Abstract
Acrolein is one of the most toxic byproducts of lipid peroxidation, and it has been shown to be associated with multiple pathological processes in trauma and diseases, including spinal cord injury, multiple sclerosis, and Alzheimer’s disease. Therefore, suppressing acrolein using acrolein scavengers has been suggested as a novel strategy of neuroprotection. In an effort to identify effective acrolein scavengers, we have confirmed that dimercaprol, which possesses thiol functional groups, could bind and trap acrolein. We demonstrated the reaction between acrolein and dimercaprol in an abiotic condition by Nuclear Magnetic Resonance (NMR) spectroscopy. Specifically, dimercaprol is able to bind to both the carbon double bond and aldehyde group of acrolein. Its acrolein scavenging capability was further demonstrated by in vitro results that showed that dimercaprol could significantly protect PC-12 cells from acrolein-mediated cell death in a dose dependent manner. Furthermore, dimercaprol, when applied systemically though intraperitoneal injection, could significantly reduce acrolein contents in spinal cord tissue following a spinal cord contusion injury in rats, a condition known to have elevated acrolein concentration. Taken together, dimercaprol may be an effective acrolein scavenger and a viable candidate for acrolein detoxification.
Keywords: oxidative stress, acrolein, dimercaprol (BAL)
Brief description
Acrolein is one of the most toxic byproducts of lipid peroxidation, and it has been shown to be associated with multiple pathological processes in trauma and diseases. Therefore, suppressing acrolein using acrolein scavengers has been suggested as a novel strategy of neuroprotection. We have shown that Dimercaprol is capable of forming acrolein-dimercaprol adduct based on nuclear magnetic resonance spectroscopy. Dimercaprol mitigates acrolein-mediated PC-12 cells toxicity and reduces acrolein accumulation in rat following spinal cord injury. Dimercaprol may be an effective acrolein scavenger and a viable candidate for acrolein detoxification.
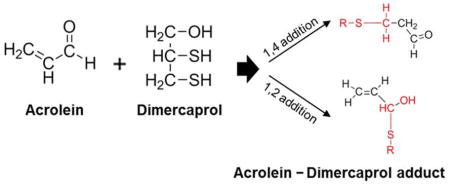
1. Introduction
Increasing evidence suggests that acrolein (2-propenal), a known product of free radical-induced lipid peroxidation (LPO), is a critical factor in perpetuating oxidative stress (Shi & Luo 2006, Shi et al. 2011a, Stevens & Maier 2008, Park et al. 2014a, Hamann & Shi 2009). Compared to other LPO-produced aldehydes such as 4-hydroxynonenal (HNE), acrolein reacts 110–150 times faster with glutathione than HNE (Esterbauer et al. 1991, Uchida 1999), and it can persist in vivo for days (Ghilarducci & Tjeerdema 1995), which is many orders of magnitude longer than the half-life of transient ROS. Furthermore, acrolein is also an instigator of LPO, capable of perpetuating oxidative stress through self-reinforcing positive feedback by direct and indirect mechanisms (Adams & Klaidman 1993, Luo et al. 2005a, Luo & Shi 2004, Luo & Shi 2005, Hamann et al. 2008a).
An extensive body of evidence exists suggesting the toxic nature of acrolein and its pathological role in a variety of disease processes, prompting the use of acrolein scavengers as a new therapeutic approach for alleviating symptoms and curtailing tissue damage in neuropathic disorders (Leung et al. 2011, Hamann & Shi 2009, Liu-Snyder et al. 2006, Park et al. 2014b, Burcham et al. 2000, Burcham & Pyke 2006, Due et al. 2014, Park et al. 2014a, Chen et al. 2016).
Previous studies have shown that acrolein levels increase significantly after spinal cord injury (SCI) (Luo et al. 2005b, Park et al. 2014b, Park et al. 2015, Due et al. 2014). Acrolein may be a key factor in secondary injury, which can expand the damage to adjacent tissues (Hamann & Shi 2009, Shi et al. 2011a, Park et al. 2014a). This results from acrolein’s capacity for destroying biomacromolecules (Kehrer & Biswal 2000, Stevens & Maier 2008), poisoning mitochondria (Luo & Shi 2005), compromising the integrity of neuronal membranes, and degrading myelin (Shi et al. 2002, Shi et al. 2011b, Luo & Shi 2004, Shi et al. 2015). Treatments targeting acrolein may be a promising strategy for alleviating post-SCI neurodegeneration (Hamann & Shi 2009, Park et al. 2014a).
To date, the most common acrolein scavengers have been the FDA-approved compounds containing a hydrazine group, such as hydralazine and phenelzine (Hamann et al. 2008b, Burcham et al. 2000, Liu-Snyder et al. 2006, Park et al. 2014b, Kaminskas et al. 2004b, Chen et al. 2016). However these compounds have potential inherent undesirable side effects when used in high concentrations (Khan 1953, Reece 1981), prompting the investigation of alternative pharmaceuticals, perhaps also FDA-approved medications, that can be repurposed to scavenge acrolein with increased efficacy and reduced risk of side effects. In this regard, the facile reactivity with unsaturated aldehydes makes thiols an attractive candidate for a new generation of acrolein scavengers (Zhu et al. 2011).
Dimercaprol, also called 2,3-dimercaptopropanol or British anti-Lewisite (BAL), was developed as an antidote for lewisite (a now-obsolete arsenic-based chemical warfare agent) by British biochemists during World War II (Peters et al. 1945). Currently, it is primarily used to treat arsenic, mercury, gold, lead, antimony, and other toxic metal poisoning (Oehme 1972). In addition, it is also used for the treatment of Wilson’s disease, a genetic disorder in which the body retains copper (Denny-Brown & Porter 1951). One important feature of this compound is that it possesses two thiol groups, each capable of binding with acrolein to create a less reactive adduct (Carleton et al. 1946). This suggests that dimercaprol could potentially serve as an effective candidate for pharmacological detoxification of acrolein in vivo. However, the direct chemical reaction between dimercaprol and acrolein or the capability of dimercaprol to reduce acrolein-mediated cell death has not been examined.
This study aimed to determine whether dimercaprol is able to react with acrolein and mitigate its toxicity and therefore reduce acrolein-mediated cell death. In addition, it is of great interest to determine whether dimercaprol could function as effectively as established acrolein scavengers, such as hydralazine, which relies on its hydrazine group to bind to acrolein (Burcham et al. 2000, Galvani et al. 2008). We first verified the reaction between acrolein and dimercaprol in an abiotic, or cell free condition using NMR Spectroscopy. Subsequently, in vitro tests using a well-established neuronal PC-12 cell tissue culture were conducted where WST-1, LDH and Trypan Blue assays could be applied effectively to evaluate the ability of dimercaprol to mitigate acrolein-mediated cell death.
Our data has clearly shown that dimercaprol is capable of binding to acrolein through both of its thiol groups based on NMR evaluation. Furthermore, cell culture tests indicate that dimercaprol could greatly reduce acrolein-mediated cell death in a dose-dependent manner, likely by directly binding to and neutralizing acrolein. Finally, we also show that dimercaprol could effectively reduce acrolein-adduct in spinal cord tissue following spinal cord injury in rat. These initial results suggest that dimercaprol is an effective acrolein scavenger, capable of offering neuroprotection in vitro, and likely in vivo as well.
2. Materials and Methods
2.1 Chemicals
Acrolein, Lactate Dehydrogenase (LDH) assay kit and Trypan Blue solution were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dimercaprol and WST-1 assay kit were purchased from Alfa Aesar (Ward Hill, MA, USA) and Roche (Indianapolis, IN, USA), respectively. Cell culture media and reagents were received from American Type Culture Collection (ATCC, Manassas, VA, USA) and Atlanta Biologicals (Flowery Branch, GA, USA). Other routine laboratory reagents were bought from Sigma-Aldrich (St. Louis, MO, USA).
Bio-Dot SF Microfiltration Apparatus was from Bio-Rad (Hercules, CA, USA). Ripa buffer was obtained from Thermo Fisher Scientific In. (Waltham, MA, USA). Rabbit anti-acrolein primary antibody was purchased from ABCAM (Cambridge, MA, USA). Casein solution, goat anti-rabbit secondary antibody and ABC-AmP reagent were from Vector Laboratories (Burlingame, CA, USA).
2.2 NMR Spectrum for Testing Chemical Reaction
The nuclear magnetic resonance (1H-NMR) spectra were recorded using a Bruker ARX300 spectrometer (300 MHz) with a QNP probe. The acrolein solution was prepared by dissolving acrolein (5 uL) in DMSO-d6 (1 mL) and the NMR spectrum for acrolein was recorded. Then, a solution of dimercaprol (50 mg) in DMSO-d6 (1 mL) was added. The mixture was thoroughly shaken and allowed to incubate for 1 hr in the dark and then the NMR spectrum for the reaction products was recorded.
