Abstract
The neuronal circuits defined by the axonal projections of pyramidal neurons in the cerebral cortex are responsible for processing sensory and other information to plan and execute behavior. Subtypes of cortical pyramidal neurons are organized across layers, with those in different layers distinguished by their patterns of axonal projections and connectivity. Those in layers 2 and 3 project between cortical areas to integrate sensory and other information with motor areas. Those in layers 5 and 6 also integrate information between cortical areas but also project to subcortical structures involved in the generation of behavior. Recent advances in neuroanatomical techniques allow one to target specific subtypes of cortical pyramidal neurons and label both their inputs and projections. Combining these methods with neurophysiological recording techniques and newly-introduced atlases of the mouse brain provide the opportunity to achieve a detailed view of the organization of cerebral cortical circuits.
Keywords: cerebral cortex, Axonal connections, Cortical neurons
The principal projection neuron type of the cerebral cortex is the pyramidal neuron that releases the excitatory neurotransmitter glutamate. Pyramidal neurons are distributed across all layers of the cerebral cortex with the exception of layer 1, with those in each layer distinguished by their patterns of long range axonal projections (Douglas and Martin, 2004; Harris and Shepherd, 2015). Those in layers 2 and 3 distribute axons principally within the cerebral cortex, while those in layer 5 project more widely. Within each of these layers there are subtypes with distinct patterns of axonal connectivity. Subtypes of layer 2 and 3 neurons, all of which distribute axons principally within the cerebral cortex, may be distinguished by patterns of cortical projections. Layer 5 cortical pyramidal neurons may be further subdivided into those with projections restricted to the cortex and striatum, bilaterally, and those with projections distributed ipsilaterally within the cortex as well as to subcortical targets including the striatum, thalamus, subthalamic nucleus, inferior and superior colliculus as well as other nuclei in the midbrain and brainstem, and to the spinal cord. These latter subtypes are located in deeper layer 5 and may be further subdivided based on the patterns of distribution of collaterals to subcortical structures. Those in layer 6 typically connect intracortically and give rise to axonal projections to the thalamus. In addition to this laminar organization, the cerebral cortex is organized into functional areas associated with specific sensory, motor or multimodal information. A major source of information entering sensory areas arises from thalamic projections to neurons in layer 4, which distribute that information primarily to layers 2 and 3 and to some extent to layer 5. Within each cortical area, pyramidal neurons in layers 2 and 3 connect with deeper layer 5 and 6 pyramidal neurons, whose axonal projections target other cortical and subcortical systems. Long distance axonal projections of pyramidal neurons across cortical layers provide the substrate for integrating information between cortical areas. The pattern of the axonal distribution of upper layer cortical neurons between cortical areas and the pattern of axonal distribution of lower layer cortical neurons that project cortically and subcortically establish coherent neural circuits that process afferent information and control behavior. Although this general architecture has been well described and provides a first-order description of cortical projections, our understanding of the fine details of this circuitry, how it varies across regions, and how it maps on to single neurons remains poorly understood.
Over the past 50 years, advances in neuroanatomical techniques have contributed to our understanding of the organization of cortical circuitry and its connectivity with subcortical systems. While technical innovation has driven our understanding of the general architecture of cortical circuitry throughout this period, the past decade has witnessed an acceleration in the development of neuroanatomical techniques. These advances have not only provided greater insight into the detailed organization of cortical connectivity but have been combined with physiological techniques to directly ascribe specific functions to individual circuit elements. Molecular genetic tools represent one of the most important recent contributions to neuroanatomical methods, and have been utilized, for example, to generate transgenic mouse lines that enable hundreds of neuronal subtypes to be specifically targeted and their roles in brain function to be interrogated (Gong et al. 2007, Gerfen et al., 2013). The continued development and increased utilization of viral vectors expressing fluorescent proteins for anterograde, retrograde, and trans-synaptic tracing (Harris et al., 2012; Wickersham et al., 2007; Wall et al., 2010) are providing ever increasing details about the specificity of inputs to and projections of distinct neuron subtypes.. These and other advances, combined with optogenetic, electrophysiological, and imaging techniques, provide the ability to determine both the identity and specific function of the components of neural circuits as they relate to behavior (Luo et al., 2008). These tools have been effectively exploited to establish databases comprehensively mapping axonal projections across an entire mammalian brain. Such atlases consist of hundreds or thousands of axonal tracing experiments and include the Allen Institute Mouse Connectivity database (Oh et al., 2014), the Mouse Connectome Project (Zingg et al. 2014) and the Mouse Brain Architecture project (Mitra, 2014). Finally, advances in imaging techniques to reconstruct the brain-wide axonal projections of individual neurons provide the ability to map the connections of cortico-cortical and cortico-subcortical circuits at the single neuron level (Economo et al., 2015).
