Abstract
Basal cell carcinoma (BCC) is the most common type of skin cancer. Microscopically, BCC can be classified into indolent-growth and aggressive-growth subtypes. Additionally, uncommon variants have been described in the literature including adamantinoid, granular, clear cell, and BCC with matrical differentiation (BCCMD). If left untreated, BCC can invade locally causing significant tissue destruction while metastatic BCC is extremely rare. There have only been rare cases of BCCMD previously reported in the literature with none exhibiting metastasis. In this report, a 76 year old male patient presented to our center with a recurrent nasal lesion. He had been diagnosed with BCC at another institution about 8 years prior. He underwent a completion rhinectomy procedure, and on microscopic examination the tumor was diagnosed as BCCMD. In view of the uncommon pathology, a PET scan was ordered, which showed a left submandibular hypermetabolic lymph node with central areas of necrosis. A fine needle aspirate from the node confirmed metastasis, and the patient underwent subsequent neck dissection. In conclusion, we have presented a very rare case of a nasal BCCMD with regional metastasis. To the best of our knowledge, this constitutes the first reported case in the English literature. This might raise the possibility of a probable metastatic potential for this lesion and subsequently a more aggressive behavior. However, it is to be noted that this is a single case report and the affirmation of any metastatic potential would still need to be confirmed through additional future reports.
Keywords: Basal cell carcinoma, Metastasis, Nose, Matrical
Introduction
Basal cell carcinoma (BCC) is the most common type of skin cancer. A study conducted in 2006 showed that NMSC is the most common malignancy in the United States [1]. Microscopically, BCC can be classified into indolent-growth subtypes (nodular and superficial) and aggressive-growth subtypes (morpheaform, infiltrative, micronodular and basosquamous) [2]. Additionally, uncommon variants have been described in the literature including adamantinoid, granular, clear cell, and BCC with matrical differentiation (BCCMD) [3].
If left untreated, BCC can invade locally causing significant tissue destruction while metastatic BCC is extremely rare [2]. To the best of our knowledge, there have only been 31 cases of BCCMD previously reported in the literature with none exhibiting metastasis [4–15]. BCCMD shows differentiation towards hair matrix and is characterized by islands of basaloid cells along with shadow cells, which are characteristic markers of pilomatricomas and also found in other entities [4]. Therefore, it is of critical importance to differentiate a suspected BCCMD from the similar malignant pilomatricoma [10].
While BCCMD has typically been described as showing similar behavior clinically to other BCCs, its own potential for metastasis is herein demonstrated. In this report, we present an extremely rare case of a nasal basal cell carcinoma with matrical differentiation that metastasized regionally to the neck. To the best of our knowledge, this represents the first case of Nasal BCCMD with metastasis reported in the English literature. A review of the pertinent literature is presented.
Case Presentation
A 76 year old male patient presented to our center with a recurrent nasal lesion. He had been diagnosed with basal cell carcinoma at another institution about 8 years prior to the current presentation. He underwent resection followed by many revision surgeries due to multiple recurrences. This resulted in a nasal defect amounting to a subtotal rhinectomy. He presented with a 3 × 2 cm nodular lesion involving the remnant right nasal sidewall and bridge (Fig. 1). It was adjacent to the right medial canthus. A biopsy was performed confirming recurrence and was labeled as BCC. He underwent a completion rhinectomy procedure. On microscopic examination the tumor was diagnosed as BCCMD.
Fig. 1.
