Abstract
Cre-loxP, as one of the site-specific genetic manipulation tools, offers a method to study the spatial and temporal regulation of gene expression/inactivation in order to decipher gene function. CRISPR/Cas9-mediated targeted genome engineering technologies are sparking a new revolution in biological research. Whether the traditional site-specific genetic manipulation tool and CRISPR/Cas9 could be combined to create a novel genetic tool for highly specific gene editing is not clear. Here, we successfully generated a CRISPR/Cas9-loxP system to perform gene editing in human cells, providing the proof of principle that these two technologies can be used together for the first time. We also showed that distinct non-homologous end-joining (NHEJ) patterns from CRISPR/Cas9-mediated gene editing of the targeting sequence locates at the level of plasmids (episomal) and chromosomes. Specially, the CRISPR/Cas9-mediated NHEJ pattern in the nuclear genome favors deletions (64%–68% at the human AAVS1 locus versus 4%–28% plasmid DNA). CRISPR/Cas9-loxP, a novel site-specific genetic manipulation tool, offers a platform for the dissection of gene function and molecular insights into DNA-repair pathways.
Keywords: CRISPR/Cas9, loxP, gene editing, NHEJ, AAVS1
Introduction
Genome editing has recently emerged as a powerful technique that allows any chosen gene to be precisely modified in a predetermined way.1, 2 The Cre-loxP system is a popular and widely used site-specific genetic manipulation tool to conditionally knockout specific genes in cell lines and animal models, which modulates expression of selected genes at a certain developmental stage or in specific tissues, greatly facilitating our understanding of gene function and developmental mechanisms3, 4, 5, 6, 7, 8 as well as Flp/FRT.8 Cre recombinase originates from P1 phage and recognizes the 34-bp loxP site (5′ATAACTTCGTATAatgtatgcTATACGAAGTTAT-3′), whereas Flp recombinase originates from the yeast 2-μm plasmid and recognizes the distinct 34-bp FRT site (5′-GAAGTTCCTATTCtctagaaaGTATAGGAACTTC-3′).8 Both Cre and Flp recombinase can excise a region of DNA surrounded by two loxP or FRT sites, respectively.
At the present time, the choice of tools for site-specific genetic manipulation is limited to just Cre-loxP, Flp-FRT, phiC31/attP, or attB and the Dre recombinase/Rox.8, 9, 10 Additional genetic tools would be of benefit in the genetic modification of cell lines, especially to achieve genetic modification of both alleles. In vivo, double-allele knock out can be obtained by the mating of single-allele knockout animals. However, this strategy is not practical for cell lines. For this reason, new site-specific genetic manipulation tools are still highly desired.
CRISPR/Cas9-mediated targeted genome engineering technologies are sparking a new revolution in biological research.11 CRISPR/Cas9 technology has been widely applied for functional genomic studies in a variety of organisms, including mouse and human cells.12, 13 It can be programmed by single-guide RNAs (sgRNAs) to cleave specific genomic loci complementary to the sgRNA with a downstream protospacer adjacent motif (PAM), where Cas9 creates double-stranded DNA breaks (DSBs). These DSBs mediate error-prone non-homologous end-joining (NHEJ) or precise homologous recombination (HR).14 The most widely used customized CRISPR/Cas9 (SpCas9) is derived from Streptococcus pyogenes, and the corresponding PAM sequence is 5′-NGG-3′, where N is any base.15
Whether the traditional site-specific genetic manipulation tool and CRISPR technologies can be combined as a novel genetic tool for specific gene knockout studies is not clear (Figure S1). To address this question, we took advantage of the double fluorescent reporter systems with CRISPR/Cas9 because it is easily detectable by flow cytometry and microscopy.16 This approach was used to test the efficiency of a CRISPR/Cas9-loxP system in human cells.