The GENSAT project produced over 200 transgenic mouse lines that used Bacterial Artificial Chromosomes (BAC) to express Cre-recombinase in specific neuronal subtypes (Gong et al., 2007). Initially BAC-Cre lines expressing in the cortex were identified with expression specific to cortical layers 2,3, 5 and 6. To identify different neuron subtypes of these layer specific lines, they were further characterized by injecting an AAV vector encoding a Cre dependent GFP construct, which labeled the axonal projections of Cre-expressing neurons (Gerfen et al., 2013). This study identified BAC-Cre transgenic lines with selective expression in subtypes of pyramidal neurons confined specifically to layers 2,3, 4, 5 and 6. As described above, cortical pyramidal neurons in layers 2 and 3 give rise to axonal projections distributed within the cerebral cortex, while layer 5 is composed of two main subtypes, inter-telencephalic neurons (IT) providing projections bilaterally to the cerebral cortex and striatum, and pyramidal tract neurons (PT) that provide inputs ipsilaterally to the cortex and subcortical structures. Layer 6 neurons provide axonal projections to other cortical areas or to the thalamus (Figure 1). Each BAC-Cre driver line is designated by the specific gene promoter that was targeted to drive Cre expression and the specific founder line that gave rise to selective expression in the specific neuron subtype. For example the BAC-Cre driver line selective for layer 2 and 3 cortical pyramidal neurons is Sepw1-NP39, in which Cre expression is linked to expression of the Sepw1 gene. A number of BAC-Cre driver lines produced selective expression in layer 5 cortical pyramidal neurons. One, Rbp4_KL100, produces Cre expression in all neocortical areas. The axonal projections of Cre-expressing cells in this line are distributed bilaterally to the cerebral cortex and striatum, indicative of expression in IT layer 5 neurons, but also include projections to the thalamus, subthalamic nucleus, superior colliculus, and pontine nuclei, indicative of labeling of PT layer 5 neurons. Thus, this line provides a BAC-Cre driver line that enables targeting of both IT and PT layer 5 neurons. The lines Tlx3_PL56, Ebf2_NP183, and Grp_KH288 produce Cre expression in layer 5 cortical pyramidal neurons whose axonal projections are distributed bilaterally in the cerebral cortex and to the striatum, but not to other subcortical structures, indicating selective labeling of neocortical layer 5 IT neurons. While Tlx3_PL56 and NP183 produce Cre expression in layer 5 IT neurons, this expression is limited primarily to neocortical areas, whereas Grp_KH288 produces Cre expression in peri-allocortical areas, including secondary motor cortex (MOs) and prefrontal areas. The lines Sim1_KJ18, Chrna2_OE25, Efr3a_NO108 and Rcan2_ON50 produce Cre expression in layer 5 cortical pyramidal neurons whose axonal projections distribute ipsilaterally within the cerebral cortex, and in the striatum, thalamus, subthalamic nucleus, superior colliculus, pontine nuclei and brainstem and spinal motor nuclei implying labeling of PT neurons. The line Ntsr1_GN220 produces labeling in layer 6 cortical pyramidal neurons, whose projections are distributed to the thalamus.
