Abstract
Paragangliomas (PG) are very rare neuroendocrine tumours, arising from neural crest derived paraganglia of the autonomic nervous system. Primary thyroid paraganglioma (PTPG) is a rare site of PG and only 45 cases have been previously reported. The preoperative diagnosis of PTPGs presents a challenge as the clinical, cytological and histological features overlap with more common primary thyroid cancers. A 55 year old male was found to have significant enlargement of the left lobe of his thyroid. Following lobectomy, the thyroid lobe showed unencapsulated tumour which was positive for synaptophysin, CD56 and S100 (sustentacular cells). Post-operative imaging demonstrated incomplete resection. There was no post-operative radiotherapy and monitoring was by 6–12 monthly MRI. 48 months after his surgery he is alive and well with no evidence of disease progression. The diagnosis of PTPG was only made postoperatively, and although rare should be considered in the differential diagnosis of a hypervascular thyroid nodule.
Keyword: Thyroid paraganglioma, CD56, Synaptophysin
Introduction
Paragangliomas (PG) are very rare neuroendocrine tumours, arising from the neural crest-derived paraganglia of the autonomic nervous system. Prominent locations of head and neck PGs include the carotid body along with the vagal, jugular, and tympanic glomus [1], and account for 0.6 % of all head and neck tumours [2]. Less common sites of head and neck PGs include the nasal cavity, orbit, larynx and thyroid gland [3]. In a recently published article, primary thyroid paragangliomas (PTPGs) accounted for 0.04 % of the final histological diagnosis of patients undergoing thyroidectomy during the period 1981–2008 [4].
To the best of our knowledge, (from reviewing the world literature written in the English language), 45 cases of PTPGs have been reported between 1965 and 2016 (Table 1) [2, 4, 5, 7, 8, 10–39]. Only 5 of these cases occurred in men [4, 5, 7, 38], suggesting the predilection of this disease towards the female sex [4, 5].
Table 1.
Overview of historical cases of PTPGs
| Author | Year | Sex | Age | Local invasion | Treatment | Resection | Mulit-centric disease | Follow up |
|---|---|---|---|---|---|---|---|---|
| Van Miert [10] | 1964 | F | 63 | NA | Radiotherapy | NA | Syn CBT | 16 mnths |
| Kay et al. [11] | 1975 | F | 51 | No | Surgical resection | Complete | No | 7.5 mnths |
| Massaioli et al. [12] | 1979 | F | 9 | NA | Subtotal thyroidectomy | Complete | No | 5 mnths |
| Banner et al. [13] | 1979 | F | 36 | No | Hemithyroidectomy | NA | No | NA |
| Haegert et al. [14] | 1980 | F | 36 | No | Hemithyroidectomy | Complete | Bilat CBT | 5 years |
| Buss et al. [15] | 1980 | F | 50 | No | Hemithyroidectomy | Complete | No | 2.5 years |
| Cayot et al. [16] | 1982 | F | 58 | No | Total thyroidectomy | NA | Bilat CBT | 7 mnths |
| Olofsson et al. [17] | 1984 | F | 44 | Yes | Hemithyroidectomy + partial pharyngeal + total laryngeal + partial tracheal resection | Complete | No | 7 years |
| Mitsudo et al. [18] | 1987 | F | 50 | Yes | Total thyroidectomy + segmental anterior tracheal resection | Complete | No | 2 years |
| de Vries and Watson [19] | 1989 | F | 73 | Yes | Hemithyroidectomy | Complete | No | 2 years |
| Brownlee and Shockley [20] | 1992 | F | 27 | Yes | Hemithyroidectomy extended to right subglottic laryngeal resection | Complete | No | 18 mnths |
| Hughes et al. [21] | 1997 | F | 31 | No | Hemithyroidectomy to completion total thyroidectomy | Complete | Met CBT | 2 years |
| LaGuette et al. [22] | 1997 | F | 56 | No | Hemithyroidectomy | Complete | No | 8 years |
| 1997 | F | 64 | No | Hemithyroidectomy | Complete | No | 4 years | |
