Abstract
The process of neurodegeneration in Parkinson's disease begins long before the onset of clinical motor symptoms, resulting in substantial cell loss by the time a diagnosis can be made. The period between the onset of neurodegeneration and the development of motoric disease would be the ideal time to intervene with disease modifying therapies. This pre-motor phase can last many years, but the lack of a specific clinical phenotype means that objective biomarkers are needed to reliably detect prodromal disease. In recent years, recognition that patients with REM sleep behaviour disorder (RBD) are at particularly high risk of future parkinsonism has enabled the development of large prodromal cohorts in which to investigate novel biomarkers, and neuroimaging has generated some of the most promising results to date. Here we review investigations undertaken in RBD and other pre-clinical cohorts, including modalities that are well established in clinical Parkinson's as well as novel imaging methods. Techniques such as high resolution MRI of the substantia nigra and functional imaging of Parkinsonian brain networks have great potential to facilitate early diagnosis. Further longitudinal studies will establish their true value in quantifying prodromal neurodegeneration and predicting future Parkinson's.
Highlights
-
•
Parkinson’s disease (PD) has a long prodromal phase prior to the emergence of motor symptoms.
-
•
Neuroimaging in this phase may aid identification of individuals at highest risk of near-term conversion to clinical PD.
-
•
Promising biomarkers include high resolution MRI of the substantia nigra and imaging of functional brain networks.
-
•
Long term follow-up studies are required to validate their use in pre-motor disease.
1. Introduction
Parkinson's Disease is currently diagnosed when the cardinal motor sign of bradykinesia appears alongside rigidity, tremor or postural instability (Reichmann, 2010). This clincal picture is caused by degeneration of dopaminergic neurons in the substantia nigra pars compacta and consequent denervation of the dorsal striatum (Galvan and Wichmann, 2008). The pathological hallmark of the disease is the presence of Lewy bodies within these structures, containing intracellular aggregations of misfolded alpha-synuclein. The anatomical origin of the disease process, however, is likely to be further afield, perhaps even outside of the brain (Braak et al., 2003, Braak and Del, 2008). Braak's hypothesis suggests that neurodegeneration is initiated in the lower brainstem and anterior olfactory structures before ascending to the basal ganglia and cortical areas in a characteristic sequence. In keeping with this, non-motor symptoms such as hyposmia, autonomic failure and sleep disturbances commonly emerge many years before motor parkinsonism (Postuma and Berg, 2016). The importance of developing reliable methods for diagnosis during this pre-motor phase is underlined by the observation that > 50% of nigral dopaminergic neurons and up to 80% of nigrostriatal synaptic activity have been lost by the time the motor phenotype emerges (Fearnley and Lees, 1991, Fuente-Fernandez et al., 2011). In the face of such extensive, irreversible neurodegeneration it may be too late to substantially alter the disease course with neuroprotective agents. However, whilst the constellation of prodromal symptoms in Parkinson's is well described, most features, for example depression and constipation, are non-specific (Noyce et al., 2012); even those with the highest predictive value for future disease development, such as REM sleep behaviour disorder, leave many questions unanswered regarding an individual's trajectory.
In recent years the terms pre-clinical and prodromal have been used to describe the period between the onset of neurodegeneration and the development of diagnostic motor signs, with pre-clinical referring to the asymptomatic phase and prodromal describing the emergence of non-motor symptoms as the disease progresses (Stern et al., 2012). Just as the natural history of established Parkinson's encompasses a broad spectrum of disease phenotypes, so the rate of progression through the pre-clinical and prodromal phases is likely to be highly variable. Post-mortem and neuroimaging studies suggest that degeneration within the basal ganglia may commence up to 7 years prior to diagnosis (Hawkes, 2008, Morrish et al., 1998), but prodromal symptoms such as hyposmia and REM sleep behaviour disorder can emerge decades earlier in some cases (Iranzo et al., 2013, Schenck et al., 2013). The potential window of opportunity that this creates for early therapeutic interventions has led to extensive research activity in search of biomarkers that can reliably detect Parkinson's during this period.
The ideal diagnostic imaging test would not only confirm the presence of prodromal Parkinson's but also give some information about the lead time to motoric disease and the rate of progression. This inevitably means that long term follow-up will be required before such tests can be validated against the gold standard of clinical diagnosis. Sensitivity to longitudinal change may provide a surrogate imaging marker and demonstration of this in serial assessments would be a valuable characteristic. Reproducibility is crucial as any neuroprotective trials are likely to require multi-site involvement to recruit sufficient numbers of prodromal subjects (Postuma et al., 2015). Finally, any imaging test to enter mainstream clinical practice must be cost efficient and widely available.
2. Pre-clinical and prodromal cohorts
When selecting populations in whom to investigate pre-motor neuroimaging tests, a balance must be struck between cohorts that are truly representative of idiopathic Parkinson's as a whole and cohorts that are sufficiently enriched for at-risk individuals to enable adequate numbers of positive results to be obtained. Population wide screening may capture the full spectrum of sporadic disease in its pre-clinical and prodromal phases, but is generally not feasible for neuroimaging studies when the outcome of interest is relatively rare. Narrowing down such cohorts by selecting for prodromal features or family history can overcome this problem, but only by selecting for a particular subtype of disease in whom findings may not be wholly generalizable.
Asymptomatic carriers of mutations that cause rare, monogenic forms of Parkinson's present the only opportunity to study a highly enriched cohort in the pre-clinical (rather than prodromal) phase. The most common monogenic cause of Parkinson's is mutation of the autosomal dominant Leucine Rich Repeat Kinase (LRRK2) gene (Paisán-Ruiz et al., 2013), and families harbouring LRRK2 mutations have been the subject of many neuroimaging studies. However, the incomplete penetrance of this mutation, ranging from 24 to 74% by age 80 in various study populations (Healy et al., 2008, Sierra et al., 2011, Marder et al., 2015), means that long term follow-up is still necessary to confirm true pre-clinical subjects. There is also evidence that the characteristics of the prodromal phase in genetic forms of Parkinson's vary according to the gene concerned, calling into question the generalizability of these findings to sporadic disease (McNeill et al., 2012, Brockmann et al., 2011a, Fernández-Santiago et al., 2016). Heterozygous carriers of genes causing recessive forms of Parkinson's have also been studied, but the risk of Parkinson's in these subjects is low and the form of Parkinson's seen in homozygotes exhibits a different pattern of neurodegeneration to sporadic disease and is therefore even less representative (McNeill et al., 2013, Scherfler et al., 2004). The size of genetic cohorts is also a limitation as large numbers of subjects are likely to be needed to power neuroprotective trials.
Selecting patients on the basis of prodromal features enables the development of large cohorts of at-risk individuals. Amongst the most consistent symptoms of prodromal disease is hyposmia: around 80% of people with Parkinson's have impaired olfaction and, in line with Braak's hypothesis, it is usually one of the earliest prodromal features to emerge (Postuma et al., 2012a). Hyposmia can be objectively quantified with standardised tests such as the self-administered University of Pennsylvania Smell Identification Test (Doty et al., 1984), or the investigator-administered Sniffin Sticks test (Hummel et al., 1997). Two prospective cohort studies have reported odds ratios of around 6 for the development of Parkinson's over a 3 to 4-year period in hyposmic individuals compared to those with normal olfaction (Ross et al., 2008, Berg et al., 2013a). The high sensitivity of hyposmia as a marker for the whole spectrum of Parkinson's phenotypes minimises bias in such prodromal cohorts. However, hyposmia is common in the population and relatively non-specific; its low predictive value means that large cohorts are required in order to capture incident Parkinson's.
Rapid eye movement sleep behaviour disorder (RBD) has emerged in recent years as the most promising prodromal marker (Boeve, 2013). This parasomnia is characterised by loss of the normal atonia that occurs during rapid eye movement (REM) sleep, causing patients to move in response to their dream content (Iranzo et al., 2016). Up to half of patients with Parkinson's have RBD (Rolinski et al., 2014), and around 20% report its onset prior to motor symptoms (Postuma and Berg, 2016). Importantly, long-term cohort studies suggest that as many as 90% of people who develop isolated RBD will go on to develop Parkinson's or another neurodegenerative disorder, most of which are also related to alpha-synuclein, such as Dementia with Lewy Bodies (DLB) or Multiple System Atrophy (MSA) (Iranzo et al., 2014a, Howell and Schenck, 2015). The positive predictive value of RBD therefore exceeds even that of LRRK2 mutation carriers. Although it could be argued that such a high conversion rate obviates the requirement for a diagnostic test in these individuals, the latency from development of RBD to diagnosis of Parkinson's is highly variable (Iranzo et al., 2013, Iranzo et al., 2014a). There remains a need for tests that can provide information regarding lead time and progression of prodromal neurodegeneration.
Whilst most prodromal neuroimaging research has been conducted in RBD cohorts, studying RBD is not without limitations. Firstly, we cannot yet predict which alpha-synucleinopathy these patients will develop in future. This may not matter if the objective is to treat with an agent that targets a common molecular pathway, such as monoclonal antibodies against alpha-synuclein. Furthermore, some promising imaging biomarkers may themselves shed light on the ultimate disease phenotype. A second limitation is that Parkinson's patients who develop RBD in the prodromal phase may represent a particular subtype of disease, with an akinetic-rigid motor phenotype and more severe non-motor symptoms such as depression, autonomic failure and cognitive impairment (Iranzo et al., 2016, Rolinski et al., 2014). Neuroimaging studies in established Parkinson's support this, with evidence of more widespread degeneration in Parkinson's patients with concomitant RBD (Kotagal et al., 2012, Boucetta et al., 2016). Finally, a male to female ratio of around 5:1 is typically seen in idiopathic RBD, compared with 2:1 in Parkinson's. Whether RBD is truly distributed in this pattern or simply more likely to be reported in men, the underrepresentation of women in these cohorts is a significant source of bias. Despite these limitations, imaging studies in RBD have generated some of the most promising prodromal biomarkers to date.
3. Neuroimaging in pre-motor Parkinson's
Development of a diagnostic imaging test for pre-clinical or prodromal Parkinson's is a significant challenge given that such a test has so far failed to supplant clinical diagnosis in established Parkinson's. However, recent expansion in the number of prodromal cohorts under investigation and the development of novel imaging techniques make this a rapidly advancing field with great near-term potential. This review will focus predominantly on those studies with diagnostic and prognostic potential for prodromal Parkinson's in general, rather than studies devoted to mechanisms of pathogenesis, phenotypic variation or cohort-specific factors, though these will be covered briefly when relevant. The wide range of imaging modalities that has been investigated can be broadly divided into three approaches: 1) those that target dopaminergic function in the basal ganglia 2) those that directly image the substantia nigra and 3) those assessing perturbations of disease-related brain networks.
3.1. Dopaminergic radiotracer imaging
Assessment of the integrity of dopaminergic circuits in the basal ganglia can be made using a range of radio-labelled ligands, detected with single photon emission computed tomography (SPECT) or positron emission tomography (PET) (Brooks, 2016). The most frequently labelled structure with SPECT is the presynaptic dopamine transporter (DAT), which is responsible for re-uptake of dopamine from the synaptic cleft by the pre-synaptic nerve terminal. Visualisation of this structure with 123I-FP-CIT (ioflupane) SPECT is currently the only imaging method to be approved by the Food and Drug Administration (FDA, United States) and the National Institute for Health and Care Excellence (NICE, United Kingdom) for the differentiation of parkinsonian syndromes from other causes of tremor. In this regard it can separate cases of parkinsonism from essential tremor with sensitivity of 95% and specificity of 93% (Benamer et al., 2000a), and consistently demonstrates greater specificity than clinical examination in diagnostically uncertain cases (Marshall et al., 2009, Jennings et al., 2004).
