Abstract
Mechanistic basis governing the extreme longevity and developmental quiescence of dauer juvenile, a “non-ageing” developmental variant of Caenorhabditis elegans, has remained largely obscure. Using a lipidomic approach comprising multiple reaction monitoring transitions specific to distinct fatty acyl moieties, we demonstrated that in comparison to other developmental stages, the membrane phospholipids of dauer larva contain a unique enrichment of polyunsaturated fatty acids (PUFAs). Esterified PUFAs in phospholipids exhibited temporal accumulation throughout the course of dauer endurance, followed by sharp reductions prior to termination of diapause. Reductions in esterified PUFAs were accompanied by concomitant increases in unbound PUFAs, as well as their corresponding downstream oxidized derivatives (i.e. eicosanoids). Global phospholipidomics has unveiled that PUFA sequestration in membrane phospholipids denotes an essential aspect of dauer dormancy, principally via suppression of eicosanoid production; and a failure to upkeep membrane lipid homeostasis is associated with termination of dauer endurance.
Keywords: Dauer larva, Phospholipids, Polyunsaturated fatty acids, Eicosanoids, Lipidomics, Caenorhabditis elegans
Graphical abstract
Highlights
-
•
Dauer contains more PUFAs in phospholipids compared to other larval stages.
-
•
PUFA accumulation throughout dauer endurance is lost prior to the end of diapause.
-
•
Marked increases in eicosanoid production precede the termination of diapause.
-
•
Changes in membrane lipid dynamics possibly underlie mechanisms of dauer diapause.
1. Introduction
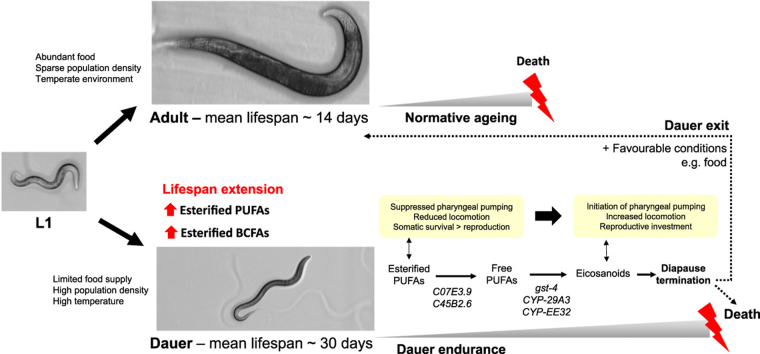
Survival in nature is characterized by constant episodes of feasts and famines. As such, a number of organisms has evolved developmental variants to survive prolonged period of harsh environmental conditions [1]. The C. elegans dauers represent a developmental variant specifically adapted to endure adverse environmental conditions (e.g. food scarcity, overcrowding) for extended duration. Dauers arrest feeding, possess thickened cuticles, often remain motionless, and ration the use of its lipid reserves across the entire period of dormancy, which on average lasts for approximately 30 days for the wild type Bristol N2 strain at 25 °C [2]. The dauer stage is perceived as “non-ageing” [3], [4], as dauer entry allows C. elegans to significantly “outlive” its normal lifespan (about two weeks) [4]; and that the dauer larva can progress normally into adult stage with unaltered lifespan when favourable conditions return.
Dauer response and survival has garnered considerable research interest, since it is mediated by common pathways that control general metabolism, aging, as well as development, such as the insulin and TGF-β pathways, that are conserved in both C. elegans and higher eukaryotes [3]. Indeed, a spatiotemporal compartmentalization of signaling pathways regulating both dauer arrest and normative aging has been previously demonstrated in C. elegans [5]. Thus, an in-depth understanding into the mechanistic basis of dauer survival may reveal novel insights to retarding tissue ageing and lifespan extension. Indeed, previous research has demonstrated that dauer entry is mediated by small molecule signals of immense structural diversities, comprising modular assemblies of carbohydrates, amino acids, lipids, as well as fatty acids collectively termed ascarosides [6]. While it is apparent that ascaroside biosynthesis per se serves to integrate nutritional inputs from major metabolic pathways, elucidation of signal perception and integration leading to dauer formation and survival has remained largely obscure, primarily owing to the structural complexities of ascarosides as well as the plethora of associated downstream phenotypes [6].
The C. elegans dauer larva thus represents an ideal model to investigate the mechanistic basis of a naturally-occurring mode of extended longevity. Biological ageing is defined as the gradual accumulation of cellular damage with age, governed by both environmental and genetic inputs [7]. Intensive research in gerontology has led to the emergence of a number of theories explaining the biological basis of ageing that are often mutually non-exclusive of one another; such as the century-old wear and tear theory, the Medawar's mutation accumulation theory that perceives ageing a “by-product” of natural selection, as well as the rate of living theory that links lifespan with the rate of oxygen consumption [7], [8]. While there is no one prevailing theory of ageing, the oxidative stress theory currently represents one of the most well-received correlative theories of the ageing process [7]. The oxidative stress theory principally postulates that ageing is related to the deleterious effects of free radicals on cellular constituents including DNA, proteins as well as membrane lipids; and these reactive oxygen species are generated as byproducts of normal metabolic process integral to life, such as oxidative phosphorylation in the mitochondria [8].
Using a lipidomic approach comprising multiple reaction monitoring (MRM) transitions specific to distinct fatty acyl moieties in individual phospholipids, we demonstrated that in comparison to other developmental stages, the dauer larva contain a unique enrichment of polyunsaturated fatty acids (PUFAs) in their membrane phospholipids. We next sought to elucidate the changes in membrane lipid dynamics throughout the course of dauer endurance, and discovered that esterified PUFAs exhibited temporal accumulation during the early phases of dauer endurance (i.e. diapause), followed by sharp reduction prior to termination of diapause. Changes in esterified phospholipids were accompanied by concomitant increases in unbound PUFAs, as well as their corresponding downstream oxidized derivatives (collectively known as eicosanoids). Global phospholipidomics has therefore unveiled that PUFA sequestration in membrane phospholipids denotes an essential aspect of dauer dormancy, and a failure to upkeep membrane lipid homeostasis is associated with termination of dauer endurance.
2. Methods
2.1. Synchronization of C. elegans developmental stages
Wild type C. elegans strain Bristol (N2) was provided by the Caenorhabditis Genetics Center (CGC, University of Minnesota, USA), which is funded by National Institute of Health Office of Research Infrastructure Programs. Nematodes were cultured and maintained at 20 °C on nematode growth medium (NGM) agar plates containing 200 µg/mL of streptomycin spotted with Escherichia coli OP50-1 as a food source, as previously described elsewhere [9]. To obtain synchronized developmental stages of C. elegans, gravid hermaphrodites were treated with sodium hypochlorite solution for fixed duration at defined volumetric ratios to obtain embryos. The embryos were then allowed to hatch on NGM agar plates overnight in the absence of food to obtain arrested first-stage larva (L1). L1 larva were washed off the plates and the density was estimated via counting the number of worms in diluted aliquots. The L1 larva were then deposited onto individual plates seeded with fixed concentration of OP-50-1 at a density of 10e4 larva per plate. The larva on individual plates were allowed to grow and develop into adults, and plates denoting each developmental stage was collected at specified time-points after the initiation of feeding [10]. Bacterial food source which may skew the C. elegans lipid profiles was removed via sucrose floatation prior to worm collection. Collected worms were frozen immediately in M9 buffer at −80 °C until lipid extraction.
