Abstract
A total of 10 species of Baylisascaris, a genus of ascaridoid nematodes, occur worldwide and 6 of them occur in the New World. Most of the Baylisascaris species have a similar life cycle with carnivorous mammals or marsupials serving as definitive hosts and a smaller prey host serving as paratenic (or intermediate) hosts. However, one species in rodents is unique in that it only has one host. Considerable research has been conducted on B. procyonis, the raccoon roundworm, as it is a well-known cause of severe to fatal neurologic disease in humans and many wildlife species. However, other Baylisascaris species could cause larva migrans but research on them is limited in comparison. In addition to concerns related to the potential impacts of larva migrans on potential paratenic hosts, there are many questions about the geographic ranges, definitive and paratenic host diversity, and general ecology of these non-raccoon Baylisascaris species. Here, we provide a comprehensive review of the current knowledge of New World Baylisascaris species, including B. columnaris of skunks, B. transfuga and B. venezuelensis of bears, B. laevis of sciurids, B. devosi of gulonids, B. melis of badgers, and B. potosis of kinkajou. Discussed are what is known regarding the morphology, host range, geographic distribution, ecoepidemiology, infection dynamics in definitive and paratenic hosts, treatment, and control of these under-studied species. Also, we discuss the currently used molecular tools used to investigate this group of parasites. Because of morphologic similarities among larval stages of sympatric Baylisascaris species, these molecular tools should provide critical insight into these poorly-understood areas, especially paratenic and definitive host diversity and the possible risk these parasites pose to the health to the former group. This, paired with traditional experimental infections, morphological analysis, and field surveys will lead to a greater understanding of this interesting and important nematode genus.
Keywords: Ascarids, Baylisascaris, Larva migrans, Wildlife parasites, Zoonoses
Graphical abstract
Highlights
-
•
Baylisascaris species other than B. procyonis are under-studied.
-
•
Many questions about the natural history of these parasites remain unanswered.
-
•
Molecular tools should be used with morphology in studying this group of parasites.
1. Genus Baylisascaris
1.1. History and relationships within Ascarididae
Baylisascaris is a genus within the family Ascarididae, comprising mostly heteroxenous nematodes with carnivorous definitive hosts. Baylisascaris procyonis, the raccoon roundworm, is by far the most well-known and extensively studied member of the genus, primarily because of its association with severe neurologic disease in humans and numerous species of animals. As a consequence, many other Baylisascaris species are relatively poorly studied compared to B. procyonis. Here, we review the life history and current knowledge of non-raccoon Baylisascaris spp. in the Americas.
The genus Baylisascaris was officially described in 1968 and was named in honor of parasitologist H. A. Baylis of the British Museum of Natural History (Sprent, 1968). This genus united some previous members of Ascaris and Toxascaris and was mainly differentiated from other ascarid genera based on the presence of pericloacal rough patches and subventral postcloacal papillae (versus the absence of subdorsal postcloacal papillae as in Toxascaris) (Sprent, 1968). Former members of Ascaris reassigned into Baylisascaris include B. devosi, B. columnaris, B. procyonis, and B. laevis, while B. transfuga and B. melis were formerly within the genus Toxascaris. While Baylisascaris and Toxocara share biological similarities and are often discussed together in the context of zoonotic ascarids, they are in different subfamilies and are well-separated within Ascarididae (Nadler and Hudspeth, 2000). Molecular phylogenetic analyses of several genetic targets also support the separation of Baylisascaris from other ascarid genera (Zhu et al., 1998, Nadler and Hudspeth, 2000, Franssen et al., 2013, Tokiwa et al., 2014).
1.2. Life cycle characteristics
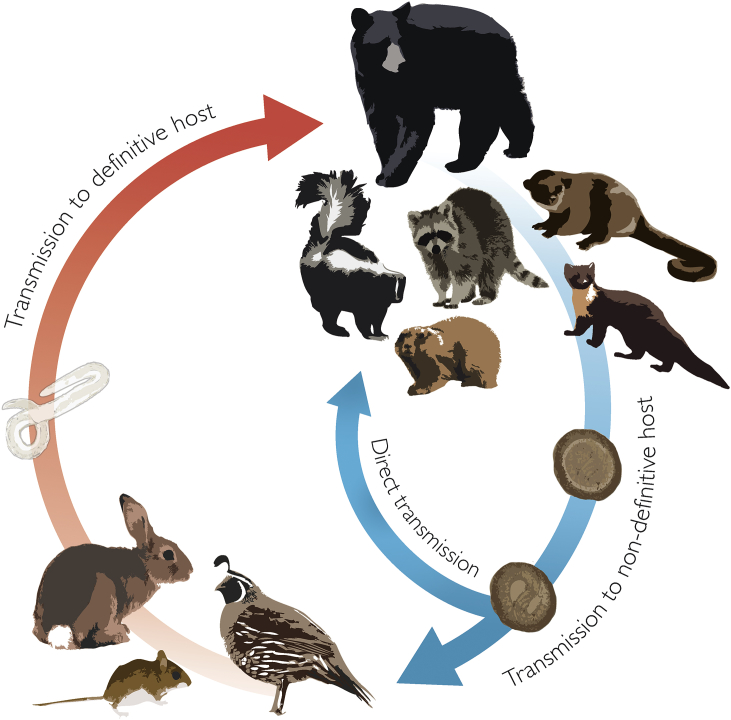
With the exception of B. laevis, all members of Baylisascaris utilize a carnivore definitive host. Most of these carnivore-infecting species also utilize a wide range of natural paratenic hosts (although there are some data to suggest these hosts are intermediate hosts, see below). Adult nematodes develop in the small intestinal lumen of the definitive host where they feed on host digesta. Females are remarkably fecund and can release >100,000 eggs/worm/day (primarily based on data from B. procyonis), which are shed in the feces (Snyder and Fitzgerald, 1987). Over a variable period of time (10–14 days under ideal conditions), the zygote within the egg develops into an infective-stage larva that may infect either definitive hosts in a direct cycle, or paratenic hosts in an indirect cycle (Fig. 1). However, it is likely that definitive hosts acquire some immunity to infection via the direct route with age; experimental infections show that egg inoculation can generally only establish infection in young definitive hosts (Kazacos, 1983, Berry, 1985). Like other ascarids, Baylisascaris spp. eggs are covered in an adhesive proteinaceous coat that confers a high degree of resilience to desiccation, freezing, heat (up to 62 C), and disinfectants, and may remain viable in the environment for years (Shafir et al., 2007, Kazacos, 2016).
Fig. 1.
Generalized life cycle scheme for Baylisascaris spp.
When ingested by paratenic hosts, larvae hatch from eggs in the small intestine, penetrate the intestinal wall, and undergo tissue migration after entering circulation. The pattern of migration and the resulting larva migrans syndromes vary among Baylisascaris spp. and host species. In B. procyonis, three larva migrans syndromes are well-described: visceral larva migrans (VLM), ocular larva migrans (OLM), and neural larva migrans (NLM), the latter of which can cause severe neurologic disease with permanent sequellae and death (Kazacos, 2016). Migrating larvae often become encapsulated in paratenic host tissues and are infective to definitive hosts upon predation. Once ingested by the definitive host, it is presumed L3 larvae mature in the mucosa of the small intestine, returning to the lumen at the L4 stage and molting into adults, as has been shown with Toxascaris, although it is not clear whether further somatic and/or tracheal migration takes place within all definitive hosts (Sprent, 1954).
It is possible that the route of infection determines whether further migration occurs within the definitive host. Assuming that the stage within the egg is L2, after egg inoculation, migration out of the gastrointestinal tract may be required for advancement to L3. However, if infection occurs via the ingestion of L3 larvae in host tissues, somatic migration may not be necessary; maturation may occur solely in the intestinal wall and lumen without migration, as has been shown with Toxascaris leonina (Sprent, 1954). Phylogenetically, Baylisascaris forms a sister clade with other Ascarididae (e.g., Toxascaris, Ascaris, Parascaris) although within this group, Toxascaris is the only member not known to undergo larva migration during development (Li et al., 2012). Experimental data on differential infection dynamics based on exposure route for Baylisascaris are lacking (Sprent, 1954, Nadler and Hudspeth, 2000). However, a skunk orally inoculated with B. columnaris eggs had an L3 recovered from skeletal muscle whereas this was not observed in other skunks in the same trial that had been inoculated with infected mouse carcasses (Berry, 1985). Migrating Baylisascaris sp. larvae have been recovered from skeletal muscle of naturally-infected wild definitive hosts but route of infection is unknown (Hoberg et al., 1990).
Debate exists as to whether hosts in which larvae migrate should be referred to as paratenic or intermediate hosts, which would be dictated by the presence of either a L2 or L3 larvae within the egg. In developmental observations of Baylisascaris tasmaniesnsis, changes in the oral region and a duplication of the cuticular sheath were observed at six days post infection in laboratory mice, which is evidence that a second molt occurs during larva migrans (i.e. L2 to L3) (Sprent et al., 1973). Additionally, only one molt was described within the egg after differentiation of the embryo of B. tasmaniensis, so it is likely that larvae within fully developed eggs of this species represent the L2 stage and not L3 (Sprent et al., 1973). Similar observations were also reported in Baylisascaris laevis (Babero, 1960a, Babero, 1960b). However, when hatched from eggs for in vitro culture, B. procyonis larvae were partially encased in a thin cuticle and did not molt further; it is not clear whether this cuticle represents a product of the first molt or a second molt within the egg (Boyce et al., 1988). Descriptions of Toxocara canis larval development suggest this thin cuticle is an artifact of the first stage cuticle, and could be misinterpreted as a second in ovo molt (Schacher, 1957). It is also possible that certain Baylisascaris species molt twice within the egg while others molt only once.
Whether or not the second molt occurs within the host or in the egg of Baylisascaris spp. and related ascaridoids, migrating Baylisascaris spp. larvae grow extensively while migrating through host tissues (depending on species, from ∼200 μm to ∼1800 μm over the course of weeks). It is possible this growth confers some advantage to larvae as infection efficiency in mature definitive hosts is remarkably higher when inoculated with larvae (i.e. infected carcasses) versus eggs and prepatent periods are shorter (Kazacos, 1983, Miyashita, 1993, Berry, 1985). This suggests that some larval development during somatic migration in non-definitive hosts.
1.3. Diagnostic features
Grossly, Baylisascaris spp. adults appear very typical of large ascarids (long cylindrical body, off-white to brown in color, three prominent lips, tail tapered to a point), with the females larger than males. Sprent (1968) described the main diagnostic features of the genus Baylisascaris as being:
-
-
Males have a roughened area anterior and posterior of the cloaca, unlike Ascaris or Toxascaris; post-cloacal papillae arranged differently from Toxascaris
-
-
Includes all characteristics within Ascarididae.
-
-
Cervical alae present, either salient or reduced.
-
-
Dorsal and sub-ventral labial papillae present in doublets.
-
-
Excretory cell U-shaped, nucleus within or behind commissural region.
-
-
Relatively short, stout spicules less than 1.0 mm in most members
-
-
Male tail papillae are segregated into pre- and post-cloacal groups with varying numbers depending on species. The post-cloacal group comprises a pairs in doublets, a single pair of closely associated singlet papillae, and one phasmid.
-
-
Eggs ovoid to round and finely pitted.
Among the majority of Baylisascaris spp., considerable overlap occurs in the morphology of eggs, larva, and many adult characteristics (Table 1). However, there are some morphologic characteristics that can be used to distinguish the various species. For example, B. transfuga and B. melis are generally thicker and more stout in overall appearance than other Baylisascaris spp., have larger spicules, and have cervical alae that are grossly visible (Table 1) (Sprent, 1968). Distinguishing among B. procyonis, B. columnaris, B. devosi, B. potosis, and B. laevis on morphology alone is difficult or impossible, although B. laevis is generally smaller than the other species and although there is overlap, there are some differences in spicule length, number of pre-anal papillae or vulval position (Table 1). Most species are identified based on definitive host alone. However, because some Baylisascaris spp. can cross-infect various definitive host species, species identification should not be based on definitive host species alone (Berry, 1985, Sprent, 1952a, Kazacos, 2016). Molecular tools for species identification are readily available and should be used when possible to confirm identifications if needed (see Section 3).
Table 1.