PET has been used with various radio-labelled ligands to interrogate aspects of dopaminergic transmission in the basal ganglia (Brooks, 2016, Stoessl, 2007). The presynaptic dopamine transporter (DAT) can be targeted with compounds such as 18F-FP-CIT, 11C-methylphenidate (11C-MP) or 18F-FE-PE2I (Sonni et al., 2016). The intracellular vesicular monoamine transporter (VMAT2) is responsible for re-packaging dopamine into vesicles, and is commonly labelled with 11C-dihydrotetrabenazine (11C-DTBZ) or 18F-fluoropropyl-dihydrotetrabenazine (18F-FP-DTBZ). 18F-DOPA is taken up by neurons in the same manner as L-DOPA, where it is a substrate for dopa decarboxylase; imaging this compound therefore reflects the brain's capacity to take up, process and store dopamine. Finally, ligands such as 11C-raclopride or 18F-fallypride can be used to measure binding to the post-synaptic dopamine D2 and D3 receptor, as can the SPECT compound 123I-IBZM (iodobenzamide).
The presynaptic DAT signal is depressed in the posterior putamen early in the course of Parkinson's disease and demonstrates characteristic asymmetry in the pattern of signal loss (Brooks and Tambasco, 2016). The level of striatal DAT binding in PD correlates with contralateral clinical measures of bradykinesia and rigidity (Benamer et al., 2000b), as well as with the degree of dopaminergic cell loss in the substantia nigra post-mortem (Kraemmer et al., 2014). It is sensitive to longitudinal changes, demonstrating a significantly faster rate of decline in patients with Parkinson's than in healthy controls (Marek et al., 2001, Pirker et al., 2003), although this must be interpreted with caution in the context of treatment with medications that might themselves lead to downregulation of DAT levels (Brooks, 2016). This potential confounding effect of medication is avoided when studying pre-motor Parkinson's, and the fact that substantial nigro-striatal cell loss occurs before the onset of motor symptoms makes dopaminergic radiotracer imaging an attractive modality to evaluate preclinical and prodromal disease. A major disadvantage of both PET and SPECT, however, is the exposure of subjects to ionising radiation. This is a particular limitation in younger subjects and in serial longitudinal studies where the cumulative risk of repeated scans may be unacceptable.
3.1.1. Dopaminergic imaging in hyposmic cohorts
Several studies using SPECT have demonstrated pathologically reduced DAT signal in a proportion of people with idiopathic hyposmia (Ponsen et al., 2004, Jennings et al., 2014, Sommer et al., 2004). In one cohort comprising relatives of Parkinson's patients, 18% of hyposmic individuals had an abnormal DAT SPECT scan, and the rate of signal loss on serial imaging was significantly higher than in subjects with normal olfaction (Ponsen et al., 2004). Of the seven hyposmic relatives with an abnormal baseline scan, the four with the lowest DAT binding developed Parkinson's within 2 years.
By far the largest study to evaluate DAT SPECT in prodromal Parkinson's is the Parkinson's Associate Risk Syndrome Study (PARS), which also selected patients on the basis of olfactory impairment (Jennings et al., 2014). 11% of 203 hyposmic subjects had abnormal DAT imaging, compared to 1% of 100 normosmic controls. The combination of hyposmia with two other known Parkinson's risk factors – male sex and constipation – increased the abnormal scan rate to > 40%. After 4 years, 51% of the hyposmic individuals with a DAT deficit converted to Parkinson's and these subjects had substantially faster decline in the DAT signal on serial imaging than hyposmic non-converters (Jennings et al., 2015). Further follow-up will establish the true predictive value of DAT SPECT in these subjects, but this already demonstrates substantial enrichment for a population-ascertained cohort. However, given the low overall incidence of PD in this group, identification of clinical features that can further risk stratify subjects prior to imaging will be important for future studies.
3.1.2. Dopaminergic imaging in REM Sleep Behaviour Disorder (RBD) cohorts
Early studies using PET and SPECT to image presynaptic and vesicular dopamine transporters in RBD confirmed that a deficit is detectable in these patients (Albin et al., 2000, Eisensehr et al., 2000), and subsequent larger cohorts comparing RBD subjects to healthy controls have demonstrated that around 20–40% of RBD patients have abnormal DAT imaging (Kim et al., 2010, Iranzo et al., 2010, Heller et al., 2016). The DAT deficit seen in RBD is less severe than in established Parkinson's (Eisensehr et al., 2003, Arnaldi et al., 2015), suggesting that dopaminergic imaging may have the potential to quantify progression through the prodromal phase. The presence of subtle motor abnormalities in RBD subjects is more predictive of a DAT deficit than hyposmia (Rupprecht et al., 2013, Stiasny-Kolster et al., 2005), consistent with longitudinal studies in RBD showing that motor signs occur late in the prodromal phase whereas olfaction is lost early (Postuma et al., 2012b). Reduced F-DOPA uptake in the putamen has also been reported in a single study of 11 RBD patients with concurrent major depressive disorder (Wing et al., 2015).
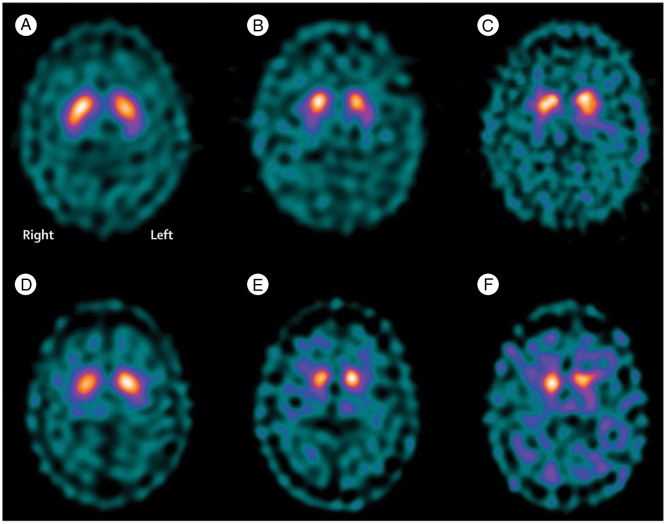
In one of the largest studies using DAT SPECT, Iranzo and colleagues found pathologically reduced 123I-FP-CIT binding in 17 out of 43 individuals with RBD (Iranzo et al., 2010). After 2.5 years, 6 of these subjects had developed an alpha-synuclein related neurodegenerative disorder (PD in 4 cases, DLB in one and MSA in one). However, two subjects with a normal SPECT scan also pheno-converted, whilst 65% of those with an abnormal scan remained disease free. A follow-up study with serial imaging in 20 RBD subjects suggests that the rate of decline in DAT signal may be a predictor of imminent conversion (Iranzo et al., 2011) (Fig. 1). Over 3 years, the DAT signal declined by an average of 6% per year in RBD subjects, compared to around 3% per year in controls. In the three subjects who converted to Parkinson's during follow-up, the rate of decline was 10%, and these three subjects had the lowest DAT signal at baseline assessment.
Fig. 1.
Serial SPECT images from two patients (A–C and D–F) with RBD, showing labelling of the basal ganglia with 123I-FP-CIT. Images are at baseline (A and D), 1.5 years (B and E) and 3 years (C and F). A greater signal decline over time is seen in the patient who developed Parkinson's (D–E) than in the patient who did not (A–C).
(Iranzo et al., 2011 reused with permission)
3.1.3. Dopaminergic imaging in genetic cohorts
The use of dopaminergic imaging as a pre-clinical biomarker in asymptomatic LRRK2 carriers has yielded some elegant results. In a cross-sectional study of a large Spanish cohort, DAT SPECT revealed pathologically reduced striatal uptake in 43.7% of 32 asymptomatic carriers (Sierra et al., 2013a). This figure closely matches the observed penetrance of the mutation and it will be interesting to see from longitudinal follow-up whether these individuals are indeed those who go on to develop clinical disease.
The use of three different PET ligands in a study of two North American kindreds revealed a pattern of progressive neurodegeneration in asymptomatic LRRK2 carriers that provides insight into the mechanism of pathogenesis at different pre-motor stages (Adams et al., 2005). Out of six asymptomatic carriers, all had normal F-DOPA uptake; four had abnormal DAT signal measured by 11C-MP, and two of these subjects had abnormal VMAT2 levels, measured by 11C-DTBZ. At follow-up after 2.5 years one of these two subjects had converted to clinical Parkinson's, and this was accompanied by the development of abnormal F-DOPA uptake (Nandhagopal et al., 2008). This intriguing result mirrors the changes seen in early Parkinson's, where compensatory mechanisms downregulate presynaptic DAT in the already reduced number of presynaptic terminals, and upregulate dopa decarboxylase (Lee et al., 2000). It follows that reduction of DAT might be the most sensitive marker of prodromal disease in the striatum, whilst loss of the ability to synthesise and store dopamine may be the critical factor that precipitates clinical motoric disease. Despite the histopathological differences that can occur between LRRK2-related and sporadic Parkinson's (Zimprich et al., 2004), affected relatives in this kindred had PET imaging findings that were indistinguishable from those seen in sporadic disease. Larger studies in other prodromal cohorts using this multiple PET ligand approach will be valuable in determining its capacity to delineate prodromal Parkinson's and predict conversion more generally.
Subtle deficits on dopaminergic radiotracer imaging have been demonstrated in asymptomatic heterozygotes carrying one copy of the parkin mutation (Pavese et al., 2010, Pavese et al., 2009) - a rare cause of recessive familial Parkinson's - and it has been suggested that there may be an increased risk of Parkinson's in these subjects. However, despite the presence of mild motor signs in some cases, the imaging and clinical deficits do not appear to deteriorate significantly with time (Khan et al., 2002). Furthermore, the pattern of striatal signal loss in homozygous individuals with parkinsonism is quite different from sporadic disease, with less sparing of the caudate nuclei (Scherfler et al., 2004). A similar story is reported with PINK1 heterozygotes, another gene causing recessive Parkinson's; despite moderate group-level reductions in F-DOPA uptake and subtle motor signs, the risk of developing overt Parkinson's appears to be low (Eggers et al., 2010). These findings, as well as the scarcity of such subjects, make recessive heterozygotes less promising as pre-clinical cohorts.
Mutations in the glucocerebrocidase (GBA) gene, which cause the autosomal recessive lysosomal storage disorder Gaucher's disease, are one of the most prevalent genetic risk factors for Parkinson's disease (Neumann et al., 2009). Heterozygous GBA mutations occur in around 4–7% of sporadic cases and are 5 times more common in PD patients than in controls (Sidransky et al., 2009). However, despite evidence of a more severe non-motor prodrome in these patients (Brockmann et al., 2011a), the only study to conduct F-DOPA PET in non-PD heterozygous carriers found no evidence of signal loss in the striatum (Goker-Alpan et al., 2012). There was, however, reduced striatal F-DOPA uptake in a small proportion of Gaucher's patients without Parkinsonism, potentially revealing a pre-parkinsonian disease state. On this evidence GBA heterozygotes in isolation are unlikely to be a useful pre-clinical cohort, but given the high frequency of GBA mutations the presence of these could potentially contribute to the risk stratification of population cohorts.
3.2. Imaging of the substantia nigra
Direct visualisation of the substantia nigra with neuroimaging has obvious appeal as a potential diagnostic approach given the specificity of degeneration in this area to parkinsonian disorders. Quantification of nigral tissue using cross-sectional imaging has been limited by the spatial resolution of these methods and the lack of sensitivity to detect subtle signal change in this area, though recent advances in MRI methodology have shown promise in overcoming these hurdles. Transcranial ultrasound on the other hand has been used for many years to image this key pathological area, and may have a role to play in pre-clinical risk stratification.
