Abstract
Thoracotomy results in a high frequency of chronic post-operative pain. Resolvins are endogenous molecules, synthesized and released by activated immune cells, effective against inflammatory and neuropathic pain. Different resolvins have differential actions on selective neuronal and glial receptors and enzymes. This paper examines the ability of intrathecal Resolvin D1 (RvD1) and Resolvin D2 (RvD2) to reduce chronic postthoracotomy pain in rats. Thoracotomy, involving intercostal incision and rib retraction, resulted in a decrease in the mechanical force threshold to induce nocifensive behavior, an enlargement of the pain-sensitive area, and an increase in the fraction of rats showing nocifensive behavior, all for at least 5 weeks. The qualitative nature of the behavioral responses to tactile stimulation changed dramatically after thoracotomy, including the appearance of vigorous behaviors, such as turning, shuddering, and squealing, all absent in naive rats. Intrathecal delivery of RvD1 (30ng/30µL), at surgery or 4 days later, halved the spread of the mechano-sensitive area, lowered by 60% the percent of rats with tactile hypersensitivity, and reduced the drop in threshold for a nocifensive response, along with a reduction in the occurrence of vigorous nocifensive responses. RvD2’s actions on threshold changes were statistically the same. These findings suggest that intrathecal resolvins, delivered pre-operatively or several days later, can prevent chronic post-operative hyperalgesia.
Keywords: resolvins, post-operative pain, hyperalgesia, inflammation resolution, intrathecal drugs, perspective
INTRODUCTION
Chronic pain after surgery remains a major medical problem.32 Despite much research, knowledge of the mechanisms of chronic post-operative pain and of agents that are effective in its prevention remain limited. Peripheral factors, such as nerve growth factor5, endothelin-1,41 and other cytokines that are released by damaged tissue,13,46 have identified importance for the induction of acute post-incisional pain. The importance of spinal glutamate receptors67,68, TRPV14, and certain mitogen activated protein kinases (MAPK), such as p38, in both glia and neurons19,28, has been shown for induction of acute post-incisional pain61 and of prolonged, retraction-induced pain25 from skin/muscle incision retraction (SMIR), of 3–4 weeks duration.17 However, there have been almost no studies on the mechanisms that are critical for the pain that persists for months or longer after surgery, although clinical data show that younger age, female sex and the presence of prolonged pre-operative pain are all predictive of chronic post-operative pain.20,31
As many as 40–50% of post-thoracotomy patients suffer enduring pain for more than 6 months.7,21 Pain occurs at rest, i.e. with shallow breathing, and also has a pronounced movement-related component during stretching or twisting, an example of mechano-hyperalgesia.14,30 There is debate about the frequency with which this pain can be classified as neuropathic,27,40,62 and neurophysiological studies of chronic post-thoracotomy pain (CPTP) patients detect evidence of peripheral nerve damage.22,55 But the fact that peri-operative epidural blocks substantially reduce the incidence of long-term post-thoracotomy pain2 points to an important role of the central nervous system in this syndrome.
Research on the mechanisms underlying CPTP have benefitted from the development of a rat model that uses surgical procedures and has post-operative symptoms that closely parallel the clinical ones.8,26,54,58 In the present work we have used this model to study the ability to prevent or to reverse CPTP of 2 different resolvins, naturally occurring lipid mediators that facilitate the resolution of inflammation49,51 and are known to have anti-hyperalgesic effects on neuropathic and inflammatory pain.29,37,65
Our analysis includes metrics for pain behavior that go beyond the stimulus threshold for eliciting a simple nocifensive response, and include a classification of more complex behavior: the Qualitative Hyperalgesic Profile (QHP), which scores the frequency of a more complex repertoire of nocifensive behaviors.58
Resolvins, endogenous anti-inflammatory and pro-resolving mediators (for review see50), have been shown to have profound anti-hyperalgesic properties after peripheral nerve injury or tissue inflammation. These arise from actions on reactive neutrophils, macrophages42,50 and pro-inflammatory cytokine production,6,16 and also a direct action on spinal neurons and glia,63,65 including effects on pre- and post-synaptic elements of neuronal glutamatergic transmission as well as MAPK activation in glia.65
Earlier we showed that intrathecal Resolvin D1 was able to prevent the development of tactile hyperalgesia following soft tissue incision and retraction17, if given at the time of or a few days following surgery; however, if given a week or two after the surgery the analgesic effects were minimal and temporary.24 In the present experiments, we investigated the actions of two different resolvins, RvD1 and RvD2, that differ in their downstream targets, on the CPTP model to determine any differences in their anti-hyperalgesic actions, both when applied peri-operatively and at different times after surgery.
MATERIALS AND METHODS
Drugs
Resolvin D1 and RvD2 were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) and was dissolved in 100% ethanol to a stock solution of 0.267mM (1 µg/10 µL) and stored at −80°C. Less than 1 hour before injection, an aqueous solution of these resolvins was prepared by evaporating the stock RvD1 to dryness under a gentle nitrogen steam, then rapidly adding vehicle solution, PBS with 0.1% ethanol, while minimizing exposure to light. Pentobarbital (Sigma–Aldrich, St. Louis, MO, USA) was injected intraperitoneally (i.p.; 60 mg/kg) to provide general anesthesia during the placement of the intrathecal (i.t.) catheter and also during thoracotomy procedures.
Animals
All procedures were approved by the Harvard Standing Committee on Animals (Harvard Medical School, Boston, MA) and are in keeping with the Guide23 for the care and use laboratory animals. Animals were grouped into treatment or control groups with n=8 in each group. In most cases single groups were processed together, analyzed and compared with each other. Sometimes, as noted in Results, two groups were merged (n=16 rats). The person who injected the compounds, and who conducted the behavioral tests, was not blind to their identity.
Male Sprague-Dawley rats were purchased, at 225–250gm, from Charles River Laboratory (Wilmington, MA) and kept in the animal housing facilities at Brigham and Women’s Hospital, with controlled relative humidity (20%–30%), at room temperature (24°C), and under a 12–12 h light-dark cycle, with free access to food and water. They were handled by a single investigator (J.C-F.W, who also conducted all behavioral tests), for 5–7 days before the procedure to familiarize them with the experimental environment, to minimize stress-induced analgesia and to establish baseline behavioral parameters for each individual animal. At the time of surgery, animals weighted 280–310 g.
