Abstract
Initial historical accounts as well as recent data suggest that emotion processing is dysfunctional in conversion disorder patients and that this alteration may be the pathomechanistic neurocognitive basis for symptoms in conversion disorder. However, to date evidence of direct interaction of altered negative emotion processing with motor control networks in conversion disorder is still lacking. To specifically study the neural correlates of emotion processing interacting with motor networks we used a task combining emotional and sensorimotor stimuli both separately as well as simultaneously during functional magnetic resonance imaging in a well characterized group of 13 conversion disorder patients with functional hemiparesis and 19 demographically matched healthy controls. We performed voxelwise statistical parametrical mapping for a priori regions of interest within emotion processing and motor control networks. Psychophysiological interaction (PPI) was used to test altered functional connectivity of emotion and motor control networks. Only during simultaneous emotional stimulation and passive movement of the affected hand patients displayed left amygdala hyperactivity. PPI revealed increased functional connectivity in patients between the left amygdala and the (pre-)supplemental motor area and the subthalamic nucleus, key regions within the motor control network. These findings suggest a novel mechanistic direct link between dysregulated emotion processing and motor control circuitry in conversion disorder.
Keywords: Amygdala, Emotion processing, fMRI, Motor network, Psychogenic paresis, Conversion disorder
Graphical abstract
Highlights
-
•
We studied emotion processing effects on motor networks in conversion disorder (CD).
-
•
Simultaneous motor and emotional stimulation resulted in enhanced amygdala activation.
-
•
Left amygdala showed increased functional connectivity with an inhibitory motor loop.
-
•
This suggests a direct link of impaired emotion processing and motor networks in CD.
1. Introduction
Rooted in the psychoanalytic view of conversion neurosis as negative affects being converted into physical symptoms (Freud, 1896) growing evidence suggests that conversion disorder is related to a dysfunctional alteration of emotion processing. Traumatic life events (Kanaan et al., 2007, Steffen et al., 2015) and the suppression of traumatic experiences (Anderson et al., 2004) are common features in conversion disorder and conversion disorder patients show a greater rate of alexithymia (Steffen et al., 2015). Findings of imaging studies suggest altered neural activity in emotion processing areas in conversion disorder, especially in the amygdala (Aybek et al., 2015, Voon et al., 2010a, Voon et al., 2011). Even if clinical data and the concept of psychotherapeutic approach indicate that psychogenic trauma is a risk factor for conversion disorder and even if altered emotional processing and motor symptoms seem to be closely linked in conversion disorder (Aybek et al., 2014, Freud, 1896), it is poorly understood which neural networks interact in this process.
For conversion disorder patients with motor symptoms diverse alterations of the motor network have been demonstrated (de Lange et al., 2010, Liepert et al., 2009). One possible anatomical link between emotion processing and the motor network could be midline prefrontal monitoring structures, especially the anterior cingulate cortex (ACC) (Bush et al., 2000). Several imaging studies showed enhanced activation in medial prefrontal cortex in motor conversion disorder patients (Cojan et al., 2009, Roelofs et al., 2006). It was proposed that the ACC may exert an excessive inhibitory effect on the motor cortex (Marshall et al., 1997) potentially caused by a disconnection between the dorsal and ventral subdivisions of the ACC and the prefrontal cortex that might provide an anatomical basis for the psychodynamic dissociation hypothesis (Ballmaier and Schmidt, 2005). Similarly, the functional-unawareness neurobiological framework (Perez et al., 2012) ascribes a central role to the perigenual ACC in motor conversion disorder. According to this framework dysfunction in the perigenual ACC and its subcortical loops such as reciprocal cingulate–amygdalar connections may result preferentially in impaired motor control motivated behavior and affect regulation. In a recent review Vuilleumier (2014) delineated a model with the ventromedial prefrontal cortex and the precuneus as key regions for internal representations and integration of memory of affective relevance being the link between emotion processing and motor symptoms.
There is also evidence for an involvement of the inferior frontal gyrus (IFG) as part of a motor inhibition network in conversion disorder. Especially the right IFG is strongly associated with motor inhibition (Aron, 2011, Criaud and Boulinguez, 2013, Sebastian et al., 2016). During active movement left IFG activity has been reported in conversion disorder patients (Stone et al., 2007). In a single case study of hysterical mutism altered IFG activity was described following speech recovery (Bryant and Das, 2012). In several imaging studies with conversion disorder patients with other than motor symptoms IFG activity was consistently observed (Kanaan et al., 2007, Mailis-Gagnon et al., 2003). One study using a Go/NoGo paradigm with neutral stimulus material in a single patient proposed, however, that the IFG is not specifically related to motor symptoms in conversion disorder (Cojan et al., 2009).
Taken together, alterations in emotion processing and motor control networks in conversion disorder patients with motor symptoms have been described. However, to date evidence of a direct interaction of altered negative emotion processing with motor control networks in conversion disorder patients with paretic symptoms is still lacking. To this end we developed a functional magnetic resonance imaging (fMRI) paradigm with separate and simultaneous emotional and sensorimotor stimulation to study the interaction of negative emotion processing with motor networks. More specifically, we aimed at assessing which of the regions previously implicated in conversion disorder, i.e. amygdala, ACC, ventromedial prefrontal cortex and precuneus, as well as motor inhibition network, are involved in the altered interaction of emotion processing and motor control networks in motor conversion disorder. Based on previous findings we expected the interaction of emotion processing and motor control in conversion disorder to be associated with hyperactivation in one or several of these regions. We recruited a comparably large sample of conversion disorder patients with sole hemiparesis to assure a high symptomatic and, hence, neural mechanistic homogeneity of the group since specific neural interaction could vary across different conversion disorder symptoms. To keep the sensorimotor stimulation as constant as possible across different degrees of paresis and, most importantly, to secure independence of patients' subjective intention and motivation to initiate and execute a movement we employed passive movements of the wrist. Passive movement activates reliably and robustly the sensorimotor network (Weiller et al., 1996) and is independent of the concurrent psychogenic paresis (Voon et al., 2010a).
