Abstract
Salivary gland-type tumors have been rarely described in the thyroid gland. Mammary Analog Secretory Carcinoma (MASC) is a recently defined type of salivary gland carcinoma characterized by a t(12;15)(p13;q25) resulting in an ETV6-NTRK3 fusion gene. We report 3 cases of MASC involving the thyroid gland without clinical evidence of a salivary gland or breast primary; the clinico-pathologic characteristics are reviewed. Assessment for rearrangement of the ETV6 (12p13) locus was conducted by fluorescence in situ hybridization (FISH) on representative FFPE sections using an ETV6 break apart probe (Abbott Molecular, Des Plaines, IL, USA). The patients were two females (52 and 55 years-old) and 1 male (74 years-old). The tumors were poorly circumscribed solid white tan nodules involving the thyroid. Histologically, they were invasive and showed solid, microcystic, cribriform, and tubular growth patterns composed of variably bland polygonal eosinophilic cells with vesicular nuclear chromatin and conspicuous nucleoli. All three cases showed metastasis to lymph nodes; one case showed lateral neck involvement. The tumor cells were positive for S100 and mammaglobin. GATA-3 and PAX-8 were positive in 2 cases, one of which only focally so. All three cases were negative for TTF-1 and thyroglobulin. Rearrangement of the ETV6 locus was confirmed in all cases and a diagnosis of MASC rendered for each case. A site of origin distinct from the thyroid gland was not identified, with a median follow up of 24 months. MASC may rarely involve the thyroid gland. The origin of these lesions is unknown; while an origin from ectopic salivary gland-type cells is entertained, a metastatic origin from an occult primary cannot be definitively excluded at this time. Given the histologic (follicular-like microcystic pattern with colloid-like secretions and papillary pattern), immunophenotypic (PAX-8), and even molecular overlap, MASC can be mistaken for papillary thyroid carcinoma and should be considered in the differential diagnosis of a thyroid mass.
Keywords: Mammary analog secretory carcinoma, Thyroid, Salivary gland-type carcinomas, Adenocarcinomas, ETV6-NTRK3
Introduction
Salivary gland-type tumors, such mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma, have been rarely described in the thyroid gland [1–4]. Mucoepidermoid-like areas may be found in follicular derived tumors [5, 6]. In the salivary glands, mammary analog secretory carcinoma (MASC) is recently defined neoplasm that is characterized by a t(12;15)(p13;q25) resulting in an ETV6-NTRK3 fusion gene [7–9]. Histologically, MASC is composed of cells with bland nuclei, prominent central nucleoli and abundant, granular to vacuolated eosinophilic cytoplasm. MASC can show a variety of histologic patterns including: microcystic, solid, tubular, and glandular [7, 9]. Associated bubbly intraluminal secretions are typically present [7]. In the salivary gland, the main differential diagnosis of MASC includes acinic cell carcinoma [8, 10]; however, depending on their histologic pattern, these tumors can resemble many other tumors, including adenocarcinoma, not otherwise specified, polymorphous low-grade adenocarcinoma, low grade salivary duct carcinoma, mucin-producing signet ring adenocarcinomas, and mucoepidermoid carcinomas [8]. MASCs are known for having a slightly better behavior than many of their mimics making their identification important [8].
In a recent large series of head and neck MASC, one case involving the thyroid gland was reported [11]. Herein, we present a clinicopathologic series of three MASCs that were centered about the thyroid gland, including two previously unreported cases.
