Abstract
Histone deacetylase 6 (HDAC6) likely is important in inflammatory diseases. However, how HDAC6 exerts its effect on inflammatory processes remains unclear. HIV-1 transactivator of transcription (Tat) activates NADPH oxidase resulting in generation of reactive oxygen species (ROS), leading to extensive neuro-inflammation in the central nervous system. We investigated the correlation of HDAC6 and NADPH oxidase in HIV-1 Tat-stimulated astrocytes. HDAC6 knockdown attenuated HIV-1 Tat-induced ROS generation and NADPH oxidase activation. HDAC6 knockdown suppressed HIV-1 Tat-induced expression of NADPH oxidase subunits, such as Nox2, p47phox, and p22phox. Specific inhibition of HDAC6 using tubastatin A suppressed HIV-1 Tat-induced ROS generation and activation of NADPH oxidase. N-acetyl cysteine, diphenyl iodonium, and apocynin suppressed HIV-1 Tat-induced expression of HDAC6 and the pro-inflammatory chemokines CCL2, CXCL8, and CXCL10. Nox2 knockdown attenuated HIV-1 Tat-induced HDAC6 expression and subsequent expression of chemokines. The collective results point to the potential crosstalk between HDAC6 and NADPH oxidase, which could be a combined therapeutic target for relief of HIV-1 Tat-mediated neuro-inflammation.
Abbreviations: Tat, transactivator of transcription; HDAC6, histone deacetylase 6; ROS, reactive oxygen species; Nox2, NADPH oxidase enzyme 2; CNS, central nervous system
Keywords: HIV-1 Tat, HDAC6, NADPH oxidase, ROS, Inflammation, Astrocytes
Graphical abstract

Highlights
-
•
HDAC6 mediates HIV-1 Tat-induced ROS generation in astrocytes.
-
•
HDAC6 is involved in HIV-1 Tat-induced activity and expression of Nox2-based NADPH oxidase.
-
•
Crosstalk between HDAC6 and NADPH oxidase exists in HIV-1 Tat-stimulated astrocytes.
1. Introduction
Human immunodeficiency virus-1 (HIV-1) infection in the central nervous system (CNS) induces chronic inflammatory responses that may contribute to the development of neurological dysfunction, which are collectively known as HIV-associated neurocognitive disorders (HAND) [1]. Infiltration of immune cells into the CNS is one of characteristic features of neuro-inflammation that is accelerated by dysregulation of pro-inflammatory mediators including cytokines, chemokines, and adhesion molecules [2], [3], [4]. HIV-1 transactivator of transcription (Tat) released from HIV-infected cells contributes to the infiltration of immune cells by up-regulating pro-inflammatory mediators including chemokines (reviewed in [5]). Upon stimulation with HIV-1 Tat, astrocytes secrete various pro-inflammatory chemokines that include CC chemokine ligand 2 (CCL2; monocyte chemoattractant protein-1), CXC chemokine ligand 8 (CXCL8; IL-8), and CXCL10 (interferon-gamma-induced protein-10) [6], [7], as well as adhesion molecules, such as VCAM-1 and ICAM-1 [8], [9].
Reactive oxygen species (ROS) play an important role during inflammatory response. Elevated levels of ROS activate redox-sensitive signal transduction pathways, such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1), resulting in expression of various pro-inflammatory cytokines/chemokines and adhesion molecules [10]. NADPH oxidase is a potential cellular source of ROS; it is a multi-complex enzyme consisting of membrane-bound subunits (gp91phox/Nox2 and p22phox) and cytosolic subunits (p40phox, p47phox, p67phox, small GTPase Rac) [11]. Homologues (Nox1~Nox5) of gp91phox/Nox2 subunits have been reported in several cell types (for review, see [12]). NADPH oxidase catalyzes NADPH-dependent reduction of oxygen to form superoxide.
HIV-1 Tat activates NADPH oxidase leading to ROS generation, resulting in expression of pro-inflammatory mediators like cytokines, chemokines, and adhesion molecules. HIV-1 Tat induces ROS generation mediated by Nox2-based NADPH oxidase in astrocytes [8] and Nox2/Nox4-based NADPH oxidase in microglia [13]. Inhibition or knockdown of NADPH oxidase suppresses HIV-1 Tat-induced expression of various pro-inflammatory mediators, including adhesion molecules and cytokines/chemokines [8].
Histone deacetylase 6 (HDAC6) is a member of the class IIb HDACs. HDAC6 consists of two homologous catalytic domains and a C-terminal ubiquitin-binding zinc-finger domain [14], [15]. HDAC6 is found mainly in the cytoplasm due to the presence of nuclear export signals and a cytoplasm-anchoring domain [16]. Identified substrates of HDAC6 include histones, α-tubulin, cortactin, peroxiredoxin (Prx), and heat shock protein 90 (HSP90) [14], [15], [17]. The varied substrates suggest that HDAC6 exerts its activity on various cellular processes. Consistent with this view, dysregulation of HDAC6 contributes to the development of various diseases, such as cancers, neurological disorders, and inflammatory diseases (reviewed in [17], [18], [19]).
Accumulating evidence supports the view that HDAC6 is an important regulator of the immune response to bacterial and viral infection [20], [21], [22]. HDAC6 mediates immune responses induced by various microbial products, such as Clostridium difficile toxin A, lipopolysaccharide, and HIV-1 Tat [23], [24], [25], [26]. HDAC6 has been suggested to be a master regulator of the expression of pro-inflammatory mediators by regulating ROS-mitogen-activated protein kinase (MAPK)-NF-κB/AP-1 pathways in macrophages [27].
Previously, we reported that HIV-1 Tat augments HDAC6 expression, which mediates the expression of pro-inflammatory genes by regulating MAPK-NF-κB/AP-1 pathways in astrocytes [26]. In addition, HIV-1 Tat induces ROS generation by Nox2-based NADPH oxidase [8]. However, the molecular correlation of HDAC6 and NADPH oxidase in the HIV-1 Tat-stimulated astrocytes has not been examined.
