Abstract
Gardner syndrome (GS) is caused by mutations in the APC and besides adenomatous colorectal polyps includes such manifestations as osteomas, epidermoid cysts (ECs) and occasionally multiple pilomatricomas. More than 50 % of ECs in patients with GS exhibit pilomatricoma-like ghost cell keratinization. The latter may be explained by the fact that the development of both GS and pilomatricoma is driven by activation of the Wnt/β-catenin signaling pathway. A 62-year-old, Caucasian male with history of GS presented with a unilocular, mixed radiopaque/radiolucent mandibular lesion causing divergence and external root resorption of involved teeth. Histopathologically, the lesion was composed of two cystic components, an orthokeratinized odontogenic cyst (OOC) and a smaller one with characteristics of keratocystic odontogenic tumor (KCOT) featuring, focally, ghost cells and an epithelial morule-like structure. Dystrophic calcifications essentially similar to those seen in pilomatricomas were observed in the fibrous connective tissue wall. The KCOT and OOC epithelia revealed strong and diffuse cytokeratin (AE1/AE3) and β-catenin immunoreactivity. CD10 positive immunostaining was seen in the keratin and superficial spinous cell layers in both OOC and KCOT. The intraepithelial and mural ghost cells showed a cytokeratin (+), β-catenin and CD10 (−) immunophenotype. The diagnosis of OOC with ghost cell calcifications in association with KCOT was rendered. The patient was lost to follow-up. Although a coincidental co-existence cannot be excluded, ghost cell calcifications mimicking pilomatricoma-like changes in an unusual odontogenic cyst combining OOC and KCOT features as seen in this patient with GS may be explained by the common molecular mechanisms underlying the pathogenesis of cutaneous pilomatricomas and GS.
Keywords: Orthokeratinized odontogenic cyst, Keratocystic odontogenic tumor, Gardner syndrome, β-catenin, Ghost cells, Pilomatricoma
Introduction
Gardner syndrome (GS) is an autosomal dominant variant of familial adenomatous polyposis (FAP) syndrome, caused by mutations in the adenomatous polyposis coli (APC) tumor suppressor gene located on chromosome 5q21-22 [1]. Clinically, GS is characterized by numerous adenomatous colorectal polyps demonstrating malignant transformation observed before age 40 in almost all patients with unresected polyps [2].
Additionally, numerous extraintestinal manifestations are encountered in GS and include epidermoid cysts (ECs), osteomas of long and cranial bones, dental abnormalities (odontomas, supernumerary and impacted teeth), congenital hypertrophy of the retinal pigment epithelium and desmoid tumors [1–3]. Less frequently, multiple, familial, cutaneous [4–6] or intraosseous pilomatricomas [7] have been described.
Cooper and Fechner [8] reported that approximately 63 % of ECs associated with GS exhibit pilomatricoma-type ghost cell keratinization. The pilomatricoma-like changes are observed either in form of columns of ghost cells adherent to the cystic epithelial lining and projecting into the lumen, or as scattered intraluminal or mural ghost cell aggregates [8]. Similar findings have been published by Narisawa and Kohda [9], and Urabe et al. [10].
Reports of odontogenic cysts or tumors in patients with GS, besides dentigerous cysts and odontomas, are scarce [11]. A review of the English literature revealed only three cases of odontogenic neoplasms developing in patients with GS, specifically two unicystic ameloblastomas [12, 13] and a case of ameloblastic carcinoma [11].
Herein, we present the histopathologic, immunohistochemical and radiographic characteristics of an odontogenic lesion in a patient with GS characterized by an orthokeratinized odontogenic cyst (OOC), a smaller keratocystic odontogenic tumor (KCOT; odontogenic keratocyst) and calcified ghost cells in the wall.
Case Report
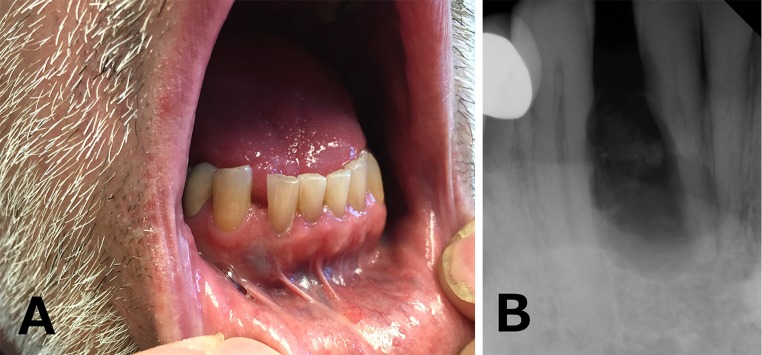
A 62-year-old, Caucasian male presented with a unilocular, generally circumscribed, mixed radiopaque/radiolucent lesion of the right anterior mandible, occupying the space between the right lateral incisor (tooth #26) and right mandibular canine (tooth #27). Clinically, bluish discoloration of the overlying oral mucosa was noticed (Fig. 1a). Radiographically divergence and external root resorption of involved teeth were observed (Fig. 1b). An excisional biopsy was performed. No further information regarding the medical history of the patient was provided at the time of tissue submission.