2. Materials and methods
2.1. Participants
2.1.1. Patients
Thirteen patients (10 women, 3 men, mean age: 38.7 ± 11.0 years; range: 21–51 years) with a diagnosis of conversion disorder according to ICD 10 criteria (WHO World Health Organization, 1992) with motor symptoms of a spastic or flaccid hemiparesis were included. The study was performed in a rehabilitation hospital where all patients underwent rehabilitation therapy in the Department of Psychotherapeutic Neurology for 3–10 weeks. All individuals were diagnosed by an experienced clinician (RS). The patients were recruited consecutively meeting the inclusion criteria of conversion disorder with hemilateral paresis, being 20 years or older, having no contraindication for MRI and agreeing to take part in the study. In all patients, extensive diagnostic procedures including MRI of brain and spinal cord, somatosensory and motor evoked potentials and EMG recordings had produced normal, non-pathological results. Patients with precedent neurologic disorders, post-traumatic stress disorder, panic disorder, major depression or other major affective or psychotic disorders were excluded from this study. Four patients had paresis on the left side, nine patients on the right side. The mean duration of symptoms were 83 weeks, range 12–177 weeks (see Table 1).
Table 1.
Clinical data.
| Patients | Gender | Age | Side of paresis | Spastic/flaccid | Duration (weeks) of symptoms |
|---|---|---|---|---|---|
| 1 | F | 21 | r | f | 80 |
| 2 | F | 28 | l | s | 136 |
| 3 | F | 26 | r | f | 19 |
| 4 | F | 40 | r | f | 15 |
| 5 | F | 48 | l | s | 12 |
| 6 | F | 45 | r | f | 177 |
| 7 | F | 36 | r | f | 25 |
| 8 | F | 41 | r | s | 93 |
| 9 | M | 51 | l | f | 129 |
| 10 | M | 22 | r | f | 55 |
| 11 | M | 46 | r | f | 58 |
| 12 | F | 51 | r | s | 108 |
| 13 | F | 48 | l | f | 179 |
| Mean | 38,7 | Mean | 83,5 | ||
| SD | 11,0 | SD | 59,3 |
Abbreviation: F: female; M: male; r: right; l: left; SD = standard deviation.
2.1.2. Controls
Nineteen healthy control participants were recruited from among the staff members of the clinic and via community advertisements (14 females, 5 males, mean age: 36.5 ± 9.4 years; range: 22–56 years). None of them had a history of neurological or psychiatric disease.
The study was approved by the Ethics Committee of the University of Konstanz, and all participants gave their written informed consent to the study following the principles and guidelines of the Declaration of Helsinki (1975).
2.2. Behavioral paradigm
To explore the impact of negative emotional stimuli processing on the motor system patients and control participants were exposed to a paradigm combining emotional visual stimulus processing with passive movements of the hands.
2.2.1. Passive movement
Participants were instructed to relax completely and not to interfere voluntarily with the passive movements. This was trained outside the scanner before the experiment. Passive flexion-extension wrist movements of 70–90° were executed at a fixed rate of 1 Hz for 16 s paced by a visual signal that was only visible from the operator's perspective. For stimulus presentation and MR scanner synchronization the software ‘Presentation’ (http://www.neurobs.com, Albany, CA, USA) was used.
2.2.2. Visual stimuli
Sixteen grayscale faces with sad or calm expression of the NimStim set of Facial Expressions (Tottenham et al., 2009) were used as emotional visual stimuli. This set of faces has been shown to be reliable and valid for accurate identification of expressions of emotion by untrained individuals (mean kappa across stimuli = 0.79 (S.D. ± 0.17); median kappa = 0.83). Sad faces were chosen as negative stimuli since sad emotions have been shown to robustly induce activity aside from other limbic areas in the medial prefrontal cortex (PFC) (especially ventral and dorsal ACC (Phan et al., 2002)). Since neutral faces have been shown to be interpreted as slightly negative (Somerville et al., 2004, Tottenham et al., 2009) we used a series of calm faces of the NimStim set as neutral stimuli. 16 faces of equal emotion (eight male, eight female) were presented centrally in a pseudorandomized order in a blocked fMRI design, each for 1 s resulting in a 16 s block.
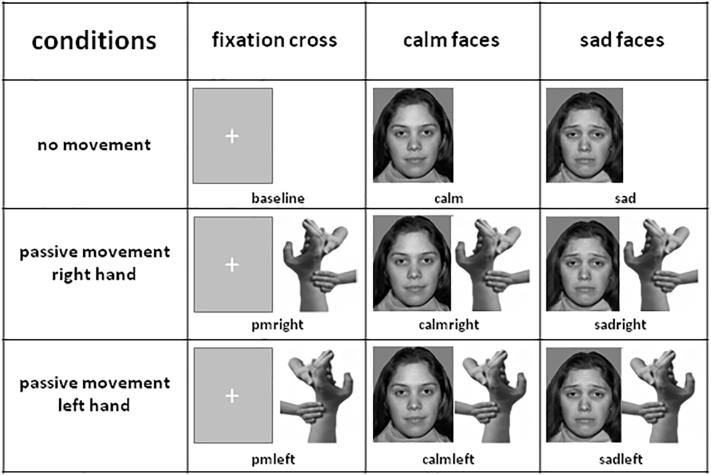
Sensorimotor stimulation (passive movement of the right hand, the left hand and no motor stimulation) was combined with emotional stimuli (sad faces, calm faces, fixation cross) resulting in a 3 × 3 design (Fig. 1). The rest condition (no motor stimulation, fixation cross) served as baseline for analysis. The remaining eight conditions were intermixed in a pseudorandomized order and interspersed with the rest condition lasting alternately 8 and 16 s. Six blocks of each condition (24 blocks in total) were performed in two runs with total duration of 23.5 min. To maintain alertness the participants were asked to count the number of small red square dots that were superimposed to a face per block and report them after the run.