Overview of Baylisascaris species endemic to the New World, including B. procyonis for comparison.
| B. procyonis | B. columnaris | B. devosi | B. laevis | B. melis | B. potosis | B. transfuga | B. venezuelensis | |
|---|---|---|---|---|---|---|---|---|
| Authority | Stefanski and Zarnowski, 1951 | Leidy, 1851 | Sprent, 1952a, Sprent, 1952b | Leidy, 1851 | Gedoelst, 1920 | Tokiwa et al., 2014 | Rudolphi, 1819 | Pérez, García & Gauta, 2015 |
| Historical synonyms | Ascaris procyonis, Ascaris columnaris “raccoon ascarid” | Ascaris alienata, Ascaris columnaris “skunk ascarid” | Ascaris gulonis, Ascaris mustelarum, Ascaris devosi | Ascaris laevis, Ascaris tarbagan | Belascaris melis, Toxascaris melis, Ascaris columnaris “badger ascarid” | N/A | Ascaris transfuga, Toxascaris transfuga, Baylisascaris multipapillata | N/A |
| Primary DH (genus or species) | Raccoons (Procyon lotor); Domestic dogs (Canis familiaris) | Skunks (Mephitis, Spilogale) | Fishers, Martens, Wolverines (Martes, Gulo) | Marmots, ground squirrels (Marmota, Spermophilus) | Badgers (Meles, Taxidea) | Kinkajou (Potos flavus) | Bears (Ursus, Melursus) | Spectacled bear (Tremarctos ornatus) |
| Adult lengtha (♂; ♀) | 46-119; 55-337 | 43-110; 72-266 | 57-123; 105-285 | 37-108; 32-212 | 120-127; 22-260 | 117-123; 214-223 | 63-120; 102-240 | 102; 250 d |
| Adult midbody width (♂; ♀) | 1.0–2.0; 1.5–2.5 | 1.3–2.5 | 1.3–2.5 | 1.0–2.3; 1.7–3.2 | up to 3; up to 5 | 1.6–1.75; 2.51–2.9 | 1.2–1.9; 1.6–4.5 | 3.0; 4.1 d |
| Esophageal length (♂; ♀) | 2.19–6.46; 2.41–7.53 | 2.69–5.21; 3.76–7.81 | 4.15–4.50; 4.00–4.83 | 2.73–5.64; 1.84–6.59 | 5.9; 7.25 d | 4.10–4.56 | 3.7–4.7; 4.0–5.1 | ND |
| Spicules | 0.49–0.71 | 0.33–0.76 | 0.39–0.54 | 0.24–0.81 | 0.80–0.90 | 0.61–0.77 | 0.80–0.93 | 0.9 |
| Vulvar positionb | 25% | 25% | 33% | 34% | 37% | 28.2% | 37% | 40% d |
| Number of pre-anal papillae | 43–67 | 36–53 | 30–40 | 41–61 | up to 63 | 44–52 | 46–70 | 44 d |
| Cervical alaec | Inconspicuous | Inconspicuous | Inconspicuous | Inconspicuous | Prominent | Inconspicuous | Prominent | Prominent |
| Average egg size | 80 × 60 μm | 73 × 63 μm | 77 × 61 μm | 75 × 62 μm | 87 × 75 μm | 83 × 73 μm | 90 × 75 μm | ND |
| Sources | Sprent, 1968, Berry, 1985, Kazacos, 2016 | Sprent, 1968, Berry, 1985 | Sprent, 1952a, Sprent, 1953b, Sprent, 1968 | Babero, 1960a, Sprent, 1968, Berry, 1985 | Gedoelst, 1920, Hartwich, 1958, Sprent, 1968 | Tokiwa et al., 2014 | Rudolphi, 1819, Sprent, 1968, Testini et al., 2011, Moudgil et al., 2014 | Pérez Mata et al., 2016 |
ND = not determined.
Measurements in mm unless otherwise specified.
Percentage of body length from anterior end.
Inconspicuous = only visible in transverse section.
Measurement represents examination of single specimen.
2. New World species of Baylisascaris
2.1. Baylisascaris columnaris
Originally identified as Ascaris alienata by Leidy (1851), the species was renamed Ascaris columnaris in 1856, and then reassigned to Baylisascaris by Sprent in 1968 (Leidy, 1851, Sprent, 1968). Morphologically, B. columnaris is highly similar to B. procyonis, with only subtle distinguishing features, including the structure of cervical support in the cuticle (a wide arch in B. procyonis compared with a narrow A-shape in B. columnaris), shape of denticles (equilateral triangles versus elongated triangles), male tail terminal shape (spike versus knob), and the number of preanal papillae (average of 40 versus 43, although there is considerable overlap in range) (Franssen et al., 2013, Sprent, 1968). However, it is likely that enough natural variability occurs between the two species such that identification based only on morphological characteristics is inadequate and molecular identification is ideal (Berry, 1985).
Skunks are the definitive host for B. columnaris. Infection has primarily been detected in the striped skunk (Mephitis mephitis), the most broadly-distributed and studied skunk species in North America, but infections have also been detected in Eastern spotted skunks (Spilogale putorius) (Table 2). Sympatric Western spotted skunks (Spilogale gracilis) and hog-nosed skunks (Conepatus sp.) and may be potential hosts as well but testing has been limited. Ascarids identified as B. columnaris have been reported in American badgers (Taxidea taxus); however, it is likely that these are actually B. melis or B. devosi (Table 2). Further surveys that utilize molecular parasite species identification tools will be useful in elucidating the full definitive host range of B. columnaris.
Table 2.
Host and locality records for Baylisascaris columnaris in North America.
| Host | Location | No. infected/no. examined (%) | Source |
|---|---|---|---|
| Striped skunk (Mephitis mephitis) | New York, USA (central) | NG/74 (>90) | Stegeman, 1939 |
| Massachusetts, USA (western) | 15/19 (84) | Rankin, 1946 | |
| Texas, USA (central) | 0/NG (0) | Tiner, 1946 | |
| Oregon, USA (northwestern) | 1/1 | Stegner and Neiland, 1955 | |
| California, USA (central) | 0/45 (0) | Mead, 1963 | |
| North Dakota, USA | 8/42 (19) | Dyer, 1970 | |
| Ontario, Canada (southern) | (62) | Berry, 1985 | |
| Illinois, USA (northeastern) | 18/73 (25) | Gehrt, 2005 | |
| Texas, USA (west-central) | 0/23 (0) | Neiswenter et al., 2006 | |
| Saskatchewan, Canada | 65/173 (38) | Wirsing et al., 2007 | |
| Eastern spotted skunk (Spilogale putorius) | Minnesota, USA | 4/23 (17) | Erickson, 1946 |
| California, USA (central) | 0/14 (0) | Mead, 1963 | |
| Arkansas, USA (western) | 1/29 (3) | Lesmeister et al., 2008 | |
| Western spotted skunk (Spilogale gracilis) | Texas, USA (central) | 0/NG (0) | Tiner, 1946 |
| Texas, USA (west-central) | 0/9 (0) | Neiswenter et al., 2006 | |
| American hog-nosed skunk (Conepatus leuconotus) | Texas, USA (central) | 0/NG (0) | Tiner, 1946 |
| Texas, USA (west-central) | 0/28 (0) | Neiswenter et al., 2006 |
NG = not given.
2.1.1. Distribution and ecology
Contemporary surveys on B. columnaris are generally lacking; more surveillance is needed to accurately characterize the distribution and eco-epidemiology of this parasite because most recent reports are from captive pet skunks (d’Ovidio et al., 2016). From published reports, B. columnaris appears to be well-established in the northeast, upper Midwest, and prairie regions of the United States and Canada, apparently uncommon in arid regions of the west and southwest (Table 2). In southern Ontario, Canada, prevalence in M. mephitis was significantly lower in spring months compared to late summer or early fall (Berry, 1985), similar to the general seasonal patterns observed for B. procyonis in northern climates (Kidder et al., 1989). Similar seasonal variation in prevalence and intensity have been observed for other gastrointestinal helminths of skunks, such as Physaloptera maxillaris (Cawthorn and Anderson, 1976). It is likely that the resource-limiting nature of skunk torpor/overwinter fasting causes the loss or developmental arrest of helminths, potentially including B. columnaris, but more research is needed to investigate this phenomenon (Dragoo, 2009).
2.1.2. Natural infections in definitive hosts
Similarly to B. procyonis in raccoons, B. columnaris infection is generally not associated with morbidity or mortality in wild skunk definitive hosts. However, peritonitis or intestinal perforation associated with high worm burdens in captive skunks have been documented (Goodey and Cameron, 1923, Nettles et al., 1978). Goodey and Cameron (1923) noted that skunks from a United Kingdom fur farm exhibited poor body condition, failure to thrive, and inferior coat quality possibly associated with high-intensity B. columnaris infection, and possibly resulting in economic losses. Few recent studies have investigated the occurrence of B. columnaris among farmed skunks or its economic impacts, although infection control should be straightforward with appropriate enclosure cleaning and regular administration of anthelminthics, most of which are highly efficacious against intestinal stages of the related parasite B. procyonis (Bauer and Gey, 1995). Given these data, it is possible that wild skunks with high worm burdens may develop disease.
In Europe, 15 of 60 (25%) pet striped skunks primarily from Germany and Italy were positive and additional infections have been reported from the Netherlands and Poland; all were genetically-confirmed as B. columnaris (Franssen et al., 2013, d’Ovidio et al., 2016, Jańczak et al., 2016). Worm burdens were not determined, but in one study, eggs per gram of feces (EPG) ranged from 150 to 14,500 (mean of 4713 EPG) (d'Ovidio et al., 2016). This is relatively low compared to natural B. procyonis infections, which may average 26,000 EPG (Kazacos, 2016). Importantly, many of these infected pet skunks were housed near or with other pet species (e.g. dogs, guinea pigs, parrots), and none of the skunks had ever received anthelminthic treatment (d’Ovidio et al., 2016). Although there are no published reports, B. columnaris infections have been diagnosed in pet skunks from the United States (Yabsley, unpublished data). Given the popularity of skunks as pets and the potential for larva migrans in various hosts, education of pet owners is needed to reduce the risk of transmission.
2.1.3. Experimental infections in definitive hosts
Experimental infections of B. columnaris in skunks have provided data on the fate of larvae within the definitive host and the development of patency. Berry (1985) experimentally infected striped skunks by feeding them mouse carcasses containing unknown numbers of L3 larvae. Two juvenile female skunks became patent ∼48 days post-inoculation (DPI) and one mature male skunk became patent at 93 DPI. Another route of exposure investigated was inoculation with larvated eggs; inoculation of an unspecified number of embryonated eggs resulted in intestinal infections in three juvenile skunks. The youngest individual (38 days old) was sacrificed at 10 DPI and one L3 larva was recovered from skeletal muscle, suggesting that at least some larvae undergo early somatic migration in the definitive host following egg inoculation. This individual also had a small number of L3 and L4 larvae within the lumen of intestine. Numerous L3 and L4 larvae were observed in the lumen of the small intestine in another juvenile “young of the year” skunk scarified at 19 DPI; additional larvae larvae were recovered via digestion of the walls of the anterior and posterior small intestine. The remaining juvenile animal was sacrificed at 139 DPI, and although immature adults were found in the intestine at necropsy, eggs were never detected in the feces. The limited number of experimental infection trials in skunks and the low sample sizes makes determining the average onset of patency difficult, and host age and route of infection may be important factors not fully investigated.
To investigate susceptibility of raccoons to B. columnaris, two juvenile raccoons were inoculated with unreported numbers of either L3 larvae (in mouse tissue) or embryonated eggs (Berry, 1985). The raccoon inoculated with larvae became infected as L4 larvae were present in the small intestine of the raccoon upon necropsy; however, infection was not allowed to proceed so it is unknown if the raccoon would have become patent. Thus it is unknown if raccoons can serve as alternative definitive host for B. columnaris. The single raccoon inoculated with embryonated B. columnaris eggs did not develop an intestinal infection (Berry, 1985).
2.1.4. Natural infections in non-definitive hosts
There are no reports of naturally-acquired B. columnaris larva migrans in wild or captive paratenic hosts. There have been suspected cases in captive animals that were linked to co-housing with infected skunks. For example, an outbreak involving a white-headed marmoset (Callithrix geoffroyi) and two species of tamarins (Saguinus nigricollis, Saguinus midas) in a zoological park in Texas was likely due to a skunk (of unknown infection status) housed in the enclosure. These primates developed signs of NLM, were treated unsuccessfully with fenbendazole, and were subsequently euthanized (Huntress and Spraker, 1985). Infection with B. columnaris in a captive emu (Dromaius novaehollindiae) in Indiana with fatal NLM was suspected based on the history of a skunk (also of unknown infection status)being previously held in the enclosure (Kazacos et al., 1982). However, species identification was not confirmed in the emu case. Raccoons are also reportedly common in the area where the emu was housed and B. procyonis is highly prevalent in Indiana (Kazacos et al., 1982).
The paratenic host range of B. columnaris is likely broad given its biological and phylogenetic similarity to B. procyonis and experimental host range. However, molecular techniques will be required in future case studies or surveys to investigate possible natural paratenic hosts. Additionally, B. columnaris could be a zoonotic parasite, given its similarities to B. procyonis and case reports in primates. It is possible that some presumed B. procyonis natural infections are actually B. columnaris, due to the extreme difficulty of species identification through adult/larval morphology, egg morphology (from environmental samples), or current serologic techniques which are cross-reactive among Baylisascaris spp. (Dangoudoubiyam et al., 2010, Berry, 1985). No human cases have been reported, and even if zoonotic, it is unlikely to represent as significant a public health threat as B. procyonis, as skunks are generally in lower densities in urban areas compared to raccoons (Gehrt, 2004). Therefore, potential human contact with skunks feces is limited. Nonetheless, individuals with frequent contact with skunks and skunk feces (pet owners, wildlife rehabilitators, fur farmers, trappers, etc.) should take precautions against potential exposure to B. columnaris. Recently, antibodies to Baylisascaris (presumed to be mostly due to B. procyonis exposure, but could be due to other species) were detected in wildlife rehabilitators (Sapp et al., 2016a).
2.1.5. Experimental infections of non-definitive hosts
B. columnaris produces disease due to larva migrans in a variety of experimentally-infected paratenic host species, particularly rodents and lagomorphs. Compared to B. procyonis, B. columnaris generally causes less mortality in experimentally-infected rodents due to slower and more limited NLM. Independent experiments by Sprent (1952b) and Tiner (1953a) demonstrated that neurological signs were generally noted between 17 and 25 days in laboratory mice inoculated with an unspecified number of eggs, compared to 7–10 days for B. procyonis. However, dose is likely important in the rate of disease development as has been shown with B. procyonis (Tiner, 1953a, Sheppard and Kazacos, 1997, Sapp et al., 2016b). Domestic rabbits (Oryctolagus cuniculus) inoculated with 100,000 eggs, considered a very high dose, rapidly developed severe neurologic disease involving seizures, epistaxis, ataxia, and dyspnea, with onset between 4 and 10 DPI (Church et al., 1975).