Fig. 1.
a Bluish discoloration of the overlying oral mucosa between the right lateral incisor (tooth #26) and right mandibular canine (tooth #27). b Periapical radiograph showing a unilocular, generally circumscribed, mixed radiopaque/radiolucent lesion of the right anterior mandible, along with divergence and external root resorption of involved teeth
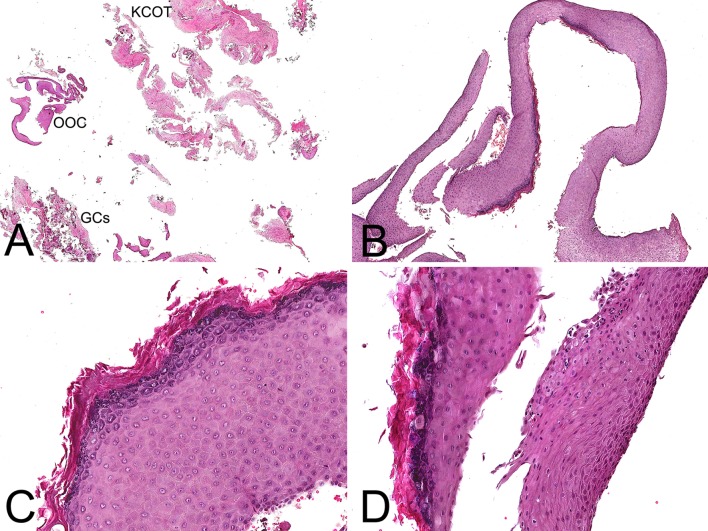
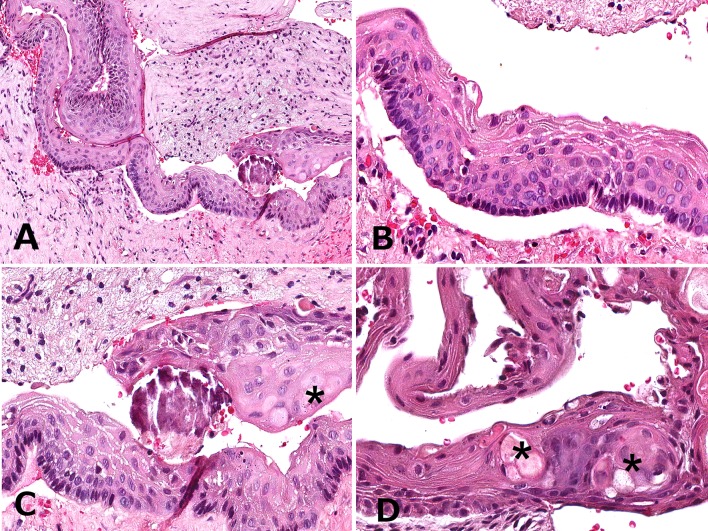
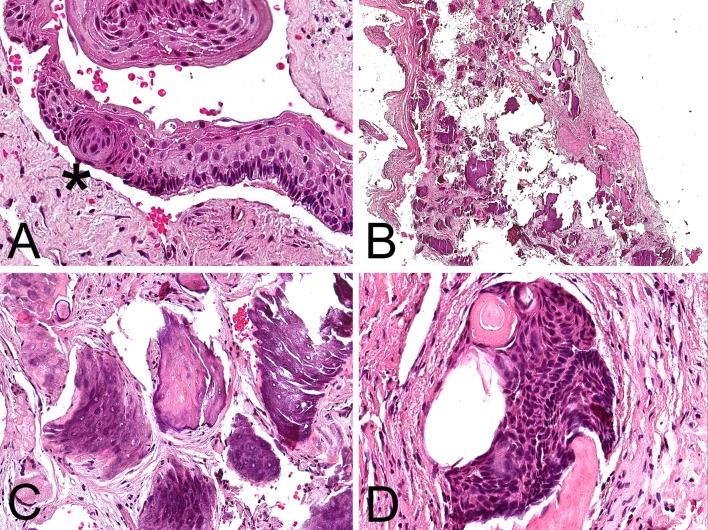
Macroscopically, the specimen consisted of multiple light tan and brown soft tissue fragments measuring 3.7 cm in greatest aggregate dimension. Histopathologic examination of multiple preparations revealed a fragmented lesion featuring cystic lining (Fig. 2a, b) that in areas was partially non-keratinized (Fig. 2b, d), focally parakeratinized and in other areas hyperorthokeratinized (Fig. 2b–d). Another part of this cystic lesion was composed of a smaller cystic cavity (Figs. 2a, 3a) where the epithelial lining was 5–7 cells in thickness exhibiting fine parakeratinization with a slightly corrugated surface and foci of nuclear hyperchromatism associated with palisading in the basal cell layer (Fig. 3b, c). Intraepithelially, large pale epidermoid cells some of which with faint eosinophilic cytoplasm and empty nucleus were noted (Fig. 3c, d), as well as one epithelial sphere or morule-like structure (Fig. 4a). We interpreted the large epidermoid cells as ghost cells. The fibrous connective tissue wall contained aggregates of ghost cells and multiple dystrophic calcifications including psammomatoid spherules (Figs. 2a, 4b, c). The calcified ghost cell deposits seen in the wall were not attached to the epithelial lining and resembled those of cutaneous pilomatricomas (Fig. 4c). A single calcifying cystic odontogenic tumor (CCOT)-like nest was seen in the initial preparation only (Fig. 4d).