Fig. 1.
3 × 3 fMRI paradigm:
fMRI paradigm in a 3 × 3 design combining sensorimotor and emotional stimulation. Passive movement was performed during presentation of a fixation cross or during simultaneous presentation of calm or sad faces from the NimStim set of Facial Expressions (Tottenham et al., 2009).
2.3. MRI data acquisition
Images were acquired on a 1.5 Tesla Philips Gyroscan (Philips Medical, Hamburg) equipped with a 8-channel head coil for parallel signal reception. Functional T2*-weighted echo echoplanar imaging (EPI) was performed (32 axial slices of 3.1 mm thickness with 1 mm gap, FOV of 230 × 230 mm, 80 × 80 matrix TR = 2.392 ms, TE = 40 ms, flip angle = 90°; 290 volumes per session). A FLAIR sequence (21 axial slices of 5 mm thickness with 1 mm gap, FOV 250 × 250 mm, 512 × 512 matrix, TR = 11,000 ms, TE = 140 ms, flip angle = 90°) and a T1 sequence (21 axial slices of 6 mm thickness with no gap, FOV 250 × 250 mm, 512 × 512 matrix, TR = 139.22 ms, TE = 2.3 ms) were acquired to exclude structural lesions.
2.4. FMRI data analysis
Statistical parametric mapping software (SPM12; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm12/) was used for fMRI data analysis. Images were realigned to the first image for motion correction. The gray matter segments of the structural FLAIR sequences were spatially normalized into the reference system of the Montreal Neurological Institute's (MNI) segmented reference brain; these normalization parameters were applied to the functional images. Finally, the normalized functional data were smoothed with a 12 mm Gaussian kernel.
BOLD effect was modeled with a box-car function and convoluted with the standard SPM12 hemodynamic response function for the eight conditions (pmright/pmleft: passive movement of the right/left hand; calm/sad: viewing calm/sad faces; calmright/calmleft: passive movement of the right/left hand while viewing calm faces; sadright/sadleft: passive movement of the right/left hand while viewing sad faces; please cf. Fig. 1). The regressors' coefficients for this voxel-based general linear model were estimated using least squares and correction for non-sphericity.
Random effects over subjects were assessed in a mixed measures ANOVA design with the above mentioned conditions as a within subject factor and two groups (patients, controls) as a between subject factor. This second level analysis was conducted in two different ways. First, to account for the different affected sides of hemiparesis all contrast images of the patients were arranged accordingly on second level, e.g. the contrast sad-affected combined the sadright contrasts of the patients with right-sided hemiparesis and the sadleft contrasts of the patients with left-sided hemiparesis (analysis of non-flipped data). Second, to assess whether effects of sensorimotor stimulation of the affected side were lateralized, the data sets of patients with paresis on the left side were flipped prior to preprocessing so that in all patients the right side (virtually) was affected (analysis of flipped data). For both types of analysis, we then computed the same contrasts.
We were specifically interested in the following contrasts assessing the interaction of negative emotion processing and motor execution: To probe the neural interaction of negative emotion with the motor system, we contrasted simultaneous passive movement of the affected side and visual input of a sad face (sad-affected) with passive movement and visual input of an emotionally neutral face (calm-affected) to control for the visual input (sad-affected > calm-affected) and compared the resulting brain activation patterns between groups resulting in the following contrast: [(sad-affected > calm-affected) × (patients > controls)]. To further test for syndrome specificity, the comparison to the unaffected side was added (sad-unaffected > calm-unaffected) in a second contrast assessing the following three way interaction [(sad-affected > calm-affected) × (sad-unaffected > calm-unaffected) × (patients > controls)]. Correction for multiple comparisons on the second level was performed using a whole brain peak voxel threshold of p < 0.05, family wise error (FWE).
In addition, small volume corrections (SVC) were performed in hypothesized, predefined regions of interest (ROI). If not stated differently, ROI masks from the AAL atlas (Tzourio-Mazoyer et al., 2002) were used. First, we aimed to assess whether the amygdala, a central emotion processing area, was implicated in the interaction of emotion processing and motor control as suggested by earlier studies (Aybek et al., 2015, Voon et al., 2010a, Voon et al., 2011). To control for multiple comparisons we combined the masks of right and left amygdalae into a combined amygdala mask. Next, to assess the hypothesis of Ballmaier and Schmidt (2005) we combined the masks of bilateral ventromedial prefrontal cortex (VMPFC), in particular the frontal superior medial cortex, the frontal medial orbital cortex, and the anterior cingulate cortex to create a single search volume for the VMPFC. To assess the model proposed by Vuilleumier (2014), we combined the VMPFC mask described above with the masks of the medial cingulate cortex as well as the bilateral precuneus. Finally, to test the proposed interaction of emotion processing with motor control networks, we combined the masks of the opercular part of the right inferior frontal gyrus (IFG), globus pallidus (GP), the (pre-) supplementary motor area (SMA) and subthalamic nucleus (STN) as essential parts of inhibitory function in the human motor control system (Aron and Poldrack, 2006). For the STN region, a coordinate-based mask was used following Aron and Poldrack (2006) with a center at MNI space x, y, z = 10, − 15, − 5. Yet, we defined the box size more restrictively at 5 × 10 × 8 mm following the dimension of STN in MRI images reported by Patel et al. (2008). Since the motor control network is known to be right lateralized (Aron, 2011, D'Alberto et al., 2017), we included only regions within the right hemisphere. Again, all masks were combined into one search volume for the motor control network. SVC activations within the respective combined ROIs were considered as significant if they survived p < 0.05, FWE–corrected.