In this study, we observed that pharmacological inhibition and knockdown of HDAC6 significantly attenuated HIV-1 Tat-induced ROS generation and activation of NADPH oxidase. The results also reveal regulatory crosstalk between HDAC6 and NADPH oxidase that is involved in mediating HIV-1 Tat-induced expression of pro-inflammatory mediators.
2. Materials and methods
2.1. Cell culture
CRT-MG human astroglial cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), penicillin G (100 U/ml), streptomycin (100 μg/ml), and L-glutamine (2 mM) at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air [8]. All animal experiments were approved by the Animal Care and Use Committee of Hallym University (Hallym 2015-60). Primary astrocyte cultures were obtained from the cerebral cortex of 1- to 2-day-old ICR mice as described previously [26]. Briefly, the cortex derived from whole brain was cut into small pieces and incubated with 0.05% trypsin- EDTA for 5 min at 37 °C. Next, the tissue was dissociated in a cell suspension by triturating through a Pasteur pipette in Dulbecco Modified Eagle Medium (DMEM) containing 10% FBS and antibiotics. The dissociated cells were seeded in 75 cm2 culture flasks (Falcon, Franklin, NJ, USA). Next day and every 3 days thereafter, the culture medium was replaced with fresh medium. After 6–7 days, microglia and oligodendrocytes were removed from astrocytes by mechanical dislodgment. Astrocyte-enriched cultures were seeded in 6-well culture plates. More than 95% of the astrocyte-enriched cultures were glial fibrillary acidic protein positive, as monitored by immunofluorescent staining.
2.2. Reagents
N-acetyl cysteine (NAC), diphenylene iodonium (DPI), apocynin (APO), and tubastatin A (TBA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dihydroethidium (DHE) was purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Primary antibodies against gp91phox/NOX2, p47phox, acetylated α-tubulin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and those against HDAC6 and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Lipofectamine 3000 transfection reagent was purchased from Invitrogen (Carlsbad, CA, USA). A luciferase assay kit was purchased from Promega (Madison, WI, USA). Oligonucleotide primers (HDAC6, CCL2, CXCL8, CXCL10, Nox2, p22phox, p47phox, and β-actin) and HDAC6 siRNA were obtained from Bioneer (Seoul, Korea).
2.3. Preparation of recombinant HIV-1 Tat protein
Recombinant HIV-1 Tat protein was prepared from the glutathione S-transferase-Tat fusion protein expressed from the GST-Tat 1 86 R plasmid obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Rockville, MD, USA) as described previously [28], [29]. Endotoxin levels for the HIV-1 Tat preparation were <0.3 EU/ml as tested using a Limulus Amoebocyte Lysate assay (BioWhitaker, Walkersville, MD, USA). The integrity and purity of the HIV-1 Tat proteins were assessed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie Blue staining. The concentration of HIV-1 Tat tested in this study is consistent with the data previously reported [30], [31], [32], [33].
2.4. Western blot analysis
Cells were incubated in lysis buffer (125 mM Tris-HCl pH 6.8, 2% SDS, 10% v/v glycerol) at 4 °C for 30 min. Cell lysates were obtained by centrifugation at 12,000×g for 15 min. Thirty micrograms of proteins were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The blots were probed with the indicated primary antibodies followed by incubation with the corresponding horseradish peroxidase-conjugated secondary antibodies. The immune-reactive bands were visualized by a chemiluminescence system (Amersham Life Sciences, Parsippany, NJ, USA) [27].
2.5. Real-time reverse transcription-polymerase chain reaction (RT-PCR) Analysis
Total RNA was prepared from cells using a TRIzol reagent kit (Invitrogen) according to the manufacturer's instructions. Two micrograms of total RNA were reverse-transcribed to cDNA using 10,000 U of reverse transcriptase and 0.5 μg/μl oligo-(dT)15 primer (Promega) [26]. The resulting cDNA was analyzed by quantitative PCR. Twenty ng of cDNA and 150 nM of each primer were mixed with iQ™ SYBR® Green Supermix (BIO-RAD, Hercules, CA, USA). Reactions were carried out in a 96-well format using an CFX Connect™ Real-Time PCR Detection System (BIO-RAD). Relative mRNA levels were evaluated using the comparative CT method and normalized to β-actin mRNA. The following set of primers (5′→3′) were used: human CCL2 sense, TGC AGA GGC TCG CGA GCT A; human CCL2 anti-sense, CAG GTG GTC CAT GGA ATC CTG A; human CXCL8 sense, GAG AGT GAT TGA GAG TGG AC; human CXCL8 anti-sense, AGA CAG AGC TCT CTT CCA TC; human CXCL10 sense, CTA GAA CTG TAC GCT GTA CC; human CXCL10 antisense, GAC ATC TCT TCT CAC CCT TC; human NOX2 sense AAG GCT TCA GGT CCA CAG AGG AAA; human NOX2 antisense, AGA CTT TGT ATG GAC GGC CCA ACT; human p47phox sense, TGA CTT TTG CAG GTA CAT GG; human p47phox antisense, TGA CTT TTG CAG GTA CAT GG; human p22phox sense, AGT GGT ACT TTG GTG CCT ACT C; human p22phox antisense, ACG GCG GTC ATG TAC TTC TG; human HDAC6 sense, CAA CTG AGA CCG TGG AGA G; human HDAC6 antisense, CCT GTG CGA GAC TGT AGC; human β-actin sense, TGA AGT GTG ACG TTG ACA TCC; and human β-actin antisense, GCC AGA GCA GTA ATC TCC TT; mouse CCL2 sense, CTT CTG GGC CTG CTG TTC A; mouse CCL2 anti-sense, CTTCTGGGCCTGCTGTTCA; mouse CXCL8 sense, CTC TTG GCA GCC TTC CTG ATT; mouse CXCL8 anti-sense, TAT GCA CTG ACA TCT AAG TTC TTT AGC A; mouse CXCL10 sense, AAG TGC TGC CGT CAT TTT CT; mouse CXCL10 antisense, GTG GCA ATG ATC TCA ACA CG; mouse NOX2 sense CCC TTT GGT ACA GCC AGT GAA GAT; mouse NOX2 antisense, CAA TCC CGC CTC CCA CTA ACA TCA; mouse p47phox sense, TGG ACT TCT TCA AAG TGC GG; mouse p47phox antisense, CCA ACC TCG CTT TGT CTT CA; mouse p22phox sense, TGG CTA CTG CTG GAC GTT TC; mouse p22phox antisense, TTC TGT CCA CAT CGC TCC AT; mouse HDAC6 sense, TTT CCC TTC TGA GGC CAC AG; mouse HDAC6 antisense, TCC TTC TGG GTA GAA CAG AG; mouse β-actin sense, AGT GTG ACG TTG ACA TCC GTA AAG A; and mouse β-actin antisense, GGA CAG TGA GGC CAG GAT GG.
