Abstract
Endometriosis is a chronic, painful condition with unknown etiology. A differential expression of microRNAs in the endometriotic tissues from women with endometriosis with pain compared to those without suggested a plausible role for miRNA or epigenetic mechanisms in the etiology of endometriotic pain. The peritoneal milieu is involved in maintenance of endometriotic lesion and nociception. We recently showed the mechanistic role for oxidized-lipoproteins (ox-LDLs) present in peritoneal fluid (PF) in endometriosis and pain. We explored the possibility of ox-LDLs modulating the expression of miRNAs in a manner similar to PF from women with endometriosis. Expression levels of miRNAs and their predicted nociceptive and inflammatory targets were determined in PF and ox-LDL treated human endometrial cell-lines. Samples from IRB-approved and consented patients with and without endometriosis or pain were used. These were compared to endometrial cell-lines treated with various forms of oxidized-lipoproteins. RNA (including miRNAs) were isolated from treated endometrial cells and expression levels were determined using commercial miRNome arrays. Cell lysates were used in immunoblotting for inflammatory proteins using a protein array. Twenty miRNAs including isoforms of miR-29, miR-181 and let-7 were mutually differentially expressed in cells treated with PF from endometriosis patients with pain and those treated with ox-LDL components. The ox-LDLs and endo-PF treatment also produced significant overexpression of microRNA predicted target genes nerve growth factor, interleukin-6 and prostaglandin E synthase and overexpression of their downstream protein targets Mip1α and MCP1. This study showed similarities between miRNA regulation in PF from endometriotic women and ox-LDLs present in abundance in the PF of these women. Key miRNAs responsible for targeting nociceptive and inflammatory molecules were downregulated in the presence of ox-LDLs and endo-PF, thus playing a role in the etiology of endometriotic pain. These redox-sensitive miRNAs can be of potential use as targets in the treatment of endometriosis-associated pain.
Abbreviations: RIPA, Radioimmunoprecipitation assay; SNORD, Small nucleolar RNAs, C/D box; RNU6, RNA, U6 Small Nuclear 2
Keywords: Oxidative stress, Peritoneal fluid, Oxidized-lipoproteins
Graphical abstract
Highlights
-
•
A mechanism for modulation of microRNA expression by oxidized-lipoproteins in endometriosis is proposed.
-
•
The mechanism impacts the expression levels of microRNA target genes and their downstream proteins.
-
•
Antioxidants and other microRNA modulating agents offer a therapeutic solution to endometriosis-associated pain.
1. Introduction
Endometriosis is a gynecological disorder that affects 5–15% of women of childbearing age and 3–5% of post-menopausal women worldwide [1], [2]. It is defined by the presence of endometrial cells implanted in an extra-uterine location and can be asymptomatic or present with a wide range of symptoms, including infertility and a number of chronic pelvic pain conditions [3], [4]. Despite the intensity of some of these symptoms, endometriosis often goes undiagnosed for several years [5], [6].
Numerous mechanisms of endometriosis-associated pain and inflammation have been proposed over the years [7], [8]. It is also known that endometriosis is a hormonal disorder, and heightened levels of estrogen are associated with increased inflammation and nociception [9], [10]. Prostaglandin E2 is an example of an overexpressed inflammatory nociceptive molecule involved in pain associated with endometriosis which can have further downstream effects [11], [12]. It is also believed that endometriotic lesions release chemotactic molecules such as monocyte chemotactic protein-1 (MCP-1) and fractalkine (CX3CL1) that attract immune cells into the peritoneal cavity [13], [14]. These cells trigger the secretion of more cytokines and growth factors such as IL-6, IL-8, and TNF-α, further promoting lesion growth [15], [16], [17], [18], [19]. All of these molecules accumulate in the peritoneal fluid (PF), creating a dynamic milieu of inflammatory and nociceptive mediators which plays a role in the etiology of endometriosis [8], [20], [21], [22].
Over the years, our laboratory has provided evidence for the role of oxidative stress in the etiology of endometriosis and its associated pain [18], [23], [24], [25]. We showed increased presence of oxidatively modified proteins in the PF and endometrium/endometriotic tissue [26], [27]. Oxidatively modified LDLs present in the PF increased the proliferation of endometrial cells and the expression of MCP-1 [15]. We recently showed the nociceptive role for oxidatively modified low-density lipoproteins (ox-LDLs) in endometriosis-associated pain [28] and the ability of antioxidant supplementation to lower inflammation and chronic pelvic pain in women with endometriosis [18], [28], [29]. Though many nociceptive molecules including ox-LDLs have been identified, the mechanism through which these molecules promote endometriosis-associated pain is still unclear.
The etiological role of epigenetics in health and disease is ever-expanding. This concept of mRNA alterations without changes to the gene sequences has become part of the paradigm in studying many disease conditions in humans [30], [31]. Often included as a regulator in epigenetics are microRNAs (miRNAs), short RNAs (about 23 nucleotides) which are capable of regulating gene expression at the transcriptional, post-transcriptional, and translational levels by binding to complementary sequences on target mRNA [32], [33]. It has long been stated that miRNA regulation occurs in one of two ways: i) the target mRNA is degraded when a miRNA seed sequence perfectly complements with the target mRNA sequence, or ii) translation is impaired when there is imperfect matching between the miRNA-mRNA sequences, leading to gene silencing [34], [35]. However, recent discoveries provide evidence that miRNAs in eukaryotes, zebrafish, and Drosophila predominantly repress translation of new mRNA targets, succeeded by deadenylation and degradation of the targets [36], [37], [38]. Interestingly, gene activation by miRNAs is also plausible. This can occur directly via targeting of the mRNA by miRNA, or indirectly by repressing nonsense-mediated RNA decay [39].
MiRNAs have a crucial role in cellular homeostasis, which explains why alterations in their expression or function have been associated with diseased states including certain cancers [40], [41], [42], neurodegenerative disorders [43], [44], [45], and cardiovascular and respiratory conditions [46], [47], [48]. Fluid-based miRNA (serum, saliva, sputum, cerebrospinal fluid, plasma, whole blood, and urine) profiling could provide invaluable information for studies where the disease is not derived from only one type of cell or a specific type of cell. This possibility has opened doors for non-invasive diagnostic techniques in various disease states [49], [50], [51], [52], [53]. Hence miRNAs are considered good therapeutic targets in cancer and cardiovascular disease [54], [55], [56].
Very few studies have explored the possible association between miRNA-mediated regulation and reproductive diseases such as endometriosis. Recent studies have speculated that endometriosis is an epigenetic disease [57], [58], [59]. MiRNAs play a major role in the development of endometriotic lesions by contributing to mechanisms involving hypoxic injury, inflammation, tissue repair, cell proliferation, extracellular matrix remodeling, and angiogenesis [60], [61]. In endometriosis, miRNA profiling studies have compared ectopic versus eutopic endometrial tissues [60], [62], [63], often concluding that many miRNAs are differentially expressed between the two groups and target genes closely associated with endometriosis. Studies investigating miRNA profiles in eutopic tissues from women with and without endometriosis [64], [65], [66] showed a trend of downregulated miRNA levels in tissues from women with endometriosis. Wang and colleagues also showed global downregulation in the circulating levels of miRNAs in the serum of women with endometriosis, with 91% of significantly differentiated miRNAs showing decreased expression in endometriosis patients [67]. There are very few studies that have measured miRNAs in the peritoneal fluid (PF), which is the most dynamic component and major player in the etiology of endometriosis [11], [68], [69].
With our continued interest in understanding the etiology of the pain associated with endometriosis, we profiled miRNAs in endometriotic tissues obtained from women with endometriosis and pain and compared it to eutopic tissue from women without endometriosis. Since we recently identified that ox-LDLs parallel nociceptive responses similar to PF from women with endometriosis-associated pain [28], we hypothesized that these lipoprotein components function through modulating miRNAs that regulate inflammatory and nociceptive genes in endometriosis. We compared the miRNA profile of PF treated endometriotic cells to Ox-LDL treated cells. We validated miRNA regulation by assessing the levels of their predicted target genes. Our results identified miRNAs that play a role in endometriosis-associated pain. Targeting these redox-sensitive miRNAs may be a novel approach to treat endometriosis-associated pain.
2. Material and methods
2.1. Human subject participants
Women ages 21–60 years undergoing tubal ligation or have non-endometriosis disorders (controls) or patients with endometriosis- endo (laparoscopically diagnosed or patients with symptoms followed by pathological confirmation) were recruited from Obstetrics-Gynecology clinic at Cabell Huntington Hospital, Joan C Edwards School of Medicine, Marshall University, in Huntington, WV. This HIPAA compliant study was approved by the Institutional Review Board of the Marshall University School of Medicine and was carried out according to the principles of the Declaration of Helsinki. All patients were consented prior to the study. All women completed a gynecologic/infertility history form, a pre-operative quality of life questionnaire and assessment of pain using a visual analog scale for assessment of endometriosis associated pain (dysmenorrhea, non-menstrual pelvic pain, dyspareunia, and dyschesia) (adapted from the validated International Pelvic Pain Society's Pelvic Assessment Form). Date of their last menstrual period was used to assess their cycle time. The inclusion criteria included women ages 21–60 years old, with normal menstrual cycles and otherwise in normal health (except for pain and endometriosis) who have not been on any hormonal medication for at least one month before sample collection. Exclusion criteria included subjects with current medical illnesses such as diabetes, cardiovascular disease, hyperlipidemia, hypertension, systemic lupus erythematosis or rheumatologic disease, positive HIV/AIDS, active infection. Subjects were asked to stop multivitamins that contain high levels of antioxidants and anti-inflammatory medications one month prior to sample collection.
