Abstract
Patients with Attention-Deficit/Hyperactivity Disorder (ADHD) and obsessive/compulsive disorder (OCD) share problems with sustained attention, and are proposed to share deficits in switching between default mode and task positive networks. The aim of this study was to investigate shared and disorder-specific brain activation abnormalities during sustained attention in the two disorders. Twenty boys with ADHD, 20 boys with OCD and 20 age-matched healthy controls aged between 12 and 18 years completed a functional magnetic resonance imaging (fMRI) version of a parametrically modulated sustained attention task with a progressively increasing sustained attention load. Performance and brain activation were compared between groups. Only ADHD patients were impaired in performance. Group by sustained attention load interaction effects showed that OCD patients had disorder-specific middle anterior cingulate underactivation relative to controls and ADHD patients, while ADHD patients showed disorder-specific underactivation in left dorsolateral prefrontal cortex/dorsal inferior frontal gyrus (IFG). ADHD and OCD patients shared left insula/ventral IFG underactivation and increased activation in posterior default mode network relative to controls, but had disorder-specific overactivation in anterior default mode regions, in dorsal anterior cingulate for ADHD and in anterior ventromedial prefrontal cortex for OCD. In sum, ADHD and OCD patients showed mostly disorder-specific patterns of brain abnormalities in both task positive salience/ventral attention networks with lateral frontal deficits in ADHD and middle ACC deficits in OCD, as well as in their deactivation patterns in medial frontal DMN regions. The findings suggest that attention performance in the two disorders is underpinned by disorder-specific activation patterns.
Keywords: ADHD, DMN, fMRI, OCD, Vigilance
Highlights
-
•
Sustained attention is associated with disorder-specific dysfunction in ADHD and OCD.
-
•
ADHD patients showed dorsolateral prefrontal and dorsal inferior frontal gyrus underactivation.
-
•
OCD patients showed underactivation in middle anterior cingulate cortex.
-
•
Patient groups had disorder-specific patterns of overactivation in default mode network.
-
•
ADHD and OCD patients shared left insula/ventral inferior frontal gyrus underactivation.
1. Introduction
1.1. Attention-Deficit/Hyperactivity Disorder and obsessive-compulsive disorder
Attention-Deficit/Hyperactivity Disorder (ADHD) affects 3–8% of children worldwide and 4% of adults (Biederman et al., 2012), and is defined by age-inappropriate problems with inattention, impulsivity and hyperactivity (American Psychiatric Association, 2013).
Obsessive-compulsive disorder (OCD) has a lifetime risk of 2–3% (Ruscio et al., 2010). The key symptoms are obsessions, defined as recurrent and intrusive thoughts (e.g., on themes of contamination, checking, orderliness and symmetry), and compulsions, i.e. repetitive, ego-dystonic and time-consuming behavioural and mental rituals (e.g., repetitive washing or checking) (American Psychiatric Association, 2013). ADHD and OCD are frequently comorbid in young people and have been shown to share familial and genetic risk factors (Geller et al., 2007, Mathews and Grados, 2011), although the majority of the genetic risk is thought to be disorder-specific, rather than shared (Pinto et al., 2016).
1.2. Sustained attention and underlying brain networks
Sustained attention refers to the ability to voluntarily maintain the focus of attention for infrequently occurring critical events (Parasuraman et al., 1998, Warm, 1984). Neurofunctionally, it is dependent on the interplay of four canonical brain networks (Menon, 2011, Metin et al., 2015). First is the “task-positive” central executive network consisting of dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), lateral parietal, and dorsal striato-thalamic regions, which is engaged during tasks requiring the active maintenance of attention toward external stimuli, mediates goal-directed selection of stimuli and responses, and is associated with adaptive performance on sustained attention tasks (Dosenbach et al., 2007, Petersen and Posner, 2012). The task-positive ventral attention network, consisting of IFG and the temporo-parietal junction (TPJ), and the salience network, consisting of anterior insula and middle/dorsal anterior cingulate cortex (mACC/dACC), are involved in detecting behaviourally relevant cues, and engage the central executive network and disengage the default mode network according to perceived environmental demands (Cai et al., 2014, Menon, 2011, Seeley et al., 2007). The “task-negative” default mode network consists of anterior/ventromedial prefrontal cortex (A/VMPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), precuneus, and inferior temporal regions which are proposed to mediate internally generated cognition such as mind-wandering and rumination (Buckner et al., 2008). This network is usually deactivated during cognitive tasks (Raichle, 2015, Raichle et al., 2001). Activation in task-positive networks is typically anti-correlated with that in the default mode network, and a failure to adequately disengage default mode network activation is associated with poorer sustained attention performance, presumably due to an increase of self-referential thoughts at the expense of exteroceptive goal-directed attention (Christakou et al., 2013, Christoff et al., 2009, Rubia, 2017).
1.3. Sustained attention in ADHD and OCD
Sustained attention has been found to be impaired in ADHD (Huang-Pollock et al., 2012, Losier et al., 1996, Malloy-Diniz et al., 2007, Mowinckel et al., 2015, Rubia et al., 2009b, Rubia et al., 2007a, Willcutt et al., 2005) and OCD (Abramovitch et al., 2013, Baykal et al., 2014, Benzina et al., 2016, Bersani et al., 2013, Morein-Zamir et al., 2010, Rajender et al., 2011, Snyder et al., 2015, Trivedi et al., 2008). Both disorders have also been linked to increased spontaneous mind-wandering (Mowlem et al., 2016, Seli et al., 2016, Seli et al., 2015), which is proposed to reflect an imbalance between task-positive and default mode networks (Christakou et al., 2013, Metin et al., 2015), and to underlie poor performance on sustained attention tasks, as attention is focused on internal thoughts, thereby limiting attention resources available for task-relevant processing (Thomson et al., 2015). Moreover, both ADHD and OCD patients self-report impaired executive attention abilities (Armstrong et al., 2011, Benatti et al., 2014, Grassi et al., 2015, Malloy-Diniz et al., 2007, Nandagopal et al., 2011, Sohn et al., 2014).
In ADHD, poor concentration is a symptom of the disorder (American Psychiatric Association, 2013) associated in particular with poor educational and workplace performance (Todd et al., 2002). In OCD, difficulty in sustaining attention toward external goal-relevant stimuli is a plausible neurocognitive mechanism which may underlie difficulties in disengaging from internally generated obsessional thoughts, which are hypothesised to be mediated by the default mode network (Seli et al., 2016, Stern et al., in press). However, the extent to which sustained attention performance is associated with shared and disorder-specific neural dysfunctions in ADHD and OCD is unknown. Shared neural dysfunction during sustained attention would suggest that alterations in sustained attention networks are a transdiagnostic mechanism in ADHD and OCD, while largely distinct neural abnormalities would suggest that disorder-specific neural mechanisms are associated with difficulties in maintaining attentional focus in the two disorders.
1.4. Neuroimaging evidence in ADHD and OCD
ADHD patients show reduced recruitment in task-positive (insula/IFG/DLPFC/striatum/cerebellum) regions and increased default mode (ACC/PCC/precuneus) activation during attention tasks (Christakou et al., 2013, Cubillo et al., 2012, Hart et al., 2013, Metin et al., 2015, Rubia et al., 2009a, Rubia et al., 2009d). The IFG, in particular, is a key region in ADHD, which has been shown to be reliably underactive across multiple tasks of cognitive and attention control (Cortese et al., 2012, Hart et al., 2012, Hart et al., 2013, Lei et al., 2015, Rubia, 2017). It has been found to be disorder-specific relative to OCD (Rubia et al., 2010a), as confirmed in a meta-analytic comparison of 541 ADHD and 287 OCD patients during inhibitory control tasks (Norman et al., 2016) as well as relative to bipolar disorder (Passarotti et al., 2010a, Passarotti et al., 2010b) and conduct disorder (Rubia, 2011, Rubia et al., 2010b, Rubia et al., 2009c, Rubia et al., 2009d) during cognitive control and attention tasks.
Patients with OCD demonstrate abnormalities in task-positive and default mode connectivity at rest (Posner et al., 2017, Stern et al., 2012, Zhu et al., 2016), and deficits in switching between default mode and task-positive networks during cognitive tasks (Cocchi et al., 2012, Stern et al., in press). In particular, increased A/VMPFC activation has been reported in OCD at rest (Menzies et al., 2008, Zhu et al., 2016), during symptom provocation (Brennan et al., 2015, Rotge et al., 2009) and during cognitive tasks (Agam et al., 2014, Brennan et al., 2015, Page et al., 2009, Stern et al., 2011, Stern et al., 2013) suggesting that OCD symptoms may be associated with a failure to adequately regulate activity in this default mode region (Agam et al., 2014, Stern et al., 2012, Stern et al., in press, Stern et al., 2011, Stern et al., 2013). Abnormalities have also been reported in the salience network, which is hyperactive to errors (Stern et al., 2011), emotional stimuli (Berlin et al., 2015), and during symptom provocation (Brennan et al., 2015), but shows decreased negative connectivity with the default mode network at rest (Posner et al., 2017, Stern et al., 2012). Furthermore, functional alterations in central executive network regions such as DLPFC, dorsal striatum and cerebellum have been reported previously in OCD during cognitive tasks (Gu et al., 2008, Kang et al., 2013, Page et al., 2009, Woolley et al., 2008), and structural abnormalities in these regions are found reliably in OCD (Carlisi et al., 2017b, de Wit et al., 2014, Norman et al., 2016). Finally, in our recent comparison of adolescents with autism, adolescents with OCD and age matched healthy controls during sustained attention, patients with OCD showed a disorder-specific pattern of progressively decreasing activation in salience/ventral attention regions including left insula/IFG, as well as progressively increasing activation in default mode region the A/VMPFC with increasing attention load, relative to autism and healthy control groups (Carlisi et al., in press-a).
