Abstract
Inflammatory myofibroblastic tumors (IMTs) are rare mesenchymal tumors initially described in the lung. About half of them exhibit expression of the ALK1 protein, generally resulting from a gene rearrangement. Paranasal sinus IMTs are extremely uncommon, and gene rearrangement of ALK1 is very rare in this localization. A 47-year-old woman presented with rapidly progressive vision loss in her left eye. Clinical and imaging work-up revealed a tumor invading the left ethmoidal and sphenoidal sinuses and extending into the nasal cavity, the orbit and the skull base. Complete tumor resection was performed using an endonasal approach. Pathological examination revealed a paranasal localization of IMT, positive for ALK1 immunostaining. FISH analysis showed an ALK1 gene rearrangement. This case illustrates the local aggressive potential for IMTs. Treatment is primarily surgical, but targeted therapies (crizotinib) might be a solution for ALK1 rearranged cases with a poor prognosis.
Keywords: Sinonasal tumor, Inflammatory myofibroblastic tumor, Anaplastic Lymphoma Kinase 1, Endoscopic approach, Targeted therapies
Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare soft tissue tumors, which have primarily been described in the lungs. Extra-pulmonary IMTs occur throughout the body. Approximately 10 % of these involve the upper aerodigestive tract, primarily the larynx. Between 36 and 60 % of IMTs show an immunoreactivity for the Anaplastic Lymphoma Kinase 1 (ALK1) antibody [1]. This over expression may be caused by different molecular abnormalities, most frequently by a gene rearrangement [1], but it may involve other molecular events.
The present study reports a case of paranasal sinus IMT with an ALK1 gene rearrangement. The patient provided informed consent for the use of her clinical data. To the best of our knowledge, this is the second case described in the literature of paranasal sinus IMT displaying an immunoreactivity for the ALK1 antibody, caused by an ALK1 gene rearrangement confirmed by FISH. Clinical, radiological and pathological findings of the tumor are described, and a review was carried out.
Case Description
A 47-year-old woman presented with a left-sided nasal obstruction associated with rapidly progressive left-sided visual loss. She complained of a long-lasting nasal obstruction before her left vision was compromised. She reported no medical history, no smoking or alcohol use, and no family history. The initial clinical examination revealed a large mass filling the entire left nasal cavity, which was responsible for a septal deviation. Ocular motility was normal, but the left visual acuity was 4/20 with a paracentral left temporal scotoma. There were no other neurologic deficits.
A head computed tomography (CT) scan revealed a lesion occupying the entire left ethmoid sinus extending to the contralateral ethmoid, with lysis of the skull base and lamina papyracea, without obvious crossing of the left periorbit. The lesion also extended to the left nasal cavity and sphenoid sinus (Fig. 1). Magnetic resonance imaging (MRI) revealed a homogeneous tumor showing an isointense signal in T1-weighted images and a hyperintensity in T2-weighted images, with intense and homogeneous enhancement after contrast injection. The optic nerve was not followed on MRI, and the lesion was in contact with the internal carotid artery (Fig. 2).
Fig. 1.
Preoperative axial (a) and coronal (b) CT scan showing a lytic mass invading the nasal fossa, the sphenoid, the ethmoid and the maxilla
Fig. 2.

Preoperative MRI in T1 (a), T2 (b), and T1 with gadolinium-weighted sequences (c axial plane; d coronal plane) showing a tumor in the left nasal fossa extended into the contralateral fossa, the two sphenoid sinuses, and the left ethmoid
Biopsies under local anesthesia were carried out, and an inflammatory myofibroblastic tumor was diagnosed. The patient underwent a semi-emergency surgery to prevent visual loss. Complete tumor resection was performed using an endonasal approach with bilateral ethmoidectomy, bilateral sphenoidectomy, access to the left cavernous sinus and dissection of the optic nerve and the internal carotid artery, which were dehiscent, and a type II nasopharyngectomy [2]. Excision showed a hemorrhagic tumor, which was mainly implanted in the left sphenoid sinus and along the left orbit.
