Abstract
Anaplastic transformation of papillary thyroid carcinoma (PTC) at distant metastatic sites is extremely rare, and there have been fewer than 20 reported cases in the literature. A 61-year-old woman presented with 1-week history of dyspnea. Her past medical history was remarkable because, 19 years ago, she underwent nearly total thyroidectomy and radical neck dissection due to PTC. Computed tomography of the chest revealed a 1.7 cm nodule in the lung and diffuse pleural thickening. Gun biopsy of the lung nodule revealed metastatic PTC with typical histology. However, the pleural biopsy predominantly showed anaplastic pleomorphic and spindle sarcomatoid carcinoma with microscopic focus of PTC. Immunohistochemical results showed both anaplastic sarcomatoid and PTC components positive for TTF-1, galectin-3 and PAX-8, thus supporting anaplastic transformation of PTC at the metastatic site. Subsequently the patient received 1 cycle of cisplatin-based chemotherapy but died from the disease 4 months after diagnosis. Although it is rare, anaplastic transformation of PTC should be considered during differential diagnosis of patients who present with exclusive sarcomatoid morphology at metastatic sites and have a history of PTC. We report another case of anaplastic transformation of PTC, found at pleural metastasis, together with the immunohistochemical profile and a literature review.
Keywords: Papillary thyroid carcinoma (PTC), Anaplastic transformation, Pleural metastasis, Anaplastic thyroid carcinoma (ATC)
Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy with a relatively favorable prognosis [1]. Many studies support the concept that anaplastic thyroid carcinoma arises from pre-existing differentiated thyroid carcinomas, such as papillary or follicular carcinoma of the thyroid [2]. Although anaplastic transformation rarely occurs, anaplastic carcinoma is one of the most aggressive cancers, and is responsible for more than half of the deaths attributed to thyroid cancers, with a mean survival time of 6 months after diagnosis [3]. Anaplastic transformation commonly occurs in the thyroid and metastatic regional lymph nodes, whereas occurrence at distant metastatic sites is extremely rare [4]. Among 677 cases of anaplastic thyroid carcinoma (ATC), collected from 38 registered institutions by the ATC Research Consortium of Japan (ATCCJ), only 6 cases showed anaplastic transformation at the metastatic sites (0.9 %), and these had the worst outcomes among all ATC cases [4].
To date, fewer than 20 cases of anaplastic transformation at the metastatic sites have been reported in the English literature [1, 4–11]. Here, we report an additional case of anaplastic transformation which developed in the pleural metastasis, combined with a literature review.
Clinical Summary
A 61-year-old Korean woman presented with a 1-week history of dyspnea. Her past medical history was remarkable as 19 years ago, she underwent nearly total thyroidectomy with preservation of about 1.5 g of the right thyroid lobe and radical neck dissection due to PTC. The tumor showed classic PTC morphology with extrathyroidal extension and 5 of 10 level VI lymph node metastases (pT3N1aM0; stage III). No anaplastic carcinoma in the thyroid or metastases in the lymph nodes were found during initial diagnosis. The patient did not consent to additional treatment including radioactive iodine therapy.
The patient had not been admitted to the hospital since the thyroidectomy. A CT scan of the chest revealed a 1.7 cm nodule in the upper lobe of the right lung and diffuse pleural thickening with pleural effusion (Fig. 1a) without a residual mass lesion in the neck. Whole body PET-CT revealed a hypermetabolic nodule in the upper lobe of the right lung and hypermetabolic confluent masses in the right pleura, and mild fludeoxyglucose (FDG) uptake in cervical lymph nodes. No other abnormalities were identified. The Chest CT and whole body PET-CT findings suggested either a primary lung malignancy with pleural seeding or a metastatic PTC to the lung and pleura.
