Abstract
Background
In men undergoing definitive radiation for prostate cancer, it is unclear whether early biochemical response can provide additional prognostic value beyond pre-treatment risk stratification.
Methods
Prostate cancer patients consecutively treated with definitive radiation at our institution by a single provider from 1993–2006 and who had an EOR PSA (n=688, median follow-up 11.2 years). We analyzed the association of an end-of-radiation (EOR) prostate-specific antigen (PSA) level, obtained during the last week of radiation, with survival outcomes. Multivariable-adjusted cox proportional hazards models were constructed to assess associations between a detectable EOR PSA (defined as ≥0.1 ng ml−1) and biochemical failure-free survival (BFFS), metastasis-free survival (MFS), prostate cancer-specific survival (PCSS), and overall survival (OS). Kaplan-Meier survival curves were constructed, with stratification by EOR PSA.
Results
At the end of radiation, the PSA level was undetectable in 30% of patients. Men with a detectable EOR PSA experienced inferior 10-year BFFS (49.7% vs. 64.4%, p<0.001), 10-year MFS (84.8% vs. 92.0%, p=0.003), 10-year PCSS (94.3% vs. 98.2%, p=0.007), and 10-year OS (75.8% vs. 82.5%, p=0.01), as compared to men with an undetectable EOR PSA. Among NCCN intermediate- and high-risk men who were treated with definitive radiation and androgen deprivation therapy (ADT), a detectable EOR PSA was more strongly associated with PCSS than initial NCCN risk level (EOR PSA: HR 5.89, 95% CI 2.37–14.65, p<0.001; NCCN risk level: HR 2.01, 95% CI 0.74–5.42, p=0.168). Main study limitations are retrospective study design and associated biases.
Conclusions
EOR PSA was significantly associated with survival endpoints in men who received treated with definitive radiation and ADT. Whether the EOR PSA can be used to modulate treatment intensity merits further investigation.
Introduction
Accurate risk stratification of men with localized prostate cancer is paramount in selecting optimal treatment intensity. As such, multiple risk stratification systems have been developed using combinations of pre-treatment prognostic factors, most commonly initial prostate-specific antigen (PSA) level, biopsy Gleason score, and clinical stage.1–5 Despite these tools, reliable identification of patients at risk for failure has remained elusive, as evidenced by heterogeneous outcomes seen within risk groupings, highlighting the limitations of available pre-treatment prognostic factors.6 In patients treated with neoadjuvant androgen deprivation therapy (ADT), emerging literature suggests that the biochemical response to neoadjuvant ADT may provide dynamic prognostic value beyond initial risk grouping and help identify men with disease that is more biologically aggressive than suggested by standard pre-treatment variables.7–18 In these men, PSA response to neoadjuvant ADT may offer an earlier time point at which to consider modification of the treatment regimen.
Similarly, in men undergoing definitive radiation for localized prostate cancer, assessment of the biochemical response at the completion of radiation may serve as a helpful biomarker but has not been previously analyzed. Indeed, an end-of-radiation (EOR) PSA level may be a more useful biomarker for guiding adjuvant treatment strategies than assessment of biochemical response prior to radiation and may also be applicable to men undergoing definitive radiation alone. At our institution, it has been the standard practice of one provider to obtain an end-of-radiation (EOR) PSA during the last week of treatment in men with localized prostate cancer undergoing definitive radiation. Herein, we examine the prognostic value of the EOR PSA in a cohort of prostate cancer patients with long-term follow-up after being treated at our institution with definitive radiation.
Materials and Methods
Study population
The study was approved by the institutional review board of Johns Hopkins Hospital (Baltimore, MD). We reviewed a prospectively acquired database of 936 patients with clinically localized prostate cancer who were consecutively treated with definitive radiation between January 1, 1993 and December 31, 2006 by a single provider (T.L. DeWeese). Biopsies which were performed at an outside hospital were reviewed by the genitourinary pathologists at our institution before treatment. Patients without complete clinical or pathologic information were excluded (n=18), as were patients with less than 24 months of follow-up (n=30). Patients who did not have an EOR PSA drawn were also excluded (n=200). Notably men lacking an EOR PSA did not differ from men with an EOR PSA with respect to age, race, initial PSA level, clinical stage, Gleason score, or National Comprehensive Care Network (NCCN) risk level (data not shown, all p>0.05). The final study population comprised 688 men with clinically localized disease.