2.6. Assessment of intracellular ROS generation
The levels of intracellular ROS, such as hydrogen peroxide (H2O2) and superoxide (O2-) were estimated by a 2′,7′ –dichlorofluorescin diacetate (DCF-DA) and dihydroethidium (DHE) assay as described previously [27], [34]. DCF-DA is oxidized by intracellular ROS and forms green fluorescent DCF. DHE is converted by superoxide into red fluorescent ethidium. The cellular fluorescent intensity for DCF was measured at 485 nm excitation and 538 nm emission using a SpectraMax M2 ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA). The fluorescence images for DHE were obtained at 590 nm excitation and 650 nm emission using an ECILIPSE 80i fluorescence microscope (Nikon, Tokyo, Japan).
2.7. Measurement of NADPH oxidase activity
The stimulated cells were scraped off and centrifuged at 1500g for 5 min at 4 °C, and re-suspended in phosphate buffered saline (PBS). NADPH oxidase activity was analyzed as described previously [8]. Briefly, cells were incubated with 250 μM NADPH and NADPH consumption was assessed by monitoring the decrease in absorbance at λ=340 for 10 min. An aliquot of cells was lysed with 2% SDS and the protein content of cell lysates was estimated. The absorption extinction coefficient used to calculate the amount of NADPH consumed was 6.22 mM −1 cm−1. Results are expressed as pmol of substrate/minute/mg of protein.
2.8. Detection of p47phox association with gp91phox
To evaluate the levels of p47phox associated with gp91phox, co-immunoprecipitation followed by Western blot analysis was performed as described previously [27]. Cellular lysates were prepared by incubating cells in a lysis buffer consisting of 50 mM HEPES (pH 7.5), 150 mM NaCl, 2 mM EDTA, 0.5% NP-40, 5% glycerol, and complete mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The cellular lysates (500 μg of protein) were incubated with anti-gp91phox antibody overnight at 4 °C on a rotating shaker. Immune complexes were recovered using protein A/G–agarose beads (Santa Cruz Biotechnology). Aliquots of immunoprecipitates were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The level of p47phox was determined by Western blot analysis using an anti-p47phox antibody.
2.9. Enzyme-linked immunosorbent assay (ELISA)
CRT-MG cells were stimulated with HIV-1 Tat (20 nM) for 24 h. The concentrations of CCL2, CXCL8, and CXCL10 chemokines in the culture media were determined using ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions.
2.10. Transient transfection
Transfection of cells with control or HDAC6 specific siRNA was performed using the Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer's protocol.
2.11. Statistical analyses
Results are expressed as the mean±standard deviation (SD) from at least three independent experiments. Statistical analysis was carried out by one-way analysis of variance, followed by Bonferroni's test using SigmaPlot 10.0 software (SYSTAT Software Inc, Chicago, IL, USA). Differences were considered significant at p<0.05.
3. Results
3.1. Effects of HDAC6 knockdown on HIV-1 Tat-induced ROS generation in astrocytes
HIV-1 Tat induces ROS generation by activation of Nox2-based NADPH oxidase, which mediates the production of pro-inflammatory mediators in endothelial cells, microglia, and astrocytes [8], [33], [35], [36]. We recently reported that HIV-1 Tat increases HDAC6 expression, which regulates MAPK-NF-κB/AP-1 signaling pathways. This results in the production of pro-inflammatory mediators in astrocytes [26]. Presently, we first investigated the effect of HDAC6 knockdown on HIV-1 Tat-induced ROS production in astrocytes. CRT-MG cells and primary mouse astrocytes were transfected with HDAC6 siRNA and stimulated with HIV-1 Tat. After 1 h, cells were stained with DCF-DA (Fig. 1A and C) and DHE (Fig. 1B and D). HDAC6 knockdown significantly inhibited HIV-1 Tat-induced increase of DCF-DA fluorescent signal in CRT-MG cells (Fig. 1A) and primary mouse astrocytes (Fig. 1C), indicating that HDAC6 regulates HIV-1 Tat-induced redox status in astrocytes. In addition, HDAC6 knockdown significantly suppressed HIV-1 Tat-induced increase of intracellular ROS levels in CRT-MG cells (Fig. 1B) and primary mouse astrocytes (Fig. 1D), as judged by DHE staining, suggesting that HDAC6 contributes to HIV-1 Tat-induced ROS production in astrocytes.
Fig. 1.
Effects of HDAC6 knockdown on HIV-1 Tat-induced ROS generation in astrocytes. CRT-MG cells (A, B) and mouse primary astrocytes (C, D) were transfected with control siRNA or HDAC6 siRNA for 48 h, followed by stimulation with HIV-1 Tat (20 nM) for 1 h. Transfected cells were stained with DCF-DA (A, C) or DHE (B, D) for 30 min. The levels of intracellular ROS probed with DCF-DA were measured using an ELISA plate reader (A, C). Data are presented as mean±SD of three independent experiments (***p<0.001, as compared with the cells treated with HIV-1 Tat after control siRNA transfection). Microphotographs of ROS levels in the transfected cells were obtained by fluorescence microscopy after DHE staining (original magnification, ×200) (B, D).
