Abstract
Carcinomas arising from thyroglossal duct remnant cysts (TGDCs) are rare, without well-defined management and staging criteria. All TGDCs (n = 685) diagnosed between 2005 and 2015 were retrospectively reviewed, with 22 carcinomas identified (3.2 % incidence). Twenty-two patients (17 females, 5 males), aged 12–64 years (mean 39.9 years; median 39 years) were identified. An anterior, superior midline neck mass was the presenting symptom in all patients. A cancer diagnosis [all papillary thyroid carcinoma (PTC)] was made after the Sistrunk procedure (SP), with a Bethesda Category V or VI classification preoperatively by fine needle aspiration in 5 of 12 cases tested. A SP was performed in all patients, with total thyroidectomy concurrently (n = 4) or subsequently (n = 12). A selected neck dissection was performed in 5 patients, with metastases found in 3. Of the patients who had a thyroidectomy, synchronous PTC was identified in 6 (thus, 6 of 22 patients had synchronous thyroid gland primaries). This supports an origin from extra-thyroidal remnants (cyst origin) rather than metastatic tumor from a thyroid gland primary. Follow-up radioactive iodine therapy was performed in 13 patients. Metastatic disease to local lymph nodes 57 months after presentation was seen in 1 patient, with all others alive and disease free (mean 3.8 years; range 0.4–10.8 years). The TGDCs ranged from 0.8 to 5 cm (mean 2.3 cm), while the PTCs ranged from 0.1 to 3.8 cm (mean 1.4 cm). All of the tumors were classical PTC, showing a sclerotic and infiltrative pattern, with a capsule present in 11. Lymphovascular invasion was detected in 11; margins were positive in 6. Using currently defined criteria, the patients were separated into AJCC stage group I (n = 21) or II (n = 1). However, if extension into the adipose tissue (n = 11), skeletal muscle (n = 10), or perineural/perivascular tissues (n = 10) were used to stage the patients, interpreted to represent the equivalent of “extrathyroidal extension” (n = 13) as defined for thyroid gland primaries, there would be 15 group I and 7 group III cases. All seven group III patients were ≥45 years. Three of four patients with lymph node metastasis also showed soft tissue extension. In conclusion, TGDC carcinomas (TGDCCa) are uncommon, with all classical PTC. For “microcarcinomas” (≤1 cm), conservative management can be used for patients <45 years (i.e., Sistrunk procedure only); for >1 cm tumors, and due to the high incidence of concurrent papillary carcinoma and higher stage at presentation in older patients, completion thyroidectomy is recommended for patients ≥45 years. Thus, even though a good prognosis can be expected for PTC developing in TGDCs, staging is advocated to more appropriately match therapeutic interventions.
Keywords: Carcinoma, papillary/pathology; Thyroglossal cyst/pathology; Thyroglossal cyst/surgery; Thyroglossal cyst/epidemiology; Incidence; Follow-up studies; Prognosis; Thyroid neoplasms/pathology; Neck dissection; Thyroidectomy
Introduction
The thyroglossal duct is part of the embryonic development of the thyroid gland, evolving as a tract, which forms during descent of the thyroid gland from the ventral aspect of the pharynx. Following caudal migration of the thyroid gland, the thyroglossal duct atrophies and disappears between the 7th and 10th weeks of gestation. In a minority of individuals, the thyroglossal duct does not undergo complete involution. Persistent remnants of the thyroglossal duct may manifest clinically as thyroglossal duct remnant cysts (TGDCs), characteristically presenting as a mobile midline anomaly in the anterior neck [1–4].
On rare occasions, malignancy may develop within a TGDC. The reported incidence of carcinoma occurring in TGDCs ranges from <1 to 19.6 % [5–16], although probably more in the 2 % range when referral and academic bias are removed from consideration [4]. The vast majority of TGDC carcinomas (TGDCCa) are papillary thyroid carcinomas (PTCs), thought to originate from the ectopic thyroid gland tissue which is commonly (approximately 70 % of cases) found in association with TGDCs [2, 4, 7, 9, 16–22]. The clinical presentation of TGDCCa do not differ significantly from that of nonneoplastic TGDC, although a fixed mass is worrisome, with most cases of malignancy diagnosed postoperatively following pathologic evaluation of the lesion [2, 17, 20, 21]. Given the rarity of TGDCCa and the frequent incidental nature of the diagnosis, clinical management of these tumors remains controversial particularly in regards to the need for thyroidectomy and the need for staging of the tumors [3, 8, 12, 16, 20, 23, 24].