2.2. Peritoneal fluid collection
Peritoneal fluid (PF) (devoid of blood contamination) was collected on ice from all women during laparoscopic surgery. Peritoneal fluid was spun at 2000xg to remove any cellular debris. The supernatant was used immediately for studies or stored in a −80 °C freezer for future use.
2.3. Endometrial tissue collection and RNA isolation
Endometrial (eutopic) tissues from control patients and ectopic endometriotic tissues from endometriosis (ovarian or peritoneal endometriosis) patients were removed during laparoscopy/laparotomy by a qualified physician. Biopsy fragments were immediately placed in RNAlater solution (Qiagen, Gaithersburg, MD) and subsequently stored in a freezer at −80 °C. RNA extraction from 100 mg of tissue (eutopic and ectopic) was carried out using Qiazol Lysis Reagent (Qiagen). Tissues were homogenized using zirconium oxide beads in a Bullet Blender® homogenizer (Next Advance, USA) and RNA was isolated using the Qiagen miRNeasy Mini Kit following the manufacturer's recommendations. The quantity and quality of RNA were measured in the NanoDrop 2000 spectrophotometer (Thermo Scientific, USA).
2.4. Endometrial cell culture and RNA and protein isolation
Ishikawa cells, a human (39-year-old woman) established endometrial cell line (Sigma-Aldrich, St. Louis, MO), were cultured in T75 flasks in complete media (DMEM/F12, 10% FBS, 1% Pen/Strep, 1% l-glutamine). These cells were used because they express characteristics similar to those of mature endometrial epithelial cells [70], [71], [72]. Approximately 70% confluent cells were treated with either 25 µg of various LDL preparations (ox-LDLs, as described previously [28]) or 1% PF from patients for 48 h in a DMEM/F12 media containing 1% charcoal-stripped FBS. Briefly, LDL isolated from plasma (human volunteers) was oxidized using copper sulfate. Extent of oxidation was determined by the formation of conjugated diene at OD 234 nm. The oxidation process was terminated at specific time points to generate various forms of ox-LDL preparations: (a) native LDL (L0), (b) minimally-modified LDL (L1, usually terminated at the end of the lag time), (c) oxidized LDL (L2, after the oxidation has reached its plateau) and (d) completely or fully oxidized LDL (L3, after 24 h of oxidation) [28], [73], [74], [75]. Patient peritoneal fluid (PF) groups were +endo/+pain (YY), +endo/-pain (YN), and –endo/-pain (NN, “control fluid”). The concentrations chosen were selected from our previous published studies [28]. At the end of 48 h, cells were collected using Qiazol reagent and RNA was isolated using the Qiagen miRNeasy Mini Kit. The quantity and quality of RNA were measured in the NanoDrop 2000 spectrophotometer. Cells were also collected in RIPA buffer containing protease inhibitors and protein concentrations were measured using a modified Lowry protocol.
2.5. RT2 miRNome ARRAY
Total RNA (which includes miRNA) isolated from the tissues and treated cells using MiRNeasy kit (Qiagen) were used. cDNA synthesis from 2 µg of each sample was performed using miScript II RT Kit (Qiagen). MiRNA expression was analyzed in the cDNA samples using the commercial Human miRNome PCR Array (MIHS-3216Z; Qiagen) on the Roche LightCycler 480 system (Roche, Indianapolis, IN). Fold change was determined using Pfaffl equation (2-ddCt) for all groups compared with eutopic tissue from control women (tissues) or media control (cells) using the manufacturer's algorithm, which uses a t-test as the default statistics to compare differences using five SNORDs and RNU6 as housekeeping genes. A p-value less than 0.05 was used to identify significantly differentially expressed miRNAs in treated Ishikawa cells or in endometriotic tissues.
2.6. Real-time PCR analysis for gene expression
cDNA synthesis from 1 µg of RNA isolated from each cell treatment was prepared using iScript cDNA Synthesis Kit (Biorad, Hercules, CA). Expression of nerve growth factor (NGF); interleukin-6 (IL-6); cannabinoid receptor 1 (CNR1); Sodium Channel, Voltage Gated, Type XI Alpha Subunit (SCN11A); and prostaglandin E synthase 3 (PTGES3) in cells were analyzed using the Applied Biosystems OneStepPlus Real-Time PCR system (Thermo Scientific). Primers used in the experiment are listed in Supplementary Table 1. Fold change was determined using Pfaffl equation (2-ddCt) for all groups compared with 1% charcoal-stripped serum media alone. A p-value less than 0.05 was used to identify significantly differentially expressed mRNAs in Ishikawa cells treated with PF and ox-LDLs compared with the charcoal-stripped media treated cells (control group).
3. Immunoblotting
Cell lysates were prepared from PF or ox-LDL-treated Ishikawa cells using RIPA buffer containing protease inhibitors. The Human Neuro Discovery Array C1 (RayBiotech, Inc., Norcross, GA), which includes 20 human neurologically relevant proteins belonging to immune response and inflammation pathways was used for the detection of changes in target proteins. This array was chosen because it includes several proteins that play a role in neuronal and peripheral nociception and inflammation. The manufacturer's suggested protocol for analysis was followed. In brief, the provided membranes were blocked for 30 min prior to sample treatment and then incubated with samples overnight at 4 °C. Following washing, the membranes were then incubated with a biotinylated detection antibody cocktail overnight (4 °C), washed, and incubated with horseradish peroxidase (HRP)-conjugated streptavidin. Following additional washing steps, the membrane was incubated in the detection buffer followed by imaging of the developed proteins using the ChemiDoc system (Biorad). Results were analyzed using the manufacturer's Analysis Tool Excel-based software (RayBiotech, Inc).
3.1. TargetScan and Ingenuity Pathway Analysis
TargetScan Human 7.0 online database (www.targetscan.org) was used to identify miRNA target genes. The list of differentially expressed miRNAs in PF and ox-LDL-treated cells was uploaded into Ingenuity Pathway Analysis (IPA, Qiagen), along with the cytokines analyzed using the protein array. IPA was used to identify any relationship among the differentially expressed miRNAs and cytokines, either via direct or indirect interactions.
3.2. Statistical analysis
Prism software (GraphPad, Inc., La Jolla, CA) was used for analysis of non-array qPCR data in human tissue and cell culture studies. All values were expressed as mean±standard error of the mean (SEM). One-way ANOVA followed by Tukey's post-hoc test was used to detect differences in relative gene expression among treatment groups. P values less than 0.05 were considered significant.
4. Results
4.1. miRNome analysis in endometrial tissues
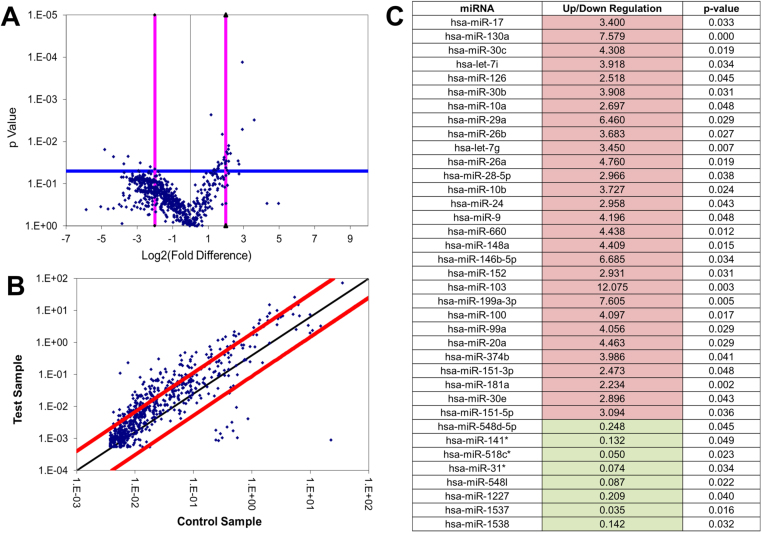
A human miRNome qPCR array consisting of primers for over 750 identified human miRNAs were used to detect changes in global miRNA expression in eutopic endometrial tissue from control women (control, n=5) and ectopic endometriotic tissues from endo women with pain (endo, n=4). Statistical analysis was performed using the online software portal available at the manufacturer's website (SA Biosciences, Valencia CA). Student's t-test (the default statistical test used by the manufacturer) showed that thirty-seven miRNAs were significantly differentially expressed (p<0.05) between control and endo tissues (Fig. 1A and B). As shown in Fig. 1C, twenty-nine of these miRNAs were upregulated in endometriotic tissues compared to controls (shown in red) while eight were downregulated (shown in green). The potential mRNA targets of the 37 significantly altered miRNAs was determined using the TargetScan Human 7.0 online database (www.targetscan.org) and Ingenuity Pathway Analysis (IPA, www.ingenuity.com), with emphasis on target genes that played a functional role in: (i) Endometriosis – Do these miRNAs target any genes that are already associated with the disease state? (ii) Pain and inflammation –Do these miRNAs target any neuropathic or inflammatory mediators or regulators? (iii) Epigenetic mechanisms – Do these miRNAs target any genes associated with epigenetic markers?