3.2. Effects of HDAC6 knockdown on HIV-1 Tat-induced NADPH oxidase activity in astrocytes
NADPH oxidase is one of the enzymatic sources of ROS production in microglia and astrocytes [37]. We previously reported that HIV-1 Tat induces ROS generation by Nox2-base NADPH oxidase in astrocytes [8]. We examined the effect of HDAC6 knockdown on HIV-1 Tat-induced NADPH oxidase activity in astrocytes. HDAC6 knockdown significantly suppressed HIV-1 Tat-induced NADPH oxidase activity in CRT-MG cells (Fig. 2A). During activation of NADPH oxidase, the p47phox cytosolic component translocates to the plasma membrane and associates with gp91phox/Nox2. A co-immunoprecipitation experiment was done to analyze the effect of HDAC6 knockdown on association of p47phox with gp91phox/Nox2 in HIV-1 Tat-stimulated CRT-MG cells. HDAC6 knockdown decreased the level of p47phox associated with gp91phox/Nox2 (Fig. 2C). Similarly, HDAC6 knockdown decreased HIV-1 Tat-induced NADPH oxidase activity and the level of p47phox associated with gp91phox/Nox2 in HIV-1 Tat-stimulated primary mouse astrocytes (Fig. 2B and D). These results suggest that HDAC6 is involved in HIV-1 Tat-induced ROS production by regulating Nox2-based NADPH oxidase in astrocytes.
Fig. 2.
Effects of HDAC6 knockdown on HIV-1 Tat-induced NADPH oxidase activity in astrocytes. CRT-MG cells (A, C) and mouse primary astrocytes (B, D) were transfected with control siRNA or HDAC6 siRNA for 48 h, followed by stimulation with HIV-1 Tat (20 nM) for 1 h. (A, B) The transfected cells were harvested and incubated with 250 µM NADPH. NADPH consumption was monitored by the decrease in absorbance at λ=340 for 10 min. Data are presented as mean±SD of three independent experiments **p<0.01, as compared with the cells treated with HIV-1 Tat after control siRNA transfection. (C, D) Cell lysates were prepared from cells and immune-precipitated with an antibody against gp91phox/NOX2, followed by Western blotting using a p47phox antibody to determine the levels of p47phox associated with gp91phox/NOX2.
3.3. Effects of HDAC6 knockdown on HIV-1 Tat-induced expression of NADPH oxidase subunits in astrocytes
The expression of NADPH oxidase subunits, such as gp91phox/Nox2, p47phox, and p22phox, are up-regulated upon various stimuli [38], [39], [40]. Under our experimental conditions, HIV-1 Tat up-regulated gp91phox/Nox2, p47phox, and p22phox mRNA by 3.1-fold, 2.2-fold, and 2.5 fold, respectively, in CRT-MG cells (Fig. 3A), and 4-fold, 2.8-fold, and 2.7 fold, respectively, in primary mouse astrocytes (Fig. 3B). The effect of HDAC6 knockdown on mRNA levels of NADPH oxidase subunits was assessed by quantitative RT-PCR in HIV-1 Tat-stimulated cells. HDAC6 knockdown significantly decreased HIV-1 Tat-induced mRNA expression of gp91phox/Nox2, p47phox, and p22phox in CRT-MG cells and primary mouse astrocytes (Fig. 3A and B). HDAC6 knockdown significantly decreased HIV-1 Tat-induced protein levels of gp91phox/Nox2 and p47phox in both cell types (Fig. 3C and D). These results suggest that HDAC6 mediates up-regulation of the expression of Nox2-based NADPH oxidase subunits in HIV-1 Tat-stimulated astrocytes.
Fig. 3.
Effects of HDAC6 knockdown on HIV-1 Tat-induced expression of NADPH oxidase subunits in astrocytes. CRT-MG cells (A, C) and mouse primary astrocytes (B, D) were transfected with control siRNA or HDAC6 siRNA for 48 h, followed by stimulation with HIV-1 Tat (20 nM) for 3 h (mRNA) or 12 h (protein). (A, B) Total RNA was extracted from cells and analyzed for mRNA of gp91phox/NOX2, p47phox, p22phox, and β-actin by quantitative RT-PCR using specific primers. Data are presented as mean±SD of three independent experiments **p<0.01, ***p<0.001, ++p<0.01, ^p<0.05, ^^^p<0.001 as compared with the cells treated with HIV-1 Tat after control siRNA transfection. (C, D) Total cell lysate was extracted from cells and analyzed for protein of gp91phox/NOX2, p47phox, p22phox, and β-actin by Western blotting using specific antibodies.
3.4. Effects of HDAC6 inhibitor on HIV-1 Tat-induced ROS generation and activity and expression of NADPH oxidase in astrocytes
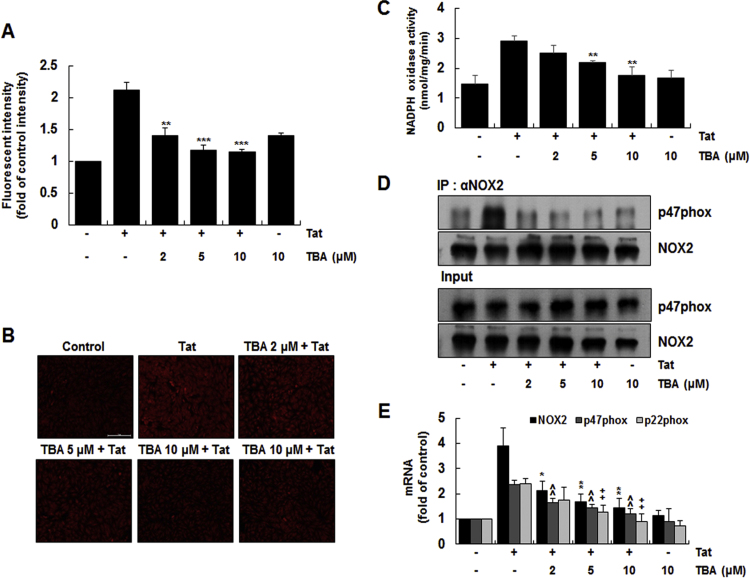
We next investigated the effect of HDAC6 inhibitor on HIV-1 Tat-induced ROS generation, and NADPH oxidase activity and expression in CRT-MG cells. We used tubastatin A (TBA), which is a selective inhibitor of HDAC6 [41], to assess the effect of TBA on HIV-1 Tat-induced ROS production in CRT-MG cells. Cells pretreated with various doses of TBA for 1 h were stimulated with 20 nM HIV-1 Tat for 1 h, and the levels of intracellular ROS was assessed by DCF-DA and DHE staining. TBA significantly inhibited HIV-1 Tat-induced increase of intracellular ROS levels in a dose-dependent manner in CRT-MG cells (Fig. 4A and B). We next examined the effect of TBA on HIV-1 Tat-induced NADPH oxidase activity in CRT-MG. TBA significantly inhibited HIV-1 Tat-induced NADPH oxidase activity in CRT-MG cells (Fig. 4C). In addition, TBA decreased the level of p47phox associated with gp91phox/Nox2 in a dose-dependent manner in a co-immunoprecipitation experiment (Fig. 4D). The effect of TBA on HDAC6-induced expression of NADPH oxidase subunits in CRT-MG cells was determined. TBA efficiently decreased HIV-1 Tat-induced mRNA expression of gp91phox/Nox2, p47phox and p22phox in a dose-dependent manner (Fig. 4E). Taken together, these results suggest that HDAC6 enzymatic activity is required for ROS production as well as activity and expression of NADPH oxidase in astrocytes.
