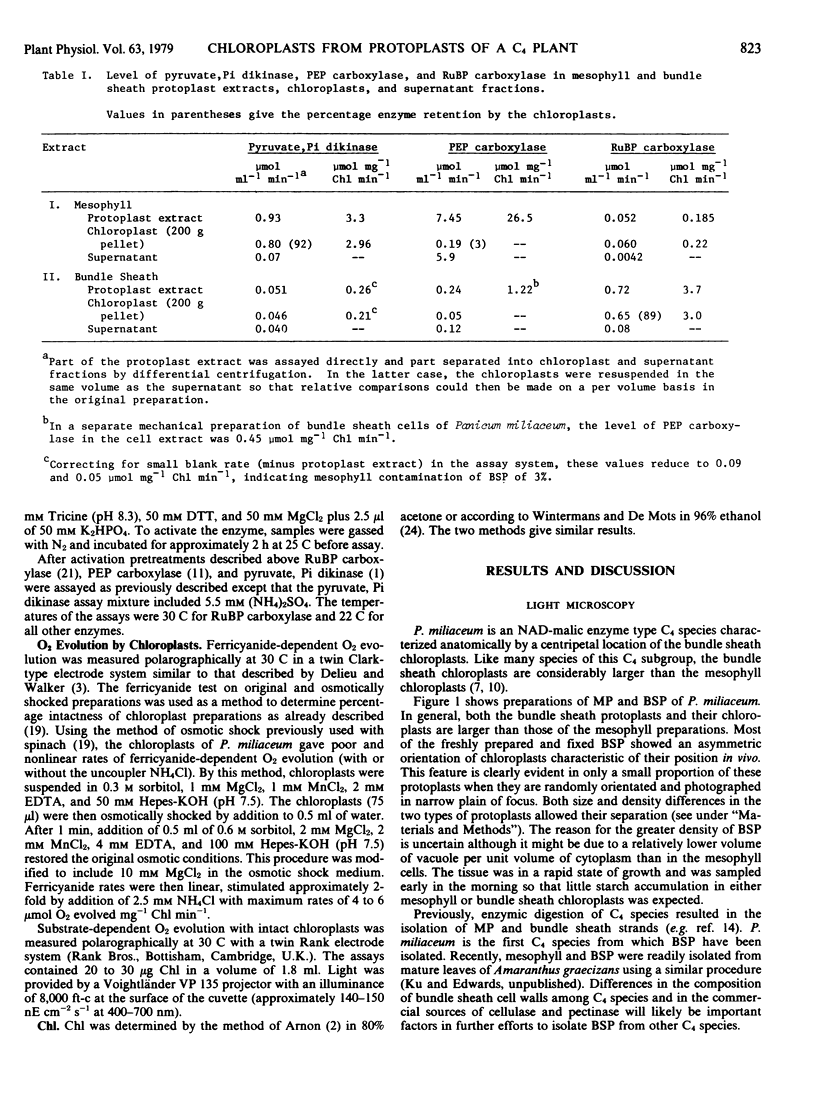
Abstract
A procedure is described for isolating and purifying mesophyll protoplasts and bundle sheath protoplasts of the C4 plant Panicum miliaceum. Following enzymic digestion of leaf tissue, mesophyll protoplasts and bundle sheath protoplasts are released and purified by density centrifugation. The lower density of mesophyll protoplasts allowed rapid separation of the two protoplast types. Evidence for separation of mesophyll protoplasts and bundle sheath protoplasts (up to 95% purity) is provided from light microscopy (based on size difference in both chloroplasts and protoplasts), levels of marker enzymes in the preparations (i.e. pyruvate, Pi dikinase and phosphoenolpyruvate carboxylase for mesophyll and ribulose-1,5-bisphosphate carboxylase for bundle sheath), and differences in substrate-dependent O2 evolution by chloroplasts isolated from protoplasts.
Chloroplasts were isolated from protoplasts by several passages of the protoplasts through a 20-micrometer nylon mesh. Mesophyll chloroplasts were judged approximately 90 to 95% intact and bundle sheath chloroplasts 80 to 90% intact based on retention of chloroplast marker enzymes and the ferricyanide test for intactness. It was necessary to include 10 millimolar MgCl2 in media for osmotically shocking the chloroplasts in order to obtain maximum and linear rates of ferricyanide-dependent O2 evolution.