Figure 1.
The Major Circuits of the Cerebral Cortex and Basal Ganglia Are Diagrammed in a Sagittal Plane. Corticostriatal inputs arise from two major subtypes: intertelencephalic (IT) corticostriatal neurons, which provide bilateral inputs to the striatum, and pyramidal tract (PT) corticofugal neurons, which project an axon ipsilaterally with collaterals to the striatum, thalamus, subthalamic nucleus (STN), superior colliculus (SC), pons, and spinal cord. Two main subtypes of projection neurons in the striatum give rise to direct and indirect pathways. The direct pathway provides direct projections to the output nuclei of the basal ganglia, the internal segment of the globus pallidus (GPi), and the substantia nigra pars reticulata (SNr). The indirect pathway projects to the external segment of the globus pallidus (GPe), which connects indirectly through the STN to the GPi and SNr. The major output of the basal ganglia originates from GABAergic neurons in the GPi and SNr, which provide inhibitory input to the thalamus, SC, and pedunculopontine nucleus (PPN) From Gerfen et al., 2013.
The GENSAT BAC-Cre driver line resource has been used in numerous studies to identify specific patterns of connectivity in subtypes of cortical pyramidal neurons, to characterize their physiological characteristics within neural circuits and to determine their functional role in behavior. For example, in a study of the visual cortex using these lines, Kim et al. (2015) characterized differences in the axonal projections of three subtypes layer 5 neurons using AAV-GFP vectors to trace anterograde projections and a modified rabies virus to label the inputs to each subtype. This study found that projections from V1 Tlx3_PL56 neurons distribute within V1, adjacent visual cortical and other sensory areas, frontal cortex and to the striatum, typical of IT cortical neurons. Projections of Efr3a neurons distributed axons locally in V1 and in adjacent visual cortical areas but not to the striatum, which distinguishes this subtype from other IT cortical neurons. On the other hand, projections of Glt25d2 neurons distribute axons only locally within VI but not to other cortical areas and project to the striatum, thalamus, superior colliculus and pontine nuclei, typical of PT pyramidal neurons. In addition to divergent axonal projection patterns, these three classes of projection neurons received presynaptic input from distinct populations. Cortical inputs to the Tlx3 and Efr3a V1 neurons arose from other visual cortical areas, other sensory areas, prefrontal, motor, and other associational areas, whereas cortical inputs to Glt25d2 PT V1 neurons originated primarily in retrosplenial and cingulate areas. Additionally, the Glt25d2 PT V1 neurons received a significantly higher proportion of inputs from the thalamus than the Tlx3 and Efr3a V1 neurons. These results illustrate how gene-linked expression of recombinases in specific mouse lines can be used to better define cell types and to define the structure of cortical circuits..
In another study using GENSAT BAC-Cre lines selective for neurons in cortical layer 2 and 3 (Sepw1-NP39), layer 5 IT and PT neurons (Rbp4_KL100) and neurons in layer 6 (Ntsr1_GN220), Denardo et al (2015) provided a comprehensive mapping of the laminar distribution of local and long distance inputs to these populations in the barrel cortex (BF) and medial prefrontal (mPF). While each region exhibited classical connectivity patterns – with deep layer neurons receiving strong input from layer 2 and 3 and layer 2 and 3 neurons in BF receiving input from layer 4 – this study also revealed an additional pathway from superficial to deep layers with layer 6 neurons in BF receiving inputs from layer 3. In addition, this study was able to provide a detailed mapping of the long distance projections from other cortical areas to the different layers of BF. Long distance inputs to BF arose from other S1 areas (body) as well as from S2, M1 and M2. Notably, they observed inputs to L2, L3, L5 and L6 from the postero-medial (VPm) and posteromedial (POm) nuclei of the thalamus, confirming previous suggestions that thalamic input to cortex is not restricted to layer 4. Long-range cortical inputs to BF were organized such that more superficial layers receive inputs from superficially-located neurons in M1 and M2, while deeper layers receive inputs from neurons located in corresponding layers. This pattern of connectivity demonstrates a distinct organization of information flow in feedback pathways to BF. BF and mPF were further found to receive long-range input from divergent cortical areas, as inputs to mPF were shown to arise primarily from other prefrontal areas rather than the somatomotor areas that connected preferentially with BF. Lastly, layer 5 neurons in mPF were found to be connected to a 2.5 fold greater proportion of GABAergic inhibitory neurons. Taken together these data demonstrate that compared to the cortical BF, which is a dedicated circuit integrating sensorimotor information, the mPFC receives inputs from a wider range of cortical areas in order to integrate information from a wide variety of functions related to cognitive function and behavior.