Materials and Methods
Three cases diagnosed as mammary analog secretory carcinoma involving the thyroid gland were identified; one case each occurring at H. Lee Moffitt Cancer Center, Tampa, FL; University of Pittsburgh, Pittsburgh, PA; and University of Alabama at Birmingham (UAB). We reviewed their clinical and pathologic information as well as their histologic and immunohistochemical findings. The case from UAB had been previously published as part of a large series of head and neck MASC [11]. Using routine methods, immunohistochemical stains were performed on formalin fixed paraffin embedded tissue using the automated immunostainers. FISH analysis was performed on representative 4 micron-thick, formalin-fixed, paraffin-embedded tissue sections on each case using the Vysis LSI ETV6 (12p13) Dual Color, Break Apart Rearrangement Probe (Abbott Molecular, Downers Grove, IL) as previously described. Strong, well-delineated hybridization signals were independently scored by two individuals in 200 interphase nuclei per specimen. An interphase cell specimen was interpreted as abnormal if splitting of the normally juxtaposed ETV6 probe signals were detected in >15 % of cells. Images were acquired using the CytoVision Image Analysis System (Applied Imaging, Santa Clara, CA).
Results
The patients were two females (52 and 55 year-old) and on male (74 years-old); the mean age of our patients was 60.3 years. Clinically, all patients presented with a thyroid mass. All patients underwent a total thyroidectomy and lymph node resection. On macroscopic examination, the size ranged from 2.4 to 4 cm; the epicenter of the first two cases was the thyroid gland and the third case involved both the trachea and thyroid gland; whether the tumor arose in the trachea or in the thyroid was not entirely definitive in this case. Extrathyroidal extension was not present in the first two cases (See Table 1). All the tumors were poorly circumscribed solid white tan nodules. Histologically, the tumors showed several overlapping features. All were invasive and showed solid, microcystic, cribriform, tubular and papillary growth patterns composed of polygonal eosinophilic cells with vacuolated cytoplasm with vesicular nuclear chromatin and conspicuous nucleoli. Nuclear grooves were present in all three cases and were frequent in the second and third case. Cellular atypia was mild to focally moderate (See Figs. 1, 2, 3, 4). The first case demonstrated high-grade histologic features in the form of an elevated mitotic rate (10 per 10 high power fields) and cellular necrosis, while the other 2 cases lacked necrosis and exhibited low mitotic rates (3 per 10 hpf and 2 per 10 hpf). The secretions varied in color from pink to pale purple and could easily be mistaken for colloid. Two of the 3 cases showed a prominent lymphoplasmacytic infiltrate. None of the tumors demonstrated a conventional papillary or follicular thyroid carcinoma component (although a separate papillary microcarcinoma was seen in one case). Ectopic benign salivary gland tissue was not seen in any case, however the last case showed tracheal salivary gland tissue adjacent to the tumor. All three carcinomas showed metastasis to lymph nodes; one case showed lateral neck involvement.
Table 1.
Clinical and pathologic findings
| Case | Age (years) | Sex | Tumor size (cm) | Tumor site | Lesion Epicenter | Papillary growth | Nuclear atypia | Nuclear grooves | Mitoses Per 10 hpf | IBT | +LN met | ENE | Treatment | Evidence of disease | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | 4.0 | RUL | Intra-thyroidal | Present, focal | Severe | Yes | 10 | No | Yes; Central, VI: 0/3 Levels II–V: 3/21 | No | Thyroidectomy and right levels II–V and right level VI LN dissection; Radiotherapy and Chemotherapy | Liver metastasis; DOD | 12 |
| 2 | 52 | F | 2.4 | Left mid inferior | Intra-thyroidal | Present | Mild | Yes | 3 | No | Yes; Paratracheal: 1/3 Lateral neck: ND | No | Thyroidectomy and paratracheal LN dissection | NED | 26 |
| 3 | 55 | F | 2.6 | Trachea/Left thyroid | Trachea and thyroid | Present, focal | Mild | Yes | 2 | Yes | Yes; Paratracheal: 5/17 Left Neck: 0/24 | No | Thyroidectomy, tracheal resection, and LN dissection; Postoperative radiation | NED | 43 |
DOD death of disease, ENE extranodal extension, F female, IBT involvement beyond thyroid, hpf high power fields, LN lymph node, M male, NED no evidence of disease, ND not done, RUL right upper thyroid lobe, +LN met, lymph node metastasis
Fig. 1.