2.2. Dauer induction and purification
N2 were grown in 500 mL of S-medium containing fixed concentration of bacterial food source for 10 days at 25 °C with constant agitation at 120 rpm. During this period of growth, food source was slowly depleted over time and overcrowding set in after repeated cycles of reproduction and multiplication, and dauers became plentiful at the end of the 10-day period. Worms were first isolated free of any remaining bacteria and tissue remnants by sucrose floatation. Next, dauers were purified by treating the isolated worms with 1% sodium dodecyl sulfate (SDS) for 15 min, followed by sucrose floatation and pelleting through 15% Ficoll [11]. These freshly isolated dauer population denotes Day 0 of our experimental time-point of dauer endurance. Purified dauers were plated on NGM plates at a density of 10e4 dauers per plate. Dauers were collected on representative time-points (Day 0, Day 7, Day 14 and Day 21) across the period of dauer endurance. Dauers were treated with 1% SDS for 15 min followed by sucrose floatation prior to collection to ensure that the collected samples were devoid of contaminating carcasses that may confound the lipid profiles.
2.3. Phospholipid extraction and HPLC-MS/MS analysis
Lipids were extracted from the C. elegans using a modified version of the Bligh and Dyer's method [12]. Briefly, frozen worm samples were first thawed on ice for 5 min, followed by the addition of chloroform:methanol 1:2 (v/v). Next, fixed amount of ceramic beads pre-cleaned with methanol to remove contaminating lipids were added, and the samples were homogenized on a bead ruptor (OMNI, USA) using a pre-optimized programme (speed =5 m/s, duration =8 s, pause =2 s, 2 cycles). Samples were then incubated at 4 °C for 1 h at 1500 rpm. At the end of the incubation, 400 µL of MilliQ water and 300 µL of chloroform were added. Samples were centrifuged at 12,000 rpm for 5 min at 4 °C, and the lower organic phase was extracted into a clean tube. The extraction procedure was repeated twice via addition of chloroform, and 50 µL of 1 M HCl was added at the last round of extraction. Extracted organic phases were pooled together into a single tube and dried under vacuum in the OH mode using the SpeedVac (Genevac, UK). Dried lipid extracts were stored at −80 °C until mass spectrometric analysis. 10e4 worms were collected per sample for analysis of phospholipidome.
Analytical methods were constructed based on high performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) as previously described [13]. Lipidomic analyses were conducted on an Exion ultra-performance liquid chromatographic system (UPLC) coupled with Sciex 6500 Plus Qtrap (Sciex, USA). Qualitative deuterated lipid standards from LIPID MAPS were pre-corrected using suitable quantitative lipid standards from the same lipid class based on molar response prior to their use for quantitation. Separation of individual phospholipid classes of polar lipids by normal phase HPLC was carried out using a Phenomenex Luna 3 µm silica column (i.d. 150×2.0 mm) with the following conditions: mobile phase A (chloroform:methanol:ammonium hydroxide, 89.5:10:0.5), B (chloroform:methanol:ammonium hydroxide:water, 55:39:0.5:5.5). Multiple reaction monitoring (MRM) transitions specific to both head groups and fatty acid chains were set up for quantitative analysis of various phospholipids. Individual lipid species will be quantified by referencing to spiked internal standards, which included PC-14:0/14:0, PC34:1-d31, LPC-d4−26:0, PE-14:0/14:0, PE34:1-d31, LPE-17:1, PS-14:0/14:0, PS-16:0/18:1-d31, LPS-17:1, PA34:1-d31, PA-17:0/17:0, LPA-17:0, PG34:1-d31, PG-14:0/14:0, PI34:1-d31, LPI-17:1 obtained from Avanti Polar Lipids and LIPIDS MAPS (Alabaster, AL, USA). Dioctanoyl phosphatidylinositol (PI, 16:0-PI) used for phosphatidylinositol quantitation was obtained from Echelon Biosciences, Inc. (Salt Lake City, UT, USA). For all LCMS analyses, individual peaks were examined and only peaks above the limit of quantitation and within the linearity range were included in the quantitation [14], [15]. Lipidomic analyses were performed in triplicates for developmental stages and in quadruplicates for dauer endurance.
2.4. Eicosanoid extraction and UPLC-MS/MS analysis
Eicosanoids were extracted from freshly collected C. elegans samples on the day of the collection, and 10e5 dauers were used for each replicate due to the endogenously low level of eicosanoids in worms. Briefly, 1 mL of phosphate-buffered saline (PBS) containing 2% formic acid (v/v), 50 µL of 0.1% (w/v) butylated hydroxytoluene (BHT) in methanol and 10 µL of 0.2 ng/µL internal standard cocktail were added to each sample and vortexed to mix thoroughly. A fixed amount of ceramic beads pre-cleaned with methanol was then added and the samples were incubated at 1500 rpm for 12 h at 4 °C to ensure efficient extraction of eicosanoids from the tissue matrix. The samples were centrifuged at 4 °C for 10 min at 12,000 rpm, and the supernatant was extracted for eicosanoids using Oasis MAX SPE columns (Waters, USA). The internal standard cocktail contained dhk-PGD2-d4, 6k-PGF1α-d4, PGD2-d4, PGE2-d4, PGF2α-d4, TXB2-d4, 9-HODE-d4,13-HODE-d4, 9,10-diHOME-d4, 12,13-diHOME-d4, FFA20:4-d8 and FFA20:5-d5 in methanol (Cayman chemicals, USA). Eicosanoid extract was transferred to tubes containing 20 µL of ethanol:glycerol 1:1 (v/v) to prevent complete desiccation, and dried under flowing stream of nitrogen gas. The dried extract was re-constituted immediately in 50 µL of water:acetonitrile:formic acid 63:37:0.02 (v/v/v) for mass spectrometric analysis. Eicosanoid analyses were conducted on an Exion UPLC coupled with Sciex 6500 Plus Qtrap (Sciex, USA). Eicosanoid analyses were conducted based on previously published protocols with modifications [16], [17]. The mobile phase comprises (A) water:acetonitrile:formic acid 63:37:0.02 (v/v/v) and (B) acetonitrile:isopropanol 1:1 (v/v), and separation was carried out on a Phenomenex Kinetex 1.7 µm C18 column (i.d. 100×2.1 mm). Eicosanoid analyses were performed in triplicates.
2.5. RNA extraction and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen). RNA (2 µg) was reversely transcribed using M-MLV Reverse Transcriptase (Invitrogen), according to the manufacturer's instructions, and subject to qPCR analysis using TransStrart® Green qPCR Supermix (Transgen) and Agilent Stratagene Mx3000p (Agilent). Act-1 was used as housekeeping gene for relative quantitation of mRNAs of interest. Sequences of PCR primers were compiled in Table 1. Experiments were performed in triplicates.
Table 1.
Primer pairs used for qPCR analyses.
| Gene name | Gene ID | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|---|
| act-1 | 179535 | TCCAAGAGAGGTATCCTTAC | CGGTTAGCCTTTGGATTGAG |
| gst-4 | 177886 | GCCAATCCGTATCATGTTTGC | CAAATGGAGTCGTTGGCTTCAG |
| rme-2 | 177329 | ATGAAGACAATAAGTGTCGGAG | CGCTTGGAGCATTAGTTTGG |
| CYP-29A3 | 189649 | TGATTCTGCCCTGTTTATTG | ACCGGGGAGCAAATCATC |
| CYP-33E2 | 185653 | ATGAATCACAACGTCTTGCC | TCAGGGAAGATTTCTGGATT |
| C45B2.6 | 180874 | TTGGTACCGGTTATGCTGAGAG | AAATCGAGCGGCAACATGAAC |
| C07E3.9 | 182374 | TCACAATCACAAAGTGCCGC | TCTCATGAGCCTGTCGATGG |
2.6. Statistical analysis
Lipid levels were expressed in terms of molar fractions normalized to total polar lipids detected in each sample for statistical analyses. Kruskal-Wallis non-parametric analysis of variance (ANOVA) were used to compare the changes in lipids and mRNA levels across the various time-points of dauer endurance. Hierarchical clustering was performed to stratify lipids that displayed similar patterns of changes across time, using the pdist() function provided in Matlab (R2012b, the MathWorks. Natick, MA, USA) with Euclidean distance metric. Details of the algorithm have been previously described elsewhere [18].