Studies combining molecular genetic neuroanatomical tracing techniques targeted to specific cortical pyramidal neuron subtypes can also provide details of the complex organization of connections between cortical areas. These tools can also be combined with neurophysiology, cellular imaging and optogenetic techniques to illuminate the functional role of identified cell types in the generation of behavior. In one such study from the Svoboda lab, Li et al. (2015) took advantage of a behavioral task in which a sensory cue was used to instruct a mouse to lick one of two lickports following a delay period to study how projection neurons in the anterior lateral motor (ALM) cortex control upcoming movements. Combining anatomical methods to dissociate intermingled IT and PT neurons in layer 5 with eletrophysiological and imaging approaches to characterize the activity of individual neurons during this task, they were able to demonstrate that intermingled populations of neurons coded for each movment direction, PT neurons in particular selectively coded for movements to the contralateral side. This demonstrates that while ALM IT neurons encode preparatory information for different possible movements, activity in ALM PT neurons likely drives the directionality of the movement. This conclusion was tested by selectively activating ALM PT neurons during the delay prior to movement initiation, a manipulation that resulted in movements directed biased to the contralateral direction, as predicted. In contrast, selective activation of IT neurons resulted in movements in both directions. This study demonstrates how novel anatomical tools can be leveraged to untangle the functional roles of parallel projection pathways originating in motor cortex.
In addition to accelerating hypothesis-driven research, technological innovation has enabled the construction of several comprehensive databases of neuroanatomical connectivity of the mouse brain. The Mouse Brain Architecture project directed by Partha Mitra at Cold Spring Harbor (http://www.brainarchitecture.org/projects-2; Mitra, 2014) and Mouse Connectome Project led by Hong-Wei Dong at the University of Southern California (http://www.mouseconnectome.org; Zingg et al, 2014), have each constructed databases of as many as 600 of tracer experiments focused on the mouse cerebral cortex. These efforts utilize classic axonal tracing methods with multiple anterograde (BDA and PHA-L) and retrograde (Fluoro-Gold and cholera toxin) axonal tracers injected into one or more cortical locations to provide a comprehensive mapping of cortico-cortical and corticofugal projections at a population level. The axonal projections and retrograde labeling for each injection case were mapped into the Allen Reference Atlas (Dong et al, 2007). Registration of the cortical connectivity data onto a reference allowed analysis of the organization of patterns of cortical circuits using cluster analysis algorithms. This analysis identified distinct cortico-cortical networks and subnetworks as well as patterns of connectivity between the networks. The somatic sensorimotor network containing connections between sensory and motor areas was shown to include four subnetworks including the orofacialpharyngeal, upper limb, lower limb/trunk and vibrissal. Separte medial visual and auditory networks were shown to have connections with the lower/limb and whisker subnetworks of the distributed somatomotor system. Two distinct networks including insular and temporal cortical areas were identified as well as a network broadly integrating the claustrum and entorhinal cortices.