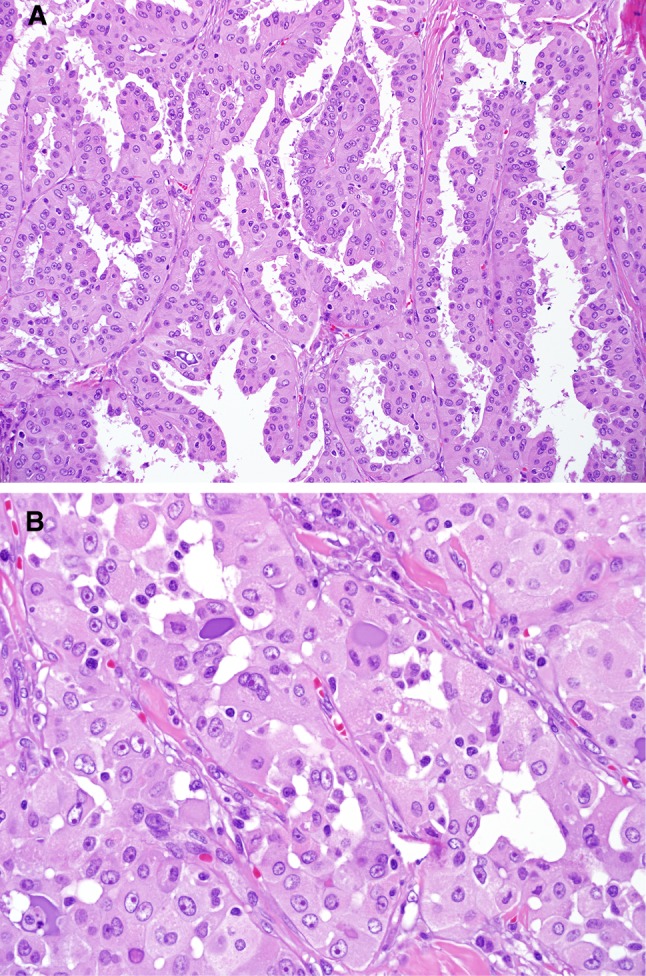
Case 1. The tumor showed focally papillary features with well-defined fibrovascular cores (a). Areas with glandular pattern were admixed with the papillary patterned areas. Areas with the “classic” pattern of MASC were also seen; these areas show dense eosinophilic to purple mucin. The nuclei showed prominent red nucleoli and open clear chromatin (b)
Fig. 2.
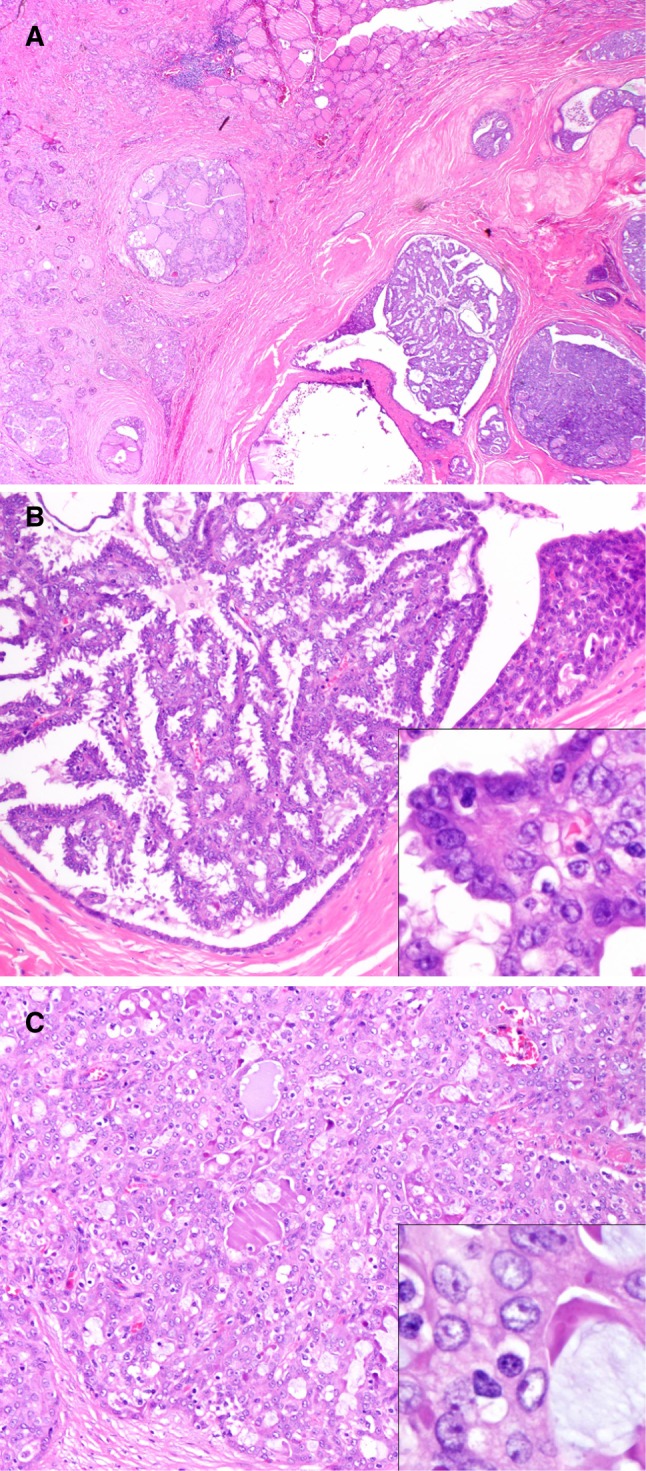
Case 2. This case showed a tumor involving the thyroid. The tumor showed a capsule and hyalinization with a variety of histologic patterns (a); areas with papillary pattern were identified (b). Additionally, the tumor cells showed nuclear clearing and small nucleoli attached to the nuclear envelop resembling a “papillary thyroid carcinoma”(inset; b); a cribriform pattern with glandular spaces filled with pale pink to eosinophilic mucinous material resembling colloid was also noted (c); the cells also showed round nuclei with prominent nucleoli (inset; c)
Fig. 3.
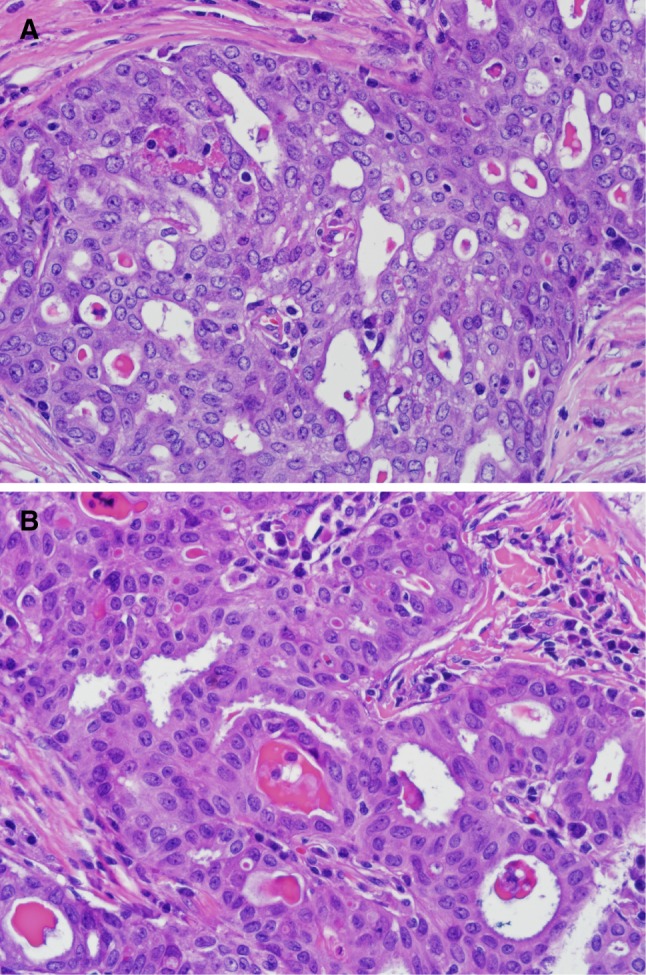
Case 3. The tumor in this case showed the classic cribriform pattern with dense eosinophilic mucin (a). The tumor cells show nuclear grooves and nuclear overlapping resembling a papillary thyroid carcinoma (b)
Fig. 4.