Treatment
Patients were treated with definitive radiation using either three-dimensional conformal radiation therapy (3D-CRT, 79%) or intensity modulated radiation therapy (IMRT, 21%), with the latter technique increasingly utilized at the end of the study period. For NCCN low- and intermediate-risk patients, treatment fields generally included the prostate and seminal vesicles, with a boost to the prostate. For NCCN high-risk men, treatment generally consisted of an initial whole pelvis field, which included the prostate, seminal vesicle, and pelvic lymph nodes, followed by a boost field to the prostate. Seminal vesicles were also included in the boost field if there was high suspicion of involvement based on clinical exam. The prescription dose for the initial field was 45–46 Gy, delivered in 1.8–2 Gy fractions. The prescription dose for the boost field varied over the study period, with higher doses administered in more recent years. Median total dose for the cohort was 70.2 Gy (range: 64.8–75.6 Gy). When administered, neoadjuvant ADT was initiated two months prior to the radiation start date and consisted of a luteinizing hormone-releasing hormone (LHRH) agonist and an oral antiandrogen. Duration of LHRH agonist administration was dictated by disease characteristics. Complete information regarding the duration of oral antiandrogen use was unavailable.
Following treatment, patients underwent routine follow-up with serial PSA measurements and digital rectal exam, generally at six month intervals. Frequency of PSA measurements and digital rectal exams was altered based on the PSA trend and clinical symptoms. Similarly, clinical imaging was obtained in the setting of concerning PSA trends or clinical symptoms. Salvage ADT was administered based on the discretion of the treating provider, but was generally influenced by PSA doubling time, co-morbidity, and life expectancy. No patients received salvage local therapy, except for one patient who underwent salvage prostatectomy at an outside institution.
Statistical Analysis
The primary endpoint of our study was prostate cancer-specific survival (PCSS). Prostate cancer-specific death was recorded if patient had a documented history of hormone-refractory metastatic prostate cancer, evidence of a rising PSA at last follow-up visit, and no other obvious cause of death. Additionally, the National Death Index (NDI) was cross-referenced to confirm cause of death. Secondary endpoints included biochemical failure-free survival (BFFS), metastasis-free survival (MFS), and overall survival (OS). Biochemical failure, defined as nadir PSA plus 2.0 ng ml−1, was based on the Radiation Therapy Oncology Group – American Society for Therapeutic Radiation Oncology Phoenix Consensus Conference definition.19 For the purpose of calculating BFFS, patients without biochemical failure were censored at time of last PSA measurement. Metastasis was defined by a radiographic abnormality on bone scan and/or computed tomography, with biopsy performed as needed for confirmation. Failure points were measured from the last day of radiation.
Differences in patient and treatment characteristics were compared between men with a detectable EOR PSA (defined as ≥0.1 ng ml−1 and measured during the last week of the radiation schedule) and men with an undetectable EOR PSA using the χ2 test. A PSA threshold of 0.1 ng ml−1 was selected based on the minimum PSA that was detectable by the assay in use at our institution during the study period. Thresholds for dichotomous variables were defined in accordance with the literature. Univariate and multivariable-adjusted hazard ratios were calculated using a Cox proportional hazards model to associations between the EOR PSA and BFFS, MFS, PCSS, and OS. Covariates in the multivariable model were based on significance in univariate analysis and included age, race, initial PSA level, clinical stage, Gleason score, perineural invasion (PNI), radiation dose, use of IMRT, and administration of ADT (data not shown). Kaplan-Meier survival curves were also constructed for all survival endpoints, with stratification by EOR PSA and comparison using the log-rank test.
All analyses were performed with Stata software (Stata/IC10.0). Two-sided significance testing was used, and a P-value of 0.05 was considered statistically significant.