Fig. 4.
Effects of HDAC6 inhibitor on HIV-1 Tat-induced ROS generation and NADPH oxidase activation in astrocytes. Cells were pretreated with tubastatin A (TBA) at the indicated concentrations for 1 h and then stimulated with HIV-1 Tat (20 nM) for 1 h. (A) Cells were stained with DCF-DA for 30 min and intracellular ROS levels were measured using an ELISA plate reader. (B) Microphotographs of superoxide levels in cells were obtained by fluorescence microscopy after DHE staining (original magnification, ×200; scale bar=100 µm). (C) To measure the NADPH oxidase activity, the treated cells were harvested and incubated with 250 µM NADPH. NADPH consumption was monitored by the decrease in absorbance at λ=340 for 10 min. Data are presented as mean±SD of three independent experiments (**p<0.01, as compared with the cells treated with HIV-1 Tat alone). (D) To evaluate the levels of p47phox associated with gp91phox, cell lysates were immune-precipitated with an antibody against gp91phox, followed by Western blotting using a p47phox antibody. (E) Total RNA was analyzed for the mRNA levels of gp91phox, p47phox, p22phox, and β-actin by RT-qPCR using specific primers. Data are presented as means±SD of three independent experiments *p<0.05, **p<0.01, ^^p<0.01, ++p<0.01 as compared with the cells treated with HIV-1 Tat alone.
3.5. HIV-1 Tat-induced expression of chemokines via a positive feedback loop between HDAC6, NADPH oxidase and ROS in astrocytes
The possibility of cross-talk between HDAC6 and NADPH oxidase expression/ROS generation was addressed. We first analyzed the effect of N-acetyl cysteine (NAC), diphenylene iodonium (DPI), and apocynin on HIV-1 Tat-induced expression of HDAC6 in CRT-MG cells. All three compounds significantly inhibited HIV-1 Tat-induced expression of HDAC6 mRNA (Fig. 5A). Similarly, each compound significantly reduced HIV-1 Tat-induced HDAC6 expression with concomitant recovery of a reduced level of acetylated α-tubulin (Fig. 5B). NAC, DPI, and apocynin significantly reduced HIV-1 Tat-induced mRNA expression (Fig. 5A) and production (Fig. 5C) of CCL2, CXCL8, and CXCL10 chemokines. These results suggest that NADPH oxidase activity and ROS generation regulate HDAC6 expression in HIV-1 Tat stimulated astrocytes. To address the involvement of ROS in HDAC6 expression, we determined the direct effect of ROS on HDAC6 expression using hydrogen peroxide. Hydrogen peroxide increased HDAC6 mRNA expression in dose- and time-dependent manners (Fig. 5D), suggesting an interplay of NADPH oxidase and ROS with HDAC6.
Fig. 5.
Effects of ROS and NADPH oxidase inhibitors on HIV-1 Tat-induced expression of HDAC6 and chemokines in astrocytes. CRT-MG cells were pre-treated with the NAC, DPI, or apocynin (Apo) for 1 h and then stimulated with HIV-1 Tat (20 nM) for 3 h (mRNA), 12 h (protein), or 24 h (ELISA). (A) Total RNA was extracted from cells and analyzed for the mRNA levels of HDAC6, NOX2, CCL2, CXCL8, CXCL10, and β-actin by RT-qPCR using specific primers. Data are presented as means±SD of three independent experiments. **p<0.01, ***p<0.001, ^^p<0.01, ^^^p<0.001, ++p<0.01, +++p<0.001, as compared with the cells treated with HIV-1 Tat alone. (B) Total cell lysate was extracted from cells and analyzed for the levels of HDAC6, acetylated α-tubulin, and β-actin by Western blotting using specific antibodies. (C) Culture supernatants of cells were harvested and analyzed for production of CCL2, CXCL8, and CXCL10 by ELISA. Data are presented as mean±SD of three independent experiments. ***p<0.001, ^^^p<0.001, +++p<0.001, as compared with the cells treated with HIV-1 Tat alone. (D) Cells were treated with hydrogen peroxide at the indicated concentrations for 3 h (mRNA) or 12 h (protein). Total RNA or cell lysate was prepared from cells and analyzed for the levels of HDAC6 mRNA by RT-qPCR (upper panel) or HDAC6, acetylated α-tubulin, and β-actin by Western blotting using specific antibodies (low panel), respectively. Data are presented as mean±SD of three independent experiments. **p<0.01, ***p<0.001, as compared with the control cells.
To further investigate the effect of NADPH oxidase on the HIV-1 Tat-induced HDAC6 expression, a Nox2 knockdown experiment was done using siRNA in CRT-MG cells. Nox2 siRNA efficiently inhibited mRNA (Fig. 6A) and protein (Fig. 6B) expression of HDAC6, with concomitant increase of acetylated α-tubulin (Fig. 6B). In addition, Nox2 siRNA significantly suppressed HIV-1 Tat-induced mRNA (Fig. 6A) and protein (Fig. 6C) expression of CCL2, CXCL8, and CXCL10. Together, these results suggest that HIV-1 Tat induces the expression of chemokines via a positive feedback loop between HDAC6, NADPH oxidase, and ROS in astrocytes (Fig. 7).