| 1997 | F | 55 | No | Total thyroidectomy | Complete | No | 7 years | |
| Bizollon et al. [23] | 1997 | F | 48 | NA | Not available | NA | No | NA |
| Tiong et al. [24] | 2000 | F | 52 | No | Hemithyroidectomy to completion total thyroidectomy 4 weeks later | Complete | No | 2 years |
| Kronz et al. [5] | 2000 | M | 55 | No | Hemithyroidectomy | Complete | No | 9 mnths |
| 2000 | F | 52 | Yes | (1) Hemithyroidectomy + tracheostomy (2) Post-op RT (3) Completion TT + total laryngeal, pharyngeal, oesophageal resection + central and bilateral neck dissection | Complete after 2nd surgery | No | 6 years | |
| Napolitano et al. [25] | 2000 | F | 47 | No | Total thyroidectomy | Complete | No | 6 mnths |
| Skiadas et al. [26] | 2001 | F | 65 | No | Total thyroidectomy | Complete | No | 22 mnths |
| Vera-Cruz et al. [27] | 2001 | F | 32 | NA | Hemithyroidectomy | NA | No | 4 years |
| Vodovnik [28] | 2002 | F | 46 | No | Hemithyroidectomy | Complete | No | NA |
| Corrado et al. [29] | 2004 | F | 46 | Yes | Hemithyroidectomy | Incomplete | No | 3 mnths |
| Zantour et al. [30] | 2004 | F | 32 | Yes | Total thyroidectomy | Complete | No | 6 years |
| Foppiani [31] | 2005 | F | 51 | No | Total thyroidectomy | Complete | No | 5 years |
| Yano et al. [7] | 2007 | M | 24 | No | Hemithyroidectomy | Complete | No | 6 mnths |
| Ashraf et al. [32] | 2008 | F | 40 | NA | Surgical resection | NA | No | NA |
| Ferri et al. [2] | 2009 | F | 63 | Yes | (1) Hemithyroidectomy (2) RT planned for residual tumour | Incomplete | No | 18 mnths |
| Gonzalez Poggioli et al. [33] | 2009 | F | 36 | No | Total thyroidectomy | Complete | No | 2 mnths |
| Erem et al. [34] | 2009 | F | 58 | No | Subtotal thyroidectomy | Complete | No | 3 mnths |
| Mun et al. [35] | 2009 | F | 40 | No | Total thyroidectomy | Complete | No | NA |
| Phitayakorn et al. [8] | 2011 | F | 41 | No | Surgical resection | Complete | No | 14 mnths |
| 2011 | F | 73 | Yes | Hemithyroidectomy | Complete | No | 13 mnths | |
| Basu and Viswanathan [36] | 2011 | F | 70 | No | Hemithyroidectomy | Complete | No | 12 mnths |
| Castelblanco et al. [37] | 2011 | F | 59 | No | Surgical resection | NA | No | NA |
| 2011 | F | 78 | No | Surgical resection | NA | No | NA | |
| 2011 | F | 51 | No | Surgical resection | NA | No | NA | |
| Armstrong et al. [4] | 2012 | F | 67 | Yes | Hemithyroidectomy + tracheal resection | Complete | No | 7 years |
| 2012 | M | 40 | Yes | Total thyroidectomy + tracheal resection | Complete | No | Lived 14 years | |
| 2012 | M | 60 | Yes | Total thyroidectomy | NA | NA | NA | |
| Yu et al. [38] | 2013 | F | 30 | Yes | Hemithyroidectomy + subtotal thyroidectomy on other side | NA | No | 39 |
| 2013 | M | 47 | Yes | Hemithyroidectomy | Complete | No | 47 | |
| 2013 | F | 37 | No | Hemithyroidectomy + isthmus + partial thyroid lobectomy on other side | Complete | No | 10 | |
| Filipovic et al. [39] | 2014 | F | 69 | Yes | Extended total thyroidectomy | Complete | No | 3 years |
| Navaratne et al. (case study) | 2016 | M | 55 | Yes | Hemithyroidectomy | Incomplete | No | 4 years |
bilat bilateral, CBT carotid body tumour, Met metachronous, NA not available, RT radiotherapy, Syn synchronous, TT total thyroidectomy
The pre-operative diagnosis of PTPGs presents a challenge to the otolaryngologist and pathologist since the clinical, cytological and histological features overlap with more common primary thyroid cancers, namely medullary thyroid carcinoma (MTC). Although rare, PTPGs should be considered in the differential diagnosis of a hypervascular thyroid nodule [8]. In this case study, we report a 55 year old male with locally invasive PTPG, its clinical, radiological and immunohistochemical features and its management.