This generally delayed onset of neurological disease compared to B. procyonis is most likely due to the relatively slower growth of L3s within paratenic hosts. In a 20 day trial of experimental infection of laboratory mice, B. columnaris larvae grew to approximately 1000 μm in length by the end of the trial, compared to B. procyonis that achieved this size by day 10 and reached an average maximal length of 1200 μm by day 20 (Tiner, 1953b). Experimental trials in laboratory mice suggest that neurological disease does not become readily apparent until larvae within the brain have reached a length of ∼1000 μm (Tiner, 1953b) In laboratory mice, B. procyonis reaches an average length of ∼1000 μm in ∼8–10 days post infection, after which survival of infected hosts fell dramatically, whereas B. columnaris took ∼16 days to reach 1000 μm in length, after which time some mortality occurred (Tiner, 1953b). In some cases, mice inoculated with B. columnaris eggs were able to recover from clinical disease or survived despite the presence of larvae in the brain (Tiner, 1953b). Similarly, B. procyonis larvae have been detected in wild-caught, presumably normally-acting, Peromyscus leucopus further suggesting that some rodents can survive infections of the brain (Page et al., 2001; Sapp and Yabsley unpublished data). The observed differences larval growth between B. columnaris and B. procyonis may also reflect differences in paratenic host species adaptation. In experimentally-infected meadow voles (Microtus pennsylvanicus), B. columnaris larvae achieved a greater average length (1570 μm) than B. procyonis (1060 μm) by 10 DPI (Berry, 1985). The study was terminated at 10 DPI for larval morphological analysis, so other infection dynamics were not assessed. The impact of larval size on disease severity could also explain why cerebral infection with other smaller larval ascarids such as Toxocara canis (that achieves a maximal length of about 500 μm in rodent brains) produces overt disease much less frequently than B. columnaris or B. procyonis, although host factors, including brain size, likely play a role in the rate and severity of neurologic disease (Tiner, 1953a, Sprent, 1955).
A large proportion of larvae in inoculated paratenic host species become encapsulated within the intestinal wall and mesentery within 1–4 DPI (Berry, 1985). Encapsulated larvae were also abundant in the lungs, heart, kidneys, and liver shortly after inoculation in laboratory mice and in a groundhog (Marmota monax), presumably due to liver-lung migration (Sprent, 1952b, Berry, 1985). Numerous larvae migrated within skeletal muscle and became encapsulated after 10 DPI in inoculated Microtus pennsylvanicus (Berry, 1985). In inoculated experimentally-infected rabbits, extensive larval granulomas with eosinophilic infiltration were observed in the lungs, liver, brain, eyes, kidneys, heart, and gastrointestinal tissues (Church et al., 1975).
Similar to other Baylisascaris species, B. columaris larvae within tissues are resistant to freezing. Encapsulated larvae in tissues remained viable and recovered substantial motility after a periods of freezing ranging from 8 to 18 weeks at −20 C, which was superior to freeze-susceptible B. transfuga and T. canis larvae in the same experiment (Sprent, 1953a). However, experimental trials to confirm infectiousness of previously-frozen Baylisascaris larvae have not been conducted.
2.2. Baylisascaris spp. of bears
2.2.1. Baylisascaris transfuga
Baylisascaris transfuga was originally described by Rudolphi (1819) as Ascaris transfuga and re-described as a Toxascaris species in 1922 (Baylis and Daubney, 1922). Ultimately Sprent (1968) formally described the Baylisascaris genus and designated Baylisascaris transfuga as the type species for this genus. Morphological characteristics and/or molecular techniques can be used to distinguish this species from other Baylisascaris spp. (Table 3). Baylisascaris transfuga adults can be morphologically distinguished from other species by their spicule length (estimated between 0.80 and 0.92 mm), having between 46 and 70 precloacal papillae, rounded posterior margin of the pericloacal area, salient alae, denticles in equilateral triangles, and a saddle shape of the median lobe of the lip (Testini et al., 2011, Sprent, 1968, Baylis and Daubney, 1922). Eggs of B. transfuga are morphologically similar to other Baylisascaris spp. eggs and are largely considered indistinguishable (Sprent, 1968, Kazacos and Turek, 1983, Testini et al., 2011) (Table 1).
Table 3.
Morphometrics of Baylisascaris transfuga adults and eggs from various hosts and geographic regions.
| Host | Location | Male length (mm) | Female length (mm) | Egg width (μm) | Egg length (μm) | Source |
|---|---|---|---|---|---|---|
| Multiple captive and wild species | Multiple | Up to 120 | Up to 240 | Up to 75.0 | Up to 90 | Sprent 1968 |
| Sloth bear (Melursus ursinus) | India | 64–94 | 138–183 | 47.0–75.2 | 65.8–94.0 | Moudgil et al., 2014 |
| Polar bear (Ursus maritimus) | Italy | 63–116 | 102–203 | NG | NG | Testini et al., 2011 |
| NG | NG | 57.6–64.0 | 60.8–73.6 | Papini and Casarosa 1994 | ||
| Multiple captive species | Pennsylvania (USA) | 81–142 | 108–166 | NG | NG | Canavan 1929 |
| Multiple captive species | Louisiana (USA) | NG | NG | 66.3–74.7 | 78.3–88.0 | Clark et al., 1969 |
NG = not given.
Of interest is that morphometrics of parasites reported as B. transfuga from numerous hosts across several continents show variation (Table 3). Measurements have been made on specimens from black bears (Ursus americanus) in Canada, multiple subspecies of brown bear (Ursus arctos) from across sites Eurasian and North American sites, multiple captive polar bears (Ursus maritimus) in various sites, sloth bears in India (Melursus ursinus), and a captive sun bear (Helarctos malayanus) (Canavan, 1929, Baylis and Daubney, 1922, Sprent, 1968, Testini et al., 2011, Moudgil et al., 2014). Sprent (1968) reported a maximal length of 120 mm for males and 240 mm for females; other studies have found smaller size ranges for both males and females (Table 3). Egg dimensions are also variable and have been reported as low as ∼57 μm wide up to ∼94 μm long, although fertilization or embyronation status may influence this morphology (Table 3).
These morphologic differences in a limited number of parasites examined across a wide geographic and host range suggest that these “B. transfuga” may represent several distinct species. Currently there are two distinct Baylisascaris species apart from B. transfuga reported within Ursidae, B. schroederi of giant pandas (Ailuropoda melanoleuca), and the recently-described B. venezuelensis from spectacled bears (Tremarctos ornatus). Further support is provided by preliminary molecular data on B. transfuga samples from Alberta, Canada and West Virginia that suggest these parasites are genetically distinct across locations (L. Camp, pers. comm.). Careful morphologic analysis combined with molecular characterization of B. transfuga samples from a diverse geographic and host range is needed to address parasite diversity in the Ursidae.
2.2.1.1. Epidemiology
Worldwide, B. transfuga has been reported in all extant species of bear in the family Ursidae excluding the spectacled bear although unidentified ascarid eggs have been detected in spectacled bear feces (Figueroa, 2015). Baylisascaris transfuga infections have been reported from American black bears (Ursus americanus), sloth bears, polar bears, brown bears (Ursus arctos), Malayan sun bears (Helarctos malayanus), Asiatic black bears (Ursus thibetanus), and giant pandas (Ailuropoda melanoleuca) although these reports are most likely B. schroederi (Sprent, 1968, Rudolphi, 1819, Canavan, 1929, Baylis and Daubney, 1922). No non-bear definitive hosts are known.
The prevalence of B. transfuga in bears varies widely among studies (Table 4). In North America, the majority of studies have been conducted on black bears but natural infections have been reported in brown bears and in captive polar bears. Prevalence of B. transfuga in black bears appears to be highest in Alberta, Canada and the Great lakes regions of the USA. In brown bears, prevalence of infection with B. transfuga was higher in the Wyoming and Montana, USA compared to Alaska and Canada.
Table 4.
Reports of Baylisascaris transfuga in free-ranging and captive bears in North America.
| Host | Location | Captive/Wild | No. infected/no. examined (%) | Method of detection | Source |
|---|---|---|---|---|---|
| Brown bear (Ursus arctos) | Northwest Territories, Canada | Wild | 3/56 (5) | Fecal flotation | Gau et al., 1999 |
| Wyoming and Montana, USA | Wild | 53/70 (76) | Necropsy | Worley et al., 1976 | |
| Canada | Wild | 16/21 (76) | Necropsy | Choquette et al., 1969 | |
| Alaska, USA | Wild | 0/28 (0) | Fecal flotation | Schaul, 2006 | |
| British Columbia and Alberta, Canada | Wild | 7/13(54) | Necropsy | Catalano et al., 2015 | |
| Black bear (Ursus americanus) | Florida, USA | Wild | 5/22 (23) | Necropsy | Foster et al., 2004 |
| New York, USA | Wild | 17/55 (31) | King et al., 1960 | ||
| Minnesota and Michigan, USA | Wild | 5/9 (56) | Necropsy | Rogers, 1975 | |
| Wisconsin, USA | Wild | 59/92 (64) 25/29 (86) |
Fecal Flotation Necropsy |
Manville, 1978. | |
| Wyoming and Montana, USA | Wild | 24/30 (80) | Necropsy | Worley et al., 1976 | |
| Minnesota, USA | Wild | 1/1 (100)(august) | Necropsy | Barnes and Rogers, 1980 | |
| Southeastern USA | Wild | 28/53 (53) | Necropsy | Crum et al., 1978 | |
| New Brunswick, Canada | Wild | 1/12 (8) | Necropsy | Duffy et al., 1994 | |
| Alberta, Canada | Wild | 35/56 (62) | Necropsy | Dies, 1979 | |
| Quebec, Canada | Wild | 17/80 (21) | Fecal flotation | Frechette and Rau 1978 | |
| Quebec, Canada | Wild | 38/168(23) 2/21(10) |
Fecal flotation Necropsy |
Frechette and Rau 1978 | |
| Ontario, Canada | Wild | 20/83(24) | Addison et al., 1978 | ||
| British Columbia and Alberta, Canada | Wild | 24/40(60) | Necropsy | Catalano et al., 2015 | |
| Northwest Territories, Canada | Wild | 12/27 (44) 18/28 (64) |
Fecal flotation Necropsy |
Johnson et al., 2013 | |
| Polar bear (U. maritimus | Massachusetts, USA | Captive | 1/1(100) | Necropsy | McOrist et al., 2002 |
| U. arctos, U. maritimus | California, USA | Captive | 2/3 (66) | Abdel-Rasoul and Fowler, 1979 | |
| Various | USA, various | Captive | 125/260 (48) | Fecal flotation | Schaul, 2006 |
A few studies have attempted to investigate seasonal trends in prevalence with some conflicting results. One, based on fecal floatation, detected a higher prevalence in spring compared to the fall in black bears in Quebec, Canada (Frechette and Rau, 1978). Another, based on collection of nematodes at necropsy in black bears and grizzly bears in western Canada observed the opposite seasonal trend with peaks in the fall but this study was (Frechette and Rau, 1978, Catalano et al., 2015). However, the seasonal association with prevalence in black bears was weakly significant (p = 0.04) and sample sizes were relatively small (n = 40); sample size for grizzly bears was too small for statistical analysis. Finally, Rausch (1954) and Rogers (1975) found that bears were shedding eggs in the spring soon after torpor and found evidence of egg shedding just prior to denning, so it remains unclear whether or not infections are cleared during winter torpor. Additional studies are needed with greater sample sizes and age class representation to accurately assess the seasonal ecology of B. transfuga in bears.
In the bear host, B. transfuga infections do not typically cause clinical disease, but heavy infections have been reported causing clinical disease or death. Reports of disease in the natural bear host include peritonitis in a brown bear in Europe and suggestions of enteric impactions (Mozgovoi, 1953, Szczepaniak et al., 2012). Subclinical effects including reduced host condition have also been reported in infected bears (Fu et al., 2011).
2.2.1.2. Non-definitive hosts
The risk of B. transfuga to cause clinical disease as a result of larva migrans in aberrant hosts is considered low compared to other Baylisascaris species. This is partly due to their slower growth rate and smaller size as well as the decreased penetration of the intestinal wall by B. transfuga larvae resulting in fewer larvae being detected in visceral organs compared to other species (Sprent, 1952b, Sprent, 1953a, Sato et al., 2004, Schaul, 2006).
Despite a reduced migratory capacity relative to other Baylisascaris spp., experimental infections in laboratory mice, Mongolian jirds (Meriones unguiculatus), guinea pigs, rabbits, and chickens indicate that B. transfuga can occasionally cause VLM, OLM, and/or NLM; however, in most hosts, clinical disease was mild or not apparent (Sprent, 1952b, Sprent, 1955, Papini et al., 1993, Papini et al., 1994, Papini et al., 1996a, Papini and Casarosa, 1994; Sato et al., 2004, Matoff and Komandarev, 1965). There is substantial variation among hosts in disease severity.Laboratory mice developed only mild clinical disease with granulomas in the brain. Mongolian jirds developed severe clinical signs with malacia and lack of host immune reaction (Sato et al., 2004). Rabbits displayed a loss of appetite, dyspnea, and depression but no neurological signs (Papini et al., 1996a). No clinical signs were observed in experimentally-inoculated chickens (Papini et al., 1993). In guinea pigs, Matoff and Komandarev (1965) showed that B. transfuga larvae migrate into the intestinal wall and either encyst in the wall, penetrate the intestinal wall and enter the abdominal cavity, or travel to the lungs, heart, and/or skeletal muscles through the lymphatics and systemic circulation. However, larvae were not noted in brains. Sprent (1953a) reported that larvae were still alive in experimentally-infected mice one year after infection.
