traits would provide a stronger test of this model. Additionally, we focused on testing a limited number of path models to simultaneously estimate regressions with multiple predictors and outcomes to decrease concerns about alpha inflation due to multiple comparisons. However, because we did conduct a number of moderation and exploratory analyses (which mostly yielded null findings), significant effects between AB and amygdala reactivity would not survive correction for all of the multiple comparisons examined in this study, and thus should be interpreted with caution. Finally, as we utilized a unique sample including adolescents at high familial risk for depression and excluding adolescents with diagnosable externalizing disorders, these relationships may differ in populations at risk for AB, as well as psychiatric or forensic populations, many of which would also have substantial amount of comorbidity for ADHD. However, studies in samples with lower levels of AB are also essential to identify whether associations are present across the range of AB and CU traits and to understand the emergence of late onset and normative, subclinical presentations of AB comorbid with internalizing disorders.
4.5. Conclusions
In sum, we found that AB was specifically associated with increased amygdala reactivity to angry facial expressions, whereas CU traits were not related to amygdala reactivity to angry or fearful facial expressions. We found evidence that these associations may generalize across sex, ethnicity, pubertal development and age in a select sample of adolescents enriched for familial risk for depression and without diagnosable levels of AB. Further, this relationship was specific to AB, even after accounting for concurrent CU traits and internalizing symptoms. The present study emphasizes the complex nature of threat processing associated with AB and CU traits, and the potential influence of methodology and sample composition on findings. These findings further demonstrate a tractable relationship between AB and amygdala reactivity that informs etiologic models of the development of AB.
Funding
The work was supported by grants to the fourth author by the National Institute on Drug Abuse [R01DA033369, R01DA031579, and R01AG049789]; grants to the fifth author by the National Institute of Alcohol Abuse and Alcoholism (R01AA016274) and the Dielmann Family; and grants to the third author by the National Institute on Drug Abuse (P30DA023026) and the National Institute on Aging (R01AG049789), and Prop. 63, the Mental Health Services Act and the Behavioral Health Center of Excellence at UC Davis.
Conflict of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.02.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- American Psychiatric Association . fifth edition. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5) [Google Scholar]
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N. Cytoarchitectonic mapping of the human amygdala: hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl.) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers A., Curtin J., Newman J. Specifying the attentional selection that moderates the fearlessness of psychopathic offenders. Psychol. Sci. 2011;2:226–234. doi: 10.1177/0956797610396227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Leibenluft E., Pine D.S. Conduct Disorder and callous–unemotional traits in youth. New Engl. J. Med. 2014;371:2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen D.M., Hicks B.M., Krueger R.F., Patrick C.J., Iacono W.G. Continuity and change in psychopathic traits as measured via normal-range personality: a longitudinal-biometric study. J. Abnorm. Psychol. 2006;115:85–95. doi: 10.1037/0021-843X.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R., Williamson D.E., Hariri A.R. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatry. 2012;169(5):515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré J.M., Fisher P.M., Manuck S.B., Hariri A.R. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc. Cogn. Affect. Neurosci. 2012;7(2):213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré J.M., Hyde L.W., Neumann C.S., Viding E., Hariri A.R. The neural signature of distinct psychopathic traits. Soc. Neurosci. 2013;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro E.F., McCloskey M.S., Fitzgerald D.A., Phan K.L. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dadds M.R., Allen J.L., Oliver B.R., Faulkner N., Legge K., Moul C. Love, eye contact and the developmental origins of empathy v. psychopathy. Br. J. Psychiatry. 2012;200:191–196. doi: 10.1192/bjp.bp.110.085720. [DOI] [PubMed] [Google Scholar]
- Dodge K.A. Social-cognitive mechanisms in the development of conduct disorder and depression. Annu. Rev. Psychol. 1993;44:559–584. doi: 10.1146/annurev.ps.44.020193.003015. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Measuring facial movement. Environ. Psychol. Nonverbal Behav. 1976;1(1):56–75. [Google Scholar]
- Elliott D., Huizinga D., Ageton S. Sage Publications; Beverly Hills, CA: 1985. Explaining Delinquency and Drug Use. [Google Scholar]
- Fairchild G., Hagan C.C., Passamonti L., Walsh N.D., Goodyer I.M., Calder A.J. Atypical neural responses during face processing in female adolescents with conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(6):677–687. doi: 10.1016/j.jaac.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E.M., Jones D.E. The high costs of aggression: public expenditures resulting from conduct disorder. Am. J. Public Health. 2005;95:1767–1772. doi: 10.2105/AJPH.2004.061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick P.J., Nigg J.T. Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annu. Rev. Clin. Psychol. 2012;8:77–107. doi: 10.1146/annurev-clinpsy-032511-143150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick P.J., Viding E. Antisocial behavior from a developmental psychopathology perspective. Dev. Psychopathol. 2009;21(04):1111–1131. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- Frick P.J., White S.F. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J. Child Psychol. Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Frick P.J., Bodin S.D., Barry C.T. Psychopathic traits and conduct problems in community and clinic-referred samples of children: further development of the Psychopathy Screening Device. Psychol. Assess. 2000;12:382–393. [PubMed] [Google Scholar]
- Frick P.J. Unpublished Rating Scale. 2004. The inventory of callous-unemotional traits. [Google Scholar]
