Abstract
Sinonasal hemangiopericytoma (HPC) is a tumor showing pericytic myoid differentiation and which arises in the nasal cavity and paranasal sinuses. CTNNB1 mutations appear to be a consistent aberration in sinonasal HPC, and nuclear expression of β-catenin has been reported. Our aim was to evaluate the frequency of β-catenin expression in sinonasal HPC and its histologic mimics in the upper aerodigestive tract. Cases were retrieved from the surgical pathology and consultation files. Immunohistochemical staining for β-catenin was performed on 50 soft tissue tumors arising in the sinonasal tract or oral cavity, and nuclear staining was recorded semiquantitatively by extent and intensity. Nuclear reactivity for β-catenin was present in 19/20 cases of sinonasal HPC; 17 showed moderate-to-strong multifocal or diffuse staining, and 2 had moderate focal nuclear reactivity. All solitary fibrous tumors (SFT) (10/10) showed focal-to-multifocal nuclear staining, varying from weak to strong in intensity. Most cases of synovial sarcoma (9/10) showed nuclear β-catenin expression in the spindle cell component, ranging from focal-weak to strong-multifocal. No cases of myopericytoma (0/10) showed any nuclear β-catenin expression. β-catenin expression is prevalent in sinonasal HPC, but is also frequent in SFT and synovial sarcoma. Our findings indicate that β-catenin is not a useful diagnostic tool in the evaluation of spindle cell tumors with a prominent hemangiopericytoma-like vasculature in the sinonasal tract and oral cavity, and that definitive diagnosis relies on the use of a broader immunohistochemical panel.
Keywords: β-Catenin, Sinonasal hemangiopericytoma, Sinonasal, Soft tissue, Solitary fibrous tumor, Synovial sarcoma, Myopericytoma
Introduction
Sinonasal hemangiopericytoma (HPC) is a rare tumor of perivascular myoid differentiation that arises uniquely in the nasal cavity and paranasal sinuses, and which constitutes <1 % of all sinonasal tumors [1–3]. Tumors arise most commonly in adults, affect women and men at approximately equal frequencies, and show relatively frequent local recurrence (up to 20 %) but virtually no metastases [1–3]. Histologically, sinonasal HPC is comprised of uniform, bland spindled and rounded tumor cells with ovoid nuclei and palely eosinophilic cytoplasm arranged in sheets, whorls, or short fascicles. Tumor cells often show concentric arrangement around thin-walled gaping or slit-like (“HPC”) vessels, which occasionally show perivascular hyalinization. In most cases, the overlying sinonasal epithelium is intact with an underlying “Grenz” zone. Perivascular myoid differentiation is evident by immunohistochemistry, as sinonasal HPC expresses SMA and HHF-35, and is typically negative for desmin, CD34, and keratin. CTNNB1 gene mutations and nuclear expression of β-catenin have recently been identified in sinonasal HPC [4, 5].
Many mass lesions in the upper aerodigestive tract are sampled by endoscopic or core needle biopsies, and definitive diagnosis is often helpful for treatment planning. Spindle cell neoplasms in small biopsies may be diagnostically challenging, and sinonasal HPC shows morphologic overlap with solitary fibrous tumor (SFT), synovial sarcoma, and myopericytoma. Our aim was to evaluate the diagnostic utility of nuclear β-catenin expression for sinonasal HPC by determining the frequency of β-catenin expression in sinonasal HPC and its histologic mimics in the upper aerodigestive tract.
Materials and Methods
Cases were retrieved from pathology archives, and hematoxylin and eosin stained slides and available immunohistochemical stains were reviewed to confirm diagnoses using standardized and widely accepted criteria. There were 20 sinonasal HPCs, 10 SFTs, 10 synovial sarcomas, and 10 myopericytomas. All tumors were located in the sinonasal tract or oral cavity.