On day one after surgery, the visual acuity of the patient recovered with persistence of a small central scotoma. A postoperative MRI showed complete resection. Control imaging 1 year after surgery (Fig. 3) showed a non-specific contrast enhancement in the right sphenoid, while a systematic biopsy was negative for tumor.
Fig. 3.
Postoperative axial (a), coronal (b) and sagittal (c) T1 with gadolinium-weighted MRI showing no recurrence 1 year after surgery
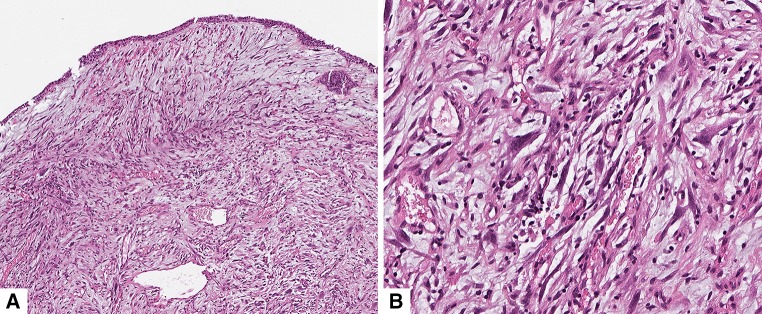
Microscopic examination of the biopsy and subsequent surgical specimen (Fig. 4) showed a spindle cell proliferation, dispersed in an abundant edematous myxoïd inflammatory stroma infiltrated by numerous lymphocytes, plasma cells and eosinophils. Vascularization was abundant with no typical hemangiopericytic feature. Immunohistochemical analysis revealed diffuse cytoplasmic reactivity for ALK1 (Fig. 5a); AE1 and AE3 keratins were weakly and very focally expressed (Fig. 5b). There was no expression of smooth muscle actin (Fig. 5c), CK5/6, CD34 or desmin. Tumor cells showed a cytoplasmic but no nuclear expression of beta catenin, and S100 protein was weakly expressed in the cytoplasm of rare tumor cells (Fig. 5d). Mitoses were scarce, but many atypia were present, and the proliferation index was high, up to 30 % in the hot-spots. Using ALK break-apart FISH, most tumor cells displayed one colocalization signal (indicating a normal ALK allele) and a split signal (indicating a rearranged ALK allele). The spacing of the split signals was large, indicating an ALK rearrangement through a translocation rather than an inversion (Fig. 6).
Fig. 4.
Histopathological analysis. a HES staining ×5 sinonasal mucosa infiltrated by a spindle cell proliferation with a highly vascular inflammatory stroma. b HES staining ×20 showing marked atypia
Fig. 5.
Immunohistochemical analysis ×20. a Cytoplasmic staining for ALK1 antibody in the tumor cells. b Very weak and focal expression of AE1/AE3 cytokeratins in the tumor cells. c No expression of smooth muscle actin in the tumor cells. d Weak and focal cytoplasmic expression of S100 protein in the tumor cells
Fig. 6.

ALK break apart Fluorescence in situ hybridization (FISH) performed on a 4 µm dewaxed tissue section using the Zytolight SPEC ALK Dual Color Break Apart probe with the Zytolight FISH-Tissue Implementation kit (Zytovision GmbH, Bremerhaven, Germany). Tumor cells had a colocalisation signal associated with a split signal, indicating ALK rearrangement (arrows). Note that non-tumor cells had only colocalisation signals, indicating that both ALK loci were normal (arrow heads)
Discussion
Inflammatory myofibroblastic tumors are solid mesenchymal tumors that were first described in the lung by Pettinato [3]. IMTs have since been reported in numerous extrapulmonary sites, including the abdomen, pelvis, urogenital system, orbit, oral cavity, and nose and paranasal sinuses. These are usually benign tumors, with local development, but malignant behavior with metastasis is described in 5 % of cases [4]. Recurrences may occur in 25 % of cases depending on completeness of surgical resection [4]. Microscopic examination reveals a proliferation of differentiated myofibroblastic spindle cells associated with an infiltrate of inflammatory cells (lymphocytes and plasma cells) in variable myxoid or collagen stroma. Coffin et al. [5] described three main histopathological subtypes:
The presence of stellate or spindle-shaped cells in myxoid edematous stroma associated with numerous polymorph inflammatory cells.
