Abstract
Squamous odontogenic tumor (SOT) is a rare benign epithelial odontogenic neoplasm of the jaws. Both intraosseous and peripheral SOTs have been described in the English language literature. While most intraosseous SOTs occur as solitary lesions, a multicentric variant has also been previously described. Although the radiographic and microscopic features are identical for both solitary and multicentric clinical presentations, there are three significant differences between them. More specifically, multicentric SOT presents at an earlier age (third decade of life), has a slightly higher male to female ratio than the solitary type and has a marked predilection for African–Americans. Here we document the eighth reported case of multicentric SOT, which was diagnosed in a 43-year-old African–American male. In addition, we feature focal sebaceous metaplasia, a heretofore unknown microscopic feature of SOT. Clinical, radiological, and histopathological findings are discussed. The differential diagnosis, biological behavior and management modalities for SOT are also addressed.
Keywords: Odontogenic, Tumor, Squamous, Multicentric
Introduction
A cascading sequence of complicated, yet precise events leads to the formation of teeth [1] Some of the embryonic structures involved in this process may persist into adulthood (i.e., odontogenic epithelium). This residual odontogenic epithelium retains the ability to proliferate and therefore, has the potential to generate odontogenic cysts and tumors that can, at times, create diagnostic and treatment challenges [2].
One such lesion is squamous odontogenic tumor (SOT) a rare benign, sometimes aggressive epithelial odontogenic neoplasm that was first recognized as a distinct entity in 1975 [3]. In 2005, the World Health Organization (WHO) classification described SOT as a benign odontogenic tumor and further defined it as “a locally infiltrative neoplasm consisting of islands of well-differentiated squamous epithelium in a fibrous stroma” [4]. SOT occurs over a wide age range, but is typically reported during the fourth decade of life [5]. The tumor shows equal distribution in the maxilla and in the mandible. Although SOT is predominantly recognized as a solitary intraosseous lesion, peripheral SOT affecting soft tissue, and SOT-like proliferation arising in odontogenic cyst variants have also been described [6–10]. As of today, only 55 cases (51 central/4 peripheral) of SOT have been published in the English language literature [3, 5, 7, 8, 10–47]. Typically, the radiographic presentation of SOT is that of a triangular or semicircular radiolucency with or without radio-dense border involving the alveolar bone. The base of the radiolucent triangle is located between the apices of the associated teeth causing root divergence. Occasionally, a multilocular presentation has been reported [5, 23]. Multicentric SOT is extremely rare; at the time of this review, only seven cases have been reported in the English language literature [3, 14, 16, 17, 26, 33, 41]. Here we report the eighth case of central multicentric SOT affecting both maxilla and mandible.
Case Report
A 43-year-old well-nourished, well-developed African–American male was referred by his general dentist for evaluation of multiple radiolucent lesions in the mandible and maxilla. Past medical history was significant only for depression and type I diabetes.
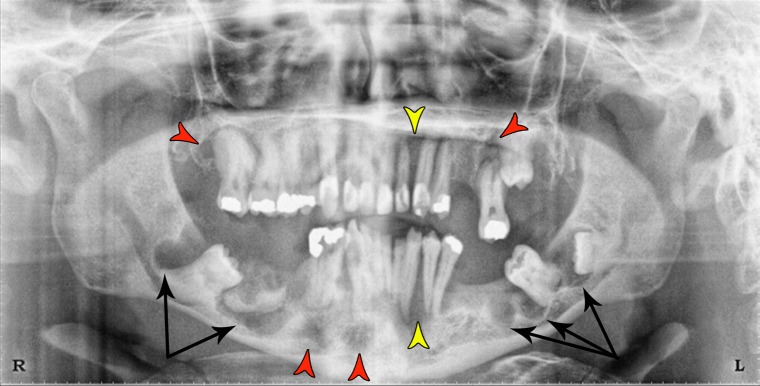
Oral examination revealed several missing and carious teeth. Irregular buccal bony expansion of the jaws was observed. Radiographic examination (Panorex film) showed several impacted teeth (#16, #17, #18 and #32) (Fig. 1). Tooth #2 exhibited hypercementosis. A total of eight radiolucent lesions displaying corticated margins were present in all quadrants. Two of them represented multilocular radiolucencies (one on the left mandible area of #17–18 extending mesially to the edentulous area of #19 and the other in the distal area of tooth #32 extending mesially to the distal area of a tooth #28). Four unilocular lesions were also noted: one distal to #2, one in the left maxilla, and two in the anterior mandible. In addition, two wedge-shaped radiolucencies were seen (between #10–11 and between 22 and 23). Furthermore, a large radiopacity suggestive of osteosclerosis was present in the right mandible amidst the multilocular lesion.