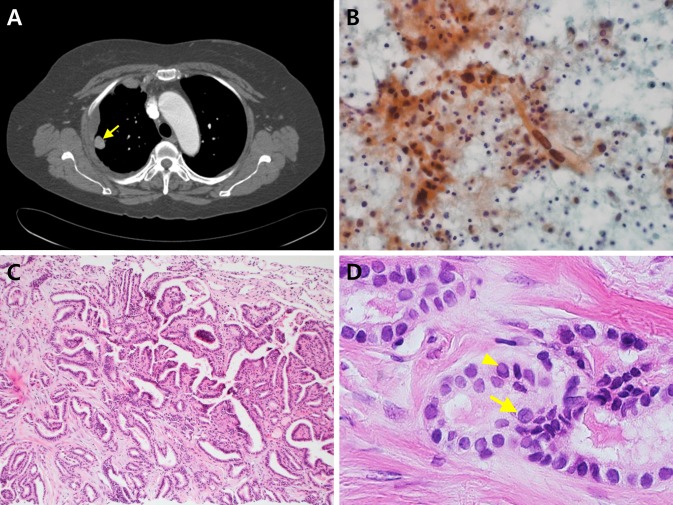
Fig. 1.
a Computed tomographic scan revealed a 1.7 cm nodule in the right upper lobe of the lung (arrow) with diffuse pleural thickening. b Pleural fluid cytology showed scattered pleomorphic spindle cells in the background of inflammatory and mesothelial cells (Pap, ×400). c Lung biopsy showed typical histological features of PTC with mixed papillary and microfollicular patterns (H–E, ×100). d The nuclear features of the tumor revealed ground-glass nuclei, nuclear grooves (arrow) and intranuclear pseudoinclusions (arrow head) (H–E, ×400)
The lung biopsy revealed metastatic PTC, but the pleural biopsy showed predominantly sarcomatoid anaplastic carcinoma with microscopic focus of PTC. Subsequently, the patient received 1 cycle of chemotherapy with pemetrexed and cisplatin, but died of metastatic ATC in 4 months after diagnosis.
Pathological Findings
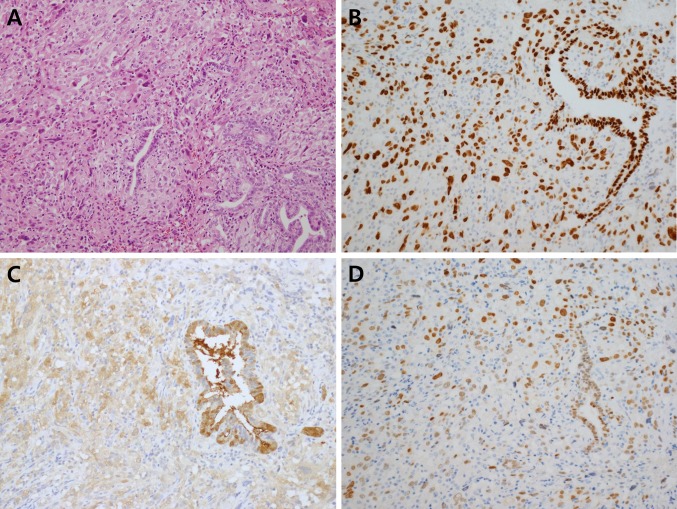
The pleural fluid cytology revealed atypical pleomorphic and spindle cells (Fig. 1b). Gun biopsy of the lung mass was performed and revealed metastatic PTC with classic histology (Fig. 1c, d). It showed mixed papillary and microfollicular growth patterns with typical PTC nuclear features, including ground-glass nuclei, nuclear grooves, and intranuclear pseudoinclusions. However, the pleural biopsy disclosed different histology from the lung biopsy, which revealed almost exclusively anaplastic cells. The tumor cells were arranged in sheets or fascicular patterns and showed marked pleomorphism with spindle and giant cell morphology, bizarre nuclei, large prominent nucleoli and brisk mitoses with atypical forms (>10 mitoses/10 high power fields). Hemorrhage, necrosis and invasion into skeletal muscle were also seen. Multiple deeper levels disclosed focal PTC component only with an abrupt transition between spindle sarcomatoid carcinoma component and differentiated PTC, strongly supporting anaplastic transformation at the metastatic site (Fig. 2a). Immunohistochemical testing for both PTC and anaplastic carcinoma was positive for transcription factor 1 (TTF-1), galectin-3, and PAX-8 (Fig. 2b–d, respectively); thyroglobulin was positive for PTC only. TTF1 and PAX8 stained positive in the nucleus only, galectin-3 tested positive in both the nucleus and cytoplasm, and thyroglobulin tested positive in the cytoplasm only. The BRAF (V600E) mutation, determined by real-time PCR, was found in both PTC and anaplastic carcinoma components.