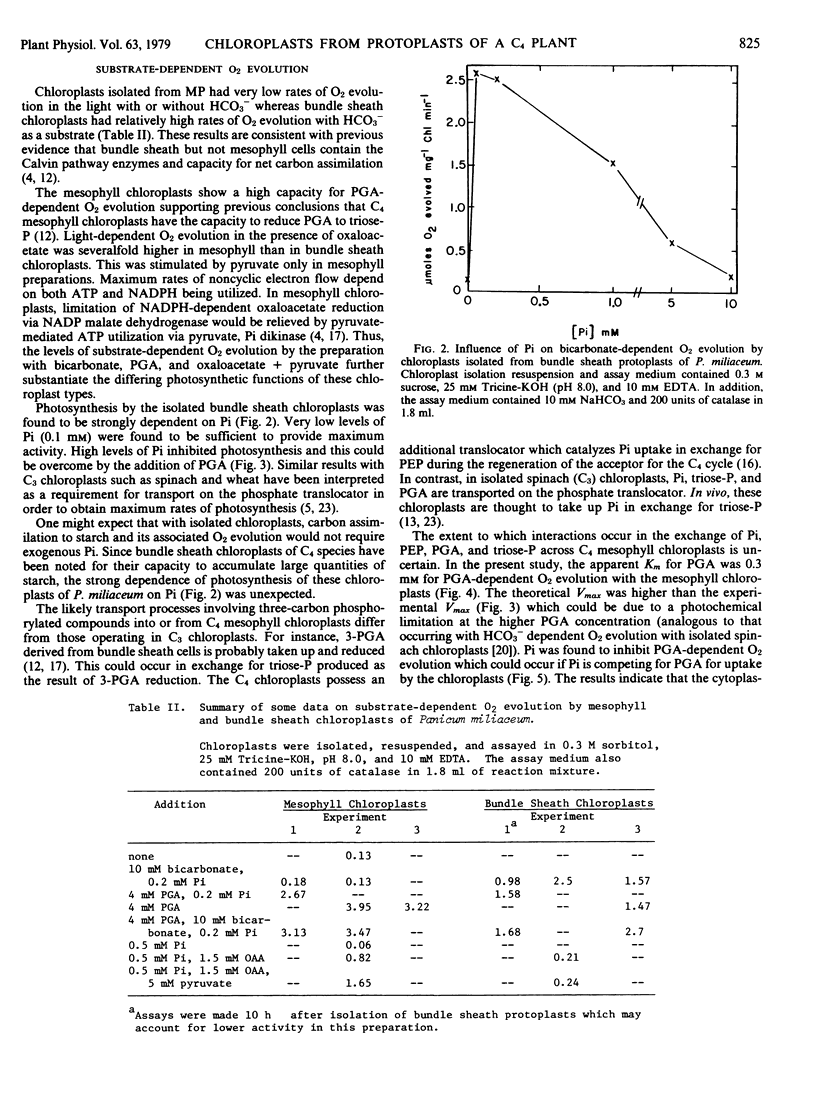
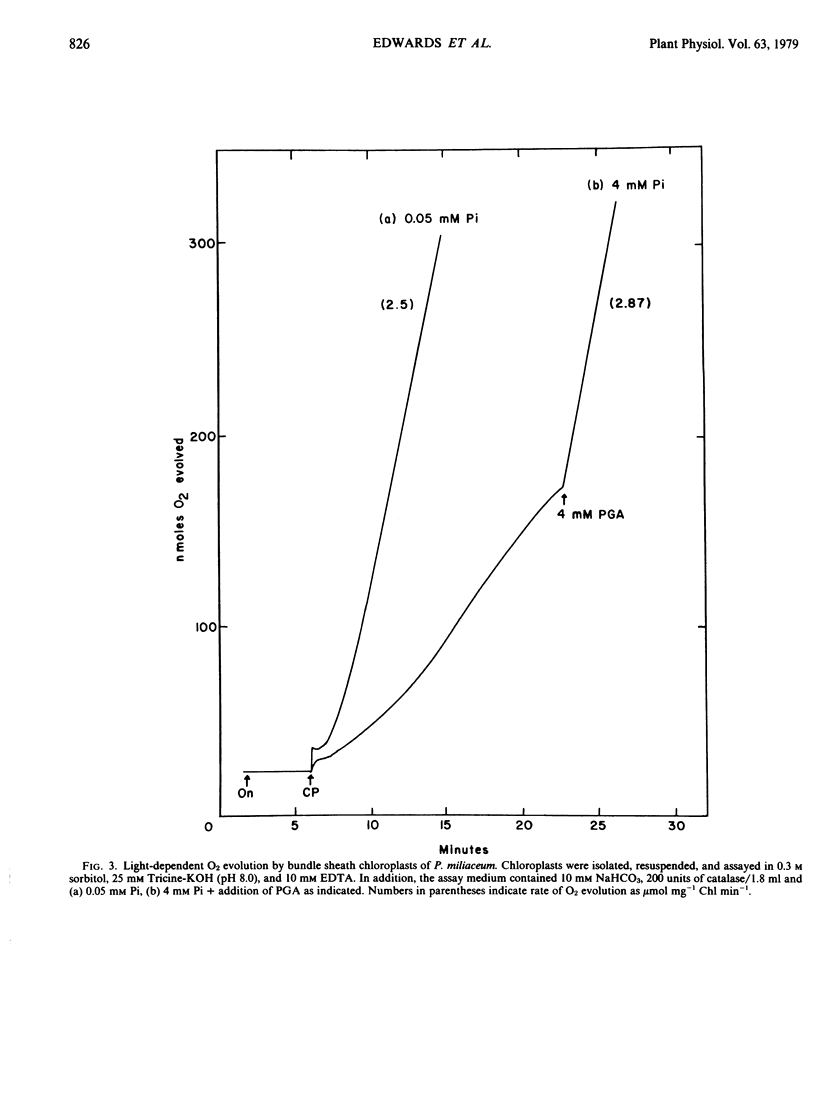
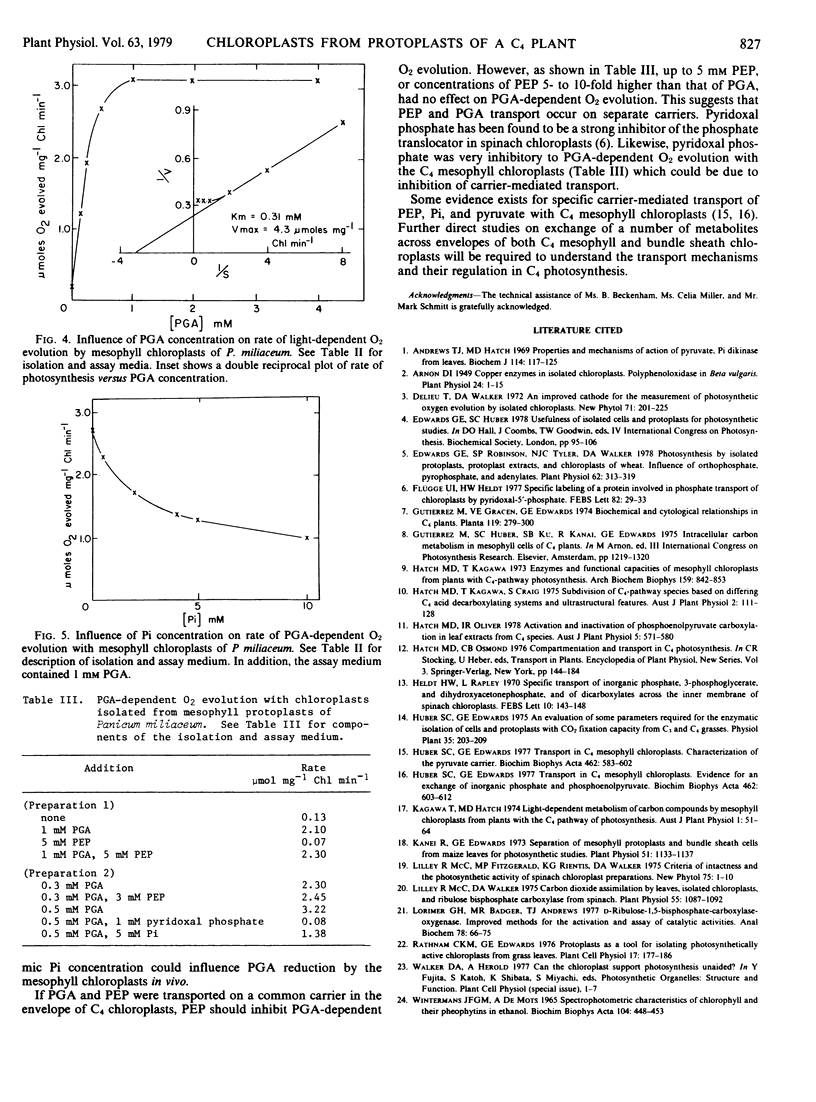
Chloroplasts isolated from mesophyll protoplast preparations had low rates of light-dependent O2 evolution in the presence of 10 millimolar NaHCO3 (0.13 micromoles per milligram chlorophyll per minute) in comparison to bundle sheath chloroplasts (1 to 2.5 micromoles per milligram chlorophyll per minute). The mesophyll chloroplasts catalyze high rates of 3-phosphoglycerate-dependent O2 evolution (2 to 4 micromoles per milligram chlorophyll per minute). Orthophosphate but not phosphoenolpyruvate inhibited the 3-phosphoglycerate-dependent O2 evolution by the mesophyll chloroplasts. Rates of O2 evolution were much higher with mesophyll than with bundle sheath chloroplasts in the presence of pyruvate plus oxaloacetate. The results are discussed in relation to the proposed function of these chloroplasts during C4 photosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Hatch M. D. Properties and mechanism of action of pyruvate, phosphate dikinase from leaves. Biochem J. 1969 Aug;114(1):117–125. doi: 10.1042/bj1140117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Heldt H. W. Specific labelling of a protein involved in phosphate transport of chloroplasts by pyridoxal-5'-phosphate. FEBS Lett. 1977 Oct 1;82(1):29–33. doi: 10.1016/0014-5793(77)80878-8. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Transport in C4 mesophyll chloroplasts characterization of the pyruvate carrier. Biochim Biophys Acta. 1977 Dec 23;462(3):583–602. doi: 10.1016/0005-2728(77)90103-7. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Transport in C4 mesophyll chloroplasts: evidence for an exchange of inorganic phosphate and phosphoenolpyruvate. Biochim Biophys Acta. 1977 Dec 23;462(3):603–612. doi: 10.1016/0005-2728(77)90104-9. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]