To test for alterations in functional connectivity, psychophysiological interactions (PPIs) (Friston et al., 1997) were calculated for the amygdala activation cluster revealed by the group interaction condition [(sad-affected > calm-affected) × (patients > controls)]. Based on coordinates of the voxel of maximal activation intensity in the left amygdala, a sphere of 4 mm radius was centered at the closest individual local maximum voxel of each single subject (volume of interest, VOI). The mean-corrected signal time course from this VOI was used as the physiological factor. The psychological factor was a vector coding for the interaction contrast (sad-affected > calm-affected) convolved with the hemodynamic response function. The contrast images of each participant were then entered into random effects group analysis with a two sample t-test. Small volume corrections were performed for all ROIs described above.
3. Results
3.1. Behavioral data
All participants were asked to report the number of small red square dots that were superimposed to the visual stimuli after each run thus providing evidence for a high level of alertness in both groups during the whole fMRI data recording. We observed no group differences. All participants reported the correct number of dots (100%).
3.2. Neural activation during combined sensorimotor and emotional stimulation
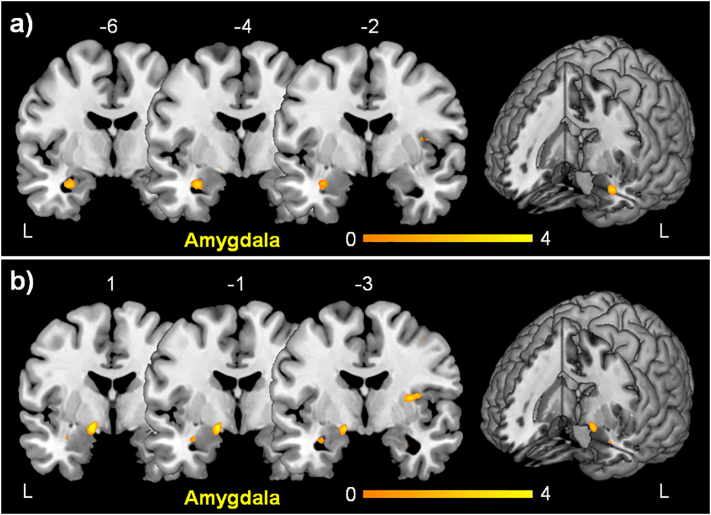
To assess brain activity in conversion disorder involved in the interaction of negative emotion with the motor system we first contrasted activation during passive movement of the affected side and simultaneous visual input of a sad face (sad-affected) with passive movement and simultaneous visual input of an emotionally neutral face (calm-affected) to control for the visual input (sad-affected > calm-affected) in the analysis of flipped data. The only significant cluster was seen in the left amygdala (Table 2 and Fig. 2a). The analysis of non-flipped data revealed no activation for this contrast.
Table 2.
Clusters of activation by simultaneous sensorimotor and emotional stimulation.
| Contrast | Region | MNI coordinates (x, y, z) | k | Z values | p values | ||
|---|---|---|---|---|---|---|---|
| Analysis of non-flipped data | |||||||
| Patients > controls: (sad-affected > calm-affected) > (sad-unaffected > calm-unaffected) | |||||||
| Left amygdala | − 16 | − 2 | − 12 | 1 | 3.41 | 0.038a | |
| Right amygdala | 30 | − 4 | − 20 | 1 | 3.19 | 0.039b | |
| Analysis of flipped data | |||||||
| Patients > controls: (sad-affected > calm-affected) > (sad-unaffected > calm-unaffected) | |||||||
| Left amygdala | − 16 | − 2 | − 12 | 10 | 3.35 | 0.049a | |
| Patients > controls: (sad-affected > calm-affected) | |||||||
| Left amygdala | − 30 | − 4 | − 22 | 11 | 3. 75 | 0.013a | |
| Patients > controls: (sad-unaffected > calm-unaffected) | |||||||
| Right angular gyrus | 54 | − 60 | 34 | 5 | 5.29 | 0.006 | |
| Functional connectivity - PPI for activation in the left amygdala | |||||||
| Patients > controls: (sad-affected > calm-affected) | |||||||
| (pre-)Supplemental motor area | 8 | − 4 | 66 | 58 | 4.24 | 0.028a | |
| Right subthalamic nucleus | 6 | − 20 | − 10 | 5 | 2.97 | 0.031b | |
Local maxima of brain activation in Montreal Neurological Institute (MNI) x-, y-, and z-coordinates with associated Z-score (pFWE < 0.05) and cluster extent in number of voxel (k). R right, L left; abbreviations: PPI = psychophysical interaction, FWE = family-wise error.
Small volume corrected.
Post-hoc analysis, small volume corrected.
Fig. 2.
Activation associated with altered interaction of emotion processing and motor control in conversion disorder.
a) Activation of left amygdala during simultaneous passive movement of right affected hand and the presentation of sad faces controlled for visual input (calm faces) in patients as compared to controls [(sad-affected > calm-affected) × (patients > controls)].
b) Activation of left amygdala resulting from a three way interaction contrast with patients vs. controls, showing syndrome specificity of amygdala activation [(sad-affected > calm-affected) × (sad-unaffected > calm-unaffected) × (patients > controls)]. For presentation purposes the statistical maps were thresholded at p < 0.005 unc. Color bar represents T-scores. The position on y-axis in the MNI-space is indicated above each coronal slice. The activation is displayed on the MRIcron template.
Next, to ensure syndrome specificity the comparison to the unaffected side was added. This resulted in a three way interaction contrast with patients vs. controls [(sad-affected > calm-affected) × (sad-unaffected > calm-unaffected) × (patients > controls)]. For the analysis of the flipped data this interaction also revealed a cluster of activation in the left amygdala (Table 2 and Fig. 2b). For the analysis of the non-flipped data this three way interaction resulted also in a cluster of activation in the left amygdala (Table 2). A post-hoc small volume correction only for the right amygdala showed also a small activation in the right amygdala (Table 2). The analysis of the flipped data revealed no activation in the right amygdala in this post-hoc analysis.