Fig. 2.
a Pleural biopsy discloses transition between papillary thyroid carcinoma and anaplastic sarcomatoid carcinoma component (H–E, ×200). Both papillary thyroid carcinoma and anaplastic carcinoma were positive for TTF-1 (b), galectin-3 (c) and PAX-8 (d). TTF1 and PAX8 staining were positive in the nucleus and galectin-3 staining was positive in both the nucleus and cytoplasm
Discussion
Thyroid cancer is the 5th most common cancer in females (49,350 new cases reported in 2016) [12] and the most common malignant tumor of endocrine organs [13]. Despite its continuous rise worldwide, mortality due to thyroid cancer is low and stable at approximately 0.5 deaths per 100,000 persons [14]. PTC is the most common subtype of thyroid cancer [15]. Metastases occur mainly to the regional lymph nodes, and are unusual beyond the neck [15]. Anaplastic transformation of PTC is very rare, and shows extremely aggressive behavior [1]. Accumulating clinical, pathological, and molecular evidence supports the concept that ATC arises from pre-existing differentiated thyroid carcinoma including papillary, follicular and Hurthle cell thyroid carcinomas [2]. PTC is the most common subtype associated with anaplastic transformation [1].
Anaplastic transformation mainly occurs in the thyroid or regional cervical lymph nodes [1]. There are fewer than 20 reported cases presenting anaplastic transformation at distant metastatic sites, and they are summarized in Table 1. All are single reports except for one multi-institutional collection of cases. Sugitani [4] reported 6 out of 677 ATC cases exhibiting anaplastic transformation at metastatic sites (incidence 0.9 %). However, no detailed demographic data are available for these reported cases. The groups of Sotome et al. [6] and Solomon et al. [7] individually reported a single case of anaplastic transformation developed within the metastatic site in the retroperitoneal region. Takeshita et al. [8] described anaplastic transformation of p53-positive papillary thyroid carcinoma, in the liver metastasis, following postoperative radioactive iodine therapy. The authors suggested that radioactive iodine therapy could play a role in anaplastic transformation.
Table 1.
Anaplastic transformation of papillary thyroid carcinoma at distant metastatic sites
| Authors | Sex/age | Site of anaplastic transformation | Duration between primary diagnosis and anaplastic transformation | Histopathological features of anaplastic carcinoma | Follow up |
|---|---|---|---|---|---|
| Sotome et al. [6] | F/82 | Retroperitoneum | 17 | Spindle cell-type anaplastic carcinoma | Died |
| Takeshita et al. [8] | F/81 | Liver | 5 | Anaplastic carcinoma | NA |
| Angeles et al. [9] | F/58 | Breast | 20 | Spindle and giant cell anaplastic carcinoma with rhabdoid inclusions | NA |
| Al-Qsous et al. [5] | M/83 | Lung | 10 | Undifferentiated carcinoma | Died |
| Kaushal et al. [10] | F/52 | Shoulder | 8 | Spindle cell-type anaplastic carcinoma | Died 2 months after Dx |
| Nakayama et al. [11] | F/55 | Pelvis | 12 | Anaplastic carcinoma | Died 2 months after Dx |
| Sugitani et al. [4] | 6 out of 677 cases | NA | NA | NA | 48 days (median survival) |
| Abe et al. [1] | M/61 | Lung | 10 | Pleomorphic and undifferentiated anaplastic carcinoma | Died 6 months after Dx |
| Solomon et al. [7] | M/64 | Retroperitoneum | 30 | Pleomorphic cells with rhabdoid features | 3 weeks after Dx |
| Present case | F/61 | Lung and pleura | 19 | Anaplastic carcinoma with pleomorphic and spindle cell type | Died 4 months after Dx |
NA not available, Dx diagnosis