1.5. Aims and hypotheses of the present study
In this study, we aimed to conduct the very first direct comparison of neurofunctional task-positive and default mode network abnormalities in paediatric ADHD and OCD during sustained attention. For this purpose, we used a parametrically modified sustained attention task with three levels of sustained attention load (Christakou et al., 2013, Murphy et al., 2014, Lim et al., 2016, Carlisi et al., 2017a). We purposely chose a relatively simple sustained attention task that is modelled on a sensorimotor vigilance task in order to probe attention networks in the context of relatively intact or minimally impaired task performance (Christakou et al., 2013, Lim et al., 2016, Murphy et al., 2014, Carlisi et al., 2017a). We hypothesised that both disorders would show reduced activation in task-positive regions, as well as decreased deactivation within the default mode network relative to controls (Carlisi et al., 2017a, Christakou et al., 2013), but that IFG underactivation would be more pronounced or disorder-specific to ADHD patients (Norman et al., 2016, Rubia et al., 2014, Rubia et al., 2010a), while A/VMPFC hyperactivation would be more pronounced or disorder-specific in OCD relative to ADHD patients (Agam et al., 2014, Brennan et al., 2015, Carlisi et al., 2017a, Page et al., 2009, Stern et al., 2011, Stern et al., 2013).
2. Materials and methods
2.1. Participants
Sixty (20 ADHD, 20 OCD, 20 controls) right handed (Oldfield, 1971) male adolescents aged between 12 and 18 years participated, with an IQ > 70 as measured by the Wechsler Abbreviated Scale of Intelligence-Revised (WASI-R) short form (Wechsler, 2008). Non-comorbid ADHD boys were recruited from local child and adolescent mental health services (CAMHS), met DSM-IV criteria for inattentive/hyperactive-impulsive combined subtype, as assessed using the standardized Maudsley diagnostic interview (Goldberg and Murray, 2006), and scored above clinical cut-off on the Conner's Parent Rating Scale-Revised (CPRS-R) (Conners et al., 1998) and the inattention/hyperactivity scale of the parent strength and difficulty questionnaire (SDQ) (Goodman, 1997). Thirteen boys were medication naïve, while 7 were receiving psychostimulant medication. Medicated ADHD patients underwent a 48 h washout period prior to scanning. A consultant psychiatrist excluded comorbidity with other disorders, including OCD. Non-comorbid OCD boys had clinical diagnoses of OCD, and were recruited from a national specialist clinic for child and adolescent OCD and local CAMHS. OCD diagnosis was made by a psychiatrist/clinical psychologist in accordance with ICD-10 criteria after an in-depth, semi-structured interview between patient and clinician was used to administer an expanded version of the Children's Yale-Brown Obsessive Compulsive Symptom checklist (Scahill et al., 1997). Absence of comorbidity, including comorbid ADHD, was confirmed by a consultant psychiatrist. Sixteen OCD boys were medication naïve, while four were being treated with SSRI medication. Further, one of these four patients was receiving risperidone as an augmentation treatment. Control participants had no diagnoses of any psychiatric conditions, scored below clinical cut-offs on the SDQ, and were recruited using local advertising. Exclusion criteria included comorbid psychiatric disorders, medical disorders affecting brain development, drug/alcohol dependency, head injury, abnormal brain structural MRI findings and MRI contraindications. Only boys were studied due to preponderance of males in adolescent ADHD and OCD populations, and to achieve greater homogeneity across participants and groups (Geller et al., 1998, Willcutt, 2012). Data for controls, patients with OCD and 12 out of 20 patients with ADHD have been published elsewhere (Carlisi et al., 2017a, Christakou et al., 2013, Murphy et al., 2014).
Ethical approval was obtained from the local Research Ethics Committee (05/Q0706/275), and the study was conducted in accordance with the Declaration of Helsinki. Study details were explained to both child and guardian. Written informed consent was obtained for all participants.
2.2. Sustained attention task
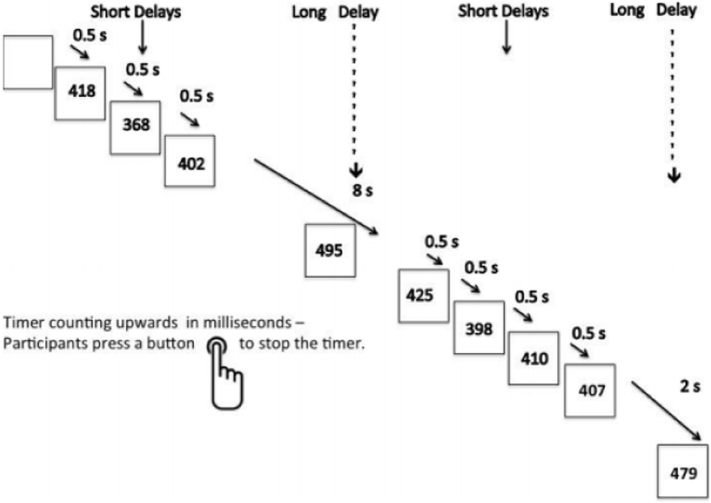
The sustained attention task (Carlisi et al., 2017a, Christakou et al., 2013, Lim et al., 2016, Murphy et al., 2014) is a variant of psychomotor/vigilance and delay tasks (Drummond et al., 2005) and requires participants to respond with a right-handed button press as quickly as possible and within 1 s to a visual stimulus (a timer counting up in milliseconds). The timer appears after either short, predictable, consecutive delays of 0.5 s in series of three to five stimuli (240 trials total) or after unpredictable delays of 2, 5, or 8 s (20 trials each). Trials involving longer delays were pseudorandomly interspersed into the 0.5 s series after at least three 0.5 s trials. The long, infrequent, unpredictable delays require greater sustained attention load which increases with increasing length of delay, while the short, predictable 0.5 s trials are typically anticipated and therefore place a higher demand on sensorimotor synchronisation (Christakou et al., 2013, Murphy et al., 2014). Each participant practiced the task once in a mock scanner before scanning (Fig. 1).
Fig. 1.
Schematic representation of the Sustained Attention Task (SAT). Participants are required to press a right-hand button as soon as they see a timer appear on the screen. The counter appears after either predictable short delays of 0.5 s in series of three to five trials or after unpredictable delays of 2, 5, or 8 s, which are pseudorandomly interspersed into the 0.5 s series after at least three short delay trials. The long, infrequent, unpredictable delays require greater sustained attention, while the predictable 0.5 s delays place a higher demand on sensorimotor synchronisation.
2.3. Analysis of performance data
To examine differences in performance, 3 (group) × 3 (sustained attention load) within-between repeated measures ANOVAs were used. The dependent variables were reaction time, reaction time variability, and omission errors.
2.4. fMRI image acquisition
The fMRI images were acquired at King's College London, Institute of Psychiatry's Centre for Neuroimaging Sciences on a 3T General Electric Signa Horizon HDx MRI scanner (GE Healthcare, UK) using the body coil for radio frequency transmission and a quadrature birdcage headcoil for radio frequency transmission and reception. In each of 22 non-contiguous planes parallel to the anterior–posterior commissure, 480 T2*-weighted MR images depicting BOLD (blood oxygen level dependent) contrast covering the whole brain were acquired with echo time (TE) = 30 ms, repetition time (TR) = 1.5 s, flip angle = 60°, in-plane voxel size = 3.75 mm, slice thickness = 5.0 mm, slice skip = 0.5 mm). A whole-brain high resolution structural scan (inversion recovery gradient echo planar image) used for standard space normalisation was also acquired in the inter-commissural plane with TE = 40 ms, TR = 3 s, flip angle = 90°, number of slices: 43, slice thickness = 3.0 mm, slice skip = 0.3 mm, in-plane voxel size = 1.875 mm, providing complete brain coverage.
2.5. fMRI data analysis
2.5.1. fMRI data analysis methods
Event-related activation data were acquired in randomized trial presentation and analysed using the non-parametric XBAM software package developed at the Institute of Psychiatry, Psychology and Neuroscience, King's College London (www.brainmap.co.uk; (Brammer et al., 1997). XBAM was used because its non-parametric approach overcomes many of the issues associated with parametric software packages (e.g., poor control of FWE-corrected false positive cluster-wise inference rates) (Bullmore et al., 1999b, Eklund et al., 2016).
2.5.2. Individual analysis
Data were first processed to minimize motion-related artefacts (Bullmore et al., 1999a). A 3-D volume consisting of the average intensity at each voxel over the entire experiment was calculated and used as a template. The 3D image volume at each time point was then realigned to this template by computing the combination of rotations (around the x, y and z axes) and translations (in x, y and z) that maximised the correlation between the image intensities of the volume in question and of the template (rigid-body registration). Following realignment, data were then smoothed using a Gaussian filter (full-width at half-maximum (FWHM) 7.2 mm) to improve the signal-to-noise ratio of the images (Bullmore et al., 1999a). Following motion correction, global detrending, spin-excitation history correction and smoothing, time series analysis for each subject was conducted based on a previously published wavelet-based resampling method for fMRI data (Bullmore et al., 2001, Bullmore et al., 1999b). At the individual-subject level, a standard general linear modelling approach was used to obtain estimates of the response size (beta) to each of the sustained attention load (2, 5 and 8 s trials) conditions against the 0.5 s blocks, which were modelled as an implicit baseline. Trials were modelled from the initiation of the delay period until the participant responded with a button press during the counter period. Only correct trials were included in the analysis. We first convolved the main experimental conditions with 2 Poisson model functions (peaking at 4 and 8 s). We then calculated the weighted sum of these 2 convolutions that gave the best fit (least-squares) to the time series at each voxel. A goodness-of-fit statistic (SSQ ratio) was then computed at each voxel consisting of the ration of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by that of the squares due to the residuals (original time series minus model time series). The appropriate null distribution for assessing significance of any given SSQ ratio was established using a wavelet-based data re-sampling method and applying the model-fitting process to the resampled data (Bullmore et al., 2001). The aim was to achieve a global (image-wide) permutation based threshold for forming clusters at p < 0.05 at the first stage of cluster analysis. We did this by permuting 20 times per voxel and combining permutations over the whole brain, resulting in 20 null parametric maps of SSQ rations for each subject, which were combined to give the overall null distribution of SSQ ratio. As there are approximately 50,000 intra-cerebral voxels in our analysis, this means that we used around 1,000,000 combined permutations to form a global threshold for the first stage of our cluster analysis. This same permutation strategy was applied at each voxel to preserve spatial correlation structure in the data. Individual SSQ ratio maps were then affine transformed into standard space, by first mapping the fMRI data onto a high-resolution inversion recovery image of the same subject, and then by normalising onto a Talairach template (Talairach and Tournoux, 1988).