2.3 Cell Culture
PC-12 cells were obtained from American Type Culture Collection (ATCC). Cell medium was made with 85% ATCC-formulated RPMI-1640 Medium (Catalog No. 30-2001), 10% horse serum and 5% fetal bovine serum. PC-12 cells were placed in 100 mm cell culture dishes in an incubator which was set at 37°C under a humidified atmospheric condition of 5% CO2 and 95% air. PC-12 cells were suspended in Krebs-Ringer solution (125 mM NaCl, 5mM HEPES, 6 mM glucose, 5 mM NaHCO3, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 2.4 mM CaCl2, PH7.4) before use, then centrifuged and made to desired concentration by cell medium.
2.4 WST-1 Cell Viability Assay
Cell viability was tested by WST-1 ([2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium]) assay. This assay is based on the enzymatic cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases present in viable cells. The amount of formazan produced represents the quantity of viable cells. This assay can be used not only for quantifying cell proliferation, but can also be employed for cytotoxicity measurement.
Both dimercaprol and acrolein were dissolved in Hank’s Balanced Salt Solution (HBSS). The final concentration of acrolein was 100 μM. The final concentrations of dimercaprol were 10, 25, 50 and 100 μM. PC-12 cells were seeded in 12-well plates at the concentration of 105 cells/well. After overnight incubation, acrolein was added into each well of acrolein group and treatment groups. Equal volumes of HBSS were added into wells of the negative control group. After incubation for 15 minutes, dimercaprol was added at the desired final concentrations (10, 25, 50 and 100 μM) into wells of treatment groups and equal volume of HBSS was added into other wells. The initial 15-minute incubation in acrolein was designed to mimic pathological conditions by allowing acrolein to enter the cells. WST-1 assay solution was added into every well after incubating 4 hrs, and then allowed to incubate for an additional 2 hrs. The resulting absorbance value was obtained using a spectrophotometer at 450 nm, which was further subtracted the background absorbance at 620 nm to derive the final value.
2.5 Lactate Dehydrogenase Assay for Cell Membrane Integrity
Lactate dehydrogenase (LDH) assay quantifies the amount of cytoplasmic LDH released into culture medium to ascertain the degree of cell membrane damage. LDH is an oxidoreductase enzyme that catalyzes the interconversion of pyruvate and lactate. Cells release LDH after tissue damage or red blood cell hemolysis. Since LDH is a fairly stable enzyme, it has been widely used to evaluate the presence of damage and toxicity of tissue and cells. The amount of LDH in the cell and the medium was measured fluorometrically as a function of the reduction of NAD by LDH to form a tetrazolium dye. The amount of product can be measured spectrophotometrically at 492 nm (SLT, Spectra). The background absorbance was measured at 660 nm and subtracted from the reading at 492 nm. A total amount of 2.5×105/well cells were seeded in 1.5 mL microcentrifuge tubes. Acrolein was added so that the final concentration was 100 μM in both acrolein and acrolein plus dimercaprol groups. The maximal cellular LDH value (LDHm) was obtained using a lysis solution that resulted in the release of all the LDH within cells. The value of background LDH (LDHb) released to the media in healthy cells was also obtained with normal uninjured cells. After acrolein exposure for 15 minutes, different concentrations of dimercaprol were added into dimercaprol-treated groups. In order to evaluate the cytotoxicity of dimercaprol, cells which were not exposed to acrolein were incubated in 50 μM or 100 μM dimercaprol for 4 or 24 hrs. The final absorbance was read and the percentages of cytotoxicity were calculated as follows:
2.6 Trypan Blue Assay for Direct observation of Cell Membrane Damage
Trypan blue is a vital stain used to identify dead tissues and cells. Live tissues and cells with intact cell membranes cannot be colored by trypan blue, because cells are very selective in the compounds that pass through the membrane. Trypan blue is a ~960 Daltons molecule that is cell membrane impermeable and therefore only enters cells with compromised membranes. After entry into the cells, trypan blue binds to intracellular proteins thereby rendering the cells a bluish color. All the cells blued by the dye were identified as dead cells, while non-stained cells were considered viable. For evaluation of the anti-acrolein effect of dimercaprol, cell suspensions (0.5 mL, 5×105 cells/mL in cell medium) were treated with acrolein and dimercaprol as stated in LDH assay. In order to determine the cytotoxicity of dimercaprol itself, the same procedures were performed but different concentrations of just dimercaprol were added. Samples were then mixed thoroughly with an equal volume (or other unit) of 0.4% tryptan blue solution and allowed to sit at room temperature for 2 minutes. A volume of 10 uL of each mixture was extracted to fill a hemocytometer on each side. The numbers of stained cells and non-stained cells were counted under the light microscope. The total number of cells of each group was the addition of stained and non-stained cells in that group. The viability percentage of each group was calculated as below:
The viability percentages were averaged by duplicate readings from both side of the hemocytometer.
2.7 Cell Morphology
For cell morphology study, cells were seeded in collagen-coated 12-well plates at a concentration of 2×106 cells/well and allowed to settle for 1 hr. The procedures for treating acrolein and dimercaprol were the same as trypan blue assay except for a 24 hrs incubation time after the applications of all drugs, in order to show a clear difference. Cell samples were mixed thoroughly with an equal volume (or other unit) of 0.4% trypan blue solution and allowed to sit at room temperature for 2 minutes before the liquid was removed by suction from each well. The images of the culture were recorded with a digital camera on a microscope with a 10X objective.
2.8 Rat Spinal Cord Contusion Injury Model
Male Sprague–Dawley rats with a body weight between 200 and 250 g (7–8 weeks old) were used at the time of surgery. Rats were obtained from Harlan Laboratory (Indianapolis, IN, USA). The animals were housed and handled in compliance with the Purdue University Animal Care and Use Committee guidelines and ARRIVE guidelines. The institutional approved protocol number for this study is 1111000095. The animals were acclimated for at least 1 week before surgery.
Rats were anesthetized by intraperitoneally (IP) injecting a mixture with ketamine (80 mg/kg) and xylazine (10 mg/kg). Lack of withdrawal response to a foot pinch was considered as complete anesthesia. Rat’s dorsal surface of spinal cord at the T-10 spinal level was exposed by a dorsal laminectomy. A New York University (NYU) impactor was used to induce contusion SCI model. A 10-gram rod was dropped from 25 mm onto the intact dura mater to generate a moderate contused SCI model. Sham animals only suffered a laminectomy at the T-10 vertebra without a spinal cord contusion. After surgery, Ketoprofen (5 mg/kg) was subcutaneously injected to provide anti-inflammatory, analgesic and antipyretic effects. A dosage of 3.0 mL of saline was administrated via subcutaneous injections to prevent dehydration. The animals were placed on a heating pad in order to maintain body temperature until consciousness was regained. For post-surgical care, bladder checks were performed to SCI rats twice a day. Manual expression of the bladder was performed, if needed, until the animal regained a reflexive bladder.
2.9 Dimercaprol Treatment
A dosage of 5 mg/kg dimercaprol was chosen to reduce acrolein levels in rats. Dimercaprol was dissolved in saline and administered through a daily IP injection for 48 hrs in injured rats, starting immediately (within 5 min) after SCI. Rats were euthanized 4 hrs after the last treatment. In addition, dimercaprol was also applied daily in a similar manner to normal rats for 6 consecutive days to assess its general toxicity.
2.10 Isolation of Spinal Cord
The animals were anesthetized with an IP injection of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). When deeply anesthetized, they were perfused with oxygenated Kreb’s solution (all in mM): 124 NaCl, 2 KCl, 1.24 KH2PO4, 26 NaHCO3, 10 ascorbic acid, 1.3 MgSO4, 1.2 CaCl2, and 10 glucose. The whole vertebral column was rapidly removed and a dorsal laminectomy was performed along the vertebral column. The spinal cord was removed and 1 cm sections were cut out around injury site for acrolein concentration determination.
2.11 Dot Immunoblotting
The extracted spinal cord segments were incubated with Ripa buffer with 0.1% protease inhibitor and then homogenized. The solution was centrifuged at 13,500 g for 30 min at 4°C after incubation on ice for at least 1 hr. The samples were then stored at −80°C before performing experiments and could be kept for up to 2 weeks. An additional round of centrifugation was performed after removing samples from storage at −80°C. A bicinchoninic acid protein assay was performed to ensure equal loading for all samples. A quantity of 200 μg of each sample was transferred to a nitrocellulose membrane using a Bio-Dot SF Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA), The membrane was then blocked for 1 hr in 1X casein solution, and then transferred to 1 : 1000 primary rabbit anti-acrolein antibody in 1X casein solution and incubated overnight at 4°C. After the primary antibody incubation, the membrane was washed with 1X casein buffer. The membrane was then transferred to 1 : 1000 secondary alkaline phosphatase conjugated goat anti-rabbit IgG antibody and incubated for 1 hr at room temperature. After secondary antibody incubation, the membrane was again washed with 1X casein solution and then incubated in VECTASTAIN ABC-AmP reagent for 30 min at room temperature. DuoLuX substrate was then added onto the membrane surface for chemiluminescent signal acquisition. The images were analyzed by AlphaView SA software (Cell Biosciences Inc.).