Finally, to test whether there were alterations in brain activity associated with the interaction of negative emotion processing and passive movement of the unaffected left hand we performed an analysis of the 2-way interaction condition for the unaffected hand [(sad-unaffected > calm-unaffected × (patients > controls)]. This resulted in a cluster in the right angular gyrus (Table 2). Small volume corrections for VMPFC and precuneus showed no significant results for all contrast specified above.
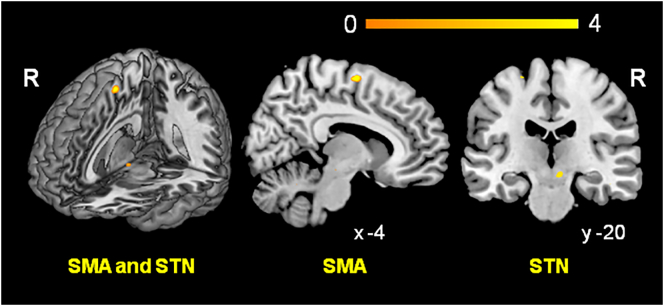
3.3. Functional connectivity
To assess the functional connectivity with the left amygdala we calculated a PPI for the amygdala activation cluster revealed by the group interaction for affected side [(sad-affected > calm-affected) × (patients > controls)] in the analysis with the flipped data. Significantly increased functional connectivity was observed with the (pre-)SMA (Table 2 and Fig. 3). Since the STN has a pivotal role in global stopping (Aron, 2011, Wessel and Aron, 2017), we assessed post-hoc whether there was evidence for functional connectivity between amygdala and STN; using only the STN mask for SVC we found evidence for increased connectivity between amygdala and STN (Table 2 and Fig. 3). Small volume corrections for VMPFC and precuneus revealed no suprathreshold clusters. The PPI within the non-flipped data set revealed no increased functional connectivity with the left or the right amygdala.
Fig. 3.
Result of the psycho-physiological interaction analysis (PPI): functional connectivity with the left amygdala as resulting from group interaction condition [(sad-affected > calm-affected) × (patients vs. controls)]. Patients showed increased functional connectivity with the (pre-)supplemental motor area (SMA) and, post-hoc, the subthalamic nucleus (STN) (see Methods). For presenting purposes the statistical maps were thresholded at p < 0.005 unc.
3.4. Activation during pure, emotionally neutral sensorimotor stimulation
The results for pure passive movement without emotional stimulation in comparison to a group of healthy controls simulating paresis are reported elsewhere (Hassa et al., 2016). Of note, during emotionally neutral passive movement conversion disorder patients displayed no altered activity in any emotion processing area. This indirectly supports our notion of an altered interaction of affective and motor networks which is only present during simultaneous affective and sensorimotor stimulation.
4. Discussion
The aim of our study was to identify the potential link between altered emotional processing and a physical symptom, here the affected motor network, in conversion disorder. To this end we applied an fMRI paradigm that stimulated simultaneously both emotional and the motor networks. Only during simultaneous negative emotional stimulation and passive movement of the affected hand conversion disorder patients as compared to healthy control participants displayed hyperactivation of the left amygdala as clear evidence for altered emotional processing in conversion disorder. The amygdala has a pivotal role in emotion processing and emotional memory (Richter-Levin, 2004). Emotional conflict itself leads to activation of the amygdala (Etkin et al., 2006) and it is functionally connected to implicit integration of affects and drives (Mega et al., 1997). The amygdala plays a key role in changing the neurochemical environment of the prefrontal cortex during stress resulting in prefrontal cortical dysfunction and changing motor habits (Arnsten, 2009). Hence, the amygdala is an appropriate structural substrate for altered emotional processing in conversion disorder and a possible link to the motor system.
Enhanced amygdala activity for emotional stimuli in conversion disorder has previously been reported. However, amygdala hyperactivity was not specific for negative emotion and not related to a motor function (Voon et al., 2010a). In line with Voon and colleagues (Voon et al., 2010a) we did not observe enhanced amygdala activity during pure negative emotional visual stimulation in conversion disorder patients. In the study by Voon and colleagues right amygdala hyperactivity was driven by increased activity related to the happy emotions (Voon et al., 2010a) pointing towards dysfunctional emotion discrimination in conversion disorder. Amygdala hyperactivity in the present study cannot reflect an unspecific effect of heightened attention due to input of two different sensory channels because the emotional stimulation was controlled for neutral visual stimuli. In the context of conversion disorder it is intriguing to think that the passive movement of a hemiplegic limb in a negative emotional situation confronts the patient with his motor deficit, links it directly to negative experience and may enhance specifically emotional processing thus reinforcing the amygdala hyperactivity to a detectable threshold.