Surgical Procedures
Endotracheal Intubation
Rats were briefly anesthetized with 4%–5% sevoflurane (Sevorane, Abbott Laboratory, North Chicago, IL, USA) before receiving intraperitoneal pentobarbital sodium (60 mg/kg). Animals were then tracheally intubated by a modified method from Weksler et al..60 The anesthetized rat was placed in the supine position with a small pillow under the neck. An otoscope (Welch Allyn, Inc., Skaneateles Falls, NY) with a number 3 speculum was introduced into the animal’s oropharynx, and the tongue was gently retracted and fixed above the speculum by left index finger compression. A guide wire (spring-wire guide:0.46 mm dia. × 25 cm; Arrow® International, Inc., Reading, PA) was introduced through the epiglottis, then vocal cords, into the trachea. The otoscope removed over the wire, a 16-gauge polyethylene Angiocath(1.7 × 51 mm Insyte™ Autoguard™ winged; BD Infusion Therapy Systems Inc., Sandy, UT) was glided over the wire to its full length. The wire was removed, the catheter was connected to a Y-connector attached to tubing from a small animal pressure controlled ventilator (model TOPO220; Kent Scientific Corporation, Torrington, CN) which was set up at a respiratory rate of 65–80/min. An isoflurane vaporizer (SurgiVet) was connected to the intake of the ventilator to deliver the output concentration 1.0%–1.5% isoflurane in oxygen when necessary. A CO2 analyzer (model CapStar-100; IITC Inc., Woodland Hills, CA) was connected to the expiratory end to monitor end tidal CO2, which was maintained at 25–40 mm Hg for the entire surgical procedure.
Thoracotomy and Rib Retraction
Surgery followed the procedure of Buvanendran et al..8,58 Following endotracheal intubation (see above), rats were put at a left decubitus position with a pillow under the contralateral armpit to elevate the surgical field. The skin below the ear line and above the superior iliac crest was shaved on both sides. A 3-cm long incision was made in the skin of the right lateral chest wall along the fourth intercostal line, beginning from 1 cm lateral to the midline to 1 cm below the inferior angle of right scapula. The superficial and deep lateral thoracic muscles covering the ribs were incised and retracted to expose the intercostal muscles. A 1.0-cm incision was made through the intercostal muscle and pleura along the cranial border of the fifth rib. The blunt tines of a small retractor (model 17003-03, Goldstein 3×3 sharp teeth depth 4.5mm, teeth width 6.5mm; FST, Inc., Foster City, CA) were placed under the fourth and fifth ribs. The retractor was opened to separate the ribs by 1 cm, and was left in place for 60 min, as previously described.8 During this time, the open wound was covered with wet-dressing gauze kept moist with sterile phosphate buffered saline (PBS). After one hour the retractor was removed and the fourth and fifth ribs were approximated and ligated tightly with 3-0 chromic gut sutures. Air was aspirated from the pleural cavity with a 5-mL syringe attached to the tubing to restore normal intrapleural pressure. The superficial muscle covering the ribs was then apposed with 4-0 Vicryl sutures (MYCO Medical, Cary, NC), and the skin was closed with 3-0 silk sutures (Angiotech, Reading, PA). The animals were allowed to recover in separate, heated cages, and the endotracheal catheter was removed once spontaneous breathing was re-established.
Implanting the Intrathecal Catheter
Direct intrathecal (i.t.) injection caused neurological problems, e.g. forepaw motor deficits, in many rats, in preliminary experiments, probably due to the narrow dimensions of the spional cord in the thoracic region. Therefore, i.t. catheters were implanted, although not without some negative consequences (see below). At specified times after the thoracotomy procedure, rats were briefly anesthetized with 4%–5% sevoflurane and intraperitoneal pentobarbital (60/mg/kg) was administered, after which the rat was mounted in a stereotaxic device. The intrathecal polyethylene catheter (PE-10 tubing, Becton Dickinson and Company, Sparks, MD) was implanted by a modification of Yaksh and Rudy’s procedure.66 A skin incision was made at the midline, beginning at the ear line and extending 2.5 cm caudally. The superficial muscle layers were separated along the midline by incision and blunt dissection to expose the atlanto-occipital membrane. A 1–2 mm longitudinal slit, beginning at the base of the skull, was made along the midline of the membrane by a No. 11 surgical blade. Success of this step was indicated by the appearance of clear cerebrospinal fluid welling up through the slit. Tubing (PE-10), prefilled with PBS, that had a loose double knot 3 cm from the tip was inserted into the spinal subarachnoid space through the slit. The catheter was then carefully advanced while rotating it back and forth between the thumb and index finger. The knot of the catheter was fixed and buried in the muscle layers with 4-0 Vicryl stitches. Leaving 4 cm tubing exposed, and the skin closed with 3-0 silk sutures. Finally, the catheter was injected with 20 µl PBS to clear any residue from the insertion, and then was occluded at the open end with a short length of 30 gauge stainless steel wire.
For the results reported here, each catheter was left in place for 1 hour after the injection, then removed. When any behavioral signs of damage to the spinal cord or root were observed, e.g., ataxia, or areflexia to forepaw pinch, which occurred in <10% of the catheterized rats, that animal was euthanized and any baseline data not included in the study.
We had originally intended to implant the catheters at the time of thoracotomy, and then to leave them in place until and after the i.t. injections were completed. However, in several preliminary experiments we observed mechano-hypersensitivity developing around the thoracic trunk in half the rats, after just 1–2 days, and persisting for the entire time the catheters were present, even when no thoracotomy surgery was performed. Therefore, all data are from rats that had only a briefly in-dwelling catheter (1–2 hrs), just long enough to deliver the liquid.
Although these data were not included in the analysis, we did note that when RvD1 was injected through these indwelling catheters 4–6 days after the implant the threshold drop was less than that when vehicle was injected, suggesting that the hypersensitivity was caused by injury or inflammation at the catheterized region, not altogether surprising considering the narrow i.t. space available in the thoracic zone.
Intrathecal resolvin administration
Either at the time of thoracotomy or 4 or 14 days afterwards, rats received either Resolvin or Vehicle, the latter serving as the Control group. All rats had intrathecal (i.t.) catheters inserted in the thoracic region for drug delivery to the thoracic spinal cord that receives inputs from the primary incisional area. Three treatment groups, each with 8 rats, were studied for delivery at the time of surgery: one group was injected intrathecally with RvD1 (30ng/30µL), another with the same dose/volume of RvD2, and a third, Control group with 30 µl Vehicle (phosphate buffered saline, PBS, at pH 7.2–7.4; see Figure 1A). Any residual drug, or vehicle, was flushed from the catheter with a subsequent delivery of 10µl of PBS. The catheter was kept in place for 1 hour, with the outer end sealed by heat.