3.2.1. Transcranial sonography (TCS)
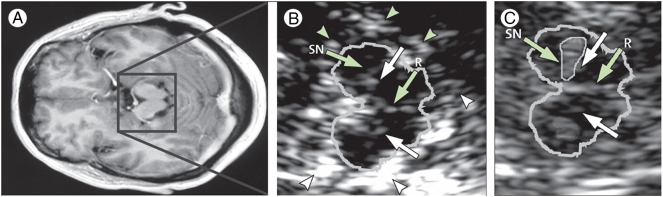
Transcranial ultrasound across the temporal bone can be used to directly visualise midbrain structures and qualitatively assess their echogenic properties. The key abnormality detected by this technique is increased echogenicity of the substantia nigra, which is consistently found in up to 90% of patients with Parkinson's disease (Berg et al., 2001, Ressner et al., 2007, Huang et al., 2007a, van de Loo et al., 2010) (Fig. 2). The origin of this signal is not fully understood but it has been shown to correlate with iron deposition (Berg et al., 2002) and microglia activation (Berg et al., 2010) in the substantia nigra. Hyperechogenicity does not appear to reflect the dynamics of the neurodegenerative process as the signal can be detected at an early age, does not change with time in established disease (Iova et al., 2004, Berg et al., 2005) and does not correlate with objective measures of disease severity (Berg et al., 2001). The diagnostic value of TCS is limited by the presence of an abnormal signal in around 10% of healthy subjects (Schroeder et al., 2013) and it does not accurately differentiate PD from other causes of parkinsonism (Bouwmans et al., 2013). Furthermore, insufficient bone windows in 10–20% of Caucasian individuals and up to 60% of people of Asian origin are an additional drawback (Berg et al., 2008). Despite these limitations, it is a safe, inexpensive and non-invasive test which makes it a good candidate for screening large unselected cohorts.
Fig. 2.
Transcranial ultrasound (TCS) of the midbrain. A: axial T1-weighted MRI at the level of the midbrain demonstrating the approximate area imaged by TCS in B and C. B: TCS of the midbrain (outlined) in an individual with normal appearances of the substantia nigra (SN). Arrowheads indicate signal from the surrounding basal cisterns. R = raphe. C: TCS from an individual with hyperechogenicity of the substantia nigra (SN, encircled within the midbrain outline). White arrows indicate the position of the red nuclei.
(Berg et al., 2008, reused with permission)
3.2.1.1. TCS in population studies
In a study using TCS in a population-ascertained cohort of 1847 older persons, 17% were found to have abnormal echogenicity of the substantia nigra (Berg et al., 2011). After 5 years, 21 individuals had developed Parkinson's, of whom 78% had an abnormal baseline signal, making the risk of Parkinson's in those with an abnormal test 20 times higher than in those without (Berg et al., 2013b). The high prevalence of hyperechogenicity in the population and relatively low conversion rates limited the positive predictive value of the test to only 6.5% at 5 years, but this study nevertheless demonstrates the ability of TCS to substantially enrich cohorts for at-risk individuals.
3.2.1.2. TCS in REM sleep behaviour disorder (RBD)
Hyperechogenicity of the substantia nigra has been demonstrated in 35–50% of patients with idiopathic RBD, in keeping with the recognised prodromal risk status in these individuals (Stockner et al., 2009, Iwanami et al., 2010, Shin et al., 2013, Iranzo et al., 2014b). As with established Parkinson's, the signal does not change with time in RBD and its ability to predict pheno-conversion in longitudinal studies is disappointing (Iranzo et al., 2014b). Several studies have found no correlation in RBD subjects between substantia nigra hyperechogenicity and dopaminergic dysfunction measured by DAT or PET (Iranzo et al., 2010, Rupprecht et al., 2013, Unger et al., 2008, Miyamoto et al., 2012). These results further suggest that the TCS finding may be a binary susceptibility marker in RBD rather than a quantifiable measure of neurodegeneration. The combination of TCS and DAT SPECT was shown to be 100% sensitive for conversion from RBD to a parkinsonian disorder in one study (Iranzo et al., 2010). Whilst this might provide some reassurance to those with normal imaging, the result is difficult to interpret given the lack of concordance between the two imaging modalities.
Interestingly, if the signal is truly static it ought to provide an estimate of the number of prodromal subjects in a given cohort, by comparing the rates of hyperechogenicity with those seen in Parkinson's and controls in the same centre. For example, Iwanami and colleagues (Iwanami et al., 2010) found an abnormal TCS signal in 14 out of 34 RBD subjects, as well as in 53% of Parkinson's patients and 10% of healthy controls. If the signal does not change over time, one could calculate that that the RBD cohort may be comprised of 25 individuals with prodromal Parkinson's and 9 without.
3.2.1.3. TCS in genetic cohorts
A number of studies have investigated TCS in asymptomatic LRRK2 carriers, the results of which highlight the complexity of genetic susceptibility factors in Parkinson's. Substantia nigra hyperechogenicity is reported in up to 90% of asymptomatic carriers (Sierra et al., 2013b, Bruggemann et al., 2011, Brockmann et al., 2011b, Vilas et al., 2015), a figure that exceeds the penetrance of the mutation. Furthermore, non-carrier relatives have markedly increased rates of hyperechogenicity compared to unrelated control subjects.(Vilas et al., 2015) These observations suggest the sonographic findings are the result of a more widespread genetic risk profile and are unlikely to be a useful predictor of future pheno-conversion in this population. Again, the limitations in generalizability using genetically predisposed cohorts are illustrated by such studies.
3.2.2. MRI of the substantia nigra
Whilst MRI is commonly used in clinical practice as an adjunctive test in the differential diagnosis of parkinsonian syndromes, reliable identification of the specific neurodegenerative signature of idiopathic PD has not been possible with routine imaging sequences (Brooks and Tambasco, 2016, Rizzo et al., 2016a). However, the capacity to resolve the key brainstem structures and identify relevant pathological changes has improved with the advent of high field strength MRI scanners and sequences targeting specific molecular processes. Some of the most promising results come from direct imaging of the substantia nigra.
Diffusion tensor imaging (DTI) has been used to assess the integrity of nigrostriatal fibres in Parkinson's in a number of studies. Reduced fractional anisotropy in the substantia nigra in PD patients compared to controls is frequently reported (Zhang et al., 2015) and reached significance in a recent meta-analysis (Cochrane and Ebmeier, 2013). However, there is substantial variation in results amongst DTI studies and as yet a consistent biomarker has not emerged that can accurately categorise individual patients (Schwarz et al., 2013). Evidence in prodromal conditions is limited to a small number of studies in RBD, which have yielded similarly mixed results. Group level changes in diffusion parameters have been reported in the substantia nigra (Unger et al., 2010, Garcia-Lorenzo et al., 2013), but this is not a consistent finding (Scherfler et al., 2010, Rahayel et al., 2015). Much of the variation between studies is a result of methodological differences and it remains to be seen whether DTI can reliably detect early nigral degeneration once this technique is further refined.
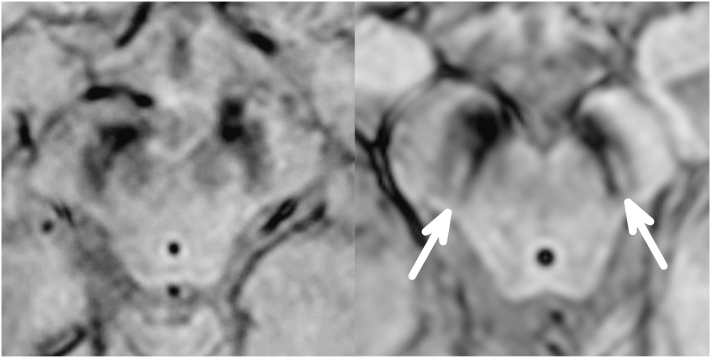
More promising has been the finding that high field strength MRI with T2*-weighted sequences reveals a characteristic high signal area within the dorsolateral substantia nigra that is present in healthy controls and lost in patients with Parkinson's. A study comparing in vivo 7-Tesla MRI with post mortem MRI and histopathology found that this signal corresponds to the anatomical location of nigrosome 1, the nigral area that sustains maximal loss of dopaminergic neurons in Parkinson's (Blazejewska et al., 2013). A follow-up study using clinical 3T MRI with a susceptibility weighted (SWI) sequence replicated this finding in a retrospective evaluation of 9 PD patients and 81 controls, and a prospective analysis of 10 PD patients and 9 controls (Schwarz et al., 2014). The authors coined the term ‘swallow tail sign’ in reference to the characteristic appearance of the presumed nigrosome 1 in healthy controls (Fig. 3). In blinded assessments of this sign by two independent neuroradiologists, accuracy against the gold standard clinical diagnosis was 96% for the retrospective series, with sensitivity of 100% and specificity of 95%. In the prospective study 8 of 9 controls and 8 of 10 PD patients were correctly classified. A subsequent study replicated the classification accuracy of this sign in PD and extended the finding to MSA and PSP, where it was 100% sensitive (Reiter et al., 2015).
Fig. 3.
Clinical high resolution 3D–T2*/SWI MRI of the midbrain showing the normal appearance of the substantia nigra (right image) with high intensity signal in the dorsolateral region, and absence of this feature (left image) in a patient with Parkinson's.
Only one study has investigated this sign in pre-clinical disease (de Marzi et al., 2016). De Marzi and colleagues performed SWI sequences in 15 RBD subjects, 104 Parkinson's patients and 42 healthy controls. The dorsal nigral hyperintensity was absent in 92% of PD cases, and present in 97% of controls. 77% of RBD patients also had absence of the sign. Such a high rate in RBD patients may indicate that this sign develops early in the prodromal phase, or that it indicates a susceptibility trait in the same manner as TCS hyperechogenicity. Further studies in larger and more diverse prodromal cohorts with longitudinal follow-up will determine whether the signal can stratify patients according to their prodromal stage, but even if it is a trait marker, the low positivity observed in controls would make its predictive value greater than that of TCS. A limitation of this technique at present is that in 5–10% of cases the images are of insufficient quality for diagnostic assessment, usually due to motion artifacts.
Another disease specific target of MRI that has emerged recently is neuromelanin, a pigment expressed in high quantities in the substantia nigra and locus coeruleus (Elstner et al., 2011). Neuromelanin is responsible for the for the dark colour of these structures and may contribute to their susceptibility to alpha-synuclein mediated neurodegeneration (Halliday et al., 2005). It is an iron chelator, which makes it visible to MR imaging due to the complex's paramagnetic properties. Modified T1-weighted sequences that are sensitive to neuromelanin display high signal intensity in areas where it is expressed and differences in this signal are observed between Parkinson's patients and controls (Sasaki et al., 2006). A recent study used an automated segmentation protocol with neuromelanin-sensitive images to make an objective assessment of focal atrophy in the substantia nigra (Castellanos et al., 2015); this technique separated PD patients from controls with an AUC of 0.93–0.94. In the context of prodromal disease, neuromelanin-sensitive sequences have been used in RBD patients, where loss of neuromelanin in the coeruleus/subcoeruleus complex is observed (Garcia-Lorenzo et al., 2013, Ehrminger et al., 2016). The technique has not yet been used to measure nigral degeneration in prodromal cohorts and it will be interesting to compare the sensitivity of this technique against SWI imaging in these subjects. Looking further ahead, new methods for automated MRI segmentation of brainstem structures may allow us to further resolve the key sites of prodromal neurodegeneration and thereby stage the disease process non-invasively (Lambert et al., 2013).