Fig. 2.
a Low-magnification photomicrograph revealing a fragmented cystic lining. Labels were added to show the location of the three components of the lesion; OOC orthokeratined odontogenic cyst, KCOT keratocystic odontogenic tumor, GCs ghost cells. b The epithelial lining showed areas of hyperorthokeratinization. c Medium-magnification photomicrograph highlighting the thick, verrucoid, orthokeratin layer as well as the pronounced granular cell layer. d Fragments of the hyperorthokeratinized cystic lining juxtaposed to non-keratinized areas (H&E; original magnification a: ×10, b: ×40, c: ×200, d: ×180)
Fig. 3.
a Low-magnification photomicrograph of the keratocystic odontogenic tumor (KCOT). b High-magnification photomicrograph demonstrating an epithelial lining of 5–7 cells in thickness with fine parakeratinization, a slightly corrugated surface, nuclear hyperchromatism and palisading of the basal cell layer. c and d High-magnification photomicrographs showing the presence of large pale epidermoid cells some of which with faint eosinophilic cytoplasm and empty nucleus (ghost cells) in the KCOT. The location of ghost cells is indicated with asterisks (H&E; original magnification a: ×180, b: ×420, c: ×420, d: ×460)
Fig. 4.
a The epithelial whorl or morule-like structure (asterisk) observed in the KCOT lining. b Low-magnification photomicrograph depicting aggregates of ghost cells and multiple dystrophic calcifications in the fibrous connective tissue wall. c Calcified ghost cell deposits resembling those of cutaneous pilomatricomas. d This singular calcifying cystic odontogenic tumor (CCOT)-like nest was present in the initial preparation only (H&E; original magnification a: ×400, b: ×150, c: ×260, d: ×420)
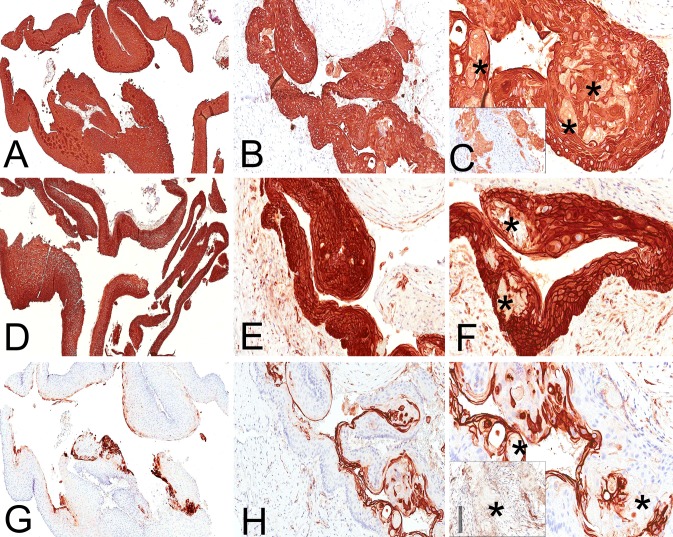
Immunohistochemical evaluation for cytokeratin (AE1/AE3, mouse monoclonal, Dako, Carpinteria, CA) revealed strong and diffuse cytoplasmic immunostaining throughout the OOC and the KCOT epithelia (Fig. 5a, b), as well as the intraepithelial (Fig. 5c) and mural ghost cells (Fig. 5c inset). Investigation of the immunohistochemical expression of β-catenin [beta-catenin (14), mouse monoclonal, Cell Marque, Rocklin, CA)] showed strong and diffuse membranous (submembranous), cytoplasmic and nuclear immunopositivity throughout the OOC (Fig. 5d), as well as the KCOT epithelial linings (Fig. 5e). The intraepithelial ghost cells of the KCOT along with the mural pilomatricoma-like ghost cells did not stain for β-catenin (Fig. 5f). Finally, strong and membranous CD10 (56C6, mouse monoclonal, Carpinteria, CA) immunoreactivity was observed in the keratin and superficial spinous cell layers in both OOC and KCOT (Fig. 5g, h), whereas the ghost cells were uniformly CD10 negative (Fig. 5i, inset). The immunohistochemical findings can be found collectively in Table 1.
Fig. 5.