β-Catenin immunohistochemistry was performed on formalin-fixed, paraffin-embedded whole-sections using a mouse monoclonal antibody (BD Bioscience, #610154) at a 1:1000 dilution after antigen retrieval by citrate buffer and pressure cook. Appropriate positive and negative controls were used throughout. β-catenin immunostains were scored by one author (V.Y.J.) blinded to the histologic diagnosis. Nuclear β-catenin staining was scored according to intensity (weak, moderate, strong) and extent according to percentage of positive tumor cells (negative; focal, <10 %; multifocal, 25–75 %; diffuse, >75 %).
Results
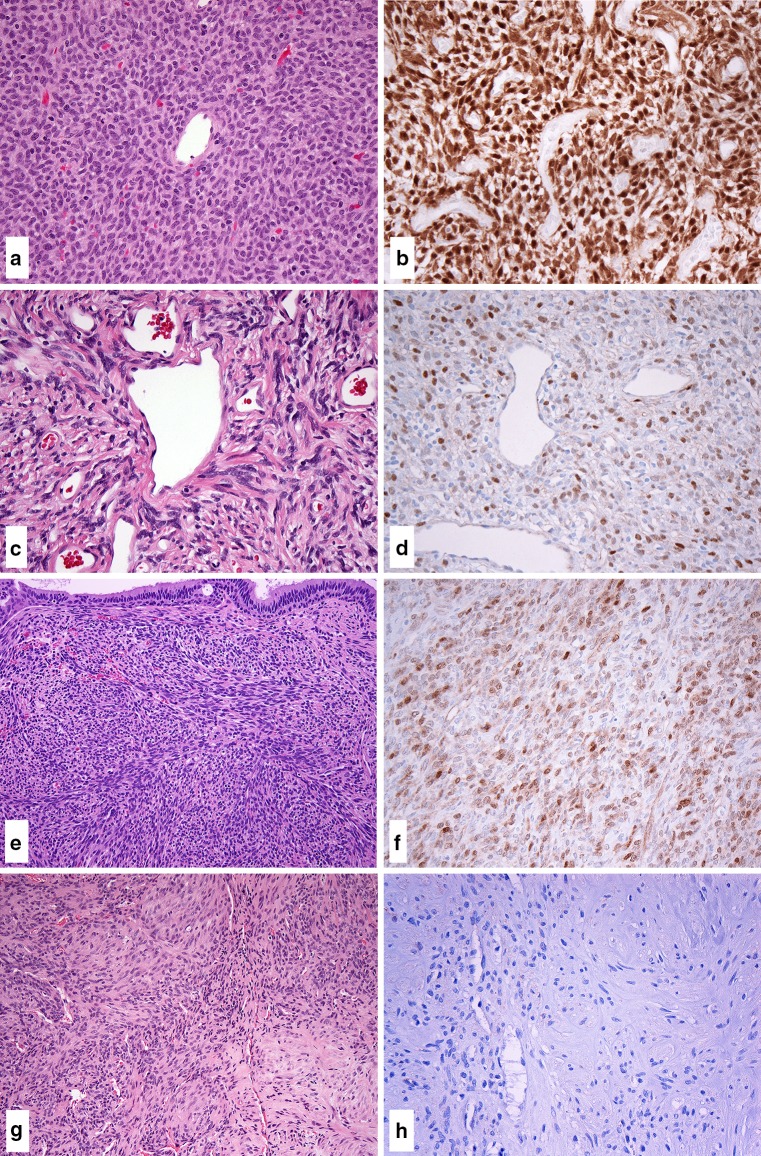
The clinical characteristics of the tumors in this study are summarized in Table 1. Nuclear reactivity for β-catenin was present in 19 of 20 cases of sinonasal HPC. Most cases (17/20) showed moderate-to-strong multifocal or diffuse nuclear staining, and 2 cases exhibited moderate focal nuclear reactivity (Fig. 1a, b).
Table 1.
Clinical characteristics of the study cases
| Tumor type | Age (years) Median (range) |
Gender (M:F) | Tumor location |
|---|---|---|---|
| Sinonasal HPC (n = 20) | 62 (31–79) | 11:9 | Sinonasal [20] |
| SFT (n = 10) | 59 (31–80) | 5:5 | Sinonasal [4] Oral cavity [6] |
| Synovial sarcoma (n = 10) | 37 (1–73) | 3:7 | Sinonasal [4] Orbit [1] Oral cavity [2] Hypopharynx [3] |
| Myopericytoma (n = 10) | 46 (11–64) | 4:6 | Sinonasal [2] Oral cavity [8] |
HPC, hemangiopericytoma; SFT, solitary fibrous tumor
Fig. 1.