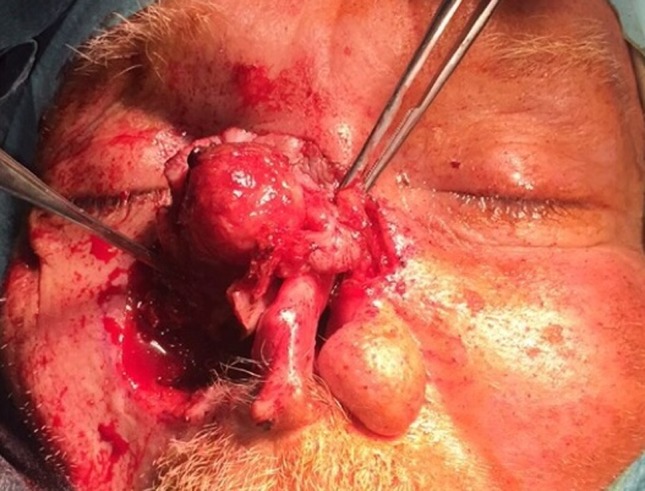
Intraoperative picture showing the extent of the lesion
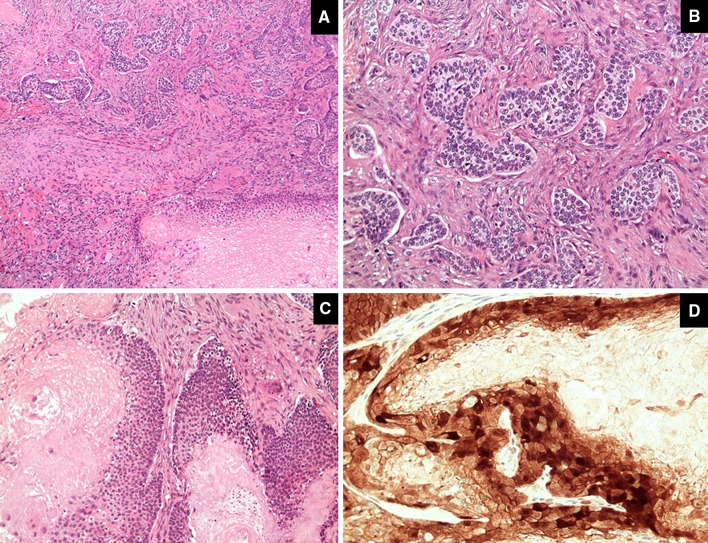
Microscopically, the tumor had dual morphology. The outer part of the tumor showed epithelial nests with palisading nuclei at their borders. These nests were separated from the surrounding stroma by mucin. This component represents the classic basal cell carcinoma. The second component exhibits matrical differentiation with basaloid tumor islands composed of follicular germinative cells at the outer part of the nests and shadow cells in the center reminiscent of the cells seen in pilomatricoma. The matrical component showed diffuse nuclear and cytoplasmic expression of beta catenin (Fig. 2).
Fig. 2.
The tumor shows dual morphology (a) with basal cell component (b) and matrical component (c). The matrical component shows diffuse nuclear and cytoplasmic expression of beta catenin (d)
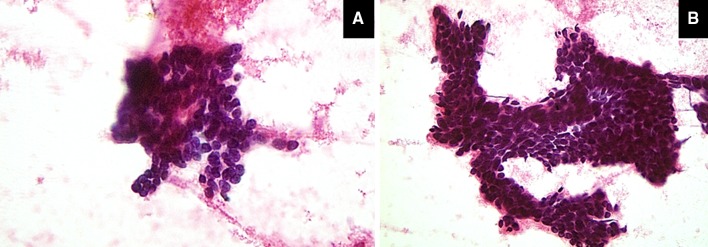
In view of the uncommon pathology, a PET scan was ordered. Interestingly, it showed a left submandibular hypermetabolic lymph node with central areas of necrosis with an SUV of 6.82 in keeping with metastasis (Fig. 3). Note that the overlying skin was completely normal. A fine needle aspirate from the node was ordered confirming BCC (Fig. 4a, b). The patient underwent a subsequent neck dissection procedure and was referred for postoperative radiotherapy afterwards.
Fig. 3.