3.3. Imaging brain network activity
Many of the imaging techniques described so far either directly or indirectly measure changes in the structural integrity of brain regions involved in disease pathogenesis and as such they reveal a degree of irreversible neurodegeneration. Disruptions of normal brain network dynamics may instead reflect early changes in synaptic function caused by the accumulation of abnormal alpha-synuclein. The possibility that such functional changes precede neuronal death makes them of particular interest in pre-clinical diagnostics. Furthermore, changes in functional brain organisation can potentially reveal patterns that are more disease specific than structural abnormalities and may therefore be more predictive of future phenotype in prodromal cohorts (Niethammer and Eidelberg, 2012). Various imaging modalities have been applied to investigate network-level changes, measuring either metabolic activity, regional cerebral blood flow or changes in blood oxygenation. Most studies record these parameters in the resting state, avoiding the potential confounding factors in task-related imaging and therefore increasing their replicability and potential for translation into the clinical arena.
3.3.1. Imaging networks through metabolism and perfusion
PET is commonly used to measure metabolic activity in the brain with the ligand 18F–fluorodeoxyglucose (FDG). This gives a readout of cellular glucose uptake, which is physiologically most correlated to afferent synaptic activity and neuronal connectivity. Patterns of brain perfusion have been investigated in pre-motor Parkinson's primarily with SPECT, using 99mTc-Ethylene Cysteinate Dimer (ECD) as a marker of regional cerebral blood flow (rCBF). These two characteristics are not analogous, particularly in the context of medications that have vasoactive properties (Niethammer and Eidelberg, 2012); nevertheless, consistent patterns of brain activity have been demonstrated using both modalities. More recently, the development of arterial spin labelled (ASL) MRI has enabled brain perfusion to be imaged non-invasively and without exposure to ionising radiation. ASL is limited by a relatively poor signal to noise ratio and its clinical potential remains somewhat uncertain, but it has demonstrated similar network patterns to PET and SPECT in established PD (Melzer et al., 2011, Teune et al., 2014).
The identification of a spatial covariance pattern specific to Parkinson's disease has been one of the most promising applications of network imaging techniques. This Parkinson's Disease Related Pattern (PDRP) is detected using a principal component analysis (PCA)-based method known as the Scaled Subprofile Model (SSM). The approach, which is described in detail elsewhere (Spetsieris and Eidelberg, 2011), involves the extraction of biologically relevant signals from pooled control and patient data using a whole brain multivariate analysis. The process is entirely data driven and as such it does not depend on a priori hypotheses regarding specific brain areas. The PDRP is characterised by increased activity in the pallido-thalamic and pontine regions with relative cortical reductions in the premotor, supplementary motor and parietal association areas (Eidelberg, 2009). It is highly reproducible within subjects and between populations (Ma et al., 2007, Moeller et al., 1999) and in established Parkinson's demonstrates progression over time and correlation with clinical severity measures (Huang et al., 2007b, Eidelberg et al., 1995). All of these factors make it a potentially valuable biomarker in clinical neuroprotective trials. Other network patterns have also been described in Parkinson's using this approach, which appear to reflect particular phenotypic features such as tremor and cognitive impairment (Huang et al., 2008, Mure et al., 2011).
3.3.1.1. Brain perfusion and metabolism in prodromal cohorts
REM sleep behaviour disorder has been the focus of most of the studies to investigate metabolic and perfusion abnormalities in prodromal disease. Group level assessments of regional blood flow in RBD patients compared to controls have generated somewhat inconsistent results, but tend to show increased flow in subcortical regions, including the pons and putamen, and decreases in cortical areas (Mazza et al., 2006, Hanyu et al., 2011). Clinical measures of cognition, olfaction and colour vision have been reported to correlate with decreased perfusion in the hippocampus, anterior parahippocampal gyrus and visual cortex respectively, suggesting that these perfusion measures have clinical relevance in prodromal disease (Vendette et al., 2011, Vendette et al., 2012). Decreases in rCBF over time have also been found with serial imaging of RBD patients, perhaps indicating a biomarker sensitive to progressive neurodegeneration (Sakurai et al., 2014). A reproducible finding in RBD patients is increased perfusion in the hippocampus compared to controls, which is supported by the demonstration of a corresponding increase in metabolic activity in this region using FDG PET (Vendette et al., 2012, Dang-Vu et al., 2012, Ge et al., 2015). In one of the few longitudinal experiments to assess the predictive value of ECD SPECT, a larger increase in hippocampal perfusion was observed in 10 RBD patients who subsequently pheno-converted to parkinsonian disorders compared to 10 RBD patients who did not (Dang-Vu et al., 2012).
Tang and colleagues investigated PDRP expression in a pseudo pre-motor situation by measuring the clinically unaffected hemisphere of early Parkinson's patients with unilateral signs (Tang et al., 2010a). They found increased PDRP expression in the ‘asymptomatic’ hemisphere, which preceded the development of clinical disease in the contralateral limbs by 2 years. However, interpretation of this finding is limited by the fact that the PDRP signal was always symmetrical, which suggests that its expression on the ‘unaffected’ side may be attributable to contralateral basal ganglia degeneration.
Evidence that PDRP expression may be able to quantify the transition from prodromal to clinical Parkinson's is presented by Wu et al. (2014) in a comparison of the network between RBD, Parkinson's and control subjects using FDG PET. They found the PDRP to be expressed at intermediate levels in RBD subjects, significantly higher than in healthy controls but lower than patients with moderate PD. However, the increase in expression from RBD subjects to early stage Parkinson's did not reach significance. The authors also identified a distinct network in RBD subjects that was not present in controls and was expressed at lower levels in PD patients. They propose that this RBD related pattern is a marker of the prodromal phase of Parkinson's that decreases with the emergence of clinical disease, though since they did not exclude the presence of RBD in their PD cohort the specific relationship of this pattern to RBD itself cannot be assessed. Further investigation with longitudinal follow-up will no doubt resolve this question.
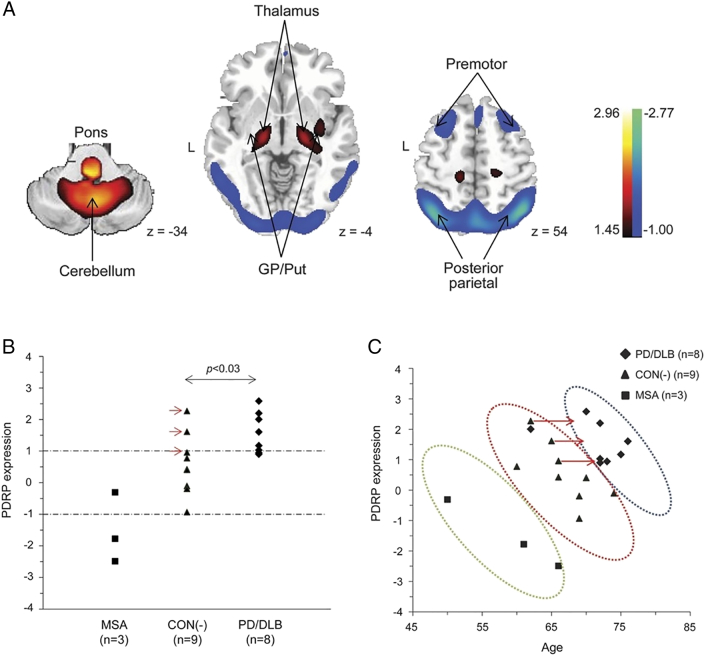
One benefit of the PDRP as a biomarker is that it can differentiate idiopathic PD and DLB from other parkinsonian disorders such as MSA and PSP (Tang et al., 2010b), something that is not possible with presynaptic dopaminergic radiotracer imaging alone (Brooks, 2016). This is important in the context of pre-motor disease as MSA in particular can exhibit a similar prodromal phenotype to Parkinson's disease. An intriguing recent study in RBD with considerable longitudinal follow up suggests that this distinction may be possible prior to the emergence of motoric disease (Holtbernd et al., 2014). Firstly, the presence of PDRP expression was confirmed in two RBD cohorts, using FDG PET and ECD SPECT respectively. The latter cohort was evaluated clinically after a 4.6-year mean follow-up period, during which 8 patients converted to either PD or dementia with Lewy Bodies (DLB). A logistic regression analysis combining baseline PDRP expression and age separated converters and non-converters into two distinct clusters. Further analysis of 3 RBD subjects who subsequently developed MSA showed that these formed a separate cluster using this model, which was largely accounted for by reduced PDRP expression compared to both non-converters and PD/DLB converters (fig. 4). The fact that converters to PD and DLB could not themselves be differentiated is unsurprising since these disorders likely represent a spectrum of common disease rather than truly distinct syndromes. It seems increasingly likely that pre-motor diagnosis will require a combination of both clinical and imaging biomarkers; this study provides encouraging evidence that the PDRP might contribute to such a model.
Fig. 4.
A: representation of the Parkinson's Disease Related spatial covariance pattern (PDRP) derived from FDG-PET imaging, with increased metabolic activity denoted in red-yellow and decreased metabolic activity in blue-green. B: baseline PDRP expression levels in 20 individuals with RBD, divided into those who remained disease free (CON(−)) and those went on to develop MSA or PD/DLB. C: A combination of PDRP expression and age was able to separate the three groups into distinct clusters. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
(Holtbernd et al., 2014 reused with permission)
3.3.2. Functional MRI (fMRI) networks
Fluctuations in the resting brain's activity can be inferred from changes in the blood oxygen level dependent (BOLD) signal detected with appropriate T2*-sensitive MRI sequences (Beckmann et al., 2005). Approaches to network characterisation generally involve either temporal correlation of this activity with a pre-defined region of interest or a data-driven approach, for example with independent component analysis (ICA) (Joel et al., 2011). The functional connectivity networks that emerge closely mirror patterns of activation observed in task-based fMRI experiments (Smith et al., 2009), suggesting that physiologically relevant interactions between brain regions can be probed in the resting state. Advantages of imaging brain connectivity with MRI are that such scans are short, non-invasive, free from ionising radiation and easy to acquire using widely-available hardware. Resting state functional MRI (rs-fMRI) can reveal perturbations of functional connectivity in neurodegenerative disorders in the absence of structural changes (Barkhof et al., 2014), in some cases decades before the onset of clinical disease (Filippini et al., 2009), suggesting that these methods are sensitive to early pathogenic processes.
Group level differences in functional connectivity (FC) between patients with established Parkinson's disease and controls have been observed in various brain regions, and the demonstration of correlations between FC and clinical measures is encouraging for the development of a quantifiable biomarker (Olde Dubbelink et al., 2014). One study has even reported a correlation between reduced FC in the sensorimotor network and reduced CSF alpha-synuclein levels, potentially indicating a more direct representation of the pathological process, since PD is associated with reduced total CSF alpha-synuclein compared to controls (Campbell et al., 2015, Mollenhauer et al., 2011). Disturbances of connectivity within the basal ganglia are a potentially disease-specific characteristic and these have been demonstrated in Parkinson's disease. Hacker et al. used a seed-based approach to assess connectivity between the striatum and brainstem, and as well as showing reduced FC in Parkinson's patients they demonstrated a connectivity gradient across the striatum that mirrors the known susceptibility of different striatal regions to PD-related neurodegeneration (Hacker et al., 2012). More recently, our group used an ICA-based approach to define a basal ganglia network (BGN) from a large group of control participants (Szewczyk-Krolikowski et al., 2014). Measurements of FC within this network differentiated Parkinson's patients from controls with 100% sensitivity and 89.5% specificity. This pattern of reduced connectivity in the BGN is robust to methodological variations (Griffanti et al., 2016) and is not seen in Alzheimer's neurodegeneration (Rolinski et al., 2015). Importantly, the reduction in FC seen in Parkinson's patients was reversed with levodopa, mirroring the clinical feature of levodopa responsiveness which forms an important part of PD diagnosis.