A compact myofibroblast cell proliferation primarily accompanied by an infiltrate of inflammatory plasma cells.
A collagen-rich stroma in which the inflammatory infiltrate is poor with rare mitoses.
He et al. [6] were the first to show that the (a) and (b) patterns were associated with poor event-free-survival in the nasal and paranasal sinuses. Malignancy is histologically suspected in cellular cases with nuclear atypia, high proliferation index, high cellularity, necrosis, and ganglion-like cells [6].
The pathogenesis of IMT is unclear. Based on the presence of infectious agents described in some IMTs, several authors have hypothesized that there is an initial infectious process that triggers an inappropriate inflammatory response and reactive myofibroblastic proliferation [7, 8]. The causative germs vary and could be bacteria, virus or fungus.
IMTs of the head and neck are predominantly located in the larynx, orbit, oral cavity and paranasal sinuses, with no age or sex predilection. IMTs of the nose and paranasal sinuses are rare. Mainly case reports, and one case series, are available in the literature. Table 1 describes the main features of these tumors [6, 9–18].
Table 1.
Paranasal inflammatory myofibroblastic tumors: literature review
| References | Gender | Age | Symptoms | Clinical appearance | Imaging | IHC | Evolution |
|---|---|---|---|---|---|---|---|
| Constantino et al. [9] | F | 66 | Unilateral NO, hyposmia, facial pain, epistaxis | Yellowish solid tumor in the middle meatus | CT scan: heterogeneous enhancement, bone lysis | NA | Recurrence 6 months after surgery |
| F | 62 | Unilateral NO, frontal pain, hyposmia, clear rhinorrhea | Red tumor filling the nasal fossa | CT scan: heterogeneous tumor | NA | No recurrence (FU: 16 months) | |
| M | 26 | Bilateral NO, epistaxis, proptosis, trigeminal hypoesthesia | Mucosal thickening in the upper nasal fossa | CT scan: ethmoidal opacity | NA | Recurrence | |
| Gadde et al. [10] | M | 40 | Facial pain, otalgia, trigeminal hypoesthesia | Nasopharyngeal mass | CT scan: homogeneous mass MRI: enhancing tumour in the foramen ovale and the Meckel’s cave |
NA | Recurrence 14 months after treatment (external radiation, 40 Gy) |
| Huang et al. [11] | F | 59 | Epistaxis, NO | Red and granular mass of the middle meatus | CT scan: mild enhancement | CD58+ | No recurrence (FU: 24 months) |
| Lee et al. [12] | F | 64 | NO | Yellow heterogeneous mass | CT scan: bone lysis and remodeling | No recurrence (FU: 24 months) | |
| Maire et al. [13] | F | 38 | Headache, proptosis, VI nerve palsy | Normal | CT scan: sphenoidal mass invading the clivus and the cavernous sinus | SMA+, CD45+ ALK1− |
No recurrence after external radiation, 40 Gy (FU: 24 months) |
| Fang et al. [14] | F | 42 | Epistaxis, facial pain | Dark red mass in the nasal fossa | CT scan: maxillary mass with bone lysis and heterogeneous enhancement | NA | NA |
| F | 63 | Hypoesthesia, facial pain | Normal | CT scan: maxillary mass | Vimentin+ SMA−, MSA−, Desmin−, CD34− |
Mandibular metastasis and local recurrence after surgery, death during postoperative radiation | |
| Soysal et al. [15] | F | 10 | NO, epistaxis, headache | Red mass filling the nasal fossa | CT scan: tumor filling the nasal fossa | Vimentin+ | No recurrence (FU: 13 months) |