The Allen Institute Mouse Connectivity Project (Oh et al., 2014) also constructed a database containing the axonal projection patterns resulting from over 1000 tracer injections throughout the brain that are also registered to the same reference space. Axonal projections were visualized using AAV-GFP vectors that labeled projections in wild-type mice as well as Cre-dependent vectors that selectively label the axonal projections of defined cell types when combined with GENSAT and other transgenic Cre lines. In this project, labeled projections were imaged using serial two-photon tomography, which integrates two-photon microscopy (Ragan et al, 2012) with automated vibratome sectioning in order to automatically acquire high resolution images in 100 um intervals across the full extent of the brain. This series of sections were then assembled across each specimen and registered to a consensus reference brain. This consensus, the Common Coordinate Framework, was constructed from the background autofluorescence images acquired from each brain in the full dataset at 10µm resolution. Individual brain structures were then annotated in this reference space using a previously introduced hierarchical ontology (Dong 2007). This dataset is publicly available via a web interface, (http://connectivity.brain-map.org/) and associated Application Programming Interface (API) and is searchable by injection structure, mouse line, projection target, and a number of other parameters.
Having injection cases that span the full extent of the mouse brain that are registered to common reference space allows for analysis of the patterns of brain connectivity. Analysis of all injection cases in the database using cluster analysis algorithms identified the functional subnetworks spanning the cortex, thalamus, hypothalamus, midbrain, and brainstem. Through the Mouse Connectivity API, this database may be queried in order to retrieve individual experiments and images. This data is then available to users for further analysis in standard imaging programs such as ImageJ (FIJI) or ITKsnap. For example, injections into different parts of the somatosensory cortex, the barrel field, upper and lower limb and mouth areas may be viewed in a single image volume to map the topographic organization of projections into the striatum, thalamus, superior colliculus, and pontine nuclei (Figure 3). The ability to view the brain in multiple planes (horizontal, coronal and sagittal) allows the topographic organization of these projections to be fully explored.
Figure 3.
Injection cases into 5 sites in the primary somatosensory cortex from the Allen Institute Mouse Connectivity Database and their axonal projections are mapped in the Common Coordinate Framework mouse reference atlas. A) Injections of 5 sites in the primary somatosensory cortex are color coded with sites in the mouth region (blue), upper limb (red), lower limb (gree) and two sites in the barrel field (yellow and purple). B) In a horizontal plane the projections within the cortex are shown to project to frontal motor areas. C and D) In the sagittal plane the projections from the cortex are shown to project through the striatum to the thalamus and superior colliculus. In the coronal plane (E to J) the topographic relationship of the somatotopically organized primary sensory cortex is shown to be maintained in the axonal projections to the striatum (in sections E and F and at higher magnification in F”), to the thalamus ( G and H and at higher magnification in H”) and to the pontine nucleus (I and at higher magnification in I”).
The Mouse Connectome Project used bulk injections of axonal tracers that label multiple neurons of all types at the injection site, whereas the Allen Institute Mouse Connectivity Project utilized techniques that also labeled multiple neurons at the injection site but labeled specific subtypes of cortical pyramidal neurons using Cre-driver lines. As discussed above, cortical pyramidal subtypes in layers 2 and 3, layer 5 IT and PT, and layer 6 neurons have distinct patterns of axonal projections, both in terms of their distribution of projections within the cortex and to subcortical structures, but also in terms of their laminar distributions within the cortex. These distinct patterns of axonal distribution are important in fully understanding the organization of cortical circuits. While the ability to target specific cortical pyramidal subtypes with Cre-driver lines provides the ability to map the patterns of connectivity of these populations, the labeling of axonal projections of individual, similarly-classified neurons has identified substantial diversity. For example, in the somatosensory cortex, individual neurons in layers 2 and 3 have been shown to project axons either to motor areas or to secondary somatosensory areas (Yamashita et al., 2013). Similarly, in the visual cortex different neurons in layer 5 have been shown to project to distinct secondary visual areas (Glickfeld et al., 2013). In each example, neurons projecting to different cortical areas encoded specific features of sensory stimuli or behavioral tasks demonstrating that parallel output pathways are embedded within intermingled cortical populations. Similarly, although the set of target structures receiving connections from populations of layer 5 pyramidal tract neurons has been well described, a surprising diversity has been revealed in the projection patterns of individual PT neurons as well. Tracing the axonal projections of single cells, Kita (2012) demonstrated that some PT neurons in secondary motor areas have axon projections to all of these areas, whereas others have axonal projections directed to specific subsets. Classifying subtypes of projection neurons based on their patterns of axon collaterals and determining how these subtypes vary across cortical areas requires tracing the axonal projections of many individual neurons across cortical areas.