Results
Performance of a CRISPR/SpCas9-eloxP System with Transient Reporter-Gene Expression
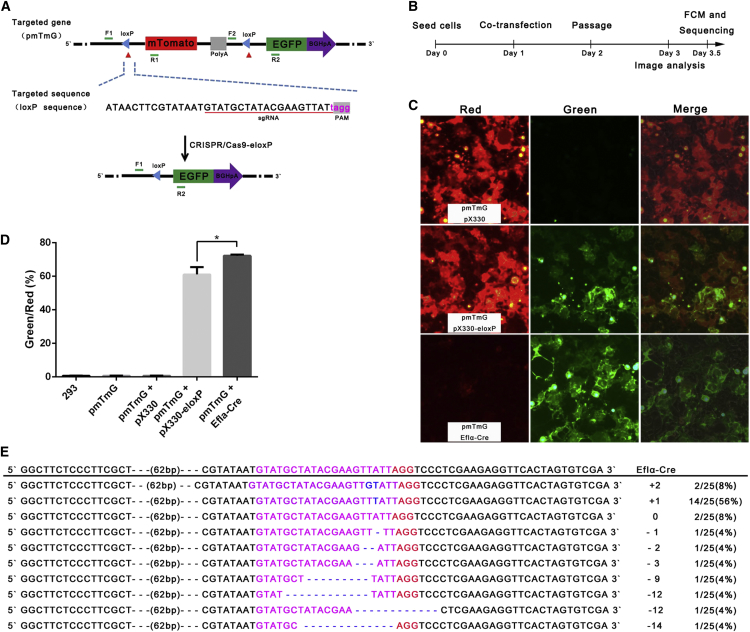
To develop a simple reporter system for visualizing CRISPR/SpCas9-loxP effects, we used the mT/mG system,16 with the structure CAG(promoter)-loxP-mTomato(mT)-loxP-Poly(A)- EGFP (mGreen, mG)-Poly(A) (Figure 1A). We hypothesized that if we introduced two CRISPR/Cas9-mediated DNA double strands break via targeting loxP flanking the mTomato cassette, the expression of the EGFP gene will be directly driven by the CAG promoter. Because there are two identical loxP sequences flanking the mTomato cassette, one sgRNA would be able to target them. However, loxP sites do not contain an “NGG” PAM sequence, and the additional tAGG sequence at the 3′ end of the loxP (5′-ATAACTTCGTATAAtgtatgcTATACGAAGTTATtagg-3′), named extended loxP (eloxP), was created (Figure 1A; Table S1). Thus, the reformed loxP with the addition of an NGG PAM sequence can be recognized by CRISPR /SpCas9. As we expected, co-transfection of two plasmids (pX330- eloxP expressing Cas9 and sgRNAs to target eloxP and pmTmG) in human HEK293 cells indeed led to EGFP expression (Figure 1C; the schematic of the protocol is shown in Figure 1B), which implied that the CRISPR/SpCas9-eloxP system can be used to excise the mTomato gene and allow the CAG promoter to drive the expression of the EGFP gene directly. Specially, in the CRISPR/SpCas9-eloxP group, 56.0%–63.6% of cells were EGFP-positive cells (Figures 1C, 1D, and S2). PCR was then used to confirm the NHEJ events triggered appropriately by CRISPR/SpCas9-eloxP (Figure 1E).
Figure 1.
Performance of CRISPR/SpCas9-eloxP System in Transient Reporter-Gene Expression Assays
(A) Schematic of the mT/mG cassette (loxP-mT-pA-loxP-mG-pA) before and after CRISPR/SpCas9-eloxP-mediated recombination. mT/mG consists of a promoter driving a loxP-flanked coding sequence of membrane-targeted tandem dimer Tomato (mT), resulting in mTomato expression with membrane localization. After CRISPR/SpCas9-eloxP-mediated recombination, the mTomato sequence is excised, allowing the promoter to drive expression of membrane-targeted EGFP (mG). (B) An illustration of the CRISPR/SpCas9-eloxP system in transient reporter-gene expression assays. (C) HEK293 cells were co-transfected with pmTmG and CRISPR/SpCas9-eloxP (0.75 ug each), and images were obtained at 48 hr post transfection (red indicates mT; green indicates mG). See also Figure S2. (D) The level of fluorescence was quantified (red indicates mT; green indicates mG). Error bars are SD (n = 3). The p value was calculated by Student’s t test; *p < 0.05. (E) NHEJ pattern of CRISPR/SpCas9-eloxP-mediated gene editing. Pink nucleotides indicate sgRNA. Blue nucleotides indicate indels.
To gain insights into the occurrence of the NHEJ events, we mapped the sequences of CRISPR/Cas9-eloxP-mediated NHEJ products. Comparing one loxP sequence left in the Cre group, notably, there was one dominant sequence (named −3T Ins, 14/25, 56%) in the CRISPR/Cas9-eloxP group, which comprises the insertion of one additional T base 3 nt upstream of the PAM (Figure 1E). Other fusion sites included one loxP sequence, an additional GT at 3 nt upstream of the PAM as well as 1-, 2-, 3-, 9-, 12-, and 14-bp multiple variable deletions at lower frequencies (Figure 1E). The majority (64%) of the fusion sites have insertions. Taken together, with plasmids (transient reporter-gene expression), our data showed that CRISPR/Cas9-eloxP system-based gene editing can achieve high excision efficiency of a specific gene between two loxP sites.