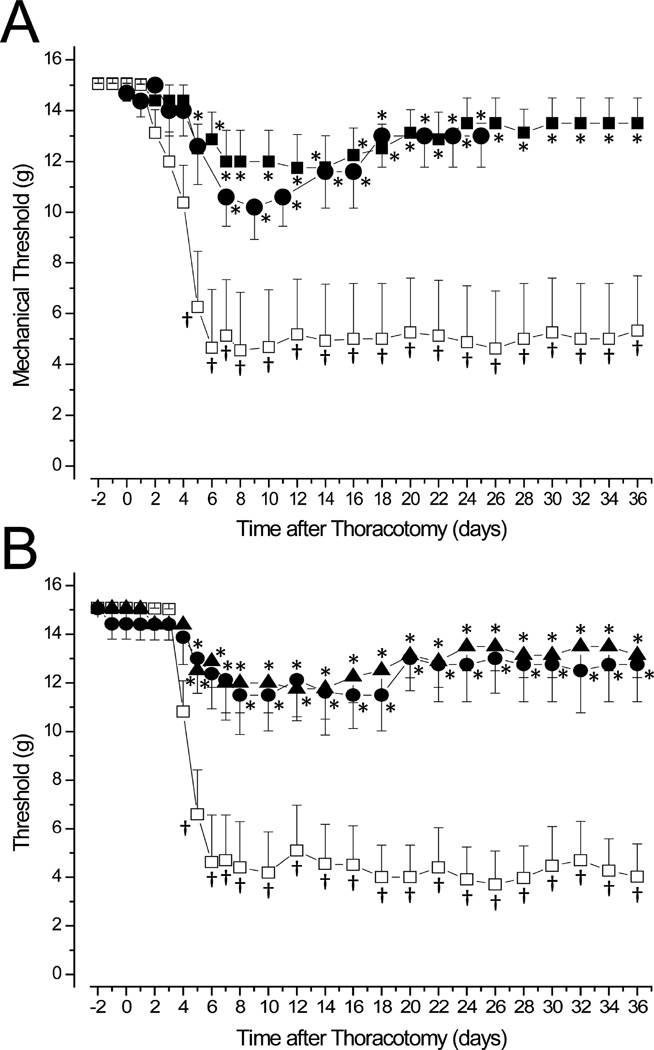
Figure 1.
Intrathecal delivery A. at the day of surgery, POD0, of RvD1 (■) or RvD2 (●), both at 30ng/30µL), slows the development and prevents the chronic stage of post-thoracotomy-induced mechano-hypersensitivity, shown for the Control group after i.t. injection of vehicle (□, 30µL PBS). B. No difference in the threshold changes from RvD1 delivery occurred when the same i.t. dose was injected on POD4 (●) or POD0 (▲). * p<0.05 for comparison with vehicle-injected rats. † p<0.05 for comparison with baseline, preoperative threshold.
In a related experiment, either RVD1(30ng/30µL), or Vehicle was injected 4 days after surgery (Figure 1B).
In a third experiment, rats that had been subject to thoracotomy 14 days beforehand were implanted with a catheter that remained for 12h and injected 4 times with RvD1 (30ng/30µL) over that time. There was no Control group in this study of delayed administration.
The person doing the injection, the same as the one conducting the behavioral assays (J C-F W), was aware of the identity of the injected substances.
Behavioral Testing
To test mechanical hyperalgesia, each rat was placed in a restricted cage and allowed to rest there for 15 min. A series of calibrated von Frey filaments (VFH; Stoelting Co, Wood Dale, IL) with bending forces ranging from 0.4 to 15 g were applied perpendicularly to the dorsal skin surface, starting from the lowest force, to calculate the threshold for a nocifensive response.9 The shaved skin was marked by an indelible pen with a grid of 1cm × 1cm squares, centered on the midline, and the VFH tip was placed at a location within each grid to allow mapping of the sensitivity of skin areas. Each VFH was applied sequentially at a particular rostro-caudal band, starting at the contralateral side, moving towards the midline, and then extending to the ipsilateral area. By this process, the skin was tested along bands at each dorso-thoracic segments, moving from the least to the most sensitive area. Each testing spot was probed twice, pressing with a 3 sec duration spaced 3 sec apart. There was a 30-sec resting period between testing each location. Rats not responding to the highest force were assigned a threshold of 15.1 g., avoiding stronger forces in order to minimize the tactile sensitization that occurs with repeated poking by stiffer VFHs.
In one analysis of tactile hypersensitivity, the threshold for nocifensive responses, determined as the minimum force to produce any response, was calculated for each experimental group.9 In a second analysis, tactile allodynia was functionally defined by the presence of any response to a VFH of 10gm force or lower, since that force never caused a response in handled animals in the pre-operative period; the fraction of (8) rats in each group that responded to a 10g VFH was termed the “allodynia index”.
In a third mode of analysis, the characteristics of nocifensive behavior were categorized by the Qualitative Hyperalgesia Profile (QHP) into four grades, according to the behavior displayed58: Grade 0= No response; Grade I= slight lateral withdrawal movement of trunk or local subcutaneous muscle contraction (the cutaneous truncii muscle response, CTMR57); Grade II= withdrawal or CTMR (as in Grade II) followed by turning around by 180° (shifting foot position to change location); Grade III= behavior of Grade II plus scratching near the test location, shuddering, or audible vocalization. Thus, in a third type of analysis each experimental group was graded according to the fraction of animals that showed Grades 0, I, II or III at the threshold VFH force for that particular rat.
Finally, the responsive area at which any of these behaviors was first induced by the weakest VFH, i.e., the threshold for the least intense nocifensive response, was mapped for each individual rat and these were graphically summed to give a “density map” showing the anatomical distribution of post-operative hypersensitivity for all the rats in each experimental group.
Behavioral evaluations were made over 2 days before thoracotomy, and averaged as the baseline, and then daily at post-operative days (PODs) 1–8, then every 2 days until day 36, at which time animals were euthanized by CO2 asphyxiation. In the cases where RvD1 was administered after surgery, behavior was evaluated at 3h after injection and then daily or every other day, as per the above-described schedule. For the cumulative dosing at late times (14d) after thoracotomy, the catheter was placed on the day of injection and RvD1 injected 4 times, at 3h intervals.
Statistical Analysis
Threshold forces are presented as mean ± SEM, and are compared for statistically significant differences among groups by multi-group ANOVA followed by Tukey’s pair-wise test. When compared to the pre-operative baseline value or to the post-operative value, these analyses are Bonferroni corrected for repeated measures. The “allodynia index” differences were assessed by Fisher’s exact test. The behavior in the QHP analysis is reported as the population distribution among the different grades in any one treatment group; significant differences among distributions in the treatment groups were determined by Fisher’s exact test with Bonferroni correction. All statistical tests were conducted using SAS version 9.3 software (SAS, Cary, N.C.). Unless specifically noted, significance occurred for p<0.05.
RESULTS
Thoracotomy-induced hypersensitivity is protected against by Resolvin D1 and D2
Thoracotomy, involving incision and 60 min of rib retraction, was followed in 2–4 days by a drop in threshold for nocifensive responses from 15.1gm to ~4–5gm, when the intrathecal catheter was inserted and vehicle injected, the catheter remaining in the thoracic area for 1h, and then removed (Figure 1A). This response of the Control group showed a low threshold value that persisted for the full 5 weeks of post-operative testing. (Sham surgery, accomplished without any catheter and involving only intercostal incision and no retraction, caused only a brief (2–3d) and small (by ~2–3g) drop in threshold, as previously reported by Buvanendran et al.8, data not shown.)
If RvD1 was delivered through the catheter 20–30 min before surgery (POD0) the resultant drop in threshold was much smaller (Figure 1A). There was a longer delay in onset and then threshold fell to ~12g from POD6 to POD16, then rose to ~14g for the remainder of the testing period, but none of these values was significantly below the pre-operative baseline level. Intrathecal delivery of the same dose of RvD2 had the same effects (Figure 1A).