2.5.3. Group analysis
A group-level activation map was produced for each group for each contrast by calculating the median observed SSQ ratios at each voxel in standard space across all subjects and testing them against the null distribution of median SSQ ratios computed from the identically transformed wavelet-resampled data (Brammer et al., 1997, Bullmore et al., 2001). ANOVAs were conducted using randomization-based tests for voxel- or cluster-wise differences (Bullmore et al., 1999b). The voxel-level threshold was first set to p < 0.05 to give maximum sensitivity and to avoid Type II errors, as in order to maximize detection power we used the highest threshold that we have shown previously to give good type I error control at cluster level under the null hypothesis (Bullmore et al., 1999b). Next, a cluster-level threshold was computed for the resulting 3D voxel clusters in such a way as to produce less than one false positive 3D cluster per map. The necessary combination of voxel and cluster level thresholds was not assumed from theory but rather was determined by direct permutation for each dataset, giving excellent type-I and type-II error control (Bullmore et al., 1999b). Cluster mass rather than a cluster extent threshold was used to minimize discrimination against possible small, strongly responding foci of activation (Bullmore et al., 1999b). For the between-group comparisons, a 3 × 3 ANOVA (three levels of sustained attention load, three groups) was conducted testing for group, sustained attention load and group by sustained attention load interaction effects. For group and group by sustained attention load interaction analyses, less than one false positive cluster per map was expected at p < 0.05 for voxel and p < 0.02 for cluster comparisons. In large clusters, we identified local maxima that were further apart than the upper bound of the likely Talairach mapping error (3 voxel radius: 10 mm) (Thirion et al., 2007), and voxels were then assigned to the nearest local maximum with a statistical value that exceeded that of the voxels.
Additional regions of interest (ROI) analyses were performed based on a priori hypotheses. A single ROI search space was based on regions expected to differ between patient groups. This included the default mode network regions A/VMPFC (Talairach coordinates: 0,50,− 4; 18 mm radius sphere) (Stern et al., 2012), and precuneus (extracted from the Talairach atlas using XBAM), the central executive and ventral attention network regions the IFG (BA 44/45/47) and DLPFC (BA 8/9/46), and the salience network regions the anterior insula (Talairach coordinates: ± 35,14,5; 18 mm radius sphere) (Stern et al., 2012) and mACC (Talairach coordinates: 0,10,47; 18 mm radius sphere) (Stern et al., 2012). Within this search space, less than one false positive cluster per map was expected at p < 0.05 for voxel and p < 0.05 for cluster comparisons.
Statistical measures of BOLD response (SSQ) for each participant were then extracted in each of the significant clusters and post-hoc least significance difference t-tests (correcting for multiple comparisons) were conducted to identify between-group differences.
2.5.4. Exploratory brain-behaviour and brain-performance correlations
Statistical BOLD response from regions that showed a significant group by delay effect were extracted, and, collapsing across 2, 5 and 8 s levels of sustained attention load, were correlated with task performance, age and symptom scores within each group. We further performed exploratory examinations within each group for significant negative correlations between activation from task-positive regions and default mode activation from regions showing a significant group by sustained attention load effect.
3. Results
3.1. Participant characteristics
There were no significant group differences in age, but IQ was significantly lower in ADHD (Table 1). This was to be expected as ADHD is associated with lower IQ (Bridgett and Walker, 2006). However, IQ was not covaried in the first instance as covarying for differences between groups that were not randomly selected violates ANCOVA assumptions (Miller and Chapman, 2001). Nonetheless, supplementary analysis was performed covarying for IQ to rule out that IQ was a confounding factor (see below).
Table 1.
Participant characteristics.
| Controls | ADHD | OCD | Sig. | ||
|---|---|---|---|---|---|
| N | 20 | 20 | 20 | – | |
| Age | 15.4 (1.43) | 15 (1.28) | 15.76 (1.43) | F(2,57) = 1.46, p = 0.24 | |
| IQ | 117.75 (12.10) | 101.7 (14.61) | 117.7 (13.36) | F(2,57) = 9.5, p < 0.001 | C,OCD > ADHD |
| SDQ hyperactivity/inattention | 2 (1.71) | 8.16 (1.38) | 4.4 (3.03) | F(2,54) = 37.78, p < 0.001 | ADHD > OCD > C |
| CY-BOCS | … | … | 22.32 (5.97) | ||
| Conner's T | … | 81.2 (9.95) | … |
Abbreviations. ADHD, Attention-Deficit/Hyperactivity Disorder; CYBOCS, Children's Yale-Brown Obsessive Compulsive Scale; IQ, intelligence quotient; OCD, obsessive/compulsive disorder; SDQ, strengths and difficulties questionnaire.
3.2. Performance data
There were no significant main effects of delay or significant group by delay interactions on reaction time, reaction time variability or omission errors (p > 0.1). There was a main effect of group for reaction time (F(2,57) = 4.66, p = 0.013), driven by ADHD boys who showed slower reaction times relative to both controls (p = 0.005) and patients with OCD (p = 0.028), and for reaction time variability (F(2,57) = 10.43, p < 0.001), which was increased in ADHD patients relative to controls (p < 0.001) and OCD patients (p = 0.002), but not for omission errors (F(2,57) = 0.871, p = 0.424). Results remained unchanged after controlling for IQ (see Supplementary Table 2 and Supplementary Figs. 1–2).
3.3. Motion
There were no significant group differences in mean Euclidian displacement of x, y, z movement parameters (F(2, 57) = 0.04, p = 0.96).
3.4. Main effect of sustained attention load
When pooled, all participants showed significant main effect of delay in bilateral DLPFC, IFG, insula, cingulate, temporal lobe, parietal lobe, cerebellum, basal ganglia, thalamus and hippocampal gyri (Supplementary Fig. 3).
3.5. Main effect of group
Whole-brain ANOVA split-plot analysis showed a significant main effect of group in bilateral cerebellum, lingual gyrus, cuneus (Supplementary Table 2, Supplementary Fig. 4). Post-hoc analyses showed that ADHD patients showed disorder-specific left cerebellum (anterior lobe, culmen) underactivation relative to controls and OCD patients. OCD patients showed disorder-specific increased activation in bilateral cerebellum (posterior lobe, culmen, declive/vermis/anterior lobe, culmen), lingual gyrus, and cuneus relative to ADHD patients and controls.
3.6. Group by sustained attention load interaction
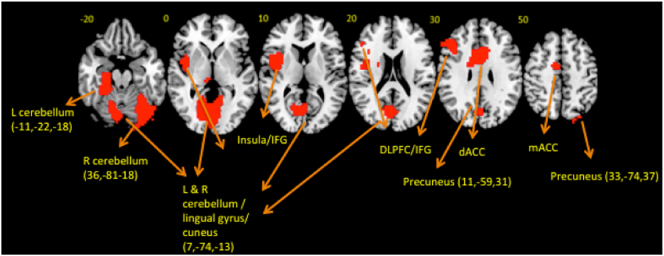
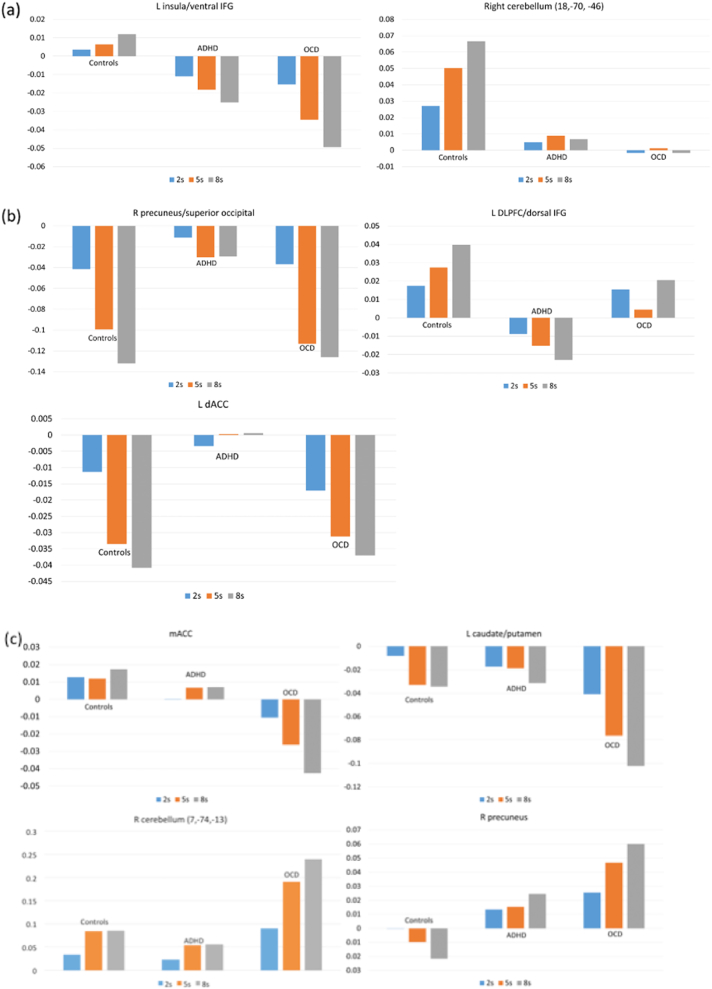
Whole-brain ANOVA split-plot analysis group by sustained attention load interaction effects were significant in left insula/ventral IFG, left DLPFC/dorsal IFG, mACC, left caudate/putamen, bilateral cerebellum/occipital/parahippocampus/hippocampus, right precuneus and bilateral dACC (Table 2, Fig. 2, Fig. 3). Follow-up t-tests revealed that decreased left insula/ventral IFG activation was shared in ADHD (p = 0.018) and OCD (p < 0.001) relative to controls, and due to progressively increased activation in this region in controls with increasing sustained attention load but progressively decreased activation with increasing sustained attention load in both patient groups. A region of right cerebellum (posterior lobe, inferior semilunar lobule) showed increasing activation with increasing sustained attention load in controls, but not in ADHD (p = 0.063, trend) and OCD (p = 0.029) patients. Decreased recruitment of left DLPFC/dorsal IFG was driven by ADHD patients who showed progressively decreased activation with increasing delays relative to controls (p = 0.001) and OCD patients (p = 0.022) who showed progressively increased activation with increasing sustained attention load. Findings in mACC (controls, p < 0.001; ADHD, p = 0.01), left caudate/putamen (controls, p = 0.011; ADHD, p = 0.007) and regions of right cerebellum (posterior lobe, uvula/declive/tonsil) were driven by disorder-specific progressively decreasing activation with increasing sustained attention load in OCD patients relative to controls (uvula, p = 0.026; declive, p = 0.027; tonsil, p = 0.003) and patients with ADHD (uvula, p = 0.006; declive, p = 0.001; tonsil, p = 0.046). A further cluster in the tonsil sub-region of the cerebellum showed a similar pattern between OCD and controls (p = 0.42), but was only trend level significant in comparison with patients with ADHD (p = 0.065). Activation in bilateral cerebellum (posterior lobe, declive/vermis)/occipital lobe was progressively increased in OCD with increasing sustained attention load relative to controls (p = 0.004) and ADHD patients (p < 0.001). Left cerebellum (anterior lobe, culmen)/parahippocampus/hippocampus was progressively increased in activation in OCD relative to ADHD (p = 0.012), and part of right precuneus showed increasing activation with increasing sustained attention load in OCD but progressively decreasing activation with increasing sustained attention load in controls (p = 0.018). Right and left dACC and right precuneus were increased in activation in ADHD relative to controls (right dACC, p = 0.066, trend; left dACC, p = 0.023; right precuneus, p = 0.005) and patients with OCD (right dACC, p = 0.005; left dACC, p = 0.023; right precuneus, p = 0.004), who showed progressive deactivation in these regions with increasing sustained attention load.