Currently, the American Joint Committee on Cancer (AJCC) staging (2010) criteria for cancer of thyroid gland tumors is not consistently applied to these tumors and has not been recommended [6, 8, 20, 22, 25, 26]. This study of a community practice based series of TGDCCa is presented with analysis of histopathologic features and recommendations of the use of staging to alter therapeutic management.
Materials and Methods
Six hundred eighty-five cases of TGDC were retrieved from the files of the Pathology Departments of the Southern California Permanente Medical Group hospitals between June 2005 and June 2015. All hematoxylin and eosin stained slides were reviewed (range 1–34 slides; mean 3 slides per case). The clinical data, treatment, and followup information were obtained from the electronic medical records augmented by the surgical pathology reports. Overall, 22 patients from this cohort had carcinoma. Fine needle aspiration was performed preoperatively in 12 of 22 patients. Imaging studies were performed (computed tomography [CT] = 17; ultrasound [US] = 5; magnetic resonance imaging [MRI] = 2; radioisotope thyroid scanning = 1) to document the extent and location of the disease. Data recorded included: tumor focality (unifocal, multifocal); tumor encapsulation (presence or absence); soft tissue extension (defined as tumor microscopically present within adipose tissue, skeletal muscle, adjacent to nerves, adjacent to medium-sized vessels); capsular invasion (tumor penetration through the capsule by >50 % of the capsule thickness); lymphovascular invasion (tumor plugging a vascular channel within or immediately beyond the tumor capsule; tumor attached to the vessel wall; presence of associated thrombus); intratumoral fibrosis; architectural pattern of growth (papillary, follicular, solid, cystic, trabecular, insular); irregularly shaped/twisted/elongated follicles; tumor necrosis; increased mitotic rate (>3/10 HPFs); presence of lymph nodes (central compartment, perithyroidal, cervical) and presence of metastatic disease. All cases were staged by the American Joint Committee on Cancer (AJCC) staging (2010) criteria [27], understanding that soft tissue extension moved the tumor to the pT3 category. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group.
Results
Clinical Features
Among 685 patients with TGDCs, 22 (3.2 %) had TGDCCa (Table 1). There were 17 females and 5 males with a mean age at diagnosis of 39.9 years (range 12–64 years). A midline neck mass was the most common initial clinical presentation, observed in 20 patients. Fifteen were located in the infrahyoid region and 7 were located above the hyoid bone. The masses were most typically mobile, though fixation to the surrounding tissues was noted in 2 patients. Clinical infection was reported in 4 patients. The duration of symptoms ranged from 0.25 to 156 months with a mean of 21.5 months.
Table 1.
Clinical and pathologic criteria of 22 thyroglossal duct cyst (remnant) carcinomas
| Case # | Age (years) | Sex | Sym dur (m) | TGDC size (cm) | T4 rem. size | T4 rem. loc. | PTC size | Total # of sync. tumors | LVI | ST ext. | + Mar | LN Mets | AJCC stage | New stage a | Tx: SP, T | A, NED | FU dur (in m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | M | 0.3 | 2.8 | 0.6 | C, S | 0.1 | 1 | A | P | A | A | I | I | S | A, NED | 4.6 |
| 2 | 16 | M | 3 | 0.8 | 0.2 | C | 0.6 | 1 | A | A | A | np | I | I | S, Tb | A, NED | 97.3 |