Results
Of the 688 men in our cohort treated with definitive radiation, 368 patients (41%) died during the study period, including 74 patients (8%) who died of prostate cancer. Median follow-up was 11.2 years (range: 2.0–20.6 years) for all patients, and 12.1 years (range: 2.0–20.6 years) for surviving patients.
Demographic, tumor, and treatment characteristics are outlined in Table 1. By the end of radiation, an undetectable PSA was achieved by 30% of patients, including 12% of NCCN low-risk patients, 39% of NCCN intermediate-risk patients, and 41% of NCCN high-risk patients. Men with a detectable EOR PSA were more likely to have had NCCN low-risk disease, including a lower Gleason score and less advanced T-stage, and less likely to have had PNI on biopsy (all p≤0.001). Men with a detectable EOR PSA also received a lower median radiation dose, were less likely to have received IMRT, and were less likely to have received neoadjuvant-concurrent ADT (all p<0.001).
Table 1.
Demographic, disease, and treatment characteristics
| Characteristic | Undetectable EOR PSA (N=206) |
Detectable EOR PSA (N=482) |
P-value |
|---|---|---|---|
|
| |||
| Median age (range), yrs | 68 (50–80) | 69 (49–82) | 0.07 |
|
| |||
| Africa-American race, n (%) | 40 (19) | 126 (26) | 0.06 |
|
| |||
| Initial PSA, ng mL−1 | 0.25 | ||
| <10, n (%) | 128 (62) | 318 (66) | |
| 10–20, n (%) | 54 (26) | 99 (21) | |
| >20, n (%) | 24 (12) | 65 (13) | |
|
| |||
| Gleason score | <0.001 | ||
| 3+3 | 84 (41) | 289 (60) | |
| 3+4 | 64 (31) | 80 (17) | |
| 4+3 | 17 (8) | 38 (8) | |
| 4+4 | 22 (11) | 30 (6) | |
| 4+5 | 14 (7) | 18 (4) | |
| 5+4 | 5 (2) | 8 (2) | |
| 5+5 | 0 (0) | 3 (1) | |
|
| |||
| T-stage, % | 0.001 | ||
| T1 | 81 (39) | 229 (48) | |
| T2 | 83 (40) | 205 (43) | |
| T3/T4 | 42 (20) | 48 (10) | |
|
| |||
| NCCN risk level | <0.001 | ||
| Low | 28 (14) | 214 (44) | |
| Intermediate | 91 (44) | 144 (30) | |
| High | 87 (42) | 124 (26) | |
|
| |||
| Perineural invasion on biopsy | 64 (31) | 71 (15) | <0.001 |
|
| |||
| Median radiation dose (range), cGy | 7380 (6840 – 7560) | 7200 (6480 – 7560) | <0.001 |
|
| |||
| Intensity modulated radiation therapy | 74 (36) | 107 (22) | <0.001 |
|
| |||
| Androgen deprivation therapy | <0.001 | ||
| None | 7 (3) | 287 (60) | |
| Neoadjuvant and concurrent only | 117 (57) | 101 (21) | |
| Neoadjuvant, concurrent, and adjuvant | 82 (40) | 94 (19) | |
Abbreviations: National Comprehensive Care Network (NCCN); prostate-specific antigen (PSA)
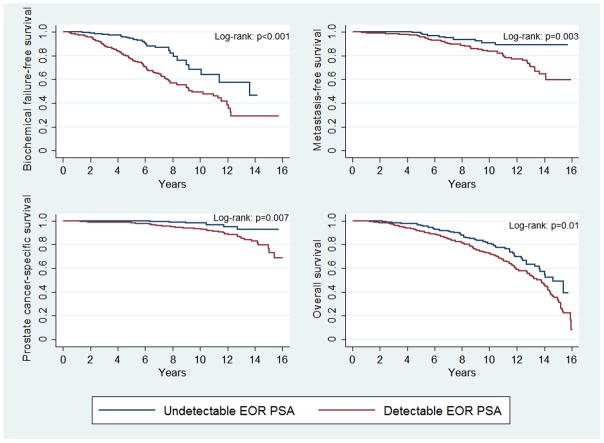
Univariate and multivariable-adjusted associations between EOR PSA and survival endpoints are summarized in Table 2 and Table 3 respectively. Hazard ratios for the associations between all covariates included in the multivariable model and survival endpoints are also summarized (Supplementary eTable 1). A detectable EOR PSA was independently associated with significantly inferior BFFS (p<0.001), MFS (p<0.001), PCSS (p<0.001), and OS (p=0.004). Figure 1 displays Kaplan-Meier survival estimates, stratified by EOR PSA level. Men with a detectable EOR PSA experienced inferior 10-year BFFS (49.7% vs. 64.4%, p<0.001), 10-year MFS (84.8% vs. 92.0%, p=0.003), 10-year PCSS (94.3% vs. 98.2%, p=0.007), and 10-year OS (75.8% vs. 82.5%, p=0.01), as compared to men with an undetectable EOR PSA.