3.3.2.1. Functional connectivity in RBD
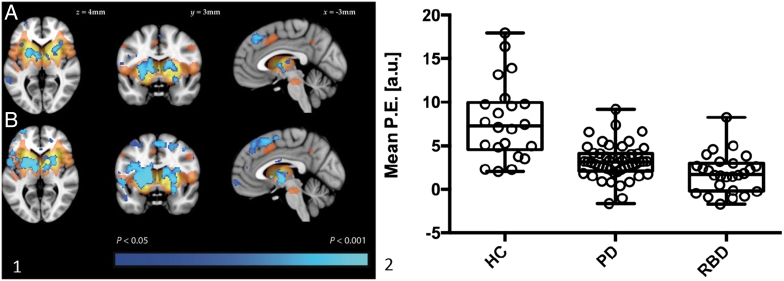
Only two studies have assessed rs-fMRI in patients with REM sleep behaviour disorder. Using the same ICA-derived template described above, we recently investigated functional connectivity in the basal ganglia network in 26 individuals with RBD compared with Parkinson's patients and healthy control participants (Rolinski et al., 2016). Interestingly, the reduction in BGN connectivity seen in RBD was of a similar magnitude to that in established Parkinson's, despite evidence of less striatal denervation in a subset of RBD patients who also underwent DAT SPECT (Fig. 5). Longitudinal follow-up is underway to assess the predictive value of the BGN signal for future pheno-conversion but this evidence suggests that connectivity disruptions may be a very early sign of pre-motor neurodegeneration. In contrast with our data, Ellmore and colleagues demonstrated an intermediate phenotype in RBD patients using a seed-based analysis to look at connectivity with the substantia nigra (SN) (Ellmore et al., 2013). The pattern was not always consistent, but correlations between left SN and putamen, and between right SN and superior occipital gyrus, were reduced in RBD patients compared to controls but not to the extent seen in PD patients. Both of these approaches require replication in other cohorts, as well as longitudinal investigations to assess the change in these signals with time.
Fig. 5.
Resting state MRI in individuals with RBD, PD and healthy controls. Panel 1 (left): resting state functional connectivity in PD (A) and RBD (B). The basal ganglia network template derived from a separate group of control participants is shown in red-yellow. Areas where functional connectivity was significantly reduced compared to controls is shown in blue. Panel 2 (right): extracted mean parameter estimates from the clusters with significantly different connectivity in both the PD versus control and RBD versus control comparisons. Box plots represent the group mean and quartiles with whiskers denoting minimum and maximum values. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
3.3.2.2. Functional connectivity in genetic cohorts
Studies in asymptomatic LRRK2 mutation carriers have also revealed pre-symptomatic changes in brain network activity. Two task-based fMRI studies have demonstrated increases in effective connectivity between basal ganglia and cortical regions including the premotor cortex (van Nuenen et al., 2012, Thaler et al., 2013), perhaps suggestive of mechanisms compensating for early neurodegeneration. Increased task-related premotor cortex activity has also been reported in heterozygous carriers of the PINK1 and parkin mutations (van Nuenen et al., 2009). Resting state connectivity using a seed-based approach in 37 asymptomatic LRRK2 carriers revealed a shift in the pattern of connectivity between the right inferior parietal cortex (IPC) and the putamen (Helmich et al., 2015). Compared to controls, the LRRK2 carriers had decreased connectivity between right IPC and dorsoposterior putamen and increased connectivity between right IPC and ventroanterior putamen, in keeping with early degeneration in the posterior regions and compensation anteriorly. Similar group-level cortico-striatal connectivity reductions have been seen in a separate LRRK2 cohort, which also reported correlations between FC and subcortical grey-matter volumes (Vilas et al., 2016).
Whilst these resting state fMRI studies support the idea that connectivity changes precede the development of motor Parkinson's, some limitations of the technique should be noted. The signal is rather sensitive to motion artifacts and shows test-retest variability within subjects. At present the reproducibility of findings amongst different cohorts remains to be established and it is uncertain whether the promising group-level results will translate in to biomarkers that can be used on an individual basis.
4. Concluding remarks and Future directions
The considerable volume of work dedicated to neuroimaging in pre-clinical and prodromal Parkinson's in recent years has generated a number of promising early biomarkers. In addition to results from imaging modalities that are well characterised in established Parkinson's, novel techniques analysing brain networks and nigral structure give particular cause for optimism. Developments in MRI biomarkers raise the tantalising possibility of a widely available, non-invasive diagnostic test for prodromal disease in the near future.
Despite this promise, some important obstacles remain to be overcome. Reproducibility is a major issue and several of the methodologies described in this review require further replication in distinct populations. Translation of group level differences into meaningful individual results is also a challenge, and is limited by the relatively small sample size in many studies. Few of the studies discussed include longitudinal follow-up, something that will be crucial both to establish the change in imaging signals over time as well as to validate putative biomarkers against clinical outcomes.
All of the imaging methods studied thus far give a somewhat indirect readout of disease status rather than a specific quantification of the underlying pathological process. This issue has led to problems in the study of other neurodegenerative diseases, particularly in the context of clinical trial design (Karran and Hardy, 2014), and given the fallibility of clinical diagnosis in up to 20% of Parkinson's disease cases (Rizzo et al., 2016b) this is an important consideration. The development of a method to image alpha-synuclein directly would address this, but there remain significant methodological obstacles to doing so. Compared to beta-amyloid, which has been successfully imaged with PET in Alzheimer's disease, alpha-synuclein is far less abundant and is predominantly intracellular (Eberling et al., 2013). If these issues are overcome, however, in vivo visualisation of the key pathological protein in Parkinson's could prove an important marker of disease progression and response to alpha-synuclein targeted therapies.
In the absence of a single diagnostic biomarker, the use of multimodal imaging also warrants further investigation. As well as providing a direct comparison of different imaging tools, the combination of these may yield more diagnostic information than either alone. There is already some evidence that combining transcranial sonography and DAT SPECT in persons with RBD increases the sensitivity for future pheno-conversion (Iranzo et al., 2010). Moreover, the sequential use of imaging tests may prove a more practical and cost effective way of screening cohorts for high-risk individuals (Sommer et al., 2004). Diagnostic algorithms that include clinical, genetic and molecular biomarkers in addition to imaging may have yet greater power to detect early disease. Such methods have been shown recently to have predictive value in subjects without evidence of dopaminergic dysfunction (SWEDDs) – individuals with parkinsonian signs but normal dopaminergic radiotracer imaging (Nalls et al., 2015).
Whichever imaging test ultimately proves most useful, methods of identifying at-risk individuals from the population to target with neuroimaging need to be both accurate and cost effective. Risk markers such as anosmia and constipation are not specific enough, whilst identification of RBD itself requires an invasive and costly diagnostic procedure. As recognition of RBD as an important clinical entity becomes more widespread, the development of new technologies to facilitate its diagnosis is clearly a priority. However, focusing solely on RBD will not capture the full spectrum of prodromal disease, and particularly neglects female patients. A more inclusive approach will require screening at the population level, which is likely to be a multi-stage process. To that end a set of new diagnostic criteria for prodromal Parkinson's proposed by the Movement Disorder Society may prove to be a valuable tool for large scale risk stratification.(Berg et al., 2015)
Alongside the advancement of imaging biomarkers must come improvements in the detection and quantification of clinical signs of parkinsonism. The current gold standard is the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) (Goetz et al., 2008), but whilst this is useful for the quantification of established PD it is likely to be insensitive to the progression of subtle motor signs in the prodromal phase and lacking in specificity in the elderly population (Bennett et al., 1996). The creation of precise, objective methods to quantify early motoric disease is another area where technology has great potential.
Finally, the advent of new diagnostic tools for pre-motor Parkinson's disease will only be worthwhile if effective disease modifying treatments become available. It is encouraging that trials of several re-purposed medications are currently underway in Parkinson's patients (https://www.cureparkinsons.org.uk/trials-repurposed-drugs, n.d.), with other disease-specific agents on the horizon (Schenk et al., 2016). The results of these promising interventions are eagerly awaited.
Acknowledgements
TB receives funding from the National Institute for Health Research Biomedical Research Centre, based at Oxford University Hospitals Trust, Oxford (grant code BRD00530). MH is funded by the OPDC Monument Discovery award (grant code AVR00610), the Oxford Biomedical Research Centre and National Institute of Health Research Clinical Research Network.
Footnotes
Financial or non-financial conflicts of interest relevant to the manuscript: None
Contributor Information
Thomas R. Barber, Email: thomas.barber@ndcn.ox.ac.uk.
Michele T.M. Hu, Email: michele.hu@ndcn.ox.ac.uk.