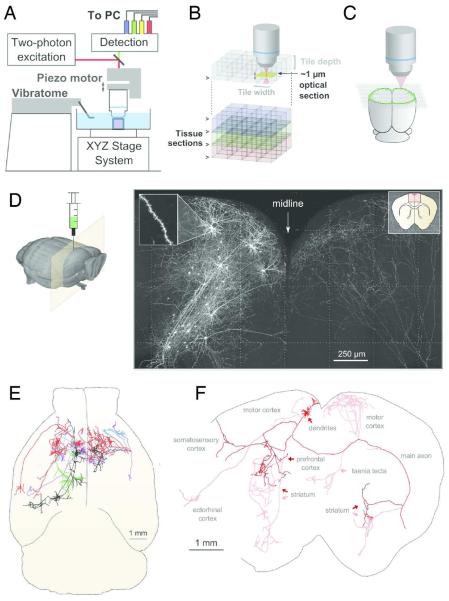
These and other studies highlight the necessity of describing projection patterns at the level of single neurons in order to better understand how information is represented and communicated between brain regions. However, reconstructing the brain-wide axonal projections of single neurons is technically challenging and has thus far been limited to a few tens of neurons in the most extensive studies. Several technologies have been recently introduced that aim to enable single-cell axonal projections of larger populations (Xiong et al., 2014, Economo et al. 2016). The MouseLight Project at the Howard Hughes Medical Institute Janelia Research Campus seeks to produce a database of single-cell projections analogous to that of the Mouse Connectome Project and Allen Institute Mouse Connectivity Project (Economo et al., 2016). In this approach, neurons and their axonal projections are sparsely labeled using genetic methods, whole-brain samples are cleared using a novel, stable immersion medium, and the entire brain volume imaged at high resolution using block face two-photon microscopy (Figure 4a). The result of this procedure is a continuous, three-dimensional image volume in which every location in the mouse brain is captured at submicron resolution with high quality. At this resolution, each whole-brain dataset is comprised of approximately 5 teravoxels of image data, necessitating efficient tools for visualization, annotation, and analysis. Similar to software packages developed for browsing large-scale electron microscopy datasets (ie, CATMAID, Saalfeld et al., 2009), the Janelia Workstation enables navigation through and annotation of large scale 3D fluorescence images. The full arbors of the long-range projections of single motor cortical pyramidal neurons were reconstructed using this approach (Figure 4), demonstrating its effectiveness. In Figure 4, the tracing of neurons in an individual brain are shown and the axonal projections of a single IT pyramidal neuron is depicted in a flattened coronal plane. The axonal projections of this layer 5 IT pyramidal neuron in the secondary motor cortex is typical of this neuron type, extending an axon collateral locally around the parent neuron and ipsilaterally to the somatosensory and ectorhinal cortex, contralaterally to the secondary motor cortex, bilaterally to multiple regions of the striatum and to the tenia tecta. Thus this single IT neuron distributes axon collaterals to 10 or more long-range targets. These advances in imaging techniques, combined with advances in automatic image segmentation and annotation of neuroanatomical data (Peng et al., 2015) are now making it possible to investigate projection pathways in the brain at the single-neuron level.
Figure 4.