Fig. 1.
Differentially expressed miRNAs in endometrial tissues: Significant differentially expressed miRNAs in ectopic endometriotic tissues (endo, n=4) compared to eutopic control endometrium (control, n=5) based on the Qiagen MiRNome qPCR array. Fold change determined by SA Biosciences software. A) Volcano plot comparing the fold change (difference) in miRNA expression between control and endo tissues, as well as the corresponding p-values. Dots above the blue horizontal line indicate p>0.05. Pink vertical lines indicate 2-fold decrease and increase in expression. B) Scatter plot comparing control and endo tissues. The black line indicates fold changes (2-ΔCt) of 1. The red lines indicate the fold-change in gene expression threshold, defined as 4. C) List of the 37 differentially expressed miRNAs. Red cells indicate upregulation of miRNA expression in endo tissues while green cells indicate downregulation of expression in endo tissues compared to control tissues. Significance determined by a p-value<0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
TargetScan and IPA analysis narrowed the list to the following miRNAs that were closely associated with the afore-mentioned pathways: hsa-miR-29a, hsa-miR-148a, hsa-miR-100, hsa-miR-548l, and hsa-let-7g (Table 1). Human miR-29a, miR-148a, miR-100, and let-7g were upregulated in endometriotic tissues compared to control tissues, while the expression of miR-548l was significantly lower in endometriotic tissues than in control tissues. Each of these miRNAs has been shown to target key genes that play a role in endometriosis, pain, and/or epigenetics.
Table 1.
List of miRNAs with functional role in endometriosis and/or nociception. TargetScan and IPA software analysis were used to identify target genes with functional role in inflammatory/nociceptive, epigenetic and endometriosis. Bolded genes were further investigated in this study. Fold change values were based on the Qiagen MiRNome qPCR array and associated SA Biosciences software. RT-qPCR analysis showed that compared to control tissues, the mRNA expression of BCL2 (fold change=0.75), DNMT3B (fold change=0.57), and OPRM1 (fold change=0.51) were lower in endometriotic tissues, as indicated by ↓. For all tissue miRNome array data, p<0.05.
| miRNA | Fold change (Tissues, array) | GENE (pain/inflamm) | GENE (epigenetic) | GENE (endometriosis) |
|---|---|---|---|---|
| hsa-miR-29a | 6.46 | CNR1, CX3CL1 | KDM5A/5 C/6B/4B, PHF21A, DNMT3A, DNMT3B ↓↓ | BCL2 ↓ |
| hsa-miR-148a | 4.41 | PTGES3 | SIRT1, COX1, KDM6B | DNMT1, DNMT3B ↓↓ |
| hsa-miR-100 | 4.10 | mTOR | mTOR, IG1R | |
| hsa-miR-548l | 0.09 | OPRM1 ↓↓ | ||
| hsa-let-7g | 3.45 | NGF, OPRM1 ↓↓, SCN11A, IL6 |
The mRNA expression of few of the miRNA target genes—B-cell lymphoma 2 (BCL2), DNA methyltransferase 3B (DNMT3B), and the mu-opioid receptor (OPRM1)—was measured using RT-qPCR. Compared to control tissues, the expression of BCL2 (fold change=0.75), DNMT3B (fold change=0.57), and OPRM1 (fold change=0.51) were all lower in tissues from endometriosis patients.
4.2. miRNome analysis of endometrial cells treated with Peritoneal fluid
Since we and others have shown a prominent role for PF in pain associated with endometriosis [8], [13], [20], [21], [28], we next determined the changes in the miRNA profile in endometrial cells treated with PF from patients with and without endometriosis and/or pain. MiRNome array showed 89 miRNAs to be differentially expressed between cells treated with PF from patients with no endometriosis (control, NN-PF) compared to PF from patients with endometriosis, with (YY-PF) and without (YN-PF) pain (Supplementary Table 2). It is interesting to note that there was upregulation of only two of the 89 differentially expressed miRNAs in cells treated with YY-PF. The majority (98% of YY-PF, 62% of YN-PF) of the miRNAs were downregulated when the patient had endometriosis.
4.3. miRNome analysis in endometrial cells treated with oxidatively-modified lipoproteins
We had recently shown that oxidatively modified LDLs (ox-LDLs) are powerful nociceptive mediators and are present in abundance in the PF of women with endometriosis [28]. We thus determined the ability of native LDL (L1) and various forms of ox-LDL preparations (minimally modified LDL-L2, oxidized LDL-L3 and fully oxidized LDL-L4) to alter the miRNA profile in endometrial cells and compared it to that seen in PF treated cells. Fig. 2 is a Venn diagram that represents the distribution of significantly differentially expressed miRNAs in ox-LDL- and PF-treated cells.
Fig. 2.
Comparison of differentially expressed miRNAs in PF and Ox-LDL treated endometrial cells: Venn diagrams indicate the numbers of miRNAs that are significantly differentially expressed in treated cells compared to media control (p<0.05). A) Distribution of miRNAs that were significantly differentially expressed PF-treated cells. The largest commonality (n=24) was between endo PF groups (YY-PF and YN-PF). B) Distribution of miRNAs that were significantly differentially expressed in ox-LDL-treated cells. C) Distribution of significant miRNAs in endo PF-treated and ox-LDL treated cells. Twenty-two miRNAs (listed) were significantly expressed in all treatment groups except NN-PF. Eleven miRNAs (listed) were only significant in YY-PF and L1 treated cells.
4.4. miRNA target genes in PF or ox-LDL treated cells
To assess the potential functional relevance of the differentially expressed miRNAs in ox-LDL or PF treated cells, RT-qPCR was performed to determine the levels of target genes of select miRNAs involved in nociceptive/inflammation pathways. Fig. 3 shows the expression of nociceptive genes, nerve growth factor (NGF), cannabinoid receptor 1 (CNR1), and sodium voltage-gated channel alpha subunit 11 (SCN11A), as well as inflammatory genes interleukin 6 (IL6) and prostaglandin E synthase 3 (PTGES3) in cells treated with ox-LDLs and PF. In general, the presence of the ox-LDLs resulted in an increase in gene expression, with the ox-LDL (L2) treatment group having significantly higher expression of NGF (p<0.001), PTGES (p=0.0113), and IL6 (p<0.001). No significant difference in the expression of these target genes were seen in cells treated with NN-PF and cells treated with YY-PF or YN-PF, but there was a trend towards higher expression of CNR1 and SCN11A in cells treated with PF from endometriosis patients (YY-PF and YN-PF). Similar trends in gene expression were observed in the mu opioid receptor (OPRM1) and fractalkine ligand (CX3CL1). No statistical significance in expression was observed among the treatment groups, but there was a 2–3-fold induction of CX3CL1 by ox-LDLs.
Fig. 3.
mRNA expression of predicted miRNA targeted genes: mRNA expression of neuropathic and inflammatory target genes in endometrial cells treated with PF and oxidatively-modified LDLs as determined by RT-qPCR. A) Expression of CNR1 (targeted by miR-29a), SCN11A (targeted by let-7g), OPRM1 (targeted by let-7 and miR-548l), and CX3CL1 (targeted by miR-29a). No significant differences in expression were observed with these genes. B)NGF (targeted by let-7g) was significantly differentially expressed among treatment groups (one-way ANOVA p<0.001). Expression in cells treated with L1 and L2 was significantly higher than expression in other treatment groups. Expression of PTGES3 (targeted by miR-148a) across sample groups was also significant (p=0.0113). Treatment with L2 resulted in overexpression of PTGES3. C) Expression of IL6 (targeted by let-7g) across sample groups (p<0.0001). Treatment with L3 resulted in IL6 expression that was significantly higher than the NN-PF and YY-PF treatment groups, while treatment with L2 resulted in significantly higher expression than all treatment groups other than L3.
4.5. Translational regulation of differentially expressed miRNAS
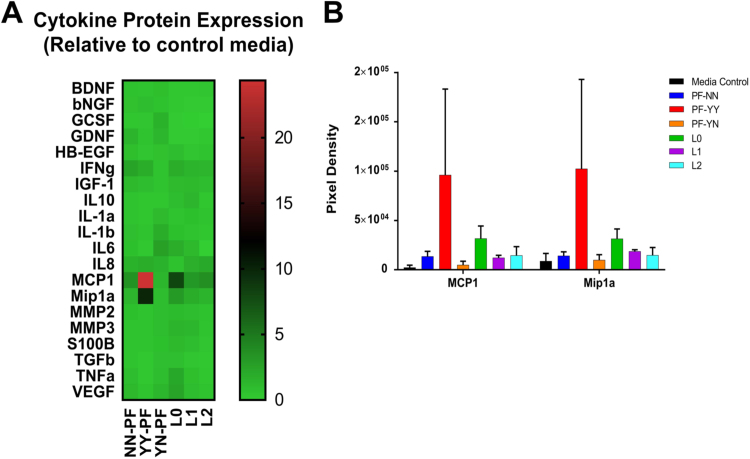
MicroRNAs modulate both the transcriptional and translational levels of their target genes, thus regulating gene pathways. The protein levels of genes involved in nociceptive and inflammatory pathways were measured in endometrial cells treated with PF or ox-LDLs using the Human Neuro Discovery Array (Ray Biotech, Inc.). As shown in the heat map (Fig. 4A), many cytokines had similar expression across all treatment groups except for MCP1 (CCL2) and monocyte inflammatory protein-Mip1α (CCL3). Densitometric analysis showed a 14.6-fold and 8.9-fold, respectively, increase in expression of MCP1 and Mip1α in cells treated with PF from patients with endometriosis and pain (YY-PF) compared to media control. Expression of MCP1 and Mip1α in YN-PF treated cells was very similar to that seen in control media (0.83-fold and 0.97-fold, respectively). These two proteins were also overexpressed by 2.78-fold and 1.08-fold in L2-treated cells, with expression trending downward as LDL oxidation increased (Fig. 4B).