Despite experimental studies suggesting a wide range of hosts may develop larva migrans, only one presumed report of natural infection of a paratenic host has been reported. Japanese macaques (Macaca fuscata) housed near American black bears at a zoo in Japan developed fatal neurological disease; however, identification of the parasite was not definitive in this case and identification of the parasite was based only on histology (Sato et al., 2005). While there are no confirmed reports of larva migrans in humans following B. transfuga infection, experimental evidence with other species shows that given a sufficiently high infection, larva migrans in people may be possible.
2.2.1.3. Treatment and control
Baylisascaris eggs present in the environment or in captive animal facilities are difficult to eliminate or kill. Similar to other Baylisascaris species, eggs of B. transfuga become infective after ∼2 weeks and can remain infective for at least 15 months under artificial conditions (Papini and Casarosa, 1994). Eggs have reported to persist in the environment for up to five years, and infected bears can pass between 100 and 19,800 eggs per gram of feces so environments can become contaminated with large number of eggs quickly (Abdel-Rasoul and Fowler, 1979, Vercruysse et al., 1976). In captivity, the prevalence of B. transfuga is higher in certain species (U. maritimus, Melursus ursinus), although this could be sampling bias, the substrate (e.g. sand or soil) used in enclosures, or the housing of bears in groups (Schaul, 2006). Strict, routine quarantine and treatment of bears in captive settings can be an effective way to reduce shedding and prevent subsequent infections.
Treatment of bears infected with B. transfuga has only been done in captive situations. Numerous anthelmintics have been used to manage B. transfuga infections in captive bears; however, efficacy is variable and dose-dependent (Clark et al., 1969, Moudgil et al., 2014). Dichlorvos (19 mg/lb) rapidly (1–2 days post treatment) reduced fecal egg counts (FEC) to zero in many bear species; however, these animals became reinfected within months after treatment, emphasizing the need to clean the environment (Clark et al., 1969). Orally-administered fenbendazole (10 mg/kg) on three consecutive days was unable to reduce fecal egg counts to zero in a sloth bear, however, this infection was cleared with 15 mg/kg for three days followed by the original treatment (Moudgil et al., 2014). Mebendazole was used successfully to treat five polar bears infected with B. transfuga (Vercruysse et al., 1976). Macrolides, benzimidazoles, and tetrahydropyrimidines have all been used in North American zoos to treat bears but efficacy data were not provided (Schaul, 2009).
Due to concerns about larva migrans, it is important to determine if larvae could be killed prior to entering the CNS. Laboratory mice given one dose of 2 mg/kg ivermectin had fewer lesions and resulted in fewer B. transfuga larvae recovered in visceral organs compared to those without treatment (Papini et al., 1996a, Papini et al., 1996b, Papini et al., 1996c). In another study, single doses of levamisole or ivermectin were administered subcutaneously and intramuscularly, respectively, to groups of inoculated laboratory mice 3 DPI or 14 DPI (Fu et al., 2011). Upon necropsy, reduced larval burdens were observed in treatment groups compared to controls. Levamisole resulted in an 81% decrease in “migrating” larvae (at 3 DPI) but only a 49% overall decrease in “encapsulated” larvae (at 14 DPI).Although ivermectin had similar activity against larvae at 3 DPI (88% reduction), it had greater activity against larvae at 14 DPI (75% reduction). Levamisole-treated mice at 14 DPI had fewer larvae within the brain (43% versus 24% in ivermectin group) and appeared to ameliorate the severity of neurologic signs in two of five mice displaying clinical disease after 17 DPI (Fu et al., 2011). These data suggest that larvicidal activity will vary depending on the age of infection, resulting in changes in susceptibility to the drugs during migration, and in encapsulation status. Pharmacokinetics of anthelmintics also influence treatment efficacy; for example, ivermectin does not cross the blood-brain barrier whereas levamisole appears to do so (Fox, 2006, Lin and Tsai, 2006). Given this differential susceptibility and drug efficacy, it seems the best option for treating B. transfuga larva migrans in paratenic hosts is to use multiple drug classes in order to maximize larvicidal activity in brain, viscera, and skeletal muscle.
2.2.2. Baylisascaris venezuelensis
A new species, Baylisascaris venezuelensis, originating from a South American spectacled (Andean) bear (Tremarctos ortnatus) in western Venezuela was recently described (Pérez Mata et al., 2016). A female spectacled bear in poor body condition was found dead and at necropsy, a large number of Baylisascaris were present in the gastrointestinal tract. Other gross pathologic findings included congestion and hemorrhagic foci in the lungs. The authors suggest that the high worm burden was the cause of mortality.
An adult male and female nematode were examined morphologically. Fewer post-cloacal papillae (n = 44) were present compared to Nearctic and Palearctic B. transfuga worms, which have an average of 66 (Sprent, 1968). Other morphologic features were similar to B. transfuga, including overall length, a stout appearance, salient cervical alae, similar length spicules, and a rounded posterior margin of the pre-cloacal area (with B. venezuelensis having a “little process” on this margin) (Pérez Mata et al., 2016). Molecular analysis supported the separation of B. venezuelensis as a separate species. Combined ITS-1 and ITS-2 sequences were only 91.8% and 90.6% similar to B. transfuga and B. schroederi, respectively (Pérez Mata et al., 2016). There were also three nucleotide differences in the highly conserved region of 5.8S rDNA that differentiated B. venezuelensis from the two other ursid-associated species and other Baylisascaris spp. Phylogenetic analysis of both ITS regions included B. venezuelensis in a clade containing the other two ursid-associated species along with B. ailuri from red panda (Pérez Mata et al., 2016).
A few instances of previously detected ascarid eggs in fecal examinations of captive and free-ranging spectacled bears may represent B. venezuelensis infections (Schaul, 2006, Figueroa, 2015). Eggs designated as “roundworm” eggs were present in 9/25 (36%) of spectacled bear fecal samples from zoos across the United States, although it is impossible to determine if this is B. venezuelensis or native B. transfuga acquired from other bears in the captive environment (Schaul, 2006). Ascarid eggs resembling those of Baylisascaris or Toxocara were reported in 6/28 (21%) of T. ornatus scats from northern Peru, but measurements of the eggs were not provided (Figueroa, 2015). It is currently unknown if B. venezuelensis is usually pathogenic for spectacled bears. The type host is believed to have died from the nematode infection, but most previously reported positive spectacled bears were presumably asymptomatic. Although most Baylisascaris spp., including B. transfuga, rarely cause mortality in their definitive hosts, B. schroederi from pandas is a major cause of morbidity and mortality so additional research on the potential risk of B. venezuelensis to spectacled bears is needed (Zhang et al., 2008).
The finding of a new, seemingly valid Baylisascaris species in a relatively isolated population of ursids further supports the idea that “B. transfuga” represents an assemblage of species globally, and highlights the need for further molecular and morphologic work to characterize possibly cryptic species. Field surveys are also necessary to determine the prevalence as well as definitive and paratenic host range of this new tropical species.
2.3. Baylisascaris laevis
Baylisascaris laevis uses rodents instead of carnivores as definitive hosts that makes it unique among the other Baylisascaris spp. in the New World (Berry, 1985). The parasite was first described in 1856 by Leidy as Ascaris laevis from naturally infected groundhogs (Marmota monax). Later it was reassigned to the genus Baylisascaris (Sprent, 1968).
Among the New World Baylisascaris species, B. laevis is generally smaller and wider than other species of Baylisascaris (Table 1). This species also has the smallest spicules and largest dorsal lip compared to other Baylisascaris spp. (Babero, 1960a, Sprent, 1968). Other differences involve the posterior end of the male and female worms with the female B. laevis tail abruptly tapered to a sharp point and the male tail narrowed mid-tail and appears swollen at the end (Tiner, 1951, Berry, 1985). Other diagnostic features included indistinct knobby protrusions near the cloacal opening of males (Tiner, 1951). The external genitalia of female B. laevis more anterior compared to B. columaris and B. procyonis (Berry, 1985).
2.3.1. Host range
The most commonly reported definitive host of B. laevis is the groundhog, a member of the Sciuridae family. Infections in other Scuridae hosts have been reported in the Alaska marmot (M. broweri), hoary marmot (M. caligata), yellow-bellied marmot (M. flaviventris), Olympic marmot (M. olympus, California ground squirrel (Otospermophilus beecheyi), Barrow ground squirrel (Spermophilus parryi barrowensis), Richardson's ground squirrel (U. richardsonii), and long-tailed ground squirrel (Urocitellus undulatus) (Berry, 1985).
2.3.2. Differences in life cycle compared to other Baylisascaris spp.
B. laevis is the only member of the genus that has a strictly monoxenous life cycle with the apparent loss of a paratenic host during evolution. Development from L2 to adult occurs entirely in the sciurid definitive host (Berry, 1985). Unlike other Baylisascaris spp., larvae which migrate throughout the body of paratenic hosts, B. laevis only migrate within the liver and lungs of their hosts (Babero, 1960b).
When larvated eggs are ingested, L2 hatch and migrate to the liver by 10–12 DPI and develop into L3. These larvae migrate to the lungs where they molt into L4 that are coughed up and swallowed. Once in the small intestine they continue to develop into adults within the wall of the small intestine (Babero, 1960b). Adults enter the intestinal lumen, mate, and produce eggs that are then shed in feces. Reinfection likely occurs when embryonated eggs adhering to fur are ingested during grooming (Berry, 1985). B. laevis can produce liver lesions in its sciurid host (Tiner, 1953b).
2.3.3. Ecology and epidemiology
Although the distribution of known B. laevis sciurid hosts extends throughout North America, the parasite has only been reported in New York, Pennsylvania, California, and Alaska. Outside the United States it has been reported in southern Ontario and Saskatchewan, Canada (Berry, 1985). Further surveillance is needed to characterize the distribution of B. laevis in North America.
A few studies have been conducted on the seasonality of B. laevis infectious. During a 2 year study in southern Ontario, Canada, B. laevis prevalence peaked in September, with intensity showing similar seasonal variation (Berry, 1985). Prevalence was lowest during winter months and increased during the spring, similar to the annual cycles observed in B. columnaris and B. procyonis (Berry, 1985). This seasonality of B. laevis seems to be primarily driven by feeding habits; groundhogs and ground squirrels continually feed throughout the spring and summer, and then in the fall begin to consume less in preparation for hibernation. In the winter, absence of (Young and Sims, 1979) and physiological changes accompanying hibernation, such as lowered temperature, heart and metabolic rate of hosts, likely prevents B. laevis from developing if acquired late in the year (Babero, 1960b). Despite lower rates of shedding in the winter, eggs can persist in the environment because they are resistant to sub-zero temperatures. Most (94%) non-embryonated eggs survived −10 C temperatures after exposure for 10 days, while >70% of embryonated L3 eggs survived −10 C temperatures for 16 days (Berry, 1985). Also, eggs deposited in the environment are often protected from extreme temperatures because they are covered by leaf litter and snow or in subterranean burrows (Berry, 1985).
2.3.4. Experimental infections
A variety of species have been assessed as experimental hosts for B. laevis using experimental infections. Babero (1959) conducted oral infection trials to assess susceptibility and pathology of B. laevis infection in eleven species including laboratory mice, laboratory rats (Rattus norvegicus), cotton rats (Sigmodon hispidus), hamsters, guinea pigs (Cavia porcellus), thirteen-lined ground squirrel (Ictidomys tridecemlineatus) and Franklin ground squirrels (Poliocitellus franklinii), opossums (Didelphis virginiana), groundhogs, domestic cats, and raccoons. Many of these experimental hosts were examined for infection via necropsy, although details on the duration of infection and stages recovered are not given so interpretation is difficult.
Some inoculated hosts developed disease, including a groundhog with signs of pneumonia due to larva migration. Two guinea pigs, who died ∼40 days post infection, exhibited dyspnea, bloody stools, ataxia and emaciation. Granulomatous liver lesions were frequently observed, sometimes containing L2 larval sheaths, as well as foci of pulmonary hemorrhage (Babero, 1959). In separate experimental infections of laboratory mice, larvae apparently did not become completely encapsulated in the liver, but lesions due to migration of the larvae through the liver were present (Tiner, 1953a). Other experimental infections on multiple hosts (groundhogs, ground squirrels, cats, mice, guinea pigs, and hamsters) showed that larvae become encapsulated in the liver. When larvae migrated to the lungs, very few were retained, and even fewer were found in the small intestine after migration was completed (Babero, 1960b).
2.4. Baylisascaris devosi
Baylisascaris devosi infects North American mustelids belonging to the clade Guloninae (martens, fishers and wolverines), which are all carnivorous mammals that inhabit forests in the northern United States and Canada (Ruggiero et al., 1994, Li et al., 2014) (Table 5). B. devosi was formally described by Sprent (1952a) showing that parasites from fisher (Martes pennant) and marten (Martes americana) were distinct from B. columnaris from striped skunks. Prior to the description of B. devosi, a specimen from a Pacific marten (Martes caurina) in Idaho was recorded as B. columnaris but this parasite was likely B. devosi (Sprent, 1952a, Marshall, 1942). Baylisascaris devosi can be distinguished from B. columnaris by several morphologic characteristics including body length, length of spicules, the position of the vulva, and the width of denticles on the dentigerous ridges. Specimens recovered from fisher were longer than those parasites recovered from martens (Sprent, 1952a). Egg sizes range from 58–77 × 51–61 μm (Sprent, 1953a). However, given potential overlap in morphometric features, species identification should be confirmed using molecular analysis.
Table 5.