Table 2.
ANOVA group by sustained attention load interaction effect on brain activation between ADHD, OCD and healthy boys.
| Brain regions of activation | BA | Peak Talairach coordinates | Voxels | Cluster p-value |
|---|---|---|---|---|
| C > ADHD,OCD | ||||
| L insula/IFGa | 13/44/45 | − 40,4,− 2 | 114 | 0.001 |
| R cerebellum (posterior lobe, inferior semilunar lobule) | 18,− 70,− 46 | 36 | 0.005 | |
| OCD < C, ADHD | ||||
| mACC | 24/23/6 | − 4,0,31 | 87 | 0.001 |
| L caudate/putamen | − 18,0,15 | 19 | 0.05 | |
| R cerebellum (posterior lobe, uvula) | 33,− 63,− 24 | 91 | 0.001 | |
| R cerebellum (posterior lobe, declive) | 36,− 81–18 | 93 | 0.001 | |
| R cerebellum (posterior lobe, cerebellar tonsil) | 33,− 19,− 46 | 31 | 0.005 | |
| R cerebellum (posterior lobe, cerebellar tonsil) | 36,− 44,− 35 | 70 | 0.001 | |
| OCD > ADHD,C | ||||
| L & R cerebellum (posterior lobe, declive/vermis), lingual gyrus/cuneus | 17/18 | 7,− 74,− 13 | 200 | 0.001 |
| L cerebellum (anterior lobe, culmen) | − 22,− 33,− 24 | 60 | 0.005 | |
| L cerebellum (anterior lobe, culmen) | − 11,− 52,− 13 | 167 | 0.001 | |
| ADHD < C, OCD | ||||
| L DLPFC/IFG | 9/46/44/45 | − 40,30,26 | 144 | 0.001 |
| OCD > C | ||||
| R precuneus | 7 | 11,− 59,31 | 55 | 0.05 |
| ADHD < OCD | ||||
| L cerebellum (anterior lobe, culmen), parahippocampus/hippocampus | − 11,− 22,− 18 | 75 | 0.05 | |
| ADHD > C,OCD | ||||
| R precuneus/superior occipitala | 7/19 | 33,− 74,37 | 55 | 0.001 |
| R dACC | 33/24 | 7,19,20 | 59 | 0.002 |
| L dACC | 33/24 | − 4,11,20 | 39 | 0.05 |
Abbreviations: ADHD, Attention-Deficit/Hyperactivity Disorder; BA, Brodmann area; C, controls; dACC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; mACC, middle anterior cingulate; OCD, obsessive/compulsive disorder.
Note: We identified local maxima that were farther apart than the upper bound of the likely Talairach mapping error (3 voxel radius: 10 mm) (Thirion et al., 2007). Voxels were then assigned to the nearest local maximum with a statistic value that exceeded that of the voxels. The probabilities for this contrast are given for sub-cluster maxima.
Indicates that this region was also found to be significant within our ROI search space.
Fig. 2.
Horizontal slices showing whole-brain split plot analysis of variance (ANOVA) effects of group by sustained attention load interactions on brain activation. Thresholded at p < 0.05 for voxel and p < 0.02 for cluster comparisons. The right side corresponds to the right side of the image.
Fig. 3.
Statistical measures of BOLD response are shown for each of the three groups for each of the brain regions that showed a significant group by sustained attention load effect. (a) Shows findings shared in ADHD and OCD, (b) shows findings disorder-specific in ADHD, and (c) shows findings disorder-specific in OCD.
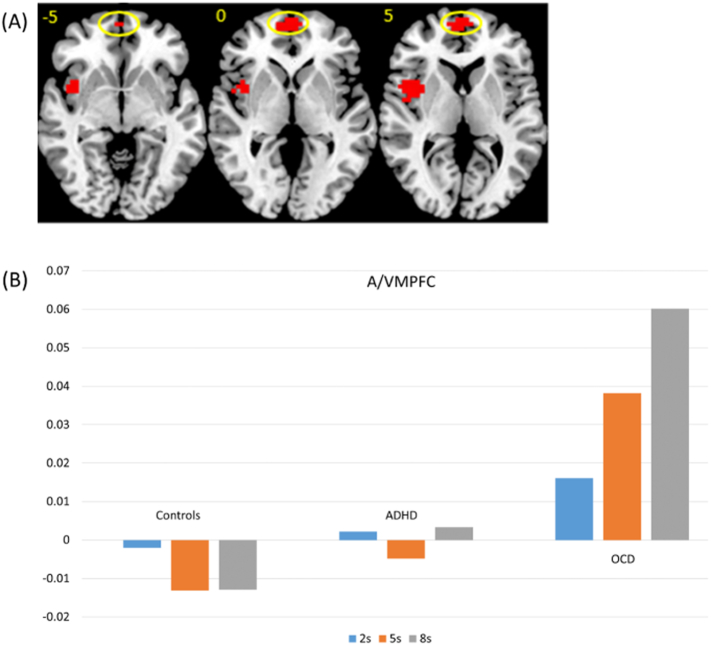
The ROI analysis showed an additional cluster in the A/VMPFC (Talaraich coordinates, 0,67,− 2; BA 10/11, 28 voxels, p < 0.05) which was disorder-specifically increased in activation with increasing sustained attention load in OCD relative to ADHD and control boys (p = 0.002). See Fig. 4.
Fig. 4.
(A) Horizontal slices showing split plot analysis of variance (ANOVA) effects of group by delay interactions within ROI search space. Circled is the A/VMPFC cluster. Thresholded at p < 0.05 for voxel and p < 0.05 for cluster comparisons The right side corresponds to the right side of the image. (B) Statistical measures of BOLD response are shown for each of the three groups for the A/VMPFC cluster that showed a significant group by delay effect within the ROI search space.
All group by sustained attention load effects remained after covarying for IQ. See Supplementary Fig. 5.
3.7. Exploratory brain-behaviour and brain-performance correlations
Collapsing across 2, 5 and 8 s levels of sustained attention load, activation in right cerebellum (posterior lobe, cerebellar tonsil) positively correlated with reaction time (r(18) = 0.789, p < 0.001) and reaction time variability (r(18) = 0.620, p = 0.004) within controls. Only the correlation with reaction time survived correction for multiple comparisons using the Benjamini and Hochberg method (Benjamini and Hochberg, 1995). Within the ADHD group, activation in left insula/ventral IFG correlated positively with reaction time (r(18) = 0.455, p = 0.044) and reaction time variability (r(18) = 0.457, p = 0.043). In OCD patients, a significant positive correlation was found between reaction time and activation in right cerebellum (posterior lobe, inferior semilunar lobule) (r(18) = 0.508, p = 0.022), left putamen/caudate (r(18) = 0.473, p = 0.035), mACC (r(18) = 0.532, p = 0.016), and left dACC (r(18) = 0.446, p = 0.049). However, these findings did not survive correction for multiple comparisons.
There were no significant correlations between CY-BOCS scores and brain activation in the group difference clusters in the OCD patients. There was a significant negative correlation between scores on the SDQ inattention/hyperactivity scale and right cerebellum (posterior lobe, cerebellar tonsil) activation in ADHD patients (r(17) = − 0.528, p = 0.02), although this did not survive correction for multiple comparisons.
Within controls, increasing age was associated with decreasing activation in right uvula region in the cerebellum (r(18) = − 0.522, p = 0.012). Within patients with OCD, increasing age was associated with decreasing activation in right cerebellum (posterior lobe, inferior semilunar lobule) (r(18) = − 0.563, p = 0.01) and left caudate/putamen (r(18) = − 0.516, p = 0.02). No findings survived correction for multiple comparisons.
To test whether task-positive network regions were anti-correlated with regions of the default mode, we conducted correlations between activation in left DLPFC/dorsal IFG, left insula/IFG, and mACC with activation in A/VMPFC, dACC and right precuneus separately within each group. Within controls, activation in DLPFC/dorsal IFG had a significant negative correlation with activity in A/VMPFC (r(18) = − 0.507, p = 0.023) and in left dACC (r(18) = − 0.467, p = 0.038), and mACC activation had a significant negative correlation with A/VMPFC (r(18) = − 0.482, p = 0.032). Greater activation in left insula/ventral IFG was associated with decreased activation in right precuneus in ADHD (r(18) = − 0.615, p = 0.004) and OCD (r(18) = − 547,p = 0.013). In ADHD patients alone, activation in left insula/ventral IFG and mACC had significant positive correlations with left dACC (left insula/IFG; r(18) = 0.573, p < 0.001, mACC; r(20) = 0.489, p = 0.029), while left insula/ventral IFG activation had a significant positive correlation with right dACC activation (r(18) = 0.550, p = 0.012). In OCD patients, activation in the mACC had a significant negative correlation with activation in right precuneus (r(18) = − 0.455, p = 0.044) and positive correlations with bilateral dACC (left; r(18) = 0.684, p < 0.001; r(18) = 0.678, p = 0.001). Only the correlations between mACC and dACC in OCD survived correction for multiple comparisons.