3. Results
3.1. Enrichment of PUFA-containing phospholipids specifically in the dauer stage of development
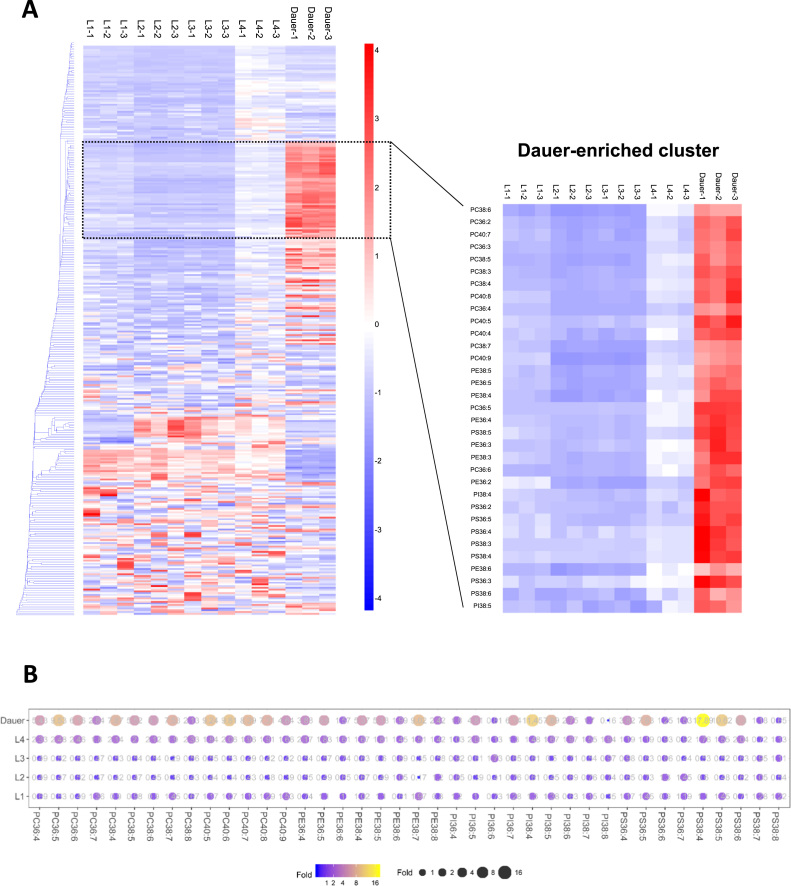
Since the C. elegans dauer larva represents a developmental variant ideated to survive prolonged periods of adverse conditions, it is likely that dauers may possess unique membrane lipid properties among other developing larval stages that render such specialised adaptations. The phospholipidome of C. elegans dauers was comprehensively profiled alongside with that of larva from the first to fourth stages of development (i.e. L1, L2, L3, L4), and the resultant lipidomic profiles were compared using Euclidean clustering. Remarkably, a distinct phospholipid cluster highly enriched in the dauer larva was uncovered, which exclusively comprised phospholipids that possessed a high degree of unsaturation in their fatty acyl chains (Fig. 1A). These highly unsaturated phospholipids exhibited close to ten-fold increases in dauers compared to larva of other developmental stages (p<0.05) (Fig. 1B). In addition, PUFA enrichment in dauers was not limited to any particular classes of phospholipids; and was observed consistently across all classes of phospholipids examined, including phosphatidylcholines (PCs), phosphatidylethanolamines (PEs,), phosphatidylinositols (PIs) and phoshatidylserines (PSs) (Fig. 1).
Fig. 1.
Unique accumulation of PUFAs in membrane phospholipids of dauers. Euclidean clustering based on the z-scores of 331 individual phospholipids across the larval developmental stages of C. elegans revealed a unique cluster comprising highly unsaturated phospholipids specific to the dauer larva (A). An illustration of the fold-changes in major unsaturated phospholipids across different larval stages. Size and colour of circles indicate magnitude of fold-changes displayed as numerical values. Fold-changes are calculated relative to the mean of L1, L2, L3 and L4 for each lipid species (B). L1: first-stage larva; L2: second-stage larva; L3: third-stage larva; L4: fourth-stage larva; experiments were performed in triplicates.
3.2. Lyso-phospholipids and saturated phospholipids displayed marked increases at stage IV of dauer endurance
To elucidate the mechanistic roles of membrane-esterified PUFAs in dauer metabolism, the phospholipidome of worms at four representative stages (I-IV) of dauer survival (i.e. endurance), which corresponded approximately to Day 0, Day 7, Day 14, and Day 21 of dauer formation, was examined. Dauer formation was induced by progressive overcrowding and food scarcity in liquid culture, which was examined daily for the presence of dauer larva. When significant enrichment of dauers was observed in liquid culture, purification of dauers were carried out using 1% SDS followed by sucrose floatation to obtain isolated populations of dauers (see Methods). The time-point of first collection corresponded to stage I of dauer endurance in our experiment, which represented worm populations that had just entered the dauer stage. The dauer population was monitored weekly until Day 21, or stage IV of dauer endurance, at which substantial deaths (>50%) had occurred that represented dauers at an advanced stage of endurance.
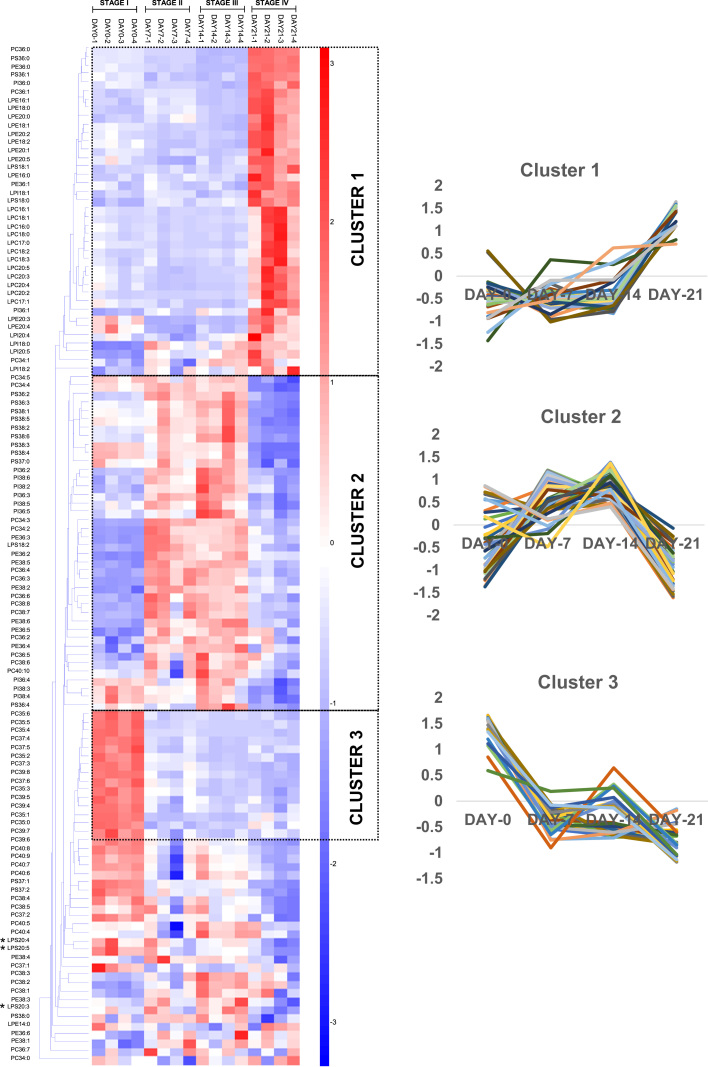
Temporal analysis of phospholipid profile changes during dauer endurance revealed that saturated phospholipids, including PC36:0, PE36:0, PS36:0 and PI36:0, which were maintained at relatively low levels across stages I-III of dauer endurance, exhibited sharp increases at stage IV (Fig. 2, cluster 1). Similarly, numerous lyso-phospholipids across different classes comprising lyso-PC (LPC), lyso-PE (LPE) and lyso-PI (LPI) exhibited were appreciably elevated at stage IV of dauer endurance, which may be indicative of enhanced phospholipase activities toward the advanced stage of dauer survival (Fig. 2, cluster 1). Notably, lyso-PS (LPS) containing C20-PUFAs i.e. LPS 20:3, LPS 20:4 and LPS 20:5, unlike their counterparts from other phospholipid classes, were appreciably reduced at stage IV (Fig. 2, asterisked).