Nuclear β-catenin staining was present in the majority of sinonasal HPC. β-catenin expression was most frequently moderate-to-strong and multifocal or diffuse (a H&E, b β-catenin). SFT showed consistent nuclear β-catenin expression, although staining intensity and extent were more variable, ranging from focal to multifocal in extent and weak to strong in intensity (c H&E, d β-catenin). Frequent nuclear β-catenin was observed in synovial sarcomas; β-catenin expression ranged from focal and weak to strong and multifocal in staining (e H&E, f β-catenin). No nuclear β-catenin expression was observed myopericytoma (g H&E, h β-catenin)
All cases of SFT (10/10) were positive, but nuclear β-catenin staining patterns were more variable. Nuclear β-catenin staining was focal to multifocal in extent, and varied from weak to strong in intensity (Fig. 1c, d). The majority of synovial sarcomas (9/10) showed nuclear β-catenin expression, which was present in the spindle cell component and ranged from focal and weak to strong and multifocal in intensity (Fig. 1e, f). No cases of myopericytoma showed nuclear β-catenin reactivity (0/10) (Fig. 1g, h).
Discussion
Our findings demonstrate that the majority of sinonasal HPC show consistent nuclear β-catenin expression (95 %), and most cases exhibit moderate-to-strong multifocal or diffuse nuclear staining. However, β-catenin immunoreactivity is also frequent in its morphologic mimics SFT and synovial sarcoma, albeit staining patterns are more variable in the latter. No β-catenin immunoreactivity was observed in myopericytoma, which may be helpful in its distinction from sinonasal HPC.
β-Catenin is encoded by the CTNNB1 gene (on 3p21). β-catenin is a cytoplasmic protein that interacts with the cytoplasmic domains of E-cadherin, functioning to stabilize E-cadherin in cell adhesion functions, and also acts as a transcription factor in the Wnt signaling pathway. Mutations in β-catenin result in its intranuclear accumulation, thereby evading ubiquitination and degradation which leads to increased transcription of Wnt pathway target genes, which are involved in cellular proliferation [6]. CTNNB1 alterations in sinonasal HPC have been consistently reported in exon 3, with missense mutations affecting codons 32, 33, 34, 35, 37, and 45 [4, 5]; mutations affecting positions 32–45 of the amino-terminal region disrupt phosphorylation-dependent degradation of β-catenin [6]. Numerous other tumor types harbor CTNNB1 mutations, most frequently desmoid-type fibromatosis [7, 8] as well as salivary basal cell adenoma [9], pilomatricoma and pilomatrix carcinoma [10], hepatocellular carcinoma [11], colorectal carcinoma [12], medulloblastoma [13], endometrial adenocarcinoma [14], Wilms tumor [15], and adrenocortical carcinoma [16].
Sinonasal HPC shares the features of a uniform spindle and ovoid cell population and HPC-like vessels with SFT, synovial sarcoma, and myopericytoma. Frequent nuclear β-catenin expression was observed in SFT and synovial sarcoma, which may possibly be secondary to Wnt pathway activation, although CTNNB1 mutations are not characteristic of these entities. SFT occurs infrequently in the sinonasal tract, and is comprised of uniform spindled and ovoid tumor cells having little cytoplasm and poorly defined cell borders; HPC-like vessels are typical. The diagnosis of SFT can be facilitated by recognition of its characteristic “patternless grown pattern” in which tumor cells are arranged haphazardly with variably prominent hyaline stromal collagen. The seemingly specific NAB2–STAT6 fusion gene has been identified in SFT; this fusion gene results from inversion of the two genes on chromosome 12q13 [17, 18] and results in STAT6 overexpression. SFT typically shows strong and diffuse cytoplasmic CD34 positivity and nuclear STAT6 expression [19, 20]; focal positivity may be observed for SMA, EMA, desmin, S-100, and cytokeratin (unpublished personal observations). β-catenin expression has previously been reported in SFT [21–23], although the mechanism for Wnt pathway activation remains to be elucidated.