Host and locality records of Baylisascaris devosi in North American mustelid species.
| Host species | Location | No. infected/No. examined (%) | Source |
|---|---|---|---|
| American marten (Martes americana) | Manitoba, Canada | 1/139 (0.7) | Poole et al., 1983 |
| Alaska, USA | 1/141 (0.7) | Scranton 1986 | |
| Washington, USA | 4/78 (5) | Hoberg et al. 1990,a | |
| Wolverine (Gulo gulo) | Northwest Territories, Canada | NG | Addison and Boles 1978 |
| Alaska, USA | 17/80 (21) | Rausch 1959 | |
| Fisher (Martes pennanti) | New Brunswick, Canada | NG | Dick and Leonard 1979 (cited pers. comm. with C. Bursey) |
| Manitoba, Canada | 52/162 (32) | Dick and Leonard 1979 | |
| Pacific marten (Martes caurina) | Idaho, USA | 6/17 (35) | Marshall 1942,b |
| Martes americana, Martes pennanti | Ontario, Canada | NG | Sprent 1952a |
NG=Not given.
Unidentified larvae authors suggested could be B. devosi were found in digestions of hind limb musculature of 10 martens in addition to the 23 adult nematodes identified as B. devosi in study.
Identified as Ascaris columnaris.
Eggs of B. devosi can become fully larvated in 12 days and remain infective for at least one year (Sprent, 1953a, Sprent, 1953b). In experimentally-inoculated laboratory mice, larvae migrated out of the intestine and by three days were found in the heart, lungs, brain and kidney. Mice showed severe symptoms of pulmonary disease between 3 and 4 DPI and lungs were dark red (Sprent, 1952b). By 8–12 DPI, larvae were present in muscular and subcutaneous tissues of the neck, shoulders and thorax. These third stage larvae remained encapsulated in muscle tissue for at least 6 months. Larvae from a mouse infected for 25 days were infective to a domestic ferret (Sprent, 1953b). Larvae appear to be tolerant to freezing as larvae recovered from mice >3 weeks post infection were motile after being frozen at −20 C for up to 8 weeks, but it is unknown if they were infectious to a definitive host (Sprent, 1953b). If larvae remain infectious following freezing, this would facilitate transmission of B. devosi from frozen carcasses that may be scavenged by a definitive host. Experimental inoculation of several adult and juvenile domestic ferrets and a single skunk via infected mouse carcasses established patent infections. An attempt to inoculate a single adult marten by the same manner was unsuccessful, but perhaps this wild-caught animal had pre-existing immunity to B. devosi (Sprent, 1953b).
Most larvae recovered from laboratory mice hosts experimentally infected with B. devosi were concentrated in the cervical and thoracic musculature (Sprent, 1953b). This localization of infection may be adaptive because marten are known to attack the head and neck region of prey and occasionally discard the rest of the carcass (Powell et al., 2003, Sprent, 1953b). If this concentration of B. devosi larvae in the anterior region occurs in natural paratenic hosts, larvae may be more likely to be ingested by the definitive mustelid host.
In the future, molecular studies on parasites recovered from free-living hosts could help more accurately identify B. devosi from other Baylisascaris species, which will provide important data on prevalence and host specificity. Further, experimental trials on B. devosi in various suspected or known natural definitive hosts would provide data on host suitability.
2.5. Baylisascaris melis
The definitive hosts of Baylisascaris melis are North American badgers (Taxidea taxus) and European badgers (Meles meles). The parasite was first described from European badgers in Belgium (Gedoelst, 1920). Morphological characteristics of B. melis are similar to those of B. transfuga; both of these species have salient alae, as opposed to the vestigial alae of other Baylisascaris spp. (Sprent, 1968). There have been multiple reports of ascarids in wild North American badgers that are assumed to be B. melis although most were reported as A. columnaris (Table 6). Contemporary studies, including molecular characterization, on this parasite are needed to confirm the identity of parasites from North American badgers and their conspecificity with B. melis from European badgers.
Table 6.
Locality records of presumed Baylisascaris melis from North American badgers (Taxidea taxus).
| Location | No. infected/No. examined | Source |
|---|---|---|
| Minnesotaa | 1/8b | Erickson 1946 |
| Iowaa | 29/NG | Wittrock and Ulmer 1974 |
| North Dakotaa | 6/17 | Leiby et al., 1971 |
| Kansasa | 10/30 | Pence and Dowler 1979 |
| Wisconsina | 2/4 | Morgan 1943 |
| South Dakotaa | NG | Jense 1968 |
| Coloradoa | NG | Leiby 1961 |
| Wyoming | 1 roadkill badger, 1 captive badger | Tiner 1953a |
NG=Not given.
Identified as Ascaris columnaris but are assumed to be B. melis.
2 of the 8 badgers had been in captivity for 2 years.
Baylisascaris melis can cause larva migrans in experimentally infected rodents. Tiner (1953a) fed 2000–3000 eggs collected from a naturally-infected badger in Wyoming to four rodent species: ground squirrel (Citellus armatus), laboratory mice, deer mice (Peromyscus maniculatus) and guinea pigs. Only the ground squirrels (5 of 7 infected) developed neurologic disease; however, larvae were found in the brains of the deer mice and encapsulated in the skeletal muscle of all four species (Tiner, 1953a). In one ground squirrel, larvae were recovered from the lungs and were widely distributed in the skeletal muscle, with greatest abundance in the intercostal spaces under the parietal pleura of the diaphragm. In P. maniculatus, B. melis larvae were encapsulated in the mesentery of the small intestine, on the epicardial surfaces, and in the brain on 3 DPI (Tiner, 1953b).
Although B. melis can cause central nervous system disease in experimentally-infected rodent species, there have been no confirmed cases of natural B. melis infections in wild rodents (Boyce et al., 1988, Kazakos, 2001, Tiner, 1953a, Tiner, 1953b). However, neurologic cases due to Baylisascaris sp. diagnosed in ground squirrels and other rodents in regions where the badger ranges overlap with raccoons could be due to B. melis (Kazakos, 2001). In future cases, identification of larvae in these cases using molecular techniques is needed to better understand the role of non-B. procyonis species in cases of neurologic disease in wildlife. Also, serum from mice infected with B. melis cross-reacted with larval excretory-secretory antigens from B. procyonis (Boyce et al., 1988), so it is important to consider B. melis as a possible etiologic agent of hosts with antibodies to Baylisascaris spp. in areas where badgers and raccoons are sympatric.
2.6. Baylisascaris potosis
A novel Baylisascaris species,B. potosis, was recently described in kinkajous (Potos flavus). Type specimens were collected from captive kinkajous that originated in Cooperative Republic of Guyana (Tokiwa et al., 2014). This species is morphologically similar to B. procyonis but was described as a new species based on genetic analysis of several gene targets (i.e., internal transcribed spacer (ITS) 2 region, 28S rRNA gene, and COX1 gene) (Taira et al., 2013; Tokiwa et al., 2014). Kinkajous are common exotic pets in the United States and other countries.
Previously, kinkajous were reported as a host of B. procyonis, both in the wild in Columbia and in captivity in the United States and Japan (Overstreet, 1970; Kazacos et al., 2011, Taira et al., 2013; Parzansky, 2015). Another possible host, the bushy-tailed olingo (Bassaricyon gabbii) passed a male Baylisascaris (reported as B. procyonis) after being fed eggs from a naturally-infected kinkajou from Columbia; however, it is not known if the olingo was infected prior to the experiment (Overstreet, 1970). However, since the description of B. potosis, these reports are questionable, and where possible should be confirmed with molecular data.
To evaluate possibile paratenic hosts that can develop larva migrans, Tokiwa et al. (2015a), experimentally inoculated Mongolian gerbils. Exposure of gerbils to 100–4000 embryonated eggs resulted in VLM, but no larvae were found in the brain. A squirrel monkey (Saimiri sciureus) inoculated with 10,000 B. potosis eggs did not develop clinical signs or gross lesions, although a few migrating larvae were recovered from liver and kidney tissues (Tokiwa et al., 2015b). Another squirrel monkey inoculated with 100,000 eggs in the same trial developed gross lesions, including liver congestion, pulmonary edema, and abundant intestinal granulomas. Small granulomatous lesions containing larvae were found in the outer layers of the cerebral cortex, without deeper invasion as is typical of B. procyonis. This animal was found dead at 30 DPI, but the animal lacked clinical signs and the authors state that no cause of death was determined. However, pulmonary edema, liver congestion, and nodular lesions containing non-degenerate larvae along the intestine were found at necropsy (Tokiwa et al., 2015b). Based on these preliminary trials, it appears that B. potosis can cause larva migrans in rodent and primate hosts, although the pathogenicity and capacity for neural invasion appears less than that of B. procyonis or B. columnaris.
Because of the recent description of this parasite and the paucity of surveillance in possible hosts in South America apart from a single infected individual, little is known about the natural history of B. potosis. Interestingly, raccoons were recently confirmed to have B. procyonis infections, based on sequence analysis, in Costa Rica (Baldi et al., 2016). It appears that B. procyonis and B. potosis are sympatric in procyonids in Central America, highlighting the need for additional research to understand these closely related parasites that may share hosts.
3. Molecular and diagnostic approaches to the study of Baylisascaris
3.1. Molecular epidemiology of Baylisascaris spp.
Microscopy has been traditionally used to identify Baylisascaris spp. based on adult morphological characters although some species can be difficult to distinguish, especially if only immature worms are found. However, because of the similarity among the sizes of eggs in feces or larvae in tissues, molecular markers have been developed to facilitate identification. For example, multiple single nucleotide polymorphisms (SNPs) in mitochondrial and nuclear gene sequences of B. columnaris, B. procyonis and B. transfuga, have been used to develop species-specific diagnostic molecular markers for rapid identification of different Baylisascaris species (Blizzard et al., 2010, Testini et al., 2011, Franssen et al., 2013). Studies have also characterized the genetic diversity, investigated the population structure and the phylogenetic relationships among Baylisascaris species, which is important for understanding the zoonotic potential and host specificity of these parasites. To discuss the phylogenetic relationships among the Baylisascaris spp. that occur in the New World in a broader context, this section includes data published on Baylisascaris spp. from Asia and Europe and Baylisascaris procyonis from raccoons.
3.1.1. Phylogenetic relationships
Based on analysis of numerous genetic targets (Table 7), Baylisascaris is most closely related to the genus Ascaris. The entire mitochondrial genome has been sequenced for four species— B. transfuga, B. ailuri, B. schroederi and B. procyonis (all samples from China) (Xie et al., 2011a, Xie et al., 2011b, Li et al., 2012). Phylogenetic analyses of these mitochondrial genomes and concatenated partial mitochondrial and nuclear genes (12S rDNA, 18S rDNA and 28S rDNA) provide the strongest support for the relatedness of the genus Baylisascaris with Ascaris and other members of the order Ascaridida (Xie et al., 2011a, Xie et al., 2011b, Li et al., 2012).
Table 7.
Summary of different PCR primers available for identification of Baylisascaris species.
| Target gene | Length of the target gene | Species | Primer | Reference |
|---|---|---|---|---|
| ATPase subunit 6 (atp6) | 600bp | B. schroederi | F (5′-CGCGGATCCTTCGATATTCGTGG CCT-3′) R (5′-CGCAAGCTTCTAATATGGTGTCTT CGG-3′) |
Xie et al., 2015 |
| cytochrome oxidase c subunit I (CO1) | 413bp | B. procyonis, B. transfuga, B. columnaris | F: (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) R: (5′-TAACGACATAACATAATGAAAATG-3′) |
Franssen et al., 2013 |
| cytochrome oxidase c subunit 2 (CO2) | 1578bp | B. schroederi | F (5′-TTTAGAGGTTGGAATGTAGGGT-3′) R (5′- CCATCCCCTTAATCTGCAAT-3′) |
Xie et al., 2015 |
| 483bp | B. procyonis, B. transfuga, B. columnaris | F: (5’-AATTTTAATTGTAGTCTTTTGTTTGG-3′) R: (5’-CTATGATTAGCACCACAAATC-3′) |
Franssen et al., 2013 | |
| mitochondrial cytochrome b cytb | ∼1500bp | B. schroederi | Cytb-1 (5′-GGTGCTATGCTCGGTTACG-3′) Cytb-2 (5′-CCACTAAGACCCTCCATT-3′) |
Zhou et al., 2013 |
| 12s rRNA | 499bp | B. schroederi, B.ailuri, B. transfuga, B. procyonis | F (5′-AGCGGAGGAAAAGAAACTAA3′) R (5′-TGATCCTTCTGCAGG TTCACCTAC-3) |
Li et al., 2012 |
| 18s rDNA | 1708bp | B. schroederi, B. ailuri, B. transfuga, B. procyonis | F (5′-AGCGGAGGAAAAGAAACTAA-3′) R (5′-TGATCCTTCTGCAGG TTCACCTAC-3′) |
Li et al., 2012 |
| 28s rDNA | 751bp | B. schroederi, B. ailuri, B. transfuga, B. procyonis | F (5′-CCCGATTGATTCTGTCGGC-3′) R (5′-TGATCCTTCTGCAGG TTCACCTAC-3′) |
Li et al., 2012 |
| 718bp | B. procyonis, B. transfuga, B. columnaris | F: (5′-CGAGGATTCCCTTAGTAACT-3′) R: (5′-TCGGATAGGTGGTCAACG-3′) |
Franssen et al., 2013 | |
| ITS1-5.8S-ITS2 | 630-650bpa | B. procyonis, B. transfuga, B. columnaris | F: (5’-ATAGTGAGTTGCACACTAATGT-3′) R: (5’-TTATATGCTTAAATTCAGCGGG-3′) ITS2-F: (5′-GCCATTTATGAATTTTCAACATGG-3′) ITS2-R: (5′-AGTTATATGCTTAAATTCAGCGG-3′) |
Franssen et al., 2013 |
| Complete mt genome- (atp6, CO1–CO3, cytb, nad1–nad6 nad4L, 22 transfer RNA (trn) Genes (small (rrnS) and large (rrnL) subunits) |
B. schroederi, B.ailuri, B. transfuga, B. procyonis | See reference for numerous sets of primers. | Xie et al., 2011a, Xie et al., 2011b | |
| ITS2 rDNA | 301 bp | B. transfuga | F (5’-TTATGAATTTTCAACATGGC-3’) R (5’-GTTAGATGCTTAAATTCAGC-3’) |
De Ambrogi et al., 2011 |
| 3′ end of the ITS- 1, complete 5.8S and ITS-2, and the 5′ end of 28S rDNA |
700bp | B. schroederi | zghu (5′- AAGGTGGAGAGAAAGCTCCTC GT-3′) NC2 (5’-TTAGTTTCTTTTCCTCCGCT-3’) |
Zhao et al., 2012 |
| ITSs (18s and 28S) | 1177bp | B. transfuga | F (5′-ACTGCTGTTTCGAGACCTTTCGAG-3′) R(5′-TAGCACCTTCTTTGGACTATAGCC-3′) |
Testini et al., 2011 |
Different length in different parasites due to insertions and tandem repeat.