| 3 | 24 | F | 3 | 3.5 | 0.3 | C | 3.0 | 2 | P | P | A | P: 3/71 | I | I | S, T | A, NED | 37.0 |
| 4 | 28 | F | 5 | 3.8 | 0.3 | C | 3.8 | 1 | A | P | A | A | I | I | S, Tb | A, NED | 5.9 |
| 5 | 31 | F | 156 | 1.8 | 0.1 | C | 1.1 | 1 | A | A | A | np | I | I | S | A, NED | 13.1 |
| 6 | 34 | F | 9 | 1.8 | 0.7 | C | 1.8 | 2 | P | P | P | P: 4/5 | I | I | S, Tb | A, NED | 24.9 |
| 7 | 37 | F | 42 | 1.2 | 0.3 | C, S | 0.5 | 1 | A | A | P | A | I | I | S, T | A, NED | 71.8 |
| 8 | 37 | F | 3 | 1.5 | 1.6 | C | 1.1 | 1 | P | A | A | P: 2/4 | I | I | S, Tb | A, NED | 129.2 |
| 9 | 37 | F | 3 | 5.0 | 0.8 | C | 2.5 | 1 | A | A | A | A | I | I | S, Tb | A, NED | 8.0 |
| 10 | 38 | F | 68 | 2.5 | 0.9 | C, S | 0.8 | 1 | A | P | A | np | I | I | S | A, NED | 29.3 |
| 11 | 38 | F | 15 | 1.2 | 0.7 | C | 1.0 | 1 | A | A | A | A | I | I | S | A, NED | 8.9 |
| 12 | 40 | F | 14 | 1.8 | 1.3 | C, Fat | 0.8 | 1 | A | A | A | A | I | I | S | A, NED | 33.6 |
| 13 | 42 | F | 2 | 1.5 | 0.3 | C | 0.2 | 1 | A | A | A | np | I | I | S | A, NED | 35.5 |
| 14 | 42 | F | 4 | 1.6 | 0.9 | C | 1.6 | 5 | P | P | A | A | I | I | S, Tb | A, NED | 9.2 |
| 15 | 45 | F | 7 | 2.0 | 0.1 | C | 1.0 | 3 | P | P | P | P: 1/1(FU) | I | III | S, T | A, NED | 119.4 |
| 16 | 45 | F | 1 | 1.9 | 0.9 | C | 1.9 | 2 | P | P | P | A | I | III | S, Tb | A, NED | 21.4 |
| 17 | 49 | M | 2 | 2.8 | 0.3 | C | 1.2 | 3 | P | A | A | A | I | I | S, Tb | A, NED | 96.3 |
| 18 | 50 | F | 120 | 2.5 | 0.3 | C | 2.5 | 1 | P | P | P | A | II | III | S, Tb | A, NED | 27.6 |
| 19 | 54 | M | 8 | 1.3 | 1.8 | C, S | 1.3 | 1 | P | P | A | A | I | III | S, T | A, NED | 118.1 |
| 20 | 57 | F | 0.5 | 3.5 | 0.2 | C | 1.2 | 1 | A | P | P | A | I | III | S, Tb | A, NED | 24.9 |
| 21 | 57 | M | 1 | 2.5 | 0.6 | S | 1.2 | 1 | P | P | A | A | I | III | S, Tb | A, NED | 31.2 |
| 22 | 64 | F | 7 | 2.7 | 0.3 | C, S | 1.1 | 3 | P | P | A | A | I | III | S, T | A, NED | 52.2 |
| Mean | 40 | 17 : 5 | 21 | 2.3 | 0.6 | 1.4 | 1.6 | n = 11 | n = 13 | n = 6 | n = 4 | 45.4 |
M male, F female, Sym dur (m) symptom duration in months, TGDC thyroglossal duct cyst size, T4 rem. thyroid remnant, T4 rem. loc. thyroid remnant location, S skeletal muscle, C cyst wall, PTC papillary thyroid carcinoma, Total # of tumors (sync.) is the number of synchronous primaries identified, including thyroidectomy, LVI lymphovascular invasion, P present, A absent, ST ext. soft tissue (fat, skeletal muscle, perineural, perivascular) extension, + mar positive margin, LN mets lymph node metastasis, np not performed, Tx: SP, T treatment by Sistrunk procedure, thyroidectomy; A, NED alive, no evidence of disease, FU follow-up
a Stage determined based on extrathyroidal extension criteria used for thyroid gland primary
b Patients had thyroidectomy as a second surgery
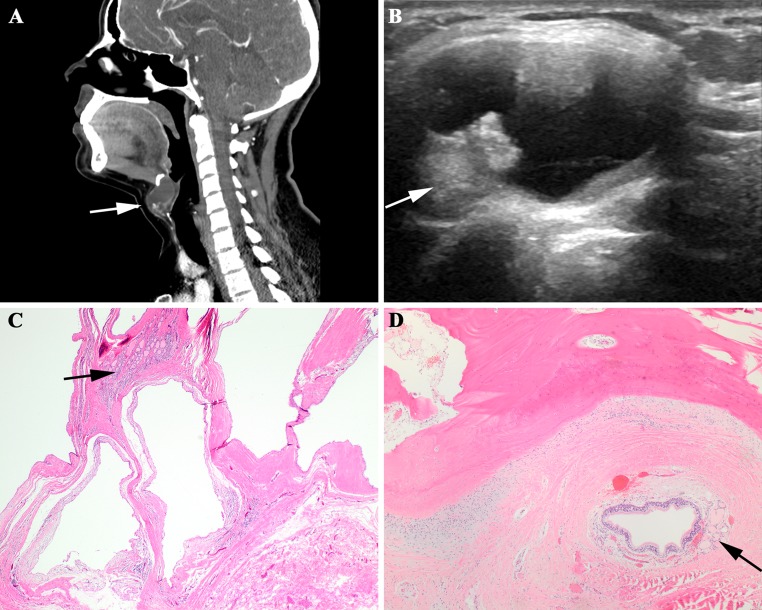
Preoperative radiographic evaluation was performed in 21 patients. The imaging modalities employed included CT only in 13 patients, US only in 2 patients, and MRI and radioisotope thyroid scanning in 1 patient each. Both CT and US were performed in 3 patients, while CT and MRI were performed in 1 patient. By imaging studies, the lesions ranged from 0.7 to 3.2 cm in greatest dimension with an average size of 1.9 cm. All were described as cystic, with an associated enhancing solid nodule present in 9 patients (Fig. 1). In 3 of these patients, the presence of calcifications was also noted within the solid component.