Fig. 4.
Protein array of inflammatory and nociceptive targets: The RayBiotech Human Neuro Discovery Array C1 was used to determine the expression of 20 human immunomodulators in treated endometrial cells. A) Heat map showing relative expression of inflammatory and nociceptive proteins in endometrial cells treated with PF and ox-LDLs (n=3). PF was obtained from patients with neither endometriosis nor pain (PF-NN, n=6), with endometriosis and pain (PF-YY, n=6), and with endometriosis and no pain (PF-YN, n=4). B) Fold change ratio for MCP1 and Mip1a was calculated in comparison to media control (n=4). While cells treated with PF-YY typically had cytokine expression that was lower than or similar to other cells treatment groups, MCP1 and Mip1α showed an exaggerated increased trend. A similar trend was seen in protein from cells treated with ox-LDLs, particularly native LDL (L0). Two-way ANOVA determined p>0.05.
4.6. Pathway analysis to identify associations between differentially expressed miRNAs and nociceptive/inflammation targets
Fig. 5 summarizes the potential interactions between the miRNAs differentially expressed in PF and ox-LDL treated cells and their predicted targets, as determined by RT-qPCR arrays or protein array. Predicted targets of let-7 family, miRNA10 a/b, −181, −98, −19 and −374 showed association in the treated cells. Mip1α, is a documented target of let-7 (TargetScan.org), of which two isoforms (let-7i/g) were significantly downregulated in PF and ox-LDL-treated endometrial cells. MCP1 is a target of miR-374 (IPA), whose decreased expression was only significant in the L2 cell treatment group.
Fig. 5.
MiRNAs targeting key inflammatory molecules in endometrial cells treated with peritoneal fluid and ox-LDLs. TargetScan and IPA analysis was used to identify associations between the inflammatory/nociceptive proteins determined using protein array and differentially expressed miRNAs as determined by the Human MiRNome array. ↓/↑ indicates significant miRNA expression in YY-PF and L1-treated cells. ↓↓/↑↑ indicates an expression change of at least 4-fold. For all noted miRNA expression differences, p<0.05. BDNF [123], [124], [125], IL10 [126], [127], IL1a [128], [129], Tnfa [130], [131], [132], NGF [91], [133], Mip1α [17], [134], MCP1 [17], [132].
5. Discussion
The role of epigenetic mechanisms, including miRNA regulation, in endometriosis is still not completely understood and is an area of intense investigation. In the past few years, there has been a tremendous interest among endometriosis researchers to identify miRNA signatures that play a role in the pathophysiology of endometriosis. This led to a series of studies demonstrating differences in miRNA expression between paired ectopic and eutopic endometrial tissues versus normal endometrium [60], [62], [63], [64], [65], [66]. The majority of these miRNAs are located in the genomically unstable sites, lending to their targeting of oncogenes, tumor suppressor genes, angiogenesis, and genes associated with inflammation or immune function [62], [76]. Functional pathway analyses of miRNA targets showed alterations in genes such as aromatase (CYP-19) and COX-2 as well as those involved in apoptosis and cell-signaling to be differentially expressed in endometriosis [60], [61], [77], [78], [79]. For example, the downregulation of migration inhibitory factor (MIF) in ectopic endometriotic lesions compared to eutopic endometrium has been attributed to the upregulation of miR-451 [80]. Similarly, miR-93, which targets MMP3 and VEGFA, genes involved in angiogenesis and shown to inhibit proliferation, invasiveness, and migration of endometrial stromal cells, was underexpressed in ectopic tissues when compared to control endometrium [81]. Validation studies using these tissues or isolated primary endometrial cells showed that several of these miRNAs were influenced by ovarian steroids [82], [83], [84]. Though these studies speculated the association between the differentially expressed miRNAs to pathophysiological changes in endometriosis, none of these studies directly delved into whether any of these miRNAs may be playing a regulatory role in pain associated with endometriosis.
There are very few studies in the literature that have shown a direct relationship between miRNA changes and pain [64], [85], [86], [87], [88]. Bai et al. recently showed down-regulation of several miRNAs in the trigeminal ganglion neurons following inflammatory muscle pain [87]. Recent investigations have shown that expression of miR-100 and miR-29a in tissues of the central nervous system (spinal cord and dorsal root ganglion, respectively) are associated with neuropathic and inflammatory pain in animal models [89], [90]. In our study, we observed that both these miRNAs were significantly upregulated in ectopic lesions compared to the control tissues, along with 27 other miRNAs. Additionally, we found that mRNA targets of these miRNAs-BCL2, DNMT3B, and OPRM1-were also downregulated in the endometriotic tissues.
The peritoneal milieu in women with endometriosis expresses several mediators, such as PGE2, that play an important role in pain [18], [91], [92], [93], [94]. The cyclooxygenase (COX-2) enzymes that synthesize prostaglandins are highly expressed in endometriotic glands [11], [95], [96], [97] and their increased expression strongly correlates with pathological abnormalities [98], [99], [100], [101]. However, the contribution of the PF milieu to the observed miRNA changes during endometriosis is not clearly known. In a recent study, exposure of primary (eutopic and ectopic) cells to PF from endometriosis patients compared to control patients, caused lower expression of a number of miRNAs that played a role in angiogenesis (e.g. VEGFA) [102]. Our study showed similar trends in relation to miRNAs that target inflammation and nociceptive pathways. We did not observe significant overlap between miRNAs altered in PF-treated cells and those that were differentially expressed in eutopic/ectopic endometriotic tissues. This lack of overlap might be related to time of exposure to PF. Most of our cell treatments are 48 h exposures; however, the endometriotic tissues obtained from patients have been exposed to PF for months or even years. However, endo PF or ox-LDL treated cells seemed to cause a global downregulation of miRNA expression compared to untreated cells. Additionally, there were many miRNAs that were similarly (up or down) regulated in cells treated with endo PF and those treated with ox-LDLs (Fig. 2). The ox-LDL treatment also produced significant overexpression of nociceptive and inflammatory genes NGF, PTGES3, and IL6. While it did not reach significance, the expression of fractalkine (CX3CL1) and OPRM1 was of interest due to the established association between these genes and the progression of endometriosis [14], [19], [103].
Pathway analysis using TargetScan and IPA analysis identified protein targets of the differentially expressed inflammatory and nociceptive genes, such as the induction of MCP-1 by IL-6 [104], [105], [106]. MCP-1, along with Mip-1α, was highly expressed in cells treated with ox-LDLs and YY-PF. Both of these signaling proteins attract macrophages and monocytes to the site of inflammation [17], [107]. Both these inflammatory molecules are also associated with nociception [108], [109], [110]. Immunoblotting indicated that ox-LDLs may increase the level of MCP-1 protein in treated cells similar to endo PF treatment, supporting our paradigm that the LDL components of the PF is responsible for the modulation of these chemokines and play a key role in nociception. Additionally, miR-374, which targets MCP-1, was also significantly downregulated in cells treated with oxidatively-modified LDLs (L2), further validating our findings.
Pathway analysis also identified potential relationships among the miRNAs that were modulated by PF or ox-LDL treatments and their predicted target genes (Fig. 5). A higher frequency of down-regulation of the let-7 family of miRNAs was seen. This is not surprising when we consider several evidences in cancer research that let-7 is a potential tumor suppressor whose altered regulation leads to many types of cancer [111], [112]. The let-7 family also targets opioid receptors and other nociceptors [113], [114], [115]. It was recently shown that the let-7 cluster of miRNAs plays a role in endometriosis. Circulating let-7 isoforms have been reported at varying levels in endometriosis patients [116]. Studies have shown that the let-7 cluster is regulated by ox-LDLs [117], [118], [119]. Increased expression of the let-7f isoform in endometrial cells decreased cell migration [71]. While the previous study only investigated isoforms let-7a-f, our finding that PF and ox-LDL treatments can also modulate let-7i-g suggest oxidative components of patient PF may also potentially play a role in endometrial cell invasion and migration.
6. Conclusions
Endometriosis is a debilitating, chronic inflammatory condition that afflicts many young women around the world with chronic pain. Knowledge of pathways involved in the pathophysiology of endometriotic pain and regulators of such pathways will be a great asset in identifying new and appropriate targets for therapy. MiRNAs have established themselves as critical epigenetic regulators in the development and progression of several diseases, including endometriosis. MiRNAs can regulate several nodal points in the complex etiology of endometriosis and its associated pain. Our data confirms earlier findings [60], [62], [64], [82], [120] that miRNAs are down-regulated in endometriosis, but also additionally provide evidence that the presence or absence of pain discriminates the miRNA signature in these women. Our studies therefore suggest pain symptoms to be a unique discriminator of miRNA fingerprint in endometriotic women.