To provide further evidence for the interaction of emotion processing and of motor control networks, we assessed the psychophysiological interaction of the left amygdala during motor execution and simultaneous processing of negative emotions. This revealed significantly increased functional connectivity between the amygdala and the (pre-)SMA in patients as compared to healthy control participants. The pre-SMA is involved in motor inhibition and is reliably recruited during stopping tasks (Bari and Robbins, 2013, Sebastian et al., 2013, Spieser et al., 2015, Watanabe et al., 2015). Beyond this, Aron and colleagues showed a white matter tract connecting the (pre-)SMA, IFG and the STN using diffusion-weighted imaging tractography which could underlie a pathway for basal ganglia control (Aron et al., 2007). FMRI revealed the same functional–anatomical network comprising right (pre-)SMA, IFG and STN during response inhibition in a stop signal paradigm referring to a model that was first presented by Nambu and colleagues (Nambu et al., 2002). Later, these findings led to a concept of different inhibitory control systems (reactive vs. proactive) with the reactive implementing a global stop system including the so-called “hyperdirect” IFG-STN pathway. The hyperdirect pathway supposedly initiates STN stimulation of GP leading to global suppression of thalamocortical circuits (Aron, 2011). Given the central role of the STN in global stopping (Aron, 2011, Wessel and Aron, 2017), we post-hoc assessed whether there was some evidence for altered connectivity between amygdala and STN in conversion disorder. Using only the STN mask for small volume correction we indeed found preliminary evidence for increased connectivity between amygdala and STN in patients as compared to healthy controls. Although this cluster did not survive more conservative corrections when using the combined search volume for the motor control network, this finding in combination with significantly increased functional connectivity between amygdala and (pre-)SMA suggests a direct interaction of the amygdala with the hyperdirect motor inhibition network and a modulation of the motor output by the amygdala. Previous studies using emotional stimulation or potentially traumatic memories have provided evidence for increased functional connectivity of amygdala with SMA, hence, increased motor planning/selection coupling (Aybek et al., 2015, Voon et al., 2010a). Similarly, a recent meta-analysis revealed alterations in conversion disorder patients as compared to healthy controls in areas implicated in motor-planning and -selection, intentional behavior, volition, and affective functions such as the frontal and prefrontal cortices, ACC, and amygdala (Boeckle et al., 2016). To our best knowledge this is the first report of detecting a direct interaction between a central emotion processing area and this motor inhibition loop in conversion disorder.
The analysis of the flipped and non-flipped data sets revealed slightly different results that might point to laterality of amygdala activation in motor conversion disorder. We observed activation of the right amygdala in a post-hoc analysis of the non-flipped data but not of the flipped data, while both analyses resulted in left amygdala activity in patients as compared to healthy participants during negative emotion processing and simultaneous passive movement of the affected hand. Of note, nine patients were affected on the right side and only four patients were affected on the left side. For the analysis of the flipped data sets, only the data of the four left affected patients were flipped. This might suggest the intriguing hypothesis that there is lateralized activation of the amygdala contralateral to the affected side and therefore in the same hemisphere as the corresponding motor network. However, the evidence for a laterality effect is quite vague and must be seen as preliminary. A positive functional connectivity of the amygdala (respectively left or right) with the lateralized, right-sided inhibition network could have supported this hypothesis but we found no evidence for that connectivity. The lacking effect in the PPI analyses could be due to the small size of the two subgroups of left and right affected patients but this stays hypothetical. Follow-up studies systematically comparing left- and right affected patients are necessary to investigate laterality effects of amygdala hyperactivation underlying the interaction of emotion processing and motor networks in motor conversion disorder.
For the interaction contrast of simultaneous emotional and sensorimotor stimulation of the unaffected hand we found increased activation in the right gyrus angularis in patients as compared to normal controls. The gyrus angularis is connected to body representation and to self-referential memory processing (Luckmann et al., 2014). Findings of activation in the gyrus angularis in conversion disorder patients are inconsistent so far. One study reported enhanced activity in the gyrus angularis during recall of negative life events (Aybek et al., 2014) which was interpreted as symptom-related alteration of body schema. In contrast, in another study hypoactivity in right gyrus angularis was interpreted as lack of appropriate sensory prediction signal leading to maladaptive attribution of self-reference of movements (Voon et al., 2010b). However, in the present study activation of the gyrus angularis was observed only in the interaction contrast concerned with the unaffected hand. Apart from this it is to stress that no activation of emotion processing areas like amygdala was observed in this contrast suggesting that the passive movement of the unaffected hand did not elicit maladaptive emotional processing.
The midline structures are perfectly suited for mediating between emotional relevant memory, internal representations and the motor system (Vuilleumier, 2014). Yet, neither simultaneous emotional and sensorimotor stimulation of the affected hand nor the functional connectivity analysis (see above) revealed activation in midline structures as the VMPFC and the precuneus. We had specifically expected to find ACC activation since this area has been suggested to provide a link between emotion processing and motor control in conversion disorder (Ballmaier and Schmidt, 2005). Yet, we found no evidence for altered activity of midline structures in conversion disorder patients. Similarly, in pure passive movement without emotional stimulation no altered activation in this area was observed (Hassa et al., 2016). These results thus are - although unexpected - in line with the bulk of the literature as only a minority of imaging studies showed altered activity in the VMPFC or the precuneus (Bell et al., 2011).
Assessing motor symptoms in motor conversion disorder bears disorder inherent difficulties. We chose to use passive movement of the wrist to keep the sensorimotor stimulation as constant as possible across different degrees of paresis and, most importantly, to secure independence of patients' subjective intention and motivation to initiate and execute a movement. Moreover, passive movement assures comparable movement across all participants included in the study, patients as well as healthy controls. It has been demonstrated that passive movement reliably and robustly activates the sensorimotor network (Weiller et al., 1996) and is independent of the concurrent psychogenic paresis (Voon et al., 2010a). This approach may, however, be inappropriate to capture conversion disorder specific characteristics pertaining to difficulties in generating movements rather than executing a movement. Therefore, others have assessed motor symptoms by means of paradigms such as action selection (Voon et al., 2011), movement observation (Burgmer et al., 2006, Liepert et al., 2011) or movement imagination (de Lange et al., 2007, Liepert et al., 2011). These approaches, however, cannot control for patients' subjective intention, motivation and effort to initiate a movement. Recently it has been shown that passive movement of the affected limb in conversion disorder patients as compared to healthy controls is associated with altered brain activity in bilateral inferior frontal gyri (Hassa et al., 2016). Altered IFG activity has been shown to be involved in conversion disorder by other studies as well (Bryant and Das, 2012, Kanaan et al., 2007, Mailis-Gagnon et al., 2003, Stone et al., 2007). This suggests that movement execution by means of passive movement can be used to capture motor symptoms in motor conversion disorder. Since studies using other approaches than passive movement have also revealed altered brain activation in conversion disorder it is reasonable to assume that both, initiation and execution of movements might be affected in motor conversion disorder (but see Vuilleumier, 2014 for an alternative view suggesting that specifically motor execution rather than motor initiation or motor imagery is affected in conversion disorder). To disentangle neural correlates of movement initiation and movement execution as well as their potential overlap in conversion disorder, future studies should combine different approaches assessing different stages of movements. Since the present results suggest that an altered interaction of motor and emotion networks is crucial for conversion disorder, these paradigms should combine motor and affective tasks to test both networks separately as well as simultaneously.