Case Report
A 55 year old military officer was found to have significant enlargement of the left lobe of his thyroid, which was picked up on a pre-discharge medical check-up. Although his presentation seemed asymptomatic, he did indeed have symptoms of an enlarged neck and some compressive type symptoms including some breathing difficulty. Previous medical and family histories were non-contributory. Ear, nose and throat examination, including direct laryngoscopy, was unremarkable. Palpation of the neck revealed a bulky left lobe of thyroid. Routine laboratory tests including thyroid function tests were all within normal limits, however he was noted by his medical officer to have persistently raised CRP (25 mg/L) and ESR (39 mm/h).
Chest radiograph was unremarkable except for right tracheal deviation. The ultrasound scan revealed a large mass replacing most of the superior aspect of the left lobe which was diffusely hypoechoic and irregular in outline. Extensive vascularity throughout the left lobe with extensive collateral vessel formation and retrosternal extension beyond the thyroid gland was also noted.
CT neck and chest confirmed the presence of a large soft tissue mass in the left side of the neck, which appeared to be growing beyond the limits of the left lobe of the thyroid. The mass measured 8.5 × 8.0 × 6.5 cm and was heterogeneously enhancing with an area of central low density suggestive of necrosis and several coarse calcifications (Figs. 1, 2). The mass extended posteriorly to contact the anterior margin of upper thoracic vertebrae and displaced the trachea to the right. There were no definite signs of invasion, enlarged local lymph nodes or metastatic disease in the chest.
Fig. 1.
Pre-operative computed tomography scan (coronal section) with contrast showing abnormal vascularity of this tumour
Fig. 2.
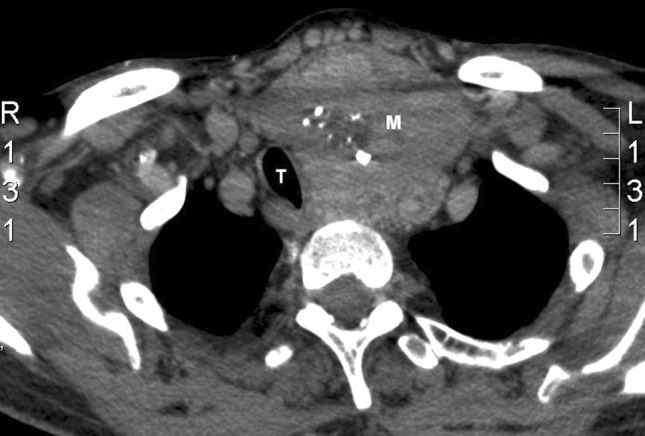
Pre-operative CT neck showing heterogeneously enhancing mass with area of central low density suggestive of necrosis and several coarse calcifications. The mass extends posteriorly to contact the anterior margin of upper thoracic vertebrae and displaces the trachea to the right. T trachea, M mass
No attempt at pre-operative diagnosis with fine needle aspiration (FNA) or core needle biopsy was made and the patient was booked for an elective left thyroid lobectomy. The mass was very large, extremely vascular and fibrotic. Excision of the mass was difficult because surgical planes could not easily be identified. The left recurrent laryngeal nerve was accidently severed during the operation and there was significant blood loss (post-operative haemoglobin concentration 82 g/l).