Our observation that ox-LDL treated cells have very similar response on miRNA profile to the endo PF treatment suggests that many of these pain-targeting miRNAs are oxidation sensitive and can be targeted by drugs that reduce oxidation. MiRNA-based therapeutics provide a possible novel way to treat endometriotic symptoms. A potential example is the let-7 cluster, which is a known tumor suppressor and apparent target of oxidative stress in the peritoneal cavity of endometriosis patients. However, the ubiquitous nature of the let-7 family and their role in cellular homeostasis makes this option extremely complex. Over the past several years, researchers in cancer biology have made key advancements toward a let-7 therapy for various cancers, but the balancing act requires extensive preliminary studies in cell and animal models [121], [122]. Based on our findings, another option would be to target the other miRNAs shown in Fig. 5, for which validation studies need to be conducted. Lastly, our findings also support the potential use of agents that will diminish the oxidative stress in the peritoneal cavity (ox-LDLs), thus alleviating chronic pelvic pain.
Funding
NS was partially supported by NCRR/NCATS (NIH) through grant UL1TR000117 (UK-CCTS-Marshall) and NIGMS under grant number P20GM103434 (WV-INBRE).
Acknowledgements
The authors would like to thank Mrs. Sandra White for coordinating patient sample collection. The authors would like to thank Dr. Robert Nerhood and Dr. David Jude (past and present Chairman, Department of OB-GYN, MUSOM) for their continuous support.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.04.037.
Appendix A. Supplementary material
Supplementary material Supplementary Table 1. Primer sequences for RT-qPCR analysis of nociceptive gene expression in endometrial cells treated with peritoneal fluid from patients with and without endometriosis or Ox-LDL components. Primers were designed using NCBI GenBank and ordered from Invitrogen.
Supplementary material Supplementary Table 2. Significant miRNAs in endometrial cells treated with peritoneal fluid (PF) from women with and without endometriosis (n=3). YY indicates that the peritoneal fluid was from patients with endometriosis and pain. YN indicates that the patients had endometriosis but lacked pain. Significance (indicated in bold) was determined by a p-value<0.05 and compared to cells treated with PF from control patients (NN-PF). Fold change determined by SA Biosciences software. Red cells indicate upregulation while green cells indicate downregulation in compared to control PF.
References
- 1.Vigano P., Parazzini F., Somigliana E., Vercellini P. Endometriosis: epidemiology and aetiological factors. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Giudice L.C. Clinical practice. Endometriosis. New Engl. J. Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor R.N., Hummelshoj L., Stratton P., Vercellini P. Pain and endometriosis: etiology, impact, and therapeutics. Middle East Fertil. Soc. J. 2012;17:221–225. doi: 10.1016/j.mefs.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene A.D., Lang S.A., Kendziorski J.A., Sroga-Rios J.M., Herzog T.J., Burns K.A. Endometriosis: where are we and where are we going? Reproduction. 2016;152:R63–R78. doi: 10.1530/REP-16-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadfield R., Mardon H., Barlow D., Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum. Reprod. 1996;11:878–880. doi: 10.1093/oxfordjournals.humrep.a019270. (published online EpubApr) [DOI] [PubMed] [Google Scholar]
- 6.Kavoussi S.K., Lim C.S., Skinner B.D., Lebovic D.I., As-Sanie S. New paradigms in the diagnosis and management of endometriosis. Curr. Opin. Obstet. Gynecol. 2016;28:267–276. doi: 10.1097/GCO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 7.Laux-Biehlmann A., d'Hooghe T., Zollner T.M. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol. Sci. 2015;36:270–276. doi: 10.1016/j.tips.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 8.McKinnon B.D., Bertschi D., Bersinger N.A., Mueller M.D. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol. Metab.: TEM. 2015;26:1–10. doi: 10.1016/j.tem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y., Gong P., Chen Y., Nwachukwu J.C., Srinivasan S., Ko C., Bagchi M.K., Taylor R.N., Korach K.S., Nettles K.W., Katzenellenbogen J.A., Katzenellenbogen B.S. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y., Yao S. Potential role of estrogen in maintaining the imbalanced sympathetic and sensory innervation in endometriosis. Mol. Cell. Endocrinol. 2016;424:42–49. doi: 10.1016/j.mce.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Hu J., Shen W., Wang J., Chen C., Han J., Zai D., Cai Z., Yu C. Peritoneal fluid of patients with endometriosis promotes proliferation of endometrial stromal cells and induces COX-2 expression. Fertil. Steril. 2011;95:1836–1838. doi: 10.1016/j.fertnstert.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Arosh J.A., Lee J., Balasubbramanian D., Stanley J.A., Long C.R., Meagher M.W., Osteen K.G., Bruner-Tran K.L., Burghardt R.C., Starzinski-Powitz A., Banu S.K. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc. Natl. Acad. Sci. USA. 2015;112:9716–9721. doi: 10.1073/pnas.1507931112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn S.H., Monsanto S.P., Miller C., Singh S.S., Thomas R., Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed. Res. Int. 2015;2015:795976. doi: 10.1155/2015/795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Fu Y., Xue S., Ai A., Chen H., Lyu Q., Kuang Y. The M2 polarization of macrophage induced by fractalkine in the endometriotic milieu enhances invasiveness of endometrial stromal cells. Int. J. Clin. Exp. Pathol. 2014;7:194–203. [PMC free article] [PubMed] [Google Scholar]
- 15.Rong R., Ramachandran S., Santanam N., Murphy A.A., Parthasarathy S. Induction of monocyte chemotactic protein-1 in peritoneal mesothelial and endometrial cells by oxidized low-density lipoprotein and peritoneal fluid from women with endometriosis. Fertil. Steril. 2002;78:843–848. doi: 10.1016/s0015-0282(02)03333-2. (published online EpubOct) [DOI] [PubMed] [Google Scholar]
- 16.Cao X., Yang D., Song M., Murphy A., Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: implications for endometriosis. Fertil. Steril. 2004;82(Suppl. 3):S999–S1007. doi: 10.1016/j.fertnstert.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Na Y.J., Lee D.H., Kim S.C., Joo J.K., Wang J.W., Jin J.O., Kwak J.Y., Lee K.S. Effects of peritoneal fluid from endometriosis patients on the release of monocyte-specific chemokines by leukocytes. Arch. Gynecol. Obstet. 2011;283:1333–1341. doi: 10.1007/s00404-010-1583-1. [DOI] [PubMed] [Google Scholar]
- 18.Santanam N., Kavtaradze N., Murphy A., Dominguez C., Parthasarathy S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl. Res.: J. Lab. Clin. Med. 2013;161:189–195. doi: 10.1016/j.trsl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X.X., Zhou W.J., Wang X.Q., Li D.J. Fractalkine/CX3CR1 is involved in the pathogenesis of endometriosis by regulating endometrial stromal cell proliferation and invasion. Am. J. Reprod. Immunol. 2016;76:318–325. doi: 10.1111/aji.12557. [DOI] [PubMed] [Google Scholar]
- 20.Bedaiwy M.A., Falcone T. Peritoneal fluid environment in endometriosis. Clinicopathological implications. Minerva Ginecol. 2003;55:333–345. (published online EpubAug) [PubMed] [Google Scholar]
- 21.Kyama C.M., Mihalyi A., Simsa P., Falconer H., Fulop V., Mwenda J.M., Peeraer K., Tomassetti C., Meuleman C., D'Hooghe T.M. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front. Biosci. 2009;1:444–454. doi: 10.2741/e40. (published online EpubJun01) [DOI] [PubMed] [Google Scholar]
- 22.Mahnert N., Morgan D., Campbell D., Johnston C., As-Sanie S. Unexpected gynecologic malignancy diagnosed after hysterectomy performed for benign indications. Obstet. Gynecol. 2015;125:397–405. doi: 10.1097/AOG.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 23.Murphy A.A., Santanam N., Parthasarathy S. Endometriosis: a disease of oxidative stress? Semin. Reprod. Endocrinol. 1998;16:263–273. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- 24.Santanam N., Murphy A.A., Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann. N.Y. Acad. Sci. 2002;955:183–198. doi: 10.1111/j.1749-6632.2002.tb02779.x. (published online EpubMar) [DOI] [PubMed] [Google Scholar]
- 25.Santanam N., Song M., Rong R., Murphy A.A., Parthasarathy S. Atherosclerosis, oxidation and endometriosis. Free Radic. Res. 2002;36:1315–1321. doi: 10.1080/1071576021000049908. (published online EpubDec) [DOI] [PubMed] [Google Scholar]
- 26.Murphy A.A., Santanam N., Morales A.J., Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J. Clin. Endocrinol. Metab. 1998;83:2110–2113. doi: 10.1210/jcem.83.6.4823. [DOI] [PubMed] [Google Scholar]