If RvD1 was injected at POD4, just at the time when hypersensitivity usually first appears58, the fall of threshold was slowed and the minimum value was reduced compared to the responses when thoracotomy occurred with no catheter (Figure 1B) or when vehicle was injected at this time (data not shown). The time-courses of the threshold changes from RvD1 injection at POD0 and POD4 were indistinguishable from each other, and there was, for both, a suggestion of recovery back towards baseline, preoperative values at about POD18-20 (Figure 1B).
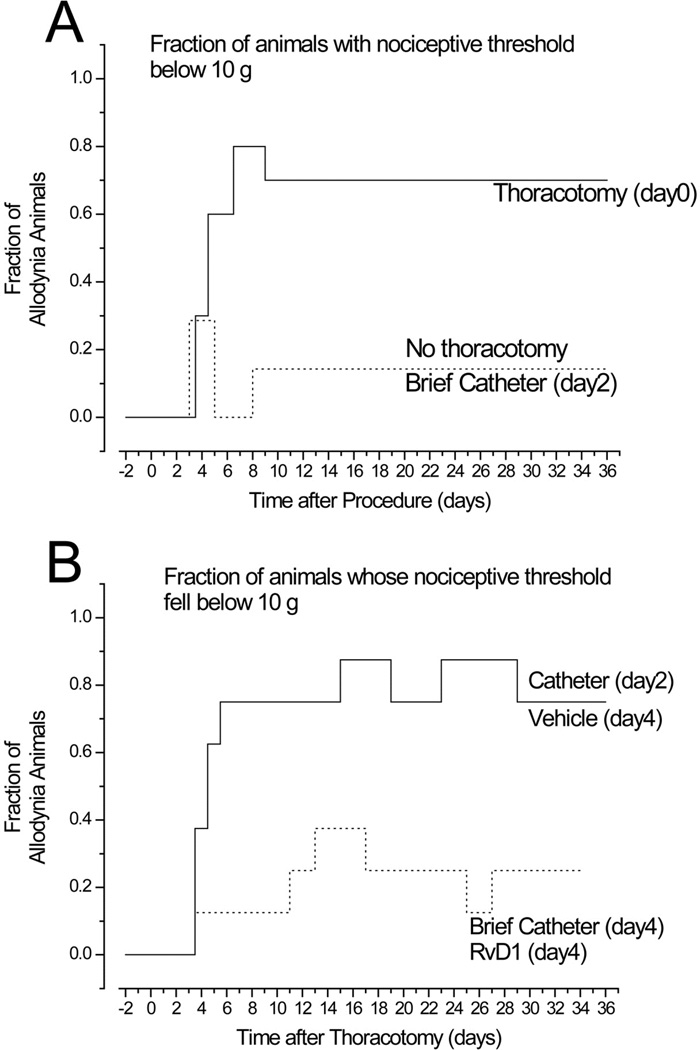
As a second measure of post-operative tactile hypersensitivity, the allodynia index was increased by thoracotomy (Figure 2). Rats with thoracotomy but never having a catheter inserted had an allodynic index of 7/10 (Figure 2A). If thoracotomy occurred followed by vehicle injection at POD4, the allodynia index ranged from 6/8 to 7/8 (Figure 2B), not different from thoracotomy with no catheter (Fisher’s exact test), so that these two groups were merged as one Control group. If the catheter was inserted, vehicle injected, then removed, but without any thoracotomy, the allodynia index was only 1/8 (Figure 2A). However, when thoracotomy was followed by RvD1 (30µg/30µL), injected through the briefly implanted catheter on POD4, the allodynia index was 0-1/7 (Figure 2B), significantly different from the index of the thoracotomy Control group (p=0,0136, Fisher’s exact test), but not different from that after a catheter was inserted briefly (on day 2) and then removed, with no thoracotomy (Figure 2A). By the “allodynia index” metric, therefore, RvD1 prevented any post-thoracotomy hypersensitivity.
Figure 2.
The allodynia index, as the fraction of animals that show a nocifensive response to tactile (VFH) stimulation at <10gm force, is A. increased after thoracotomy (with no treatment, ________), but not changed by insertion and removal of an i.t. catheter when no surgery was performed (- - - -). B. If catheter is inserted and vehicle injected, thoracotomy still causes a high score on the allodynia index (_________), but if RvD1 (30ng/30µL) is delivered i.t. on POD4 (- - - - -) the index is the same as if no thoracotomy had occurred and the values lie significantly below that from thoracotomy alone or after i.t. vehicle (p=0.0136, Fisher’s exact test).
(An indwelling catheter alone, left in the thoracic spinal space for 4 days or longer, without thoracotomy, raised the allodynia index to 5/10 (graphical data not shown), confirming the drop in threshold that occurred with indwelling catheters, see above).
Anatomical distribution of elevated pain responses after thoracotomy
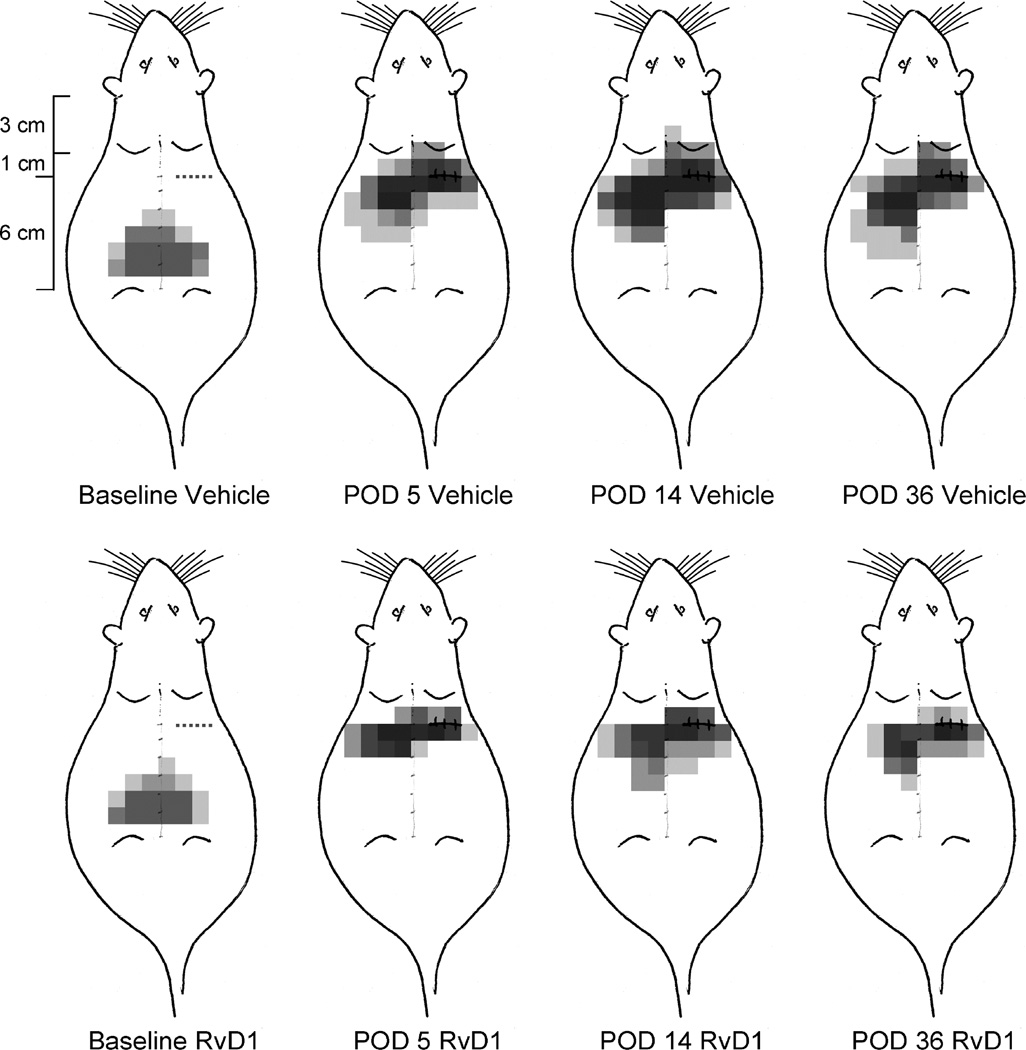
Before thoracotomy the area of lowest threshold was centered at the midline, around the L3–L5 vertebra and extending 2–4 cm bilaterally (Figure 3). Many of the rats showed no response, even to a 15g VFH, and none had the lowest threshold at the site of future incision/retraction, shown by the dotted line in the “Baseline” schematics of Figure 3. Five days after surgery (POD5) the most sensitive area had shifted to a more rostral location, on the ipsilateral side, around the incision/retraction site and, importantly, on the contralateral side, and slightly more caudal. This pattern