4. Discussion
This study investigated shared and disorder-specific neurofunctional abnormalities in patients with ADHD and with OCD during a sustained attention task. Patients with ADHD and OCD shared underactivation in left insula/ventral IFG and right cerebellum. However, they also showed disorder-specific brain dysfunction in frontal regions of attention networks as well as in frontal regions of the default mode network. Patients with ADHD showed disorder-specific progressively decreased activation with increasing delay relative to healthy control and OCD boys in key task-relevant central executive regions of left DLPFC/dorsal IFG while OCD patients showed progressively decreased activation with increasing delay in salience network region, the mACC. In dACC, controls and OCD patients showed progressively decreased activation with increasing delays while ADHD patients did not show this pattern. On the other hand, in A/VMPFC, OCD patients showed progressively increased activation with progressive delays, while controls showed progressive deactivation with increasing delays in this region.
4.1. Task-positive dysfunction in paediatric ADHD and OCD
Decreased recruitment of task-positive DLPFC/dorsal IFG regions in ADHD is in line with previous studies of sustained attention in the disorder (Christakou et al., 2013, Cubillo et al., 2012, Rubia et al., 2009a, Rubia et al., 2009d), as well as with evidence that decreased IFG grey matter volume (GMV) predicts poor sustained attention performance in ADHD (Pironti et al., 2014). Previous research has shown disorder-specificity in ADHD in the IFG during attention tasks relative to conduct disorder (Rubia et al., 2009c, Rubia et al., 2009d), and during inhibitory control tasks relative to conduct disorder (Rubia et al., 2010b), bipolar disorder (Passarotti et al., 2010a, Passarotti et al., 2010b) and OCD (Norman et al., 2016, Rubia et al., 2010a), while underactivation of left DLPFC during sustained attention has been found previously relative to patients with autism (Christakou et al., 2013). The current results extend these previous findings of disorder-specific underactivation in ADHD patients in left lateral prefrontal regions by demonstrating disorder-specificity relative to OCD in left DLPFC/dorsal IFG during sustained attention, and provide further evidence that IFG underactivation during attention and inhibition tasks is a biomarker more closely associated with ADHD than to other childhood disorders (Rubia et al., 2014). Findings of shared underactivation in ventral IFG, but disorder-specific underactivation in dorsal IFG in ADHD, are consistent with evidence that sub-regions of the IFG have distinct patterns of structural and functional connectivity, with the dorsal portions of the IFG being more closely integrated with central executive network regions, including adjacent DLPFC (Barredo et al., 2016). Underactivation in left DLPFC/dorsal IFG may be related to a maturational delay, as these regions have been found to be delayed in structure and function in ADHD patients (Rubia et al., 2014, Shaw et al., 2012, Sripada et al., 2014).
Patients with OCD showed disorder-specific underactivation relative to ADHD in mACC, extending previous evidence for decreased activation during cognitive tasks and GMV in this region compared to healthy controls (Carmona et al., 2007, Kang et al., 2013, Page et al., 2009, Rubia et al., 2010a, Woolley et al., 2008) by showing disorder-specificity relative to ADHD. Disorder-specific underactivation in this region relative to ADHD is in line with our recent meta-analysis which found medial prefrontal underactivation and decreased GMV to be disorder-specific in OCD relative to ADHD (Norman et al., 2016). Findings of disorder-specific mACC underactivation in OCD and DLPFC/dorsal IFG underactivation in ADHD therefore support accounts proposing that primary prefrontal deficits in ADHD are in dorsal and especially inferior lateral prefrontal cortex, while primary prefrontal deficits in OCD are within medial prefrontal cortex (Norman et al., 2016, Rubia et al., 2014).
Both ADHD and OCD boys shared underactivation in left insula extending into ventral IFG. The insula and ventral IFG are key task-positive regions and the central hubs of the salience and ventral attention networks involved in switching from the default mode network to the central executive network on the basis of stimuli indicating the need for external attention (Cai et al., 2014, Menon, 2011, Menon and Uddin, 2010, Seeley et al., 2007), or based on a need to supress mind-wandering and remain vigilant for upcoming stimuli (Hasenkamp et al., 2012, Langner and Eickhoff, 2013). In ADHD, insufficient activation of the insula and ventral IFG has been reported previously during sustained attention (Cubillo et al., 2012, Rubia et al., 2009a, Rubia et al., 2009d), attention allocation (Rubia et al., 2009c, Rubia et al., 2007b), and inhibitory control tasks (Cortese et al., 2012, Hart et al., 2013, Lei et al., 2015, Rubia et al., 2010a, Rubia et al., 2009c), and has been proposed to underlie decreased task-related salience and resultant heightened distractibility (Norman et al., 2016). In OCD, heightened insula related activation is reported to errors (Stern et al., 2011) during affective processing (Berlin et al., 2015), and during symptom provocation (Brennan et al., 2015, Rotge et al., 2008), although there is some evidence for decreased activation during cognitive tasks (Carlisi et al., 2017a, Gu et al., 2008, Huyser et al., 2011, Woolley et al., 2008). Findings are also in line with resting-state fMRI evidence showing decreased negative connectivity between insula and default mode network regions including A/VMPFC (Posner et al., 2017, Stern et al., 2012) and decreased insula, but increased A/VMPFC activity at rest in OCD (Zhu et al., 2016).
Interestingly, we showed recently in our comparative meta-analysis that underactivation of the insula/ventral IFG during inhibitory control was disorder-specific to patients with ADHD relative to patients with OCD (Norman et al., 2016), while a posterior insula/putamen cluster was increased in activation in OCD, whereas in the current study reduced insula/ventral IFG activation was shared. Discrepant findings are likely due to differences in task conditions. Insula/ventral IFG deficits in OCD may be dependent on conditions that invite greater DMN, such as long delays (Christoff et al., 2009, Hasenkamp et al., 2012), an interpretation which is supported by findings of decreases in insula activation and increases in default mode activation with increasing delays in OCD.
4.2. Default mode network dysfunction in paediatric ADHD and OCD
Both groups showed increased activation within the default mode network, supporting accounts wherein deficient switching from default mode to task-positive networks underlies problems with sustained attention in ADHD and OCD (Carlisi et al., 2017a, Christakou et al., 2013, Metin et al., 2015, Sonuga-Barke and Castellanos, 2007, Stern et al., 2012, Stern et al., in press). Findings are consistent with a role for attention lapses caused by poor control over default mode network mediated task-unrelated thoughts (Raichle, 2015), which have been shown to occur more often in both patient groups (Mowlem et al., 2016, Seli et al., 2016, Seli et al., 2015), and to be associated with poor sustained attention performance (Thomson et al., 2015). In ADHD, disorder-specific reduced deactivation was seen in dACC. In contrast, in OCD, disorder-specific alterations were found in A/VMPFC, which showed disorder-specific increases in activation with increasing delays. Both patient groups showed increased activation relative to controls in portions of the precuneus. Patients with ADHD have previously shown reduced deactivation of the ACC during cognitive tasks (Fassbender et al., 2009, Liddle et al., 2011, Metin et al., 2015, Peterson et al., 2009). Shared hyperactivity in the precuneus in both disorders is in line with previous research in ADHD (Christakou et al., 2013, Cubillo et al., 2012, Durston et al., 2003, Liddle et al., 2011, Rubia et al., 2009d) and OCD (Kang et al., 2013, Page et al., 2009, Roth et al., 2007). The A/VMPFC is a key region of DMN that is also a commonly implicated region in OCD. It has shown increased activity at rest (Menzies et al., 2008, Zhu et al., 2016), during symptom provocation (Brennan et al., 2015, Rotge et al., 2008), and during cognitive tasks (Agam et al., 2014, Brennan et al., 2015, Page et al., 2009, Stern et al., 2011, Stern et al., 2013) in OCD patients. Furthermore, in a meta-analytic comparison of VBM studies, disorder-specific increased GMV was found in paediatric OCD relative to paediatric ADHD (Norman et al., 2016). Given its role in internally generated thought and its hyperactivation in OCD, it could be hypothesised that A/VMPFC is a plausible correlate of intrusive obsessions, which is poorly controlled by task-positive networks during sustained attention. In sum, increased activation in posterior default mode network region the precuneus during sustained attention may be a shared feature of both disorders, although perturbations in separate anterior default mode network regions appear to be largely disorder-specific during sustained attention.
4.3. Cerebellar dysfunction in paediatric ADHD and OCD
Patients with OCD showed increased activation relative to controls and patients with ADHD in medial cerebellum, but showed decreased activation in lateral cerebellum, whereas ADHD patients showed disorder-specific decreased activation in a distinct portion of medial cerebellum. As in previous studies of attention tasks in ADHD, cerebellar underactivation correlated with ADHD symptoms (Cubillo et al., 2011, Rubia et al., 2007b), although his finding did no survive correction for multiple comparisons. Patient groups shared underactivation in a portion of inferior cerebellum relative to controls. The cerebellum forms part of central executive, ventral attention, salience and sensorimotor networks, although its role in each of these is poorly understood, making the current findings difficult to interpret (Habas et al., 2009). Patients with OCD and with ADHD have shown both increased and decreased cerebellar activation in previous studies using cognitive tasks (Kang et al., 2013, Page et al., 2009, Rubia et al., 2009b, Rubia et al., 2009d, Woolley et al., 2008), and these findings, along with the ones presented here, are likely a result of as yet poorly defined cerebellar functional heterogeneity.
4.4. Behavioural findings
Unlike our initial work using the SAT, we did not find a significant main effect of sustained attention load on reaction time in patient groups or controls (Christakou et al., 2013). We showed recently that reaction time in the SAT decreases with increasing age (Murphy et al., 2014), and samples in the earlier study were, on average, younger than the ones included here. Discrepant behavioural finding might therefore be explained by differences in age between studies, and the current negative findings are consistent with recent reports using this task (Carlisi et al., 2017a, Lim et al., 2016).