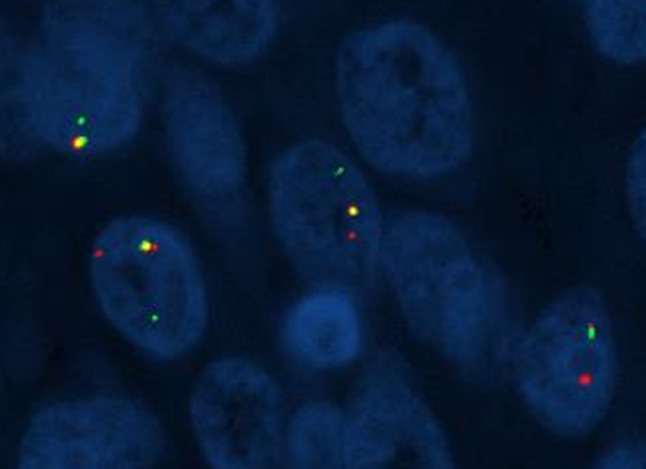
Case 1. ETV6 break-apart probe of the tumor involving the thyroid showing disruption of the orange and green signals and indicating rearrangement of the ETV6 (12p15) locus (Fluorescent in situ hybridization; high power)
The secretions were focally positive for mucicarmine and PAS with and without diastase digestion. Mucicarmine stain showed focally positive intracytoplasmic vacuoles. Immunophenotypically, the tumor cells were positive for S100 and mammaglobin in all cases. PAX-8 was diffusely positive in a case and focally positive in another, and negative in the third. All these cases were negative for TTF-1 and thyroglobulin. Rearrangement of the ETV6 locus was confirmed in all cases by molecular cytogenetic analysis and a diagnosis of MASC rendered for each case (See Table 2). In all the cases, a site of origin distinct from the thyroid gland was not identified. One patient (case 1) developed liver metastasis and died of disease; and two patients are currently alive without evidence of disease, with a mean follow up of 27 months (range: 12–43; median: 26 months).
Table 2.
Immunohistochemical stains and fluorescent in situ hybridization results
| Case | TTF-1 | TG | PAX-8 | S100 | Mammaglobin | GATA-3 | DOG-1 | ETV6 FISH |
|---|---|---|---|---|---|---|---|---|
| 1 | − | − | +, f | + | + | +, f | +, f | + |
| 2 | − | − | − | + | + | ND | ND | + |
| 3 | − | − | + | + | + | + | + | + |
f focal, ND not done, − negative, + positive, TG Thyroglobulin, TTF-1 thyroid transcription factor-1
Discussion
The presence of t(12;15)(p12;q25) translocation resulting in the ETV6-NTRK3 gene fusion was initially identified in congenital fibrosarcoma and later in breast secretory carcinoma [12–15]. The protein product of this abnormality is a constitutively active protein-tyrosine kinase with transformation activity [16]. In 2010, a series of tumors arising from the salivary gland harboring the same translocation were identified; due to their characteristic morphologic features and the similarity to the secretory carcinoma of the breast this tumor was designated as mammary analog secretory carcinoma (MASC) of salivary gland [7]. Later on, additional MASC cases were identified either retrospectively or prospectively [8, 11, 17–21]. Before the MASC characterization, these tumors were previously classified as acinic cell carcinoma, adenocarcinoma, not otherwise specified, mucoepidermoid carcinomas [8, 17, 22] and cystadenomas [23]. We present three distinct cases of MASC involving the thyroid gland, including two previously unreported examples.