References
- https://www.cureparkinsons.org.uk/trials-repurposed-drugs
- Adams J.R., van Netten H., Schulzer M. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain. 2005;128:2777–2785. doi: 10.1093/brain/awh607. [DOI] [PubMed] [Google Scholar]
- Albin R.L., Koeppe R.A., Chervin R.D., Consens F.B., Wernette K., Frey K.A. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55(9):1410–1412. doi: 10.1212/wnl.55.9.1410. [DOI] [PubMed] [Google Scholar]
- Arnaldi D., de Carli F., Picco A., Ferrara M., Accardo J., Bossert I. Nigro-caudate dopaminergic deafferentation: a marker of REM sleep behavior disorder? Neurobiol. Aging. 2015;36(12):3300–3305. doi: 10.1016/j.neurobiolaging.2015.08.025. [DOI] [PubMed] [Google Scholar]
- Barkhof F., Haller S., Rombouts S.A. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014 Jul;272(1):29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005 May 29;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamer T.S., Patterson J., Grosset D.G. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT imaging: the [123I]-FP-CIT study group. Mov. Disord. 2000;15:503–510. [PubMed] [Google Scholar]
- Benamer H.T.S., Patterson J., Wyper D.J., Hadley D.M., Macphee G.J.A., Grosset D.G. Correlation of Parkinson's disease severity and duration with I-123-FP-CIT SPECT striatal uptake. Mov. Disord. 2000;15(July):692–698. doi: 10.1002/1531-8257(200007)15:4<692::aid-mds1014>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Bennett D.A., Beckett L.A., Murray A.M., Shannon K.M., Goetz C.G., Pilgrim D.M., Evans D.A. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N. Engl. J. Med. 1996 Jan 11;334(2):71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Berg D., Siefker C., Becker G. Echogenicity of the substantia nigra in Parkinson's disease and its relation to clinical findings. J. Neurol. 2001;248:684–689. doi: 10.1007/s004150170114. [DOI] [PubMed] [Google Scholar]
- Berg D., Roggendorf W., Schröder U., Klein R., Tatschner T., Benz P., Tucha O., Preier M., Lange K.W., Reiners K., Gerlach M., Becker G. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch. Neurol. 2002;59:999–1005. doi: 10.1001/archneur.59.6.999. [DOI] [PubMed] [Google Scholar]
- Berg D., Merz B., Reiners K., Naumann M., Becker G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson's disease. Mov. Disord. 2005 Mar;20(3):383–385. doi: 10.1002/mds.20311. [DOI] [PubMed] [Google Scholar]
- Berg D., Godau J., Walter U. Transcranial sonography in movement disorders. Lancet Neurol. 2008 Nov;7(11):1044–1055. doi: 10.1016/S1474-4422(08)70239-4. [DOI] [PubMed] [Google Scholar]
- Berg D., Godau J., Riederer P., Gerlach M., Arzberger T. Microglia activation is related to substantia nigra echogenicity. J. Neural Transm. 2010;117:1287–1292. doi: 10.1007/s00702-010-0504-6. [DOI] [PubMed] [Google Scholar]
- Berg D., Seppi K., Behnke S., Liepelt I., Schweitzer K., Stockner H., Wollenweber F., Gaenslen A., Mahlknecht P., Spiegel J., Godau J., Huber H., Srulijes K., Kiechl S., Bentele M., Gasperi A., Schubert T., Hiry T., Probst M., Schneider V., Klenk J., Sawires M., Willeit J., Maetzler W., Fassbender K., Gasser T., Poewe W. Enlarged substantia nigra hyperechogenicity and risk for Parkinson disease: a 37-month 3-center study of 1847 older persons. Arch. Neurol. 2011 Jul;68(7):932–937. doi: 10.1001/archneurol.2011.141. [DOI] [PubMed] [Google Scholar]
- Berg D., Godau J. The PRIPS study: screening battery for subjects at risk for Parkinson's disease. Eur. J. Neurol. 2013;20:102–108. doi: 10.1111/j.1468-1331.2012.03798.x. [DOI] [PubMed] [Google Scholar]
- Berg D., Behnke S., Seppi K., Godau J., Lerche S., Mahlknecht P., Liepelt-Scarfone I., Pausch C., Schneider N., Gaenslen A., Brockmann K., Srulijes K., Huber H., Wurster I., Stockner H., Kiechl S., Willeit J., Gasperi A., Fassbender K., Gasser T., Poewe W. Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson's disease. Mov. Disord. 2013 Feb;28(2):216–219. doi: 10.1002/mds.25192. [DOI] [PubMed] [Google Scholar]
- Berg D., Postuma R.B., Adler C.H. MDS research criteria for prodromal Parkinson's disease. Mov. Disord. 2015 Oct;30(12):1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- Blazejewska A.I., Schwarz S.T., Pitiot A. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology. 2013;81:534–540. doi: 10.1212/WNL.0b013e31829e6fd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve B.F. Idiopathic REM sleep behaviour disorder in the development of Parkinson's disease. Lancet Neurol. 2013 May;12(5):469–482. doi: 10.1016/S1474-4422(13)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucetta S., Salimi A., Dadar M., Jones B.E., Collins D.L., Dang-Vu T.T. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson's disease. Sci. Rep. 2016 Jun 1;6:26782. doi: 10.1038/srep26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmans A.E., Vlaar A.M., Mess W.H., Kessels A., Weber W.E. Specificity and sensitivity of transcranial sonography of the substantia nigra in the diagnosis of Parkinson's disease: prospective cohort study in 196 patients. BMJ Open. 2013 Apr 2;4(3) doi: 10.1136/bmjopen-2013-002613. (pii: e002613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Del T.K. Invited article: nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Braak H. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brockmann K., Srulijes K., Hauser A.K. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011 Jul 19;77(3):276–280. doi: 10.1212/WNL.0b013e318225ab77. [DOI] [PubMed] [Google Scholar]
- Brockmann K., Gröger A., di Santo A. Clinical and brain imaging characteristics in leucine-rich repeat kinase 2-associated PD and asymptomatic mutation carriers. Mov. Disord. 2011;26:2335–2342. doi: 10.1002/mds.23991. [DOI] [PubMed] [Google Scholar]
- Brooks D.J. Molecular imaging of dopamine transporters. Ageing Res. Rev. 2016 Sep;30:114–121. doi: 10.1016/j.arr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Brooks D.J., Tambasco N. Imaging synucleinopathies. Mov. Disord. 2016 Jun;31(6):814–829. doi: 10.1002/mds.26547. [DOI] [PubMed] [Google Scholar]
- Bruggemann N., Hagenah J., Stanley K. Substantia nigra hyperechogenicity with LRRK2 G2019S mutations. Mov. Disord. 2011;26(5):885e888. doi: 10.1002/mds.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M.C., Koller J.M., Snyder A.Z., Buddhala C., Kotzbauer P.T., Perlmutter J.S. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology. 2015;84:2413–2421. doi: 10.1212/WNL.0000000000001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos G., Fernández-Seara M.A., Lorenzo-Betancor O., Ortega-Cubero S., Puigvert M., Uranga J., Vidorreta M., Irigoyen J., Lorenzo E., Muñoz-Barrutia A., Ortiz-de-Solorzano C., Pastor P., Pastor M.A. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson's disease. Mov. Disord. 2015 Jun;30(7):945–952. doi: 10.1002/mds.26201. [DOI] [PubMed] [Google Scholar]
- Cochrane C.J., Ebmeier K.P. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013 Feb 26;80(9):857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu T.T., Gagnon J.F., Vendette M., Soucy J.P., Postuma R.B., Montplaisir J. Hippocampal perfusion predicts impending neurodegeneration in REM sleep behavior disorder. Neurology. 2012;79(24):2302–2306. doi: 10.1212/WNL.0b013e318278b658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marzi R., Seppi K., Högl B., Müller C., Scherfler C., Stefani A., Iranzo A., Tolosa E., Santamarìa J., Gizewski E., Schocke M., Skalla E., Kremser C., Poewe W. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann. Neurol. 2016 Jun;79(6):1026–1030. doi: 10.1002/ana.24646. [DOI] [PubMed] [Google Scholar]
- Doty R.L., Shaman P., Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Eberling J.L., Dave K.D., Frasier M.A. α-Synuclein imaging: a critical need for Parkinson's disease research. J Parkinsons Dis. 2013;3(4):565–567. doi: 10.3233/JPD-130247. [DOI] [PubMed] [Google Scholar]
- Eggers C., Schmidt A., Hagenah J., Brüggemann N. Progression of subtle motor signs in PINK1 mutation carriers with mild dopaminergic deficit. Neurology. 2010 Jun 1;74(22):1798–1805. doi: 10.1212/WNL.0b013e3181e0f79c. [DOI] [PubMed] [Google Scholar]
- Ehrminger M., Latimier A., Pyatigorskaya N., Garcia-Lorenzo D., Leu-Semenescu S., Vidailhet M. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016 Apr;139(Pt 4):1180–1188. doi: 10.1093/brain/aww006. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D., Moeller J.R., Ishikawa T. Assessment of disease severity in parkinsonism with fluorine-18-fluorodeoxyglucose and PET. J. Nucl. Med. 1995;36:378–383. [PubMed] [Google Scholar]
- Eisensehr I., Linke R., Noachtar S., Schwarz J., Gildehaus F.J., Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder. Comparison with Parkinson's disease and controls. Brain. 2000;123(Pt 6):1155–1160. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- Eisensehr I., Linke R., Tatsch K., Kharraz B., Gildehaus J.F., Wetter C.T. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease, and controls. Sleep. 2003;26(5):507–512. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- Ellmore T.M., Castriotta R.J., Hendley K.L., Aalbers B.M., Furr-Stimming E., Hood A.J. Altered nigrostriatal and nigrocortical functional connectivity in rapid eye movement sleep behavior disorder. Sleep. 2013;36:1885–1892. doi: 10.5665/sleep.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner M., Müller S.K., Leidolt L., Laub C., Krieg L., Schlaudraff F., Liss B., Morris C., Turnbull D.M., Masliah E., Prokisch H., Klopstock T., Bender A. Neuromelanin, neurotransmitter status and brainstem location determine the differential vulnerability of catecholaminergic neurons to mitochondrial DNA deletions. Mol Brain. 2011 Dec 21;4:43. doi: 10.1186/1756-6606-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley J.M., Lees A.J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fernández-Santiago R., Iranzo A., Gaig C. Absence of LRRK2 mutations in a cohort of patients with idiopathic REM sleep behavior disorder. Neurology. 2016 Mar 15;86(11):1072–1073. doi: 10.1212/WNL.0000000000002304. [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U. S. A. 2009 Apr 28;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente-Fernandez R. Age-specific progression of nigrostriatal dysfunction in Parkinson's disease. Ann. Neurol. 2011;69:803–810. doi: 10.1002/ana.22284. [DOI] [PubMed] [Google Scholar]
- Galvan A., Wichmann T. Pathophysiology of parkinsonism. Clin. Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lorenzo D., Longo-Dos Santos C., Ewenczyk C., Leu-Semescu S., Gallea C., Quattrocchi G. The locus coeruleus/subcoeruleus complex in rapid eye movement sleep behavior disorders in Parkinson's disease: a 3T MRI study. Brain. 2013;136:2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J., Wu P., Peng S., Yu H., Zhang H., Guan Y. Assessing cerebral glucose metabolism in patients with idiopathic rapid eye movement sleep behavior disorder. J. Cereb. Blood Flow Metab. 2015;35(12):2062–2069. doi: 10.1038/jcbfm.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008 Nov 15;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O., Masdeu J.C., Kohn P.D., Ianni A., Lopez G., Groden C., Chapman M.C., Cropp B., Eisenberg D.P., Maniwang E.D., Davis J., Wiggs E., Sidransky E., Berman K.F. The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain. 2012 Aug;135(Pt 8):2440–2448. doi: 10.1093/brain/aws174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Rolinski M., Szewczyk-Krolikowski K., Menke R.A., Filippini N., Zamboni G., Jenkinson M., Hu M.T., Mackay C.E. Challenges in the reproducibility of clinical studies with resting state fMRI: An example in early Parkinson's disease. NeuroImage. 2016 Jan 1;124(Pt A):704–713. doi: 10.1016/j.neuroimage.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain. 2012;135:3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G.M., Ophof A., Broe M., Jensen P.H., Kettle E., Fedorow H., Cartwright M.I., Griffiths F.M., Shepherd C.E., Double K.L. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease. Brain. 2005 Nov;128(Pt 11):2654–2664. doi: 10.1093/brain/awh584. (Epub 2005 Jul 6) [DOI] [PubMed] [Google Scholar]
- Hanyu H., Inoue Y., Sakurai H. Regional cerebral blood flow changes in patients with idiopathic REM sleep behavior disorder. Eur. J. Neurol. 2011;18:784–788. doi: 10.1111/j.1468-1331.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- Hawkes C.H. The prodromal phase of sporadic Parkinson's disease: does it exist and if so how long is it? Mov. Disord. 2008;23:1799–1807. doi: 10.1002/mds.22242. [DOI] [PubMed] [Google Scholar]