Fig. 1.
Panoramic radiograph shows multiple radiolucent areas in the maxilla and the mandible. Two multilocular radiolucencies are present in the left and right mandible (black arrows). Four unilocular radiolucencies are observed in the right and left maxilla, as well as the anterior mandible (red arrowheads). Two wedge-shaped radiolucencies are seen; one in the maxilla between the left lateral incisor and canine and the other between the left mandibular canine and first bicuspid (yellow arrowheads). Note the radiopaque area in the right mandible amidst the multilocular radiolucency
The patient was taken to the operating room suite and under general anesthesia, teeth #15, #16, #17, #18, #20, #21, and #32 were extracted. Full thickness incisions were done on the left maxilla, and the left, right and anterior mandible. The excised tissue appeared as solid masses that extended beyond the radiographic borders. At this point, removal of all the lesions was considered imprudent, as the patient did not anticipate a complete edentulous outcome. Extensive curettage followed by peripheral ostectomy of the surgical sites was done. The areas were copiously irrigated with normal saline and sutured with 3–0 chromic gut stitches. The specimens were submitted for H &E examination.
Microscopic examination of the specimens from intervened sites presented identical features. The specimens were characterized by the presence of numerous islands of irregularly shaped, bland squamous epithelium of varying size displaying central microcystic degeneration amidst a dense fibrous connective tissue (Fig. 2a). Cord-like epithelial structures showing central dystrophic calcification were also noted (Fig. 2b). Basal cell nuclear palisading and reverse polarization of peripheral keratinocytes were conspicuously absent (Fig. 2c). Occasionally, some islands of squamous odontogenic epithelium showed cystic transformation and intraluminal laminated calcification while other islands displayed clusters of central cells reminiscent of ghost cells (Fig. 2d). Mucous metaplasia and focal sebaceous differentiation were occasionally present in some of the islands (Fig. 2e, f). In addition, a large island of vital dense lamellar bone consistent with reactive osteosclerosis was present in the surgical specimen harvested from the right mandible. The microscopic findings were consistent with squamous odontogenic tumor involving multiple sites. At the post-surgical follow-up visit, the patient was informed at length that he required close follow up and that additional surgical procedures were needed. He was also told that extraction of all the remaining teeth followed by construction of immediate complete dentures was indicated. Unfortunately, the patient disappointed multiple times and was lost to long-term follow-up.
Fig. 2.
a Islands of well-differentiated squamous epithelium showing cystic degeneration (H&E, original magnification X200). b Interspersed cord-like structures of epithelial cells supported by fibrotic stroma. Dystrophic calcification within the epithelium is seen in one of the islands (arrow) (H&E, original magnification X200). c Absence of peripheral nuclear palisading and reverse polarization of the basal cell layer (H&E, original magnification X200). d Occasional tumor islands showing central laminar calcification (arrow), cluster of ghost-cell like aggregates (arrowhead) and intracellular keratinization (white arrow) (H&E, original magnification X200). e Mucous metaplasia (arrowheads) (H&E, original magnification X400). f A cluster of sebocytes is noted in epithelial islands (H&E, original magnification X400)
Multicentric Squamous Odontogenic Tumor
Only eight cases (including this one) of multicentric SOT have been documented in the English language literature (Table 1). An analysis of the demographics associated with multicentric SOT revealed three main differences when compared with the solitary presentation of SOT. First, the multicentric SOT presents a decade (third) earlier than the solitary type (age range from 22 to 43 years; mean age = 27.5). Second, although both solitary and multicentric SOTs have a male predilection, the multicentric variant shows a higher male to female ratio (M:F=1.6:1) than the solitary variant (M:F=1.2:1) [36]. Given the relatively small number of documented cases of multicentric SOT, this tenuous difference in gender predilection should be viewed with caution until larger series are published. Third, the multicentric variant shows a marked predilection for African-Americans (7 African-Americans and one Caucasian). Interestingly, two of the multicentric cases presented in siblings [16].