5. Conclusion
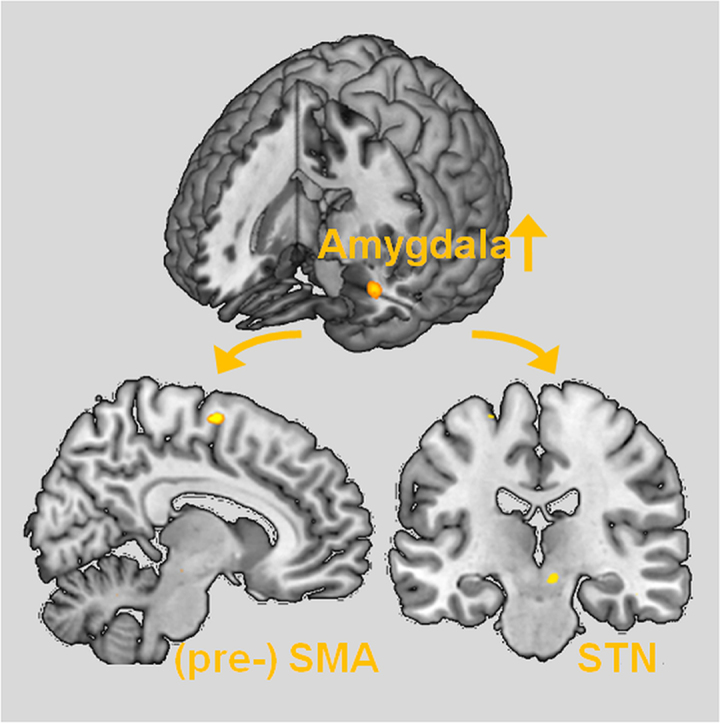
Simultaneous motor and emotional stimulation induces enhanced amygdala activation reflecting altered emotional processing in motor conversion disorder. This amygdala hyperactivation is functionally connected to a network known for motor inhibition. We therefore provide first evidence for a direct influence of altered emotional processing on a motor control circuitry, hence, “converting” emotional dysregulation, i.e. emotion and symptom specific amygdala hyperactivity, into motor inhibition thereby eliciting conversion symptoms in conversion disorder patients.
Declaration of interest
The authors report no financial or other relationship relevant to the subject of this article.
Financial support
This study was funded by Stiftung Schmieder für Wissenschaft und Forschung.
Acknowledgements
Author contributions: Dr. Hassa had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Hassa, Sebastian, Liepert, Weiller, Schmidt, Tüscher.
Analysis and interpretation of data: Hassa, Sebastian, Liepert, Schmidt, Tüscher.
Drafting of the article: Hassa, Sebastian, Schmidt, Tüscher.
Critical revision of the manuscript for important intellectual content: Hassa, Sebastian, Liepert, Weiller, Schmidt, Tüscher.
References
- Anderson M.C., Ochsner K.N., Kuhl B., Cooper J., Robertson E., Gabrieli S.W., Glover G.H., Gabrieli J.D. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Behrens T.E., Smith S., Frank M.J., Poldrack R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., Zelaya F., O'Daly O.G., Craig T.J., David A.S., Kanaan R.A. Neural correlates of recall of life events in conversion disorder. JAMA Psychiat. 2014;71:52–60. doi: 10.1001/jamapsychiatry.2013.2842. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., O'Daly O., Zelaya F., Kanaan R.A., David A.S. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M., Schmidt R. Conversion disorder revisited. Funct. Neurol. 2005;20:105–113. [PubMed] [Google Scholar]
- Bari A., Robbins T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bell V., Oakley D.A., Halligan P.W., Deeley Q. Dissociation in hysteria and hypnosis: evidence from cognitive neuroscience. J. Neurol. Neurosurg. Psychiatry. 2011;82:332–339. doi: 10.1136/jnnp.2009.199158. [DOI] [PubMed] [Google Scholar]
- Boeckle M., Liegl G., Jank R., Pieh C. Neural correlates of conversion disorder: overview and meta-analysis of neuroimaging studies on motor conversion disorder. BMC Psychiatry. 2016;16:195. doi: 10.1186/s12888-016-0890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Das P. The neural circuitry of conversion disorder and its recovery. J. Abnorm. Psychol. 2012;121:289–296. doi: 10.1037/a0025076. [DOI] [PubMed] [Google Scholar]
- Burgmer M., Konrad C., Jansen A., Kugel H., Sommer J., Heindel W., Ringelstein E.B., Heuft G., Knecht S. Abnormal brain activation during movement observation in patients with conversion paralysis. NeuroImage. 2006;29:1336–1343. doi: 10.1016/j.neuroimage.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cojan Y., Waber L., Carruzzo A., Vuilleumier P. Motor inhibition in hysterical conversion paralysis. NeuroImage. 2009;47:1026–1037. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Criaud M., Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- D'Alberto N., Funnell M., Potter A., Garavan H. A split-brain case study on the hemispheric lateralization of inhibitory control. Neuropsychologia. 2017;99:24–29. doi: 10.1016/j.neuropsychologia.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Freud S. S. Fischer Verlag; 1896. Zur Ätiologie der Hysterie. Hysterie und Angst; pp. 51–82. [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Hassa T., de Jel E., Tuescher O., Schmidt R., Schoenfeld M.A. Functional networks of motor inhibition in conversion disorder patients and feigning subjects. Neuroimage Clin. 2016;11:719–727. doi: 10.1016/j.nicl.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R.A., Craig T.K., Wessely S.C., David A.S. Imaging repressed memories in motor conversion disorder. Psychosom. Med. 2007;69:202–205. doi: 10.1097/PSY.0b013e31802e4297. [DOI] [PubMed] [Google Scholar]
- de Lange F.P., Roelofs K., Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia. 2007;45:2051–2058. doi: 10.1016/j.neuropsychologia.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lange F.P., Toni I., Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48:1782–1788. doi: 10.1016/j.neuropsychologia.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Liepert J., Hassa T., Tuscher O., Schmidt R. Abnormal motor excitability in patients with psychogenic paresis. A TMS study. J. Neurol. 2009;256:121–126. doi: 10.1007/s00415-009-0090-4. [DOI] [PubMed] [Google Scholar]