In the case we report, the patient underwent surgery only (she didn’t receive additional treatment including radioactive iodine therapy). Angeles et al. [9] reported that anaplastic transformation in the breast metastasis occurred 20 years after surgically-treated primary PTC. Autopsies were performed in the cases reported by Al-Qsous et al. [1] and Abe et al. [5]. Both cases presented anaplastic transformation in addition to PTC in the lung metastases. Malignant pleural effusion was developed in the case reported by Abe et al. [5]. However, the case reported by Al-Qsous et al. [1] revealed benign mesothelial cells with no evidence of malignancy in the pleural fluid cytology. Kaushal et al. [10] reported that anaplastic transformation developed in the shoulder 8 years after diagnosis of PTC, and, clinically and pathologically, it mimicked soft tissue sarcoma. Nakayama et al. [11] reported anaplastic transformation of follicular carcinoma in pelvic metastasis 12 years after initial diagnosis. The ATCCJ was established to compile data and analyze treatment outcomes of ATC cases from Japan [4]. Analysis of the ATCCJ cohort study data showed that anaplastic transformation at a distant metastatic site had the worst prognosis (median survival: 48 days) compared to anaplastic transformation in primary or regional lymph node metastatic sites [4]. Cause-specific survival rates and treatment outcomes differed significantly depending on UICC staging. The authors concluded that UICC staging and prognostic parameters such as the site of anaplastic transformation should be considered to determine treatment strategy [4].
Six females and three males exhibited anaplastic transformation of PTC among fewer than 20 reported cases including the current one (data not available for the 6 cases recorded in the ATCCJ cohort study). Ages ranged between 52 and 83 years with a mean of 68 years, and the time interval between primary diagnosis and anaplastic transformation at metastatic sites ranged from 5 to 30 years. Metastases developed at lung/pleura, liver, retroperitoneum, breast, shoulder and pelvis. Our case and others indicated that anaplastic transformation rarely occurs at metastatic sites. Also metastases developed in all the patients after extended periods, therefore long-term follow up is recommended for patients with PTC. Seven patients were available for follow up and all died from the disease. The six cases recorded by ATCCJ also showed a dismal prognosis with a median survival of 48 days only.
There are significant difficulties in distinguishing ATC from other malignant neoplasms at metastatic sites due to partial or complete loss of morphologic and immunohistochemical differentiation, and a high degree of morphological overlap with other anaplastic sarcomatoid malignant neoplasms [16]. Presence of differentiated thyroid carcinoma component or demonstration of the thyroid specific markers, including positive testing for thyroglobulin and TTF-1, supports diagnosis of ATC. Because ATC cells usually are not immunoreactive for thyroglobulin or TTF-1 [17], ATC diagnosis can be difficult in the absence of PTC component. Whereas PAX-8, a transcription factor seems to have a useful diagnostic role in this setting, having been found immunoreactivity in 79 % of ATC cells [18]. In the present case, there was transition between differentiated and anaplastic tumor components and anaplastic tumor component lost thyroglobulin immunoreactivity, but retained positivity for TTF1, galectin-3 and PAX-8, supporting anaplastic transformation of pre-existing PTC at the metastatic site. Our case also illustrated that PAX-8 immunostaining can be useful in confirming the anaplastic sarcomatoid component of PTC.
BRAF mutation is the most common one found during molecular evaluation of PTC [16]. Several gene mutations including BRAF, RAS, catenin beta 1 (β-catenin), PIK3CA and TP53 have been reported in association with ATC [16], but the complete molecular pathway of anaplastic transformation has not been determined. Our case had the BRAF (V600E) mutation in both ATC and PTC components, further supporting the presence of anaplastic transformation arising from the differentiated PTC component besides morphologic transition of ATC from the PTC component.