Generation of a Double-Fluorescent Reporter System at the Human AAVS1 Locus
Because our system showed high excision efficiency on the specific gene between two loxP sites at the plasmid co-transfection level, we wondered whether it would also be functional at human chromosomal loci. To address this, we sought to generate a double-fluorescent reporter system at the human AAVS1 locus. AAVS1 (also known as the PPP1R12C locus) on human chromosome 19 is a well-validated “safe harbor” for hosting DNA transgenes.17, 18, 19 It has an open chromatin structure and contains native insulators that prevent the integrated genes silencing.17 Most importantly, there are no known adverse effects on cells as a result of DNA fragment insertion. For the above reasons, we selected AAVS1 as a targeting site for harboring the double-fluorescent reporter system.
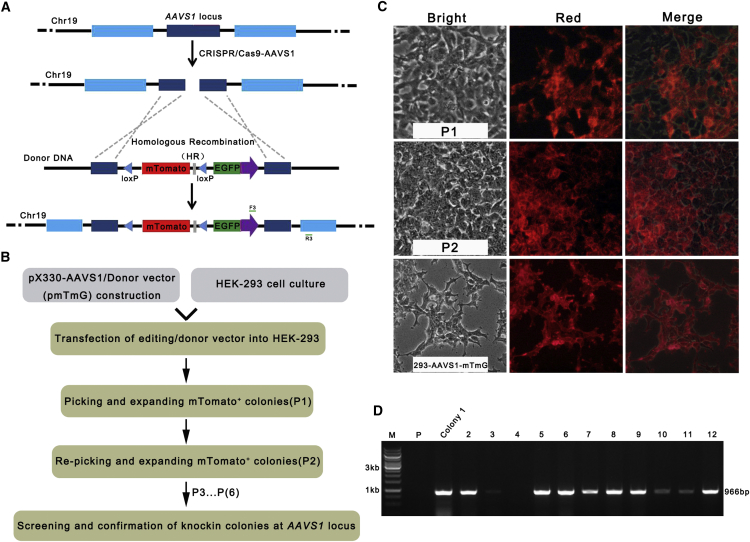
We designed one sgRNA targeting the AAVS1 locus to introduce DSBs and the pmTmG plasmid, which carries two arms homologous to the AAVS1 locus, as the donor vector to knock in a double-fluorescent reporter gene at the human AAVS1 locus (Figures 2A and 2B). To screen positive colonies, which maintain robust mTomato gene expression under the microscope (Figure 2C), we initially picked colonies that showed red fluorescence under the microscope. Then, we re-picked the colonies among the red fluorescent candidates and assumed that most random integration events will lose the red fluorescent signal, whereas the knockin cell line would maintain red fluorescence. After six passages of the re-picked candidate colonies, we screened them with gene-specific PCR (Figure 2D; Table S2). Because one primer anneals to sequences located outside of the homologous arm, all the positive colonies should contain the homologous recombinant knockin gene. 8 of the 12 colonies selected were positive ones. The four PCR-negative colonies should be due to random integration of the cassette, even if it has mTomato gene expression, which would not be suitable for further experiments.
Figure 2.
Generation of a Double-Fluorescent Reporter System at the Human AAVS1 Locus
(A) Human AAVS1 locus knockin via CRISPR/Cas9. (B) Flowchart outlining generation of gene knockin cells. (C) Images of the cells for picking up positive clones that specifically integrated mT/mG cassette into the AAVS1 locus at chromosome 19. From the top to the bottom, it shows the stable knockin colonies were generated. P1, passage 1; P2, passage 2. (D) Genotyping of the mT/mG cassette knockin candidate colonies with F3/R3 primers.
For further studies, one of the positive colonies was named 293-AAVS-mTmG, which has red fluorescence and also has a clean homogeneous genetic background. 293-AAVS-mTmG should only be a monoallelic, but not biallelic, knockin cell line because we could amplify the fragment of the wild-type AAVS1 allele with the expected size, which indicates there is one intact wild-type AAVS1 allele (Figure S3).