Fig. 6.
Effects of NOX2 knockdown on the HIV-1 Tat-induced expression of HDAC6 and chemokines in astrocytes. CRT-MG cells were transfected with control siRNA or NOX2 siRNA for 48 h, followed by stimulation with HIV-1 Tat (20 nM) for 3 h (mRNA), 12 h (protein), or 24 h (ELISA). (A) Total RNA was extracted from cells and analyzed for the mRNA levels of HDAC6, NOX2, CCL2, CXCL8, CXCL10, and β-actin by RT-qPCR using specific primers. Data are presented as mean±SD of three independent experiments. ***p<0.001, ^^p<0.01, ^^^p<0.001, +++p<0.001, as compared with the cells treated with HIV-1 Tat after control siRNA transfection. (B) Total cell lysate was extracted from cells and analyzed for the levels of HDAC6, acetylated α-tubulin, NOX2, and β-actin by Western blotting using specific antibodies. (C) Culture supernatants of cells were harvested and analyzed for production of CCL2, CXCL8, and CXCL10 by ELISA. Data are presented as mean±SD of three independent experiments. **p<0.01, ***p<0.001, as compared with the cells treated with HIV-1 Tat after control siRNA transfection.
Fig. 7.
Schematic diagram showing crosstalk between HDAC6 and NADPH oxidase in HIV-1 Tat-induced inflammatory response in astrocytes. HIV-1 Tat induces the expression of HDAC6 that activates Nox2-dependent NADPH oxidase leading to ROS generation, resulting in expression of pro-inflammatory chemokines, such as CCL2, CXCL8, and CXCL10, in astrocytes. NADPH oxidase-derived ROS can affect HDAC6 expression, supporting the evidence of a positive feedback circuit via HDAC6/NADPH oxidase/ROS axis in HIV-1 Tat-induced inflammatory response.
4. Discussion
HIV-1 Tat contributes to extensive neuro-inflammation via ROS production that mediates the up-regulation of various pro-inflammatory mediators including cytokines, chemokines, and adhesion molecules. However, the molecular mechanisms of HIV-1 Tat-induced expression of pro-inflammatory mediators in the CNS remain unclear. In this study, we show that HDAC6 mediates HIV-1 Tat-induced ROS generation by regulating the activity and expression of Nox2-based NADPH oxidase in astrocytes. We further establish that crosstalk between HDAC6 and NADPH oxidase exists in HIV-1 Tat-stimulated astrocytes.
Stimulation of astrocytes with HIV-1 Tat induces the excessive ROS generation that activates intracellular signaling pathways, such as NF-κB and AP-1, resulting in expression of pro-inflammatory mediators including CCL2, CXCL8, and CXCL10 chemokines [8]. Blockage of ROS production with selected antioxidants inhibits HIV-1 Tat-induced expression of pro-inflammatory mediators [42]. We recently reported that HDAC6 is a master regulator in expressing pro-inflammatory mediators by regulating ROS-dependent pathways in macrophages [27]. Overexpression of HDAC6 can significantly induce ROS generation in macrophages. We also reported that HIV-1 Tat augments the expression of HDAC6, which is involved in the expression of pro-inflammatory genes, by regulating the MAPK-NF-κB/AP-1 pathways in astrocytes [26]. Although HDAC6 was demonstrated to mediate immune responses induced by HIV-1 Tat, it is not clear whether HDAC6 plays a direct role in HIV-1 Tat-induced ROS generation in astrocytes. In this study, we observed that genetic knockdown and pharmacological inhibition of HDAC6 suppressed HIV-1 Tat-induced ROS production and subsequent expression of pro-inflammatory CCL2, CXCL8, and CXCL10 chemokines. These results suggest that HDAC6 plays a role in HIV-1 Tat-induced ROS production in astrocytes.
NADPH oxidase is a primary cellular source of ROS generation that leads to activation of various signal transduction processes in astrocytes [43], [44]. Different Nox families including Nox1-5 and Duox1/2 play a role in ROS generation after various routes of stimulation in several cell types [44]. Previous studies have demonstrated that Nox2-based NADPH oxidase was responsible for HIV-1 Tat-induced ROS generation in astrocytes [8]. HIV-1 Tat increases expression of chemokines like CXCL10 via Nox-2-based NADPH oxidase in interferon-gamma and tumor necrosis factor-alpha stimulated human astrocytes [33]. Nox2-based NADPH oxidase consists of plasma membrane-bound subunits (gp91phox/Nox2 and p22phox) and cytosolic subunits (p40phox, p47phox, p67phox, small GTPase Rac1) [11]. Activation of the NADPH oxidase requires the translocation of the cytosolic component p47phox to the plasma membrane and the subsequent association of p47phox with gp91phox/Nox2, leading to the generation of superoxide [45]. We recently reported that HDAC6 is capable of regulating the activity and expression of Nox2-based NADPH oxidase in macrophages [27].
Presently, knockdown of HDAC6 using siRNA inhibited HIV-1 Tat-induced enzymatic activity of NADPH oxidase in CRT-MG cells and primary mouse astrocytes. In addition, HDAC6 knockdown decreased the level of HIV-1 Tat-induced p47phox association with gp91phox/Nox2. We also observed that HIV-1 Tat up-regulated the expression of NADPH oxidase subunits, such as gp91phox/Nox2, p47phox, and p22phox, in CRT-MG cells and primary mouse astrocytes. HDAC6 knockdown significantly decreased HIV-1 Tat-induced expression of gp91phox/Nox2, p47phox, and p22phox, suggesting a possible role of HDAC6 in up-regulation of Nox2-based NADPH oxidase subunits in HIV-1 Tat-stimulated astrocytes.
TBA is a specific inhibitor of HDAC6. We observed that TBA significantly inhibited HIV-1 Tat-induced ROS generation as well as activity and expression of Nox2-based NADPH oxidase. These results indicate that HDAC6 enzymatic activity is required for HIV-1 Tat-induced activation of NADPH oxidase and subsequent ROS production in astrocytes. Along with its deacetylase activity, HDAC6 possesses several functional domains including an ubiquitin binding domain [14], [15]. We cannot rule out the possible contribution of other domains of HDAC6 in ROS generation. Taken together, these results suggest that HDAC6 is involved in HIV-1 Tat-induced ROS production by regulating Nox2-based NADPH oxidase in astrocytes.