2.12 Statistical Analysis
All of above assays were repeated more than four times. Data from WST-1, LDH, Trypan Blue assays, and acrolein-lysine level in spinal cord are given as the mean ± SEM. The one-way ANOVA was used to determine the significance between control groups and treated groups. p<0.05 indicates significance.
3. Results
3.1 Cytotoxicity of Dimercaprol
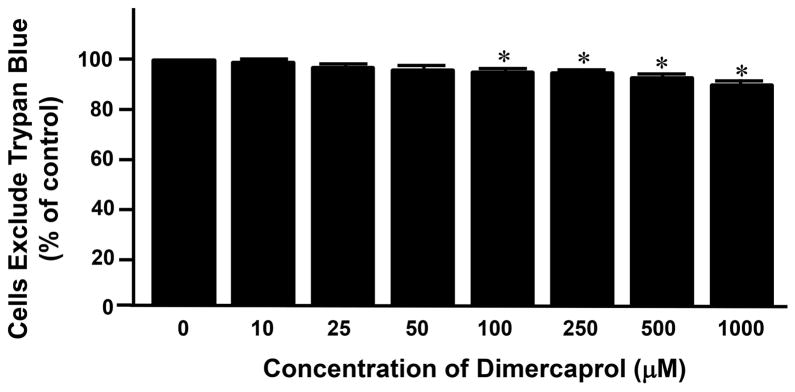
We first examined the potential cytotoxicity of dimercaprol by co-incubating it with PC-12 cells. Seven different concentrations, ranging from 10 to 1000 μM, were used to determine the cytotoxicity of varying doses of the compound. The cell viability of control (no dimercaprol) was considered as 100%. The viability of the cells that exposed to dimercaprol at other concentrations were normalized to control and expressed as percentages of survival over that in control. As shown in Figure 1, the survival rate of cell exposed to dimercaprol at 10, 25, 50, 100, 250, 500 and 1,000 μM were 98.6 ± 0.9%, 97.0 ± 0.8%, 95.8 ± 0.8%, 94.4 ± 1.0%, 93.8 ± 1.2%, 92.8 ± 1.4%, and 88.8 ± 2.3% respectively. As it indicated, for the highest concentration of 1 mM, close to 90% cells still remained viable and healthy. Notably, at a concentration of 100 μM, the highest concentration used for acrolein scavenging in our tests, around 95% cells were still alive, although the cell mortality reached a statistical significance when compared to control (p<0.05). The highest concentration that caused no significant cell death is 50 μM. From these in vitro results, it seems that dimercaprol is a safe drug when the dosage is no higher than 100 μM.
Figure 1.
Cytotoxicity of dimercaprol was tested by Trypan Blue assay. PC-12 cells were exposed to different concentrations of dimercaprol for 4 hrs. Cell viability was determined by trypan blue dye exclusion assay. Viable cells were the cells that excluded the dye. The cell viability of control (dimercaprol at 0 μM) was considered 100%. The viability of cell of other groups was expressed as the percentages of the control. It appears that dimercaprol began to induce significant cell death when its concentration was at and higher than 100 μM (* p<0.05, ANOVA). All data were expressed as mean ± SEM, n=5.
3.2 Dimercaprol Reacts with Acrolein in an Abiotic Condition
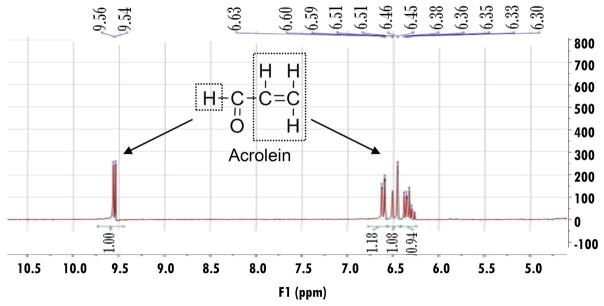
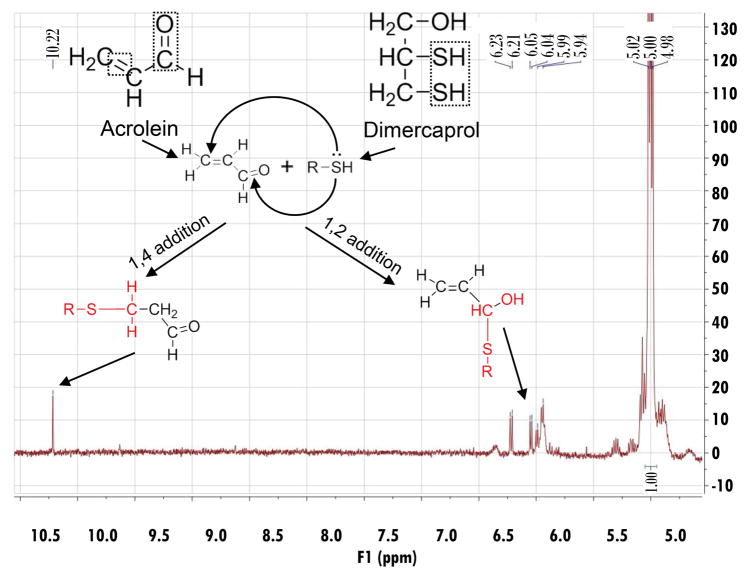
Next, we attempted to investigate the chemical reaction between dimercaprol and acrolein using NMR spectroscopy. The structure and NMR spectrum of acrolein are shown in Figure 2. The doublet peak in the downfield (δ 9.55) corresponds to the aldehyde proton, whereas the three alkene protons are located in δ 6.63–6.30. The NMR spectrum of the acrolein-dimercaprol reaction products are shown in Figure 3. In this assay, the dimercaprol was used in excess (200 mM) compared to acrolein (3.74 mM) to produce adequate acrolein-dimercaprol adducts to achieve measurable signals using NMR. According to the spectrum, the characteristic peaks for the aldehyde proton of acrolein was shifted to the downfield (δ 10.22), which indicates the formation of another new aldehyde. The peaks for the alkene protons of acrolein were shifted to the upfield (δ 6.23–5.94), which indicates the formation of another new alkene.
Figure 2.
Structural analysis of acrolein using 1H-NMR spectroscopy. Acrolein (0.5 μL) was dissolved in 1 mL DMSO-d6 and the 1H-NMR spectrum for acrolein was recorded by a 300 MHz Bruker NMR spectrometer. The chemical structure of acrolein and the NMR peak assignments are illustrated. The doublet peak in the downfield (δ 9.55) corresponds to the aldehyde proton, whereas the three alkene protons are located in δ 6.63–6.30.
Figure 3.
The 1H-NMR spectrum of acrolein-dimercaprol reaction products. After recorded pure acrolein 1H-NMR spectrum in DMSO-d6, a solution of dimercaprol (50 mg) in DMSO-d6 (1 mL) was added. The mixture was incubated for 1 hr in the dark. The spectrum was obtained with a 300 MHz Bruker NMR spectrometer. The possible reaction mechanisms between dimercaprol and acrolein, and the structures of possible products were shown. In this assay, the dimercaprol was used in excess (final concentration was approximately 200 mM) compared to acrolein (final concentration was approximately 3.74 mM). This was designed to produce adequate acrolein-dimercaprol adducts to achieve measureable signals using NMR. The characteristic peaks for the aldehyde proton of acrolein were shifted downfield (δ 10.22), which indicates the formation of another new aldehyde. The peaks for the alkene protons of acrolein were shifted upfield (δ 6.23–5.94), which indicates the formation of another new alkene.
Two possible reactions (1,2-addition and 1,4-addition) could account for the observed NMR spectrum of acrolein-dimercaprol reaction products. In 1,4-addition, the thiol group of dimercaprol attacks the conjugated C=C bond and C=O group, forms an unstable olefinic alcohol which converts to the corresponding stable aldehyde, which corresponds to the peak at δ 10.22. In 1,2-addition, the thiol group of dimercaprol directly attacks the carbonyl group of acrolein to form the hemithioacetal, whose alkene protons correspond to the peaks at δ 6.23–5.94. Therefore, based on the NMR spectrum, both 1,4-addition and 1,2-addition products are formed and contributed to acrolein neutralization by dimercaprol. No trace peaks of acrolein were observed based on NMR spectrum, indicating that the consumption of acrolein by dimercaprol was complete.