Macroscopically, the specimen measured 11 × 8.0 × 5.2 cm and weighed 122 g. On slicing, the parenchyma appeared calcified and showed a vaguely nodular cut surface. Pale fibrotic and calcified areas adjacent to the brown, fleshy homogenous thyroid parenchyma were seen. Microscopically, the thyroid lobe showed an unencapsulated tumour with lobular architecture and intersecting bands of fibrovascular connective tissue (Fig. 3a). The tumour islands demonstrated an alveolar and organoid arrangement (‘zellballen’) of neoplastic cells with pink granular cytoplasm and round to ovoid nuclei (Fig. 3b). Scattered tumour cell nuclei appeared enlarged, hyperchromatic with bizarre shapes. The tumour was noted to be highly vascularised (Fig. 3c). Importantly, the tumour was seen close to blood vessels, however no tumour fragments were seen within vascular lumina. Tumour did extend to the resection margins and was seen to extend outside the thyroid. The differential diagnosis at this stage included a medullary thyroid carcinoma (MTC), a paraganglioma and a carcinoid tumour.
Fig. 3.
Histology. a Low power (×2) of tumour with inked resection margin showing a vague lobular architecture of the tumour and intersecting bands of fibrous tissue. b Medium power (×10) of tumour showing alveolar and organoid cellular arrangements of neoplastic cells embedded within hyalinized stroma. c High power (×40) showing vascularity of the tumour and the neoplastic cells with eosinophilic cytoplasm and round/ovoid nuclei with granular chromatin material
Immunohistochemically the tumour was positive for CD56 and synaptophysin (Fig. 4a, b). S100 highlighted scattered cells within the tumour and had a nuclear and cytoplasmic staining pattern (sustentacular cells) (Fig. 4c). The tumour was negative for cytokeratin AE1/3, CAM5.2, thyroid transcription factor 1 (TTF-1), inhibin, calcitonin, thyroglobulin and carcinoembryonic antigen (CEA). The overall morphological and immunohistochemical features were those of a paraganglioma, highlighting the essential role of immunohistochemistry in its diagnosis [2, 9, 22].
Fig. 4.
Immunohistochemistry. a CD56 (neuroendocrine marker) positive (×20). b Synaptophysin (neuroendocrine marker) positive (×20). c Spindle-shaped sustentacular cells positive for S-100 protein (×40)
The patient was seen in clinic 11 days later. He had poor voice quality and was quite ‘breathy’. Nasendoscopic examination confirmed left vocal cord palsy, with the cord in the abducted position.
Post-operative CT scans and magnetic resonance imaging (MRI) of the neck with gadolinium identified residual enhancing tissue, with ill-defined margins, anterolateral to the left side of the trachea measuring 4.5 × 3.2 cm in maximal axial dimensions. There was less compression on the trachea compared to pre-operative imaging and no cervical lymphadenopathy was noted. Positron emission tomography–computed tomography (PET–CT) scan showed no evidence of metastatic disease.
The patient is being followed up at the tertiary referral centre, where he has been closely monitored by 6–12 monthly MRI. 48 months after his surgery he is alive and well with no evidence of disease progression. No plans for radiotherapy have been made at this stage.
Discussion
Thyroid paragangliomas are extremely rare tumours. To date, including this case, 46 cases of PTPGs have been reported in the literature (Table 1). Forty of these cases occurred in females, with the majority between the ages of 40–60 at diagnosis (mean 48.7 years, range 9–78 years). We report a case of PTPG in a male patient which showed local invasion into surrounding tissues. The whole tumour could not be resected which will have further implications on management of this patient. This case emphasises the need to consider PTPG in the differential diagnosis of any thyroid mass (even in those involving men or behaving in a locally aggressive fashion).
The exact cell origin of PTPG is unclear as paraganglionic tissue is not native to the thyroid gland. Displacement of the inferior laryngeal paraganglion to within the thyroid gland as a normal anatomical variant may be the cause of the rare finding of a primary intrathyroidal paraganglioma. Indeed, it has been documented that paraganglia of the inferior larynx have been found within the thyroid capsule [40]. Moreover, one of the cases included in Table 1 was originally reported as a laryngeal paraganglioma with a large portion of the tumour present within the left lobe of the thyroid [17]. This hypothesis is also supported by the matching female predominance of laryngeal paragangliomas to PTPGs [5].
The majority of cases present as a neck mass, often slowly enlarging, which can rarely be tender [14, 15, 28]. Eight cases presented as a solitary thyroid nodule [14, 15, 21–23, 26, 31, 36], of which only one was documented as being a hot nodule detected by 99mTechnetium pertechnetate (99mTC) scintigraphy [31]. Even though neck mass was the most common presentation, only 5 cases reported compressive symptoms, which mainly included tracheal compression [4, 5, 19, this case study]. Interestingly, 8 cases describe a history of hypertension [2, 5, 10, 18, 22, 26, 28, 29], with one reporting a normalisation of pulse and blood pressure after resection of the PTPG [26].