occurred whether an i.t. catheter had been briefly placed and then removed (at POD0) or not placed at all. There was a concomitant lateral spread in the area of lowest threshold, both ipsilaterally and contralaterally. At this time all of the rats responded to at least one of the filaments of 15g or less, as shown by the darker pain “density map” (Figure 3). By the 14th post-operative day, the area of heightened responsiveness had increased slightly over that at POD5 and the fraction of responsive rats (indicated by the darkness due to the overlapping sensitive regions) had reached maximum, although the overall anatomical distribution was centered on the same loci as in POD5. At POD36 the area of pain had somewhat regressed but the same fraction of rats responded to a tactile stimulus applied near the wound.
Figure 3.
Schematic diagram of the anatomical distribution of the areas of lowest threshold for nocifensive responses in rats receiving thoracotomy, with i.t. vehicle on POD0 (top row) or RvD1 (30ng/30µL) on POD0 (bottom row). The area on the shaved back was marked by a grid with 1cm × 1 cm squares, and stimuli were applied within those squares. A single gray square shows the area of lowest threshold for response in any one rat, and the superposition of these squares for all the 8 rats in the two treatment groups (vehicle and RvD1) results in the density distribution pattern seen here. When no response occurred at the highest applied force (15 gm VFH), then no square was darkened.
The nature of nocifensive responses is altered by thoracotomy
The Qualitative Hyperalgesic Profile (QHP) was used as an indicator of the perceived intensity of the nocifensive response. As reported previously, pre-operative tactile stimulation of handled rats caused reactive behavior characterized by equal populations with no response58, Grade 0, or a weak, local response, Grade I (Figure 4; see Methods). Thoracotomy, with vehicle injected through a removed catheter, resulted in the appearance of the more intense behaviors of Grades II and III, respectively a rotation of the body (II) and scratching, shuddering or squeaking (III). For the 2 groups of 8 rats used for vehicle and RvD1 treatment in this study (groups were merged for n=16 per treatment), none remained unresponsive (with Grade 0) after the operation. This profile shift in the vigor of response was fairly constant through PODs 5, 14 and 36, with an increasing % of the rats showing Grade II and Grade III behavior (Figure 4A).
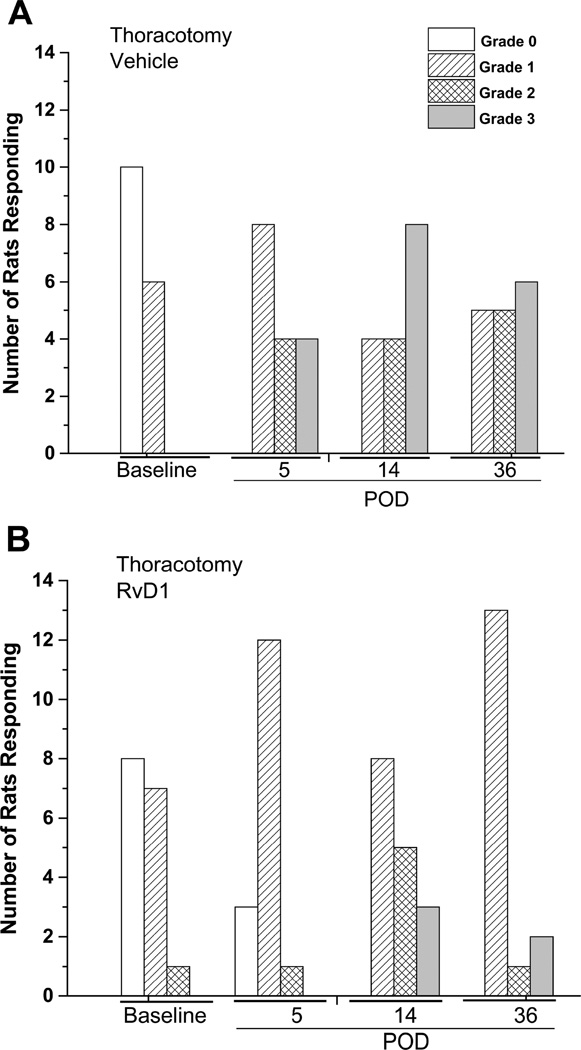
Figure 4.
The Qualitative Hyperalgesia Profile for 5 weeks after thoracotomy A. with vehicle injection on POD0 shows a shift from the baseline (BL) pattern, where either no response (empty bar) or Grade I (diagonal stripes) was observed, to a pattern where all of the rats respond, many with the vigorous nocifensive behaviors of Grade II (cross-hatched) or Grade III (shaded). In contrast, when thoracotomy is preceded by RvD1 (30ng/30µL, i.t.) the majority of rats show the much milder Grade I behavior (see Methods for description). Jonkheere-Terpstra statistics comparing the distributions on the same post-operative day between Thoracotomy with vehicle and that with RvD1 give p values (two-sided) of: Baseline:1.000; POD5:0.687; POD14:0.204; POD36:0.010.