Within the Baylisascaris genus, phylogenetic relationships have been investigated using several molecular targets (i.e., numerous mitochondrial genes, nuclear 5.8S, and second internal transcribed spacer (ITS-2) rDNA sequences). The three ursid-specific Baylisascaris species (B. transfuga, B. ailuri and B. schroederi) are more closely related to each other than to B. procyonis and B. ailuri is more similar to B. transfuga than to B. schroederi (Xie et al., 2011a, Xie et al., 2011b, Li et al., 2012). Evidence from nuclear 5.8S and ITS-2 rDNA sequences also showed higher genetic similarity between B. transfuga and B. schroederi compared to B. procyonis (Zhao et al., 2012).
Although no molecular data are available for B. laevis, B. melis or B.devosi; mitochondrial and nuclear genes of B. columnaris and B. potosis have recently been characterized and their phylogenetic relationship with other Baylisascaris species has been examined. The first phylogenetic analysis of B. columnaris from pet skunks in Europe showed closer affinity to B. procyonis compared to B. transfuga (Franssen et al., 2013) based on mitochondrial cytochrome c oxidase 1 and 2 (CO1 and CO2), ribosomal ITS1-5.8S-ITS2 and ribosomal 28S genes. This result was expected because B. columnaris was previously shown to be very similar to B. procyonis based on partial mitochondrial CO2 gene sequences (Danguodobiyam et al., 2009). Phylogenetic analyses of the mitochondrial CO1 and ITS2 rDNA gene sequences showed that B. potosis had high genetic similarity to B. procyonis and B. columnaris (Tokiwa et al., 2014).
A comprehensive phylogenetic assessment of different Baylisascaris species at common gene targets would facilitate a better understanding of genetic similarities/differences between the different species. Given that many species of Baylisascaris span a very wide geographic scale, genetic differences likely exist among these populations suggesting the existence of cryptic species. For example, B. melis is endemic to both Eurasia and the Americas and B. transfuga similarly has a large geographic and host range. The morphologic variability in B. transfuga also should be examined using molecular tools to assess if these indicate the presence of multiple species. Genetic studies are critically needed to evaluate species validity and both fine-scale and broad-scale geographic variability for all Baylisascaris species.
3.1.2. Population structure
Genetic markers are widely used to assess population structure and provide important insights into host-parasite transmission dynamics. For example, giant panda (Ailuropoda melanoleuca) populations were genetically distinct across the three mountain ranges in China but B. schroederi were not, suggesting little co-evolution between hosts and parasites and high levels of parasite gene flow (Zhou et al., 2013, Xie et al., 2015). High genetic variation within the parasite populations on each mountain range was observed, but the lack ofpopulation diversity across the mountain ranges suggested a homogenous parasite population, based on the complete mitochondrial cytb, atp6 and cox1 gene targets. In contrast, use of microsatellite markers revealed two genetic clusters in B. procyonis across the Grand River in Western Michigan, USA (Sarkissian et al., 2015). Lack of population structure in B. schroederi parasites across the mountain ranges in China indicates the fast evolving rate of parasites compared to their hosts and microsatellite markers may be able to further confirm if there is any recent genetic divergence between the parasite populations. Thus, choice of appropriate genetic marker is important while assessing parasite population structure and understanding host-parasite evolutionary dynamics.
Low genetic diversity was found within B. columnaris in the Netherlands. Multi-locus genetic analysis revealed four distinct genotypes, possibly owing to the differences in the host or geographic origin of these parasites (Franssen et al., 2013). However, a wider sampling of infected hosts from other geographical regions is warranted to reveal the true genetic diversity of these parasites.
3.2. Diagnostic considerations
As discussed throughout this review, accurately distinguishing species based only on morphologic characteristics can be a challenge. Not only does considerable overlap in morphometry exist (Table 1), but host species associations may not be as strict as often assumed as limited experimental data suggest that B. columnaris can infect raccoons and B. procyonis and B. devosi can infect skunks (Berry, 1985, Sprent, 1953a). Although the prevalence of these cross-infections in naturally-infected hosts is not understood, parasite species identity should not be assumed based on host species. When possible, molecular identification of species should be used for species confirmation of eggs, larvae, and possibly adult nematodes if only female or immature worms are present. Until this is widely implemented, our understanding of host specificity among Baylisascaris spp. will remain limited.
Additionally, current serologic methods cannot distinguish between species of Baylisascaris causing larva migrans in paratenic hosts. Antisera from animals infected with different Baylisascaris spp. all show reactivity to crude B. procyonis excretory-secretory (ES) antigen fractions as well as a recently developed recombinant antigen (BpRAG-1) (Boyce et al., 1988, Dangoudoubiyam et al., 2010). Further work is needed to determine if species-level differences in ES or other antigen targets exist, and if these differences would be sufficiently different to allow the development of species-specific serodiagnostics. This is a potentially difficult goal as serologic differentiation between two other related ascarids, Toxocara canis and Toxocara cati, has not been successful with current platforms, and these are both important zoonotic parasites (Poulsen et al., 2015). Therefore molecular identification of recovered larvae from paratenic hosts, if possible, is the ideal method of confirming species. Some serologic assays may be useful in determining exposure to parasites in the genus, given that the target antigen is not cross-reactive with other ascarids.
4. Conclusion
The genus Baylisascaris is diverse with importance to wildlife, domestic animal, and public health. However, many knowledge gaps exist regarding species other than B. procyonis, which is largely driven by a scarcity of contemporary surveys and application of molecular tools to investigate the ecology of these parasites Field studies elucidating important life history characteristics are critically lacking for these other, “neglected” Baylisascaris spp. Ideally, these future field efforts will incorporate modern molecular approaches along with traditional morphologic examination to better ascertain species diversity, species validity, host range, and disease caused by these parasites in wild definitive and paratenic hosts.
Contributor Information
Sarah G.H. Sapp, Email: sgsapp@uga.edu.
Michael J. Yabsley, Email: myabsley@uga.edu.
References
- Abdel-Rasoul I.C., Fowler M. Denver, CO. American Association of Zoo Veterinarians; Philadelphia, PA: 1979. Epidemiology of Ascarid Infection in Captive Carnivores. Proc Am Assoc Zoo Vet, October 1979; pp. 165–169. [Google Scholar]
- Addison E.M., Boles B. Helminth parasites of wolverine, Gulo gulo, from the district of mackenzie, Northwest territories. Can. J. Zool. 1978;56:2241–2242. doi: 10.1139/z78-304. [DOI] [PubMed] [Google Scholar]
- Addison E.M., Pybus M.J., Rietveld H.J. Helminth and arthropod parasites of black bear, Ursus americanus, in central Ontario. Can. J. Zool. 1978;56 doi: 10.1139/z78-288. 122–2126. [DOI] [PubMed] [Google Scholar]
- Babero B.B. Pathology resulting from experimental infections by Ascaris laevis Leidy. Trans. Am. Microsc. Soc. 1959;78:330–335. [Google Scholar]
- Babero B.B. Studies on the larval morphology of Ascaris laevis Leidy, 1856. Am. Midl. Nat. 1960;64:349–361. [Google Scholar]
- Babero B.B. On the migration of Ascaris laevis Leidy, 1856 in some experimentally infected hosts. Trans. Am. Microsc. Soc. 1960;79:439–442. [Google Scholar]
- Baldi M., Alvarado G., Smith S., Santoro M., Bolanos N., Jiminez C., Hutter S., Walzer C. Baylisacaris procyonis parasites in raccoons, Costa Rica, 2014. Emerg. Infect. Dis. 2016;22:1503–1505. doi: 10.3201/eid2208.151627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D.M., Rogers L.L. Clostridial myonecrosis in a black bear associated with drug administration. J. Wildl. Dis. 1980;16:315–317. doi: 10.7589/0090-3558-16.3.315. [DOI] [PubMed] [Google Scholar]
- Bauer C., Gey A. Efficacy of six anthelmintics against luminal stages of Baylisascaris procyonis in naturally infected raccoons (Procyon lotor) Vet. Parasitol. 1995;60:155–159. doi: 10.1016/0304-4017(94)00774-7. [DOI] [PubMed] [Google Scholar]
- Baylis H.A., Daubney R. Report on the parasitic nematodes in the collection of the zoological survey of India. Mem. Indian Mus. 1922;7:263–289. [Google Scholar]
- Berry J. Unviersity of Guelph; 1985. Phylogenetic Relationship between Baylisascaris Spp. Sprent, 1968 (Nematoda: Ascarididae) from Skunks, Raccoons, and Groundhogs in Southern Ontario. (MS Thesis) [Google Scholar]
- Blizzard E.L., Davis C.D., Henke S., Long D.B., Hall C.A., Yabsley M.J. Distribution, prevalence, and genetic characterization of Baylisascaris procyonis in selected areas of Georgia. J. Parasitol. 2010;96:1128–1133. doi: 10.1645/GE-2518.1. [DOI] [PubMed] [Google Scholar]
- Boyce W.M., Branstetter B.A., Kazacos K.R. Comparative analysis of larval excretory-secretory antigens of Baylisascaris procyonis, Toxocara canis and Ascaris suum by Western blotting and enzyme immunoassay. Int. J. Parasitol. 1988;18:109–113. doi: 10.1016/0020-7519(88)90044-6. [DOI] [PubMed] [Google Scholar]
- Canavan W.P.N. Nematode parasites of vertebrates in the philadelphia zoological garden and vicinity. Parasitol. 1929;21:63–102. [Google Scholar]
- Catalano S., Lejeune M., Tizzani P., Verocai G.G., Schwantje H., Nelson C., Duignan P.J. Helminths of grizzly bears (Ursus arctos) and American black bears (Ursus americanus) in Alberta and British Columbia, Canada. Can. J. Zool. 2015;93:765–772. [Google Scholar]
- Cawthorn R.J., Anderson R.C. Seasonal population changes of Physaloptera maxillaris (Nematoda: Physalopteroidea) in striped skunk (Mephitis mephitis) Can. J. Zool. 1976;54:522–525. doi: 10.1139/z76-058. [DOI] [PubMed] [Google Scholar]
- Choquette L.P., Gibson G.G., Pearson A.M. Helminths of the grizzly bear, Ursus arctos L., in northern Canada. Can. J. Zool. 1969;47:167–170. doi: 10.1139/z69-038. [DOI] [PubMed] [Google Scholar]
- Church E., Wyand D., Lein D. Experimentally induced cerebrospinal nematodiasis in rabbits (Oryctolagus cuniculus) Am. J. Vet. Res. 1975;36:331–335. [PubMed] [Google Scholar]
- Clark J.D., Loew F.M., Burns K.F. The use of dichlorvos as an anthelmintic in naturally parasitized bears. J. Am. Vet. Med. Assoc. 1969;155:1093–1097. [PubMed] [Google Scholar]
- Crum J.M., Nettles V.F., Davidson W.R. Studies on the endoparasites of the black bear (Ursus americanus) in the southeastern United States. J. Wildl. Dis. 1978;14:178–186. doi: 10.7589/0090-3558-14.2.178. [DOI] [PubMed] [Google Scholar]
- Dangoudoubiyam S., Vemulapalli R., Kazacos K.R. PCR assays for detection of Baylisascaris procyonis eggs and larvae. J. Parasitol. 2009;95:571–577. doi: 10.1645/GE-1905.1. [DOI] [PubMed] [Google Scholar]
- Dangoudoubiyam S., Vemulapalli R., Hancock K., Kazacos K.R. Molecular cloning of an immunogenic protein of Baylisascaris procyonis and expression in Escherichia coli for use in developing improved serodiagnostic assays. Clin. Vac. Immunol. 2010;17:1933–1939. doi: 10.1128/CVI.00404-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ambrogi M., Aghazadeh M., Hermosilla C., Huber D., Majnaric D., Reljic S., Elson-Riggins J. Occurrence of Baylisascaris transfuga in wild populations of European brown bears (Ursus arctos) as identified by a new PCR method. Vet. Parasitol. 2011;179:272–276. doi: 10.1016/j.vetpar.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Dick T.A., Leonard R.D. Helminth parasites of fisher (Martes pennanti) from Manitoba. Can. J. Wildl. Dis. 1979;15:409–412. doi: 10.7589/0090-3558-15.3.409. [DOI] [PubMed] [Google Scholar]