CRISPR/SpCas9-eloxP-Mediated Site-Specific Genome Editing in 293-AAVS-mTmG Cells
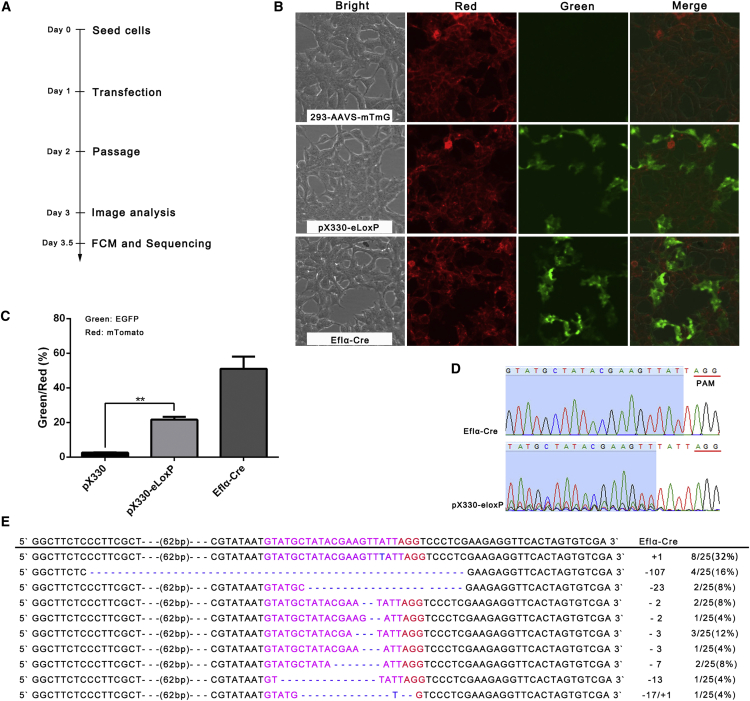
The plasmids for the CRISPR/SpCas9 expression system targeting eloxP were transfected into 293-AAVS-mTmG, and the level of effective genome editing was measured by identifying EGFP-positive cells with flow cytometry (Figure 3A). We observed a dose-dependent effect of plasmid transfection. Specifically, transfection of 0.5, 1.5, and 2.5 μg of plasmid per well of a six-well plate achieved approximately 15.1%, 20.4%, and 24.5% recombination efficiency (Figures 3B, 3C, and S4), respectively. DNA sequence chromatograms confirmed the occurrence of NHEJ in these cells (Figure 3D). These results showed that CRISPR/SpCas9-eloxP-mediated site-specific genome editing has been achieved at the human AAVS1 locus.
Figure 3.
CRISPR/SpCas9-eloxP-Mediated Site-Specific Gene Editing in 293-AAVS-mTmG Cells
(A) An illustration of the CRISPR/SpCas9-eloxP system that mediated site-specific gene editing in 293-AAVS-mTmG cells. (B) 293-AAVS-mTmG cells were transfected with 2.0 ug plasmids (plasmids coding for Cre or CRISPR/SpCas9-eloxP), and images were obtained at 48 hr post-transfection (red indicates mT; green indicates mG). (C) The level of green fluorescence was quantified. Error bars are SD (n = 3). The p value was calculated by Student’s t test; **p < 0.01. (D) Targeting DNA sequence chromatograms of cells transfected with CRISPR/SpCas9-eloxP are clustered together compared with one loxP remaining in the Cre group. (E) CRISPR/SpCas9-eloxP-mediated NHEJ pattern at the human AAVS1 locus. Pink nucleotides indicate sgRNA. Blue nucleotides indicate indels.
We then sought to ask whether there was the same NHEJ pattern between the plasmid-based NHEJs and reporter genes at the human AAVS1 locus. After transfection and isolation of the corresponding genomic DNA from 293-AAVS-mTmG cells, fragments harboring NHEJ sites were amplified and sequenced. Surprisingly, at the human AAVS1 locus, we did not identify the fusion sequence (one intact loxp sequence), which was present at the group of plasmid-based NHEJs (Figures 3E and S5). The recurrent dominant sequence (−3T Ins) was with the insertion of an additional T base 3 nt upstream of the PAM and was detected at high frequency (8/25, 32%). Also, 107-, 3-, and 2-bp deletions were the major NHEJs in addition to −3T Ins (Figure 3E). Notably, the majority (64%) of the NHEJs have deletion at the human AAVS1 locus, whereas the majority (64%) of plasmid (transient gene expression)-based NHEJs have insertion. These results implied that the target sequence under a different environment (plasmid and at the chromosome) may affect the formation of the NHEJ pattern of CRISPR/SpCas9.
Our previous study clearly demonstrated that NAG may not be the universally predominant non-canonical PAM for CRISPR/Cas9-mediated DNA cleavage in human cells.20. We also tested NGA or NAG PAM, and the results showed that CRISPR/Cas9-loxP with NGA non-canonical PAM, but not NAG, could achieve relative highly efficient (10%) excision of a specific gene between two loxP sites (Figure S6).
