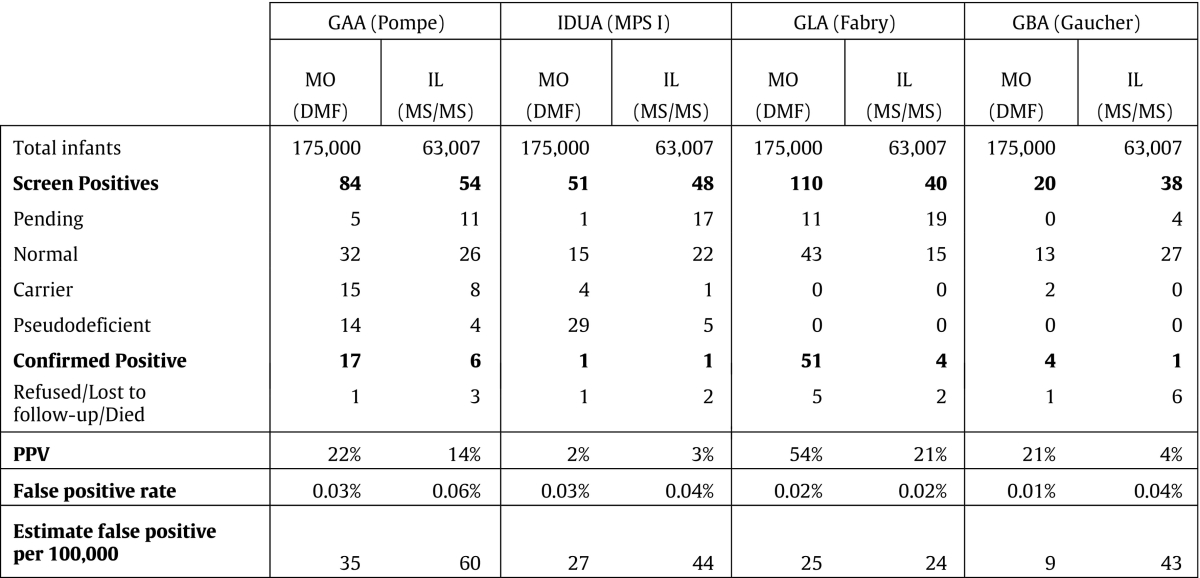
Sir – Newborn screening (NBS) programs are actively considering investment in one of two available platforms for multiple lysosomal storage disease (LSD) enzyme testing – tandem mass spectrometry (MS/MS) or digital microfluidic fluorometry (DMF) system. Both use reagent kits supplied by commercial vendors (Perkin-Elmer Life Sciences and Baebies, Inc., respectively) that are inexpensive and readily available. However, the enormous cost differential for infrastructure, capital investment, personnel and ongoing maintenance between MS/MS and DMF is difficult to justify unless there is substantial evidence of superior performance by the more expensive platform (MS/MS) [1]. We are alarmed that proponents of MS/MS repeatedly make claims to that effect without relevant supporting evidence [1], [2], [3], [4]. The clinical decision point (high risk for an LSD) is made near the LSD assay's low limit of quantification, where pre-analytical factors, including leukocyte count, pseudodeficiency alleles and sample quality cause low enzyme activity that overlaps the high-risk range [5]. In this context, the “analytical/dynamic range” of a method [3], [4] is irrelevant; the appropriate metric for assay performance is the ability to discriminate normal from confirmed positive cases when screening prospectively. Rich data sets (Table 1) are available from two programs screening for LSDs prospectively: Missouri (DMF) [6] and Illinois (UPLC-MS/MS) [7]. These and more recently presented data [8], [9] do not support claims of superior performance by MS/MS. Particularly noteworthy is that the confirmatory rate reported for Fabry disease in Illinois using MS/MS was lower than in an earlier pilot in Illinois using DMF [10]; the latter rate being similar to that reported in neighboring Missouri. We conclude that DMF is at least as effective as MS/MS for high throughput screening of multiple LSDs and recommend that NBS programs consult the Missouri and Illinois programs as part of their due diligence before making a decision that will affect their costs for many years to come.
Table 1.
Summary of published results for Fabry disease, Pompe disease, Gaucher disease and Hurler syndrome (MPS I) from programs prospectively screening for multiple LSDs. Generally, DMF has lower false-positive rates and significantly higher PPVs compared with MS/MS. Confirmed positive cases include infantile onset, late onset and undetermined phenotypes (e.g. genotype of unknown significance, unknown onset). Positive predictive value (PPV) is calculated as: the number of confirmed positive/ number of (normal + carrier + pseudodeficient).
References
- 1.Peake R.W.A., Marsden D.L., Bodamer O.A., Gelb M.H., Millington D.S., Wijburg F. Newborn screening for lysosomal storage disorders: Quo Vadis? Clin. Chem. 2016;62:1430–1438. doi: 10.1373/clinchem.2016.258459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peake R.W.A., Bodamer O.A. Newborn screening for lysosomal storage disorders. J. Pediatr. Genet. 2017;63:51–60. doi: 10.1055/s-0036-1593843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelb M.H., Scott C.R., Turecek F. Newborn screening for lysosomal storage disorders. Clin. Chem. 2015;61:335–346. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schielen P.C.J.I., Kemper E.A., Gelb M.H. Newborn screening for lysosomal storage diseases: a concise review of the literature on screening methods, therapeutic possibilities and regional programs. Int. J. Neonatal Screen. 2017;3:6. doi: 10.3390/ijns3020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chace D.H., De Jesus V.R., Spitzer A.R. Clinical chemistry and dried blood spots: increasing laboratory utilization by improved understanding of quantitative challenges. Bioanalysis. 2014;6:2791–2794. doi: 10.4155/bio.14.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins P. Lysosomal Storage Disorders Workshop; 2015. LSD Pilot Screening in Missouri for Pompe, Gaucher, Fabry and MPS-I Disorders. Association of Public Health Laboratories.https://www.aphl.org/programs/newborn_screening/Documents/LSDs-2/LSD-Screening-with-DMF_Patrick-Hopkins_4-17-15.pdf [Google Scholar]
- 7.Burton B.K., Hoganson G.E., Charrow J., Tinkle B., Dimmock D., Wagoner D., Grange D., Nash C., Becker J., Shao R., Basheeruddin D., Dizikes G. Newborn screening for lysosomal storage disorders in Illinois. Mol. Genet. Metab. 2016:S14–S124. [Google Scholar]
- 8.Hopkins P. WORLD LSD; 2017. State-wide Newborn Screening for 4 Lysosomal Storage Disorders Reveals High Incidence Rate for Pompe and Fabry Diseases. [Google Scholar]
- 9.Burton B.K., Kishnani P.S., Wasserstein M. WORLD LSD; 2017. Newborn Screening for Lysosomal Diseases: Recent Progress and Unanswered Questions. [Google Scholar]
- 10.Burton B., Charrow J., Angle B., Widera B., Waggoner D. A pilot newborn screening program for lysosomal storage disorders in Illinois. Mol. Genet. Metab. 2012;105:S23–S24. [Google Scholar]