Fig. 2.
Temporal changes in phospholipidome throughout dauer endurance based on head group-specific transitions. Euclidean clustering based on the z-scores of 121 major phospholipids in dauers revealed three individual clusters with distinct patterns of changes over the course of diapause. Experiments were performed in quadruplicates.
3.3. PUFA-containing phospholipids exhibited drastic reductions at stage IV of dauer endurance
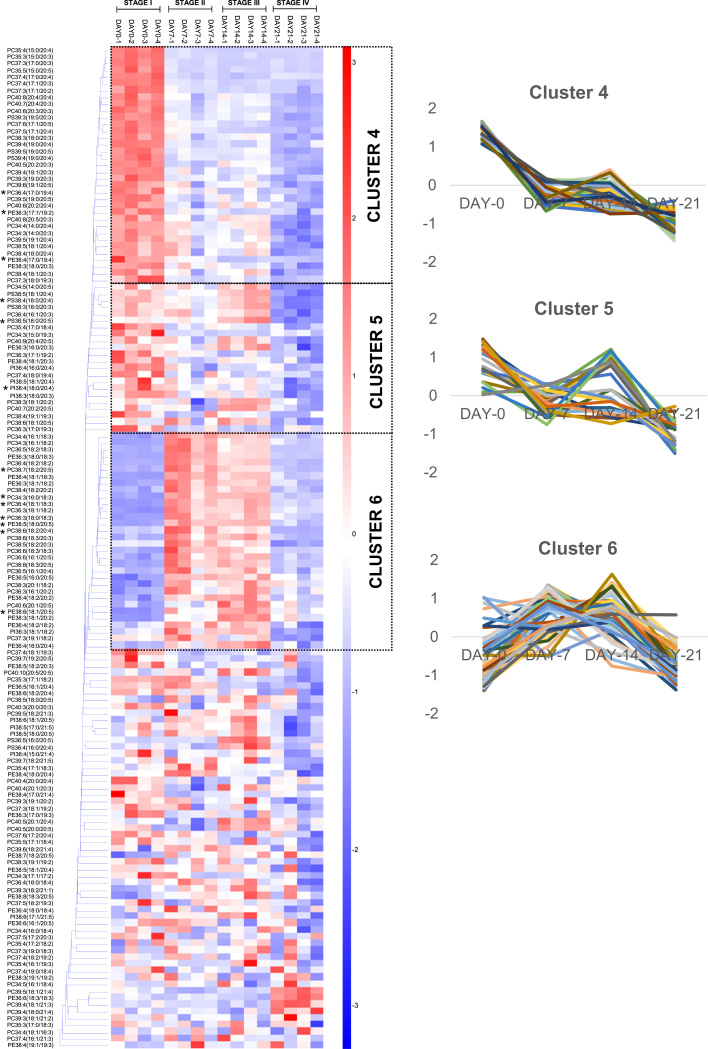
PUFA-containing PCs and PEs demonstrated significant accumulation from stage I through stage III of dauer endurance, followed by steep reductions at stage IV (Fig. 2, cluster 2). In contrast, PS and some PI comprising PUFAs were maintained at steadily high levels throughout stages I-III of dauer endurance, but were similarly reduced at stage IV (Fig. 2, cluster 2). In order to decipher a greater resolution of fatty acyl-dependent changes throughout the course of dauer endurance, MRM transitions specific to individual fatty acyls within phospholipids were constructed, and the results were presented in Fig. 3. Using fatty acyl-based MRMs with a greater resolution in terms of fatty acid identities, PUFA-PCs and PUFA-PEs of relatively high abundances, such asPC34:3(16:0/18:3), PC36:3(18:0/18:3), PC36:4(18:1/18:3), PC38:6(18:1/20:5), PC38:6(18:2/20:4), PC38:7(18:2/20:5), PE38:5(18:0/20:5) and PE38:6(18:1/20:5), were markedly increased from stage I through stage III of dauer endurance (Fig. 3, cluster 6, asterisked). Thus, a preferential accumulation of PUFAs including FFA18:2 (linoleic acid, LA), FFA18:3 (α or γ-linolenic acid, ALA or GLA), FFA20:4 (arachidonic acid, AA) and FFA20:5 (eicosapentaenoic acid, EPA) was observed in major membrane lipid classes of PCs and PEs during dauer endurance. On another note, major PS and PI species containing AA and EPA, including PS38:4(18:0/20:4), PS38:5(18:0/20:5) and PI38:4(18:0/20:4), which were maintained at relatively high levels from stages I to III, were segregated into a distinct cluster (Fig. 3, cluster 5, asterisked). Nonetheless, notable reductions in PUFA-containing species across all individual classes of phospholipids examined were unanimously observed at stage IV of dauer endurance (Fig. 3, clusters 5 and 6), indicating that a loss of membrane esterified PUFAs may be associated with termination of dauer endurance.
Fig. 3.
Temporal changes in phospholipidome throughout dauer endurance based on fatty acyl-specific transitions. Euclidean clustering based on the z-scores of 148 major phospholipids comprising PUFAs in dauers revealed three individual clusters with distinct patterns of changes over the course of diapause. Experiments were performed in quadruplicates.
3.4. Phospholipids containing odd-chain/branched-chain fatty acids were sharply decreased from stage II of dauer endurance
Apart from membrane esterified PUFAs, another interesting cluster emerged from our extensive investigation of the phospholipidome. It was observed that PCs comprising odd or branched-chain fatty acids (BCFAs) were distinctly segregated into a cluster that exhibited sharp reductions from stage I to stage II, and was subsequently maintained at low level throughout the course of dauer endurance (Fig. 2, cluster 3). Results from fatty acyl-based MRM analysis vindicated this observation, and a distinct cluster constituting predominantly of PCs with BCFAs (C15, C17, and C19) once again emerged (Fig. 3, cluster 4). Interestingly, PC species with an even total number of carbons, but comprising odd-chain fatty acids at both sn-1 and sn-2 positions, such as PC36:4(17:0/19:4), PC36:3(17:1/19:2) and PC36:4(17:0/19:4) were also found in this cluster (Fig. 3, cluster 4, asterisked). These shown that the levels of membrane-esterified BCFAs were indeed preferentially reduced at the early stages of dauer endurance.