Table 1.
Summary of reported multicentric squamous odontogenic tumors
| Publications | Race | Gender | Age | Location | X-ray features |
|---|---|---|---|---|---|
| Elmuradi et al. (present case) | AA | M | 43 | Maxilla | Well-defined wedge, multi and unilocular radiolucencies |
| Right | |||||
| Left | |||||
| Mandible | Radiopacitya | ||||
| Right/left | |||||
| Mid line | |||||
| Leider et al. [16] | AA | M | 29 | Maxilla | Multiple radiolucent “wedge” areas and single radiolucency producing a “floating tooth” appearance (#16) |
| Left | |||||
| Mandible | |||||
| Right | |||||
| Left | |||||
| AA | M | 25 | Maxilla | Multiple radiolucent “wedge” areas and single radiolucency on anterior maxilla | |
| Left | |||||
| Right | |||||
| Mandible | |||||
| Left | |||||
| Mills et al. [26] | AA | M | 26 | Maxilla | Ill-defined radiolucent areas |
| Left | |||||
| Right | |||||
| Mandible | |||||
| Left | |||||
| Norris et al. [17] | AA | M | 26 | Maxilla | Well-defined radiolucencies |
| Left | |||||
| Right | |||||
| Hopper et al. [14] | W | F | 22 | Maxilla | Diffuse radiolucent lesion with indistinct borders |
| Right/anterior | |||||
| Mandible | Well-circumscribed radiolucency | ||||
| Left | |||||
| McNeill et al. [33] | AA | F | 26 | All four quadrants | Severe alveolar bone loss in all four quadrants |
| Pullon et al. [3] | AA | F | 23 | All four quadrants | Radiolucent area |
M Male, F Female, AA African-American, W White, R Right, L Left
aMicroscopic findings consistent with reactive osteosclerosis
Discussion
SOT is a benign, slow growing, locally infiltrative tumor thought to be of odontogenic epithelial origin, although other odontogenic epithelial origins are also described in the literature. However, the pathogenesis of this tumor is still unclear [48]. Intraosseous SOT is thought to originate from epithelial rests of Malassez, while dental lamina and gingival epithelium are suspected to be the origins of peripheral SOT [10, 19].
A number of genetic, molecular and immunohistochemical studies have attempted to elucidate the mechanism involved in the development of SOT. For instance, mutation of the ameloblastin gene has been documented in SOT, as well as in adenomatoid odontogenic tumor and ameloblastoma [49, 50]. This gene is located in chromosome 4q21 [49]. It expresses a crucial protein that orchestrates epithelial-mesenchymal interactions. In addition, the heparanase gene codes the heparanase enzyme that plays a role in the cytodifferentiation of SOT. This enzyme regulates tumor invasion and dissemination by modulating extracellular matrix. Mutation of this gene is linked to the development of SOT [37]. Moreover, studies show that Notch receptors 1, 3, 4 and their ligands, Jagged1 and Delta1 may play a role in the cellular differentiation and transformation events of epithelial rests leading to the development of SOT [10]. Recent studies suggest that the expression of metallothionein, a protein responsible for cellular homeostasis of essential metals, cellular differentiation, and proliferation, also plays a role in SOT development [51]. Little is known about the immunohistochemical expression of cytokeratin in SOT [19]. The intermediate filament proteins, cytokeratin (CK8) and (CK19), are expressed during odontogenesis and also identified in SOT. More specifically, intense and diffuse signals for CK8 and CK19 are reported for the tumor epithelial cells of SOT [52].
Central solitary SOT occurs over a wide age range, but is mainly reported during the fourth decade of life [34, 36]. Males are slightly more affected than females (1.2:1) and no ethnic predilection is observed [36]. In the mandible, the posterior region is more commonly affected while maxillary lesions have a tendency to occur in the anterior-canine to premolar area [36, 37, 41]. In rare instances, maxillary tumors may exhibit a locally aggressive behavior [36, 46]. SOT may present as asymptomatic or symptomatic lesions. [37, 41]. The symptomatic lesions are associated with bone expansion and teeth mobility [37].