Immunohistochemical findings. Strong and diffuse immunopositivity for cytokeratin AE1/AE3 throughout the OOC (a) and the KCOT (b), as well as the intraepithelial (c) and mural ghost cells (inset). The asterisks highlight the ghost cells. Strong and diffuse expression of β-catenin in the OOC (d) and KCOT (e). The ghost cells (asterisks) were β-catenin negative (f). CD10 immunoreactivity was seen in the keratin and superficial spinous cell layers in both OOC (g) and KCOT (h). The intraepithelial (i) and mural ghost cells (inset), indicated with asterisks, were uniformly CD10 negative (immunoperoxidase stain; original magnification a: ×50, b: ×150, c: ×300 (inset: ×280), d: ×40, e: ×180, f: ×280, g: ×60, h: ×160, i: ×280 (inset: ×260)
Table 1.
Collective presentation of the immunohistochemical features of the current odontogenic lesion associated with Gardner syndrome (GS)
| Antibodies | Orthokeratinized odontogenic cyct (OOC) | Keratocystic odontogenic tumor (KCOT) | Ghost cells (GCs) |
|---|---|---|---|
| Keratin AE1/AE3 | Strongly positive in all epithelial layers | Strongly positive in all epithelial layers | Positive in both intraepithelial and mural GCs |
| β-catenin | Strongly positive in all epithelial layers | Strongly positive in all epithelial layers | Negative in both intraepithelial and mural GCs |
| CD10 | Strongly positive in keratin and superficial spinous cell layers | Strongly positive in keratin and superficial spinous cell layersa | Negative in both intraepithelial and mural GCs |
aThe epithelial sphere or morule-like structure seen in the KCOT lining was not present in the tissue section stained for CD10
The contributor was contacted and a panoramic radiograph taken shortly before the biopsy was obtained (Fig. 6), along with two full-mouth sets of periapical radiographs taken in 2009 and 2014 (Fig. 1b represents a portion of the full-mouth set of periapical radiographs taken in 2014), respectively. A comparison of the periapical radiographs revealed a substantial increase in the size of the cystic lesion during the 5-year period. Interestingly, evaluation of the panoramic X-ray revealed the presence of multiple circumscribed radiopacities consistent with osteomas (Fig. 6). This finding, raised the clinical suspicion of GS which was subsequently confirmed by the patient.
Fig. 6.
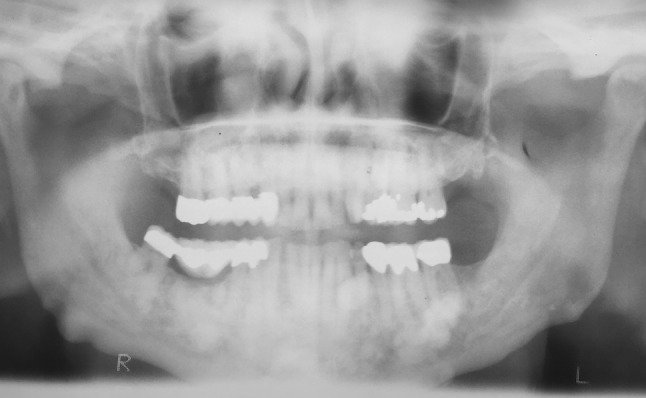
Panoramic X-ray revealing the presence of multiple circumscribed radiopacities consistent with osteomas
Based on the microscopic and radiographic findings the descriptive diagnosis of odontogenic cystic lesion with combined features of OOC and KCOT with ghost cell keratinization and calcifications was rendered. The patient has not answered the surgeon’s multiple requests for follow-up.
Discussion
GS is caused by inactivating mutations in the APC, a tumor suppressor gene located on chromosome 5q21-22. APC encodes for the approximately 300 kD APC protein involved in cell proliferation, migration, adhesion and cytoskeletal stabilization [2]. APC functions in the canonical (β-catenin-dependent) Wnt signaling pathway and regulates the cytoplasmic pool of β-catenin protein [14, 15].
In the absence of an activating Wnt ligand signal, cytoplasmic β-catenin is phosphorylated at the conserved serine/threonine residues of the amino (N)-terminal region by the destruction complex composed primarily by APC, AXIN, casein kinase I, and glycogen synthase kinase-3β factors. The N-terminal phosphorylation leads β-catenin to ubiquitination and proteasomal degradation [16]. When activating Wnt ligands are present, they bind to the frizzled and LRP5/6 (low density lipoprotein-related proteins 5 and 6) cognate receptor complex located at the cell surface resulting in inhibition of β-catenin phosphorylation and/or ubiquitination by the destruction complex [15, 17]. Consequently, destruction of the free pool of β-catenin is abrogated, active β-catenin accumulates in the cytoplasm and translocates in the nucleus, where it complexes with the DNA binding proteins of the TCF (T cell factor family)/Lef (lymphoid enhancer family) family, functioning as a transcriptional co-activator [18, 19]. The TCF/β-catenin complex regulates the transcriptional activation of various target genes including cell proliferation- and growth-associated proto-oncogenes such as CMYC and CCND1 (cyclin D1) [10, 14].