3.5. Free PUFAs and downstream oxidized derivatives (eicosanoids) were elevated at stage IV of dauer endurance
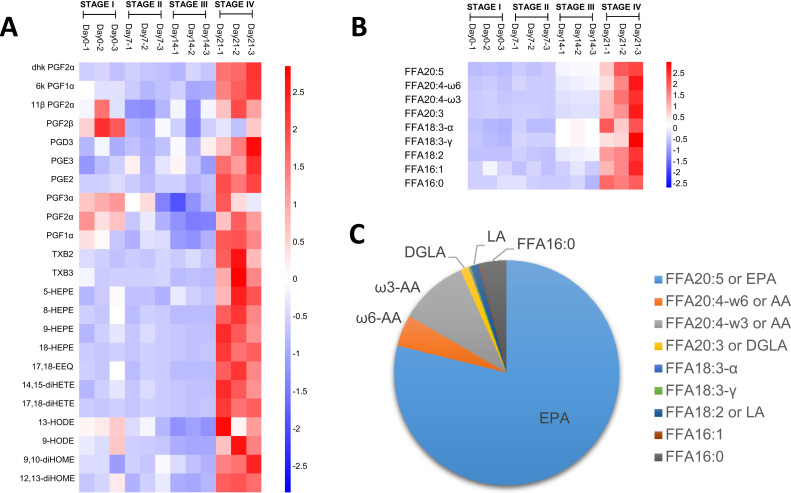
In view of the drastic reductions in membrane esterified PUFAs at stage IV of dauer endurance, we next investigated whether the downstream oxidized derivatives of PUFAs (i.e. eicosanoids) were significantly altered. Accordingly, oxidized EPA-derivatives, including various isomers of hydroxy-eicosapentaenoic acids (HEPE), epoxy-eicosatetraenoic acids (EEQ) and dihydroxy-eicosatetraenoic acids (diHETE); as well as derivatives of LA, such as hydroxyl-octadecadienoic acid (HODE) and dihydroxy-octadecenoic acid (diHOME), were markedly elevated at stage IV of dauer endurance (Fig. 4A). Furthermore, the levels of various prostaglandin (PG) species derived from FFA20:3 (di-γ-linolenic acid, DGLA), AA and EPA were also significantly raised at the advanced stage of dauer endurance (Fig. 4A). In line with these observations, the unbound PUFA precursors were appreciably elevated at stage IV as well (Fig. 4B). Most of the detected eicosanoids were derivatives of EPA, which represented the predominant unesterified PUFA in C. elegans dauers (Fig. 4C), consistent with previous observations in adult worms [19].
Fig. 4.
Temporal changes in eicosanoids and free fatty acids throughout dauer endurance. Heatmaps illustrate the z-scores of 23 eicosanoid species (A) and nine individual fatty acids (B) quantitated over the course of dauer diapause. Pi-chart displays the proportional abundances of individual free fatty acids detected in dauer larva (C). Experiments were performed in triplicates.
3.6. Temporal changes in the expression of genes implicated in PUFA metabolism throughout dauer endurance
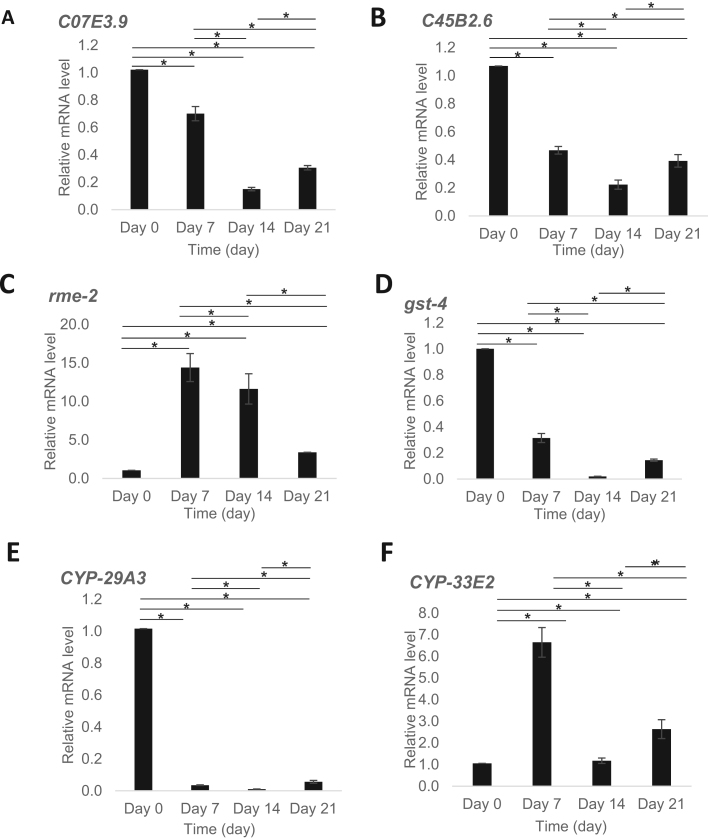
The expression of selected genes encoding enzymes implicated in the release and transport of membrane esterified PUFAs, as well as downstream eicosanoid biosynthesis, was investigated using qRT-PCR. Based on our lipidomic observations, we speculate an enhanced PUFA release from membrane lipids precedes the termination of dauer endurance. In corroboration with this, the mRNA levels of two genes orthologous to human phospholipases A2 (PLA2) [20], namely C07E3.9 and C45B2.6, which were progressively reduced throughout the course of dauer endurance (p<0.05), shown marked elevations at stage IV compared to stage III (p<0.05) (Fig. 5A-B). The expression of rme-2, which encodes a low-density lipoprotein (LDL) receptor implicated in the endocytosis of PUFAs transported from the intestines into the oocytes of adult worms [21], was markedly elevated at stage II, followed by progressive reductions throughout the investigated period of dauer endurance (p<0.05) (Fig. 5C). The expression of gst-4, which encodes glutathione S-transferase enzymes related to PG synthases [22]; as well as that of CYP-29A3, one of C. elegans’ NADPH-cytochrome P450 (CYP) reductases contributing to eicosanoid biosynthesis [23], were progressively reduced from stages I-III, followed by significant elevation at stage IV (p<0.05) (Fig. 5D-E). On the other hand, CYP-33E2, another CYP-reductase implicated in eicosanoid biosynthesis in worms, were markedly elevated at both stages II and IV (p<0.05) (Fig. 5F). Nonetheless, in contrast to gst-4 and CYP-29A3 that exhibited dauer-enriched expression [24], the expression of CYP-33E2 was not specific to dauers across development stages.
Fig. 5.
Changes in mRNA levels of selected genes throughout dauer endurance quantitated using qRT-PCR. The expression of phospholipases including C07E3.9 (A) and C45B2.6 (B), LDL receptor rme-2 (C), as well as genes implicated in eicosanoid biosynthesis including glutathione S-transferase gst-4 (D) and NADPH-cytochrome P450 reductases CYP-29A3 (E) and CYP-33E2 (F) were quantitated using qRT-PCR. Relative mRNA levels were determined by qRT-PCR and normalized over act-1 mRNA levels as a housekeeper; mean of Day 0 was set to 1. Data were presented as mean ± SEM. (* p<0.05, Kruskal-Wallis’ test). Experiments were performed in triplicates.
4. Discussion
Despite being perceived as a “non-ageing” developmental variant of the C.elegans, mechanistic basis governing the extreme longevity and developmental quiescence of dauer juvenile has remained largely obscure. Stark changes in membrane lipid composition of dauers compared to other larval stages had prompted us to comprehensively investigate global alterations in the phospholipidome of dauer larva throughout the course of their survival, which we termed dauer endurance. In contrast to a myriad of preceding works on dauer larva that adopted a principally genome-oriented approach [25], [26], an investigation based upon lipidomics could reveal potentially important lipid pathways possibly controlled by enzymes with functional paralogs, or that implicating metabolites are not directly encoded by DNA [27], [28], [29].