In conclusion, here we report a rare case of anaplastic transformation in the pleural metastasis 19 years after diagnosis of PTC. Anaplastic transformation of PTC should be considered during differential diagnosis when lesions with anaplastic sarcomatoid morphology are exclusively present in patients with a history of PTC, and PAX-8 immunostaining can be used as a useful diagnostic tool.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Abe T, Suzuki M, Shimizu K, Shinagawa N, Oizumi S, Matsuno Y, Miyazaki M, Tanino M, Tanaka S, Nishimura M. Anaplastic transformation of papillary thyroid carcinoma in multiple lung metastases presenting with a malignant pleural effusion: a case report. J Med Case Rep. 2014;8:460. doi: 10.1186/1752-1947-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson T, Nanthakumar S, Bugis S, Wiseman SM. Extrathyroidal anaplastic transformation. Cancer Ther. 2008;6:491–494. [Google Scholar]
- 3.Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014;2014:790834. doi: 10.1155/2014/790834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC research consortium of Japan cohort study of 677 patients. World J Surg. 2012;36:1247–1254. doi: 10.1007/s00268-012-1437-z. [DOI] [PubMed] [Google Scholar]
- 5.Al-Qsous W, Miller ID. Anaplastic transformation in lung metastases of differentiated papillary thyroid carcinoma: an autopsy case report and review of the literature. Ann Diagn Pathol. 2010;14:41–43. doi: 10.1016/j.anndiagpath.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Sotome K, Onishi T, Hirano A, Nakamaru M, Furukawa A, Miyazaki H, Morozumi K, Tanaka Y, Iri H, Mimura Y. A rare case of anaplastic transformation within the metastatic site of the retroperitoneal region in a patient 17 years after total thyroidectomy for papillary carcinoma of the thyroid beginning with multiple bone metastases. Thyroid. 2007;17:1309–1311. doi: 10.1089/thy.2006.0322. [DOI] [PubMed] [Google Scholar]
- 7.Solomon JP, Wen F, Jih LJ. Anaplastic transformation of papillary thyroid cancer in the retroperitoneum. Case Rep Pathol. 2015;2015:241308. doi: 10.1155/2015/241308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeshita Y, Takamura T, Minato H, Misu H, Ando H, Yamashita T, Ikeda H, Nakanuma Y, Kaneko S. Transformation of p53-positive papillary thyroid carcinoma to anaplastic carcinoma of the liver following postoperative radioactive iodine-131 therapy. Int Med. 2008;47:1709–1712. doi: 10.2169/internalmedicine.47.1190. [DOI] [PubMed] [Google Scholar]
- 9.Angeles-Angeles A, Chable-Montero F, Martinez-Benitez B, Albores-Saavedra J. Unusual metastases of papillary thyroid carcinoma: report of 2 cases. Ann Diagn Pathol. 2009;13:189–196. doi: 10.1016/j.anndiagpath.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Kaushal S, Sharma MC, Mathur SR, Rastogi S, Bal CS, Chumber S. Anaplastic transformation of metastatic papillary thyroid carcinoma at shoulder mimicking soft tissue sarcoma. Indian J Pathol Microbiol. 2011;54:796–799. doi: 10.4103/0377-4929.91512. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama R, Horiuchi K, Susa M, Hosaka S, Hayashi Y, Kameyama K, Suzuki Y, Yabe H, Toyama Y, Morioka H. Anaplastic transformation of follicular thyroid carcinoma in a metastatic skeletal lesion presenting with paraneoplastic leukocytosis. Thyroid. 2012;22(2):200–204. doi: 10.1089/thy.2011.0258. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 13.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 15.LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(Suppl 2):S1–S9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 16.Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiscer S, Asa SL. Application of immunohistochemistry to thyroid neoplasm. Arch Pathol Lab Med. 2008;132:359–372. doi: 10.5858/2008-132-359-AOITTN. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011;42:1873–1877. doi: 10.1016/j.humpath.2011.02.004. [DOI] [PubMed] [Google Scholar]