- Healy D.G., Falchi M. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008 Jul;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J., Brcina N., Dogan I., Holtbernd F., Romanzetti S., Schulz J.B., Schiefer J., Reetz K. Brain imaging findings in idiopathic REM sleep behavior disorder (RBD) - a systematic review on potential biomarkers for neurodegeneration. Sleep Med. Rev. 2016 Jun 25 doi: 10.1016/j.smrv.2016.06.006. (pii: S1087-0792(16)30057-0) [DOI] [PubMed] [Google Scholar]
- Helmich R.C., Thaler A., van Nuenen B.F. Reorganization of corticostriatal circuits in healthy G2019S LRRK2 carriers. Neurology. 2015;84(4):399–406. doi: 10.1212/WNL.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtbernd F., Gagnon J.F., Postuma R.B., Ma Y., Tang C.C., Feigin A. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–627. doi: 10.1212/WNL.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M.J., Schenck C.H. Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol. 2015 Jun;72(6):707–712. doi: 10.1001/jamaneurol.2014.4563. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Jeng J.S., Tsai C.F., Chen L.L., Wu R.M. Transcranial imaging of substantia nigra hyperechogenicity in a Taiwanese cohort of Parkinson's disease. Mov. Disord. 2007;22:550–555. doi: 10.1002/mds.21372. [DOI] [PubMed] [Google Scholar]
- Huang C., Tang C., Feigin A., Lesser M., Ma Y., Pourfar M., Dhawan V., Eidelberg D. Changes in network activity with the progression of Parkinson's disease. Brain. 2007;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Mattis P., Perrine K. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16 pt 2):1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T., Sekinger B., Wolf S.R., Pauli E., Kobal G. ‘Sniffin’ Sticks': olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem. Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Iova A. Postnatal decrease in substantia nigra echogenicity. Implications for the pathogenesis of Parkinson's disease. J. Neurol. 2004;251:1451–1454. doi: 10.1007/s00415-004-0556-3. [DOI] [PubMed] [Google Scholar]
- Iranzo A., Lomena F., Stockner H., Valldeoriola F., Vilaseca I., Salamero M. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idio- pathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2010;9(11):1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- Iranzo A., Valldeoriola F., Lomena F., Molinuevo J.L., Serradell M., Salamero M. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797–805. doi: 10.1016/S1474-4422(11)70152-1. [DOI] [PubMed] [Google Scholar]
- Iranzo A., Tolosa E., Gelpi E., Molinuevo J.L., Valldeoriola F., Serradell M., Sanchez-Valle R., Vilaseca I., Lomeña F., Vilas D., Lladó A., Gaig C., Santamaria J. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013 May;12(5):443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- Iranzo A., Fernández-Arcos A., Tolosa E., Serradell M., Molinuevo J.L., Valldeoriola F., Gelpi E., Vilaseca I., Sánchez-Valle R., Lladó A., Gaig C., Santamaría J. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014 Feb 26;9(2) doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A., Stockner H., Serradell M., Seppi K., Valldeoriola F., Frauscher B., Molinuevo J.L., Vilaseca I., Mitterling T., Gaig C., Vilas D., Santamaria J., Högl B., Tolosa E., Poewe W. Five-year follow-up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder. Mov. Disord. 2014 Dec;29(14):1774–1780. doi: 10.1002/mds.26055. [DOI] [PubMed] [Google Scholar]
- Iranzo A., Santamaria J., Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016 Apr;15(4):405–419. doi: 10.1016/S1474-4422(16)00057-0. [DOI] [PubMed] [Google Scholar]
- Iwanami M., Miyamoto T., Miyamoto M., Hirata K., Takada E. Relevance of substantia nigra hyperechogenicity and reduced odor identification in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11:361–365. doi: 10.1016/j.sleep.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Jennings D.L., Seibyl J.P., Oakes D., Eberly S., Murphy J., Marek K. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in parkinsonian syndrome: unmasking an early diagnosis. Arch. Neurol. 2004;61:1224–1229. doi: 10.1001/archneur.61.8.1224. [DOI] [PubMed] [Google Scholar]
- Jennings D. Imaging prodromal Parkinson's disease: the Parkinson associated risk syndrome study. Neurology. 2014;83:1739–1746. doi: 10.1212/WNL.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings D., Stern M., Siderowf A., Marek K. Longitudinal imaging and phenoconversion in the PARS prodromal cohort [abstract] Neurodegener. Dis. 2015;15(Suppl. 1):242. [Google Scholar]
- Joel S.E., Caffo B.S., van Zijl P.C., Pekar J.J. On the relationship between seed-based and ICA-based measures of functional connectivity. Magn. Reson. Med. 2011 Sep;66(3):644–657. doi: 10.1002/mrm.22818. (Epub 2011 Mar 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E., Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol. 2014 Aug;76(2):185–205. doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.L., Brooks D.J., Pavese N., Sweeney M.G., Wood N.W., Lees A.J. Progression of nigrostriatal dysfunction in a parkin kindred: an [18F]dopa PET and clinical study. Brain. 2002;125:2248–2256. doi: 10.1093/brain/awf237. [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Yoon I.Y., Kim J.M., Jeong S.H., Kim K.W., Shin Y.K. The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. Eur. J. Neurol. 2010;17(3):487–492. doi: 10.1111/j.1468-1331.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- Kotagal V., Albin R.L., Müller M.L., Koeppe R.A., Chervin R.D., Frey K.A., Bohnen N.I. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann. Neurol. 2012 Apr;71(4):560–568. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemmer J., Kovacs G.G., Perju-Dumbrava L., Pirker S., Traub-Weidinger T., Pirker W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov. Disord. 2014 Dec;29(14):1767–1773. doi: 10.1002/mds.25975. [DOI] [PubMed] [Google Scholar]
- Lambert C., Lutti A., Helms G., Frackowiak R., Ashburner J. Multiparametric brainstem segmentation using a modified multivariate mixture of Gaussians. NeuroImage: Clinical. 2013 May 16;2:684–694. doi: 10.1016/j.nicl.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Samii A., Sossi V., Ruth T.J., Schulzer M., Holden J.E. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Ann. Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- van de Loo S., Walter U., Behnke S. Reproducibility and diagnostic accuracy of substantia nigra sonography for the diagnosis of Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2010;8 doi: 10.1136/jnnp.2009.196352. [DOI] [PubMed] [Google Scholar]
- Ma Y., Tang C., Spetsieris P., Dhawan V., Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J. Cereb. Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder K., Wang Y., Alcalay R.N. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology. 2015 Jul 7;85(1):89–95. doi: 10.1212/WNL.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek K., Innis R., van Dyck C. [123I]β-CIT SPECT imaging assessment on the rate of Parkinson's disease. Neurology. 2001;57:2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- Marshall V.L., Reininger C.B., Marquardt M., Patterson J., Hadley D.M., Oertel W.H., Benamer H.T., Kemp P., Burn D., Tolosa E., Kulisevsky J., Cunha L., Costa D., Booij J., Tatsch K., Chaudhuri K.R., Ulm G., Pogarell O., Hoffken H., Gerstner A., Grosset D.G. Parkinson's disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov. Disord. 2009;24:500–508. doi: 10.1002/mds.22108. [DOI] [PubMed] [Google Scholar]
- Mazza S., Soucy J.P., Gravel P. Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology. 2006;67:1618–1622. doi: 10.1212/01.wnl.0000242879.39415.49. [DOI] [PubMed] [Google Scholar]
- McNeill A., Duran R., Hughes D.A. A clinical and family history study of Parkinson's disease in heterozygous glucocerebrosidase mutation carriers. J. Neurol. Neurosurg. Psychiatry. 2012;83(8):853–854. doi: 10.1136/jnnp-2012-302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A., Wu R.M., Tzen K.Y., Aguiar P.C. Dopaminergic neuronal imaging in genetic Parkinson's disease: insights into pathogenesis. PLoS One. 2013 Jul 23;8(7) doi: 10.1371/journal.pone.0069190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer T.R., Watts R., MacAskill M.R. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain. 2011 Mar;134(Pt 3):845–855. doi: 10.1093/brain/awq377. (Epub 2011 Feb 9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Miyamoto T., Iwanami M., Muramatsu S., Asari S., Nakano I., Hirata K. Preclinical substantia nigra dysfunction in rapid eye movement sleep behaviour disorder. Sleep Med. 2012 Jan;13(1):102–106. doi: 10.1016/j.sleep.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Moeller J.R., Nakamura T., Mentis M.J. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J. Nucl. Med. 1999;40:1264–1269. [PubMed] [Google Scholar]
- Mollenhauer B., Locascio J.J., Schulz-Schaeffer W., Sixel-Döring F., Trenkwalder C., Schlossmacher M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011 Mar;10(3):230–240. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- Morrish P.K., Rakshi J.S., Bailey D.L., Sawle G.V., Brooks D.J. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J. Neurol. Neurosurg. Psychiatry. 1998;64:314–319. doi: 10.1136/jnnp.64.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure H., Hirano S., Tang C.C. Parkinson's disease tremor-related metabolic network: characterization, progression, and treatment effects. NeuroImage. 2011;54:1244–1253. doi: 10.1016/j.neuroimage.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls M.A., McLean C.Y., Rick J. Diagnosis of Parkinson's disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol. 2015 Oct;14(10):1002–1009. doi: 10.1016/S1474-4422(15)00178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhagopal R., Mak E., Schulzer M., McKenzie J., McCormick S., Sossi V., Ruth T.J., Strongosky A., Farrer M.J., Wszolek Z.K., Stoessl A.J. Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology. 2008 Nov 25;71(22):1790–1795. doi: 10.1212/01.wnl.0000335973.66333.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Bras J., Deas E., O'Sullivan S.S., Parkkinen L. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009 Jul;132(Pt 7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M., Eidelberg D. Metabolic brain networks in translational neurology: concepts and applications. Ann. Neurol. 2012 Nov;72(5):635–647. doi: 10.1002/ana.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce A.J., Bestwick J.P., Silveira-Moriyama L., Hawkes C.H., Giovannoni G., Lees A.J., Schrag A. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol. 2012 Dec;72(6):893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nuenen B.F., Weiss M.M., Bloem B.R., Reetz K., van Eimeren T., Lohmann K., Hagenah J., Pramstaller P.P., Binkofski F., Klein C., Siebner H.R. Heterozygous carriers of a Parkin or PINK1 mutation share a common functional endophenotype. Neurology. 2009 Mar 24;72(12):1041–1047. doi: 10.1212/01.wnl.0000338699.56379.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nuenen B.F., Helmich R.C., Ferraye M., Thaler A. Cerebral pathological and compensatory mechanisms in the premotor phase of leucine-rich repeat kinase 2 parkinsonism. Brain. 2012 Dec;135(Pt 12):3687–3698. doi: 10.1093/brain/aws288. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T., Schoonheim M.M., Deijen J.B., Twisk J.W., Barkhof F., Berendse H.W. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. 2014;83:2046–2053. doi: 10.1212/WNL.0000000000001020. [DOI] [PubMed] [Google Scholar]
- Paisán-Ruiz C., Lewis P.A., Singleton A.B. LRRK2: cause, risk, and mechanism. J Parkinsons Dis. 2013;3(2):85–103. doi: 10.3233/JPD-130192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N., Khan N.L., Scherfler C., Cohen L., Brooks D.J., Wood N.W., Bhatia K.P., Quinn N.P., Lees A.J., Piccini P. Nigrostriatal dysfunction in homozygous and heterozygous parkin gene carriers: an 18F-dopa PET progression study. Mov. Disord. 2009 Nov 15;24(15):2260–2266. doi: 10.1002/mds.22817. [DOI] [PubMed] [Google Scholar]
- Pavese N., Moore R.Y., Scherfler C., Khan N.L. In vivo assessment of brain monoamine systems in parkin gene carriers: a PET study. Exp. Neurol. 2010;222:120–124. doi: 10.1016/j.expneurol.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Pirker W., Holler I., Gerschlager W., Asenbaum S., Zettinig G., Brücke T. Measuring the rate of progression of Parkinson's disease over a 5-year period with β-CIT SPECT. Mov. Disord. 2003;18:1266–1272. doi: 10.1002/mds.10531. [DOI] [PubMed] [Google Scholar]