Relatedly, only patients with ADHD showed a performance difference relative to controls, with slower response times and greater reaction time variability, consistent with previous research (Christakou et al., 2013, Huang-Pollock et al., 2012). Previous meta-analyses and a systematic review of sustained attention tasks in OCD adults have reported significant behavioural deficits relative to controls (Abramovitch et al., 2013, Benzina et al., 2016, Shin et al., 2008). However, the current negative result is in line with previous studies that have examined sustained attention in OCD youths (Lucke et al., 2015, Shin et al., 2014). Neuropsychological impairments are more prominent in adult than paediatric OCD (Abramovitch et al., 2013, Abramovitch et al., 2015), which may reflect altered developmental trajectories and a failure to maintain age normative cognitive performance with increasing demands (Abramovitch et al., 2015). Alternatively, negative findings may be due to the relatively simplified nature of the SAT when compared with the more commonly used continuous performance task, which also features distractor stimuli and places higher demands on working memory and inhibitory control (Christakou et al., 2013, Rubia et al., 2009a, Rubia et al., 2009d). The sustained attention task is modelled on a sensorimotor vigilance task and was purposely designed to elicit brain activation during different attention loads while being easy enough to thus allow for attention networks to be probed in the context of relatively intact task performance (Christakou et al., 2013, Murphy et al., 2014, Lim et al., 2016, Carlisi et al., 2017a). Deficits in brain activation may be a more sensitive measure of abnormalities in cognitive brain networks than behavioural performance in this simplified task, as normal performance in OCD patients can be maintained when demands are low despite neural dysfunction (Page et al., 2009, Woolley et al., 2008).
4.5. Potential implications
Although both disorders show alterations in default mode and task-positive functional brain networks, patterns of dysfunction were largely disorder-specific with impairments in different regions of these networks. The findings thus suggest that attention performance in the two disorders is underpinned by different neurofunctional substrates. Consequently, broadly defined shared alterations in networks supporting sustained attention are unlikely to explain heightened comorbidity between disorders. The findings of largely disorder-specific neurofunctional abnormalities during attention functions also have treatment implications for pharmacological or non-pharmacological therapies such as neuromodulation in the form of fMRI neurofeedback and brain stimulation, as they would imply different neurofunctional targets for the two disorders.
4.6. Limitations
Most previous neuroimaging studies in OCD have investigated adult samples, and those which have tested OCD in children and adolescents have tended to use highly comorbid (Fitzgerald et al., 2010, Huyser et al., 2010, Huyser et al., 2011) or largely medicated (Britton et al., 2010, Fitzgerald et al., 2010, Huyser et al., 2010, Huyser et al., 2011, Rubia et al., 2010a, Rubia et al., 2011, Woolley et al., 2008) samples. The current study therefore contributes to the field by examining neural abnormalities associated with OCD, without the confounding effects of comorbid disorders, long-term symptom exposure, and medication exposure on neural structure and function. However, maturational effects on brain development are in progress over the course of adolescence and early adulthood (Blakemore, 2012, Giedd and Rapoport, 2010, Paus et al., 2001, Rubia, 2013, Rubia et al., 2010c, Rubia et al., 2013), and increasing age during the transition between adolescence to adulthood is associated with increasing task-positive and decreasing DMN activation during sustained attention in healthy subjects (Murphy et al., 2014, Smith et al., 2011). Moreover, meta-analytic evidence suggests that underactivation in lateral prefrontal cortex may be more widespread in adult than paediatric ADHD (Hart et al., 2013, Norman et al., 2016), while evidence of greater neuropsychological performance deficits in adult OCD compared with paediatric OCD may be indicative of worsening neural deficits over the course of development in the disorder (Abramovitch et al., 2013, Abramovitch et al., 2015). Recent research has also indicated altered structural development in ADHD and OCD patients (Boedhoe et al., 2016, Norman et al., 2016, Shaw et al., 2012). Potential alterations in developmental trajectories therefore mean that the current findings are not generalizable beyond paediatric patients with ADHD and OCD, and future work should examine developmental trajectories of functional brain development in ADHD and OCD and also provide comparisons between the disorders across the lifespan.
A further limitation of the study is that the ADHD group had a lower mean IQ relative to the other groups. However, lower IQ is typical for this population (Bridgett and Walker, 2006), and the results remained when we covaried for IQ. Second, although an advantage of the study is that only four (20%) of the OCD patients were receiving medication, seven (35%) patients with ADHD were receiving psychostimulant medication, which may have mitigated group differences (Norman et al., 2016, Rubia et al., 2014), although patients received a 48-hour washout period, which is > 10 times the half-life of the drug. Due to preponderance of males in adolescent OCD and especially ADHD populations, only male participants were included in the current analysis (Geller et al., 1998, Willcutt, 2012). Differences in structural and functional brain development exist between males and females (Blakemore, 2012, Giedd and Rapoport, 2010, Paus et al., 2001, Rubia, 2013, Rubia et al., 2010c, Rubia et al., 2013), and gender differences in brain structure and function abnormalities have been reported in ADHD (Onnink et al., 2014, Seymour et al., 2017, Valera et al., 2010). Therefore, while including only boys increased homogeneity of the samples, it also means that findings are not generalizable to girls with ADHD or OCD. A further point is that we interpret failures of default mode deactivation based on neuroanatomical overlap with previous work (Stern et al., 2012, Metin et al., 2015, Raichle, 2015), as well as findings in the current study that these brain regions showed deactivation during task performance and/or significant negative correlations with task-positive regions in controls. However, alternative explanations, for instance that increased activation served as compensatory strategies in patient groups, are also plausible. Finally, although the sample size of twenty per group is relatively large compared with much of the existing paediatric ADHD and OCD fMRI literature, it may still be underpowered, and future work should aim to confirm these findings in larger samples.
4.7. Summary and conclusions
In summary, sustained attention performance in patients with ADHD and OCD was associated with largely disorder-specific patterns of activation abnormalities in task-positive attention control and default mode network regions. Consistent with previous research, deficits in lateral prefrontal (DLPFC/IFG) regions important for goal-directed attention and behaviour were disorder-specific to ADHD relative to controls and OCD, while deficits in mACC were disorder-specific to OCD. In the default mode network, both groups showed overactivation in different regions of the precuneus, while dACC overactivation was disorder-specific to ADHD and A/VMPFC overactivation was disorder-specific to OCD. Shared underactivation in salience and ventral attention network regions the insula and ventral IFG suggests shared deficits in switching attention from interoceptive to exteroceptive focus. The results are consistent with findings originating from the field of behaviour genetics, according to which ADHD and OCD symptom liability appears to be determined to a greater extent by disorder-specific genetic influences, underlining that these disorders are not alternative phenotypic expressions of the same underlying genetic liability (Pinto et al., 2016).
Author disclosure
K.R. has received funding from Lilly for another project and speaker's honoraria from Lilly, Shire, Novartis and Medice. D.M. has received funding for another project from Lilly. C.M. has received funding from Lilly for another project and speaker's honoraria from Flynn Pharma. The other authors have no conflict of interests to declare.
Acknowledgments
This study was supported by Grants from the Medical Research Council (MRC GO300155) to Prof. Katya Rubia. Prof. Katya Rubia and Dr. Andy Simmons receive funding from National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King's College London. Christina Carlisi was supported by a NIHR BRC PhD studentship. Dr. Anastasia Christakou was supported by a post-doctoral fellowship from Medical Research Council (MRC G0300155). Luke Norman was supported by a Ph.D. studentship from the Institute of Psychiatry, Psychology and Neuroscience, King's College London. This paper represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.04.013.