Table 2.
Univariate associations between EOR PSA and survival endpoints for entire cohort and within NCCN risk groupings
| Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|
|
| ||
| Entire cohort | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 2.69 (1.77 – 4.08) | <0.001 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 2.62 (1.36 – 5.04) | 0.004 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 3.08 (1.33 – 7.10) | 0.008 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 1.52 (1.14 – 2.03) | 0.004 |
|
| ||
| Low risk | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | 6.86 (0.93 – 50.83) | 0.06 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | 2.04 (0.26 – 16.20) | 0.50 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | *Not enough events | --- |
|
| ||
| Overall survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | 1.17 (0.53 – 2.58) | 0.69 |
|
| ||
| Intermediate risk | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 1.98 (0.95 – 4.12) | 0.07 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 3.65 (0.76 – 17.59) | 0.11 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 4.98 (0.59 – 41.78) | 0.14 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 0.74 (0.44 – 1.25) | 0.26 |
|
| ||
| High risk | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 4.21 (2.20 – 8.09) | <0.001 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 4.04 (1.82 – 8.94) | 0.001 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 4.15 (1.56 – 11.08) | 0.004 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 2.69 (1.58 – 4.60) | <0.001 |
Abbreviations: National Comprehensive Care Network (NCCN); end-of-radiation (EOR); prostate-specific antigen (PSA)
Table 3.
Multivariable-adjusted associations between EOR PSA and survival endpoints for entire cohort and within NCCN risk groupings
| Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|
|
| ||
| Entire cohort | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 3.56 (2.24 – 5.65) | <0.001 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 5.32 (2.58 – 10.97) | <0.001 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 6.02 (2.44 – 14.84) | <0.001 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 1.67 (1.18 – 2.35) | 0.004 |
|
| ||
| Low risk | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | 8.02 (1.06 – 60.73) | 0.04 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | 3.19 (0.37 – 27.28) | 0.29 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | *Not enough failure events | --- |
|
| ||
| Overall survival | ||
| EOR PSA ≥1.0 ng ml−1 vs. <1.0 ng ml−1 | 1.10 (0.05 – 2.43) | 0.81 |
|
| ||
| Intermediate risk | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 1.77 (0.82 – 3.80) | 0.15 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 6.36 (0.98 – 39.59) | 0.06 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 6.49 (0.74 – 56.73) | 0.09 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 0.87 (0.51 – 1.46) | 0.59 |
|
| ||
| High risk | ||
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 3.69 (1.79 – 7.60) | <0.001 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 5.76 (2.31 – 14.39) | <0.001 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 8.99 (2.74 – 29.50) | <0.001 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 2.60 (1.46 – 4.64) | 0.001 |
Abbreviations: National Comprehensive Care Network (NCCN); end-of-radiation (EOR); prostate-specific antigen (PSA)
Multivariable model adjusted for age, race, initial PSA, Gleason score, T-stage, perineural invasion on biopsy, radiation dose, use of intensity modulated radiation, and duration of androgen deprivation therapy
Figure 1.