The origin of the MASCs involving the thyroid gland is unknown, but there are three possibilities:
One possibility is that these MASCs represent thyroid follicular epithelium-derived tumors that are simply mimicking a salivary type carcinoma; after all, ETV6-NTRK3 rearrangement has been reported in papillary thyroid carcinoma, especially in tumors associated with exposure to iodine -131, and there is some morphologic overlap (see discussion below) [24, 25]. Indeed, papillary thyroid carcinomas with features of salivary gland tumors (e.g., mucoepidermoid carcinoma and adenoid cystic carcinoma) have been reported [5, 6, 26]. On the other hand, in the reported papillary thyroid carcinomas with areas mimicking salivary-type neoplasms, there were at least some foci where the tumor is recognizable as a thyroid follicular derived tumor such as conventional-appearing papillary thyroid carcinoma [5, 6, 26]. This is not the case in these three MASCs; all three cases had a MASC-like appearance throughout. Moreover, the three MASCs were not only histologically identical to MASC of salivary glands, but also demonstrated the same immunophenotype with diffuse S100 and mammaglobin positivity and a complete absence of TTF-1 or thyroglobulin immunoreactivity, therefore making this possibility unlikely. In these cases, the PAX-8 expression may be not specific at all.
Salivary type carcinomas may involve the thyroid gland secondarily, either by direct extension from the larynx or trachea or via distant metastasis. None of the three patients with thyroid MASC, however, had a history of malignancies elsewhere. Given the somewhat limited follow-up, however, it is not yet possible to entirely exclude the possibility of a distant metastasis from a breast or salivary gland MASC or the possibility of a MASC arising from adjacent tracheal minor salivary glands in case 3.
Finally, a third possibility as that the MASCs were truly primary to the thyroid gland. Thyroid tumors with morphology similar to their counterparts in the salivary gland are seen occasionally seen in the thyroid; these include pleomorphic adenoma and mucoepidermoid carcinoma [1–4, 27]. An origin on ectopic rests of benign salivary gland tissue may be entertained as they are rarely seen within the thyroid gland [28]. None of the three MASCs, however, demonstrated evidence of origin from metaplastic or ectopic tissues in the reviewed materials.
Thyroid MASC are too rare to comment on site specific biologic features as their prognosis is not yet known. Here, all three cases demonstrated regional lymph node metastases, and one case exhibited extrathyroidal extension and one case showed high-grade histologic features. This patient died having developed liver metastasis and the other two patients are currently alive without evidence of disease. Additional follow up will be needed to determine their behavior.
A recent case of MASC apparently arising in the thyroid has been reported [29]. Similarly to our cases, this tumor showed microcystic, solid, glandular, and papillary architecture; and the cells nuclei showed prominent nucleoli, nuclear grooves, and rare nuclear pseudoinclusions [29]. This tumor was initially diagnosed as “papillary thyroid carcinoma”; on follow up, after local recurrence and widespread metastasis, the tumor cells were found to be positive for GATA-3 and mammaglobin and negative for thyroglobulin and TTF-1. Additionally, this tumor was positive for the ETV6-NTRK3 fusion by RT-PCR [29].