- Dies K.D. Helminths recovered from black bears in the peace river region in Northwestern Alberta. J. Wildl. Dis. 1979;15:49–50. doi: 10.7589/0090-3558-15.1.49. [DOI] [PubMed] [Google Scholar]
- d’Ovidio D., Pantchev N., Noviello E., Del Prete L., Maurelli M.P., Cringoli G., Rinaldi L. Survey of Baylisascaris spp. in captive striped skunks (Mephitis mephitis) in some European areas. Parasitol. Res. 2016:1–4. doi: 10.1007/s00436-016-5307-8. [DOI] [PubMed] [Google Scholar]
- Dragoo J.W. Nutrition and behavior of striped skunks. Vet. Clin. North Am. Exot. Anim. Pract. 2009;12:313–326. doi: 10.1016/j.cvex.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Duffy M.S., Greaves T.A., Burt M.D.B. Helminths of the black bear, Ursus americanus, in New Brunswick. J. Parasitol. 1994;80:478–480. [PubMed] [Google Scholar]
- Dyer W.G. Helminths of the striped skunk, Mephitis mephitis Schreber, in North Dakota. Proc. Helminthol. Soc. Wash. 1970;37:92–93. [Google Scholar]
- Erickson A.B. Incidence of worm parasites in Minnesota Mustelida and host lists and keys to North American species. Am. Midl. Nat. 1946;36:494–509. [Google Scholar]
- Figueroa J. New records of parasites in free-ranging Andean bears from Peru. Ursus. 2015;26:21–27. [Google Scholar]
- Foster G.W., Cunningham M.W., Kinsella J.M., Forrester D.J. Parasitic helminths of black bear cubs (Ursus americanus) from Florida. J. Parasitol. 2004;90:173–175. doi: 10.1645/GE-127R. [DOI] [PubMed] [Google Scholar]
- Fox L.M. Ivermectin: uses and impact 20 years on. Curr. Op. Infect. Dis. 2006;19:588–593. doi: 10.1097/QCO.0b013e328010774c. [DOI] [PubMed] [Google Scholar]
- Franssen F., Xie K., Sprong H., van der Giessen J. Molecular analysis of Baylisascaris columnaris revealed mitochondrial and nuclear polymorphisms. Parasit. Vectors. 2013;6:124. doi: 10.1186/1756-3305-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechette J.L., Rau M.E. Seasonal changes in the prevalence of ova of Diphylobothrium ursi and Baylisascaris transfuga in the feces of the black bear (Ursus americanus) J. Wildl. Dis. 1978;14:342–344. doi: 10.7589/0090-3558-14.3.342. [DOI] [PubMed] [Google Scholar]
- Fu Y., Nie H.M., Niu L.L., Xie Y., Deng J.B., Wang Q., Yang G.Y., Bin Gu X., Wang S.X. Comparative efficacy of ivermectin and levamisole for reduction of migrating and encapsulated larvae of Baylisascaris transfuga in mice. Korean J. Parasitol. 2011;49:145–151. doi: 10.3347/kjp.2011.49.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau R.J., Kutz S., Elkin B.T. Parasites in grizzly bears from the central Canadian arctic. J. Wildl. Dis. 1999;35:618–621. doi: 10.7589/0090-3558-35.3.618. [DOI] [PubMed] [Google Scholar]
- Gedoelst C.R. Sur une espece nouvelle d’ascaride, parasite du blaireau. C. R. Seanc. Soc. Biol. 1920;83:1291–1292. [Google Scholar]
- Gehrt S.D. Ecology and management of striped skunks, raccoons, and coyotes in urban landscapes. In: Fascione N., Delach A., Smith M.E., editors. People and Predators: from Conflict to Coexistence. Island Press; Washington, DC: 2004. pp. 81–104. [Google Scholar]
- Gehrt S.D. Seasonal survival and cause-specific mortality of urban and rural striped skunks in the absence of rabies. J. Mammal. 2005;86:1164–1170. [Google Scholar]
- Goodey T., Cameron T.W.M. Observations on the morphology and life history of Ascaris columnaris Leidy, a nematode parasite of the skunk. J. Helminthol. 1923;1:1. [Google Scholar]
- Hartwich G. Der Dachsspulwurm ‘Belascaris melis' Gedoelst. 1920, (Nematoda, Ascaridoidea) und seine systematische Stellung. Mitt. Zool. Mus. Berl. 1958;34:57–61. [Google Scholar]
- Hoberg E.P., Aubry K.B., Brittell J.D. Helminth parasitism in martens (Martes americana) and ermines (Mustela erminea) from Washington, with comments on the distribution of Trichinella spiralis. J. Wildl. Dis. 1990;26:447–452. doi: 10.7589/0090-3558-26.4.447. [DOI] [PubMed] [Google Scholar]
- Huntress, S., Spraker, T., 1985. Baylisascaris infection in the marmoset. In: Proceedings of the American Association of Zoo Veterinarians. p. 78.
- Jańczak D., Rożej-Bielicka W., Gołąb E. Baylisascaris collumnaris [sic] infection in a pet skunk in Warsaw. Ann. Parasitol. 2016;62:197. [Google Scholar]
- Jense G.K. South Dakota State University; MS Thesis: 1968. Food Habits and Energy Utilization of Badgers. [Google Scholar]
- Johnson D., Larter N.C., Elkin B., Allaire D.G. An opportunistic parasitological and serological examination of nuisance black bears in the Dehcho region of the Northwest Territories. Dept Env Nat Res; Gov N. W. T. 2013 Manuscript Report No. 227. [Google Scholar]
- Kazacos K., Winterfield R., Thacker H. Etiology and epidemiology of verminous encephalitis in an emu. Avian Dis. 1982;26:389–391. [PubMed] [Google Scholar]
- Kazacos, K.R., 1983. Life cycle studies on Baylisascaris procyonis in raccoons. In: Annual Meeting of the Conference of Research Workers in Animal Disease. p. 24.
- Kazacos K.R., Turek J.J. Scanning electron microscopy of the eggs of Baylisascaris procyonis, B. transfuga, and Parascaris equorum and their comparison with Toxocara canis and Ascaris suum. Proc. Helminthol. Soc. Wash. 1983;50:36–42. [Google Scholar]
- Kazakos K.R. Baylisascaris procyonis and related species. In: Samuel W.M., editor. Parasitic Diseases of Wild Mammals. second ed. 2001. pp. 301–341. [Google Scholar]
- Kazacos K., Zimmerman K., Parman B., Lane T., Carpenter L., Green A., Mann P., Murphy T., Bertucci B., Gray A., Cunningham M., Stanek D., Blackmore C., Yabsley M., Montgomery S., Bosserman E. Raccoon roundworms in pet kinkajous–three states, 1999 and 2010. Morb. Mortal. Wkly. Rep. 2011;60:302–305. [PubMed] [Google Scholar]
- Kazacos K.R. Baylisascaris larva migrans - circular 1412. U.S. Geol. Surv. 2016 [Google Scholar]
- Kidder J.D., Wade S.E., Richmond M.E., Schwager S.J. Prevalence of patent Baylisascaris procyonis infection in raccoons (Procyon lotor) in Ithaca, New York. J. Parasitol. 1989;75:870–874. [PubMed] [Google Scholar]
- King J.J., Black H.C., Hewit O.H. Pathology, parasitology, and hematology of the black bear in New York, N.Y. Game Fish. J. 1960;7:99–111. [Google Scholar]
- Leiby P.D. Intestinal helminths of some Colorado mammals. J. Parasitol. 1961;47:311. [Google Scholar]
- Leiby P.D., Sitzmann P.J., Kritsky D.C. Studies on helminths of North Dakota. II. Parasites of the badger, Taxidea taxus (Schreber) Proc. Helminthol. Soc. Wash. 1971;38:225–228. [Google Scholar]
- Leidy J. Contributions to. Helminthol. Proc. Natl. Acad. Sci. Phila. 1851;5:205–206. [Google Scholar]
- Lesmeister D.B., Millspaugh J.J., Wade S.E., Gompper M.E. A survey of parasites identified in the feces of eastern spotted skunks (Spilogale putorius) in western Arkansas. J. Wildl. Dis. 2008;44:1041–1044. doi: 10.7589/0090-3558-44.4.1041. [DOI] [PubMed] [Google Scholar]
- Li Y., Niu L., Wang Q., Zhang Z., Chen Z., Gu X., Xie Y., Yan N., Wang S., Peng X., Yang G. Molecular characterization and phylogenetic analysis of ascarid nematodes from twenty-one species of captive wild mammals based on mitochondrial and nuclear sequences. Parasitology. 2012;139:1329–1338. doi: 10.1017/S003118201200056X. [DOI] [PubMed] [Google Scholar]
- Li B., Wolsan M., Wu D., Zhang W., Xu Y., Zeng Z. Mitochondrial genomes reveal the pattern and timing of marten (Martes), wolverine (Gulo), and fisher (Pekania) diversification. Mol. Phylogenet. Evol. 2014;80:156–164. doi: 10.1016/j.ympev.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Lin L.C., Tsai T.H. Pharmacokinetics and brain distribution of unbound levamisole in the anesthetized rats using microdialysis and microbore column liquid chromatography. Anal. Chim. Acta. 2006;569:145–150. [Google Scholar]
- Manville A.M. Ecto- and endoparasites of the black bear in northern Wisconsin. J. Wildl. Dis. 1978;14:97–101. doi: 10.7589/0090-3558-14.1.97. [DOI] [PubMed] [Google Scholar]
- Marshall W.H. University of Michigan; MS Thesis: 1942. The Biology and Management of the Pine Marten in Idaho. [Google Scholar]
- Matoff K., Komandarev S. Comparative studies on the migration of the larvae Toxascaris leonina and Toxascaris transfuga. Parasitol. Res. 1965;25:538–555. [Google Scholar]
- McOrist S., Tseng F., Jakowski R., Keating J., Pearson C. Cerebral arteriosclerosis in an aged captive polar bear (Ursus maritimus) J. Zoo. Wildl. Med. 2002;33:381–385. doi: 10.1638/1042-7260(2002)033[0381:CAIAAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mead R.A. Some aspects of parasitism in skunks of the Sacramento Valley of California. Am. Midl. Nat. 1963;70:164–167. [Google Scholar]
- Miyashita M. Prevalence of Baylisascaris procyonis in raccoons in Japan and experimental infections of the worm to laboratory animals. J. Urban Living Heal. Assoc. 1993;37:137–151. [Google Scholar]
- Morgan B.B. New host records of nematodes from Mustelidae (Carnivora) J. Parasitol. 1943;29:158–159. [Google Scholar]
- Moudgil A.D., Singla L.D., Singh M.P. First report on molecular identification and fenbendazole resistance against Baylisascaris transfuga infection in Melursus ursinus (sloth bear) Helminthologia. 2014;51:262–268. [Google Scholar]
- Mozgovoi A.A. Essentials of Nematology. Vol. II. Keter Press; Jerusalem: 1953. Ascaridia of animals and man and the diseases caused by them; pp. 214–219. [Google Scholar]
- Nadler S.A., Hudspeth D.S.S. Phylogeny of the Ascaridoidea (Nematoda : Ascaridida) based on three genes and morphology: hypotheses of structural and Sequence evolution. J. Parasitol. 2000;86:380–393. doi: 10.1645/0022-3395(2000)086[0380:POTANA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Neiswenter S.A., Pence D.B., Dowler R.C. Helminths of sympatric striped, hog-nosed, and spotted skunks in west-central Texas. J. Wildl. Dis. 2006;42:511–517. doi: 10.7589/0090-3558-42.3.511. [DOI] [PubMed] [Google Scholar]
- Nettles V.F., Davidson W.R., Doster G.L. Peritonitis due to intestinal perforation by ascarids in a skunk. J. Am. Vet. Med. Assoc. 1978;173:1227–1228. [PubMed] [Google Scholar]
- Overstreet R.M. Baylisascaris procyonis (Stefanski and Zarnowski, 1951) from the kinkajou, Potos flavus, in Colombia. Proc. Helminthol. Soc. 1970;37:192–195. [Google Scholar]
- Page L.K., Swihart R.K., Kazacos K.R. Changes in transmission of Baylisascaris procyonis to intermediate hosts as a function of spatial scale. Oikos. 2001;93:213–220. [Google Scholar]
- Papini R., Renzoni G., Malloggi M., Casarosa L. Visceral larva migrans in mice experimentally infected with Baylisascaris transfuga (Ascarididae: Nematoda) Parasitologia. 1994;36:321–329. [PubMed] [Google Scholar]
- Papini R., Casarosa L. Observations on the infectivity of Baylisascaris transfuga eggs for mice. Vet. Parasitol. 1994;51:283–288. doi: 10.1016/0304-4017(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Papini R., Cavicchio P., Casarosa L. Experimental infection in chickens with larvae of Baylisascaris transfuga (Nematoda: Ascaridoidea) Folia Parasitol. 1993;40:141–143. [PubMed] [Google Scholar]
- Papini R., Demi S., Croce G.D. Observations on the migratory behaviour of Baylisascaris transfuga larvae in rabbits. Rev. Vet. Med. (Toulouse) 1996;147:893–896. [Google Scholar]
- Papini R., Piccolo M.S., Casarosa L. Effects of ivermectin on the migration of Baylisascaris transfuga larvae into the brain of mice. Folia Parasitol. 1996;43:157–158. [PubMed] [Google Scholar]
- Papini R., Renzoni G., Lo Piccolo S., Casarosa L. Ocular larva migrans and histopathological lesions in mice experimentally infected with Baylisascaris transfuga embryonated eggs. Vet. Parasit. 1996;61:315–320. doi: 10.1016/0304-4017(95)00825-x. [DOI] [PubMed] [Google Scholar]
- Parzansky M.C. Purdue University; MS Thesis: 2015. Baylisascaris Spp. In Non-raccoon Procyonid Hosts and Assessment of Potential Risk of Human Exposure. [Google Scholar]