Survival outcomes for cohort stratified by end-of-radiation (EOR) prostate-specific antigen (PSA) level, including (a) biochemical failure-free survival, (b) metastasis-free survival, (c) prostate cancer-specific survival, and (d) overall survival. Red curves represent men with a detectable EOR PSA. Blue curves represent men with an undetectable EOR PSA.
Given that EOR PSA was significantly influenced by the use of neoadjuvant-concurrent ADT, which was primarily dictated by NCCN risk level, we performed a subset analysis to explore associations between EOR PSA and survival endpoints within NCCN risk groups, restricting the analysis to men who received ADT in accordance with modern standards. Notably, among NCCN low-risk men who received radiation alone (n=204), only 2% achieved an undetectable EOR PSA, precluding analysis in this subset. We did analyze whether higher EOR PSA thresholds offered prognostic utility in this subset, but results were not significant (data not shown). Similarly, among NCCN intermediate-risk patients who received neoadjuvant-concurrent ADT (N=168), a detectable EOR PSA was not associated with BFFS, MFS, PCSS, or OS, which may again reflect too few events in this subset. However, in NCCN high-risk men who received neoadjuvant-concurrent and long-term adjuvant ADT (n=188), a detectable EOR PSA was significantly associated with inferior BFFS (HR 3.82, 95% CI: 1.80–8.09, p<0.001), MFS (HR 5.20, 95% CI: 2.06–13.10, p<0.001), PCSS (HR 9.88, 95% CI: 2.84–34.44, p<0.001), and OS (HR 2.69, 95% CI: 1.51–4.83, p=0.001). Furthermore, when NCCN intermediate- and high-risk men who received neoadjuvant-concurrent ADT were analyzed together, a detectable EOR PSA was more strongly associated with survival outcomes than initial NCCN risk level, as illustrated in Table 4. Figure 2 displays Kaplan-Meier survival endpoints stratified by EOR PSA in NCCN intermediate- or high-risk men who received neoadjuvant-concurrent ADT; men with a detectable EOR PSA experienced inferior 10-year BFFS (43.5% vs. 59.1%, p<0.001), 10-year MFS (74.3% vs. 88.4%, p<0.001), 10-year PCSS (87.2% vs. 96.8%, p=0.002), and 10-year OS (66.2% vs. 78.4%, p<0.001), as compared to men with an undetectable EOR PSA.
Table 4.
Multivariable-adjusted associations between EOR PSA, initial NCCN risk grouping, and survival endpoints in NCCN intermediate-risk and high-risk patients undergoing neoadjuvant-concurrent androgen deprivation therapy
| Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|
|
| ||
| Biochemical failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 3.25 (2.00 – 5.28) | <0.001 |
| NCCN risk group | 1.46 (0.84 – 2.55) | 0.18 |
|
| ||
| Metastasis failure-free survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 4.56 (2.20 – 9.45) | <0.001 |
| NCCN risk group | 2.40 (1.00 – 5.78) | 0.05 |
|
| ||
| Prostate cancer-specific survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 5.89 (2.37 – 14.65) | <0.001 |
| NCCN risk group | 2.01 (0.74 – 5.42) | 0.17 |
|
| ||
| Overall survival | ||
| EOR PSA ≥0.1 ng ml−1 vs. <0.1 ng ml−1 | 1.60 (1.10 – 2.31) | 0.01 |
| NCCN risk group | 0.88 (0.56 – 1.39) | 0.59 |
Abbreviations: National Comprehensive Care Network (NCCN); end-of-radiation (EOR); prostate-specific antigen (PSA)
Multivariable model adjusted for age, race, perineural invasion on biopsy, radiation dose, use of intensity modulated radiation, and duration of androgen deprivation therapy
Figure 2.
Survival outcomes for NCCN intermediate- and high-risk men treated with neoadjuvant-concurrent androgen deprivation therapy (ADT) and neoadjuvant-concurrent and long-term ADT respectively, stratified by end-of-radiation (EOR) prostate-specific antigen (PSA) level, including (a) biochemical failure-free survival, (b) metastasis-free survival, (c) prostate cancer-specific survival, and (d) overall survival. Red curves represent men with a detectable EOR PSA. Blue curves represent men with an undetectable EOR PSA.