- 27.Shanti A., Santanam N., Morales A.J., Parthasarathy S., Murphy A.A. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil. Steril. 1999;71:1115–1118. doi: 10.1016/s0015-0282(99)00145-4. (published online EpubJun) [DOI] [PubMed] [Google Scholar]
- 28.Ray K., Fahrmann J., Mitchell B., Paul D., King H., Crain C., Cook C., Golovko M., Brose S., Golovko S., Santanam N. Oxidation-sensitive nociception involved in endometriosis-associated pain. Pain. 2015;156:528–539. doi: 10.1097/01.j.pain.0000460321.72396.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santanam N., Zoneraich N., Parthasarathy S. Myeloperoxidase as a potential target in women with endometriosis undergoing IVF. Reprod. Sci. 2016 doi: 10.1177/1933719116667225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 31.Calicchio R., Doridot L., Miralles F., Mehats C., Vaiman D. DNA methylation, an epigenetic mode of gene expression regulation in reproductive science. Curr. Pharm. Des. 2014;20:1726–1750. doi: 10.2174/13816128113199990517. [DOI] [PubMed] [Google Scholar]
- 32.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen H.H., Duroux M., Gazerani P. MicroRNAs as modulators and biomarkers of inflammatory and neuropathic pain conditions. Neurobiol. Dis. 2014;71:159–168. doi: 10.1016/j.nbd.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Deng S., Calin G.A., Croce C.M., Coukos G., Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle. 2008;7:2643–2646. doi: 10.4161/cc.7.17.6597. [DOI] [PubMed] [Google Scholar]
- 35.Mari-Alexandre J., Sanchez-Izquierdo D., Gilabert-Estelles J., Barcelo-Molina M., Braza-Boils A., Sandoval J. miRNAs Regulation and Its Role as Biomarkers in Endometriosis. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bethune J., Artus-Revel C.G., Filipowicz W. Kinetic analysis reveals successive steps leading to miRNA-mediated silencing in mammalian cells. EMBO Rep. 2012;13:716–723. doi: 10.1038/embor.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazzini A.A., Lee M.T., Giraldez A.J. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336:233–237. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djuranovic S., Nahvi A., Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasudevan S., Tong Y., Steitz J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 40.Liu C., Eng C., Shen J., Lu Y., Yoko T., Mehdizadeh A., Chang G.J., Rodriguez-Bigas M.A., Li Y., Chang P., Mao Y., Hassan M.M., Wang F., Li D. Serum exosomal miR-4772-3p is a predictor of tumor recurrence in stage II and III colon cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin M., Zhang T., Liu C., Badeaux M.A., Liu B., Liu R., Jeter C., Chen X., Vlassov A.V., Tang D.G. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res. 2014;74:4183–4195. doi: 10.1158/0008-5472.CAN-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X.Y., Luo Q.F., Wei C.K., Li D.F., Li J., Fang L. MiRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancer. Int. J. Clin. Exp. Med. 2014;7:32–40. [PMC free article] [PubMed] [Google Scholar]
- 43.Miller B.H., Zeier Z., Xi L., Lanz T.A., Deng S., Strathmann J., Willoughby D., Kenny P.J., Elsworth J.D., Lawrence M.S., Roth R.H., Edbauer D., Kleiman R.J., Wahlestedt C. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar P., Dezso Z., MacKenzie C., Oestreicher J., Agoulnik S., Byrne M., Bernier F., Yanagimachi M., Aoshima K., Oda Y. Circulating miRNA biomarkers for Alzheimer's disease. PLoS One. 2013;8:e69807. doi: 10.1371/journal.pone.0069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majidinia M., Mihanfar A., Rahbarghazi R., Nourazarian A., Bagca B., Avci C.B. The roles of non-coding RNAs in Parkinson's disease. Mol. Biol. Rep. 2016;43:1193–1204. doi: 10.1007/s11033-016-4054-3. [DOI] [PubMed] [Google Scholar]
- 46.JF O.S., Neylon A., McGorrian C., Blake G.J. miRNA-93-5p and other miRNAs as predictors of coronary artery disease and STEMI. Int. J. Cardiol. 2016;224:310–316. doi: 10.1016/j.ijcard.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y., Yang Q., Xu H., Zhang J., Deng H., Gao H., Yang J., Zhao D., Liu F. miRNA-221-3p enhances the secretion of Interleukin-4 in mast cells through the phosphatase and tensin homolog/p38/Nuclear Factor-kappaB Pathway. PloS One. 2016;11:e0148821. doi: 10.1371/journal.pone.0148821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Du J., Li H.H. The role of miRNA-155 in cardiovascular diseases. Sheng li ke xue jin zhan [Progress. Physiol.] 2013;44:377–380. (published online EpubOct) [PubMed] [Google Scholar]
- 49.Leidinger P., Keller A., Borries A., Huwer H., Rohling M., Huebers J., Lenhof H.P., Meese E. Specific peripheral miRNA profiles for distinguishing lung cancer from COPD. Lung Cancer. 2011;74:41–47. doi: 10.1016/j.lungcan.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Bhomia M., Balakathiresan N.S., Wang K.K., Papa L., Maheshwari R.K. A panel of serum MiRNA biomarkers for the diagnosis of severe to mild traumatic brain injury in humans. Sci. Rep. 2016;6:28148. doi: 10.1038/srep28148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajasekaran S., Pattarayan D., Rajaguru P., Sudhakar Gandhi P.S., Thimmulappa R.K. MicroRNA regulation of acute lung injury and acute respiratory distress syndrome. J. Cell. Physiol. 2016;231:2097–2106. doi: 10.1002/jcp.25316. [DOI] [PubMed] [Google Scholar]
- 52.Igaz I., Igaz P. Diagnostic relevance of microRNAs in other body fluids including urine, feces, and saliva. Exs. 2015;106:245–252. doi: 10.1007/978-3-0348-0955-9_11. [DOI] [PubMed] [Google Scholar]
- 53.Umemura T., Kuroki C. Circulating MicroRNAs as biomarkers of colorectal cancer. Rinsho yori. Jpn. J. Clin. Pathol. 2015;63:336–346. (published online EpubMar) [PubMed] [Google Scholar]
- 54.Tsai L.M., Yu D. MicroRNAs in common diseases and potential therapeutic applications. Clin. Exp. Pharmacol. Physiol. 2010;37:102–107. doi: 10.1111/j.1440-1681.2009.05269.x. [DOI] [PubMed] [Google Scholar]
- 55.Li C., Feng Y., Coukos G., Zhang L. Therapeutic microRNA strategies in human cancer. AAPS J. 2009;11:747–757. doi: 10.1208/s12248-009-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra P.K., Tyagi N., Kumar M., Tyagi S.C. MicroRNAs as a therapeutic target for cardiovascular diseases. J. Cell. Mol. Med. 2009;13:778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo S.W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009;15:587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 58.Izawa M., Taniguchi F., Terakawa N., Harada T. Epigenetic aberration of gene expression in endometriosis. Front. Biosci. 2013;5:900–910. doi: 10.2741/e669. (published online EpubJun01) [DOI] [PubMed] [Google Scholar]
- 59.Borghese B., Zondervan K.T., Abrao M.S., Chapron C., Vaiman D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 2016 doi: 10.1111/cge.12897. [DOI] [PubMed] [Google Scholar]
- 60.Ohlsson Teague E.M., Van der Hoek K.H., Van der Hoek M.B., Perry N., Wagaarachchi P., Robertson S.A., Print C.G., Hull L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009;23:265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mari-Alexandre J., Garcia-Oms J., Barcelo-Molina M., Gilabert-Aguilar J., Estelles A., Braza-Boils A., Gilabert-Estelles J. MicroRNAs and angiogenesis in endometriosis. Thromb. Res. 2015;135(Suppl 1):S38–S40. doi: 10.1016/S0049-3848(15)50439-8. [DOI] [PubMed] [Google Scholar]
- 62.Pan Q., Chegini N. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin. Reprod. Med. 2008;26:479–493. doi: 10.1055/s-0028-1096128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filigheddu N., Gregnanin I., Porporato P.E., Surico D., Perego B., Galli L., Patrignani C., Graziani A., Surico N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J. Biomed. Biotechnol. 2010;2010:369549. doi: 10.1155/2010/369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burney R.O., Hamilton A.E., Aghajanova L., Vo K.C., Nezhat C.N., Lessey B.A., Giudice L.C. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009;15:625–631. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laudanski P., Charkiewicz R., Kuzmicki M., Szamatowicz J., Charkiewicz A., Niklinski J. MicroRNAs expression profiling of eutopic proliferative endometrium in women with ovarian endometriosis. Reprod. Biol. Endocrinol.: RB&E. 2013;11:78. doi: 10.1186/1477-7827-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawkins S.M., Creighton C.J., Han D.Y., Zariff A., Anderson M.L., Gunaratne P.H., Matzuk M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L., Huang W., Ren C., Zhao M., Jiang X., Fang X., Xia X. Analysis of serum microRNA profile by solexa sequencing in women with endometriosis. Reprod. Sci. 2016;23:1359–1370. doi: 10.1177/1933719116641761. [DOI] [PubMed] [Google Scholar]
- 68.Loh F.H., Bongso A., Fong C.Y., Koh D.R., Lee S.H., Zhao H.Q. Effects of peritoneal macrophages from women with endometriosis on endometrial cellular proliferation in an in vitro coculture model. Fertil. Steril. 1999;72:533–538. doi: 10.1016/s0015-0282(99)00292-7. (published online EpubSep) [DOI] [PubMed] [Google Scholar]