- Liepert J., Hassa T., Tuscher O., Schmidt R. Motor excitability during movement imagination and movement observation in psychogenic lower limb paresis. J. Psychosom. Res. 2011;70:59–65. doi: 10.1016/j.jpsychores.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Luckmann H.C., Jacobs H.I., Sack A.T. The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Prog. Neurobiol. 2014;116:66–86. doi: 10.1016/j.pneurobio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Mailis-Gagnon A., Giannoylis I., Downar J., Kwan C.L., Mikulis D.J., Crawley A.P., Nicholson K., Davis K.D. Altered central somatosensory processing in chronic pain patients with “hysterical” anesthesia. Neurology. 2003;60:1501–1507. doi: 10.1212/wnl.60.9.1501. [DOI] [PubMed] [Google Scholar]
- Marshall J.C., Halligan P.W., Fink G.R., Wade D.T., Frackowiak R.S. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–B8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Mega M.S., Cummings J.L., Salloway S., Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. J. Neuropsychiatry Clin. Neurosci. 1997;9:315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- Nambu A., Tokuno H., Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci. Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Patel N.K., Khan S., Gill S.S. Comparison of atlas- and magnetic-resonance-imaging-based stereotactic targeting of the subthalamic nucleus in the surgical treatment of Parkinson's disease. Stereotact. Funct. Neurosurg. 2008;86:153–161. doi: 10.1159/000120427. [DOI] [PubMed] [Google Scholar]
- Perez D.L., Barsky A.J., Daffner K., Silbersweig D.A. Motor and somatosensory conversion disorder: a functional unawareness syndrome? J. Neuropsychiatry Clin. Neurosci. 2012;24:141–151. doi: 10.1176/appi.neuropsych.11050110. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G. The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist. 2004;10:31–39. doi: 10.1177/1073858403259955. [DOI] [PubMed] [Google Scholar]
- Roelofs K., de Bruijn E.R., Van Galen G.P. Hyperactive action monitoring during motor-initiation in conversion paralysis: an event-related potential study. Biol. Psychol. 2006;71:316–325. doi: 10.1016/j.biopsycho.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sebastian A., Pohl M.F., Kloppel S., Feige B., Lange T., Stahl C., Voss A., Klauer K.C., Lieb K., Tuscher O. Disentangling common and specific neural subprocesses of response inhibition. NeuroImage. 2013;64:601–615. doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Sebastian A., Jung P., Neuhoff J., Wibral M., Fox P.T., Lieb K., Fries P., Eickhoff S.B., Tuscher O., Mobascher A. Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Struct. Funct. 2016;221:1635–1651. doi: 10.1007/s00429-015-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Kim H., Johnstone T., Alexander A.L., Whalen P.J. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol. Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spieser L., van den Wildenberg W., Hasbroucq T., Ridderinkhof K.R., Burle B. Controlling your impulses: electrical stimulation of the human supplementary motor complex prevents impulsive errors. J. Neurosci. 2015;35:3010–3015. doi: 10.1523/JNEUROSCI.1642-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A., Fiess J., Schmidt R., Rockstroh B. “That pulled the rug out from under my feet!” - adverse experiences and altered emotion processing in patients with functional neurological symptoms compared to healthy comparison subjects. BMC Psychiatry. 2015;15:133. doi: 10.1186/s12888-015-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Zeman A., Simonotto E., Meyer M., Azuma R., Flett S., Sharpe M. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom. Med. 2007;69:961–969. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C., Ameli R., Roelofs K., LaFrance W.C., Jr., Hallett M. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Gallea C., Hattori N., Bruno M., Ekanayake V., Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C., Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov. Disord. 2011;26:2396–2403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol. Clin. 2014;44:323–337. doi: 10.1016/j.neucli.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hanajima R., Shirota Y., Tsutsumi R., Shimizu T., Hayashi T., Terao Y., Ugawa Y., Katsura M., Kunimatsu A., Ohtomo K., Hirose S., Miyashita Y., Konishi S. Effects of rTMS of pre-supplementary motor area on fronto basal ganglia network activity during stop-signal task. J. Neurosci. 2015;35:4813–4823. doi: 10.1523/JNEUROSCI.3761-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C., Juptner M., Fellows S., Rijntjes M., Leonhardt G., Kiebel S., Muller S., Diener H.C., Thilmann A.F. Brain representation of active and passive movements. NeuroImage. 1996;4:105–110. doi: 10.1006/nimg.1996.0034. [DOI] [PubMed] [Google Scholar]
- Wessel J.R., Aron A.R. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron. 2017;93:259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Health Organization, W.H.O . WHO; Geneva, Switzerland: 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. [Google Scholar]