| Dimitrakopoulos et al. [16] | M | 70 | NO, headache, fever | Normal | CT scan: maxillary mass with bone lysis | Vimentin+, SMA+ Keratin−, Desmin−, pS100−, ALK1− |
No recurrence (FU: 12 months) |
| Karakok et al. [17] | M | 59 | Jugal mass, proptosis, trigeminal hypoesthesia | Submucosal mass | CT scan: maxillary mass with bone lysis | Vimentin+, SMA+, CD68+, CD53+, pS100- | Recurrence 6 months after surgery |
| Lawson et al. [18] | F | 7 | Bilateral NO, epistaxis | Mass filling the nasal fossa | CT scan: maxillary and ethmoidal mass with lysis of the skull base | NA | Recurrence 2 months after surgery |
| He et al. [6] Case-series (n = 25) |
F (64 %) M (36 %) |
≈41.2 [2–74] | NO (36 %), facial pain (32 %), toothache (20 %), headache (16 %), facial swelling (16 %), decreased vision (16 %) | NA | CT scan: soft tissue mass with wall erosion and bone resorption | Vimentin+ (100 %), SMA+ (100 %), Fibronectin+ (68 %), MSA+ (60 %), Calponin+ (56 %), 1 case ALK1+ (4 %) | 75 % recurrence after surgery (24 % of histological malignant transformation), 25 % death One case of cervical lymph node metastasis |
IHC immunohistochemistry, F female, M Male, NO nasal obstruction, FU follow-up SMA smooth muscular actin, ALK1 Anaplastic Lymphoma Kinase 1, MSA muscular-specific actin, NA not available
Clinically, a non-specific nasal tumor syndrome gradually develops with unilateral nasal obstruction, facial pain, and epistaxis. Less frequently, the tumor is revealed by cranial nerve palsy, facial edema, hyposmia, or proptosis. Unlike in other locations, systemic inflammatory anomalies (fever, weight loss, anemia, hyper-gammaglobulinemia, and ESR and CRP increase) are rare in the head and neck. The imaging (CT scan and MRI) shows a homogeneous strongly enhancing tumor after injection of contrast. The CT scan reveals a mass syndrome with bone lysis and sometimes demonstrates calcifications in the tumor [14].
The treatment of these tumors relies on surgical excision with the goal of complete resection. Some authors have reported treatment with external radiation. In the series of Lee et al. [19], 6 patients with skull base IMTs received small dose radiation (20 Gy) with no effect, while Sasagawa et al. [20] report local control after 20 Gy radiation. In the cases of paranasal sinus IMTs reported in the literature (Table 1), one patient relapsed after treatment with 40 Gy radiation [10], and a second is currently in complete remission 2 years after the end of treatment with 20 Gy radiation [13]. As reported in the literature, 20 out of 37 patients who underwent surgery for paranasal sinus IMTs experienced tumor recurrence, two cases showed metastatic evolution [6, 14], and 6 patients died of the tumor [6, 14]. Necrosis, a high proliferation index, the presence of ganglion-like cells, and more than four relapses were shown to be poor prognostic factors [6].
The IMTs belong to a group of myofibroblastic tumors that include many subtypes [21]. In the paranasal sinuses, the best known is nasopharyngeal angiofibroma. Table 2 describes the typical features of myofibroblastic tumors in these locations.
Table 2.