- 69.Castro J., Torres M., Sovino H., Fuentes A., Boric M.A., Johnson M.C. P450Arom induction in isolated control endometrial cells by peritoneal fluid from women with endometriosis. Fertil. Steril. 2010;94:2521–2527. doi: 10.1016/j.fertnstert.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 70.Nishida M., Kasahara K., Kaneko M., Iwasaki H., Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai zasshi. 1985;37:1103–1111. (published online EpubJul) [PubMed] [Google Scholar]
- 71.Cho S., Mutlu L., Zhou Y., Taylor H.S. Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil. Steril. 2016 doi: 10.1016/j.fertnstert.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bulun S.E., Cheng Y.H., Yin P., Imir G., Utsunomiya H., Attar E., Innes J., Kim J. Julie. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol. Cell. Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 73.Parthasarathy S., Auge N., Santanam N. Implications of lag time concept in the oxidation of LDL. Free Radic. Res. 1998;28:583–591. doi: 10.3109/10715769809065814. (published online EpubJun) [DOI] [PubMed] [Google Scholar]
- 74.Parthasarathy S., Raghavamenon A., Garelnabi M.O., Santanam N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parthasarathy S., Santanam N., Auge N. Oxidized low-density lipoprotein, a two-faced Janus in coronary artery disease? Biochem. Pharmacol. 1998;56:279–284. doi: 10.1016/s0006-2952(98)00074-4. (published online EpubAug01) [DOI] [PubMed] [Google Scholar]
- 76.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. (published online EpubJan23) [DOI] [PubMed] [Google Scholar]
- 77.Long M., Wan X., La X., Gong X., Cai X. MiR-29c is downregulated in the ectopic endometrium and exerts its effects on endometrial cell proliferation, apoptosis and invasion by targeting c-Jun. Int. J. Mol. Med. 2015;35:1119–1125. doi: 10.3892/ijmm.2015.2082. [DOI] [PubMed] [Google Scholar]
- 78.Hirakawa T., Nasu K., Abe W., Aoyagi Y., Okamoto M., Kai K., Takebayashi K., Narahara H. MiR-503, a microRNA epigenetically repressed in endometriosis, induces apoptosis and cell-cycle arrest and inhibits cell proliferation, angiogenesis, and contractility of human ovarian endometriotic stromal cells. Hum. Reprod. 2016;31:2587–2597. doi: 10.1093/humrep/dew217. [DOI] [PubMed] [Google Scholar]
- 79.Okamoto M., Nasu K., Abe W., Aoyagi Y., Kawano Y., Kai K., Moriyama M., Narahara H. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum. Reprod. 2015;30:632–641. doi: 10.1093/humrep/deu332. [DOI] [PubMed] [Google Scholar]
- 80.Graham A., Falcone T., Nothnick W.B. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum. Reprod. 2015;30:642–652. doi: 10.1093/humrep/dev005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lv X., Chen P., Liu W. Down regulation of MiR-93 contributes to endometriosis through targeting MMP3 and VEGFA. Am. J. Cancer Res. 2015;5:1706–1717. [PMC free article] [PubMed] [Google Scholar]
- 82.Pan Q., Luo X., Toloubeydokhti T., Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol. Hum. Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 83.Nothnick W.B., Healy C. Estrogen induces distinct patterns of microRNA expression within the mouse uterus. Reprod. Sci. 2010;17:987–994. doi: 10.1177/1933719110377472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toloubeydokhti T., Pan Q., Luo X., Bukulmez O., Chegini N. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod. Sci. 2008;15:993–1001. doi: 10.1177/1933719108324132. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Chehade M., Sampson H.A. Epidemiology and etiology of eosinophilic esophagitis. Gastrointest. Endosc. Clin. North Am. 2008;18:33–44. doi: 10.1016/j.giec.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Bezerra M.M., Lima V., Girao V.C., Teixeira R.C., Graca J.R. Antinociceptive activity of sildenafil and adrenergic agents in the writhing test in mice. Pharmacol. Rep.: PR. 2008;60:339–344. (published online EpubMay-Jun) [PubMed] [Google Scholar]
- 87.Bai G., Ambalavanar R., Wei D., Dessem D. Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol. Pain. 2007;3:15. doi: 10.1186/1744-8069-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchez Freire V., Burkhard F.C., Kessler T.M., Kuhn A., Draeger A., Monastyrskaya K. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am. J. Pathol. 2010;176:288–303. doi: 10.2353/ajpath.2010.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kynast K.L., Russe O.Q., Geisslinger G., Niederberger E. Novel findings in pain processing pathways: implications for miRNAs as future therapeutic targets. Expert Rev. Neurother. 2013;13:515–525. doi: 10.1586/ern.13.34. [DOI] [PubMed] [Google Scholar]
- 90.Qureshi R.A., Tian Y., McDonald M.K., Capasso K.E., Douglas S.R., Gao R., Orlova I.A., Barrett J.E., Ajit S.K., Sacan A. Circulating microRNA signatures in rodent models of pain. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9281-4. [DOI] [PubMed] [Google Scholar]
- 91.Barcena de Arellano M.L., Arnold J., Vercellino F., Chiantera V., Schneider A., Mechsner S. Overexpression of nerve growth factor in peritoneal fluid from women with endometriosis may promote neurite outgrowth in endometriotic lesions. Fertil. Steril. 2011;95:1123–1126. doi: 10.1016/j.fertnstert.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 92.Barcena de Arellano M.L., Mechsner S. The peritoneum–an important factor for pathogenesis and pain generation in endometriosis. J. Mol. Med. 2014;92:595–602. doi: 10.1007/s00109-014-1135-4. [DOI] [PubMed] [Google Scholar]
- 93.Morotti M., Vincent K., Brawn J., Zondervan K.T., Becker C.M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update. 2014;20:717–736. doi: 10.1093/humupd/dmu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neziri A.Y., Bersinger N.A., Andersen O.K., Arendt-Nielsen L., Mueller M.D., Curatolo M. Correlation between altered central pain processing and concentration of peritoneal fluid inflammatory cytokines in endometriosis patients with chronic pelvic pain. Reg. Anesth. Pain. Med. 2014;39:181–184. doi: 10.1097/AAP.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi C., Chishima F., Sugitani M., Ichikawa G., Nakazawa-Watanabe T., Sugita K., Suzuki M., Nemoto N., Yamamoto T. Relationship between Toll-like receptor-4 and mPGES-1 gene expression in local lesions of endometriosis patients. Am. J. Reprod. Immunol. 2013;69:231–239. doi: 10.1111/aji.12056. [DOI] [PubMed] [Google Scholar]
- 96.Kilico I., Kokcu A., Kefeli M., Kandemir B. Regression of experimentally induced endometriosis with a new selective cyclooxygenase-2 enzyme inhibitor. Gynecol. Obstet. Investig. 2014;77:35–39. doi: 10.1159/000356686. [DOI] [PubMed] [Google Scholar]
- 97.Jana S., Chatterjee K., Ray A.K., DasMahapatra P., Swarnakar S. Regulation of matrix metalloproteinase-2 activity by COX-2-PGE2-pAKT axis promotes angiogenesis in endometriosis. PLoS One. 2016;11:e0163540. doi: 10.1371/journal.pone.0163540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buchweitz O., Staebler A., Wulfing P., Hauzman E., Greb R., Kiesel L. COX-2 overexpression in peritoneal lesions is correlated with nonmenstrual chronic pelvic pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;124:216–221. doi: 10.1016/j.ejogrb.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 99.Cho S., Park S.H., Choi Y.S., Seo S.K., Kim H.Y., Park K.H., Cho D.J., Lee B.S. Expression of cyclooxygenase-2 in eutopic endometrium and ovarian endometriotic tissue in women with severe endometriosis. Gynecol. Obstet. Investig. 2010;69:93–100. doi: 10.1159/000261017. [DOI] [PubMed] [Google Scholar]
- 100.Kim H.Y., Cho S., Choi Y.S., Yang H.I., Lee K.E., Seo S.K., Lee B.S. Cyclooxygenase-2 (COX −2) gene-765G/C polymorphism and advanced-stage endometriosis in Korean women. Am. J. Reprod. Immunol. 2012;68:238–243. doi: 10.1111/j.1600-0897.2012.01151.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang H., Sun L., Jiang M., Liu L., Wang G. −1195 A/G promoter variants of the cyclooxygenase-2 gene increases the risk of pain occurrence in endometriotic women. Clin. Exp. Obstet. Gynecol. 2016;43:254–257. [PubMed] [Google Scholar]
- 102.Braza-Boils A., Salloum-Asfar S., Mari-Alexandre J., Arroyo A.B., Gonzalez-Conejero R., Barcelo-Molina M., Garcia-Oms J., Vicente V., Estelles A., Gilabert-Estelles J., Martinez C. Peritoneal fluid modifies the microRNA expression profile in endometrial and endometriotic cells from women with endometriosis. Hum. Reprod. 2015 doi: 10.1093/humrep/dev204. [DOI] [PubMed] [Google Scholar]
- 103.Shimoya K., Zhang Q., Temma-Asano K., Hayashi S., Kimura T., Murata Y. Fractalkine in the peritoneal fluid of women with endometriosis. Int. J. Gynaecol. Obstet.: Off. Organ Int. Fed. Gynaecol. Obstet. 2005;91:36–41. doi: 10.1016/j.ijgo.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Arendt B.K., Velazquez-Dones A., Tschumper R.C., Howell K.G., Ansell S.M., Witzig T.E., Jelinek D.F. Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia. 2002;16:2142–2147. doi: 10.1038/sj.leu.2402714. [DOI] [PubMed] [Google Scholar]