These more vigorous nocifensive responses were suppressed by i.t. RvD1, also with n=16 rats. Delivery on POD4, through a briefly inserted catheter, reduced the number of post-operative animals with Grades II and III (Figure 4B) and concomitantly increased the number of rats responding with Grades 0 and I behavior. The small fraction of rats that displayed thresholds for response greater than 10 gm after RvD1 and thoracotomy (see Figure 2B), were still predominantly characterized by Grade I behavior. Because naïve, pre-operative rats showed only Grades 0 & I behavior, whereas postoperative rats with no treatment or receiving only vehicle were the only ones to show Grades II & III behavior, we distributed the animals in the vehicle and in the RvD1 groups into two categories: those with Grades 0 & I (“naïve”, as in Baseline behavior) and those with Grades II & III. Comparison of the two treatment groups on the respective post-operative days, using Fisher’s exact test, gave p values of 1 (baseline), 0.0155 (POD5), 0.273 (POD14), and 0.0113 (POD36), showing that RvD1 significantly reduced the intense pain behavior classified by QHP analysis on post-operative days 5 and 36, but not on day 14 (Figure 4).
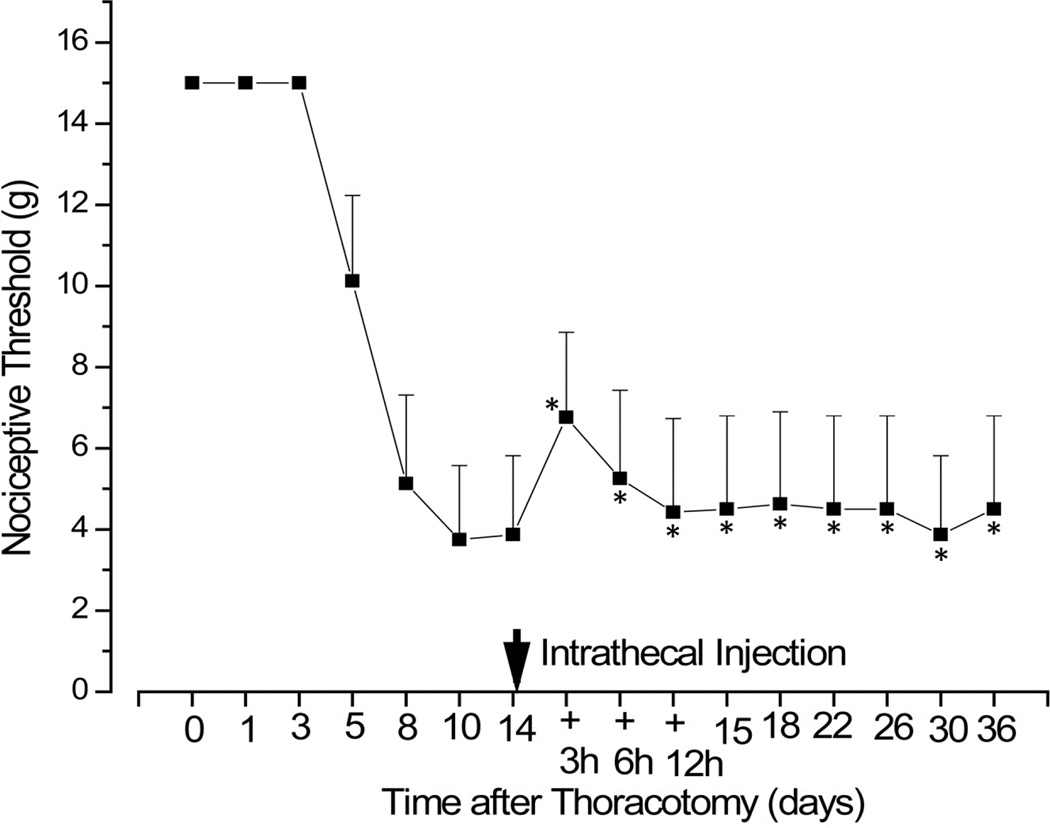
When RvD1 (30ng/30µL) was injected i.t. 14 days after thoracotomy, through a catheter implanted on POD12, only a partial and very transient reversal of hypersensitivity was observed (Figure 5). Three subsequent injections of this same dose over the next 12 h produced no further relief, as if the initial response had desensitized.
Figure 5.
When i.t. delivery of RvD1 (30ng/30µL) was delayed until POD14 (via a catheter implanted on that day), no significant reversal of hypersensitivity occurred, either with the single bolus dose, or after 3 subsequent doses given over the next 12 hours. * p>0.05 for comparison with threshold on POD 14, before the first RvD1 inject.
DISCUSSION
The results of this investigation show that RvD1 and RvD2 are equally effective in preventing the chronic pain that follows experimental thoracotomy. Thoracotomy – induced tactile hypersensitivity, shown by reductions of mechanical threshold for nocifensive responses, the fraction of rats with allodynia and responding with pain-like behavior to mechanical stimuli that normally cause no such behavior, and the appearance of vigorous nocifensive reactions such as turning, shuddering and squealing, are either absent or strongly reduced in rats that received intrathecal resolvins. These strong anti-hyperalgesic affects were present if the resolvins were delivered at the time of surgery or as late as 4 days post-operatively, although a long delay in delivery of the same dose of RvD1, or of a sequence of 3 doses, at 2 weeks after surgery was ineffective. In contrast to this transient effect of intrathecal resolvins on existing pain, the delivery of Neuroproectin/Protectin D1 to the injury location results in long-term pain relief from chronic constriction injury-induced pain, although RvE1 had only a transient anti-hyperalgesic effect.64
Thoracotomy is one surgical procedure that results in a high incidence, ca. 50%, or chronic pain, reported at 3 and 6 months post-operative.7 Such pain is refractory to the usual pain medications, such as opiates, which themselves have adverse side effects. The model of experimental, rodent thoracotomy used here has been employed in earlier studies to show that spontaneous pain is present26 and that vigorous pain behavior beyond that of a simple, brief nocifensive withdrawal, follows this surgery.58 Furthermore, surgically-associated nerve injury, underlying neuropathic pain, has been diagnosed in a large fraction of those individuals suffering from chronic post-thoracotomy pain22,40 and injury to both large fibers8; and non-myelinated, small fibers58 occurs after experimental art thoracotomy. Therefore, the findings here are likely to have high relevance to the clinical phenomenon of chronic post-operative pain.
These current findings on experimental thoracotomy are quite similar to our earlier findings with i.t. RvD1 on SMIR-induced pain. There we found that delivery 30 min before surgery or 2 days afterwards had the same strong anti-hyperalgesic action, although there was a weaker effect on allodynia, measured with weak force VFHs (4–6 gm), than the hyperalgesia measured with strong ones (10–15 gm;).24 The two surgical procedures differ in important ways, however. Skin incision and retraction of the soft tissue skin and muscle in the SMIR procedure does not injure the peripheral nerve, and the post-operative tactile hypersensitivity resolves after 3–4 weeks17, whereas thoracotomy-induced pain persists undiminished for at least 9 weeks8 and is accompanied by extensive damage to intercostal peripheral nerves8,58 which contribute to much of the chronic post-operative pain.40,62 Experimental thoracotomy induces cold allodynia8,54, but neither cold nor heat allodynia follows SMIR.17 Tactile hypersensitivity in both models is similarly abolished by systemic morphine or gabapentin given 1–2 weeks after surgery.8,18
Despite these differences, the two surgical models produce pain behaviors that are about equally responsive to i.t. resolvins. What might be the common pathways for hyperalgesia that is targeted by intrathecal resolvins? Although the spinal cord is the intended region targeted by intrathecal delivery of solutions, diffusion of agents down spinal nerves to reach the dorsal root ganglia (DRG) has been documented, so primary afferent neurons as well as spinal cells are possible target locations. Different cell types in both locations express receptors for resolvins and have physiological responses to these compounds.