Myofibroblastic tumor possibly located in the paranasal sinuses
| Epidemiology | Location | Clinical features | Evolution | Microscopic examination | Immunohistochemistry | |
|---|---|---|---|---|---|---|
| Desmoid tumor | Children and young adults (20–40 year old) Gardner syndrome |
Neck > face > oral cavity > scalp > paranasal sinuses > orbit | Deep tumors with slow growth, infiltration and compression of neighboring structures | Recurrence: 20–50 % | Myofibroblasts in long bundles in a collagen rich matrix Lymphoid clusters |
Actin+; Calponin+; Nuclear Beta-catenin+ Desmin± |
| Nasopharyngeal angiofibroma | Male teenager Rare associations with FAP |
Rhinopharynx >>> maxillary or ethmoidal sinus | NO, recurring epistaxis, headache, facial deformation | Recurrence: 20 % Locally aggressive |
Spindle or stellate fibroblastic/myofibroblastic cells in a dense, low cellular fibrous tissue Numerous vessels and hemorrhagic areas |
Nuclear Beta-catenin+ Actin± ; Androgen receptor± |
| Solitary fibrous tumor | Mean aged adult M = W |
Orbit > lacrymal glands > thyroid > salivary > sinonasal> scalp | Slow growth tumor, symptoms depending on location. Hypoglycemia due to insulin-like growth factor secretion |
Recurrence 10–15 % Metastasis 10 % |
Two variants: fibrous/cellular Spindle or oval-shaped cells Hyaline fibrous collagen-rich area |
CD34+; STAT6+; CD99+ Bcl-2±; EMA±; Actin± |
| Myofibroblastic inflammatory tumors | Adult | Orbit > oral cavity > trachea, larynx >sinonasal > tonsil > salivary glands | NO depending on location Rare systemic symptoms |
Recurrence 25 % Metastasis 5 % |
Myofibroblasts with enlarged nuclei in a myxoid stroma Mainly lymphocytic infiltrate and plasma cells, sometimes neutrophils and eosinophils |
Vimentin+ Actin±; Desmin±; Calponin± |
| Myofibroblastic sarcoma | Mean aged adult M = W |
Mouth, tong > face > maxilla and mandible | Painless and slow growth mass | Recurrence 30 % Metastasis 7 % |
Regular spindle cells + enlarged hyperchromatic cells with atypias Inflammatory matrix made of mast cells and neutrophils |
Actin+ Calponin±; Desmin± CD34−; Cytokeratin−; pS100− |
| Adult fibrosarcoma | 45–60 years old | Nasal fossa and paranasal sinuses | Slow growth mass rarely painful. Epistaxis, NO | Recurrence 60 % Metastasis 15 %. 5-year survival 75 % |
Homogeneous beams arranged in a “herringbone” made of elongated spindle cells | Vimentin+ (non specific) |
M male, W female, NO nasal obstruction, FAP familial adenomatous polyposis
Our case is the second paranasal sinus IMT with ALK1 immunohistochemical expression described in the literature. There appears to be a good correlation between immunohistochemical expression of ALK1 and molecular abnormalities [1]. In our case, the ALK1 gene rearrangement was confirmed by FISH analysis. The Anaplastic Lymphoma Kinase 1 (ALK1) gene encodes a tyrosine kinase receptor that belongs to the insulin receptor family, which is normally present in a few neuronal cells. Its expression is most frequently caused by a rearrangement of the ALK1 gene (on the short arm of chromosome 2 in the p21–p23 region) with different possible partners. The fusion product is responsible for ALK1 protein overexpression. It was first described in anaplastic large cell lymphoma, with a favorable prognosis [22].
Griffin et al. [23] first suggested that the recurrent translocation seen in inflammatory IMTs could involve ALK. The literature reports that between 9 and 63 % of IMTs have a mutation activating the ALK1 receptor [24]. The presence of this mutation confirms the neoplastic nature of this lesion. No obvious link is reported between ALK1 rearrangement and the inappropriate inflammatory response hypothesis in IMT. According to Coffin [24] and Chan [25], tumors expressing the mutation tend to occur in younger patients (under 40 years of age). ALK1 expression may impact the presentation and prognosis of IMTs: ALK1-positive IMTs seem to present a higher risk of local recurrence than ALK1-negative IMTs [24, 25]. Only one metastatic case with an ALK1 rearrangement is reported in the literature [26]. ALK1-positive IMTs seem to show more atypia and less mitosis [25]. Our case supports this finding; there was no noticeable mitotic activity, but marked atypia and a proliferation index up to 30 % was observed in the hot-spots.