CRISPR/SaCas9-eloxP-Mediated Site-Specific Genome Editing in 293-AAVS-mTmG Cells
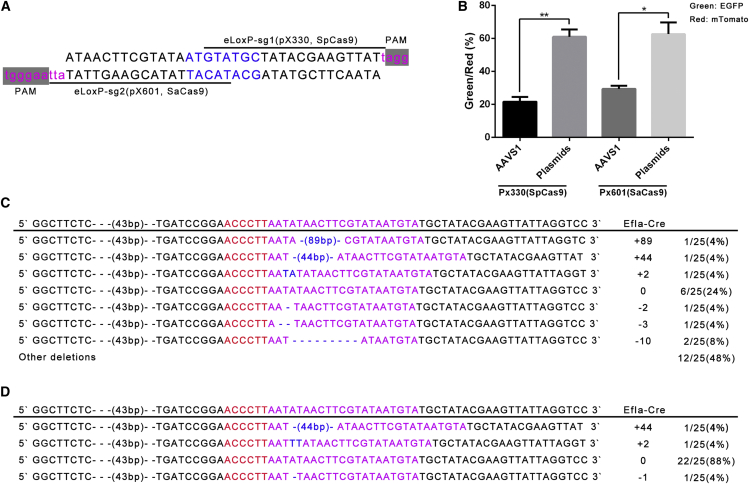
Recently, a novel type of Cas9 (SaCas9) was derived from Staphylococcus aureus, and it can edit the genome with efficiencies similar to those of SpCas9, despite being 1 kb shorter.21 Its smaller size allows packaging into a single AAV vector, with its sgRNA expression cassette, for in vivo genome editing. The canonical PAM sequence in SaCas9 is “NNGRR(T),” and the corresponding eloxP sequence is ATAACTTCGTATAatgtatgcTATACGAAGTTAT attAAGGGT (Figure 4A).
Figure 4.
CRISPR/SaCas9-eloxP-Mediated Site-Specific Gene Editing in 293-AAVS-mTmG Cells
(A) Targeting sequence and corresponding PAMs for CRISPR/SpCas9 (pX330) and CRISPR/SaCas9 (pX601). (B) Efficiency comparison of CRISPR/SaCas9 and SpCas9-eloxP mediated gene editing. The p value was calculated by Student’s t test; *p < 0.05; **p < 0.01. (C) CRISPR/SaCas9-eloxP-mediated NHEJ pattern at the human AAVS1 locus. Pink nucleotides indicate sgRNA. Blue nucleotides indicate indels. See also Figure S5. (D) CRISPR/SaCas9-eloxP-mediated NHEJ pattern in transient reporter-gene expression assays. Pink nucleotides indicate sgRNA. Blue nucleotides indicate indels.
Because site-specific genome editing is very important in vivo, here, we sought to test the performance of the CRISPR/SaCas9-eloxP-mediated novel site-specific genome editing in 293-AAVS-mTmG cells. We observed a relatively high efficiency of CRISPR/SaCas9-eloxP-mediated genome editing (27.6%–31.4% versus 19.5%–24.9%; Figures 4B and S7). Also, we found co-transfection of CRISPR/Cas9- and pmTmG-based gene editing could achieve a higher efficiency comparing genome editing at the AAVS1 locus (Figure 4B). Like the CRISPR/SpCas9-eloxP at AAVS1 locus, the majority (64%) of the fusion site (NHEJs) has deletion, including 2- to 37-bp deletion (Figures 4C and S8). The results of the NHEJ patterns of transient CRISPR/SaCas9-eloxP expression showed that the minority (8%) of the fusion site is deletion, which is different from that of the CRISPR/SaCas9-eloxP at AAVS1 locus (64% of the fusion sites have deletions).
Taken together, our study demonstrated that CRISPR/Cas9-loxP (Sa Cas9 or Sp Cas9) could be used as a tool to perform site-specific genome editing at the human AAVS1 locus. Meanwhile, CRISPR/Cas9-mediated NHEJ patterns may be modulated by the environment of targeting sequence.
Off-Target of CRISPR/SaCas9-loxP-Mediated Site-Specific Genome Editing
Off-target effects of CRISPR/Cas9 remain a key issue for its application in genome editing, including PAM-related off-target effects.20, 22 In cell lines, it is not practical to re-correct the off-target effects, although these could be minimized through outcrossing in animals or plants. New strategies using double nickase (DN) and FokI-dCas9 have been proposed,23, 24 but the presence of off-target effects due to Cas9/sgRNA may still exist. In the present study, we sought to know the off-targets of CRISPR/Cas9-eloxP. With online software (http://www.rgenome.net/cas-offinder/), we predicted the potential off-target sites in the human genome. 20 fragments harboring potential off-target sites for CRISPR/SaCas9-eloxP and CRISPR/SpCas9-eloxP have been amplified and sequenced (Tables S3 and S4). Compared with the parental cells, no additional multi-peaks in the chromatogram have been observed around the potential off-target sites (Table S4). This indicated that there are non-detectable off-target effects of CRISPR/Cas9-eloxP by Sanger sequencing. Further studies also need to characterize additional potential genome modifications inducing off-target CRISPR/Cas9-eloxP with a genome analysis tool, i.e., whole genome sequencing. Meanwhile, it is meaningful to screen and identify specific Cas9 mutants, which are optimized for genome engineering to minimize the off-target effects.25 Recently, novel high-fidelity Cas9s have been reported, which may strengthen the present novel CRISPR/Cas9-eloxP tool.26, 27
Discussion
In the present study, we successfully generated a CRISPR/Cas9-loxP system to perform site-specific genome editing in human cells, at the level of both plasmids (episomal) and chromosomes. It provides the proof of principle that these two technologies (traditional site-specific genetic manipulation tool and CRISPR technologies) can be used together. We also showed that distinct NHEJ patterns from CRISPR/Cas9-mediated gene editing of the targeting sequence locates at the level of plasmids (episomal) and chromosomes.