Along with supporting data that HDAC6 is involved in HIV-1 Tat-induced ROS generation and activation of NADPH oxidase, we found that ROS and NADPH oxidase can affect HDAC6 expression vice versa. NAC, a ROS scavenger antioxidant, efficiently suppressed HIV-1 Tat-induced HDAC6 expression, as evidenced by the concomitant increase of acetylated α-tubulin. DPI and apocynin are NADPH oxidase inhibitors. Both also efficiently suppressed HIV-1 Tat-induced HDAC6 expression. Antioxidant and NADPH oxidase inhibitors significantly suppressed HIV-1 Tat-induced expression of the CCL2, CXCL8, and CXCL10 chemokines. In this study, we found that hydrogen peroxide increased HDAC6 expression with concomitant decrease of acetylated α-tubulin, further supporting the notion that NADPH oxidase-derived ROS can affect HDAC6 expression. We also observed that Nox2 knockdown decreased HIV-1 Tat-induced HDAC6 expression and subsequent expression of the CCL2, CXCL8, and CXCL10 chemokines. Thus, these results provide the first evidence that HIV-1 Tat induces expression of chemokines via a positive feedback loop between HDAC6, NADPH oxidase, and ROS in astrocytes. Although knockdown or inhibitors of HDAC6 regulate activity and expression of NADPH oxidase, it is not clear whether HDAC6 directly or indirectly regulates NADPH oxidase. Further studies are required for identifying the mechanism by which HDAC6 affect NADPH oxidase at the molecular level. We provided evidence that hydrogen peroxide increased HDAC6 expression at the transcriptional level, further studies are needed to elucidate upstream mediators in hydrogen peroxide-induced HDAC6 expression.
In conclusion, these results provide evidence that HDAC6 mediates the HIV-1 Tat-induced expression of chemokines by regulating ROS-Nox2-based NADPH oxidase pathways in astrocytes. Furthermore, there is crosstalk between HDAC6 and NADPH oxidase in HIV-1 Tat-induced chemokine expression in astrocytes, which reveals potential therapeutic targets to combat HIV-1 Tat-associated neuro-inflammation.
Acknowledgments
This research was supported by a Basic Science Research Program Grant (2015R1D1A1A01060275) and by a Priority Research Centers Program Grant (2009–0093812) through the National Research Foundation of Korea, funded by the Ministry of Education. This work was also supported by a grant (HRF-201607-010) from Hallym University.
References
- 1.Rumbaugh J.A., Nath A. Developments in HIV neuropathogenesis. Curr. Pharm. Des. 2006;12:1023–1044. doi: 10.2174/138161206776055877. [DOI] [PubMed] [Google Scholar]
- 2.Carpentier P.A., Begolka W.S., Olson J.K., Elhofy A., Karpus W.J., Miller S.D. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 3.Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J. Biosci. 2005;30:391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport J., Joseph J., Croul S., Alexander G., Del, Valle L., Amini S., Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J. Leukoc. Biol. 1999;65:458–465. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- 5.Bagashev A., Sawaya B.E. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol. J. 2013;10:358. doi: 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irish B.P., Khan Z.K., Jain P., Nonnemacher M.R., Pirrone V., Rahman S., Rajagopalan N., Suchitra J.B., Mostoller K., Wigdahl B. Molecular mechanisms of neurodegenerative diseases induced by human retroviruses: a review. Am. J. Infect. Dis. 2009;5:231–258. doi: 10.3844/ajidsp.2009.231.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao H., Bethel-Brown C., Li C.Z., Buch S.J. HIV neuropathogenesis: a tight rope walk of innate immunity. J. Neuroimmune Pharmacol. 2010;5:489–495. doi: 10.1007/s11481-010-9211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H.Y., Ju S.M., Seo W.Y., Goh A.R., Lee J.K., Bae Y.S., Choi S.Y., Park J. Nox2-based NADPH oxidase mediates HIV-1 Tat-induced up-regulation of VCAM-1/ICAM-1 and subsequent monocyte adhesion in human astrocytes. Free Radic. Biol. Med. 2011;50:576–584. doi: 10.1016/j.freeradbiomed.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Woodman S.E., Benveniste E.N., Nath A., Berman J.W. Human immunodeficiency virus type 1 TAT protein induces adhesion molecule expression in astrocytes. J. Neurovirol. 1999;5:678–684. doi: 10.3109/13550289909021296. [DOI] [PubMed] [Google Scholar]
- 10.Gloire G., Legrand-Poels S., Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Lambeth J.D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadhav V.S., Krause K.H., Singh S.K. HIV-1 Tat C modulates NOX2 and NOX4 expressions through miR-17 in a human microglial cell line. J. Neurochem. 2014;131:803–815. doi: 10.1111/jnc.12933. [DOI] [PubMed] [Google Scholar]
- 14.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.F., Yao T.P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Gilquin B., Khochbin S., Matthias P. Two catalytic domains are required for protein deacetylation. J. Biol. Chem. 2006;281:2401–2404. doi: 10.1074/jbc.C500241200. [DOI] [PubMed] [Google Scholar]
- 16.Bertos N.R., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J. Biol. Chem. 2004;279:48246–48254. doi: 10.1074/jbc.M408583200. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Shin D., Kwon S.H. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 2013;280:775–793. doi: 10.1111/febs.12079. [DOI] [PubMed] [Google Scholar]