Expression of ALK1 paves the way for personalized treatment and molecular-targeted therapy. While the therapeutic use of inhibitors of tyrosine kinases is being tested for ALK1 rearranged neuroblastoma, glioblastoma, lung cancer and breast cancer [27], there are some recent reports concerning the use of targeted therapies for IMTs. Crizotinib (an inhibitor of ALK tyrosine kinase) was tested in one patient with ALK1-positive IMT by Butrynski et al. [28]. He responded to treatment, with a reduction of more than 50 % of the tumor volume. More recently, a complete remission was obtained with crizotinib in a patient with metastatic ALK1-positive IMT [26]. In the case of deep-seated lesions, crizotinib should therefore be considered as a therapeutic option for recurrent tumors.
As shown by the case reported here, at least some sinonasal IMTs are related to ALK1 gene rearrangement, as demonstrated by immunohistochemical expression of the ALK protein and signal split in the FISH analysis. Although complete surgical resection seems to be the best treatment for these tumors when possible, targeted therapies with tyrosine kinase inhibitors may represent an alternative solution for ALK1 large or recurring tumors, which are not amenable for surgery.
References
- 1.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14(6):569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 2.Castelnuovo P, Nicolai P, Turri-Zanoni M, Battaglia P, BolzoniVillaret A, Gallo S, Bignami M, Dallan I. Endoscopic endonasal nasopharyngectomy in selected cancers. Otolaryngol Head Neck Surg. 2013;149(3):424–430. doi: 10.1177/0194599813493073. [DOI] [PubMed] [Google Scholar]
- 3.Pettinato G, Manivel J, De Rosa N, Dehner L. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;95(5):538–546. doi: 10.1093/ajcp/94.5.538. [DOI] [PubMed] [Google Scholar]
- 4.Coffin C, Fletcher J. Inflammatory myofibroblastic tumor. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. Lyon, France: IARC Press; 2002. pp. 91–93. [Google Scholar]
- 5.Coffin C, Watterson J, Priest J, Dehner L. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinico-pathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19(8):859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 6.He C, Dong G, Yang D, Liu H. Inflammatory myofibroblastic tumors of the nasal cavity and paranasal sinus: a clinicopathologic study of 25 cases and review of literature. Eur Arch Otorhinolaryngol. 2015;272(4):789–797. doi: 10.1007/s00405-014-3026-2. [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Fales W, McCracken W, O’Bryan M, Jarnagin J, Payeur J. Inflammatory pseudotumor in a cat with cutaneous mycobacteriosis. Vet Pathol. 1999;36(2):161–163. doi: 10.1354/vp.36-2-161. [DOI] [PubMed] [Google Scholar]
- 8.Priebe-Richter C, Ivanyi P, Buer J, Langer F, Lotz J, Hertenstein B, Ganser A, Franzke A. Inflammatory pseudotumor of the lung following invasive aspergillosis in a patient with chronic graft-vs. -host disease. Eur J Haematol. 2005;75(1):68–72. doi: 10.1111/j.1600-0609.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- 9.Constantino G, Sasaki F, Tavares R, Voegels R, Butugan O. Inflammatory pseudotumor of the paranasal sinuses. Rev Bras Otorhinolaryngol. 2008;74(2):297–302. doi: 10.1016/S1808-8694(15)31104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadde J, Franck B, Liu X, Teixido M, Rizk H. Inflammatory pseudotumor of the nasopharynx with spread along the trigeminal nerve. Am J Otolaryngol. 2013;34(3):252–254. doi: 10.1016/j.amjoto.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Huang W-H, Dai Y-C. Inflammatory pseudotumor of the nasal cavity. Am J Otolaryngol. 2006;27(4):275–277. doi: 10.1016/j.amjoto.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-M, Choi G, Choi CS, Kim CH, Lee SH. Inflammatory pseudotumor of the maxillary sinus. Otolaryngol–Head Neck Surg. 2001;125(5):565–566. doi: 10.1067/mhn.2001.117165. [DOI] [PubMed] [Google Scholar]