In terms of the natural history of PTPGs, of the 46 cases described, only sixteen distinctly describe local invasion of the tumour beyond the thyroid gland and there have been four cases reporting the presence of multicentric disease [10, 14, 16, 21]. Although there have been no reports of metastases from PTPGs [2, 4–8, 10–39], due the limited number of cases, we still recommend full staging of this tumour, including screening for other head and neck PGs. Although local extension is not regarded as a malignant feature in PTPGs [5], the frequency of this has perhaps been underestimated in the past. Eight cases report extension into the trachea or larynx [4, 5, 17–20, 39], with three invading the oesophagus [2, 5, 19] and three invading the recurrent laryngeal nerve [2, 8, 38]. Interestingly, three case reports describe vascular invasion by the tumour [4, 38]. Only two of these cases were followed up (39 months in a 30 year old female and 8 years in a 67 year old female) with no evidence of local recurrence or metastases reported. A study of the pathologic features of 120 adrenal and extra-adrenal paragangliomas identified that vascular invasion and/or extensive local invasion occurred more frequently in malignant tumours, however was not shown to be amongst the four most predictive features of malignancy [41].
Pre-operative investigation of a thyroid mass usually takes the form of radiological assessment with or without FNA. In Fig. 1, a contrast enhanced CT scan of the neck, the abnormal vasculature demonstrated should alert the clinician to consider a wide differential diagnosis. Appreciation of the prolific vascular anatomy in the pre-operative scans may have avoided or helped predict the complication of blood loss endured in this case. From the cases where FNA were performed, the outcome appears to be either non-diagnostic or yield an excessive amount of blood, atypical features suspicious for malignancy, or incorrectly diagnose MTC. Indeed, there has only been one report of diagnosing paraganglioma on FNA of a thyroid mass [6]. FNA diagnosis should be made with caution and include immunohistochemical exclusion of MTC and other thyroid tumours. FNA should not be dismissed on the basis of technical failure or misinterpretation as previously reported in PTPG. Indeed technically good FNA with sufficient material could be of help in the diagnosis of PTPG so long as the pathologist remains open to its diagnosis and performs the appropriate additional immunostains.
In keeping with many of the cases reported in the literature, histopathological features usually indicate MTC. This is because microscopically both diagnoses may show clustering of cells with granular cytoplasm and a richly vascularized stroma. In fact, these tumours may also be reported as hyalinizing trabecular adenoma, ‘atypical’ follicular adenoma and carcinoid tumours that have metastasized to the thyroid, again highlighting the essential role of immunohistochemistry in deriving the correct diagnosis [2, 9, 22]. Negative staining for cytokeratin AE1/3, calcitonin, thyroglobulin, TTF-1, inhibin and CEA but positive for chromogranin A, synaptophysin and S100 (highlighting sustentacular cells) confirms the diagnosis.
Due to the rarity of this tumour, few case reports and that the mean follow up period from the literature is only 31 months; our knowledge of the natural history of PTPGs is limited. First line management is complete resection of the tumour, however this is not always possible. There have been no reports of local recurrence following complete excision of PTPG in the literature. Besides our case, there are only two other case reports where the tumour was incompletely excised [2, 29]. Radiotherapy was planned post-operatively in a 63 year-old female, who had incomplete excision of a PTPG that had infiltrated surrounding tissues, including the recurrent laryngeal nerve and oesophagus [2]. No mention was made of whether this actually took place or its success. There are very limited reports of the use of radiotherapy (either as primary or adjunctive therapy) in PTPG, and in fact only 3 cases are documented. In the first case, radiotherapy was the only treatment modality, with the patient being alive and well at 16 months [10]. The second case, describes a 52 year old female with extensive local invasion of PTPG [5]. The patient underwent radiotherapy treatment between two separate surgeries. As the second surgery achieved clear resection margins, it is difficult to comment on the effectiveness of the radiotherapy treatment in this case. Finally, the third case is mentioned above [2]. A recent systematic review of the efficacy of external beam radiotherapy (EBRT) in jugular and vagal PGs in 461 patients showed that long-term control of disease was achieved in nearly 90 % of cases [42]. Even though risk of radiation-induced malignancy is low, acute and late toxicities secondary to radiotherapy contribute to further morbidity [43].