The salutary actions of resolvins and other pro-resolving mediators on pain and inflammation are well established.29,37,38,47,49,51 Resolvins have direct actions on the recruitment and migration of neutrophils and macrophages, underlying the resolution phase of inflammation48,51–53, and they also alter peripheral and central nervous system responses to inflammation and injury, through direct actions on neurons and glia.65 Since the drugs that are traditionally used to treat inflammatory and post-operative pain, namely opiates and cyclo-oxygenase-2 (COX-2) inhibitors, have unwanted side effects, there is a need to find new approaches and compounds that will be more selective in their actions and have fewer adverse actions.
The primary target is one of the GPCRs known to bind resolvins; for RvD1 the receptor in rodents is ALX/FPR211,15,42,48 and for RvD2 the receptor is GPR18.10 In macrophages RvD2 activation of GPR18 stimulates intracellular increases in cAMP without increasing intracellular Ca2+ while RvD1 activation of ALX/FPR2 stimulates phosphorylation rather than increase in intracellular Ca2+ or cAMP. These in turn regulate several downstream targets, some that are involved in acute central sensitization that induces persistent hyperalgesia, and others that act through microRNAs to alter transcription and effect a more long-lasting genomic response. The rapid responders include transient receptor potential (TRP) channels3,43,44, TNF-α activity1,33,39, mitogen-activated protein kinase (MAPKinase) activation, particularly p38 and ERK and jnk phosphorylation64,65, and NMDA receptors.16 The slower responses that are longer lasting include changes in microRNAs that regulate receptor and ligand expression in inflammatory cells35,36 and are likely to also participate in such regulation in neurons and glia.
One class of target molecule that contributes importantly to regulate spinal neurotransmitter release is the TRP channels12,34,45, particularly TRPV1 and TRPA1. RvD1 and RvD2 are selective inhibitors of TRP channels via activation of GPRC that regulate these channels, with RvD2 having more potent actions and inhibiting both TRPV1 and TRPA144, while RvD1 selectively inhibits only TRPA1.3 Therefore, their equi-effectiveness in preventing chronic post-thoracotomy hypersensitivity suggests that the spinal action is directed at TRPA1. The finding that TRPA1 is associated with mechanical hyperalgesia59, the modality of post-operative hypersensitivity tested here, is further support for this hypothesis. However, there are other potential target sites that might be altered by the spinal resolvins, and the identification of ALX/FPR2 receptor on spinal astrocytes, the corresponding inhibition of MAPK activation by LXA456 and the observation that jnk activation is associated with prolonged pain19 suggest that this pathway may also be important for preventing chronic pain.
In conclusion, intrathecal RvD1 and RvD2 are both effective in preventing the development of post-thoracotomy pain in male rats, when given around the time of surgery, and far less effective if given much later. The cellular targets of thse anti-hyperalgesic actions are not known, but it is likely that such small doses of these molecules are acting within the spinal cord or roots. A broader region of activity might be accessed by systemic drugs, from a distribution through oral administration of specialized pro-resolving mediators.50 Clearly, more research is needed to identify the mechanisms of action of resolvins and other pro-resolving mediators in their remarkable ability to prevent chronic post-operative pain.
HIGHLIGHTS.
Thoracotomy in rats causes chronic touch-evoked pain lasting for months
Spinally delivered compounds Resolvin D1 and Resolvin D2 prevent this pain
Pain prevention occurs when resolvins are given at or 4 days after surgery
Little reversal of existing pain occurs when Resolvin is given 2 weeks after surgery
PERSPECTIVE.
In studies of rats, the injection of the pro-resolving compounds of the Resolvin-D series into spinal fluid, before or just after thoracotomy surgery, prevents the occurrence of acute and chronic pain. If these chemicals, which have shown no side-effects, were used in humans it might greatly reduce chronic post-operative pain.
Acknowledgments
Advice in the writing of this article from Professor Charles Serhan, Center for Experimental Therapeutics and Reperfusion Injury, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, is gratefully acknowledged, as is support in drawing the figures from Mr. James Bell and Dr. Alla Khodorova. Dr. Chin-Chuan Huang provided assistance with the statistical analyses.
This work was partially supported by b a grant from the National Institutes of Health: R-01 CA080153 (to GS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare that they have no personal, financial or relational conflicts of interest with this work.
References
- 1.Abdelmoaty S, Wigerblad G, Bas DB, Codeluppi S, Fernandez-Zafra T, El-Awady el S, Moustafa Y, Abdelhamid Ael D, Brodin E, Svensson CI. Spinal actions of lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced mechanical hypersensitivity and spinal TNF release. PLoS One. 2013;8:e75543. doi: 10.1371/journal.pone.0075543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111:711–720. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple antinociception. Br J Pharmacol. 2010;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain. 2009;141:41–51. doi: 10.1016/j.pain.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Bannenberg GL, Chiang N, Ariel A, Makoto A, Tjonahen E, Gotlinger KH, Song H, Serhan CN. Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 7.Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: Meta-analysis. J Pain. 2014;15:887–897. doi: 10.1016/j.jpain.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Buvanendran A, Kroin JS, Kern JM, Nagalla SNK, Tuman KJ. Characterization of a new animal model for evaluation of persistent post-thoracotomy pain. Anesth Analg. 2004;99:1453–1460. doi: 10.1213/01.ANE.0000134806.61887.0D. [DOI] [PubMed] [Google Scholar]
- 9.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 10.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiurchiù V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Science Transl Med. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SI, Lim JY, Yoo S, Kim H, Hwang SW. Emerging Role of Spinal Cord TRPV1 in Pain Exacerbation. Neural Plast (Article) 2016;5954890:1–10. doi: 10.1155/2016/5954890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiol. 2006;104:1274–1282. doi: 10.1097/00000542-200606000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Dajczman E, Gordon A, Dreisman H, Wolkove N. Long-term post-thoracotomy pain. Chest. 1991;99:270. doi: 10.1378/chest.99.2.270. [DOI] [PubMed] [Google Scholar]
- 15.Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, Smith RD, Wheway K, Watkins B, Roche L, Carr AJ. Inflammation activation and resolution in human tendon disease. Sci Transl Med. 2015;7:311ra173. doi: 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng QX, Feng F, Feng XY, Li SJ, Wang SQ, Liu ZX, Zhang XJ, Zhao QC*, Wang W. Resolvin D1 reverses chronic pancreatitis-induced mechanical allodynia, phosphorylation of NMDA receptors, and cytokines expression in the thoracic spinal dorsal horn. BMC Gastroenterol. 2012;12:148. doi: 10.1186/1471-230X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flatters SJL. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Pain. 2008;135:119–130. doi: 10.1016/j.pain.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flatters SJL. Effect of analgesic standards on persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Neurosci Lett. 2010;477:43–47. doi: 10.1016/j.neulet.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008;437:180–183. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottschalk A, Ochroch EA. Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. Clin J Pain. 2008;24:708–716. doi: 10.1097/AJP.0b013e318174badd. [DOI] [PubMed] [Google Scholar]