4.1. Sequestration of BCFAs in PCs may mediate the early phase of dauer-diapause
Our analyses revealed that a distinct accumulation of BCFA-containing PCs was observed at the early phase of dauer endurance. The discovery of BCFAs in C. elegans lipidome is not new, as numerous reports had previously demonstrated the critical roles that BCFAs elicit in C. elegans development [30], [31], [32]. In particular, BCFAs were shown to regulate the expression of several genes that control post-embryonic development in C. elegans, and suppression of BCFA biosynthesis led to growth arrest at the L1 stage [30], [32]. Furthermore, it was demonstrated that BCFAs exert their effect in promoting post-embryonic growth and development via serving as precursors for the formation of glucosylceramides comprising branched-chain sphingoid bases [31]. The C. elegans L1 larval stage represents a state of suspended animation that is, to a certain extent, akin to the dauer diapause. L1s, with attenuated levels of global gene expression, can be maintained for several days in the absence of food [33]. It is therefore plausible that dauers deploy a similar mechanism to initiate diapause via sequestration of BCFAs in phospholipids, thereby halting downstream formation of branched-chain glucosylceramides and subsequent activation of genes mediating normal growth and development. Since BCFAs are present in substantial quantities in various genera of bacteria [34], such a mechanism governing diapause entry could represent an effective means to sense/monitor food availability for C. elegans to adopt the optimal developmental decision that uplifts their chances of survival in nature. Nonetheless, the precise fate of these PC-esterified BCFAs and their potential role(s) throughout the course of dauer endurance remain to be determined.
4.2. Release of membrane-bound PUFAs and enhanced eicosanoid production precedes termination of dauer endurance
Our analyses revealed a notable and progressive accumulation of PUFAs in phospholipids throughout the course of dauer endurance. The accretion of PUFA-phospholipids in dauers was lost at stage IV prior to the termination of the endurance period, which coincided with marked elevations in saturated phospholipids as well as increases in various lyso-phospholipids (Fig. 2, Fig. 3). The augmented levels of gene expression for phospholipases (C07E3.9 and C45B2.6) at stage IV (Fig. 5A-B) therefore indicated an increased phospholipase activity that promoted the release of free PUFAs prior to the end of diapause. In addition, the mRNA levels of genes responsible for eicosanoid biosynthesis from unbound PUFA precursors, including gst-4, CYP-29A3 and CYP-33E2, which were generally maintained at low levels throughout stages I-III, exhibited significant increases at stage IV (Fig. 5D-F). In line with this, the levels of free fatty acids and various eicosanoids including PG, HEPE, EEQ, diHETE, HODE and diHOME principally derived from the oxidation of LA, AA and EPA, were markedly elevated at stage IV (Fig. 4). The lipidomic and mRNA data therefore cumulatively point to an enhanced phospholipase-mediated release of free PUFAs and their subsequent oxidation to eicosanoids prior to the termination of dauer endurance. As 1% SDS treatment was carried out prior to worm collection at all time-points investigated in this study, this implied that the observed biochemical changes preceded the loss of specialized cuticle and initiation of pharyngeal pumping that confer SDS resistance, which also represent hallmark features of dauer diapause [1].
Orthologs of cyclooxygenases (COX) and lipooxygenases (LOX) that are respectively responsible for the production of PG and hydroxylated PUFAs in mammals, were lost in C. elegans during the course of evolution [35]. Nonetheless, the genome of C. elegans contains 75 full length CYP genes, some of which were demonstrated to possess NADPH-CYP reductase activities, with CYP-29A3 and CYP-33E2 being identified as the major isoforms responsible for EPA metabolism in C. elegans [23]. Previous works in C. elegans have shown that a heterogeneous mixture of F-series PGs function redundantly in fertilization to guide the unidirectional movement of sperms towards mature oocyte [36]. Furthermore, it was demonstrated in adult C. elegans that under environmental conditions that are suboptimal, an attenuated secretion of DAF-7 ligand by pheromone-sensitive neurons inhibits COX-independent PG biosynthesis in the ovary, leading to a failure in sperm guidance and a decline in reproductive output [37]. Thus, eicosanoids serve as mediators in relaying environmental cues, possibly perceived in the form of pheromones via pheromone-responsive sensory neurons, to fine-tune reproductive output in the ovary [36], [37]. An intriguing possibility emerged from this study as to whether ascarosides, which are pheromone signals known to regulate dauer entry, also act to suppress eicosanoid production to promote long-term diapause. Also, the cyp-31A2 and cyp-31A3 mutants exhibited defects in embryo development characterized by osmotic sensitivity and perturbed polarity establishment, further suggesting that eicosanoids may be crucial in reproductive development [38].
Thus, the suppression of eicosanoid biosynthesis through stages I-III of dauer endurance via [1] sequestration of PUFA precursors in membrane phospholipids, and [2] downregulation of genes for eicosanoid biosynthesis may serve to repress germline development and divert the limiting resources towards somatic survival. In this light, it has been shown in adult C. elegans that PUFA supplementation preferentially diverts fats to somatic tissues over the adult germline, leading to increased somatic resilience and improved stress response beneficial for survival [39]. While the authors have proposed that downstream eicosanoid production may promote somatic survival over germline proliferation, no direct measurement of endogenous eicosanoid levels nor fatty-acyl specific changes in phospholipids following PUFA supplementation were conducted [39]. Since the ultimate fate of supplemented PUFAs was not determined in the study aforementioned, it could also be plausible that enhanced esterification of PUFAs into phospholipids may mediate somatic survival instead. Previous analyses have shown that the ovarian tissues represent a principal site of eicosanoid production in adult worms, utilizing PUFAs transported from the intestines obtained through endocytosis of vitellogenins [21]. In particular, the loss of RME-2 receptor resulted in suppressed eicosanoid production and a loss of directional motility in sperms [21]. The mRNA level of rme-2 was, however, not particularly increased at stage IV of dauer endurance (Fig. 5C), indicating that alternative site(s) of eicosanoid biosynthesis may exist in dauers.
Apart from their roles in mediating reproductive development and fertilization, CYP-derived eicosanoids were also reported to serve as secondary messengers in regulating pharyngeal pumping and food uptake in C. elegans. Cessation in pharyngeal pumping represents a hallmark feature of dauers, which are non-feeding with a covered buccal opening [40]. In this aspect, 17, 18-EEQ had been reported to promote pharyngeal pumping in both wild type and mutant worms [19]. The stark increases in 17, 18-EEQ, and possibly other EPA-derived eicosanoids, observed at stage IV of dauer endurance (Fig. 4A) may thus signal the initiation of pharyngeal pumping and termination of dauer endurance. Besides, CYP-generated eicosanoid signaling was also implicated in the behavioral response to oxygen availability following hypoxia in C. elegans, which is characterized by sharp increase in the speed of locomotion [41]. Thus, suppression of eicosanoid biosynthesis during dauer endurance may also explain the generally low level of motility observed in dauers.
4.3. Changes in membrane lipid compositions may underlie the basis of dauer-specific phenotypes
While eicosanoids are traditionally perceived as potent signaling molecules mediating downstream cellular events [42], the biological significance of enhanced PUFA-esterification in membrane lipids throughout the course of dauer endurance should not be overlooked as well. Indeed, the incorporation of AA into membrane phospholipids has been demonstrated to enhance neuronal mechanics governing the sensation of touch in C. elegans [37]. The authors proposed that PUFA-enriched membrane domains may function to dilute cholesterol-rich microdomains, inducing membrane thinning and subsequent activation of mechanoelectrical transduction (MeT) channels that relay the sensation of touch [43]. In this light, despite often lying motionless to conserve energy expenditure for locomotion, dauers display rapid response to external stimuli [1]. We speculate that enhanced PUFA-esterification in membrane lipids may also serve to “sensitize” MeT channels in dauers to boost their response to external vibration (e.g. passing danger, medium for dispersal), increasing their chances of survival in nature.