In this case of incomplete resection following surgery, we propose an alternative management plan of careful long-term follow-up with imaging at 6 monthly intervals initially, increasing to yearly studies to monitor residual disease. From limited cases, it is generally accepted that there is a good prognosis following complete excision of the tumour, however it is unclear how residual tumour following debulking will behave.
Compliance with Ethical Standards
Conflict of interest
None.
Contributor Information
L. Navaratne, Email: lalin.navaratne@doctors.org.uk
R. G. Mathew, Email: rgmathew@hotmail.com
G. Kousparos, Email: george.kousparos@fhft.nhs.uk
A. McCombe, Email: amcco79794@aol.com
References
- 1.Offergeld C, Brase C, Yaremchuk S, Mader I, Rischke HC, Glasker S, et al. Head and neck paragangliomas: clinical and molecular genetic classification. Clinics. 2012;67(S1):19–28. doi: 10.6061/clinics/2012(Sup01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferri E, Manconi R, Armato E, Ianniello F. Primary paraganglioma of thyroid gland: a clinicopathologic and immunohistochemical study with review of the literature. Acta Otorhinolaryngol Ital. 2009;29:97–102. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Barich F, Karnell LH, Robinson RA, Zhen WK, Gantz BJ, Hoffman HT. National cancer data base report on malignant paragangliomas of the head and neck. Cancer. 2002;94:730–737. doi: 10.1002/cncr.10252. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Chiosea SI, Carty SE, Hodak SP, Yip L. Thyroid paragangliomas are locally aggressive. Thyroid. 2012;2012(22):88–93. doi: 10.1089/thy.2011.0110. [DOI] [PubMed] [Google Scholar]
- 5.Kronz JD, Argani P, Udelsman R, Silverberg L, Westra WH. Paraganglioma of the thyroid: two cases that clarify and expand the clinical spectrum. Head Neck. 2000;22:621–625. doi: 10.1002/1097-0347(200009)22:6<621::AID-HED12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Schmit GD, Gorman B, van Heerden JA, Gharib H. Inferior laryngeal paraganglioma mimicking a primary thyroid tumour. Endocr Pract. 2006;12:432–435. doi: 10.4158/EP.12.4.432. [DOI] [PubMed] [Google Scholar]
- 7.Yano Y, Nagahama M, Sugino K. Ito Ku, Kameyama K, Ito Ko. Paraganglioma of the thyroid: report of a male case with ultrasonographic imagings, cytologic, histologic and immunohistochemical features. Thyroid. 2007;17:575–578. doi: 10.1089/thy.2006.0284. [DOI] [PubMed] [Google Scholar]
- 8.Phitayakorn R, Faquin W, Wei N, Barbesino G, Stephen AE. Thyroid-associated paragangliomas. Thyroid. 2011;21:725–733. doi: 10.1089/thy.2010.0362. [DOI] [PubMed] [Google Scholar]
- 9.Korat O, Trojanowski JQ, LiVolsi VA, Merino MJ. Antigen expression in normal paraganglia and paragangliomas. Surg Pathol. 1988;1:33–40. [Google Scholar]
- 10.Vanmiert PJ. The treatment of chemodectomas by radiotherapy. Proc R Soc Med. 1964;57:946–951. [PubMed] [Google Scholar]
- 11.Kay S, Montague JW, Dodd RW. Nonchromaffin paraganglioma (chemodectoma) of thyroid region. Cancer. 1975;36:582–585. doi: 10.1002/1097-0142(197508)36:2<582::AID-CNCR2820360238>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Massaioli N, Balbo G, Fausone G, Negro D. Endothyroid (non-chromaffin) branchiomeric paraganglioma. Description of a clinical case. Minerva Chir. 1979;34:867–874. [PubMed] [Google Scholar]
