Abstract
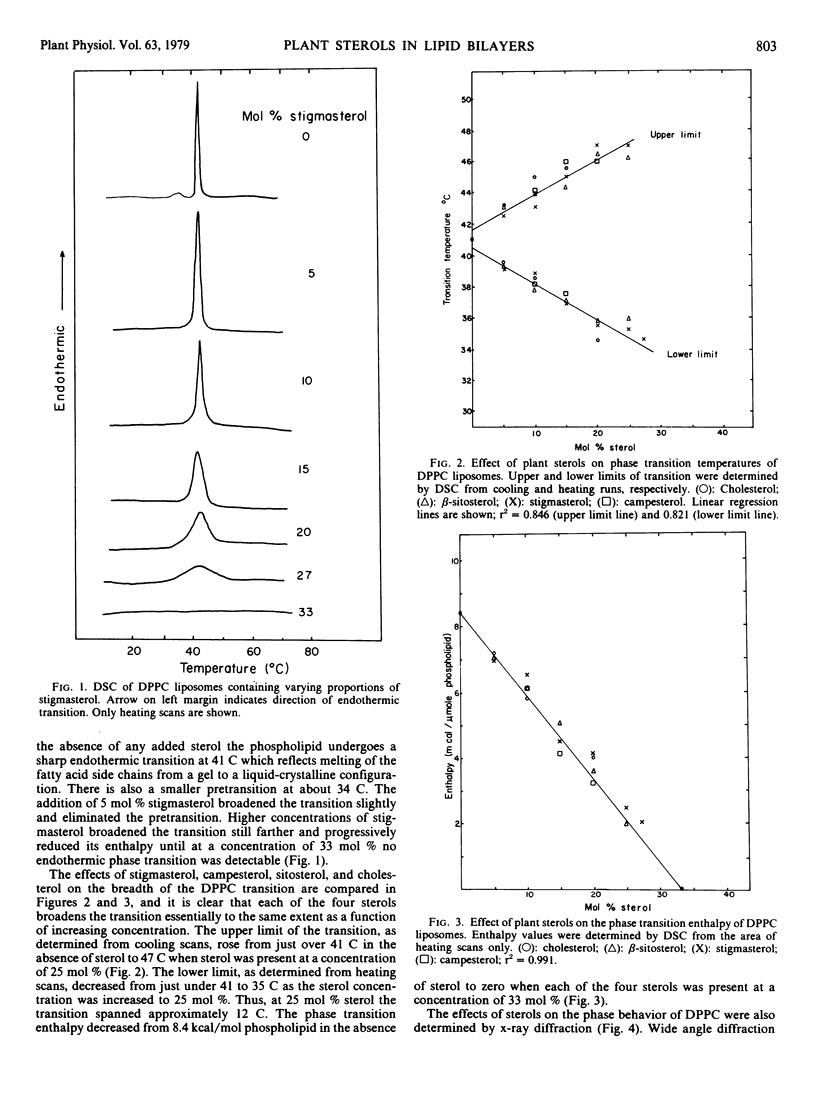
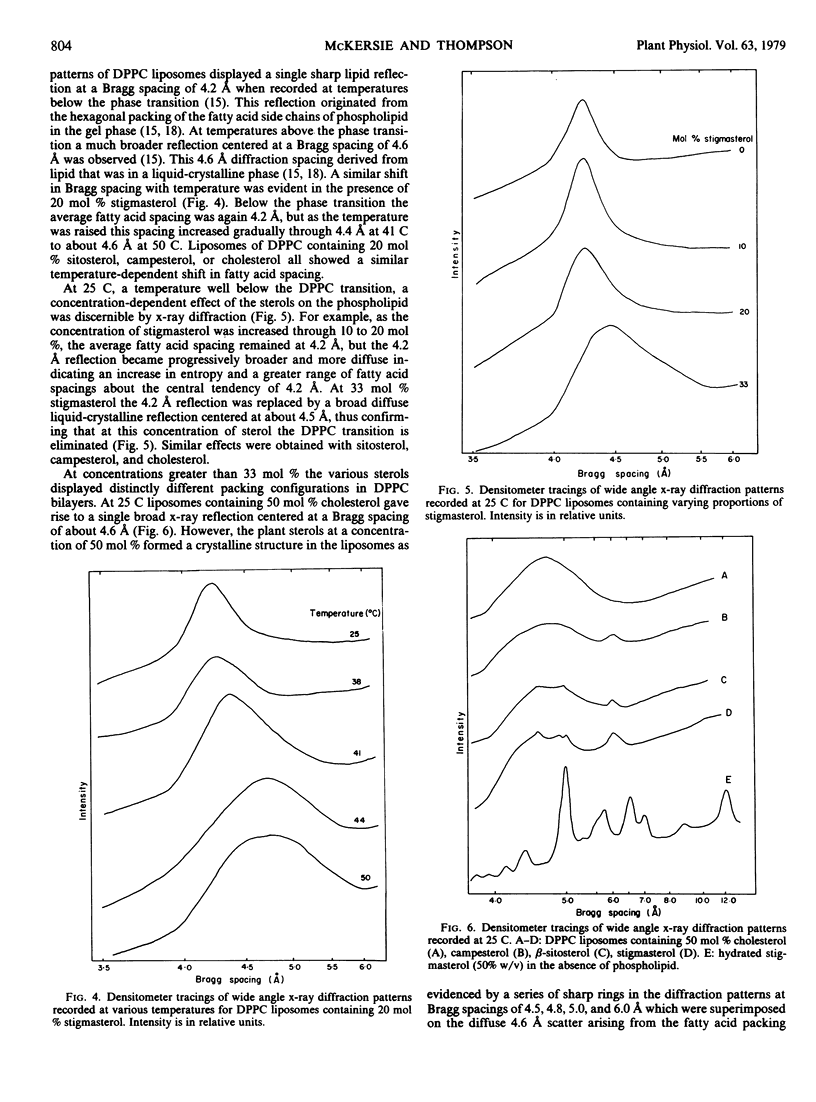
The effects of stigmasterol, sitosterol, campesterol, and cholesterol on the phase properties of dipalmitoylphosphatidylcholine bilayers have been compared by differential scanning calorimetry and x-ray diffraction. The sterols were equally effective at progressively reducing the cooperativity and the enthalpy of the dipalmitoylphosphatidylcholine phase transition as their concentrations in the bilayer were increased. Moreover, both differential scanning calorimetry and x-ray diffraction indicated that the dipalmitoylphosphatidylcholine transition was eliminated by each of the sterols when they were present at a concentration of 33 mole%. This indicates that the interaction between phospholipid and both plant and animal sterols is stoichiometric, each sterol associating with two phospholipid molecules. At concentrations above 33 mole% the sterols were no longer completely solvated by the phospholipid, and sterol-sterol interaction resulted. Cholesterol, even at concentrations as high as 50 mole%, did not disrupt the lamellar structure of the bilayer. When these high concentrations of plant sterols were intercalated into the phospholipid, crystallinity, which presumably derives from sterol-sterol interaction, was detectable in the bilayer by x-ray diffraction. This observation is consistent with previous reports to the effect that the C17 chains of the plant sterols render them less soluble in phospholipid than is cholesterol. It is clear that this solvation difference is of insufficient magnitude to affect the stoichiometry of dipalmitoylphosphatidylcholine-sterol interaction, but it could well account for the less effective modulation of lipid bilayer permeability exhibited by plant sterols in comparison with cholesterol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Cros D. Influence of sterols on ion transport through lipid bilayer membranes. Biochim Biophys Acta. 1978 Jan 19;506(2):265–280. doi: 10.1016/0005-2736(78)90397-8. [DOI] [PubMed] [Google Scholar]