3.3 Rescue of PC-12 Cells From Acrolein-mediated Cell Death by Dimercaprol-WST-1 assay
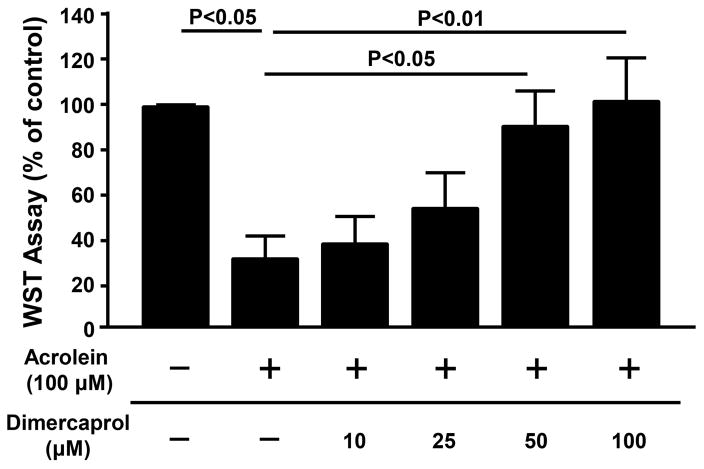
Following the confirmation of scavenging acrolein by dimercaprol in a cell free, abiotic condition, we turned our attention to verifying the ability of dimercaprol to reduce acrolein-mediated cytotoxicity in PC-12 cell cultures. We first employed a WST-1 assay to quantify viable cells. For each experiment, the final WST-1 measurement for each group was expressed as the percentage of control group (non-treated group). Specifically, as shown in Figure 4, cell survival decreased to only 31.5 ± 10.5% of the control value (or 68.5% of cells death) after exposure to 100 μM acrolein for 4 hrs (p< 0.05, n=7). A delayed application of increasing concentrations of dimercaprol, 10, 25 and 50 μM, respectively, increased the percentages of viable cells to 38.3 ± 12.7%, 55.4 ± 15.5% and 92.4 ± 15.5% (p< 0.05, n=7). At the concentration of 100 μM of dimercaprol, the cell viability was comparable to the control group. Based on the results of WST-1 assay, it seems that dimercaprol can mitigate the loss of PC-12 cells due the exposure of acrolein.
Figure 4.
Dimercaprol protected PC-12 cells from acrolein-mediated cell death based on WST-1 assay. PC-12 cells were exposed to 100 μM acrolein for 4 hrs. Some of them were treated with additional different concentrations of dimercaprol after 15 min of acrolein exposure. Cell viability was tested by WST-1 assay. The cell viability of control (no acrolein or dimercaprol) was considered 100%. The cell viability of other groups was expressed as the percentages of the control. Dimercaprol was capable of significantly protecting PC-12 cells from acrolein-mediated cell death in a dosage-dependent manner starting at a concentration of 50 μM (p<0.05 for 50 μM and p<0.01 for 100 μM when compared to acrolein only, ANOVA). All data were expressed as mean ± SEM, n=7.
3.4 Dimercaprol Protects PC-12 Cells from Acrolein-mediated Cell Membrane Damage-LDH and Trypan Blue Assays
Acrolein has the ability to react with various biomolecules including proteins, DNA and phospholipids, and thus can disrupt the functions of these biomolecules (Luo et al. 2005a, Esterbauer et al. 1991, Kehrer & Biswal 2000, Shi et al. 2002, Shi et al. 2011a, Shi et al. 2015). Phospholipids are the main constituents of cell membrane. As such, LDH and Trypan Blue assays, assessments of membrane integrity, were employed to verify the hypothesis that acrolein-mediated membrane damages can be mitigated by dimercaprol.
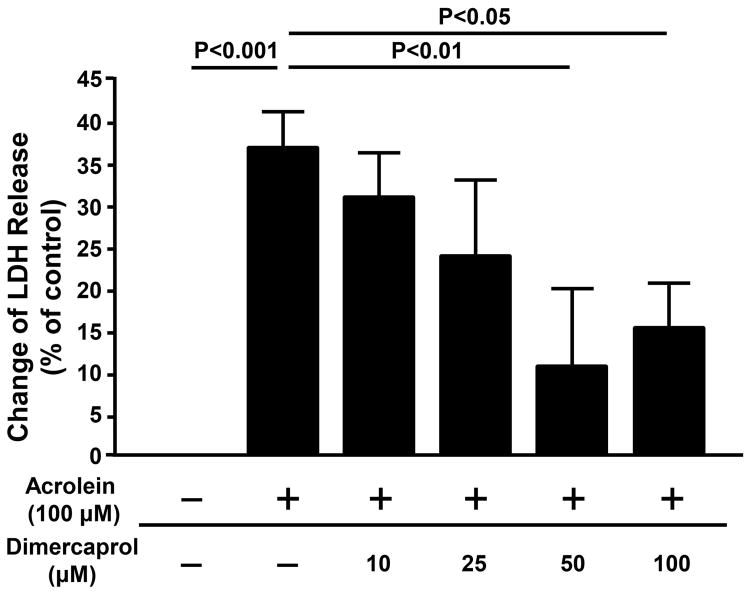
As shown in Figure 5, the LDH in control cells (no acrolein or dimercaprol) is defined as baseline. The release of LDH for various groups were expressed as the percentage of changes in relation to control. As it indicates, LDH released in cell medium increased by 37.0 ± 5.4% after exposure to acrolein at 100 μM for 4 hrs, indicating that acrolein can damage cell membrane of PC-12 cells and facilitate LDH permeability. However, the LDH level reduced to 31.7 ± 5.8%, 24.7 ± 9.0%, 11.9 ± 9.5% and 16.2 ± 5.7% with the application of dimercaprol at 10, 25, 50, and 100μM respectively. The reductions of LDH permeability due to dimercaprol at 50 and 100 μM showed significant difference comparing to that of acrolein only (p< 0.01 for 50 μM and p< 0.05 for 100 μM).
Figure 5.
Dimercaprol protected PC-12 cells from acrolein-mediated plasma membrane damage and cell death based on LDH assay. PC-12 cells were exposed to 100 μM acrolein for 4 hrs. Some of them were treated with additional different concentrations of dimercaprol after 15 min of acrolein exposure. The protection effects of dimercaprol were examined by LDH releasing assay. The percentage of LDH release was normalized to 0% for the control group (no acrolein or dimercaprol). The LDH release for other groups was expressed as the percentages of LDH level when all LDH was released from cells. Treatment with dimercaprol significantly reduced acrolein-mediated cell membrane damage (p<0.05 or p<0.01, ANOVA). All data were expressed as mean ± SEM, n=5.
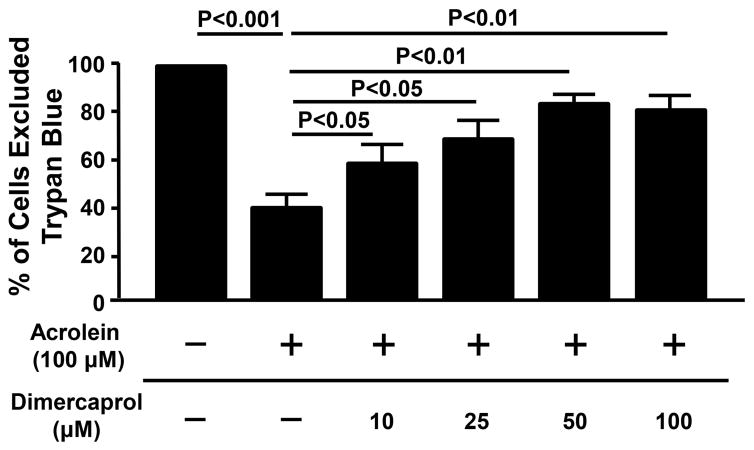
The cell viability based on the exclusion of trypan blue in the control group (non-treated group) was set as 100%, and results of other groups were expressed as percentages of the control group. As shown in Figure 6, exposing PC-12 cells to 100 μM acrolein resulted in only 41.1 ± 5.1% of cells excluding trypan blue (P < 0.001) Upon the administration of increasing dosages of dimercaprol at 10, 25, 50, and 100 μM, there was a dosage-dependent increase of cells excluding trypan blue, which are 60.4 ± 8.3%, 71.3 ± 7.8%, 85.1 ± 3.0% and 82.6 ± 6.0% respectively (p<0.05 or p<0.01). Taken together, both the LDH and Trypan Blue assays demonstrate that acrolein-mediated membrane damage can be mitigated by dimercaprol in a dose dependent manner.
Figure 6.
Dimercaprol protected PC-12 cells from acrolein-mediated membrane damage and cell death based on the Trypan Blue assay. PC-12 cells were exposed to 100 μM acrolein for a total of 4 hrs. Some of the cells were treated with additional different concentrations of dimercaprol after 15 min of acrolein exposure. The viability was determined by the Trypan Blue assay. The cell viability of the control (no acrolein or dimercaprol) was considered 100%. The viability of other groups was expressed as the percentages of the control. The treatment with dimercaprol was similar to the previous LDH assay. Note that dimercaprol reduced acrolein-mediated trypan blue membrane permeability in a dosage-dependent manner. (p<0.05 or p<0.01, ANOVA). All data were expressed as mean ± SEM, n=4.