- Ponsen M.M., Stoffers D., Booij J., van Eck-Smit B.L., Wolters E., Berendse H.W. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann. Neurol. 2004;56:173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- Postuma R.B., Berg D. Advances in markers of prodromal Parkinson disease. Nat. Rev. Neurol. 2016 Oct 27;12(11):622–634. doi: 10.1038/nrneurol.2016.152. [DOI] [PubMed] [Google Scholar]
- Postuma R.B. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov. Disord. 2012;27:617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- Postuma R.B., Lang A.E., Gagnon J.F., Pelletier A., Montplaisir J.Y. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012;135:1860–1870. doi: 10.1093/brain/aws093. [DOI] [PubMed] [Google Scholar]
- Postuma R.B., Gagnon J.F., Bertrand J.A. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015 Mar 17;84(11):1104–1113. doi: 10.1212/WNL.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahayel S., Montplaisir J., Monchi O., Bedetti C., Postuma R.B., Brambati S., Carrier J., Joubert S., Latreille V., Jubault T., Gagnon J.F. Patterns of cortical thinning in idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2015 Apr 15;30(5):680–687. doi: 10.1002/mds.25820. (Epub 2014 Feb 22) [DOI] [PubMed] [Google Scholar]
- Reichmann H. Clinical criteria for the diagnosis of Parkinson's disease. Neurodegener. Dis. 2010;7(5):284–290. doi: 10.1159/000314478. [DOI] [PubMed] [Google Scholar]
- Reiter E., Mueller C., Pinter B., Krismer F., Scherfler C., Esterhammer R., Kremser C., Schocke M., Wenning G.K., Poewe W., Seppi K. Dorsolateral nigral hyperintensity on 3.0T susceptibility-weighted imaging in neurodegenerative parkinsonism. Mov. Disord. 2015 Jul;30(8):1068–1076. doi: 10.1002/mds.26171. [DOI] [PubMed] [Google Scholar]
- Ressner P., Skoloudik D., Hlustik P., Kanovsky P. Hyperechogenicity of the substantia nigra in Parkinson's disease. J. Neuroimaging. 2007;17:164–167. doi: 10.1111/j.1552-6569.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Rizzo G., Zanigni S., de Blasi R., Grasso D., Martino D., Savica R., Logroscino G. Brain MR contribution to the differential diagnosis of parkinsonian syndromes: an update. Parkinsons Dis. 2016;2016:2983638. doi: 10.1155/2016/2983638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G., Copetti M., Arcuti S., Martino D., Fontana A., Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016 Feb 9;86(6):566–576. doi: 10.1212/WNL.0000000000002350. [DOI] [PubMed] [Google Scholar]
- Rolinski M., Szewczyk-Krolikowski K., Tomlinson P.R., Nithi K., Talbot K., Ben-Shlomo Y., Hu M.T. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2014 May;85(5):560–566. doi: 10.1136/jnnp-2013-306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolinski M., Griffanti L., Szewczyk-Krolikowski K., Menke R.A.L., Wilcock G.K., Filippini N. Aberrant functional connectivity within the basal ganglia of patients with Parkinson's disease. NeuroImage: Clinical. 2015;8:126–132. doi: 10.1016/j.nicl.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolinski M., Griffanti L., Piccini P., Roussakis A.A., Szewczyk-Krolikowski K., Menke R.A., Quinnell T., Zaiwalla Z., Klein J.C., Mackay C.E., Hu M.T. Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson's disease. Brain. 2016 Aug;139(Pt 8):2224–2234. doi: 10.1093/brain/aww124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G.W., Petrovitch H., Abbott R.D., Tanner C.M., Popper J., Masaki K., Launer L., White L.R. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann. Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- Rupprecht S., Walther B., Gudziol H., Steenbeck J., Freesmeyer M., Witte O.W. Clinical markers of early nigrostriatal neurodegeneration in idiopathic rapid eye movement sleep behavior disorder. Sleep Med. 2013;14(11):1064–1070. doi: 10.1016/j.sleep.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Sakurai H., Hanyu H., Inoue Y., Kanetaka H., Nakamura M., Miyamoto T. Longitudinal study of regional cerebral blood flow in elderly patients with idiopathic rapid eye movement sleep behavior disorder. Geriatr. Gerontol. Int. 2014;14(1):115–120. doi: 10.1111/ggi.12068. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Shibata E., Tohyama K., Takahashi J., Otsuka K., Tsuchiya K., Takahashi S., Ehara S., Terayama Y., Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006 Jul 31;17(11):1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- Schenck C.H., Boeve B.F., Mahowald M.W. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013 Aug;14(8):744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Schenk D.B., Koller M., Ness D.K., Griffith S.G., Grundman M., Zago W., Soto J., Atiee G., Ostrowitzki S., Kinney G.G. First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov. Disord. 2016 Nov 25 doi: 10.1002/mds.26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C., Khan N.L., Pavese N., Eunson L., Graham E., Lees A.J., Quinn N.P., Wood N.W., Brooks D.J., Piccini P.P. Striatal and cortical pre- and postsynaptic dopaminergic dysfunction in sporadic parkin-linked parkinsonism. Brain. 2004 Jun;127(Pt 6):1332–1342. doi: 10.1093/brain/awh150. [DOI] [PubMed] [Google Scholar]
- Scherfler C., Frauscher B., Schocke M., Iranzo A., Gschliesser V., Seppi K. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann. Neurol. 2010;69:400–407. doi: 10.1002/ana.22245. [DOI] [PubMed] [Google Scholar]
- Schroeder U. Substantia nigra hyperechogenicity in healthy controls: a [18Fluoro] Dopa-PET follow-up study. J. Neurol. 2013;260:1907–1911. doi: 10.1007/s00415-013-6904-4. [DOI] [PubMed] [Google Scholar]
- Schwarz S.T., Abaei M., Gontu V., Morgan P.S., Bajaj N., Auer D.P. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: a region-of-interest and voxel-based study at 3T and systematic review with meta-analysis. NeuroImage: Clinical. 2013 Oct 14;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S.T., Afzal M., Morgan P.S., Bajaj N., Gowland P.A., Auer D.P. The ‘swallow tail’ appearance of the healthy nigrosome—a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.Y., Joo E.Y., Kim S.T., Dhong H.J., Cho J.W. Comparison study of olfactory function and substantia nigra hyperechogenicity in idiopathic REM sleep behavior disorder, Parkinson's disease and normal control. Neurol. Sci. 2013 Jun;34(6):935–940. doi: 10.1007/s10072-012-1164-0. [DOI] [PubMed] [Google Scholar]
- Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R. Multicenter analysis of glucocerebrosidase mutations in Parkinson disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M., González-Aramburu I., Sánchez-Juan P. High frequency and reduced penetrance of LRRK2 G2019S mutation among Parkinson's disease patients in Cantabria (Spain) Mov. Disord. 2011;26:2343–2346. doi: 10.1002/mds.23965. [DOI] [PubMed] [Google Scholar]
- Sierra M., Sánchez-Juan P., Martínez-Rodríguez M.I., González-Aramburu I., García-Gorostiaga I., Quirce M.R., Palacio E., Carril J.M., Berciano J., Combarros O., Infante J. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology. 2013 Feb 12;80(7):621–626. doi: 10.1212/WNL.0b013e31828250d6. [DOI] [PubMed] [Google Scholar]
- Sierra M., Sanchez-Juan P., Martinez-Rodriguez M.I. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology. 2013;80(7):621–626. doi: 10.1212/WNL.0b013e31828250d6. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009 Aug 4;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer U., Hummel T., Cormann K., Mueller A., Frasnelli J., Kropp J., Reichmann H. Detection of presymptomatic Parkinson's disease: combining smell tests, transcranial sonography, and SPECT. Mov. Disord. 2004 Oct;19(10):1196–1202. doi: 10.1002/mds.20141. [DOI] [PubMed] [Google Scholar]
- Sonni I., Fazio P., Schain M., Halldin C., Svenningsson P., Farde L., Varrone A. Optimal acquisition time window and simplified quantification of dopamine transporter availability using 18F-FE-PE2I in healthy controls and Parkinson disease patients. J. Nucl. Med. 2016 Oct;57(10):1529–1534. doi: 10.2967/jnumed.115.171231. [DOI] [PubMed] [Google Scholar]
- Spetsieris P.G., Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. NeuroImage. 2011;54:2899–2914. doi: 10.1016/j.neuroimage.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.B., Lang A., Poewe W. Toward a redefinition of Parkinson's disease. Mov. Disord. 2012;27:54–60. doi: 10.1002/mds.24051. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K., Doerr Y., Moller J.C., Hoffken H., Behr T.M., Oertel W.H. Combination of 'idiopathic' REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128(Pt 1):126–137. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- Stockner H., Iranzo A., Seppi K. Midbrain hyperechogenicity in idiopathic REM sleep behavior disorder. Mov. Disord. 2009;24:1906–1909. doi: 10.1002/mds.22483. [DOI] [PubMed] [Google Scholar]
- Stoessl A.J. Positron emission tomography in premotor Parkinson's disease. Parkinsonism Relat. Disord. 2007;13(Suppl. 3):S421–S424. doi: 10.1016/S1353-8020(08)70041-5. [DOI] [PubMed] [Google Scholar]
- Szewczyk-Krolikowski K., Menke R.A., Rolinski M., Duff E., Salimi- Khorshidi G, Filippini N, et al. Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology. 2014;83:208–214. doi: 10.1212/WNL.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.C., Poston K.L., Dhawan V., Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J. Neurosci. 2010;30:1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.C., Poston K.L., Eckert T. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9:149–158. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune L.K., Renken R.J., de Jong B.M. Parkinson's disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging. NeuroImage: Clinical. 2014 Jul 3;5:240–244. doi: 10.1016/j.nicl.2014.06.007. (eCollection 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler A., Mirelman A., Helmich R.C. Neural correlates of executive functions in healthy G2019S LRRK2 mutation carriers. Cortex. 2013;49(9):2501–2511. doi: 10.1016/j.cortex.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Unger M.M., Möller J.C., Stiasny-Kolster K., Mankel K., Berg D., Walter U., Hoeffken H., Mayer G., Oertel W.H. Assessment of idiopathic rapid-eye-movement sleep behavior disorder by transcranial sonography, olfactory function test, and FP-CIT-SPECT. Mov. Disord. 2008 Mar 15;23(4):596–599. doi: 10.1002/mds.21908. [DOI] [PubMed] [Google Scholar]
- Unger M.M., Belke M., Menzler K., Heverhagen J.T., Keil B., Stiasny-Kolster K. Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions. Sleep. 2010;33:767–773. doi: 10.1093/sleep/33.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendette M., Gagnon J.F., Soucy J.P. Brain perfusion and markers of neurodegeneration in rapid eye movement sleep behavior disorder. Mov. Disord. 2011;26:1717–1724. doi: 10.1002/mds.23721. [DOI] [PubMed] [Google Scholar]
- Vendette M., Montplaisir J., Gosselin N., Soucy J.P., Postuma R.B., Dang-Vu T.T. Brain perfusion anomalies in rapid eye movement sleep behavior dis- order with mild cognitive impairment. Mov. Disord. 2012;27(10):1255–1261. doi: 10.1002/mds.25034. [DOI] [PubMed] [Google Scholar]
- Vilas D., Ispierto L., Álvarez R., Pont-Sunyer C., Martí M.J., Valldeoriola F., Compta Y., de Fabregues O., Hernández-Vara J., Puente V., Calopa M., Jaumà S., Campdelacreu J., Aguilar M., Quílez P., Casquero P., Lomeña F., Ríos J., Tolosa E. Clinical and imaging markers in premotor LRRK2 G2019S mutation carriers. Parkinsonism Relat. Disord. 2015 Oct;21(10):1170–1176. doi: 10.1016/j.parkreldis.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Vilas D., Segura B., Baggio H.C., Pont-Sunyer C., Compta Y., Valldeoriola F., José Martí M., Quintana M., Bayés A., Hernández-Vara J., Calopa M., Aguilar M., Junqué C., Tolosa E. Nigral and striatal connectivity alterations in asymptomatic LRRK2 mutation carriers: A magnetic resonance imaging study. Mov. Disord. 2016 Sep 21 doi: 10.1002/mds.26799. [DOI] [PubMed] [Google Scholar]
- Wing Y.K., Lam S.P., Zhang J., Leung E., Ho C.L., Chen S., Cheung M.K., Li S.X., Chan J.W., Mok V., Tsoh J., Chan A., Ho C.K. Reduced striatal dopamine transmission in REM sleep behavior disorder comorbid with depression. Neurology. 2015 Feb 3;84(5):516–522. doi: 10.1212/WNL.0000000000001215. [DOI] [PubMed] [Google Scholar]
- Wu P., Yu H., Peng S., Dauvilliers Y., Wang J., Ge J., Zhang H., Eidelberg D., Ma Y., Zuo C. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2014 Dec;137(Pt 12):3122–3128. doi: 10.1093/brain/awu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu I.W., Buckley S. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson's disease. Mov. Disord. 2015;30:1229–1236. doi: 10.1002/mds.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A., Biskup S., Leitner P. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]