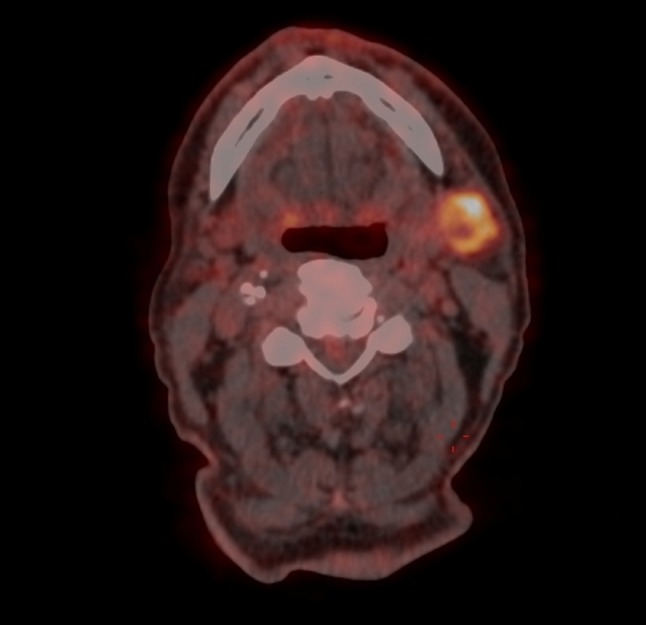
Positron Emission Tomography scan showing a left submandibular hypermetabolic lymph node with central areas of necrosis with an SUV of 6.82 in keeping with metastasis
Fig. 4.
The prepared smears from the fine needle aspirate were cellular and show tight clusters of basaloid cells (a [magnification ×20], and b [magnification ×40], both stained by H&E).”
Discussion
BCCMD is a tumor characterized by foci of shadow cells interspersed within islands of basaloid cells; shadow cells within the skin represent a form of cell death in which a faulty attempt at follicular differentiation and formation of a hair shaft takes place [7]. They are described as fully keratinized polyhedral cells with well-defined borders, abundant eosinophilic cytoplasm, and a central unstainable empty space that corresponds to its lost nucleus [4, 7]. Shadow cells are typically seen in matrical epithelial tumors such as pilomatricoma and pilomatrix carcinoma, however there are numerous other entities in which they may be seen, including: alopecia areata, condyloma acunimatum, anychomycosis, trichoepithelioma, proliferating trichilemmal tumor, trichilemmal cyst, chondroid syringoma, keratoacanthoma, epidermal cysts of Gardner’s syndrome, squamous cell carcinoma, and other unclassified hair follicle neoplasms [4, 11].
The first three cases of BCCMD were reported in 1988 by Aloi, Molinero and Pippione, who also proposed the currently accepted name for the lesion [4]. To date, there have only been 32 cases of BCCMD reported in the literature including the present case [4–15] (Table 1). Fifteen of the 32 cases were retrieved from larger studies looking at many different cutaneous tumors and did not focus exclusively on the BCCMD cases [12, 14]. Based on the little information available on this tumor, it seems to generally behave like most BCCs and is locally invasive, with no previously reported metastases. For the following observances, 11 cases from the Battistella et al. study [14] were omitted since no details about the cases were provided.
Table 1.
Cases of basal cell carcinoma with matrical differentiation as reported in the literature
| Case | References | Sex | Age | Location | Presentation | Duration | Size (cm) | Clinical diagnosis | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aloi et al. [4] | F | 79 | R Forearm | Noduloulcerative | 6 yr | 1 × 1.5 | Epithelioma | Excision | 4 yr |
| 2 | Aloi et al. [4] | M | 62 | Lumbar | Plaque w/central nodule | 10 yr | 5 × 10 | – | Excision | 3 yr |
| 3 | Aloi et al. [4] | M | 72 | R Temporal | Noduloulcerative | 1 yr | 1 × 1 | BCC | Excision | 3 yr |
| 4 | Ambrojo et al. [5] | M | 73 | C Forehead | Nodule | 20 yr | 0.8 | BCC | Excision | 1 yr |
| 5 | Ambrojo et al. [5] | M | 64 | C Forehead | Nodule | 4 yr | 0.5 | BCC | Excision | 3 yr |
| 6 | Ambrojo et al. [5] | F | 63 | C Forehead | Nodule | 1 yr | 2 × 1 | BCC | Local Radiotherapy | 8 yr |
| 7 | Ambrojo et al. [5] | F | 60 | L Preauricular | Nodule | 2 yr | 1 × 1.5 | BCC | Local Radiotherapy | 6 yr |
| 8 | Sağol and Özer [6] | M | 78 | L Forehead | Noduloulcerative | 10 yr | 1.5 × 1 × 0.5 | – | Excision | – |
| 9 | Kwittken [7] | M | 64 | R Temple | Papuloulcerative | 1 yr | 0.8 | – | Excision | – |
| 10 | Kim et al. [8] | M | 45 | L Arm | Scaly Papule | 1 yr | 1.0 × 0.8 | Actinic keratosis/psoriasis vulgaris | Excision | – |
| 11 | Ali et al. [9] | M | 58 | L Dorsal Hand | Plaque | 4–5 mo | – | Keratoacanthoma/SCC | – | |
| 12 | Haskell et al. [10] | M | 78 | R Ala Nasi | Nodule | Several mo | 0.7 | – | Excision | 18 mo |
| 13 | Del Sordo et al. [11] | F | 68 | Forehead | Noduloulcerative | – | 0.6 | – | Excision | 6 yr |
| 14 | Del Sordo et al. [11] | M | 77 | Back | Nodule | – | 0.7 | – | Excision | 1 yr |