Steric incompatibility between highly chaotic PUFA-enriched membrane domains and the relatively compact and rigid regions of cholesterol-enriched domains (i.e. lipid rafts) has been previously proposed to serve as competing platforms that differentially regulate protein functions in vivo [44]. It is noteworthy that the CYP enzymes, principally responsible for eicosanoid biosynthesis in C. elegans [23], are membrane-bound hemoproteins [45]. Thus, enhanced PUFA-esterification in membrane lipids may serve dual roles in sequestering PUFA precursors and regulating CYP enzymatic activity to attenuate eicosanoid biosynthesis throughout the course of dauer endurance. The differential membrane dynamics in dauer larva may constitute the basis of altered metabolism associated with the dauer-specific “non-ageing” phenotype. In this aspect, we have also previously observed temporal decline in PUFA-esterification and elevated levels of raft-associated lipids, including long-chain sphingomyelins and cholesterols, in the neuronal membranes of Rhesus macaques during normative ageing [13], implying that changing membrane dynamics may constitute essential mechanisms governing tissue ageing that are conserved from metazoans to mammals.
The preceding observations indicated that the dauer larva underwent a period of acute oxidative stress, characterized by sharp increases in endogenous PUFAs and the consequent biosynthesis of eicosanoids, prior to the termination of diapause. In this sense, the esterification of PUFAs in membrane phospholipids throughout dauer endurance may, at least partly, serve as an effective means to minimize oxidative stress induced by the excessive accumulation of endogenous PUFAs in dauers in order to achieve extended longevity. The stark accumulation of PUFAs in membrane lipids, nonetheless, may also represent an integral aspect of dauer dormancy that serves other physiological functions (e.g. via altering membrane dynamics and downstream signaling pathways) as aforementioned. Our lipidomic observations in the “non-ageing” dauer larva are therefore aligned with the oxidative stress theory of ageing, which primarily proposes ageing to be the result of a failure in maintaining oxidative defenses [7]. While eicosanoid production may underlie the basis of diapause termination in dauers, the precise mechanism leading to cellular senescence and death at the end of dauer endurance remains to be elucidated.
Notably, 4-hdyroxy-2-nonenal (4-HNE) denotes a predominant product of lipid peroxidation that is generated by the oxidative degradation of omega-6 PUFAs including AA and LA [46], which is relatively stable and can diffuse from its site of origin to elicit systemic effects [46]. It was shown that endogenous 4-HNE may determine cell fate in a bi-modular manner that is concentration dependent [47], [48], [49]. In human osteosarcoma cells, for example, increasing concentrations of 4-HNE was found to inhibit cellular proliferation and differentiation [48], and progressive accumulation of 4-HNE was observed in vitro during the ageing of human facial skin fibroblasts [49]. While low but supraphysiological levels are associated with cellular proliferation and tumorigenesis [47], exceedingly high levels of 4-HNE had been shown cytotoxic to HeLa cells [50]. It remains an intriguing possibility if overproduction of 4-HNE represents a key determinant driving cellular death at the end of dauer diapause. In Saccharomyces cerevisiae, excessive PUFA production was shown to result in elevated levels of 4-HNE. The PUFA-producing yeast strain displayed higher initial sensitivity to oxidative stress relative to wild type, but became more resistant (i.e. adapted) to oxidative stress over extended cultivation, for which the authors attributed to an elevated peroxisomal catalase activity [51]. Similarly, treating yeast cells with 4-HNE led to cell cycle arrest that was uplifted only when cellular glutathione content exceeded that of control cells by twofold [52]. Therefore, detoxification of 4-HNE may denote a key factor governing cellular fate in the event of 4-HNE overproduction following endogenous PUFA accumulation. It may thus be interesting to investigate if the controlled release and subsequent detoxification of 4-HNE may essentially underlie the mechanistic differences between diapause termination and death as compared to dauer exit and progression into normal development; since both events would presumably lead to high endogenous PUFA production stemming from phospholipase-mediated release of membrane-esterified PUFAs. Finally, perturbed levels of oxidized lipid derivatives have been reported in the human placenta of small-for-gestational age newborns compared to controls [53], raising the possibility that oxidative stress may alter human reproductive investment, for which the C elegans may represent an ideal model for elucidation of the implicated mechanisms.
Acknowledgements
This work was financially supported by grants from and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB13000000) and the National Natural Science Foundation of China (31371515, 3150040263, 31671226). This work was also financially supported by the Special Financial Grant awarded to SML from the China Postdoctoral Science Foundation (Grant No: 2014T70137). Wild type C. elegans strain Bristol N2 strain was provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
- 1.Cassada R.C., Russell R.L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 2.Narbonne P., Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 3.Butcher R.A., Fujita M., Schroeder F.C., Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 4.Klass M., Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- 5.P.J. Hu, Dauer. WormBook : the online review of C. elegans biology, 1-19, 2007. [DOI] [PMC free article] [PubMed]
- 6.Ludewig A.H., Schroeder F.C. Ascaroside signaling in C. elegans. WormBook: Online Rev. C. elegans Biol. 2013:1–22. doi: 10.1895/wormbook.1.155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekaran A., Idelchik M.D., Melendez J.A. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91–102. doi: 10.1016/j.redox.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohn A., Weber D., Jung T., Ott C., Hugo M., Kochlik B., Kehm R., Konig J., Grune T., Castro J.P. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porta-de-la-Riva M., Fontrodona L., Villanueva A., Ceron J. Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis. Exp. 2012;e4019 doi: 10.3791/4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karp X. Ascaroside signaling in C. elegans. WormBook: Online Rev. C. elegans Biol. 2016:1–19. [Google Scholar]
- 12.Lam S.M., Wang Y., Duan X., Wenk M.R., Kalaria R.N., Chen C.P., Lai M.K., Shui G. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol. Aging. 2014;35:2369–2381. doi: 10.1016/j.neurobiolaging.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam S.M., Chua G.H., Li X.J., Su B., Shui G. Biological relevance of fatty acyl heterogeneity to the neural membrane dynamics of rhesus macaques during normative aging. Oncotarget. 2016;7:55970–55989. doi: 10.18632/oncotarget.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam S.M., Tong L., Duan X., Petznick A., Wenk M.R., Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014;55:289–298. doi: 10.1194/jlr.M044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam S.M., Tong L., Duan X., Acharya U.R., Tan J.H., Petznick A., Wenk M.R., Shui G. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. J. Lipid Res. 2014;55:1959–1969. doi: 10.1194/jlr.P051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y., Zhu M., Li Z., Sa R., Chu Q., Zhang Q., Zhang H., Tang W., Zhang M., Yin H. Mass spectrometry-based metabolomic profiling identifies alterations in salivary redox status and fatty acid metabolism in response to inflammation and oxidative stress in periodontal disease. Free Radic. Biol. Med. 2014;70:223–232. doi: 10.1016/j.freeradbiomed.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Armando A.M., Quehenberger O., Yan C., Dennis E.A. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J. Chromatogr. A. 2014;1359:60–69. doi: 10.1016/j.chroma.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shui G., Bendt A.K., Jappar I.A., Lim H.M., Laneelle M., Herve M., Via L.E., Chua G.H., Bratschi M.W., Zainul Rahim S.Z., Michelle A.L., Hwang S.H., Lee J.S., Eum S.Y., Kwak H.K., Daffe M., Dartois V., Michel G., Barry C.E., 3rd, Wenk M.R. Mycolic acids as diagnostic markers for tuberculosis case detection in humans and drug efficacy in mice. EMBO Mol. Med. 2012;4:27–37. doi: 10.1002/emmm.201100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Falck J.R., Rothe M., Schunck W.H., Menzel R. Role of CYP eicosanoids in the regulation of pharyngeal pumping and food uptake in Caenorhabditis elegans. J. Lipid Res. 2015;56:2110–2123. doi: 10.1194/jlr.M061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camon E., Magrane M., Barrell D., Binns D., Fleischmann W., Kersey P., Mulder N., Oinn T., Maslen J., Cox A., Apweiler R. The gene ontology annotation (GOA) project: implementation of GO in SWISS-PROT, TrEMBL, and InterPro. Genome Res. 2003;13:662–672. doi: 10.1101/gr.461403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubagawa H.M., Watts J.L., Corrigan C., Edmonds J.W., Sztul E., Browse J., Miller M.A. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell Biol. 2006;8:1143–1148. doi: 10.1038/ncb1476. [DOI] [PubMed] [Google Scholar]