The Janelia Research Mouse Light Project to trace axonal projections of individual neurons. A) Schematic of the apparatus used for automated volumetric two-photon tomography. B) To image the whole mouse brain volume a collection of three-dimensional image staces covering the full volume of the mouse brain are acquired serially. Tiles are overlapped in all three dimensions aid in image registration. C) The area of the brain for each section is imaged by first tracing the outline of the section and then imaging all tiles internal to the traced region. D) Diagram of the injection of AAV-GFP vector into the cortex and resulting sparse labeling of neurons imaged. E) Top down view of axonal tracing of neurons, including pyramidal neurons in layer 2 (blue,purple), layer 5 ( red, black) and layer 6 (green). F) Illustration of axonal and dendritic reconstruction of one layer 5 IT neuron (red) shown in the coronal plane collapsed in the z-axis. Adapted from Economo et al., 2016.
The neuroanatomical techniques now available for mapping the axonal projections of cortical pyramidal neurons provide the unprecedented ability to analyze the detailed organization of cortical circuits responsible for the generation of behavior. Techniques utilizing transgenic Cre driver lines provide the ability to target specific cortical pyramidal neuron subtypes both for analyzing cortical and subcortical neuroanatomical circuits and for analyzing their functional roles in behavior. Databases of the axonal projections of hundreds of injection cases in the cerebral cortex, registered to a reference atlas provide comprehensive mapping of cortical and subcortical projections and enable quantitative descriptions of the organization of these circuits. Many decades of research have found that within a given cortical area, intermingled neurons code for distinct aspects of sensory stimuli, movements, and other behaviorally-relevant variables. Recent studies have established that these distinct patterns of activity are often related to specific patterns of axonal projections so that specific information streams may be transmitted to distinct targets (Glickfeld et al., 2013; Yamashita et al., 2013). Thus, the ability to map the axonal projections of hundreds of individual cortical pyramidal neurons offers the promise of establishing the details of information processing within cortical circuits responsible for the generation of behavior. To date, databases providing information on the axonal projections of populations of cortical neurons have enjoyed great success in furthering our understanding of neural circuit organization. Efforts such as the Janelia MouseLight Project hope to enable the construction of comparable databases of neuronal connectivity at an unprecedented level of precision.
Figure 2.
BAC-Cre driver lines from the GENSAT project with selective expression in layer and subtype specific cortical pyramidal neurons. Selective Cre-expression is demonstrated by crossing to a Rosa26_EGFP reporter that labels pyramidal neurons in specific cortical layers. Injections of AAV-Cre-dependent EGP vectors into the cortex label the axonal projections of the specific cortical neuron subtype. The Cre line, Sim1_KJ18 expresses in layer 5b pyramidal tract (PT) neurons, which have axonal projections to the ipsilateral striatum (caudate-putamen, CP), the thalamus, subthalamic nucleus (STN), superior colliculus (SC) and pontine nuclei (pons). The Cre line, Tlx3_PL56 expresses in layer 5a inter-telencephalic (IT) pyramidal neurons, which have axonal projections bilaterally within the cortex and the striatum (CP), but do not project to other subcortical structures. The Cre line, Sepw1_NP39, expresses in layer 2/3 pyramidal neuron, which also project bilaterally within the cortex and to the striatum (CP), but not to other subcortical structures. The Cre line Grp_KH288 expresses in layers 2/3 and 5a of secondary motor cortex (MOs) pyramidal neurons, which express bilaterally to the cortex and striatum (CP), but not to other subcortical structures. (From Gerfen et al.,2013)
SIGNIFICANCE STATEMENT.
The neuronal circuits defined by the axonal projections of pyramidal neurons in the cerebral cortex are responsible for processing sensory and other information to plan and execute behavior. Recent advances in neuroanatomical techniques allow one to target specific subtypes of cortical pyramidal neurons and label both their inputs and projections. Combining these methods with neurophysiological recording techniques and newly-introduced atlases of the mouse brain provide the opportunity to achieve a detailed view of the organization of cerebral cortical circuits.