Pilomatricoma represents a benign neoplasm of trichilemmal differentiation occurring primarily in young patients, although middle-aged adults may be affected as well [20–22]. The most common site of involvement is the head and neck [7, 21, 22]. Multiple pilomatricomas can be associated with myotonic dystrophy, GS, or Turner and Rubinstein-Taybi syndromes [4–6, 23–25]. At the molecular level, pilomatricomas demonstrate activating mutations of CTNNB1 (β-catenin gene) [26, 27]. Point mutations in exon 3 encoding for the N-terminal serine/threonine phosphorylation region result in production of oncogenic β-catenin proteins that are resistant to phosphorylation/degradation by the destruction complex and which activate β-catenin/TCF transcription [17, 26, 28]. Hence, regardless the mutation locus—APC for GS or CTNNB1 for pilomatricoma—both pathologic entities exhibit activation of the Wnt/β-catenin signaling pathway leading to aberant cellular proliferation, differentiation and survival by inhibition of programmed cell death.
ECs are observed in 65 % of patients with GS [1, 3] and frequently precede the intestinal polyps [29]. Cooper and Fechner [8] have reported that 36/57 (63 %) ECs in patients with GS showed extensive pilomatricoma-like changes in the form of columns of ghost cells (21/57, 37 %), scattered intraluminal masses of ghost cells (29/57, 51 %), or pericystic (mural) ghost cells aggregates (20/57, 35 %) [8]. A multi- layered, transitional zone of basophilic epithelial cells was present at the base of the columnar aggregates of ghost cells [8]. This basophilic/transitional cell zone was not observed in the ghost cell deposits scattered within the connective tissue wall of our odontogenic lesion. Calcifications were seen in 8/20 (40 %) of ECs with mural pilomatricoma-like changes but in none of the cases with luminal deposits of ghost cells [8]. Interestingly, in the study by Cooper and Fechner [8] the pericystic deposits of shadow cells were invariably associated with a giant cell reaction of foreign body type. A giant cell reaction was not seen in the examined preparations of the current case. Pilomatricoma-like characteristics in ECs in GS have also been reported by Narisawa and Kohda [9] and Urabe et al. [10].
A compressed and apparently KCOT-like in appearance cystic area was identified in the fibrous connective tissue wall. The presence of intraepithelial ghost cells in KCOTs is unusual. To the best of our knowledge after thorough review of the literature we could not find a well-documented reference of combined OOC and KCOT. In the current case, the two cystic cavities were separate with no continuity of their epithelial linings. Previously, de Fátima Bernardes et al. [30] described a unique case of CCOT associated with OOC in the mandible of a 24-year-old woman, characterized by a singular epithelial lining showing transition from CCOT to OOC. In our case only an isolated odontogenic nest with CCOT-like features and presence of ghost cells and calcification was identified. This finding was seen in the initial preparation only and, therefore, was not considered frank evidence of CCOT.
The KCOT also featured an intraepithelial, squamoid morule-like formation. This kind of epithelial sphere or whorl can be observed in a wide variety of odontogenic cysts including lateral periodontal, botryoid, glandular, adult gingival as well as dentigerous cysts [31]. Alternatively, histologically similar morular structures have been reported in the cribriform-morular variant of papillary thyroid carcinoma (CMVPTC) [32]. Although sporadic examples of CMVPTC have been reported, this rare histopathologic subtype is strongly associated with FAP syndrome [33, 34]. Specifically, 1–2 % of the patients with FAP develop papillary thyroid carcinoma and 20–40 % of these tumors are diagnosed as CMVPTC [35, 36]. The presence of a cribriform-morular architectural pattern in thyroid cancer has been considered an indicator for occult FAP necessitating appropriate patient evaluation [34, 37]. It appears possible that germline or acquired mutations in genes of the Wnt/β-catenin pathway, such as APC, may correlate with a morular phenotype in a variety of benign or malignant neoplasms arising in the thyroid gland or odontogenic tissues. Immunohistochemically, the morules demonstrate positivity for β-catenin, cyclin D1, bcl-2, PTEN and CD10 but are consistently negative for thyroglobulin, estrogen and progesterone receptors and CK19 [32, 33, 38]. We attempted to further characterize the CD10 immunoprofile of the whorl or morule-like structure seen in the KCOT, however this spherical formation was not present in the tissue section stained for CD10.
Dental anomalies, odontomas, supernumerary and impacted teeth, are frequently encountered in GS [2, 3]. With the exception of dentigerous cysts, other types of odontogenic cysts and neoplasms are scarce in patients with GS [11]. Patel and Rees [12] and Bates et al. [13] presented one case each of unicystic ameloblastoma affecting a 14-year-old male and a 15-year-old female patient, respectively. One should note that the report by Patel and Rees [12] does not include illustrations of the histopathology of the lesion. In both cases the clinical and radiological features were consistent with a dentigerous cyst [12, 13]. Fitzpatrick et al. [11] reported of a tumor combining, predominantly, characteristics of ameloblastic carcinoma and ghost cell odontogenic carcinoma in a 37-year-old male African American. The patient exhibited numerous dental abnormalities in addition to osteomas of the mandible, therefore, GS was strongly suspected but could not be confirmed [11]. A collective presentation of the clinico-pathologic characteristics of previously reported cases of odontogenic neoplasms associated with GS is provided in Table 2.
Table 2.