- Pence D.B., Dowler R.C. Helminth parasitism in the badger, Taxidea taxus (Schreber, 1778), from the western Great plains. Proc. Helminthol. Soc. Wash. 1979;46:245–253. [Google Scholar]
- Pérez Mata A., Pérez H.G., Parra G.J. Morphological and molecular description of Baylisascaris venezuelensis, n. sp., from a natural infection in the South American spectacled bear Tremarctos ornatus (Cuiver, 1825) in Venezuela. Neotrop. Helminthol. 2016;10:85–103. [Google Scholar]
- Poole B.C., Chadee K., Dick T.A. Helminth parasites of pine marten, Martes americana, from Manitoba, Canada. J. Wildl. Dis. 1983;19:10–13. doi: 10.7589/0090-3558-19.1.10. [DOI] [PubMed] [Google Scholar]
- Poulsen C.S., Skov S., Yoshida A., Skallerup P., Maruyama H., Thamsborg S.M., Nejsum P. Differential serodiagnostics of Toxocara canis and T. cati - is it possible? Parasite Immunol. 2015;37 doi: 10.1111/pim.12181. [DOI] [PubMed] [Google Scholar]
- Powell R.A., Buskirk S.W., Zielinski W.J. Fisher and marten. In: Feldhamer G.A., Thompson B.C., Chapman J.A., editors. Wild Mammals of North America: Biology, Management, and Conservation. second ed. 2003. pp. 635–649. [Google Scholar]
- Rankin J. Helminth parasites of birds and mammals in western. Mass. Am. Midl. Nat. 1946;35:756–768. [Google Scholar]
- Rausch R. Studies on the helminth fauna of Alaska. XXI. Taxonomy, morphological variation, and ecology of Diphyllobothrium usri n. sp. provis. on Kodiak Island. J. Parasitol. 1954;40:540–563. [PubMed] [Google Scholar]
- Rausch R. Studies on the helminth fauna of Alaska. XXXVI. Parasites of the wolverine, Gulo gulo L., with observations on the biology of Taenia twitchelli Schwartz, 1924. J. Parasitol. 1959;45:465–484. [PubMed] [Google Scholar]
- Rogers L. Parasites of black bears of the Lake Superior region. J. Wildl. Dis. 1975;11:189–192. doi: 10.7589/0090-3558-11.2.189. [DOI] [PubMed] [Google Scholar]
- Rudolphi C.A. 1819. Entozoorum synopsis cui accedunt mantissa duplex et indices locupletissimi. (Berolini) [Google Scholar]
- Ruggiero L.F., Aubry K.B., Buskirk S.W., Lyon L.J., Zielinski W.J. vol. 184. USDA, FS, Rocky Mountain Forest and Range Experiment Station; Fort Collins, CO: 1994. (The Scientific Basis for Conserving Forest Carnivores: American Marten, Fisher, lynx and Wolverine in the Western United States. General Technical Report RM-GTR-254). [Google Scholar]
- Sarkissian C.A., Campbell S.K., Dharmarajan G., Jacquot J., Page L.K., Graham D.H. Microgeographic population genetic structure of Baylisascaris procyonis (Nematoda: Ascaroidae) in western Michigan indicates the Grand River is a barrier to gene flow. J. Parasitol. 2015;101:671–676. doi: 10.1645/15-767. [DOI] [PubMed] [Google Scholar]
- Sapp S.G.H., Rascoe L.N., Wilkins P.P., Handali S., Gray E.B., Eberhard M., Woodhall D.M., Montgomery S.P., Bailey K.L., Lankau E.W., Yabsley M.J. Baylisascaris procyonis roundworm seroprevalence among wildlife rehabilitators, United States and Canada, 2012-2015. Emerg. Infect. Dis. 2016;22:2012–2015. doi: 10.3201/eid2212.160467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp S.G.H., Weinstein S.B., McMahan C.S., Yabsley M.J. Variable infection dynamics in four Peromyscus species following experimental inoculation with Baylisascaris procyonis. J. Parasitol. 2016;102:538–544. doi: 10.1645/16-57. [DOI] [PubMed] [Google Scholar]
- Sato H., Matsuo K., Osanai A., Kamiya H., Akao N., Owaki S., Furuoka H. Larva migrans by Baylisascaris transfuga: fatal neurological diseases in Mongolian jirds, but not in mice. J. Parasitol. 2004;90:774–781. doi: 10.1645/GE-3330. [DOI] [PubMed] [Google Scholar]
- Sato H., Une Y., Kawakamin S., Saito S., Kamiya E., Akao H., Furuoka H. Fatal Baylisascaris larva migrans in a colony of Japanese macaques kept by a safari-style zoo in Japan. J. Parasitol. 2005;91:716–719. doi: 10.1645/GE-3374RN. [DOI] [PubMed] [Google Scholar]
- Schacher J.F. A contribution to the life history and larval morphology of Toxocara canis. J. Parasitol. 1957;43:599–612. [PubMed] [Google Scholar]
- Schaul J.C. Ohio State University; PhD Thesis: 2006. Baylisascaris Transfuga in Captive and Free-ranging Populations of Bears (Ursidae) [Google Scholar]
- Scranton C.R. Montana State University; MS Thesis: 1986. Parasites of Pine Marten, Marten Americana, in Northeastern Alaska. [Google Scholar]
- Shafir S.C., Wang W., Sorvillo J., Wise M., Moore L., Sorvillo T., Eberhard M. Thermal death point of Baylisascaris procyonis Eggs. Emerg. Infect. Dis. 2007;13:172–173. doi: 10.3201/eid1301.060966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard C.H., Kazacos K.R. Susceptibility of Peromyscus leucopus and Mus musculus to infection with Baylisascaris procyonis. J. Parasitol. 1997;83:1104–1111. [PubMed] [Google Scholar]
- Snyder D.E., Fitzgerald P.R. Contaminative potential, egg prevalence, and intensity of Baylisascaris procyonis–infected raccoons (Procyon lotor) from Illinois, with a comparison to worm intensity. Proc. Helminthol. Soc. Wash. 1987;54:141–144. [Google Scholar]
- Sprent J.F.A. On an Ascaris parasite of the fisher and marten, Ascaris devosi sp. nov. Proc. Helm. Soc. Wash. 1952;19:27–37. [Google Scholar]
- Sprent J.F.A. On the migratory behavior of the larvae of various Ascaris species in white mice: I. Distribution of larvae in tissues. J. Infect. Dis. 1952;90:165–176. doi: 10.1093/infdis/90.2.165. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. On the migratory behavior of the larvae of various Ascaris species in white mice: II. Longevity. J. Infect. Dis. 1953;92:114–117. doi: 10.1093/infdis/92.2.114. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. On the life history Ascaris devosi and its development in the white mouse and the domestic ferret. J. Parasitol. 1953;42:244–258. doi: 10.1017/s0031182000084493. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. The life cycles of nematodes in the family Ascarididae Blanchard 1896. J. Parasitol. 1954;40:608–617. [PubMed] [Google Scholar]
- Sprent J.F.A. On the invasion of the central nervous system by nematodes: II. Invasion of the nervous system in ascariasis. Parasitol. 1955;45:41–55. doi: 10.1017/s0031182000027414. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. Notes on Ascaris and Toxascaris, with a definition of Baylisascaris gen.nov. Parasitol. 1968;58:185–198. doi: 10.1017/s0031182000073534. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A., Lamina J., McKeown A. Observations on the migratory behavior and development of Baylisascaris tasmaniensis. Parasitol. 1973;67:67–83. doi: 10.1017/s0031182000046291. [DOI] [PubMed] [Google Scholar]
- Stegeman L.C. Some parasites and pathological conditions of the skunk (Mephitis mephitis nigra) in Central New York. J. Mammal. 1939;20:493–496. [Google Scholar]
- Stegner C.M., Neiland K.A. Helminth parasites of some fur-bearers of Oregon. J. Parasitol. 1955;41:637–638. [Google Scholar]
- Szczepaniak K., Listos P., Lopuszynski W., Skrzypek T., Kazimierczak W. Granulomatous peritonitis in a European brown bear caused by Baylisascaris transfuga. J. Wildl. Dis. 2012;48:517–519. doi: 10.7589/0090-3558-48.2.517. [DOI] [PubMed] [Google Scholar]
- Taira K., Une Y., Šnábel V., Sugiyama H. Baylisascaris sp. infection in a pet kinkajou Potos flavus. Helminthologia. 2013;50:238–243. [Google Scholar]
- Testini G., Papini R., Lia R.P., Parisi A., Dantas-Torres F., Traversa D., Otranto D. New insights into the morphology, molecular characterization and identification of Baylisascaris transfuga (Ascaridida, Ascarididae) Vet. Parasitol. 2011;175:97–102. doi: 10.1016/j.vetpar.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Tiner J.D. Some helminth parasites of skunks in Texas. J. Mammal. 1946;27:82–83. [Google Scholar]
- Tiner J.D. The morphology of Ascaris laevis Leidy, 1856, and notes on ascarids in rodents. Proc. Helm. Soc. Wash. 1951;18:126–131. [Google Scholar]
- Tiner J.D. Fatalities in rodents caused by larval ascaris in the central nervous system. J. Mammal. 1953;34:153–167. [Google Scholar]
- Tiner J.D. The migration, distribution in the brain, and growth of ascarid larvae in rodents. J. Infect. Dis. 1953;92:105–113. doi: 10.1093/infdis/92.2.105. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Nakamura S., Taira K., Une Y. Baylisascaris potosis n. sp., a new ascarid nematode isolated from captive kinkajou, Potos flavus, from the Cooperative Republic of Guyana. Parasitol. Int. 2014;63:591–596. doi: 10.1016/j.parint.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Taira K., Une Y. Experimental infection of mongolian gerbils with Baylisascaris potosis. J. Parasitol. 2015;101:114–115. doi: 10.1645/14-541.1. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Tsugo K., Nakamura S., Taira K., Une Y. Larva migrans in squirrel monkeys experimentally infected with Baylisascaris potosis. Parasitol. Int. 2015;64:284–287. doi: 10.1016/j.parint.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Kumar V., Ceulemans F., Mortelmans J. Chemotherapy of helminthiasis among wild mammals II. Baylisascaris transfuga infection of polar bear. Acta. Zool. Pathol. Antverp. 1976;64:115–119. [PubMed] [Google Scholar]
- Wirsing A.J., Azevedo F.C.C., Larivière S., Murray D.L. Patterns of gastrointestinal parasitism among five sympatric prairie carnivores: are males reservoirs? J. Parasitol. 2007;93:504–510. doi: 10.1645/GE-1067R.1. [DOI] [PubMed] [Google Scholar]
- Wittrock D.D., Ulmer M.J. Helminths of badgers, Taxidea taxus (Schreber), in northwest Iowa. Iowa State J. Res. 1974;48:319–327. [Google Scholar]
- Worley D.E., Fox J.C., Winters J.B., Jacobson R.H., Greer K.R. vol. 40. U.S.S.R. IUCN Publications New Series no; Binghamton, New York, USA, and Moscow: 1976. Helminth and Arthropod Parasites of Grizzly and Black Bears in Montana and Adjacent Areas. Third International Conference on Bear Research and Management; pp. 455–464. [Google Scholar]
- Xie Y., Zhang Z., Niu L., Wang Q., Wang C., Lan J., Deng J., Fu Y., Nie H., Yan N., Yang D. The mitochondrial genome of Baylisascaris procyonis. PloS One. 2011;6(10) doi: 10.1371/journal.pone.0027066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhang Z., Wang C., Lan J., Li Y., Chen Z., Fu Y., Nie H., Yan N., Gu X., Wang S. Complete mitochondrial genomes of Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga from giant panda, red panda and polar bear. Gene. 2011;482:59–67. doi: 10.1016/j.gene.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhou X., Zhang Z., Wang C., Sun Y., Liu T., Gu X., Wang T., Peng X., Yang G. Absence of genetic structure in Baylisascaris schroederi populations, a giant panda parasite, determined by mitochondrial sequencing. Parasit. Vectors. 2015;7:1. doi: 10.1186/s13071-014-0606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.A., Sims E.A. The woodchuck, Marmota monax, as a laboratory animal. Lab. Anim. Sci. 1979;29:770–780. [PubMed] [Google Scholar]
- Zhang J.S., Daszak P., Huang H.L., Yang G.Y., Kilpatrick A.M., Zhang S. Parasite threat to panda conservation. EcoHealth. 2008;5:6–9. doi: 10.1007/s10393-007-0139-8. [DOI] [PubMed] [Google Scholar]
- Zhao G.H., Li H.M., Ryan U.M., Cong M.M., Hu B., Gao M., Ren W.X., Wang X.Y., Zhang S.P., Lin Q., Zhu X.Q. Phylogenetic study of Baylisascaris schroederi isolated from Qinling subspecies of giant panda in China based on combined nuclear 5.8 S and the second internal transcribed spacer (ITS-2) ribosomal DNA sequences. Parasit. Int. 2012;61:497–500. doi: 10.1016/j.parint.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Zhou X., Xie Y., Zhang Z.H., Wang C.D., Sun Y., Gu X.B., Wang S.X., Peng X.R., Yang G.Y. Analysis of the genetic diversity of the nematode parasite Baylisascaris schroederi from wild giant pandas in different mountain ranges in China. Parasit. Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Gasser R.B., Chilton N.B. Differences in the 5.8S rDNA sequences among ascarid nematodes. Int. J. Parasitol. 1998;28:617–622. doi: 10.1016/s0020-7519(97)00214-2. [DOI] [PubMed] [Google Scholar]