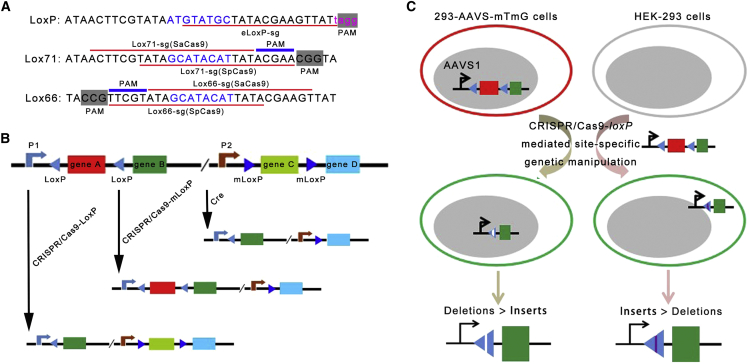
Although CRISPR/Cas9-eloxP-based knockout results are certainly encouraging, improvements still need be made. For example, if we place a specifically mutated loxP flanking the targeting gene, which Cre cannot recognize, with the specific sgRNA-targeting mutated loxP, it could be used to perform conditional knockouts, and thus could be an attractive alternative genetic tool for the compensation of Cre-loxP. The same strategy may be applied for the Flp-FRT or even two identical sequences at different locations in the genome. In the present study, we used eloxP for CRISPR/Cas9. There are also two loxP mutants, loxP66 and loxP71, which both have NGG sequences or NNGRR(T) (Figure 5A).28 Thus, these two additional loxP mutants may be more suitable for CRISPR/Cas9-loxP-mediated genome editing. Also, the wild-type and loxP mutants could be combined and the specific gene flanking them could be excised. We propose that additional CRISPR/SaCas9-loxP genetic tools could be developed (Figure 5B). For example, as illustrated in Figure 5B, genes A–D were flanked by different loxP sites. With Cre-mediated gene recombination, genes A and C could be excised. Additional sgRNAs targeting loxP or its mutants could allow different genes to be selectively excised or activated. Meanwhile, tissue-specific or inducible promoter could be used to drive Cas9 expression for conditional gene knockouts for animal models and in human embryonic stem cell or induced pluripotent stem cell studies.29, 30 These tools will be useful for conditional knockout and studies of gene-gene interaction.
Figure 5.
CRISPR/Cas9-loxP-Mediated Gene Editing as a Novel Site-Specific Genetic Manipulation Tool
(A) Schematics illustrating the wild loxP and mutant loxP pair lox66/lox71 sequence and the position of the guide RNA. (B) Schematic strategy of the switch gene expression employing the CRISPR/Cas9-loxP system. (C) Distinct NHEJ patterns from CRISPR/Cas9-mediated gene editing of the targeting sequence located at the level of plasmids (episomal) and chromosomes.
We also showed that there is a difference between the CRISPR/Cas9-mediated NHEJ patterns of the targeting sequence located at the level of plasmids (episomal) and chromosomes. In particular, the CRISPR/Cas9-mediated NHEJ pattern in the nuclear genome is in favor of deletions (64%–68% at the human AAVS1 locus versus 8%–28% plasmid DNA). We speculate that the difference between these two groups may be due to one of the following mechanisms (Figure 5C). First, the nuclear genome and plasmid DNA exist in different genetic environments, with the nuclear genome heavily regulated by histones and other DNA-interacting proteins,31 whereas the non-nuclear genome, including plasmids as episomes, are naked, with very few proteins binding. Second, different NHEJ-related enzymes may be involved or they may be at different concentrations in the two scenarios. This study may provide insights for NHEJ-related DNA repair and provides a platform for the comparison of chromosomal and extra-chromosomal DNA repair.
It is challenging but of interest to compare CRISPR/Cas9 editing of the same DNA sequence integrated into the nuclear genome (at the chromosomal level) and extra-chromosomal DNA (such as plasmids transiently existing in the cells). Here, we demonstrated that with the same DNA target sequence and CRISPR/Cas9, in the same cell type, extra-chromosomal genome CRISPR/Cas9-based gene editing could achieve a high efficiency compared with gene editing at chromosomes (55.0%–69.0% versus 19.5%–31.4% at the AAVS1 locus). We rationalize the results from these two groups are not readily comparable because there are still several factors that affect the comparison, especially target sequence copy number, which is higher in the case of plasmid transfection. This still has important implications for the future extensive use of this genome engineering to edit additional DNA outside the nuclear genome, such as in virus-related disease32 and mitochondrial diseases.33
In summary, we demonstrated that CRISPR/Cas9-loxP, a novel site-specific genetic manipulation tool, offers a genetic platform for the dissection of gene function and molecular insights into DNA-repair pathways.