Radiographically, solitary SOT classically presents as a triangular or semicircular unilocular radiolucency between the roots of adjacent teeth, and in some cases it can be associated with an impacted tooth [3, 16, 17, 41]. In addition, SOT may also present as multilocular radiolucencies [5, 23]. The peripheral variant may produce ‘saucerization’ of the underlying bone as a result of localized tumor pressure [4, 37, 42]. Furthermore, the radiographic appearance of SOT may vary from well to ill-defined radiolucencies with or without cortication [5, 14, 43]. Occasionally, these cases may show severe alveolar bone loss that makes the teeth look as if they were floating [16]. Solid radiopaque areas are not typically described in association with SOT. Our case presented a radiopacity in the right mandible that most likely represents reactive osteosclerosis, as microscopic examination of the specimen harvested from the right mandible revealed the presence of large fragments of vital dense lamellar bone.
The microscopic features of SOT in solitary and multicentric lesions are virtually identical. Typically, SOT presents as cords and islands of benign appearing, bland squamous epithelium of variable size, supported by mature connective tissue. The peripheral basal cell layer consists of flat to cuboidal keratinocytes with no nuclear palisading, polarization, or stellate reticulum-like areas [16, 47]. The squamous islands may exhibit central vacuolization, leading to microcyst formation. Keratin pearl formation, single cell keratinization, dystrophic calcification and mucous metaplasia of the squamous epithelium have been reported in SOT [12, 44]. Our case showed the typical microscopic features of SOT. However, this is the first reported SOT featuring focal sebaceous differentiation, a heretofore, unknown microscopic characteristic of SOT. This sebaceous differentiation underlines the pluripotentiality of odontogenic epithelium.
In this case, the microscopic differential diagnosis of SOT could include central mucoepidermoid carcinoma and ameloblastoma. Unlike SOT, central mucoepidermoid carcinoma typically has two cell types, epidermoid and mucous producing cells. Occasionally, intermediate cells can be identified. The neoplastic mucous cells are usually large, ovoid in shape and have abundant foamy cytoplasm resembling mucous acini [53]. In our case, the bland appearing squamous islands and scattered goblet cells of uniform size militated against a diagnosis of mucoepidermoid carcinoma. Acanthomatous ameloblastoma can be separated from SOT by virtue of the absence of nuclear palisading/reverse polarization and cytoplasmic vacuolization of peripheral basal cells, the sine qua non microscopic features of ameloblastoma [54]. These features are minimally expressed in desmoplastic ameloblastoma, but not in SOT. In addition, desmoplastic ameloblastoma shows a much more collagenized stroma than SOT, resulting in the compression of the proliferating epithelium forming thin caliber cords.
The mainstay of treatment for SOT is enucleation and curettage along with extraction of involved teeth [13, 36, 43, 46]. This is particularly appropriate for mandibular lesions where the density of the bone appears to restrict the spread of the tumor [14]. In contrast, the porous nature of the maxillary bone allows for an easier spread of the lesion and thus, it may require a more aggressive intervention such as en bloc resection. [14, 47].
References
- 1.Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18(13):75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorlin RJ, Chaudhry AP, Pindborg JJ. Odontogenic tumors. Classification, histopathology, and clinical behavior in man and domesticated animals. Cancer. 1961;14(1):73–101. doi: 10.1002/1097-0142(196101/02)14:1<73::AID-CNCR2820140111>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Pullon PA, Shafer WG, Elzay RP, Kerr DA, Corio RL. Squamous odontogenic tumor: report of six cases of a previously undescribed lesion. Oral Surg Oral Med Oral Pathol. 1975;40(5):616–630. doi: 10.1016/0030-4220(75)90372-2. [DOI] [PubMed] [Google Scholar]