3.5 Extended Beneficial Effects and Cytotoxicity of Anti-acrolein Treatment with Dimercaprol Based on LDH Assay
In addition to 4 hour incubations, we have also examined the beneficial effects of anti-acrolein treatment with dimercaprol with 24 hours of exposure time, examined through LDH assay (Figure 7). Specifically, PC-12 cells were exposed to 100 μM acrolein for an extended time of 24 hrs which resulted in a significant elevation of LDH released in cell medium (62.1 ± 7.8%, p<0.001 compared to uninjured control). Two additional groups were treated with either 50 μM or 100 μM dimercaprol, in addition to and after 15 min of acrolein exposure. Our results indicate that while acrolein induced significant membrane damage when incubated with cells for 24 hours, dimercaprol at both 50 and 100 μM significantly reduced acrolein-mediated cell membrane damage (2.4 ± 0.7%, 7.6 ± 1.1% respectively, p<0.001 compared to acrolein only for both concentrations).
Figure 7.
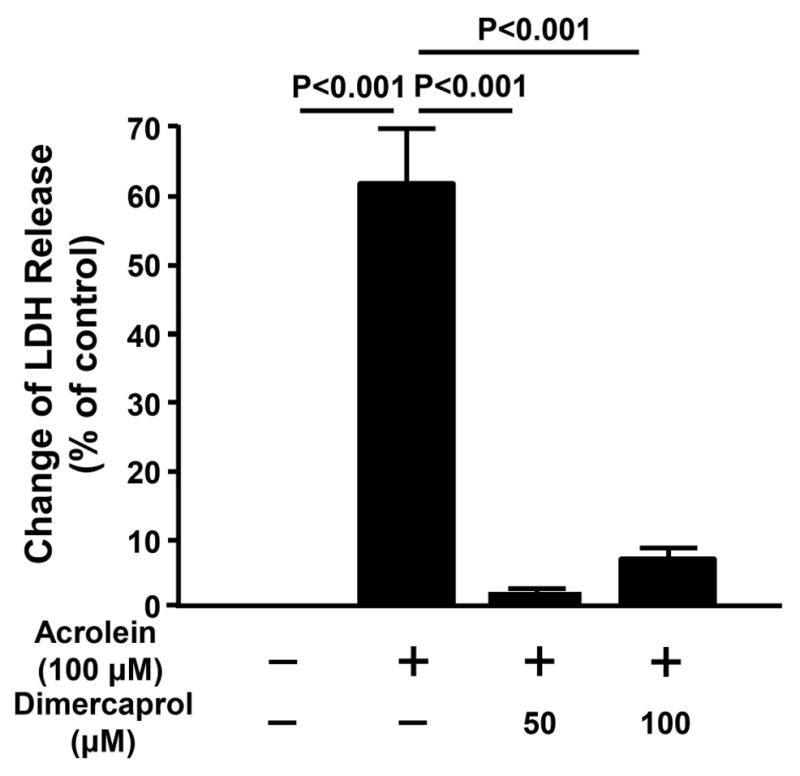
Extended effects of anti-acrolein treatment with dimercaprol based on LDH assay. PC-12 cells were exposed to 100 μM acrolein for 24 hrs. Some of them were treated with either 50 μM or 100 μM dimercaprol after 15 min of acrolein exposure. The anti-acrolein effects of dimercaprol were examined by LDH releasing assay. The release of LDH in control group (no acrolein or dimercaprol) was normalized to 0%. The LDH release for other groups was expressed as the percentages of LDH level when all LDH was released from cells. Dimercaprol significantly reduced acrolein-mediated cell membrane damage (p <0.001 for both dimercaprol concentrations, ANOVA). All data were expressed as mean ± SEM, n=8.
Next, we also assessed the cytotoxicity of dimercaprol in both short term (4 hrs) and long term (24 hrs) exposures, examined by LDH assay (Figure 8). Dimercaprol produced minimal changes in membrane leakage at 50 μM (4 and 24 hrs) and 100 μM (4 hrs) (1.2 ± 0.4%, 3.1 ± 0.5%; 2.7 ± 0.3%; respectively, p>0.05 when compared to control). However, incubation in 100 μM dimercaprol for 24 hours caused a significant elevation of LDH leakage (8.1 ± 1.2%, p<0.05).
Figure 8.
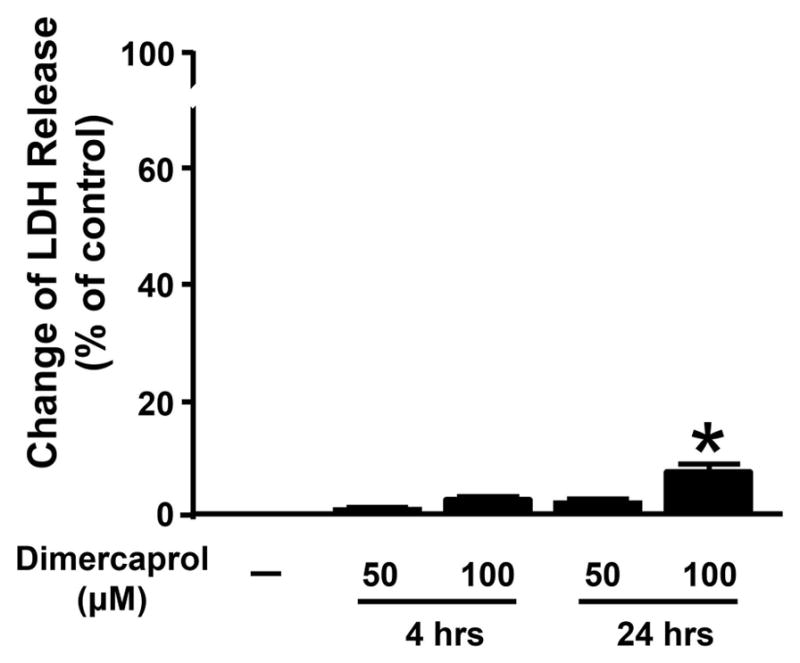
Extended assessment of cytotoxicity of dimercaprol examined by LDH assay. PC-12 cells were exposed to 50 μM and 100 μM dimercaprol for 4 or 24 hrs. Cell viability was determined by LDH releasing assay. The LDH release in control group (without dimercaprol treatment) was normalized to 0%. The LDH release in other groups was expressed as the percentages of LDH level when all LDH was released from cells. Dimercaprol caused significant cell death when its concentration was at 100 μM when exposure time was 24 hours (* p<0.05, ANOVA). All data were expressed as mean ± SEM, n=5 in 4-hour study and n=8 in 24-hour study.
3.6 Cell Morphology Study
Trypan blue was used to identify viable cells in this study. The cells in the control group appeared to attach tightly to substrates. Most of them were non-stained and protuberant, which indicates they were alive and healthy (Figure 9A). However, when treated by acrolein at 100 μM for 24 hrs, a significant portion of the cells detached from substrates and were removed along with liquid, indicating that they were dead or damaged. Some remaining cells were expended but not embossed, and stained by trypan blue (Figure 9B), which suggests they were not viable. In contrast, a significant number of cells excluded trypan blue following application of dimercaprol at 100 μM. The surviving cells were plump and morphologically indistinguishable from the control cells (Figure 9C). There were several cells labeled by Trypan Blue in the dimercaprol-exposed (no acrolein) group (Figure 9D), indicating there was little cytotoxicity of dimercaprol in this treatment.
Figure 9.
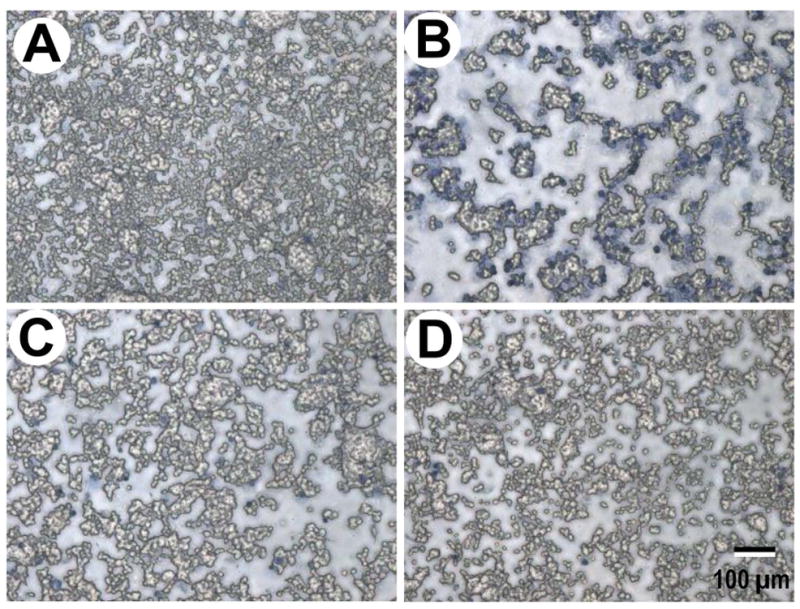
Photographic images of PC-12 cells and their plasma membrane damage and cell death induced by acrolein (100 μM) and mitigation by dimercaprol (100 μM). A) Control uninjured cells. Note almost all the PC-12 cells excluded the trypan blue dye, indicating health intact membrane. B) Acrolein-treated cells. PC12 cells were exposed to 100 μM acrolein for 24 hrs. Note most of the PC-12 cells were labeled by trypan blue, indicating membrane damage and increase of permeability to trypan blue. C) PC-12 cells were exposed to acrolein, followed by a delayed (15 min) treatment of dimercaprol (100 μM) for a total incubation time of 24 hrs. Notice the significant number of PC-12 cells were protected by dimercaprol and excluded trypan blue. D) PC12 cells were exposed to 100 μM dimercaprol (no acrolein) for 24 hrs. Note few PC-12 cells were labeled by trypan blue, indicating dimercaprol had little cytotoxicity to PC-12 cells. The scale bar represents 100 μm (for A, B, C, and D).