- Han T., Alders G.L., Greening S.G., Neufeld R.W.J., Mitchell D.G.V. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Soc. Cogn. Affect. Neurosci. 2011;7:958–968. doi: 10.1093/scan/nsr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Herpertz S.C., Huebner T., Marx I., Vloet T.D., Fink G.R., Stoecker T. Emotional processing in male adolescents with childhood-onset conduct disorder. J. Child Psychol. Psychiatry. 2008;49(7):781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Hinshaw S.P., Lahey B.B., Hart E.L. Issues of taxonomy and comorbidity in the development of conduct disorder. Dev. Psychopathol. 1993;5(1–2):31–49. [Google Scholar]
- Hinshaw S.P. Impulsivity, emotion regulation, and developmental psychopathology: specificity versus generality of linkages. Ann. N. Y. Acad. Sci. 2003;1008(1):149–159. doi: 10.1196/annals.1301.016. [DOI] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Hariri A.R. Neuroscience, developmental psychopathology and youth antisocial behavior: review, integration, and directions for research. Dev. Rev. 2013;33:168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Byrd A.L., Votruba-Drzal E., Hariri A.R., Manuck S.B. Amygdala reactivity and negative emotionality: divergent correlates of antisocial personality and psychopathy traits in a community sample. J. Abnorm. Psychol. 2014;123(1):214. doi: 10.1037/a0035467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Burt S.A., Shaw D.S., Donnellan M.B., Forbes E.E. Early starting, aggressive and callous-unemotional? Examining overlap and predictive utility of antisocial behavior subtypes. J. Abnorm. Psychol. 2015;124:329–342. doi: 10.1037/abn0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Murray L., Gard A., Hariri A.R., Forbes E.E. Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young low-income, urban men. Clin. Psychol. Sci. 2016;4(3):527–544. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.P., Laurens K.R., Herba C.M., Gareth J.B., Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am. J. Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kahn R.E., Frick P.J., Youngstrom E., Findling R.L., Youngstrom J.K. The effects of including a callous–unemotional specifier for the diagnosis of conduct disorder. J. Child Psychol. Psychiatry. 2012;53:271–282. doi: 10.1111/j.1469-7610.2011.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U.M.A., Flynn C., Moreci P. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Krueger R.F., Markon K.E., Patrick C.J., Benning S.D., Kramer M.D. Linking antisocial behavior, substance use: and personality: an integrative quantitative model of the adult externalizing spectrum. J. Abnorm. Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld S.O. Conceptual problems in the assessment of psychopathy. Clin. Psychol. Rev. 1994;14:17–38. [Google Scholar]
- Lorenz A.R., Newman J.P. Do emotion and information processing deficiencies found in Caucasian psychopaths generalize to African-American psychopaths? Pers. Individ. Diff. 2002;32:1077–1086. [Google Scholar]
- Lozier L.M., Cardinale E.M., VanMeter J.W., Marsh A.A. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G.V., Reid M.E., Sims C., Kosson D.S. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44(235):291. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Caspi A., Harrington H., Milne B.J. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Dev. Psychopathol. 2002;14(01):179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Jaffee S.R., Kim Cohen J., Koenen K.C., Odgers C.L. Research review: DSM V conduct disorder: research needs for an evidence base. J. Child Psychol. Psychiatry. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol. Rev. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Moul C., Killcross S., Dadds M.R. A model of differential amygdala activation in psychopathy. Psychol. Rev. 2012;119:789–806. doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. Muthén & Muthén; Los Angeles, CA: 2014. Mplus. [Google Scholar]
- Nock M.K., Kazdin A.E., Hiripi E., Kessler R.C. Prevalence, subtypes: and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychol. Med. 2006;36:699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers C.L., Caspi A., Broadbent J.M., Dickson N., Hancox R.J., Harrington H.L. Prediction of differential adult health burden by conduct problem subtypes in males. Arch. Gen. Psychiatry. 2007;64:476. doi: 10.1001/archpsyc.64.4.476. [DOI] [PubMed] [Google Scholar]
- Passamonti L., Fairchild G., Goodyer I.M., Hurford G., Hagan C.C., Rowe J.B. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch. Gen. Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. J. Abnorm. Child Psychol. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Satorra A. Springer; US: 2000. Scaled and Adjusted Restricted Tests in Multi-Sample Analysis of Moment Structures; pp. 233–247. [Google Scholar]
- Schafer J.L., Graham J.W. Missing data: our view of the state of the art. Psychol. Methods. 2002;7(2):147. [PubMed] [Google Scholar]
- Sebastian C.L., McCrory E.J.P., Cecil C.A.M., Lockwood P.L., De Brito S.A., Fontaine N.M. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch. Gen. Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., McCrory E.J., Dadds M.R., Cecil C.A.M., Lockwood P.L., Hyde Z.H. Neural responses to fearful eyes in children with conduct problems and varying levels of callous–unemotional traits. Psychol. Med. 2014;44(1):99–109. doi: 10.1017/S0033291713000482. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Williamson D.E., Hariri A.R. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am. J. Psychiatry. 2015;172(3):276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., Blair R.J.R., Moffitt T.E., Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J. Child Psychol. Psychiatry. 2005;46(6):592–597. doi: 10.1111/j.1469-7610.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- Viding E., Fontaine N.M.G., McCrory E.J. Antisocial behaviour in children with and without callous-unemotional traits. J. R. Soc. Med. 2012;105:195–200. doi: 10.1258/jrsm.2011.110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., Lockwood P.L., Cecil C.A.M., De Brito S.A. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am. J. Psychiatry. 2014;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- White M.G., Bogdan R., Fisher P.M., Munoz K.E., Williamson D.E., Hariri A.R. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity Genes. Brain. Behav. 2012;11(7):869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.F., Marsh A.A., Fowler K.A., Schechter J.C., Adalio C., Pope K. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am. J. Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.