Appendix A. Supplementary data
Supplementary material
References
- Abramovitch A., Abramowitz J.S., Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin. Psychol. Rev. 2013;33:1163–1171. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Abramovitch A., Abramowitz J.S., Mittelman A., Stark A., Ramsey K., Geller D.A. Research review: neuropsychological test performance in pediatric obsessive-compulsive disorder–a meta-analysis. J. Child Psychol. Psychiatry. 2015;56:837–847. doi: 10.1111/jcpp.12414. [DOI] [PubMed] [Google Scholar]
- Agam Y., Greenberg J.L., Isom M., Falkenstein M.J., Jenike E., Wilhelm S., Manoach D.S. Aberrant error processing in relation to symptom severity in obsessive–compulsive disorder: a multimodal neuroimaging study. NeuroImage: Clinical. 2014;5:141–151. doi: 10.1016/j.nicl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Armstrong T., Zald D.H., Olatunji B.O. Attentional control in OCD and GAD: specificity and associations with core cognitive symptoms. Behav. Res. Ther. 2011;49:756–762. doi: 10.1016/j.brat.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J., Verstynen T.D., Badre D. Organization of cortico-cortical pathways supporting memory retrieval across subregions of the left ventrolateral prefrontal cortex. J. Neurophysiol. 2016 doi: 10.1152/jn.00157.2016. (jn.00157.02016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykal S.K., Karabekiroğlu K., Şenses A., Karakurt M., Çalik T., Yüce M. Neuropsychological and clinical profiles of children and adolescents diagnosed with childhood obsessive compulsive disorder. Arch. Neuropsychiatr. 2014;51:334–343. doi: 10.5152/npa.2014.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti B., Dell'Osso B., Arici C., Hollander E., Altamura A.C. Characterizing impulsivity profile in patients with obsessive-compulsive disorder. Int. J. Psychiatry Clin. Pract. 2014;18:156–160. doi: 10.3109/13651501.2013.855792. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Benzina N., Mallet L., Burguière E., N'Diaye K., Pelissolo A. Cognitive dysfunction in obsessive-compulsive disorder. Curr. Psychiatry Rep. 2016;18:1–11. doi: 10.1007/s11920-016-0720-3. [DOI] [PubMed] [Google Scholar]
- Berlin H.A., Schulz K.P., Zhang S., Turetzky R., Rosenthal D., Goodman W. Neural correlates of emotional response inhibition in obsessive-compulsive disorder: a preliminary study. Psychiatry Res. 2015;234:259–264. doi: 10.1016/j.pscychresns.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Bersani G., Quartini A., Ratti F., Pagliuca G., Gallo A. Olfactory identification deficits and associated response inhibition in obsessive-compulsive disorder: on the scent of the orbitofronto–striatal model. Psychiatry Res. 2013;210:208–214. doi: 10.1016/j.psychres.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Biederman J., Petty C.R., Woodworth K.Y., Lomedico A., Hyder L.L., Faraone S.V. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J. Clin. Psychiatry. 2012;73:941–950. doi: 10.4088/JCP.11m07529. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. Imaging brain development: the adolescent brain. NeuroImage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Boedhoe P.S., Schmaal L., Abe Y., Ameis S.H., Arnold P.D., Batistuzzo M.C., Benedetti F., Beucke J.C., Bollettini I., Bose A., Brem S., Calvo A., Cheng Y., Cho K.I., Dallaspezia S., Denys D., Fitzgerald K.D., Fouche J.P., Gimenez M., Gruner P., Hanna G.L., Hibar D.P., Hoexter M.Q., Hu H., Huyser C., Ikari K., Jahanshad N., Kathmann N., Kaufmann C., Koch K., Kwon J.S., Lazaro L., Liu Y., Lochner C., Marsh R., Martinez-Zalacain I., Mataix-Cols D., Menchon J.M., Minuzzi L., Nakamae T., Nakao T., Narayanaswamy J.C., Piras F., Piras F., Pittenger C., Reddy Y.C., Sato J.R., Simpson H.B., Soreni N., Soriano-Mas C., Spalletta G., Stevens M.C., Szeszko P.R., Tolin D.F., Venkatasubramanian G., Walitza S., Wang Z., van Wingen G.A., Xu J., Xu X., Yun J.Y., Zhao Q., Thompson P.M., Stein D.J., van den Heuvel O.A. Distinct subcortical volume alterations in pediatric and Adult OCD: a worldwide meta- and mega-analysis. Am. J. Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.16020201. (appiajp201616020201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammer M.J., Bullmore E.T., Simmons A., Williams S.C., Grasby P.M., Howard R.J., Woodruff P.W., Rabe-Hesketh S. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn. Reson. Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Brennan B.P., Tkachenko O., Schwab Z.J., Juelich R.J., Ryan E.M., Athey A.J., Pope H.G., Jenike M.A., Baker J.T., Killgore W.D., Hudson J.I., Jensen J.E., Rauch S.L. An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacology. 2015;40:1866–1876. doi: 10.1038/npp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett D.J., Walker M.E. Intellectual functioning in adults with ADHD: a meta-analytic examination of full scale IQ differences between adults with and without ADHD. Psychol. Assess. 2006;18:1–14. doi: 10.1037/1040-3590.18.1.1. [DOI] [PubMed] [Google Scholar]
- Britton J.C., Rauch S.L., Rosso I.M., Killgore W.D.S., Price L.M., Ragan J., Chosak A., Hezel D.M., Pine D.S., Leibenluft E., Pauls D.L., Jenike M.A., Stewart S.E. Cognitive inflexibility and frontal-cortical activation in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:944–953. doi: 10.1016/j.jaac.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Brammer M.J., Rabe-Hesketh S., Curtis V.A., Morris R.G., Williams S.C., Sharma T., McGuire P.K. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum. Brain Mapp. 1999;7:38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., Brammer M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Long C., Suckling J., Fadili J., Calvert G., Zelaya F., Carpenter T.A., Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum. Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Ryali S., Chen T., Li C.S., Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J. Neurosci. 2014;34:14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisi C.O., Norman L., Murphy C.M., Christakou A., Chantiluke K., Giampietro V., Simmons A., Brammer M., Murphy D.G., Mataix-Cols D., Rubia K. Disorder-specific and shared brain abnormalities during vigilance in autism and obsessive-compulsive disorder. Biol. Psychiatry: Cogn. Neuroscie. Neuroimaging. 2017 doi: 10.1016/j.bpsc.2016.12.005. (in press-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisi C.O., Norman L.J., Lukito S.S., Radua J., Mataix-Cols D., Rubia K. Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol. Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.10.006. (in press-b) [DOI] [PubMed] [Google Scholar]
- Carmona S., Bassas N., Rovira M., Gispert J.D., Soliva J.C., Prado M., Tomas J., Bulbena A., Vilarroya O. Pediatric OCD structural brain deficits in conflict monitoring circuits: a voxel-based morphometry study. Neurosci. Lett. 2007;421:218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Christakou A., Murphy C.M., Chantiluke K., Cubillo A.I., Smith A.B., Giampietro V., Daly E., Ecker C., Robertson D., Consortium M.A., Murphy D.G., Rubia K. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol. Psychiatry. 2013;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Gordon A.M., Smallwood J., Smith R., Schooler J.W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Harrison B.J., Pujol J., Harding I.H., Fornito A., Pantelis C., Yucel M. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Hum. Brain Mapp. 2012;33:1089–1106. doi: 10.1002/hbm.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K., Sitarenios G., Parker J.D., Epstein J.N. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Giampietro V., Taylor E., Rubia K. Fronto-striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatry Res. 2011;193:17–27. doi: 10.1016/j.pscychresns.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Smith A., Taylor E., Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond S.P., Bischoff-Grethe A., Dinges D.F., Ayalon L., Mednick S.C., Meloy M.J. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.M., Yang Y., Ulug A.M., Casey B.J. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016:201602413. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K.D., Stern E.R., Angstadt M., Nicholson-Muth K., Maynor M., Welsh R.C., Hanna G.L., Taylor S.F. Altered function and connectivity of the medial frontal cortex in pediatric obsessive compulsive disorder. Biol. Psychiatry. 2010;68:1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D., Biederman J., Jones J., Park K., Schwartz S., Shapiro S., Coffey B. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37:420–427. doi: 10.1097/00004583-199804000-00020. [DOI] [PubMed] [Google Scholar]
- Geller D., Petty C., Vivas F., Johnson J., Pauls D., Biederman J. Examining the relationship between obsessive-compulsive disorder and attention-deficit/hyperactivity disorder in children and adolescents: a familial risk analysis. Biol. Psychiatry. 2007;61:316–321. doi: 10.1016/j.biopsych.2006.03.083. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D.P., Murray R. Oxford University Press; USA: 2006. The Maudsley Handbook of Practical Psychiatry. [Google Scholar]
- Goodman R. The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Grassi G., Pallanti S., Righi L., Figee M., Mantione M., Denys D., Piccagliani D., Rossi A., Stratta P. Think twice: impulsivity and decision making in obsessive–compulsive disorder. J. Behav. Addict. 2015;4:263–272. doi: 10.1556/2006.4.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B.M., Park J.Y., Kang D.H., Lee S.J., Yoo S.Y., Jo H.J., Choi C.H., Lee J.M., Kwon J.S. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Radua J., Mataix-Cols D., Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neurosci. Biobehav. Rev. 2012;36:2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W., Wilson-Mendenhall C.D., Duncan E., Barsalou L.W. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. NeuroImage. 2012;59:750–760. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C.L., Karalunas S.L., Tam H., Moore A.N. Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J. Abnorm. Psychol. 2012;121:360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyser C., Veltman D.J., Wolters L.H., de Haan E., Boer F. Functional magnetic resonance imaging during planning before and after cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1238–1248. doi: 10.1016/j.jaac.2010.08.007. (e1235) [DOI] [PubMed] [Google Scholar]
- Huyser C., Veltman D.J., Wolters L.H., de Haan E., Boer F. Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a flanker task before and after CBT. J. Child Psychol. Psychiatry. 2011;52:1251–1260. doi: 10.1111/j.1469-7610.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- Kang D.H., Jang J.H., Han J.Y., Kim J.H., Jung W.H., Choi J.S., Choi C.H., Kwon J.S. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;40:340–346. doi: 10.1016/j.pnpbp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Langner R., Eickhoff S.B. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant Attention. Psychol. Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D., Du M., Wu M., Chen T., Huang X., Du X., Bi F., Kemp G.J., Gong Q. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology. 2015;29:874–881. doi: 10.1037/neu0000200. [DOI] [PubMed] [Google Scholar]
- Liddle E.B., Hollis C., Batty M.J., Groom M.J., Totman J.J., Liotti M., Scerif G., Liddle P.F. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J. Child Psychol. Psychiatry. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Hart H., Mehta M.A., Simmons A., Mirza K., Rubia K. Neurofunctional abnormalities during sustained attention in severe childhood abuse. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losier B.J., McGrath P.J., Klein R.M. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. J. Child Psychol. Psychiatry. 1996;37:971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Lucke I.M., Lin C., Conteh F., Federline A., Sung H., Specht M., Grados M.A. Continuous performance test in pediatric obsessive-compulsive disorder and tic disorders: the role of sustained attention. CNS Spectr. 2015;20:479–489. doi: 10.1017/S1092852914000467. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L., Fuentes D., Leite W.B., Correa H., Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J. Int. Neuropsychol. Soc. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Mathews C.A., Grados M.A. Familiality of Tourette syndrome, obsessive-compulsive disorder, and attention-deficit/hyperactivity disorder: heritability analysis in a large sib-pair sample. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:46–54. doi: 10.1016/j.jaac.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin B., Krebs R.M., Wiersema J.R., Verguts T., Gasthuys R., van der Meere J.J., Achten E., Roeyers H., Sonuga-Barke E. Dysfunctional modulation of default mode network activity in attention-deficit/hyperactivity disorder. J. Abnorm. Psychol. 2015;124:208–214. doi: 10.1037/abn0000013. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S., Craig K.J., Ersche K.D., Abbott S., Muller U., Fineberg N.A., Bullmore E.T., Sahakian B.J., Robbins T.W. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology. 2010;212:357–367. doi: 10.1007/s00213-010-1963-z. [DOI] [PubMed] [Google Scholar]