- 4.Reichart PA, et al. Squamous Odontogenic Tumor. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and genetics. Head and neck tumors. Lyon: World Health Organization Classification of Tumors. IARC Press; 2005. p. 301. [Google Scholar]
- 5.Philipsen HP, Reichart PA. Squamous odontogenic tumor (SOT): a benign neoplasm of the periodontium. J Clin Periodontol. 1996;23(10):922–926. doi: 10.1111/j.1600-051X.1996.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 6.Buchner A, Sciubba JJ. Peripheral epithelial odontogenic tumors: a review. Oral surg Oral med Oral Pathol. 1987;63(6):688–697. doi: 10.1016/0030-4220(87)90372-0. [DOI] [PubMed] [Google Scholar]
- 7.Malathi N, Radhika T, Thamizh CH, Nandakumar N. Peripheral squamous odontogenic tumor. Indi J Dent Res. 2012;23(2):286. doi: 10.4103/0970-9290.100443. [DOI] [PubMed] [Google Scholar]
- 8.Saxby MS, Rippin JW, Sheron JE. Case report: squamous odontogenic tumor of the gingiva. J Periodontol. 1993;64(12):1250–1252. doi: 10.1902/jop.1993.64.12.1250. [DOI] [PubMed] [Google Scholar]
- 9.Wright JM. Squamous odontogenic tumorlike proliferations in odontogenic cysts. Oral Surg Oral Med Oral Pathol. 1979;47(4):354–358. doi: 10.1016/0030-4220(79)90259-7. [DOI] [PubMed] [Google Scholar]
- 10.Siar CH, Nakano K, Ng KH, Tomida M, Nagatsuka H, Kawakami T. Squamous odontogenic tumor of the mandible: a case report demonstrating immunoexpression of Notch1, 3, 4, Jagged1 and Delta1. Eur J Med Res. 2010;15(4):180–184. doi: 10.1186/2047-783X-15-4-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leventon GS, Happonen R-P, Newland JR. Squamous odontogenic tumor: report of two cases and review of the literature. Am J Surg Pathol. 1981;5(7):671–678. doi: 10.1097/00000478-198110000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Carr R, Carlton D, Jr, Marks R. Squamous odontogenic tumor: report of case. J Oral Surg (American Dental Association: 1965) 1981;39(4):297–298. [PubMed] [Google Scholar]
- 13.Goldblatt LI, Brannon RB, Ellis GL. Squamous odontogenic tumor: report of five cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1982;54(2):187–196. doi: 10.1016/0030-4220(82)90216-X. [DOI] [PubMed] [Google Scholar]
- 14.Hopper TL, Sadeghi EM, Pricco DF. Squamous odontogenic tumor: report of a case with multiple lesions. Oral Surg Oral Med Oral Pathol. 1980;50(5):404–410. doi: 10.1016/S0030-4220(80)80006-5. [DOI] [PubMed] [Google Scholar]
- 15.Doyle J, Grodjesk J, Dolinsky H, Rafel S. Squamous odontogenic tumor: report of three cases. J Oral Surg (American Dental Association: 1965) 1977;35(12):994. [PubMed] [Google Scholar]
- 16.Leider AS, Jonker LA, Cook HE. Multicentric familial squamous odontogenic tumor. Oral Surg Oral Med Oral pathol. 1989;68(2):175–181. doi: 10.1016/0030-4220(89)90189-8. [DOI] [PubMed] [Google Scholar]
- 17.Norris LH, Baghael-Rad M, Maloney PL, Simpson G, Guinta J. Bilateral maxillary squamous odontogenic tumors and the malignant transformation of a mandibular radiolucent lesion. J Oral Maxillofac Surg. 1984;42(12):827–834. doi: 10.1016/0278-2391(84)90355-0. [DOI] [PubMed] [Google Scholar]
- 18.Swan RH, McDaniel RK. Squamous odontogenic proliferation with probable origin from the rests of malassez (early squamous odontogenic tumor?) J Periodontol. 1983;54(8):493–496. doi: 10.1902/jop.1983.54.8.493. [DOI] [PubMed] [Google Scholar]
- 19.Tatemoto Y, Okada Y, Mori M. Squamous odontogenic tumor: immunohistochemical identification of keratins. Oral surg Oral med Oral pathol. 1989;67(1):63–67. doi: 10.1016/0030-4220(89)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Yaacob HB. Squamous odontogenic tumor. J Nihon Univ Sch Dent. 1990;32(3):187–191. doi: 10.2334/josnusd1959.32.187. [DOI] [PubMed] [Google Scholar]