Covariates included in the multivariable model were based on significance in univariate analysis, but the inclusion of too many covariates risks overfitting.20 As such, we repeated the analysis, reducing the number of covariates to one per ten prostate cancer deaths by eliminating from the model the least significant covariates of age, race, and radiation technique, with similar results (Supplementary eTables 2 & 3).
Discussion
In a large cohort of men who underwent definitive radiation at our institution for localized prostate cancer, an EOR PSA level, measured during the last week of radiation therapy, was significantly associated with BFFS, MFS, PCSS, and OS; moreover, these associations persisted after controlling for standard pre-treatment prognostic factors. Notably, in the subset of men with NCCN intermediate- or high-risk disease who underwent neoadjuvant-concurrent ADT, achievement of an undetectable PSA level was more strongly associated with survival outcomes than initial NCCN risk grouping. Whether the EOR PSA can be used to dictate treatment intensification in NCCN intermediate- and high-risk men undergoing neoadjuvant-concurrent ADT merits further investigation. In contrast, the utility of the EOR PSA was less pronounced in men with NCCN low-risk disease who were treated with definitive radiation alone. As only 2% of NCCN low-risk patients treated with definitive radiation alone achieved an undetectable PSA, the last week of radiation likely represents too early of a time point to yield useful information in the NCCN low-risk population.
Currently, the duration of androgen suppression for men with localized prostate cancer undergoing definitive radiation is dictated by pre-treatment tumor characteristics, with salvage therapy generally based on the follow-up PSA trend. While PSA failure serves as an excellent surrogate for risk of eventual metastasis and death from prostate cancer, current definitions of PSA failure often require considerable time to elapse before the criteria for failure have been satisfied, during which the window of opportunity for effective escalation of therapy may have passed.19 In other disease sites, treatment modulation based on mid-treatment response assessment has allowed for individualization of therapy earlier in the treatment course.21 Whether such a paradigm can be applied to prostate cancer through earlier PSA response assessment is unclear. To date, several studies have shown prognostic value in the PSA response to neoadjuvant ADT, which may serve as a helpful surrogate for tumor biology.7–18,22 Indeed, a detectable PSA level after neoadjuvant ADT may reflect persistent androgen signaling, which may undermine radio-sensitization from concurrent ADT. These studies have led some authors to postulate the use of newer agents that target androgen signaling through alternate pathways in men with persistently elevated PSA levels after neoadjuvant ADT.17
Less understood, however, is the prognostic value of an EOR PSA level drawn during the last week of radiation therapy. An EOR PSA may offer more optimal timing beyond the pre-radiation post-ADT PSA level, with potential implications on the dose of radiation administered and selection of adjuvant therapy. Recently, a preclinical and clinical study suggested that radiation can durably upregulate the androgen receptor (AR) pathway, providing a potential explanation for the benefit of adjuvant ADT, and the EOR PSA biomarker allows for integration of this radiation-AR axis, not allowed by the pre-radiation post-ADT PSA.23 While two prior studies have shown prognostic value to the first follow-up PSA after completion of radiation,24,25 this study to our knowledge represents the first analysis of PSA levels drawn at EOR.