- 13.Banner B, Morecki R, Eviatar A. Chemodectoma in the mid-thyroid region. J Otolaryngol. 1979;8:271–273. [PubMed] [Google Scholar]
- 14.Haegert DG, Wang NS, Farrer PA, Seemayer TA, Thelmo W. Non-chromaffin paragangliomatosis manifestating as a cold thyroid nodule. Am J Clin Pathol. 1974;61:561–570. doi: 10.1093/ajcp/61.4.561. [DOI] [PubMed] [Google Scholar]
- 15.Buss DH, Marshall RB, Baird FG, Myers RT. Paraganglioma of the thyroid gland. Am J Surg Pathol. 1980;4:589–593. doi: 10.1097/00000478-198012000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Cayot F, Bastien H, Justrabo E, Mottot C, Cuisenier J, Bruchon Y, et al. Multiple paragangliomas of the neck localized in the thyroid region. Papillary thyroid cancer associated with parathyroid adenoma. Sem Hop. 1982;58:2004–2007. [PubMed] [Google Scholar]
- 17.Olofsson J, Grontoft O, Sokjer H, Risberg B. Paraganglioma involving the larynx. ORL J Otorhinolaryngol Rel Spec. 1984;46:57–65. doi: 10.1159/000275687. [DOI] [PubMed] [Google Scholar]
- 18.Mitsudo SM, Grajower MD, Balbi H, Silver C. Malignant paraganglioma of the thyroid gland. Arch Pathol Lab Med. 1987;111:378–380. [PubMed] [Google Scholar]
- 19.de Vries EJ, Watson CG. Paraganglioma of the thyroid. Head Neck. 1989;11:462–465. doi: 10.1002/hed.2880110514. [DOI] [PubMed] [Google Scholar]
- 20.Brownlee RE, Shockley WW. Thyroid paraganglioma. Ann Otol Rhinol Laryngol. 1992;101:293–299. doi: 10.1177/000348949210100402. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JH, El-Mofty S, Sessions D, Liapis H. Primary intrathyroidal paraganglioma with matachronous carotid body tumour: report of a case and review of literature. Pathol Res Pract. 1997;193:791–796. doi: 10.1016/S0344-0338(97)80059-3. [DOI] [PubMed] [Google Scholar]
- 22.LaGuette J, Matias-Guiu X, Rosai J. Thyroid paraganglioma: A clinicopathologic and immunohistochemical study of three cases. Am J Surg Pathol. 1997;2:748–753. doi: 10.1097/00000478-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bizollon MH, Darreye G, Berger N. Thyroid paraganglioma: report of a case. Ann Pathol. 1997;17:416–418. [PubMed] [Google Scholar]
- 24.Tiong HY, White SA, Roop L, Furness PN, Nicholson ML. Paraganglioma: an unusual solitary nodule of the thyroid. Eur J Surg Oncol. 2000;26:720–721. doi: 10.1053/ejso.2000.0990. [DOI] [PubMed] [Google Scholar]
- 25.Napolitano L, Francomano F, Angelucci D, Napolitano AM. Thyroid paraganglioma: report of a case and review of the literature. Ann Ital Chir. 2000;71:511–513. [PubMed] [Google Scholar]
- 26.Skiadas PK, Kavavoulis TN, Gikonti IJ. Normalisation of blood pressure and heart rate after excision of a thyroid paraganglioma. Eur J Surg. 2001;167:392–394. doi: 10.1080/110241501750215320. [DOI] [PubMed] [Google Scholar]
- 27.Vera-Cruz P, Zagalo C, Felix A, Pratas S, Rosa Santos J. Paraganglioma tiroideo. Caso clinico. Rev Chil Anat. 2001;19:331–334. doi: 10.4067/S0716-98682001000300016. [DOI] [Google Scholar]
- 28.Vodovnik A. Fine needle aspiration cytology of primary thyroid paraganglioma. Report of a case with cytologic, histologic and immunohistochemical features and differential diagnostic considerations. Acta Cytol. 2002;46:1133–1137. doi: 10.1159/000327120. [DOI] [PubMed] [Google Scholar]