- 22.Guastella V, Mick G, Soriano C, Vallet L, Escande G, Dubray C, Eschalier A. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain. 2011;152:74–81. doi: 10.1016/j.pain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Guide for the Care and Use of Laboratory Animals. National Research Council of the National Academies. 8th. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 24.Huang L, Wang CF, Serhan CN, Strichartz G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Gao Y-J, Wang J, Strichartz G. Shifts in cell-type expression accompany a diminishing role of spinal p38-MAPKinase activation over time during prolonged postoperative pain. Anesthesiol. 2011;115:1281–1290. doi: 10.1097/ALN.0b013e31823499cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung C-H, Wang JC-F, Strichartz G. Spontaneous chronic pain after experimental thoracotomy revealed by conditioned place preference: morphine differentiates tactile evoked pain from spontaneous pain. J Pain. 2015;16:903–912. doi: 10.1016/j.jpain.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito N, Obata H, Saito S. Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiol. 2009;111:640–648. doi: 10.1097/ALN.0b013e3181b05f42. [DOI] [PubMed] [Google Scholar]
- 28.Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji RR, Xu ZZ, Strichartz GR, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmakar MK, Ho AM. Post-thoracotomy pain syndrome. Thorac Surg Cli. 2004;14:345–352. doi: 10.1016/S1547-4127(04)00022-2. [DOI] [PubMed] [Google Scholar]
- 31.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 32.Kissin I, Gelman S. Chronic postsurgical pain: still a neglected topic? J Pain Res. 2012;5:473–489. doi: 10.2147/JPR.S35145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein CP, Sperotto ND, Maciel IS, Leite CE, Souza AH, Campos MM. Effects of D-Series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology. 2014;86:57–66. doi: 10.1016/j.neuropharm.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 34.Kosugi M1, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci. 2007;27:4443–4451. doi: 10.1523/JNEUROSCI.0557-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Dalli J, Chiang N, Baron RM, Quintana C, Serhan CN. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by MicroRNA and proresolving mediators. Immunity. 2013;39:885–898. doi: 10.1016/j.immuni.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JY, Park C-K, Hwang SW. Biological Roles of Resolvins and Related Substances in: The Resolution of Pain. BioMed Rsch International. 2015;2015 doi: 10.1155/2015/830930. Article ID 830930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu ZH, Miao GS, Wang JN, Yang CX, Fu ZJ, Sun T. Resolvin D1 Inhibits Mechanical Hypersensitivity in Sciatica by Modulating the Expression of Nuclear Factor-kB, Phospho-extracellular Signal-regulated Kinase, and Pro- and Anti-inflammatory Cytokines in the Spinal Cord and Dorsal Root Ganglion. Anesthesiol. 2016;124:934–944. doi: 10.1097/ALN.0000000000001010. [DOI] [PubMed] [Google Scholar]
- 39.Lobo BW, Lima CK, Teixeira MS, Silva NL, Takiya CM, Ramos MF, Miranda AL, Dellamora-Ortiz GM. Fish oil attenuates persistent inflammatory pain in rats through modulation of TNF-a and resolvins. Life Sci. 2016;152:30–37. doi: 10.1016/j.lfs.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 40.Maguire MF, Latter JA, Mahajan R, Beggs FD, Duffy JP. A study exploring the role of intercostal nerve damage in chronic pain after thoracic surgery. Eur J Cardio-Thor Surgery. 2006;29:873–879. doi: 10.1016/j.ejcts.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 41.Mujenda FH, Duarte AM, Reilly EK, Strichartz GR. Cutaneous endothelin-A receptors elevate post-incisional pain. Pain. 2007;133:161–173. doi: 10.1016/j.pain.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocytes recruitment to inflammatory loci: receptor dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park CK, Lü N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1- and TNF-alpha-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quartu M, Serra MP, Boi M, Poddighe L, Picci C, Demontis R, Del Fiacco M. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J Anat. 2016;12529 doi: 10.1111/joa.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain. 2009;145:341–349. doi: 10.1016/j.pain.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito T, Hasegawa-Moriyama M, Kurimoto T, Yamada T, Inada E, Kanmura Y. Resolution of inflammation by Resolvin D1 is essential for peroxisome proliferator-activated receptor-γ-mediated analgesia during postincisional pain development in Type 2 diabetes. Anesthesiol. 2015;123:1420–1434. doi: 10.1097/ALN.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 48.Schmid M, Gemperle C, Rimann N, Hersberger M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J Immunol. 2016;196:3429–3437. doi: 10.4049/jimmunol.1501701. [DOI] [PubMed] [Google Scholar]
- 49.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin JW, Pancaro C, Wang CF, Gerner P. The effects of resiniferatoxin in an experimental rat thoracotomy model. Anesth Analg. 2010;110:228–232. doi: 10.1213/ANE.0b013e3181c5c89a. [DOI] [PubMed] [Google Scholar]
- 55.Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9:955–961. doi: 10.1016/j.jpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theriault E, Diamond J. Nociceptive cutaneous stimuli evoke localized contractions in a skeletal muscle. J Neurophysiol. 1988;60:446–462. doi: 10.1152/jn.1988.60.2.446. [DOI] [PubMed] [Google Scholar]
- 58.Wang JC-F, Hung C-H, Gerner P, Ji R-R, Strichartz GR. The Qualitative Hyperalgesia Profile: A New Metric to Assess Chronic Post-Thoracotomy Pain. Open Pain J. 2013;6:190–198. doi: 10.2174/1876386301306010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei H, Hämäläinen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiol. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- 60.Weksler B, Ng B, Lenert J, Burt M. A simplified method for endotracheal intubation in the rat. J Appl Physiol. 1994;76:1823–1835. doi: 10.1152/jappl.1994.76.4.1823. [DOI] [PubMed] [Google Scholar]
- 61.Wen Y-R, Suter MR, Ji R-R, Yeh G-C, Wu Y-S, Wang K-C, Kohno T, Sun W-Z, Wang C-C. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiol. 2009;110:155–165. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 62.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J CardiothoracSurg. 2009;36:170–180. doi: 10.1016/j.ejcts.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Xu ZZ, Berta T, Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu ZZ, Liu XJ, Berta T, Park CK, Lü N, Serhan CN, Ji RR. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol. 2013;74:490–495. doi: 10.1002/ana.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 67.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 68.Zahn PK, Sluka KA, Brennan TJ. Excitatory amino acid release in the spinal cord caused by plantar incision in the rat. Pain. 2002;100:65–76. doi: 10.1016/s0304-3959(02)00241-5. [DOI] [PubMed] [Google Scholar]