- 21.Baden E, Doyle J, Mesa M, Fabié M, Lederman D, Eichen M. Squamous odontogenic tumor: report of three cases including the first extraosseous case. Oral Surg Oral Med Oral Pathol. 1993;75(6):733–738. doi: 10.1016/0030-4220(93)90432-4. [DOI] [PubMed] [Google Scholar]
- 22.Hietanen J, Lukinmaa P-L, Ahonen P, Krees R, Calonius P. Peripheral squamous odontogenic tumour. Br J Oral Maxillofac Surg. 1985;23(5):362–365. doi: 10.1016/0266-4356(85)90009-9. [DOI] [PubMed] [Google Scholar]
- 23.Reichart PA, Philipsen HP. Squamous odontogenic tumor. J Oral Pathol Med. 1990;19(5):226–228. doi: 10.1111/j.1600-0714.1990.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 24.Monteil R, Terestri P. Squamous odontogenic tumor related to an unerupted lower canine. J Oral Maxillofac Surg. 1985;43(11):888–895. doi: 10.1016/0278-2391(85)90229-0. [DOI] [PubMed] [Google Scholar]
- 25.Favia G, Di Alberti L, Scarano A, Piattelli A. Squamous odontogenic tumour: report of two cases. Oral Oncol. 1997;33(6):451–453. doi: 10.1016/S0964-1955(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 26.Mills WP, Davila MA, Beuttenmuller EA, Koudelka BM. Squamous odontogenic tumor: report of a case with lesions in three quadrants. Oral Surg Oral Med Oral Pathol. 1986;61(6):557–563. doi: 10.1016/0030-4220(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 27.Cataldo E, Less WC, Giunta JL. Squamous odontogenic tumor: a lesion of the periodontium. J Periodontol. 1983;54(12):731–735. doi: 10.1902/jop.1983.54.12.731. [DOI] [PubMed] [Google Scholar]
- 28.Kusama K, Kawashima A, Nagai H, Tajima M, Tsuchiya H, Yamaguchi H, et al. Squamous odontogenic tumor of the maxilla: report of a case. J Oral Sci. 1998;40(3):119–122. doi: 10.2334/josnusd.40.119. [DOI] [PubMed] [Google Scholar]
- 29.Pant H, Pathak S. Squamous odontogenic tumor-exceedingly rare neoplasm. Indian J Basic Appl Med Res. 2013;8(2):1023–26. [Google Scholar]
- 30.Singh A, Agarwal N, Sinha A, Singh G, Srivastava S, Prasad RK. Squamous odontogenic tumor of the maxilla: a case report and review of the literature. Oral Radiol. 2015;31(2):129–134. doi: 10.1007/s11282-014-0180-6. [DOI] [Google Scholar]
- 31.Agostini T, Sacco R, Bertolai R, Acocella A, Colafranceschi M, Lazzeri D. Peri-implant squamous odontogenic tumor. J Craniofac Surg. 2011;22(3):1151–1157. doi: 10.1097/SCS.0b013e318210bb4d. [DOI] [PubMed] [Google Scholar]
- 32.Warnock GR, Pierce GL, Correll R, Baker DA. Triangular-shaped radiolucent area between roots of the mandibular right canine and first premolar. J Am Dent Assoc (1939) 1985;110(6):945–946. doi: 10.14219/jada.archive.1985.0010. [DOI] [PubMed] [Google Scholar]
- 33.McNeill J, Pierce H, Stoker N. Squamous odontogenic tumor: report of case with long-term history. J Oral Surg. (American Dental Association: 1965) 1980;38(6):466–471. [PubMed] [Google Scholar]
- 34.Bansal S, Joshi SK. Squamous odontogenic tumor with unusual localization and appearance: a rare case report. C Rep Med. 2013;2013:407967. doi: 10.1155/2013/407967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cillo JE, Ellis E, Kessler HP. Pericoronal squamous odontogenic tumor associated with an impacted mandibular third molar: a case report. J Oral Maxillofac Surg. 2005;63(3):413–416. doi: 10.1016/j.joms.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Mohr B, Winter J, Wahl G, Janska E. Recurrent squamous odontogenic tumor: A case report and review of the literature. Oncol lett. 2015;10(5):2713–2722. doi: 10.3892/ol.2015.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K, Mintz SM, Stevens J. Squamous odontogenic tumor causing erosion of the lingual cortical plate in the mandible: a report of 2 cases. J Oral Maxillofac Surg. 2007;65(6):1227–1231. doi: 10.1016/j.joms.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen S, Andersen J, Jacobsen P. Squamous odontogenic tumour: review of the literature and a new case. J Laryngol Otol. 1985;99(09):919–924. doi: 10.1017/S0022215100097930. [DOI] [PubMed] [Google Scholar]