Synovial sarcoma is characterized by uniform spindle cells arranged in cellular sheets and fascicles; tumor cells have overlapping ovoid nuclei with palely eosinophilic cytoplasm and indistinct cells borders. Although wiry stromal collagen and HPC-like vessels are characteristic of synovial sarcoma, the fascicles are longer and more cellular than those in sinonasal HPC. Synovial sarcoma may also show prominent stromal hyalinization and calcifications. Biphasic examples of synovial sarcoma can be recognized by foci of epithelial/glandular differentiation. Synovial sarcoma shows positivity for TLE1 [24] and epithelial markers cytokeratin and EMA, even in the spindle cell component, but does not typically express SMA or HHF-35. Similar to prior studies [22, 25], we observed frequent β-catenin expression in synovial sarcoma. Synovial sarcoma is characterized by translocation t(X; 18)(p11; q11) [26], resulting in SS18 gene rearrangement with one of three SSX gene fusion partners (SSX1, SSX2, or SSX4); SS18–SSX activation of the Wnt signaling pathway has been demonstrated using in vitro and in vivo studies [27–29].
Myopericytoma (including myofibroma) is a benign tumor of perivascular/pericytic differentiation, and appears as a well-circumscribed nodular or lobular growth of uniform spindle or ovoid cells showing frequent perivascular concentric growth around HPC-like vessels. Myopericytoma shows variable cellularity and a collagenous or sometimes myxoid stroma, and may also show bulging subendothelial proliferation of tumor cells within vessels walls. Consistent genetic alterations have not been identified in myopericytoma, apart from a unique variant characterized by translocation t(7; 12)(p22; q13) which results in ACTB–GLI1 fusion [30–32] and a report of BRAF V600E mutations in a subset of myopericytomas [33]. Similar to a prior report [34], nuclear β-catenin expression is consistently absent in myofibroma and is useful in the distinction between sinonasal HPC and myopericytoma.
In summary, β-catenin is frequently positive in sinonasal HPC and its morphologic mimics SFT and synovial sarcoma, but is negative in myopericytoma. β-catenin in sinonasal HPC is secondary to CTNNB1 exon 3 missense mutations, and the variable patterns of β-catenin expression in SFT and synovial sarcoma may possibly be secondary to alternative mechanisms of Wnt pathway activation. Certain morphologic features may be helpful in distinguishing sinonasal HPC from SFT, synovial sarcoma, and myopericytoma, although evaluation may be more challenging in small biopsies. Our findings indicate that β-catenin alone is not a useful diagnostic tool in the evaluation of spindle cell tumors with a prominent hemangiopericytoma-like vasculature in the sinonasal tract and oral cavity, and the diagnostic evaluation should include a broader immunohistochemical panel.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest.
Footnotes
These results were presented in part at the 105th Annual Meeting of the US-Canadian Academy of Pathology, Seattle, March 2015.