Collective presentation of the clinico-pathologic characteristics of the reported cases of odontogenic neoplasms associated with Gardner syndrome (GS)
| Authors | Age | Gender | Diagnosis of GS | Clinical features/location | Radiographic features | Histopathologic diagnosis | Treatment/follow-up |
|---|---|---|---|---|---|---|---|
| Patel and Rees [12] | 14 | Male | Confirmed | Slowly enlarging swelling of the right posterior mandible | Radiolucency extending from right mandibular 2nd permanent premolar to right 1st permanent molar | Unicystic ameloblastoma | Enucleation with extraction of involved teeth/No recurrence after 18 months |
| Bates et al. [13] | 15 | Female | Confirmed | Right posterior mandible | Unilocular radiolucency centred on the crown of the unerupted right 2nd permanent molar causing displacement of the tooth towards the lower border of the mandible | Unicystic ameloblastoma | Enucleation with extraction of involved tooth/No follow-up |
| Fitzpatrick et al. [11] | 37 | Male | GS was strongly suspected but not confirmed | Slowly growing ulcerated mass of the right anterior maxilla | Multilocular mixed radiolucency extending from impacted 2nd right premolar to impacted left canine causing perforation of the buccal and lingual cortices as well as invasion of the maxillary sinus | Ameloblastic carcinoma with features of ghost cell odontogenic carcinoma | Total excision along with associated teeth via hemi-maxillectomy/Uneventful healing after 2-weeks |
| Current case | 62 | Male | Confirmed | Slowly increasing lesion of the right anterior mandible along with bluish discoloration of the overlying oral mucosa | Unilocular mixed radiolucency occupying the space between the right lateral incisor and right mandibular canine causing divergence and external root resorption of involved teeth | Orthokeratinized odontogenic cyst with an associated keratocystic odontogenic tumor and ghost cell calcifications | Enucleation/No follow-up |
We investigated the AE1/AE3 and β-catenin immunohistochemical profile of the lesions and observed that both KCOT and OOC cyst linings showed strong and diffuse cytoplasmic, as well as membranous, cytoplasmic and nuclear immunopositivity for AE1/AE3 and β-catenin, respectively, throughout the thickness of the epithelium. Leonardi et al. [39] reported that sporadic primary KCOTs showed weaker staining for β-catenin, confined to the basal and para-basal layers only, compared to sporadic recurrent and nevoid basal cell carcinoma syndrome (NBCCS)-associated KCOTs, which showed expression throughout all epithelial layers. Interestingly, nuclear expression of β-catenin was found exclusively in recurrent and NBCCS-associated KCOTs [39]. Contradictory results were published by Hakim et al. [40] showing significantly higher loss of β-catenin in syndromic than in sporadic KCOTs. Furthermore, β-catenin nuclear, cytoplasmic and membranous expression has been documented in basaloid/basal cells of pilomatricoma, as well as transitional and basal cells of craniopharyngioma and CCOT [41]. In our case, both intraepithelial and mural ghost cells demonstrated a AE1/AE3 positive, β-catenin negative immunophenotype. Similar findings were reported by Rumayor et al. [41] and Hassanein et al. [42]. Bilodeau et al. [43] identified coexpression of LEF-1, a nuclear transcription factor of the Wnt signaling pathway, and nuclear β-catenin in all LEF-1 positive CCOTs.
In conclusion, we report the clinicopathologic characteristics of an unusual odontogenic lesion in a patient with GS featuring combined OOC and KCOT characteristics and ghost cell keratinization and calcifications in the wall. The current case highlights the diverse histomorphodifferentiation potential of the odontogenic epithelium especially under the constant influence of a proliferation trigger such as the Wnt/β-catenin oncogenic signal. Although we cannot definitely exclude a coincidental co-appearance of ghost cell calcifications in an odontogenic lesion with combined OOC and KCOT features in this patient with GS, molecular commonalities may be a plausible explanation of the overlapping histopathologic features between cutaneous pilomatricomas and syndromic epidermoid and odontogenic cysts.