In light of our findings, the EOR PSA may be a useful tool for developing clinical trials that explore the incorporation into the non-metastatic setting of newer agents that have proven successful in the metastatic setting.26, 27 For example, integrating these agents into the adjuvant management of men with a detectable EOR PSA after definitive radiation with neoadjuvant-concurrent ADT may be worthy of investigation. Use of the EOR PSA may be particularly useful in men with higher risk disease.28 Dose-escalation, perhaps with a brachytherapy boost, many offer another method for treatment intensification in these patients.29
A number of study limitations must be acknowledged. Foremost, the retrospective study design risks unaccounted biases in patient selection. The wide study interval, for example, may have introduced biases associated with the evolution of treatment techniques and technologies over time. However, we tried to account for such biases in our multivariable model by including those treatment variables that may have changed over time, namely radiation dose and planning technique. Of note, the wide study interval also led to a high proportion of patients with advanced clinical stage, which is less representative of the modern presentation of patients. However, the study interval allowed for excellent long-term follow-up, which is critical when analyzing long-term endpoints such as PCSS. That all patients were treated by a single provider likely reduced variation in practice patterns, but it also limits the generalizability of results. As such, external validation of EOR PSA in a definitive radiation cohort should be pursued. Lastly, the median radiation dose was 70.2 Gy, which is less than contemporary doses of >75 Gy. Nevertheless, while dose-escalated radiotherapy has improved biochemical control, it has yet to be associated with improvements in more relevant clinical endpoints such as PCSS.39
Conclusions
In conclusion, a detectable EOR PSA after definitive radiation for localized prostate cancer is associated with poor survival outcomes. The prognostic value of the EOR PSA likely applies to NCCN intermediate- and high-risk men undergoing definitive radiation and neoadjuvant-concurrent ADT, with less clear prognostic value in NCCN low-risk men undergoing definitive radiation. Further validation of the EOR PSA should be pursued, as should investigation into the potential use of the EOR PSA for dictating optimal treatment intensity in men with localized prostate cancer.
Supplementary Material
Acknowledgments
PT Tran was supported by the Keeling Family; The Motta Family; The Irene and Bernard L. Schwartz Scholar Award from the Patrick C. Walsh Prostate Cancer Research Fund; A Movember-Prostate Cancer Foundation award; Grant numbers: DoD W81XWH-11-1-0272; Sidney Kimmel Foundation SKF-13-021; ACS 122688-RSG-12-196-01-TBG; NIH/NCI R01CA166348.
Footnotes
Conflict of Interset
The authors declare no conflicts of interest.
References
- 1.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, Davis BJ, Eastham JA, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016 Jan;14(1):19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 3.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S, Schaeffer EM, Trock BJ, Epstein JI, Humphreys EB, Walsh PC. What are the outcomes of radical prostatectomy for high-risk prostate cancer? Urology. 2010;76(3):710–4. doi: 10.1016/j.urology.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Lyass O, Fuks Z, Wolfe T, Burman C, Ling CC, et al. Predictors of improved outcome for patients with localized prostate cancer treated with neoadjuvant androgen ablation therapy and three-dimensional conformal radiotherapy. J Clin Oncol. 1998 Oct;16(10):3380–5. doi: 10.1200/JCO.1998.16.10.3380. [DOI] [PubMed] [Google Scholar]
- 8.Sumi M, Ikeda H, Tokuuye K, Kagami Y, Murayama S, Tobisu K, et al. The external radiotherapy with three-dimensional conformal boost after the neoadjuvant androgen suppression for patients with locally advanced prostatic carcinoma. Int J Radiat Oncol Biol Phys. 2000;48(2):519–28. doi: 10.1016/s0360-3016(00)00614-3. [DOI] [PubMed] [Google Scholar]