3.7 Dimercaprol Effectively Decreases Acrolein Level in Spinal Cord of SCI Rat
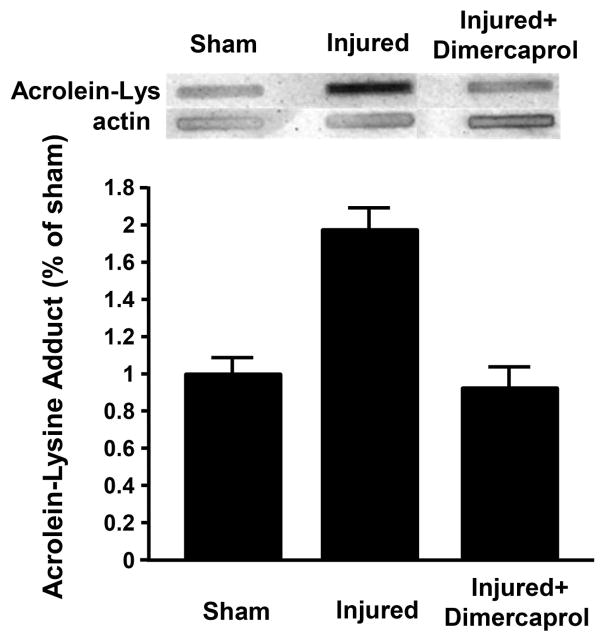
After demonstrating the anti-acrolein activity of dimercaprol on the cellular level in vitro, we tested the hypothesis that dimercaprol may reduce acrolein in the CNS in vivo using dot immunoblotting method. Acrolein-lysine concentration was measured 48 hrs post SCI. Dimercaprol was administered three times during this period, immediately after injury and then daily. The last injection of dimercaprol was given 4 hrs before euthanizing the rats. As indicated in Figure 10, acrolein-lysine level increased significantly 48 hrs after SCI in the non-treated injured group comparing to the sham, which is around 1.79 ± 0.07 folds of sham. The treatment with Dimercaprol reduced acrolein concentration to 0.93 ± 0.10 folds of sham. This result demonstrates that dimercaprol could effectively reduce acrolein levels following SCI injury. In addition, we have also shown that systemic application of dimercaprol for 6 days had little influence on the weight of the rats when compared to saline-treated rats (Fig. 11). Specifically, two groups of rats (n=5 in each group) received daily IP injection of either 5mg/kg dimercaprol or saline (equal volume). There was no statistically significant difference between the weight of saline-treated rats and dimercaprol-treated rats, in any of days during the experiments (p > 0.05).
Figure 10.
Elevation of acrolein-lysine level in spinal cord tissue post SCI. Rats were treated by 5mg/kg dimercaprol right after surgery and once a day until 48 hrs after SCI. Photographic dot immunoblotting images (top) show representative blots for each experimental condition. Bar graph (bottom) presents the overall acrolein-lysine conjugate levels for different groups, including sham, non-treated and dimercaprol treated groups. There was a 1.79 ± 0.07 fold elevation of acrolein in the non-treated SCI group comparing to sham group (p<0.005, ANOVA). Dimercaprol treatment reduced acrolein level back to 0.93± 0.10 folds of sham (p<0.001, ANOVA). All data were expressed as mean ± SEM. n = 3–5 in all three groups.
Figure 11.
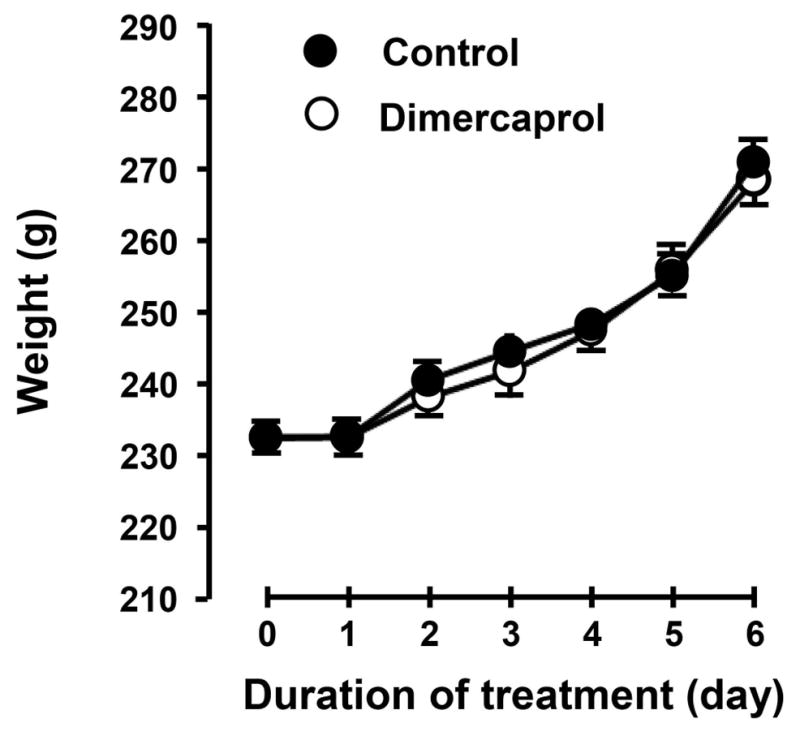
The effect of 6-day consecutive systemic application of dimercaprol on the weight of rats. Specifically, two groups of rats (n=5 in each group) received daily IP injection of either 5mg/kg dimercaprol or saline (equal volume). Note there is little difference between the average weights between these two groups over the course of 6 days.
4. Discussion
Many studies have established that acrolein participates in the pathologies of various diseases due to its highly toxic nature, high reactivity, and long half-life (Esterbauer et al. 1991, Shi et al. 2015, Shi et al. 2011a, Stevens & Maier 2008). The pathological role of acrolein is particularly apparent in neurodegenerative diseases and secondary injury following CNS trauma. This is likely because the lipid-rich nervous system provides a virtually unlimited source of compounds for acrolein generation and reaction (Shi et al. 2011a, Tully & Shi 2013, Shi et al. 2015). Neurons are particularly vulnerable to acrolein-mediated injury due to the lack of specific enzymes that are critical in eliminating acrolein such as some subtypes of aldehyde dehydrogenase (ALDH) (Hamann & Shi 2009, O’Brien et al. 2005). Therefore, elucidating the pathological role of and establishing means to neutralize acrolein is of great interest. We have found that dimercaprol was indeed capable of binding acrolein based on a cell free 1H-NMR spectrum study. Furthermore, dimercaprol was able to significantly reduce acrolein-mediated cell death and membrane damage in PC-12 cells, when examined at both 4 and 24 hours after acrolein exposure and dimercaprol treatment. Finally, we have demonstrated that dimercaprol was able to reduce the post-injury acrolein elevation in a rat contusion spinal cord injury model when examined at 48 hrs post injury and dimercaprol treatment. These data indicate that dimercaprol is an effective acrolein scavenger, capable of removing acrolein and providing neuroprotection.