| 15–18 | Kazakov et al. [12] | 2 M 2F | 47–94 | 2 Head/Neck 2 Torso | – | – | – | – | – | – |
| 19 | Murphy and Elaba [13] | F | 50 | Upper Chest | Erythematous Lesion | Several mo | 0.8 | BCC | Ablation | – |
| 20–30 | Battistella et al. [14] | – | – | – | – | – | – | – | – | – |
| 31 | Goldman-Levy et al. [15] | F | 74 | R Dorsal Hand | Crusted Papule | 2 mo | 0.5 | – | Excision | 6 mo |
M male, F female, R right, L left, C center, yr years, mo months, BCC basal cell carcinoma, SCC squamous cell carcinoma
As the most common type of skin cancer, BCC has been well characterized in terms of its epidemiological patterns. Studies have shown that in general, BCC has a higher incidence in men than women, and the risk of being diagnosed with BCC increases with age and sun exposure [2]. Similarly, in analyzing the reported cases of BCCMD, it was found that 13 out of 21 cases (62 %) occurred in men compared with 8 (38 %) that occurred in women. Also, it was found that the mean age (n = 21) of the documented reports at diagnosis was 67.4 years, which is consistent with what is already known about BCC overall. The duration of the lesion prior to presentation for each of the cases varied anywhere from 2 months to 20 years, and none of the previously reported lesions exceeded 1.5 cm in any dimension.
In 13 out of 16 well documented case reports (81 %), the lesion was described as either a nodule or papule, with or without crust or ulceration. This is consistent with the description of nodular BCC, along with its prevalence. Two of the remaining cases described the lesions as plaques, and one vaguely as an erythematous lesion.
The most common site of BCC is on the face, which is exemplary of its correlation to sun exposure [2]. Other less common sites include the trunk and on the extremities. Analysis of the BCCMD cases (n = 21) revealed that 12 lesions (57 %) were located on the head/neck, 5 (24 %) were located on the trunk, and 4 (19 %) were located on the extremities, which is relatively consistent with what is known about BCC from the literature. The majority of the cases were treated with surgical excision, with the exception of 1 case where ablation was performed and 2 cases where radiotherapy was utilized. It is notable that other than the present case, there was only one case of BCCMD located on the nose, which was treated with surgical excision using Mohs [10].
Amongst the risk factors for developing a BCC, it has been shown that there is a significantly higher risk of developing a subsequent BCC after developing a first one, and the risk increases with every subsequent BCC [16]. It was noted that 6 out of 21 cases (28.6 %) of patients with BCCMD had a history of at least one, and often multiple, NMSC, most commonly of the BCC type.
The present case is the first reported case in the English literature of a metastatic BCCMD. Metastatic basal cell carcinoma (mBCC) itself is exceedingly rare with varying incidence rates ranging between 0.003 and 0.1 % of all BCCs [2]. As a result, there is very little information available regarding mBCC. According to the Lattes and Kessler criteria, diagnosis of mBCC requires documented history of a prior BCC as well as confirmation of metastasis via histology [17]. There are currently no immunohistochemical or molecular markers that predict which BCC subtypes have a higher risk of metastasis, however it has been reported that, similarly to primary BCC, mBCC may involve mutation or loss of heterozygosity of the PTCH1 tumor suppressor [18, 19]. Also, some risk factors for mBCC have been reported including infiltrating/morphaeform subtypes, perineural invasion, tumor persistence over several years, tumors resistant to therapy, radiation exposure and primary tumors larger than 2 cm [19].