- Mowinckel A.M., Pedersen M.L., Eilertsen E., Biele G. A meta-analysis of decision-making and attention in adults with ADHD. J. Atten. Disord. 2015;19:355–367. doi: 10.1177/1087054714558872. [DOI] [PubMed] [Google Scholar]
- Mowlem F.D., Skirrow C., Reid P., Maltezos S., Nijjar S.K., Merwood A., Barker E., Cooper R., Kuntsi J., Asherson P. Validation of the mind excessively wandering scale and the relationship of mind wandering to impairment in adult ADHD. J. Atten. Disord. 2016 doi: 10.1177/1087054716651927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C.M., Christakou A., Daly E.M., Ecker C., Giampietro V., Brammer M., Smith A.B., Johnston P., Robertson D.M., Murphy D.G., Rubia K. Abnormal functional activation and maturation of fronto-striato-temporal and cerebellar regions during sustained attention in autism spectrum disorder. Am. J. Psychiatry. 2014;171:1107–1116. doi: 10.1176/appi.ajp.2014.12030352. [DOI] [PubMed] [Google Scholar]
- Nandagopal J.J., Fleck D.E., Adler C.M., Mills N.P., Strakowski S.M., DelBello M.P. Impulsivity in adolescents with bipolar disorder and/or attention-deficit/hyperactivity disorder and healthy controls as measured by the Barratt Impulsiveness Scale. J. Child Adolesc. Psychopharmacol. 2011;21:465–468. doi: 10.1089/cap.2010.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman L.J., Carlisi C., Lukito S., Hart H., Mataix-Cols D., Radua J., Rubia K. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73:815–825. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onnink A.M., Zwiers M.P., Hoogman M., Mostert J.C., Kan C.C., Buitelaar J., Franke B. Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur. Neuropsychopharmacol. 2014;24:397–409. doi: 10.1016/j.euroneuro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Page L.A., Rubia K., Deeley Q., Daly E., Toal F., Mataix-Cols D., Giampietro V., Schmitz N., Murphy D.G. A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res. 2009;174:202–209. doi: 10.1016/j.pscychresns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Parasuraman R., Warm J.S., See J.E. Brain systems of vigilance. In: Parasuraman R., editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. pp. 221–256. [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J. Int. Neuropsychol. Soc. 2010;16:106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Collins D.L., Evans A.C., Leonard G., Pike B., Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res. Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.S., Potenza M.N., Wang Z., Zhu H., Martin A., Marsh R., Plessen K.J., Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am. J. Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R., Monzani B., Leckman J.F., Ruck C., Serlachius E., Lichtenstein P., Mataix-Cols D. Understanding the covariation of tics, attention-deficit/hyperactivity, and obsessive-compulsive symptoms: a population-based adult twin study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016 doi: 10.1002/ajmg.b.32436. [DOI] [PubMed] [Google Scholar]
- Pironti V.A., Lai M.-C., Müller U., Dodds C.M., Suckling J., Bullmore E.T., Sahakian B.J. Neuroanatomical abnormalities and cognitive impairments are shared by adults with attention-deficit/hyperactivity disorder and their unaffected first-degree relatives. Biol. Psychiatry. 2014;76:639–647. doi: 10.1016/j.biopsych.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Song I., Lee S., Rodriguez C.I., Moore H., Marsh R., Blair Simpson H. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2017;38:678–687. doi: 10.1002/hbm.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98 doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajender G., Bhatia M.S., Kanwal K., Malhotra S., Singh T.B., Chaudhary D. Study of neurocognitive endophenotypes in drug-naive obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr. Scand. 2011;124:152–161. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- Rotge J.Y., Guehl D., Dilharreguy B., Cuny E., Tignol J., Bioulac B., Allard M., Burbaud P., Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J. Psychiatry Neurosci. 2008;33:405–412. [PMC free article] [PubMed] [Google Scholar]
- Rotge J.-Y., Guehl D., Dilharreguy B., Tignol J., Bioulac B., Allard M., Burbaud P., Aouizerate B. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol. Psychiatry. 2009;65 doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Roth R.M., Saykin A.J., Flashman L.A., Pixley H.S., West J.D., Mamourian A.C. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol. Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol. Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. ADHD brain function. In: Banaschweski T., Coghill D., Sadile A., editors. Oxford Handbook of ADHD. 2017. (in press) [Google Scholar]
- Rubia K., Smith A., Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol. Psychiatry. 2007;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.-M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Smith A.B., Mohammad M., Scott S., Brammer M.J. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J. Child Psychol. Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Halari R., Matsukura F., Mohammad M., Taylor E., Brammer M.J. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am. J. Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rubia K., Cubillo A., Smith A.B., Woolley J., Heyman I., Brammer M.J. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum. Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.M., Scott S., Brammer M. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Hum. Brain Mapp. 2010;31:1823–1833. doi: 10.1002/hbm.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Hyde Z., Halari R., Giampietro V., Smith A. Effects of age and sex on developmental neural networks of visual–spatial attention allocation. NeuroImage. 2010;51:817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Rubia K., Cubillo A., Woolley J., Brammer M.J., Smith A. Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum. Brain Mapp. 2011;32:601–611. doi: 10.1002/hbm.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Lim L., Ecker C., Halari R., Giampietro V., Simmons A., Brammer M., Smith A. Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. NeuroImage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Rubia K., Alegria A., Brinson H. Imaging the ADHD brain: disorder-specificity, medication effects and clinical translation. Expert. Rev. Neurother. 2014;14:519–538. doi: 10.1586/14737175.2014.907526. [DOI] [PubMed] [Google Scholar]
- Ruscio A., Stein D., Chiu W., Kessler R. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L., Riddle M.A., McSwiggin-Hardin M., Ort S.I., King R.A., Goodman W.K., Cicchetti D., Leckman J.F. Children's Yale-Brown obsessive compulsive scale: reliability and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27 doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P., Smallwood J., Cheyne J.A., Smilek D. On the relation of mind wandering and ADHD symptomatology. Psychon. Bull. Rev. 2015;22:629–636. doi: 10.3758/s13423-014-0793-0. [DOI] [PubMed] [Google Scholar]
- Seli P., Risko E.F., Purdon C., Smilek D. Intrusive thoughts: linking spontaneous mind wandering and OCD symptomatology. Psychol. Res. 2016 doi: 10.1007/s00426-016-0756-3. [DOI] [PubMed] [Google Scholar]
- Seymour K.E., Tang X., Crocetti D., Mostofsky S.H., Miller M.I., Rosch K.S. Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Res. 2017;261:20–28. doi: 10.1016/j.pscychresns.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Malek M., Watson B., Sharp W., Evans A., Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M.S., Choi H., Kim H., Hwang J.W., Kim B.N., Cho S.C. A study of neuropsychological deficit in children with obsessive-compulsive disorder. Eur. Psychiatry. 2008;23:512–520. doi: 10.1016/j.eurpsy.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Shin N.Y., Lee T.Y., Kim E., Kwon J.S. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol. Med. 2014;44:1121–1130. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- Smith A.B., Halari R., Giampetro V., Brammer M., Rubia K. Developmental effects of reward on sustained attention networks. NeuroImage. 2011;56:1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Snyder H.R., Kaiser R.H., Warren S.L., Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin. Psychol. Sci. 2015;3:301–330. doi: 10.1177/2167702614534210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn S.Y., Kang J.I., Namkoong K., Kim S.J. Multidimensional measures of impulsivity in obsessive-compulsive disorder: cannot wait and stop. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sripada C.S., Kessler D., Angstadt M. Lag in maturation of the brain's intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14259–14264. doi: 10.1073/pnas.1407787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Welsh R.C., Fitzgerald K.D., Gehring W.J., Lister J.J., Himle J.A., Abelson J.L., Taylor S.F. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol. Psychiatry. 2011;69:583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Fitzgerald K.D., Welsh R.C., Abelson J.L., Taylor S.F. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Welsh R.C., Gonzalez R., Fitzgerald K.D., Abelson J.L., Taylor S.F. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum. Brain Mapp. 2013;34:1956–1970. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Muratore, A.F., Taylor, S.F., Abelson, J.L., Hof, P.R., Goodman, W.K., Switching between internally and externally focused attention in obsessive-compulsive disorder: Abnormal visual cortex activation and connectivity. Psychiatry Res. Neuroimaging (in press). [DOI] [PMC free article] [PubMed]
- Talairach J., Tournoux P. Thieme; New York: 1988. Coplanar Stereotaxic Atlas of the Human Brain, a 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Thirion B., Pinel P., Meriaux S., Roche A., Dehaene S., Poline J.B. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. NeuroImage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Thomson D.R., Besner D., Smilek D. A resource-control account of sustained attention: evidence from mind-wandering and vigilance paradigms. Perspect. Psychol. Sci. 2015;10:82–96. doi: 10.1177/1745691614556681. [DOI] [PubMed] [Google Scholar]
- Todd R.D., Sitdhiraksa N., Reich W., Ji T.H., Joyner C.A., Heath A.C., Neuman R.J. Discrimination of DSM-IV and latent class attention-deficit/hyperactivity disorder subtypes by educational and cognitive performance in a population-based sample of child and adolescent twins. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:820–828. doi: 10.1097/00004583-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Trivedi J.K., Dhyani M., Goel D., Sharma S., Singh A.P., Sinha P.K., Tandon R. Neurocognitive dysfunction in patients with obsessive compulsive disorder. Afr. J. Psychiatry (Johannesbg) 2008;11:204–209. doi: 10.4314/ajpsy.v11i3.30270. [DOI] [PubMed] [Google Scholar]
- Valera E.M., Brown A., Biederman J., Faraone S.V., Makris N., Monuteaux M.C., Whitfield-Gabrieli S., Vitulano M., Schiller M., Seidman L.J. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am. J. Psychiatry. 2010;167:86–94. doi: 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm J.S. An introduction to vigilance. In: Warm J.S., editor. Sustained Attention in Human Performance. Wiley; Chichester: 1984. pp. 1–14. [Google Scholar]
- Wechsler D. NCS Pearson; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV) [Google Scholar]
- Willcutt E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- de Wit S.J., Alonso P., Schweren L., Mataix-Cols D., Lochner C., Menchon J.M., Stein D.J., Fouche J.P., Soriano-Mas C., Sato J.R., Hoexter M.Q., Denys D., Nakamae T., Nishida S., Kwon J.S., Jang J.H., Busatto G.F., Cardoner N., Cath D.C., Fukui K., Jung W.H., Kim S.N., Miguel E.C., Narumoto J., Phillips M.L., Pujol J., Remijnse P.L., Sakai Y., Shin N.Y., Yamada K., Veltman D.J., van den Heuvel O.A. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatry. 2014;171:340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- Woolley J., Heyman I., Brammer M., Frampton I., McGuire P.K., Rubia K. Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br. J. Psychiatry. 2008;192:25–31. doi: 10.1192/bjp.bp.107.036558. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Fan Q., Zhang H., Qiu J., Tan L., Xiao Z., Tong S., Chen J., Li Y. Altered intrinsic insular activity predicts symptom severity in unmedicated obsessive-compulsive disorder patients: a resting state functional magnetic resonance imaging study. BMC Psychiatry. 2016;16:1–9. doi: 10.1186/s12888-016-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material