It is well established that acrolein can damage neuronal tissues by attacking proteins, lipids and DNA. Acrolein has been shown to cause the dysfunction of mitochondria, loss of the integrity of neuronal membrane, and degradation of myelin (Shi et al. 2011b, Luo & Shi 2004, Luo & Shi 2005). While it is has been postulated that adenine nucleotide translocase (ANT) is a specific target of acrolein-mediated mitochondria damage (Luo & Shi 2005), the exact mechanisms of acrolein-mediated membrane damage are still not clear. Since acrolein is known to directly attach to proteins by forming acrolein-protein adduct, it is likely that damage to membrane bound proteins could play a critical role in acrolein-inflicted disruption of lipid membrane (Luo & Shi 2004, Shi et al. 2011a, Hamann & Shi 2009, Esterbauer et al. 1991). In addition, acrolein-mediated elevation of reactive oxygen species (ROS) could also further damage neuronal membrane through the peroxidation of polyunsaturated fatty acids in lipid membrane (Luo & Shi 2004). Taken together, acrolein likely damages the lipid membrane through direct destruction of membrane bound proteins, as well as through lipid peroxidation by ROS. It has been suggested that acrolein plays a pathological role in not only neuronal trauma, such as SCI and traumatic brain injury (TBI) (Hamann et al. 2008a, Luo et al. 2005b, Walls et al. 2016), but also degenerative diseases, such as multiple sclerosis (MS) and Alzheimer’s disease (Leung et al. 2011, Calingasan et al. 1999), cancers (Cohen et al. 1992, Feng et al. 2006), and chemotherapy-induced neuropathy (Conklin 2004, Barrera 2012). Therefore, removing acrolein by acrolein scavengers such as dimercaprol will likely mitigate neuronal damage and encourage repairing process through endogenous mechanisms in a variety of pathological conditions.
The highly electrophilic reactivity of acrolein is attributed to its two reactive moieties: a C=C double bond and a C=O carbonyl group in a conjugated C=C-C=O system. Both groups are capable of reacting with nucleophilic amino acid residues of proteins, i.e. cysteine, histidine and lysine, and with deoxyguanosine (dG) of DNA (Esterbauer et al. 1991, Stevens & Maier 2008, Shi et al. 2011a, Shi et al. 2015). The chemical mechanism of acrolein scavenging by hydralazine, a well-known acrolein scavenger, is mainly through reaction at C=O group of acrolein and formation of adducts (Zhu et al. 2011, Burcham et al. 2002, Burcham et al. 2000, Kaminskas et al. 2004b). However, it is also known that although acrolein-hydralazine adduct poses significantly less toxicity than free acrolein, the adduct demonstrates toxic properties when present in concentrations exceeding 10 μM. (Kaminskas et al. 2004a). It is also worth noting that remaining C=C group of acrolein may still pose toxicity (Kaminskas et al. 2004a).
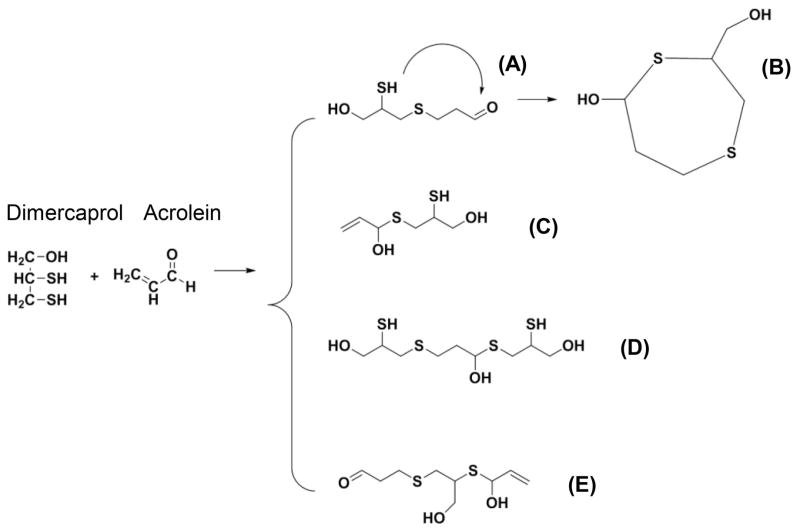
Dimercaprol, on the other hand, as demonstrated by the NMR spectrum in Figure 3, is capable of reacting and thus blocking both C=O and C=C groups through the formation of 1,2-addition and 1,4-addition of acrolein. In addition, dimercaprol possesses two thiol groups that are capable of reacting with both the C=O and C=C groups of acrolein (Figure 3 and 12), while hydralazine possesses only one hydrazine group that can only react with one reactive site of acrolein at a time. Taken together, this group of evidence suggests that each molecule of dimercaprol can potentially trap up to two equivalents of acrolein while each hydralazine can only trap one equivalent of acrolein.
Figure 12.
Scheme depicts the predicted chemical compounds which could result from acrolein-dimercaprol reaction. Each structure represents one possible mode of a reaction. A) acrolein and dimercaprol 1:1 reaction by 1,4-addition. B) Two thiol groups of dimercaprol react with both C=C and C=O groups of acrolein. C) acrolein and dimercaprol 1:1 reaction by 1,2-addition. (D) acrolein and dimercaprol reaction by 1:2 ratio. (E) acrolein and dimercaprol reaction by 2:1 ratio.
The specific manner in which dimercaprol reacts with acrolein is currently unknown. Here we speculate that there will be at least five possible forms of dimercaprol-acrolein adducts. As indicated in Figure 12, among five hypothetical structures, one interesting possibility is the formation of a cyclic structure (Figure 12B), where one molecule of dimercaprol can react with both C=O and C=C groups of acrolein through its two thiol groups, although this possibility remains to be confirmed. Hence, although it is known that dimercaprol could indeed react with acrolein, it is not clear what exactly the binding mechanisms are. Further study is clearly necessary to confirm the existence of these hypothetical structures.
In addition to the evidence in an abiotic, cell free system, the ability of dimercaprol to neutralize acrolein was further confirmed by in vitro tests using a PC-12 cell culture system and in vivo animal study through contusion SCI rat model. Based on our knowledge, there is no report of dimercaprol being a reparative agent capable of repairing damage inflicted by acrolein. In addition, we have already shown that dimercaprol can bind and neutralize acrolein. Therefore, the most plausible explanation of the neuroprotective effect of dimercaprol in cell culture study is through scavenging acrolein.
Based on the above mentioned putative structural advantages of dimercaprol in acrolein-scavenging compared to better known exogenous compounds such as hydralazine, it appears that dimercaprol might have higher capacity in scavenging acrolein. This speculation seems consistent with the findings in the PC-12 cell studies. Using PC-12 cells, hydralazine can afford 50% reduction of cell death, but only with a dosage that is at least as twice as that of acrolein (Liu-Snyder et al. 2006). However, in the current study using the same cell culture preparation, dimercaprol, with only the half of the concentration of acrolein, can produce more than 50% of reduction in acrolein-mediated cell death. This seems to be consistent with the structure-based prediction that one molecule of dimercaprol can bind and scavenge more than one acrolein molecule (structure E in Figure 12). Future studies that allow direct comparison could reveal the comparative efficacy of dimercaprol and hydralazine in scavenging acrolein.
Based on the available data, the safe dosage range of hydralazine which effectively reduces acrolein in vivo in rat is from 5 – 25 mg/kg IP. At this dosage range, no significant drop of blood pressure was found following application of hydralazine, an FDA-approved hypertensive medication (Pandit 1984, Khan 1953, Leung et al. 2011, Zheng et al. 2013, Chen et al. 2016). In the case of dimercaprol, the reported dosage employed for in vivo application to alleviate heavy metal-induced tissue damage was 2.5–50 mg/kg (Jindal et al. 1974, Coveney & Robbins 1986, Wenzel & Beckloff 1958, Cherian 1980). There are two main reasons that we chose the level of 5 mg/kg for dimercaprol to be used in the current investigation. First, 5 mg/kg is in the low end of the dosage range for in vivo application, which minimizes the possible side effects of dimercaprol in vivo (Flora & Pachauri 2010). Second, 5 mg/kg is also the level that hydralazine is known to be safe and effective in scavenging acrolein in vivo, therefore facilitating the comparison of these two scavengers in acrolein reduction in vivo (Zheng et al. 2013, Park et al. 2014b). In addition, we have also confirmed that systemic application of dimercaprol at such concentration for 6 days had no significant influence on body weight when compared to saline-treated rats (Figure 11), further indicating its general safety in longer period of usage in vivo. Therefore, based on our findings in the current study, IP application of 5 mg/kg of dimercaprol appears to be a safe dosage with no detectable side effects, as well as an effective level capable of scavenging acrolein.
At the dosage of 5 mg/kg through IP injection, dimercaprol is shown to efficiently reduce acrolein-adducts to a level that is comparable to that of sham injured rats in spinal cord tissue following SCI, an efficacy comparable to that of hydralazine (Park et al. 2014b). This also indicates that dimercaprol could gain access to CNS, cross the BBB, and effectively trap acrolein in the acute stage of SCI. Similar to hydralazine, dimercaprol has also been shown to have excellent penetration through the BBB (McEvoy 1991, Snider et al. 1990). Therefore, our data indicates that dimercaprol could be an effective and safe acrolein scavenger in vivo and potentially an effective neuroprotective agent for CNS trauma and diseases. In light of this initial confirmation of dimercaprol’s acrolein scavenging capacity, future studies could examine its dose dependent efficacy of trapping acrolein.