Acknowledgments
The authors are grateful to Mr. Brian Dunnette (University of Minnesota) for his assistance with the illustrations.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Juhn E, Khachemoune A. Gardner syndrome: skin manifestations, differential diagnosis and management. Am J Clin Dermatol. 2010;11:117–122. doi: 10.2165/11311180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Wijn MA, Keller JJ, Giardiello FM, Brand HS. Oral and maxillofacial manifestations of familial adenomatous polyposis. Oral Dis. 2007;13:360–365. doi: 10.1111/j.1601-0825.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- 3.Herford AS, Stoffella E, Tandon R. Osteomas involving the facial skeleton: a report of 2 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e1–e6. doi: 10.1016/j.oooo.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Pujol RM, Casanova JM, Egido R, Pujol J, de Moragas JM. Multiple familial pilomatricomas: a cutaneous marker for Gardner syndrome? Pediatr Dermatol. 1995;12:331–335. doi: 10.1111/j.1525-1470.1995.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 5.Trufant J, Kurz W, Frankel A, Muthusamy V, McKinnon W, Greenblatt M, Lazar A, Cook D, Bosenberg M. Familial multiple pilomatrixomas as a presentation of attenuated adenomatosis polyposis coli. J Cutan Pathol. 2012;39:440–443. doi: 10.1111/j.1600-0560.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- 6.Kraft S, Granter SR. Molecular pathology of skin neoplasms of the head and neck. Arch Pathol Lab Med. 2014;138:759–787. doi: 10.5858/arpa.2013-0157-RA. [DOI] [PubMed] [Google Scholar]
- 7.Ishida T, Abe S, Miki Y, Imamura T. Intraosseous pilomatricoma: a possible rare skeletal manifestation of Gardner syndrome. Skeletal Radiol. 2007;36:693–698. doi: 10.1007/s00256-006-0264-4. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PH, Fechner RE. Pilomatricoma-like changes in the epidermal cysts of Gardner’s syndrome. J Am Acad Dermatol. 1983;8:639–644. doi: 10.1016/S0190-9622(83)70071-X. [DOI] [PubMed] [Google Scholar]
- 9.Narisawa Y, Kohda H. Cutaneous cysts of Gardner’s syndrome are similar to follicular stem cells. J Cutan Pathol. 1995;22:115–121. doi: 10.1111/j.1600-0560.1995.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 10.Urabe K, Xia J, Masuda T, Moroi Y, Furue M, Matsumoto T. Pilomatricoma-like changes in the epidermoid cysts of Gardner syndrome with an APC gene mutation. J Dermatol. 2004;31:255–257. doi: 10.1111/j.1346-8138.2004.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick SG, Hirsch SA, Listinsky CM, Lyu DJ, Baur DA. Ameloblastic carcinoma with features of ghost cell odontogenic carcinoma in a patient with suspected Gardner syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:e241–e245. doi: 10.1016/j.oooo.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Patel H, Rees RT. Unicystic ameloblastoma presenting in Gardner’s syndrome: a case report. Br Dent J. 2005;198:747–748. doi: 10.1038/sj.bdj.4812413. [DOI] [PubMed] [Google Scholar]
- 13.Bates T, Keith D, Sloan P. Unicystic ameloblastoma presenting in Gardner’s syndrome. Head Neck Pathol. 2014;8:239–240. doi: 10.1007/s12105-013-0484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Sakamoto N, Wu R, Liu JY, Wiese A, Green ME, Green M, Akyol A, Roy BC, Zhai Y, Cho KR, Fearon ER. Tissue-specific effects of reduced β-catenin expression on adenomatous polyposis coli mutation-instigated tumorigenesis in mouse colon and ovarian epithelium. PLoS Genet. 2015;11:e1005638. doi: 10.1371/journal.pgen.1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 16.Brocardo M, Henderson BR. APC shuttling to the membrane, nucleus and beyond. Trends Cell Biol. 2008;18:587–596. doi: 10.1016/j.tcb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Mosimann C, Hausmann G, Basler K. β-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 19.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 20.Julian CG, Bowers PW. A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998;39:191–195. doi: 10.1016/S0190-9622(98)70073-8. [DOI] [PubMed] [Google Scholar]
- 21.Lan MY, Lan MC, Ho CY, Li WY, Lin CZ. Pilomatricoma of the head and neck: a retrospective review of 179 cases. Arch Otolaryngol Head Neck Surg. 2003;129:1327–1330. doi: 10.1001/archotol.129.12.1327. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor N, Patel M, Umar T, Macpherson DW, Ethunandan M. Head and neck pilomatricoma: an analysis of 201 cases. Br J Oral Maxillofac Surg. 2011;49:354–358. doi: 10.1016/j.bjoms.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Berberian BJ, Colonna TM, Battaglia M, Sulica VI. Multiple pilomatricomas in association with myotonic dystrophy and a family history of melanoma. J Am Acad Dermatol. 1997;37:268–269. doi: 10.1016/S0190-9622(97)80138-7. [DOI] [PubMed] [Google Scholar]