- 22.Edmonds J.W., Prasain J.K., Dorand D., Yang Y., Hoang H.D., Vibbert J., Kubagawa H.M., Miller M.A. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell. 2010;19:858–871. doi: 10.1016/j.devcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulas J., Schmidt C., Rothe M., Schunck W.H., Menzel R. Cytochrome P450-dependent metabolism of eicosapentaenoic acid in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 2008;472:65–75. doi: 10.1016/j.abb.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Jeong P.Y., Kwon M.S., Joo H.J., Paik Y.K. Molecular time-course and the metabolic basis of entry into dauer in Caenorhabditis elegans. PLoS One. 2009;4:e4162. doi: 10.1371/journal.pone.0004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Kim S.K. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- 26.Jones S.J., Riddle D.L., Pouzyrev A.T., Velculescu V.E., Hillier L., Eddy S.R., Stricklin S.L., Baillie D.L., Waterston R., Marra M.A. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Genome Res. 2001;11:1346–1352. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- 27.Lam S.M., Shui G. Lipidomics as a principal tool for advancing biomedical research. J. Genet. Genom. = Yi Chuan Xue Bao. 2013;40:375–390. doi: 10.1016/j.jgg.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Lam S.M., Tian H., Shui G. Lipidomics, en route to accurate quantitation. Biochim. Biophys. Acta. 2017 doi: 10.1016/j.bbalip.2017.02.008. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Lam S.M., Wang Y., Li B., Du J., Shui G. Metabolomics through the lens of precision cardiovascular medicine. J. Genet. Genom. = Yi Chuan Xue Bao. 2017;44:127–138. doi: 10.1016/j.jgg.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Kniazeva M., Crawford Q.T., Seiber M., Wang C.Y., Han M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2004;2:E257. doi: 10.1371/journal.pbio.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H., Shen H., Sewell A.K., Kniazeva M., Han M. A novel sphingolipid-TORC1 pathway critically promotes postembryonic development in Caenorhabditis elegans. eLife. 2013;2:e00429. doi: 10.7554/eLife.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kniazeva M., Euler T., Han M. A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans. Genes Dev. 2008;22:2102–2110. doi: 10.1101/gad.1692008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padilla P.A., Ladage M.L. Suspended animation, diapause and quiescence: arresting the cell cycle in C. elegans. Cell Cycle. 2012;11:1672–1679. doi: 10.4161/cc.19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annous B.A., Becker L.A., Bayles D.O., Labeda D.P., Wilkinson B.J. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan D., Zou Q., Yu T., Song C., Huang S., Chen S., Ren Z., Xu A. Ancestral genetic complexity of arachidonic acid metabolism in Metazoa. Biochim. Biophys. Acta. 2014;1841:1272–1284. doi: 10.1016/j.bbalip.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Hoang H.D., Prasain J.K., Dorand D., Miller M.A. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet. 2013;9:e1003271. doi: 10.1371/journal.pgen.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKnight K., Hoang H.D., Prasain J.K., Brown N., Vibbert J., Hollister K.A., Moore R., Ragains J.R., Reese J., Miller M.A. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science. 2014;344:754–757. doi: 10.1126/science.1250598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benenati G., Penkov S., Muller-Reichert T., Entchev E.V., Kurzchalia T.V. Two cytochrome P450s in Caenorhabditis elegans are essential for the organization of eggshell, correct execution of meiosis and the polarization of embryo. Mech. Dev. 2009;126:382–393. doi: 10.1016/j.mod.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Lynn D.A., Dalton H.M., Sowa J.N., Wang M.C., Soukas A.A., Curran S.P. Omega-3 and -6 fatty acids allocate somatic and germline lipids to ensure fitness during nutrient and oxidative stress in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2015;112:15378–15383. doi: 10.1073/pnas.1514012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow D.K., Glenn C.F., Johnston J.L., Goldberg I.G., Wolkow C.A. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp. Gerontol. 2006;41:252–260. doi: 10.1016/j.exger.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma D.K., Rothe M., Zheng S., Bhatla N., Pender C.L., Menzel R., Horvitz H.R. Cytochrome P450 drives a HIF-regulated behavioral response to reoxygenation by C. elegans. Science. 2013;341:554–558. doi: 10.1126/science.1235753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buczynski M.W., Dumlao D.S., Dennis E.A. Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasquez V., Krieg M., Lockhead D., Goodman M.B. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Rep. 2014;6:70–80. doi: 10.1016/j.celrep.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wassall S.R., Brzustowicz M.R., Shaikh S.R., Cherezov V., Caffrey M., Stillwell W. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem. Phys. Lipids. 2004;132:79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Palrasu M., Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr. Drug Targets. 2017 doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- 46.Csala M., Kardon T., Legeza B., Lizak B., Mandl J., Margittai E., Puskas F., Szaraz P., Szelenyi P., Banhegyi G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta. 2015;1852:826–838. doi: 10.1016/j.bbadis.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Gasparovic A.C., Milkovic L., Sunjic S.B., Zarkovic N. Cancer growth regulation by 4-hydroxynonenal. Free Radic. Biol. Med. 2017 doi: 10.1016/j.freeradbiomed.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 48.Sunjic S.B., Cipak A., Rabuzin F., Wildburger R., Zarkovic N. The influence of 4-hydroxy-2-nonenal on proliferation, differentiation and apoptosis of human osteosarcoma cells. BioFactors. 2005;24:141–148. doi: 10.1002/biof.5520240117. [DOI] [PubMed] [Google Scholar]
- 49.Jorgensen P., Milkovic L., Zarkovic N., Waeg G., Rattan S.I. Lipid peroxidation-derived 4-hydroxynonenal-modified proteins accumulate in human facial skin fibroblasts during ageing in vitro. Biogerontology. 2014;15:105–110. doi: 10.1007/s10522-013-9482-z. [DOI] [PubMed] [Google Scholar]
- 50.Kreuzer T., Zarkovic N., Grube R., Schaur R.J. Inhibition of HeLa cell proliferation by 4-hydroxynonenal is associated with enhanced expression of the c-fos oncogene. Cancer Biotherapy Radiopharm. 1997;12:131–136. doi: 10.1089/cbr.1997.12.131. [DOI] [PubMed] [Google Scholar]
- 51.Cipak A., Jaganjac M., Tehlivets O., Kohlwein S.D., Zarkovic N. Adaptation to oxidative stress induced by polyunsaturated fatty acids in yeast. Biochim. Biophys. Acta. 2008;1781:283–287. doi: 10.1016/j.bbalip.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Wonisch W., Kohlwein S.D., Schaur J., Tatzber F., Guttenberger H., Zarkovic N., Winkler R., Esterbauer H. Treatment of the budding yeast Saccharomyces cerevisiae with the lipid peroxidation product 4-HNE provokes a temporary cell cycle arrest in G1 phase. Free Radic. Biol. Med. 1998;25:682–687. doi: 10.1016/s0891-5849(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 53.Gveric-Ahmetasevic S., Sunjic S.B., Skala H., Andrisic L., Stroser M., Zarkovic K., Skrablin S., Tatzber F., Cipak A., Jaganjac M., Waeg G., Gveric T., Zarkovic N. Oxidative stress in small-for-gestational age (SGA) term newborns and their mothers. Free Radic. Res. 2009;43:376–384. doi: 10.1080/10715760902783285. [DOI] [PubMed] [Google Scholar]