Materials and Methods
Plasmid Information
The vector plasmid pmTmG contains the following: mTomato, EGFP, two loxP sites, and two homologous arms (Figures 1A and S1). It is originally from Dr. Murry Charles (University of Washington). The Cre-expression vector was generated using the Cre gene downstream of the elongation factor-1 alpha (EFIα) promoter. Plasmids pX330 and pX601 were gifts from Feng Zhang (Addgene plasmid # 42230 and # 61591). sgRNA oligos were annealed and cloned into the pX330 or pX601 vectors using a standard protocol. Plasmid DNA was isolated by standard techniques. DNA sequencing confirmed the desired specific sequences in the constructs.
Cells and Cell Culture
HEK293 cells were cultured as previously described.21 To generate AAVS1 knockin cell lines containing mT/mG expression cassettes, HEK293 cells were seeded on day 0 at 2.5 × 105 cells in six-well plates, and on day 1, pmTmG and pX330-AAVS1 plasmids were transfected by the calcium-phosphate precipitation method. Individual clonony with the expression of red fluorescent reporter genes were picked under the microscope at day 15. PCR was used for the confirmation of the gene knockin at the AAVS1 locus with specific primers, which is shown in Figure 2. The cell line with mT/mG expression cassette knockin at the AAVS1 locus was named 293-AAVS-mTmG.
Images and Flow Cytometry Analysis
On day 0, 5 × 105 293-AAVS-mTmG cells were seeded in six-well plates. On day 1, the cells were transfected with pX330-eLoxP or EfIα-Cre plasmids by the calcium-phosphate precipitation method. On day 2, the transfected 293-AAVS-mTmG cells were treated with trypsin and replated in a six-well plate. On day 3, the expression of red/green fluorescent reporter genes was observed under the microscope. On day 3.5, cells were harvested for flow cytometry and genomic DNA isolation (Figure 2A). Quantification was based on relative fluorescent frequencies. Green/red percentage was determined by the formula 100 × (a/(1−b)), where a is the green fluorescent frequencies and b is the no fluorescent frequencies.
Purification of Genomic DNA and Sequencing
Genomic DNA was purified from cells using the standard phenol/chloroform extraction protocols. The reporter gene sequence flanking the CRISPR target site was PCR amplified, and products were cloned into the vector pJET1.2 (CloneJET PCR Cloning Kit, Thermo Fisher Scientific). The PCR products and vectors were purified and then sequenced on an ABI PRISM 3730 DNA Sequencer (sequencing primers are shown in Table S1).
Off-Target Analysis for CRISPR/SpCas9-eloxP
We examined the possibility that CRISPR/Cas9-eloxP induced off-target mutations in 293-AAVS-mTmG. The potential off-target sites were predicted using online software (http://www.rgenome.net/cas-offinder/). The fragments harboring potential off-target sites have been amplified (primer information in Tables S3 and S4) and sequenced on an ABI PRISM 3730 DNA Sequencer.
Author Contributions
F.G. conceived the idea; F.Y., C.L., D.C., M.T., H.X., H.S., X.G., L.T., and J.Z. performed the experiments; J.L., Z.S., J.Q., and F.G. performed data analyses; and F.G. wrote the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
All authors declare that they have no competing interests.
Acknowledgments
This work was supported by grants from the Chinese National Program on Key Basic Research Project (973 Program, 2013CB967502 to F.G.), the Natural Science Foundation of China (81201181 to F.G., 81670840 to J.L., and 81473295 and 81670882 to Z.S.), the Zhejiang Provincial and Ministry of Health research fund for medical sciences (WKJ2013-2-023 to F.G., 2016KYA145 to X.G., and 2016KYA146 to D.C.), the Science Technology project of Zhejiang Province (2017C37176 to F.G.), Wenzhou City (Y20140633 to F.G., Y20150071 to D.C., and Y20160055 to J.L.), the Wenzhou Medical University (QTJ12011 to F.G.), and the Eye Hospital at Wenzhou Medical University (YNZD201602 to F.G.).
Footnotes
Supplemental Information includes eight figures and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.omtn.2017.04.018.