It was reported that the metabolic degradation and renal excretion of dimercaprol are complete within 6 to 24 hours based animal studies (Catsch & Harmuth-Hoene 1976), which supports the safe application of daily injection. However, although IP injection of dimercaprol for 6 days did not result in noticeable side effects, such as body weight changes, further studies are needed to examine the safety of extended period of usage beyond 6 days.
The effectiveness of dimercaprol in acrolein scavenging seen in our in vitro examination, likely caused by the presence of multiple nucleophilic groups, suggests that dimercaprol dosage could potentially be reduced while achieving comparable therapeutic benefits to hydralazine. One additional advantage is that dimercaprol is not known to elicit a reduction of blood pressure. Therefore, in the event that hydralazine is not applicable in a subset of patients due to risk of hypotension, dimercaprol could be a viable alternative treatment. Taken together, based on these data, it is reasonable to speculate that dimercaprol is likely an effective acrolein scavenger in vivo and, an alternative choice of anti-acrolein in addition to hydralazine, particularly when maintaining blood pressure is vital, such as in both spinal cord injury and traumatic brain injury.
Acknowledgments
This work was supported by the Indiana State Department of Health (Grant # 204200 to RS), National Institutes of Health (Grant # NS073636 to RS), Indiana CTSI Collaboration in Biomedical Translational Research (CBR/CTR) Pilot Program Grant (Grant # RR025761 to RS). We thank Jonathan Tang and Breanne Butler for critically reading of the manuscript.
Abbreviations
- SCI
Spinal cord injury
- HNE
4-hydroxynonenal
- LDH
Lactate dehydrogenase
- LPO
Lipid peroxidation
- IP
Intraperitoneal
- ATCC
American Type Culture Collection
- FDA
Food and Drug Administration
- BBB
Blood Brain Barrier
- HBSS
Hank’s Balanced Salt Solution
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Riyi Shi is the co-founder of Neuro Vigor, a star-up company with business interests of developing effective therapies for CNS neurodegenerative diseases and trauma.
References
- Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radical Biology & Medicine. 1993;15:187–193. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–236. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. Journal of Neurochemistry. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Carleton AB, Peters RA, et al. Clinical uses of 2,3-dimercaptopropanol (BAL); the treatment of complications of arseno-therapy with BAL (British anti-lewisite) J Clin Invest. 1946;25:497–527. doi: 10.1172/JCI101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsch A, Harmuth-Hoene AE. Pharmacology and therapeutic applications of agents used in heavy metal poisoning. Pharmac Ther A. 1976;1:1–118. [Google Scholar]
- Chen Z, Park J, Butler B, et al. Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury. J Neurochem. 2016;138:328–338. doi: 10.1111/jnc.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian MG. Chelation of cadmium with BAL and DTPA in rats. Nature. 1980;287:871–872. doi: 10.1038/287871a0. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Garland EM, St John M, Okamura T, Smith RA. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992;52:3577–3581. [PubMed] [Google Scholar]
- Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- Coveney JR, Robbins MS. Biodistribution of radiomercury in rabbits and efficacy of dimercaptopropanesulfonic acid (DMPS) and dimercaprol (BAL) to reduce tracer-level kindey (kid) burden of radiomercury in rats. Fed Proc, Fed Am Soc Exp Biol. 1986;45:3. [Google Scholar]
- Denny-Brown D, Porter H. The effect of BAL (2,3-dimercaptopropanol) on hepatolenticular degeneration (Wilson’s disease) N Engl J Med. 1951;245:917–925. doi: 10.1056/NEJM195112132452401. [DOI] [PubMed] [Google Scholar]
- Due MR, Park J, Zheng L, Walls M, Allette YM, White FA, Shi R. Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem. 2014;128:776–786. doi: 10.1111/jnc.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7:2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani S, Coatrieux C, Elbaz M, et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic Biol Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008a;107:712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem. 2008b;104:708–718. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- Jindal MN, Kelkar VV, Vaishnav UH. Effect of drugs on rat paw oedema induced by mercury. Br J Pharmacol. 1974;52:609–612. doi: 10.1111/j.1476-5381.1974.tb09731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org Biomol Chem. 2004a;2:2578–2584. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J Pharmacol Exp Ther. 2004b;310:1003–1010. doi: 10.1124/jpet.104.067330. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicological Sciences. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Khan MA. Effect of hydralazine in hypertension. Br Med J. 1953;1:27–29. doi: 10.1136/bmj.1.4800.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience. 2011;173:150–155. doi: 10.1016/j.neuroscience.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J Neurosci Res. 2006;84:219–227. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int. 2005a;47:449–457. doi: 10.1016/j.neuint.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int. 2004;44:475–486. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46:243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005b;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- McEvoy GK. AHFS Drug information 91. American Society of Hospital Pharmacists; 1991. [Google Scholar]
- O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Oehme FW. British anti-lewisite (BAL), the classic heavy metal antidote. Clin Toxicol. 1972;5:215–222. doi: 10.3109/15563657208991000. [DOI] [PubMed] [Google Scholar]
- Pandit RB. Long term propranolol and hydralazine in hypertension. J Assoc Physicians India. 1984;32:199–202. [PubMed] [Google Scholar]
- Park J, Muratori B, Shi R. Acrolein as a novel therapeutic target for motor and sensory deficits in spinal cord injury. Neural regeneration research. 2014a;9:677–683. doi: 10.4103/1673-5374.131564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zheng L, Acosta G, Vega-Alvarez S, Chen Z, Muratori B, Cao P, Shi R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J Neurochem. 2015;135:987–997. doi: 10.1111/jnc.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zheng L, Marquis A, et al. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem. 2014b;129:339–349. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RA, Stocken LA, Thompson RH. British anti-lewisite (BAL) Nature. 1945;156:616–619. doi: 10.1038/156616a0. [DOI] [PubMed] [Google Scholar]
- Reece PA. Hydralazine and related compounds: chemistry, metabolism, and mode of action. Med Res Rev. 1981;1:73–96. doi: 10.1002/med.2610010105. [DOI] [PubMed] [Google Scholar]
- Shi R, Luo J, Peasley MA. Acrolein inflicts axonal membrane disruption and conduction loss in isolated guinea pig spinal cord. Neuroscience. 2002;115:337–340. doi: 10.1016/s0306-4522(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Shi R, Luo L. The role of acrolein in spinal cord injury. Applied Neurology. 2006;2:22–27. [Google Scholar]
- Shi R, Page JC, Tully M. Molecular mechanisms of acrolein-mediated myelin destruction in CNS trauma and disease. Free Radic Res. 2015;49:888–895. doi: 10.3109/10715762.2015.1021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Rickett T, Sun W. Acrolein-mediated injury in nervous system trauma and diseases. Mol Nutr Food Res. 2011a;55:1320–1331. doi: 10.1002/mnfr.201100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sun W, McBride JJ, Cheng JX, Shi R. Acrolein induces myelin damage in mammalian spinal cord. J Neurochem. 2011b;117:554–564. doi: 10.1111/j.1471-4159.2011.07226.x. [DOI] [PubMed] [Google Scholar]
- Snider TH, Wientjes MG, Joiner RL, Fisher GL. Arsenic distribution in rabbits after Lewisite administration and treatment with British anti-Lewisite (BAL) Fundamental and applied toxicology : official journal of the Society of Toxicology. 1990;14:262–272. doi: 10.1016/0272-0590(90)90206-y. [DOI] [PubMed] [Google Scholar]
- Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully M, Shi R. New Insights in the Pathogenesis of Multiple Sclerosis—Role of Acrolein in Neuronal and Myelin Damage. International journal of molecular sciences. 2013;14:20037–20047. doi: 10.3390/ijms141020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends in Cardiovascular Medicine. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Walls MK, Race N, Zheng L, Vega-Alvarez SM, Acosta G, Park J, Shi R. Structural and biochemical abnormalities in the absence of acute deficits in mild primary blast-induced head trauma. J Neurosurg. 2016;124:675–686. doi: 10.3171/2015.1.JNS141571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel DG, Beckloff GN. The effect of dimercaprol on amine oxidase and amino acid decarboxylase of the normotensive and hypertensive rat kidney. J Am Pharm Assoc Am Pharm Assoc. 1958;47:844–846. doi: 10.1002/jps.3030471203. [DOI] [PubMed] [Google Scholar]
- Zheng L, Park J, Walls M, Tully M, Jannasch A, Cooper B, Shi R. Determination of Urine 3-HPMA, a Stable Acrolein Metabolite in a Rat Model of Spinal Cord Injury. J Neurotrauma. 2013;30:1334–1341. doi: 10.1089/neu.2013.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Sun Z, Jiang Y, Chen F, Wang M. Acrolein scavengers: reactivity, mechanism and impact on health. Mol Nutr Food Res. 2011;55:1375–1390. doi: 10.1002/mnfr.201100149. [DOI] [PubMed] [Google Scholar]