MASC involving the thyroid gland has a significant histologic overlap with a primary thyroid carcinoma, particularly papillary thyroid carcinoma, and represents a significant diagnostic pitfall, as seen in the recently reported case [29]. MASC demonstrates follicular, papillary, and microcystic patterns, along with eosinophilic secretions that may resemble colloid. Moreover, considering how common papillary thyroid carcinoma is compared to how rare MASC involving the thyroid gland is, mistaking the latter for the former would be understandable. Nevertheless, it is important to identify MASC in the thyroid gland because it could represent distant metastatic disease from a breast of salivary gland primary tumor. Even if MASC of the thyroid gland is presumed to be primary, it would likely be treated differently since it would likely not respond to radioactive iodine. The first step in correctly diagnosing MASC in the thyroid gland is recognizing that it may occur in this site. The clues for the diagnosis are the glandular morphology, the bluish pink intraglandular material, and the cytology details. The cells may show grooves; however, the nuclei are round than oval with vesicular chromatin and the cytoplasm may show vacuoles. This histologic, immunophenotypic, and molecular findings are otherwise typical for MASC. Second, immunohistochemistry must be employed. MASC is positive for mammaglobin and usually GATA3, while thyroid follicular epithelial neoplasms are usually negative for these markers. Conversely, MASC is consistently negative for TTF-1 and thyroglobulin. Caution must be used in interpretation of S100 and PAX8; as S100 is often positive in papillary thyroid carcinomas [30, 31], and PAX8 staining was found in 2 of these 3 MASCs involving thyroid gland.
In conclusion, MASC may rarely involve the thyroid gland where it mimics papillary thyroid carcinoma. Given some morphologic, immunophenotypic, and even the potential FISH results overlap between the two tumors, a high index of suspicion and judicious use of immunostains (primarily mammaglobin, GATA3, TTF-1, and thyroglobulin) are needed to distinguish them. Additional follow up will be needed to more conclusively determine their origin (primary vs. metastatic) and significance of MASC in the thyroid gland.
References
- 1.Franssila KO, Harach HR, Wasenius VM. Mucoepidermoid carcinoma of the thyroid. Histopathology. 1984;8(5):847–860. doi: 10.1111/j.1365-2559.1984.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 2.Wenig BM, Adair CF, Heffess CS. Primary mucoepidermoid carcinoma of the thyroid gland: a report of six cases and a review of the literature of a follicular epithelial-derived tumor. Hum Pathol. 1995;26(10):1099–1108. doi: 10.1016/0046-8177(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 3.Rhatigan RM, Roque JL, Bucher RL. Mucoepidermoid carcinoma of the thyroid gland. Cancer. 1977;39(1):210–214. doi: 10.1002/1097-0142(197701)39:1<210::AID-CNCR2820390133>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Baloch ZW, Solomon AC, LiVolsi VA. Primary mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: a report of nine cases. Mod Pathol. 2000;13(7):802–807. doi: 10.1038/modpathol.3880140. [DOI] [PubMed] [Google Scholar]
- 5.Soares J, Limbert E, Sobrinho-Simoes M. Diffuse sclerosing variant of papillary thyroid carcinoma. A clinicopathologic study of 10 cases. Pathol Res Pract. 1989;185(2):200–206. doi: 10.1016/S0344-0338(89)80252-3. [DOI] [PubMed] [Google Scholar]
- 6.Miranda RN, Myint MA, Gnepp DR. Composite follicular variant of papillary carcinoma and mucoepidermoid carcinoma of the thyroid. Report of a case and review of the literature. Am J Surg Pathol. 1995;19(10):1209–1215. doi: 10.1097/00000478-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 8.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61(3):387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 9.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36(1):27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 10.Lei Y, Chiosea SI. Re-evaluating historic cohort of salivary acinic cell carcinoma with new diagnostic tools. Head Neck Pathol. 2012;6(2):166–170. doi: 10.1007/s12105-011-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015;28(8):1084–1100. doi: 10.1038/modpathol.2015.64. [DOI] [PubMed] [Google Scholar]