- 9.Ludgate CM, Bishop DC, Pai H, Eldridge B, Lim J, Berthelet E, et al. Neoadjuvant hormone therapy and external-beam radiation for localized high-risk prostate cancer: the importance of PSA nadir before radiation. Int J Radiat Oncol Biol Phys. 2005;62(5):1309–15. doi: 10.1016/j.ijrobp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AS, Mydin A, Jones SO, Christie J, Lim JT, Truong PT, et al. Extreme-risk prostate adenocarcinoma presenting with prostate-specific antigen (PSA)> 40 ng/ml: prognostic significance of the preradiation PSA nadir. Int J Radiat Oncol Biol Phys. 2011;81(5):e713–9. doi: 10.1016/j.ijrobp.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell DM, McAleese J, Park RM, Stewart DP, Stranex S, Eakin RL, et al. Failure to achieve a PSA level ≤1 ng/ml after neoadjuvant LHRHa therapy predicts for lower biochemical control rate and overall survival in localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(5):1467–71. doi: 10.1016/j.ijrobp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Foo M, Lavieri M, Pickles T. Impact of neoadjuvant prostate-specific antigen kinetics on biochemical failure and prostate cancer mortality: results from a prospective patient database. Int J Radiat Oncol Biol Phys. 2013;85(2):385–92. doi: 10.1016/j.ijrobp.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 13.McGuire SE, Lee AK, Cerne JZ, Munsell MF, Levy LB, Kudchadker RJ, et al. PSA response to neoadjuvant androgen deprivation therapy is a strong independent predictor of survival in high-risk prostate cancer in the dose-escalated radiation therapy era. Int J Radiat Oncol Biol Phys. 2013;85(1):e39–46. doi: 10.1016/j.ijrobp.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelefsky MJ, Gomez DR, Polkinghorn WR, Pei X, Kollmeier M. Biochemical response to androgen deprivation therapy before external beam radiation therapy predicts long-term prostate cancer survival outcomes. Int J Radiat Oncol Biol Phys. 2013;86(3):529–33. doi: 10.1016/j.ijrobp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Zilli T, Jorcano S, Escude L, Linero D, Rouzaud M, Dubouloz A, et al. Hypofractionated external beam radiotherapy to boost the prostate with ≥85 Gy/equivalent dose for patients with localised disease at high risk of lymph node involvement: feasibility, tolerance and outcome. Clin Oncol. 2014;26(6):316–22. doi: 10.1016/j.clon.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Crook J, Ludgate C, Malone S, Perry G, Eapen L, Bowen J, et al. Final report of multicenter Canadian Phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(2):327–33. doi: 10.1016/j.ijrobp.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 17.Alexander A, Crook J, Jones S, Malone S, Bowen J, Truong P, et al. Is biochemical response more important than duration of neoadjuvant hormone therapy before radiotherapy for clinically localized prostate cancer? An analysis of the 3-versus 8-month randomized trial. Int J Radiat Oncol Biol Phys. 2010;76(1):23–30. doi: 10.1016/j.ijrobp.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Heymann JJ, Benson MC, O’Toole KM, Malyszko B, Brody R, Vecchio D, et al. Phase II study of neoadjuvant androgen deprivation followed by external-beam radiotherapy with 9 months of androgen deprivation for intermediate- to high-risk localized prostate cancer. J Clin Oncol. 2007;25(1):77–84. doi: 10.1200/JCO.2005.05.0419. [DOI] [PubMed] [Google Scholar]
- 19.Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins, et al. The problem of overfitting. J Chem Inf Comput Sci. 2004;44(1):1–12. doi: 10.1021/ci0342472. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114(10):2051–9. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan CJ, Smith A, Lal P, Satagopan J, Reuter V, Scardino P, et al. Persistent prostate-specific antigen expression after neoadjuvant androgen depletion: an early predictor of relapse or incomplete androgen suppression. Urology. 2006;68(4):834–9. doi: 10.1016/j.urology.2006.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015;75(22):4688–4696. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Amico AV, Chen M, de Castro M, Loffredo M, Lamb DS, Steigler A, et al. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: an analysis of two randomised trials. Lancet Oncol. 2012;13(2):189–95. doi: 10.1016/S1470-2045(11)70295-9. [DOI] [PubMed] [Google Scholar]
- 25.Cury FL, Hunt D, Roach M, Shipley W, Gore E, Hsu I, et al. Prostate-specific antigen response after short-term hormone therapy plus external-beam radiotherapy and outcome in patients treated on Radiation Therapy Oncology Group study 9413. Cancer. 2013;119(11):1999–2004. doi: 10.1002/cncr.28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narang AK, Gergis C, Robertson SP, He P, Ram AN, McNutt TR, et al. Very High-Risk Localized Prostate Cancer: Outcomes Following Definitive Radiation. Int J Radiat Oncol Biol Phys. 2016;94(2):254–62. doi: 10.1016/j.ijrobp.2015.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris WJ, Tyldesley S, Pai HH, Halperin R, McKenzie MR, Duncan G, et al. ASCENDE-RT*: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. ASCO Annual Meeting Proceedings. 2015 [Google Scholar]
- 30.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.