- 18.Aldana-Masangkay G.I., Sakamoto K.M. The role of HDAC6 in cancer. J. Biomed. Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vishwakarma S., Iyer L.R., Muley M., Singh P.K., Shastry A., Saxena A., Kulathingal J., Vijaykanth G., Raghul J., Rajesh N., Rathinasamy S., Kachhadia V., Kilambi N., Rajgopal S., Balasubramanian G., Narayanan S. Tubastatin, a selective histone deacetylase 6 inhibitor shows anti-inflammatory and anti-rheumatic effects. Int. Immunopharmacol. 2013;16:72–78. doi: 10.1016/j.intimp.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Chattopadhyay S., Fensterl V., Zhang Y., Veleeparambil M., Wetzel J.L., Sen G.C. Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators. MBio. 2013;4 doi: 10.1128/mBio.00636-12. (e00636-12) (e00636-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S.J., Lee H.C., Kim J.H., Park S.Y., Kim T.H., Lee W.K., Jang D.J., Yoon J.E., Choi Y.I., Kim S., Ma J., Kim C.J., Yao T.P., Jung J.U., Lee J.Y., Lee J.S. HDAC6 regulates cellular viral RNA sensing by deacetylation of RIG-I. EMBO J. 2016;35:429–442. doi: 10.15252/embj.201592586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J., Coyne C.B., Sarkar S.N. PKC alpha regulates Sendai virus-mediated interferon induction through HDAC6 and β-catenin. EMBO J. 2011;30:4838–4849. doi: 10.1038/emboj.2011.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam H.J., Kang J.K., Kim S.K., Ahn K.J., Seok H., Park S.J., Chang J.S., Pothoulakis C., Lamont J.T., Kim H. Clostridium difficile toxin A decreases acetylation of tubulin, leading to microtubule depolymerization through activation of histone deacetylase 6, and this mediates acute inflammation. J. Biol. Chem. 2010;285:32888–32896. doi: 10.1074/jbc.M110.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B., Rao Y.H., Inoue M., Hao R., Lai C.H., Chen D., McDonald S.L., Choi M.C., Wang Q., Shinohara M.L., Yao T.P. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat. Commun. 2014;5:3479. doi: 10.1038/ncomms4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan B., Xie S., Liu Z., Ran J., Li Y., Wang J., Yang Y., Zhou J., Li D., Liu M. HDAC6 deacetylase activity is critical for lipopolysaccharide-induced activation of macrophages. PLoS One. 2014;9:e110718. doi: 10.1371/journal.pone.0110718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn G.S., Ju S.M., Choi S.Y., Park J. HDAC6 mediates HIV-1 tat-induced proinflammatory responses by regulating MAPK-NF-kappaB/AP-1 pathways in astrocytes. Glia. 2015;63:1953–1965. doi: 10.1002/glia.22865. [DOI] [PubMed] [Google Scholar]
- 27.Youn G.S., Lee K.W., Choi S.Y., Park J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radic. Biol. Med. 2016;97:14–23. doi: 10.1016/j.freeradbiomed.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann C.H., Rice A.P. Specific interaction of the human immunodeficiency virus Tat proteins with a cellular protein kinase. Virology. 1993;197:601–608. doi: 10.1006/viro.1993.1634. [DOI] [PubMed] [Google Scholar]
- 29.Rhim H., Echetebu C.O., Herrmann C.H., Rice A.P. Wild-type and mutant HIV-1 and HIV-2 Tat proteins expressed in Escherichia coli as fusions with glutathione S-transferase. J. Acquir. Immune Defic. Syndr. 1994;7:1116–1121. [PubMed] [Google Scholar]
- 30.Bethel-Brown C., Yao H., Callen S., Lee Y.H., Dash P.K., Kumar A., Buch S. HIV-1 Tat-mediated induction of platelet-derived growth factor in astrocytes: role of early growth response gene 1. J. Immunol. 2011;186:4119–4129. doi: 10.4049/jimmunol.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi K., Pu H., Andras I.E., Eum S.Y., Yamauchi A., Hennig B., Toborek M. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J. Cereb. Blood Flow Metab. 2006;26:1052–1065. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- 32.Kutsch O., Oh J., Nat h.A., Benveniste E.N. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J. Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams R., Yao H., Peng F., Yang Y., Bethel-Brown C., Buch S. Cooperative induction of CXCL10 involves NADPH oxidase: implications for HIV dementia. Glia. 2010;58:611–621. doi: 10.1002/glia.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorina R., Sanfeliu C., Galitó A., Messeguer A., Planas A.M. Exposure of glia to pro-oxidant agents revealed selective Stat1 activation by H2O2 and Jak2-independent antioxidant features of the Jak2 inhibitor AG490. Glia. 2007;55:1313–1324. doi: 10.1002/glia.20542. [DOI] [PubMed] [Google Scholar]
- 35.Turchan-Cholewo J., Dimayuga V.M., Gupta S., Gorospe R.M., Keller J.N., Bruce-Keller A.J. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid. Redox Signal. 2009;11:193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu R.F., Ma Z., Myers D.P., Terada L.S. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J. Biol. Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 37.Nayernia Z., Jaquet V., Krause K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S.Y., Lee J.G., Cho W.S., Cho K.H., Sakong J., Kim J.R., Chin B.R., Baek S.H. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol. Cell Biol. 2010;88:197–204. doi: 10.1038/icb.2009.87. [DOI] [PubMed] [Google Scholar]
- 39.Deng B., Xie S., Wang J., Xia Z., Nie R. Inhibition of protein kinase C β(2) prevents tumor necrosis factor-α-induced apoptosis and oxidative stress in endothelial cells: the role of NADPH oxidase subunits. J. Vasc. Res. 2012;49:144–159. doi: 10.1159/000332337. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L., Pang S., Deng B., Qian L., Chen J., Zou J., Zheng J., Yang L., Zhang C., Chen X., Liu Z., Le Y. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int. J. Biochem. Cell Biol. 2012;44:629–638. doi: 10.1016/j.biocel.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Dallavalle S., Pisano C., Zunino F. Development and therapeutic impact of HDAC6-selective inhibitors. Biochem. Pharmacol. 2012;84:756–765. doi: 10.1016/j.bcp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Song H.Y., Ryu J., Ju S.M., Park L.J., Lee J.A., Choi S.Y., Park J. Extracellular HIV-1 Tat enhances monocyte adhesion by up-regulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-kappaB activation in astrocytes. Exp. Mol. Med. 2007;39:27–37. doi: 10.1038/emm.2007.4. [DOI] [PubMed] [Google Scholar]
- 43.Abramov A.Y., Jacobson J., Wientjes F., Hothersall J., Canevari L., Duchen M.R. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J. Neurosci. 2005;40:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandes R.P., Weissmann N., Schröder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]