- 105.Biswas P., Delfanti F., Bernasconi S., Mengozzi M., Cota M., Polentarutti N., Mantovani A., Lazzarin A., Sozzani S., Poli G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–265. (published online EpubJan1) [PubMed] [Google Scholar]
- 106.Choi J.M., Rotimi O.O., O'Carroll S.J., Nicholson L.F. IL-6 stimulates a concentration-dependent increase in MCP-1 in immortalised human brain endothelial cells. F1000Research. 2016;5:270. doi: 10.12688/f1000research.8153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wickstrom K., Stavreus-Evers A., Vercauteren O., Olovsson M., Edelstam G. Effect of lignocaine on IL-6, IL-8, and MCP-1 in peritoneal macrophages and endometriotic stromal cells. Reprod. Sci. 2017;24:382–392. doi: 10.1177/1933719116657188. [DOI] [PubMed] [Google Scholar]
- 108.Dauvergne C., Molet J., Reaux-Le Goazigo A., Mauborgne A., Melik-Parsadaniantz S., Boucher Y., Pohl M. Implication of the chemokine CCL2 in trigeminal nociception and traumatic neuropathic orofacial pain. Eur. J. Pain. 2014;18:360–375. doi: 10.1002/j.1532-2149.2013.00377.x. [DOI] [PubMed] [Google Scholar]
- 109.Menetski J., Mistry S., Lu M., Mudgett J.S., Ransohoff R.M., Demartino J.A., Macintyre D.E., Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 110.Kwiatkowski K., Piotrowska A., Rojewska E., Makuch W., Jurga A., Slusarczyk J., Trojan E., Basta-Kaim A., Mika J. Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Progress. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;64:68–78. doi: 10.1016/j.pnpbp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Yang J., Zhang Q., Dong J.Q., Chang X.H., He X.J. Overexpression of high mobility group A2 and its correlation with microRNA let-7 family in serous ovarian cancers. Beijing da xue xue bao. Yi xue ban=J. Peking. Univ. Health Sci. 2012;44:749–754. (published online EpubOct18) [PubMed] [Google Scholar]
- 112.Tsai C.H., Lin L.T., Wang C.Y., Chiu Y.W., Chou Y.T., Chiu S.J., Wang H.E., Liu R.S., Wu C.Y., Chan P.C., Yang M.H., Chiou S.H., Liao M.J., Lee Y.J. Over-expression of cofilin-1 suppressed growth and invasion of cancer cells is associated with up-regulation of let-7 microRNA. Biochim. Et. Biophys. Acta. 2015;1852:851–861. doi: 10.1016/j.bbadis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 113.He Y., Yang C., Kirkmire C.M., Wang Z.J. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30:10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He Y., Wang Z.J. Let-7 microRNAs and opioid tolerance. Front. Genet. 2012;3:110. doi: 10.3389/fgene.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park C.K., Xu Z.Z., Berta T., Han Q., Chen G., Liu X.J., Ji R.R. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seifer B.J., Su D., Taylor H.S. Circulating miRNAs in murine experimental endometriosis: decreased abundance of let-7a. Reprod. Sci. 2016 doi: 10.1177/1933719116667228. [DOI] [PubMed] [Google Scholar]
- 117.Ding Z., Wang X., Khaidakov M., Liu S., Mehta J.L. MicroRNA hsa-let-7g targets lectin-like oxidized low-density lipoprotein receptor-1 expression and inhibits apoptosis in human smooth muscle cells. Exp. Biol. Med. 2012;237:1093–1100. doi: 10.1258/ebm.2012.012082. [DOI] [PubMed] [Google Scholar]
- 118.Qin B., Xiao B., Liang D., Li Y., Jiang T., Yang H. MicroRNA let-7c inhibits Bcl-xl expression and regulates ox-LDL-induced endothelial apoptosis. BMB Rep. 2012;45:464–469. doi: 10.5483/BMBRep.2012.45.8.033. [DOI] [PubMed] [Google Scholar]
- 119.Tang Y., Jin X., Xiang Y., Chen Y., Shen C.X., Zhang Y.C., Li Y.G. The lncRNA MALAT1 protects the endothelium against ox-LDL-induced dysfunction via upregulating the expression of the mir-22-3p target genes CXCR2 and AKT. FEBS Lett. 2015;589:3189–3196. doi: 10.1016/j.febslet.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 120.Teague E.M., Print C.G., Hull M.L. The role of microRNAs in endometriosis and associated reproductive conditions. Hum. Reprod. Update. 2010;16:142–165. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 121.Wu L., Nguyen L.H., Zhou K., de Soysa T.Y., Li L., Miller J.B., Tian J., Locker J., Zhang S., Shinoda G., Seligson M.T., Zeitels L.R., Acharya A., Wang S.C., Mendell J.T., He X., Nishino J., Morrison S.J., Siegwart D.J., Daley G.Q., Shyh-Chang N., Zhu H. Precise let-7 expression levels balance organ regeneration against tumor suppression. eLife. 2015;4:e09431. doi: 10.7554/eLife.09431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Q.Z., Lv Y.H., Gong Y.H., Li Z.F., Xu W., Diao Y., Xu R. Double-stranded Let-7 mimics, potential candidates for cancer gene therapy. J. Physiol. Biochem. 2012;68:107–119. doi: 10.1007/s13105-011-0124-0. [DOI] [PubMed] [Google Scholar]
- 123.Wessels J.M., Kay V.R., Leyland N.A., Agarwal S.K., Foster W.G. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil. Steril. 2016;105:119–128. doi: 10.1016/j.fertnstert.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Wessels J.M., Leyland N.A., Agarwal S.K., Foster W.G. Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors. Hum. Reprod. 2015;30:925–936. doi: 10.1093/humrep/dev018. [DOI] [PubMed] [Google Scholar]
- 125.Greaves E., Temp J., Esnal-Zufiurre A., Mechsner S., Horne A.W., Saunders P.T. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am. J. Pathol. 2015;185:2286–2297. doi: 10.1016/j.ajpath.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malutan A.M., Drugan C., Walch K., Drugan T., Ciortea R., Mihu D. The association between interleukin-10 (IL-10) −592C/A, −819T/C, −1082G/A promoter polymorphisms and endometriosis. Arch. Gynecol. Obstet. 2017;295:503–510. doi: 10.1007/s00404-016-4269-5. [DOI] [PubMed] [Google Scholar]
- 127.Suen J.L., Chang Y., Chiu P.R., Hsieh T.H., Hsi E., Chen Y.C., Chen Y.F., Tsai E.M. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am. J. Pathol. 2014;184:464–471. doi: 10.1016/j.ajpath.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 128.Yin L.R., Sun J.J., Ma H.D., Mi S.L., Guo S.J., Shi Y. Expression of interleukin1-alpha, beta and interferon-gamma in macrophages from endometrium of women with endometriosis. Zhonghua fu chan ke za zhi. 2006;41:295–298. (published online EpubMay) [PubMed] [Google Scholar]
- 129.Sapkota Y., Low S.K., Attia J., Gordon S.D., Henders A.K., Holliday E.G., MacGregor S., Martin N.G., McEvoy M., Morris A.P., Takahashi A., Scott R.J., Kubo M., Zondervan K.T., Montgomery G.W., Nyholt D.R. Association between endometriosis and the interleukin 1A (IL1A) locus. Hum. Reprod. 2015;30:239–248. doi: 10.1093/humrep/deu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McKinnon B., Bersinger N.A., Wotzkow C., Mueller M.D. Endometriosis-associated nerve fibers, peritoneal fluid cytokine concentrations, and pain in endometriotic lesions from different locations. Fertil. Steril. 2012;97:373–380. doi: 10.1016/j.fertnstert.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 131.Scholl B., Bersinger N.A., Kuhn A., Mueller M.D. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecol. Endocrinol.: Off. J. Int. Soc. Gynecol. Endocrinol. 2009;25:701–706. doi: 10.3109/09513590903159680. [DOI] [PubMed] [Google Scholar]
- 132.Pizzo A., Salmeri F.M., Ardita F.V., Sofo V., Tripepi M., Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol. Obstet. Investig. 2002;54:82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 133.Anaf V., Simon P., El Nakadi I., Fayt I., Simonart T., Buxant F., Noel J.C. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum. Reprod. 2002;17:1895–1900. doi: 10.1093/humrep/17.7.1895. (published online EpubJul) [DOI] [PubMed] [Google Scholar]
- 134.Yu J., Wang Y., Zhou W.H., Wang L., He Y.Y., Li D.J. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum. Reprod. 2008;23:1614–1626. doi: 10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplementary Table 1. Primer sequences for RT-qPCR analysis of nociceptive gene expression in endometrial cells treated with peritoneal fluid from patients with and without endometriosis or Ox-LDL components. Primers were designed using NCBI GenBank and ordered from Invitrogen.
Supplementary material Supplementary Table 2. Significant miRNAs in endometrial cells treated with peritoneal fluid (PF) from women with and without endometriosis (n=3). YY indicates that the peritoneal fluid was from patients with endometriosis and pain. YN indicates that the patients had endometriosis but lacked pain. Significance (indicated in bold) was determined by a p-value<0.05 and compared to cells treated with PF from control patients (NN-PF). Fold change determined by SA Biosciences software. Red cells indicate upregulation while green cells indicate downregulation in compared to control PF.