Acknowledgments
Support:
CRG: NIMH IRP (MH002497-25) National Institute of Mental Health
MNE and JC: Howard Hughes Medical Institute
References
- Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013 doi: 10.1126/science.1236425. (1) 341-1591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denardo LA, Berns BS, DeLoach K, Luo L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-snyaptic tracing. Nature Neuroscience. 2015;18:1687–1697. doi: 10.1038/nn.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W. The Allen Reference Atlas: A Digital Color Brain Atlas Of The C57BL/6J Male Mouse. John Wiley and Sons, Inc.; Hoboken: 2007. [Google Scholar]
- Douglas RJ, Martin KAC. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Economo MN, Clack NG, Lavis LD, Gerfen CR, Svoboda K, Myers EW, Chandrashekar J. A (2016)platform for brain-wide imaging and reconstruction of individual neurons. Elife. 2016 Jan 20;5:e10566. doi: 10.7554/eLife.10566. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC Cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfield LL, Andermann ML, Bonin V, Reid RC. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nature Neuroscience. 2013;16:219–226. doi: 10.1038/nn.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting CRE recombinase to specific neuron populations with Bacterial Artificial Chromosome constructs. Journal of Neuroscience. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Li N, Huber D, Pphir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of cortical activity underlying a tactile decision in mice. Neuron. 2014;81:179–194. doi: 10.1016/j.neuron.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Oh SW, Zeng H. Adeno-associated viral vecotrs for anterograde axonal tracing with fluorescent proteins in nontransgenic and Cre drive mice. Curr. Protoc. Neurosci. 2012;59:1.20.1–1.20.18. doi: 10.1002/0471142301.ns0120s59. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Juavinett AL, Kyubwa EM, Jacobs MW, Callaway EM. Three Types of Cortical Layer 5 Neurons That Differ in Brain-wide Connectivity and Function. Neuron. 2015;88:1253–67. doi: 10.1016/j.neuron.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci. 2012;32:5990–9. doi: 10.1523/JNEUROSCI.5717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Chen TW, Guo Z, Gerfen CR, Svoboda K. Flow of information in motor cortex circuit driving voluntary movement. Nature. 2015;519:51–6. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic Dissection of Neural Circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Shepherd GMG. The neocortical circuit: themes and variations. Nature Neuroscience. 2015;18:170–181. doi: 10.1038/nn.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP. The circuit architecture of the whole brain a the mesoscopic scale Neuron. 2014;83:1273–1283. doi: 10.1016/j.neuron.2014.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–14. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Hawrlycz M, Roskams J, Hill S, Spruston N, Meijering E, Ascoli GA. BigNeuron: Large-scale 3D neuron reconstruction from optical microscopy images Neuron. 2015;87:252–256. doi: 10.1016/j.neuron.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J, Arganda-Carreras I, Kim Y, Seung HS, Osten P. Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat Methods. 2012;9:255–8. doi: 10.1038/nmeth.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfeld S, Cardona A, Hartenstein V, Tomančák P. CATMAID: collaborative annotation toolkit for massive amounts of image data. Bioinformatics. 2009;25:1984–1986. doi: 10.1093/bioinformatics/btp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl. Acad. Sci. USA. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–47. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Pala A, Pedrido L, Kremer Y, Welker E, Petersen CC. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron. 2013;80:1477–90. doi: 10.1016/j.neuron.2013.10.059. [DOI] [PubMed] [Google Scholar]
- Xiong H, Zhou Z, Zhu M, Lv X, Li A, Li S, Li L, Yang T, Wang S, Yang Z, Xu T, Luo Q, Gong H, Zeng S. Chemical reactivation of quenched fluorescent protein molecules enables resin-embedded fluorescence microimaging. Nat Commun. 2014;5:3992. doi: 10.1038/ncomms4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Hintiryan H, Gou L, Song MY, Bay M, Bienkowski MS, Foster NN, Yamashita S, Bowman I, Toga AW, Dong HW. Neural Networks of the Mouse Neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]