- 24.Cambiaghi S, Ermacora E, Brusasco A, Canzi L, Caputo R. Multiple pilomatricomas in Rubinstein–Taybi syndrome: a case report. Pediatr Dermatol. 1994;11:21–25. doi: 10.1111/j.1525-1470.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 25.Wood S, Nguyen D, Hutton K, Dickson W. Pilomatricomas in Turner syndrome. Pediatr Dermatol. 2008;25:449–451. doi: 10.1111/j.1525-1470.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 27.Kajino Y, Yamaguchi A, Hashimoto N, Matsuura A, Sato N, Kikuchi K. β-catenin gene mutation in human hair follicle-related tumors. Pathol Int. 2001;51:543–548. doi: 10.1046/j.1440-1827.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 28.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 29.Tsao H. Update on familial cancer syndromes and the skin. J Am Acad Dermatol. 2000;42:939–969. doi: 10.1067/mjd.2000.104681. [DOI] [PubMed] [Google Scholar]
- 30.de Fátima Bernardes V, de Lacerda JC, de Aguiar MC, Gomez RS. Calcifying odontogenic cyst associated with an orthokeratinized odontogenic cyst. Head Neck Pathol. 2008;2:324–327. doi: 10.1007/s12105-008-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowler CB, Brannon RB, Kessler HP, Castle JT, Kahn MA. Glandular odontogenic cyst: analysis of 46 cases with special emphasis on microscopic criteria for diagnosis. Head Neck Pathol. 2011;5:364–375. doi: 10.1007/s12105-011-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon MJ, Rho YS, Jeong JC, Shin HS, Lee JS, Cho SJ, Nam ES. Cribriform-morular variant of papillary thyroid carcinoma: a study of 3 cases featuring the PIK3CA mutation. Hum Pathol. 2015;46:1180–1188. doi: 10.1016/j.humpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Xu B, Yoshimoto K, Miyauchi A, Kuma S, Mizusawa N, Hirokawa M, Sano T. Cribriform-morular variant of papillary thyroid carcinoma: a pathological and molecular genetic study with evidence of frequent somatic mutations in exon 3 of the β-catenin gene. J Pathol. 2003;199:58–67. doi: 10.1002/path.1225. [DOI] [PubMed] [Google Scholar]
- 34.Levy RA, Hui VW, Sood R, Fish S, Markowitz AJ, Wong RJ, Guillem JG. Cribriform-morular variant of papillary thyroid carcinoma: an indication to screen for occult FAP. Fam Cancer. 2014;13:547–551. doi: 10.1007/s10689-014-9732-5. [DOI] [PubMed] [Google Scholar]
- 35.Donnellan KA, Bigler SA, Wein RO. Papillary thyroid carcinoma and familial adenomatous polyposis of the colon. Am J Otolaryngol. 2009;30:58–60. doi: 10.1016/j.amjoto.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Cameselle-Teijeiro J, Menasce LP, Yap BK, Colaco RJ, Castro P, Celestino R, Ruíz-Ponte C, Soares P, Sobrinho-Simões M. Cribriform-morular variant of papillary thyroid carcinoma: molecular characterization of a case with neuroendocrine differentiation and aggressive behavior. Am J Clin Pathol. 2009;131:134–142. doi: 10.1309/AJCP7ULS0VSISBEB. [DOI] [PubMed] [Google Scholar]
- 37.Liyanapathirana N, Seneviratne SA, Samarasekera DN. A distinct variant of papillary thyroid carcinoma indicating familial adenomatous polyposis (FAP): a case report and brief review. BMC Res Notes. 2015;8:795. doi: 10.1186/s13104-015-1736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakazawa T, Celestino R, Machado JC, Cameselle-Teijeiro JM, Vinagre J, Eloy C, Benserai F, Lameche S, Soares P, Sobrinho-Simões M. Cribriform-morular variant of papillary thyroid carcinoma displaying poorly differentiated features. Int J Surg Pathol. 2013;21:379–389. doi: 10.1177/1066896912473355. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi R, Matthews JB, Loreto C, Musumeci G, Campisi G, Lo Muzio L, dos Santos JN, Pastorino L, Bufo P, Pannone G. β-catenin and survivin expression in keratocystic odontogenic tumor (KCOT). A comparative immunohistochemical study in primary, recurrent and nevoid basal cell carcinoma syndrome (NBCCS)-associated lesions. Histol Histopathol. 2013;28:1175–1184. doi: 10.14670/HH-28.1175. [DOI] [PubMed] [Google Scholar]
- 40.Hakim SG, Kosmehl H, Sieg P, Trenkle T, Jacobsen HC, Attila Benedek G, Ribbat J, Driemel O. Altered expression of cell–cell adhesion molecules β-catenin/E-cadherin and related Wnt-signaling pathway in sporadic and syndromal keratocystic odontogenic tumors. Clin Oral Investig. 2011;15:321–328. doi: 10.1007/s00784-010-0388-8. [DOI] [PubMed] [Google Scholar]
- 41.Rumayor A, Carlos R, Kirsch HM, de Andrade BA, Romañach MJ, de Almeida OP. Ghost cells in pilomatrixoma, craniopharyngioma, and calcifying cystic odontogenic tumor: histological, immunohistochemical, and ultrastructural study. J Oral Pathol Med. 2015;44:284–290. doi: 10.1111/jop.12234. [DOI] [PubMed] [Google Scholar]
- 42.Hassanein AM, Glanz SM, Kessler HP, Eskin TA, Liu C. β-catenin is expressed aberrantly in tumors expressing shadow cells. Pilomatricoma, craniopharyngioma, and calcifying odontogenic cyst. Am J Clin Pathol. 2003;120:732–736. doi: 10.1309/EALEG7LD6W7167PX. [DOI] [PubMed] [Google Scholar]
- 43.Bilodeau EA, Acquafondata M, Barnes EL, Seethala RR. A comparative analysis of LEF-1 in odontogenic and salivary tumors. Hum Pathol. 2015;46:255–259. doi: 10.1016/j.humpath.2014.10.018. [DOI] [PubMed] [Google Scholar]