- 12.Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2(5):367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 13.Euhus DM, Timmons CF, Tomlinson GE. ETV6-NTRK3–Trk-ing the primary event in human secretory breast cancer. Cancer Cell. 2002;2(5):347–348. doi: 10.1016/S1535-6108(02)00184-8. [DOI] [PubMed] [Google Scholar]
- 14.Diallo R, Schaefer KL, Bankfalvi A, Decker T, Ruhnke M, Wulfing P, et al. Secretory carcinoma of the breast: a distinct variant of invasive ductal carcinoma assessed by comparative genomic hybridization and immunohistochemistry. Hum Pathol. 2003;34(12):1299–1305. doi: 10.1016/S0046-8177(03)00423-4. [DOI] [PubMed] [Google Scholar]
- 15.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18(2):184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 16.Lannon CL, Martin MJ, Tognon CE, Jin W, Kim SJ, Sorensen PH. A highly conserved NTRK3 C-terminal sequence in the ETV6-NTRK3 oncoprotein binds the phosphotyrosine binding domain of insulin receptor substrate-1: an essential interaction for transformation. J Biol Chem. 2004;279(8):6225–6234. doi: 10.1074/jbc.M307388200. [DOI] [PubMed] [Google Scholar]
- 17.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36(3):343–350. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 18.Kratochvil FJ, 3rd, Stewart JC, Moore SR. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases in the lips. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):630–635. doi: 10.1016/j.oooo.2012.07.480. [DOI] [PubMed] [Google Scholar]
- 19.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013;44(10):1982–1988. doi: 10.1016/j.humpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishop JA, Yonescu R, Batista DA, Westra WH, Ali SZ. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol. 2013;121(5):228–233. doi: 10.1002/cncy.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith CC, Stelow EB, Saqi A, Khalbuss WE, Schneider F, Chiosea SI, et al. The cytological features of mammary analogue secretory carcinoma: a series of 6 molecularly confirmed cases. Cancer Cytopathol. 2013;121(5):234–241. doi: 10.1002/cncy.21249. [DOI] [PubMed] [Google Scholar]
- 22.Bishop JA, Yonescu R, Batista D, Eisele DW, Westra WH. Most nonparotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am J Surg Pathol. 2013;37(7):1053–1057. doi: 10.1097/PAS.0b013e3182841554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams L, Chiosea SI. Mammary analogue secretory carcinoma mimicking salivary adenoma. Head Neck Pathol. 2013;7(4):316–319. doi: 10.1007/s12105-013-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research N Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120(6):799–807. doi: 10.1002/cncr.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baloch ZW, Segal JP, Livolsi VA. Unique growth pattern in papillary carcinoma of the thyroid gland mimicking adenoid cystic carcinoma. Endocr Pathol. 2011;22(4):200–205. doi: 10.1007/s12022-011-9174-7. [DOI] [PubMed] [Google Scholar]
- 27.Levy GH, Marti JL, Cai G, Kayne RD, Udelsman R, Hammers LW, et al. Pleomorphic adenoma arising in an incidental midline isthmic thyroid nodule: a case report and review of the literature. Hum Pathol. 2012;43(1):134–137. doi: 10.1016/j.humpath.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Cameselle-Teijeiro J, Varela-Duran J. Intrathyroid salivary gland-type tissue in multinodular goiter. Virchows Arch: Int J Pathol. 1994;425(3):331–334. doi: 10.1007/BF00196158. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds S, Shaheen M, Olson G, Barry M, Wu J, Bocklage T. A case of primary mammary analog secretory carcinoma (MASC) of the thyroid masquerading as papillary thyroid carcinoma: potentially more than a one off. Head Neck Pathol. 2016 doi: 10.1007/s12105-016-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitselou A, Vougiouklakis TG, Peschos D, Dallas P, Boumba VA, Agnantis NJ. Immunohistochemical study of the expression of S-100 protein, epithelial membrane antigen, cytokeratin and carcinoembryonic antigen in thyroid lesions. Anticancer Res. 2002;22(3):1777–1780. [PubMed] [Google Scholar]
- 31.McLaren KM, Cossar DW. The immunohistochemical localization of S100 in the diagnosis of papillary carcinoma of the thyroid. Hum Pathol. 1996;27(7):633–636. doi: 10.1016/S0046-8177(96)90390-1. [DOI] [PubMed] [Google Scholar]