- 39.Jones BE, Sarathy AP, Ramos MB, Foss RD. Squamous odontogenic tumor. Head Neck Pathol. 2011;5(1):17–19. doi: 10.1007/s12105-010-0198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ide F, Shimoyama T, Horie N, Shimizu S. Intraosseous squamous cell carcinoma arising in association with a squamous odontogenic tumour of the mandible. Oral Oncol. 1999;35(4):431–434. doi: 10.1016/S1368-8375(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 41.Badni M, Nagaraja A, Kamath V. Squamous odontogenic tumor: a case report and review of literature. J Oral Maxillofac Pathol. 2012;16(1):113. doi: 10.4103/0973-029X.92986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haghighat K, Kalmar JR, Mariotti AJ. Squamous odontogenic tumor: diagnosis and management. J Periodontol. 2002;73(6):653–656. doi: 10.1902/jop.2002.73.6.653. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira MG, Carrard VC, Carrard CC, Danesi CC, Rados PV, Sant’Ana Filho M. Squamous odontogenic tumor: with recurrence and 12 years of follow-up. Rev ciênc méd,(Campinas) 2007;16(1):51–56. [Google Scholar]
- 44.Barrios TJ, Sudol JC, Cleveland DB. Squamous odontogenic tumor associated with an erupting maxillary canine: case report. J Oral Maxillofac Surg. 2004;62(6):742–744. doi: 10.1016/j.joms.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Gardner DG. Peripheral squamous odontogenic tumor. Oral Sur Oral Med Oral Pathol. 1987;64(5):609. doi: 10.1016/0030-4220(87)90068-5. [DOI] [PubMed] [Google Scholar]
- 46.Ruhin B, Raoul G, Kolb F, Casiraghi O, Lecomte-Houcke M, Ghoul S, et al. Aggressive maxillary squamous odontogenic tumour in a child: histological dilemma and adaptative surgical behaviour. Int J Oral Maxillofac Surg. 2007;36(9):864–866. doi: 10.1016/j.ijom.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Lin Y-L, White DK. Squamous odontogenic tumor. Oral Maxillofac Surg Clin N Am. 2004;16(3):355–357. doi: 10.1016/j.coms.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 48.do Rosário Mardones N, de Oliveira Gamba T, Flores IL, de Almeida SM, de Castro Lopes SLP. Squamous Odontogenic Tumor: literature Review Focusing on the Radiographic Features and Differential Diagnosis. Op. Dent J. 2015;9:154. doi: 10.2174/1874210601509010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mardh CK, Backman B, Simmons D, Golovleva I, Gu TT, Holmgren G, et al. Human ameloblastin gene: genomic organization and mutation analysis in amelogenesis imperfecta patients. Europ J Oral Sci. 2001;109(1):8–13. doi: 10.1034/j.1600-0722.2001.00979.x. [DOI] [PubMed] [Google Scholar]
- 50.Perdigao P, Gomez R, Pimenta F, De Marco L. Ameloblastin gene (AMBN) mutations associated with epithelial odontogenic tumors. Oral Oncol. 2004;40(8):841–846. doi: 10.1016/j.oraloncology.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Johann ACBR, Caldeira PC, Souto GR, Abreu MHNG, Aguiar MCF, Mesquita RA. Metallothionein immunoexpression in selected benign epithelial odontogenic tumors. J Oral Pathol Med. 2014;43(3):177–182. doi: 10.1111/jop.12122. [DOI] [PubMed] [Google Scholar]
- 52.Yarlagadda K, Kamath VV, Satelur K. Immunohistochemical expression of keratins 8 and 19 in odontogenic cysts and tumors. J Cranio Max Dis. 2015;4(2):128. doi: 10.4103/2278-9588.163254. [DOI] [Google Scholar]
- 53.Ellis G, Auclair P. Malignant epithelial neoplasms. In Tumors of the salivary glands. AFIP atlas of tumor pathology. Silver Spring: ARP Press; 2008. pp. 180–182. Series 4.
- 54.Vickers RA, Gorlin RJ. Ameloblastoma: delineation of early histopathologic features of neoplasia. Cancer. 1970;26(3):699–710. doi: 10.1002/1097-0142(197009)26:3<699::AID-CNCR2820260331>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]