References
- 1.El-Naggar AK, Batsakis JG, Garcia GM, et al. Sinonasal hemangiopericytomas. A clinicopathologic and DNA content study. Arch Otolaryngol Head Neck Surg. 1992;118:134–137. doi: 10.1001/archotol.1992.01880020026010. [DOI] [PubMed] [Google Scholar]
- 2.Tse LL, Chan JK. Sinonasal haemangiopericytoma-like tumour: a sinonasal glomus tumour or a haemangiopericytoma? Histopathology. 2002;40:510–517. doi: 10.1046/j.1365-2559.2002.01396.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27:737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Haller F, Bieg M, Moskalev EA, et al. Recurrent mutations within the amino-terminal region of beta-catenin are probable key molecular driver events in sinonasal hemangiopericytoma. Am J Pathol. 2015;185:563–571. doi: 10.1016/j.ajpath.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Lasota J, Felisiak-Golabek A, Aly FZ, et al. Nuclear expression and gain-of-function beta-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod Pathol. 2015;28:715–720. doi: 10.1038/modpathol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salas S, Chibon F, Noguchi T, et al. Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosomes Cancer. 2010;49:560–568. doi: 10.1002/gcc.20766. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara A, Harada H, Abe H, et al. Nuclear beta-catenin expression in basal cell adenomas of salivary gland. J Oral Pathol Med. 2011;40:460–466. doi: 10.1111/j.1600-0714.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 10.Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148–157. doi: 10.1111/j.0303-6987.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 11.Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 13.Jones DT, Jager N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Bueno G, Hardisson D, Sanchez C, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21:7981–7990. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 15.Scott RH, Murray A, Baskcomb L, et al. Stratification of Wilms tumor by genetic and epigenetic analysis. Oncotarget. 2012;3:327–335. doi: 10.18632/oncotarget.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assie G, Letouze E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46:607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2–STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2–STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–395. doi: 10.1038/modpathol.2013.247. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer L, Koelsche C, Sahm F, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2–STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 21.Rakheja D, Molberg KH, Roberts CA, et al. Immunohistochemical expression of beta-catenin in solitary fibrous tumors. Arch Pathol Lab Med. 2005;129:776–779. doi: 10.5858/2005-129-776-IEOCIS. [DOI] [PubMed] [Google Scholar]
- 22.Ng TL, Gown AM, Barry TS, et al. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- 23.Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509–514. doi: 10.1111/j.1365-2559.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- 24.Foo WC, Cruise MW, Wick MR, et al. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011;135:839–844. doi: 10.1309/AJCP45SSNAOPXYXU. [DOI] [PubMed] [Google Scholar]
- 25.Horvai AE, Kramer MJ, O’Donnell R. Beta-catenin nuclear expression correlates with cyclin D1 expression in primary and metastatic synovial sarcoma: a tissue microarray study. Arch Pathol Lab Med. 2006;130:792–798. doi: 10.5858/2006-130-792-CNECWC. [DOI] [PubMed] [Google Scholar]
- 26.Crew AJ, Clark J, Fisher C, et al. Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J. 1995;14:2333–2340. doi: 10.1002/j.1460-2075.1995.tb07228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barham W, Frump AL, Sherrill TP, et al. Targeting the Wnt pathway in synovial sarcoma models. Cancer Discov. 2013;3:1286–1301. doi: 10.1158/2159-8290.CD-13-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trautmann M, Sievers E, Aretz S, et al. SS18–SSX fusion protein-induced Wnt/beta-catenin signaling is a therapeutic target in synovial sarcoma. Oncogene. 2014;33:5006–5016. doi: 10.1038/onc.2013.443. [DOI] [PubMed] [Google Scholar]
- 29.Cironi L, Petricevic T, Fernandes Vieira V, et al. The fusion protein SS18–SSX1 employs core Wnt pathway transcription factors to induce a partial Wnt signature in synovial sarcoma. Sci Rep. 2016;6:22113. doi: 10.1038/srep22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahlen A, Fletcher CD, Mertens F, et al. Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12) Am J Pathol. 2004;164:1645–1653. doi: 10.1016/S0002-9440(10)63723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahlen A, Mertens F, Mandahl N, et al. Molecular genetic characterization of the genomic ACTB–GLI fusion in pericytoma with t(7;12) Biochem Biophys Res Commun. 2004;325:1318–1323. doi: 10.1016/j.bbrc.2004.10.172. [DOI] [PubMed] [Google Scholar]
- 32.Bridge JA, Sanders K, Huang D, et al. Pericytoma with t(7;12) and ACTB–GLI1 fusion arising in bone. Hum Pathol. 2012;43:1524–1529. doi: 10.1016/j.humpath.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadow PM, Priolo C, Nanni S, et al. Role of BRAFV600E in the first preclinical model of multifocal infiltrating myopericytoma development and microenvironment. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, et al. Nuclear beta-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol. 2005;29:653–659. doi: 10.1097/01.pas.0000157938.95785.da. [DOI] [PubMed] [Google Scholar]