In conclusion, we have presented a very rare case of a nasal BCCMD with regional metastasis. To the best of our knowledge, this constitutes the first reported case in the English literature. This might raise the possibility of a probable metastatic potential for this lesion and subsequently a more aggressive behavior. However, it is to be noted that this is a single case report and the affirmation of any metastatic potential would still need to be confirmed through additional future reports. Still, recognizing this rare variant is of utmost importance as it might prove to have a direct impact on management.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Rogers H, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Marzuka A, Book S. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology and management. Yale J Biol Med. 2015;88:167–179. [PMC free article] [PubMed] [Google Scholar]
- 3.Elder D. Histopathology of the skin. 8. Philadelphia: JB Lippincott; 1997. [Google Scholar]
- 4.Aloi FG, Molinero A, Pippione M. Basal cell carcinoma with matrical differentiation. Am J Dermatopathol. 1988;10(6):509–513. doi: 10.1097/00000372-198812000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ambrojo P, Aguilar A, Simon P, Requena L, Sanchez E. Basal cell carcinoma with matrical differentiation. Am J Dermatopathol. 1992;14(4):293–297. doi: 10.1097/00000372-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Sagol O, Ozer E. Basal cell carcinoma of the skin with matrical differentiation: a case report. East J Med. 1999;4(1):37–38. [Google Scholar]
- 7.Kwittken J. Shadow cell basal carcinoma with acantholysis. Cutis. 2002;69(1):57–60, 63–5. [PubMed]
- 8.Kim SH, Lee MG, Lee KG. Basal cell carcinoma with matrical differentiation. Yonsei Med J. 2003;44(3):523–525. doi: 10.3349/ymj.2003.44.3.523. [DOI] [PubMed] [Google Scholar]
- 9.Ali F, Brown A, Gottwald L, Thomas J. Basal cell carcinoma with matrical differentiation in a transplant patient: a case report and review of the literature. J Cutan Pathol. 2005;32:445–448. doi: 10.1111/j.0303-6987.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 10.Haskell H, Haynes H, McKee P, Redston M, Granter S, Lazar AJF. Basal cell carcinoma with matrical differentiation: case study with analysis of beta-catenin. J Cutan Pathol. 2005;32:245–250. doi: 10.1111/j.0303-6987.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Del Sordo R, Cavaliere A, Sidoni A. Basal cell carcinoma with matrical differentiation: expression of.-catenin and osteopontin. Am J Dermatopathol. 2007;29:470–474. doi: 10.1097/DAD.0b013e318156dceb. [DOI] [PubMed] [Google Scholar]
- 12.Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (beta-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248–255. doi: 10.1097/DAD.0b013e318198922a. [DOI] [PubMed] [Google Scholar]
- 13.Murphy M, Elaba Z. Basal cell carcinoma with matrical differentiation. Int J Dermatol. 2011;50:485–496. doi: 10.1111/j.1365-4632.2009.04391.x. [DOI] [PubMed] [Google Scholar]
- 14.Battistella M, Carlson JA, Osio A, Langbein L, Cribier B. Skin tumors with matrical differentiation: lessons from hair keratins, beta-catenin and PHLDA-1 expression. J Cutan Pathol. 2014;41:427–436. doi: 10.1111/cup.12313. [DOI] [PubMed] [Google Scholar]
- 15.Goldman-Levy G, Frouin E, Soubeyran I, Maury G, Guillot B, Costes V. Basal cell carcinoma with matrical differentiation. Ann Pathol. 2015;35:159–163. doi: 10.1016/j.annpat.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:15241530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 17.Lattes R, Kessler RW. Metastasizing basal-cell epithelioma of the skin: report of two cases. Cancer. 1951;4:866–878. doi: 10.1002/1097-0142(195107)4:4<866::AID-CNCR2820040424>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.McCusker M, Basset-Sequin N, Dummer R, et al. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50:774–783. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho CT, Pinto MM, Placido AN, Martins A. Metastatic basal cell carcinoma: a frequent disease with rare clinical evolution. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-208904. [DOI] [PMC free article] [PubMed] [Google Scholar]