- 13.Maire J, Eimer S, Galli FS, Franco-vidal V, Galland-girodet S, Huchet A, Darrouzet V. Case report inflammatory myofibroblastic tumour of the skull base. Case Rep Otolaryngol. 2013;2013:1–6. [DOI] [PMC free article] [PubMed]
- 14.Fang S, Dong D, Jin M. Inflammatory myofibroblastic tumour of the maxillary sinus: CT appearance, clinical and pathological findings. Eur J Radiol Extra. 2006;60(1):5–9. doi: 10.1016/j.ejrex.2006.06.006. [DOI] [Google Scholar]
- 15.Soysal V, Yigitbasi O, Kontas O, Kahya H, Guney E. Inflammatory myofibroblastic tumor of the nasal cavity: a case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2001;61(2):161–165. doi: 10.1016/S0165-5876(01)00561-4. [DOI] [PubMed] [Google Scholar]
- 16.Dimitrakopoulos I, Psomaderis K, Iordanidis F, Karakasis D. Inflammatory myofibroblastic tumor of the maxillary sinus: a case report. J Oral Maxillofac Surg. 2007;65(2):323–326. doi: 10.1016/j.joms.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 17.Karakok M, Enver O, Sari I, Mumbuc S, Aydin A, Kanlikama M, Kervancioglu R. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the maxillary sinus mimicking malignancy: a case report of an unusual location (is that a true neoplasm?) Auris Nasus Larynx. 2002;29(4):383–386. doi: 10.1016/S0385-8146(02)00024-X. [DOI] [PubMed] [Google Scholar]
- 18.Lawson SLA, Azoumah DK, Lawson-Evi K, N’Timon B, Savi de Tove HM, Yehouessi-Vignikin B, Kpemissi E. Tumeur myofibroblastique inflammatoire chez une fillette de 7 ans. Arch Pédiatr. 2010;17(1):34–37. doi: 10.1016/j.arcped.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Lee DK, Cho Y-S, Hong SH, Chung WH, Ahn YC. Inflammatory pseudotumor involving the skull base: response to steroid and radiation therapy. Otolaryngol Head Neck Surg. 2006;135(1):144–148. doi: 10.1016/j.otohns.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Sasagawa Y, Akai T, Itou S, Iizuka H. Multiple intraosseous inflammatory myofibroblastic tumors presenting with an aggressive clinical course: case report. Neurosurgery. 2011;69(4):1010–1015. doi: 10.1227/NEU.0b013e318223b651. [DOI] [PubMed] [Google Scholar]
- 21.Saïji E, Guillou L. Tumeurs fibroblastiques et myofibroblastiques de la tête et du cou. Ann Pathol. 2009;29(4):335–346. doi: 10.1016/j.annpat.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a Kinase Gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s Lymphoma”. Science. 1994;263(5151):1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 23.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Advances in brief recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59(12):2776–2780. [PubMed] [Google Scholar]
- 24.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 25.Chan J, Cheuk W, Shimizu M. Anaplastic Lymphoma Kinase expression in inflammatory pseudotumors. Am J Surg Pathol. 2011;25(6):761–768. doi: 10.1097/00000478-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Jacob S, Reith J, Kojima A, Williams DW, Liu C, Vila Duckworth L. An unusual case of systemic inflammatory myofibroblastic tumor with successful treatment with ALK-Inhibitor. Case Rep Pathol. 2014;2014:1–5. [DOI] [PMC free article] [PubMed]
- 27.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 28.Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu C, Jhanwar S, Ladanyi M, Capelletti M, Rodig S, Ramaiya N, Kwak E, Clark J, Wilner KD, Christensen JG, Jänne P, Maki RG, Demetri GD, Shapiro GI. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. New Engl J Med. 2010;363(18):1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]