Supplemental Information
References
- 1.Aubrey B.J., Kelly G.L., Kueh A.J., Brennan M.S., O’Connor L., Milla L., Wilcox S., Tai L., Strasser A., Herold M.J. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep. 2015;10:1422–1432. doi: 10.1016/j.celrep.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Genovese P., Schiroli G., Escobar G., Di Tomaso T., Firrito C., Calabria A., Moi D., Mazzieri R., Bonini C., Holmes M.C. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skarnes W.C., Rosen B., West A.P., Koutsourakis M., Bushell W., Iyer V., Mujica A.O., Thomas M., Harrow J., Cox T. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flemr M., Bühler M. Single-step generation of conditional knockout mouse embryonic stem cells. Cell Rep. 2015;12:709–716. doi: 10.1016/j.celrep.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Gao Z., Lee P., Stafford J.M., von Schimmelmann M., Schaefer A., Reinberg D. An AUTS2-Polycomb complex activates gene expression in the CNS. Nature. 2014;516:349–354. doi: 10.1038/nature13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu L., Gao X., Jiang X., Chien K.R., Wang Z. Targeted conditional gene knockout in human embryonic stem cells. Cell Res. 2010;20:379–382. doi: 10.1038/cr.2010.23. [DOI] [PubMed] [Google Scholar]
- 7.Nern A., Pfeiffer B.D., Svoboda K., Rubin G.M. Multiple new site-specific recombinases for use in manipulating animal genomes. Proc. Natl. Acad. Sci. USA. 2011;108:14198–14203. doi: 10.1073/pnas.1111704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki E., Nakayama M. VCre/VloxP and SCre/SloxP: new site-specific recombination systems for genome engineering. Nucleic Acids Res. 2011;39:e49. doi: 10.1093/nar/gkq1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anastassiadis K., Fu J., Patsch C., Hu S., Weidlich S., Duerschke K., Buchholz F., Edenhofer F., Stewart A.F. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis. Model. Mech. 2009;2:508–515. doi: 10.1242/dmm.003087. [DOI] [PubMed] [Google Scholar]
- 10.Eroshenko N., Church G.M. Mutants of Cre recombinase with improved accuracy. Nat. Commun. 2013;4:2509. doi: 10.1038/ncomms3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H., Wang H., Shivalila C.S., Cheng A.W., Shi L., Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 15.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 17.Gantz J.A., Palpant N.J., Welikson R.E., Hauschka S.D., Murry C.E., Laflamme M.A. Targeted genomic integration of a selectable floxed dual fluorescence reporter in human embryonic stem cells. PLoS ONE. 2012;7:e46971. doi: 10.1371/journal.pone.0046971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Zhang W.Y., Hu S., Lan F., Lee A.S., Huber B., Lisowski L., Liang P., Huang M., de Almeida P.E. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circ. Res. 2012;111:1494–1503. doi: 10.1161/CIRCRESAHA.112.274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardo A., Cesana D., Genovese P., Di Stefano B., Provasi E., Colombo D.F., Neri M., Magnani Z., Cantore A., Lo Riso P. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat. Methods. 2011;8:861–869. doi: 10.1038/nmeth.1674. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Ge X., Yang F., Zhang L., Zheng J., Tan X., Jin Z.B., Qu J., Gu F. Comparison of non-canonical PAMs for CRISPR/Cas9-mediated DNA cleavage in human cells. Sci. Rep. 2014;4:5405. doi: 10.1038/srep05405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., Zetsche B., Shalem O., Wu X., Makarova K.S. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ran F.A., Hsu P.D., Lin C.Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilinger J.P., Thompson D.B., Liu D.R. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat. Biotechnol. 2014;32:577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., Gonzales A.P., Li Z., Peterson R.T., Yeh J.R. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., Joung J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberdoerffer P., Otipoby K.L., Maruyama M., Rajewsky K. Unidirectional Cre-mediated genetic inversion in mice using the mutant loxP pair lox66/lox71. Nucleic Acids Res. 2003;31:e140. doi: 10.1093/nar/gng140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z.P., Xing H.L., Dong L., Zhang H.Y., Han C.Y., Wang X.C., Chen Q.J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015;16:144. doi: 10.1186/s13059-015-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lian X., Hsiao C., Wilson G., Zhu K., Hazeltine L.B., Azarin S.M., Raval K.K., Zhang J., Kamp T.J., Palecek S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu V.T., Weber T., Wefers B., Wurst W., Sander S., Rajewsky K., Kühn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 2015;33:543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- 32.Seeger C., Sohn J.A. Targeting Hepatitis B virus with CRISPR/Cas9. Mol. Ther. Nucleic Acids. 2014;3:e216. doi: 10.1038/mtna.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lightowlers R.N., Taylor R.W., Turnbull D.M. Mutations causing mitochondrial disease: What is new and what challenges remain? Science. 2015;349:1494–1499. doi: 10.1126/science.aac7516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.