- 29.Corrado S, Montanini V, De Gaetani C, Borghi F, Papi G. Primary paraganglioma of the thyroid gland. J Endocrinol Invest. 2004;27:788–792. doi: 10.1007/BF03347525. [DOI] [PubMed] [Google Scholar]
- 30.Zantour B, Guilhaume B, Tissier F, Louvel A, Jeunemaitre X, Gimenez-Roqueplo AP, Bertagna X. A thyroid nodule revealing a paraganglioma in a patient with a new germline mutation in the succinate dehydrogenase B gene. Eur J Endocrinol. 2004;151:433–438. doi: 10.1530/eje.0.1510433. [DOI] [PubMed] [Google Scholar]
- 31.Foppiani L. Thyroid paraganglioma manifesting as hot toxic nodule. J Endocrinol Invest. 2005;28:479–480. doi: 10.1007/BF03347231. [DOI] [PubMed] [Google Scholar]
- 32.Ashraf MJ, Azarpira N, Vasei M, Tavakol MH, Khademi B. Thyroid paraganglioma: diagnostic pitfall in fine needle aspiration biopsy. Acta Cytol. 2008;52:745–747. doi: 10.1159/000325637. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez Poggioli N, Amado ML, Pimentel MTY. Paraganglioma of the thyroid gland: a rare entity. Endocr Pathol. 2009;20:62–65. doi: 10.1007/s12022-009-9066-2. [DOI] [PubMed] [Google Scholar]
- 34.Erem C, Kocak M, Nuhoglu I, Cobanoglu U, Ucuncu O, Ikatan BK. Primary thyroid paraganglioma presenting with double thyroid nodule: a case report. Endocrine. 2009;36:368–371. doi: 10.1007/s12020-009-9238-3. [DOI] [PubMed] [Google Scholar]
- 35.Mun KS, Pailoor J, Chan KS, Pillay B. Extra-adrenal paraganglioma: presentation in three uncommon locations. Malays J Pathol. 2009;31:57–61. [PubMed] [Google Scholar]
- 36.Basu S, Viswanathan S. Primary paraganglioma of thyroid presenting as solitary thyroid mass. J Can Res Ther. 2011;7:385–387. doi: 10.4103/0973-1482.87028. [DOI] [PubMed] [Google Scholar]
- 37.Castelblanco E, Gallel P, Ros S, Gatius S, Valls J, De-Cubas AA, et al. Thyroid paraganglioma. Report of 3 cases and description of an immunohistochemical profile useful in the differential diagnosis with medullary thyroid carcinoma, based on complementary DNA array results. Hum Pathol. 2012;43:1103–1112. doi: 10.1016/j.humpath.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Yu B, Sheng W, Wang J. Primary paraganglioma of thyroid gland: A clinicopathologic and immunohistochemical analysis of three cases with a review of the literature. Head Neck Pathol. 2013;7:373–380. doi: 10.1007/s12105-013-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filipovic A, Vuckovic L, Pejakov L. Paraganglioma of the thyroid gland: a case report. Vojnosanit Pregl. 2014;71:875–878. doi: 10.2298/VSP130420043F. [DOI] [PubMed] [Google Scholar]
- 40.Zak FG, Lawson W. Glomic (paraganglionic) tissue of the larynx and capsule of the thyroid gland. Mt Sinai J Med. 1972;39:82–90. [PubMed] [Google Scholar]
- 41.Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol. 1990;21:1168–1180. doi: 10.1016/0046-8177(90)90155-X. [DOI] [PubMed] [Google Scholar]
- 42.Suarez C, Rodrigo JP, Bodeker CC, Llorente JL, Silver CE, et al. Jugular and vagal paragangliomas: Systemic study of management with surgery and radiotherapy. Head Neck. 2013;35:1195–1204. doi: 10.1002/hed.22976. [DOI] [PubMed] [Google Scholar]
- 43.Chino JP, Sampson JH, Tucci DL, Brizel DM, Kirkpatrick JP. Paraganglioma of the head and neck: long-term local control with radiotherapy. Am J Clin Oncol. 2009;32:304–307. doi: 10.1097/COC.0b013e318187dd94. [DOI] [PubMed] [Google Scholar]