- Blok M. C., Van Deenen L. L., De Gier J. The effect of cholesterol incorporation on the temperature dependence of water permeation through liposomal membranes prepared from phosphatidylcholines. Biochim Biophys Acta. 1977 Feb 4;464(3):509–518. doi: 10.1016/0005-2736(77)90026-8. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim Biophys Acta. 1972 Jan 17;255(1):311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. The effect of sterol structure on the permeability of lipomes to glucose, glycerol and Rb + . Biochim Biophys Acta. 1972 Jan 17;255(1):321–330. doi: 10.1016/0005-2736(72)90031-4. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Gilbert D. B., Tanford C., Reynolds J. A. Cholesterol in aqueous solution: hydrophobicity and self-association. Biochemistry. 1975 Jan 28;14(2):444–448. doi: 10.1021/bi00673a035. [DOI] [PubMed] [Google Scholar]
- Grunwald C. Sterol distribution in intracellular organelles isolated from tobacco leaves. Plant Physiol. 1970 Jun;45(6):663–666. doi: 10.1104/pp.45.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Sterol molecular modifications influencing membrane permeability. Plant Physiol. 1974 Oct;54(4):624–628. doi: 10.1104/pp.54.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1572–1576. doi: 10.1073/pnas.68.7.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Lipid crystallization in senescent membranes from cotyledons. Plant Physiol. 1977 May;59(5):803–807. doi: 10.1104/pp.59.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruyff B., Demel R. A., van Deenen L. L. The effect of cholesterol and epicholesterol incorporation on the permeability and on the phase transition of intact Acholeplasma laidlawii cell membranes and derived liposomes. Biochim Biophys Acta. 1972 Jan 17;255(1):331–347. doi: 10.1016/0005-2736(72)90032-6. [DOI] [PubMed] [Google Scholar]