Fig. 1.
a A sagittal computed tomography scan shows a large cyst just below the hyoid bone, with a luminal solid area (arrow). b The ultrasound demonstrates intracystic growth of a solid tumor (arrow). c A thyroglossal duct cyst, showing a small component of normal thyroid tissue (arrow). d Adjacent to the hyoid bone is a small respiratory lined cyst associated with thyroid follicles (arrow)
Twelve patients underwent fine needle aspiration (FNA) of the cystic mass prior to surgery. Utilizing the Bethesda System for reporting thyroid cytopathology, the aspirates were classified as follows: Category I (n = 2), Category II (n = 4), Category IV (n = 1), Category V (n = 2), Category VI (n = 3).
Pathologic Features
On gross examination, the lesions were described as cysts that ranged from 0.8 to 5.0 cm in greatest dimension (mean 2.3 cm). Associated solid or nodular areas were observed in 12 cases, but the cystic component of the lesion was always the dominant finding. All neoplasms were PTCs that varied from 0.1 to 3.8 cm (mean 1.4 cm). Seven (32 %) were “microscopic” measuring 1.0 cm or less in diameter. Histologically, characteristic nuclear features of PTC were present including enlargement, crowding, irregular contours, grooves, chromatin clearing, and pseudoinclusions (Figs. 1, 2, 3). The tumors exhibited a papillary and/or follicular architecture with stromal sclerosis and an infiltrative pattern of growth.
Fig. 2.
Papillary carcinoma adjacent to thyroglossal duct cysts (a–d). Psammoma bodies were present (a and b). Degenerative changes were noted as a post FNA effect (c). The histologic features were those of a classical papillary thyroid carcinoma, with papillary and follicular growth. The arrows designate the uninvolved thyroid tissue of the TGDC
Fig. 3.
a A sclerotic papillary carcinoma (arrow) associated with thyroid tissue remnants. b Classical nuclear features of papillary carcinoma. c and d Metastatic papillary carcinoma to a lymph node, including a psammoma body (d, arrow)
A fibrous connective tissue capsule was present in 11 tumors, with capsular invasion identified in 9. Eleven tumors showed lymphovascular invasion. Extension of tumor into the adjacent soft tissue was observed in 13 cases (Fig. 4), involving adipose tissue (n = 11), skeletal muscle (n = 10), and/or perineural/perivascular tissues (n = 10). The margins were positive in 6 cases, 5 of which exhibited soft tissue extension by tumor. None of the tumors exhibited histologic features of other recognized variants of PTC.
Fig. 4.
Soft tissue extension. a Extension into soft tissues, including skeletal muscle and fat. b Tumor follicles within skeletal muscle (arrow). c Large soft tissue vessels (arrow) surrounded by papillary carcinoma follicles. d A large soft tissue smooth muscle-walled vessel surrounded by papillary carcinoma
All carcinomas were associated with TGDCs lined by squamous epithelium (n = 8), ciliated respiratory type epithelium (n = 6), or a combination of the two (n = 8). Ectopic nonneoplastic thyroid gland tissue was present in all cases (Figs. 1, 2, 3), which ranged from 0.1 to 1.8 cm (mean 0.6 cm). The thyroid gland tissue was predominantly localized to the cyst wall (n = 15), but was also observed in surrounding skeletal muscle (n = 6) and adipose tissue (n = 1).
Total thyroidectomy was performed in 16 patients. A synchronous malignancy was identified in the thyroid gland of 7 patients. The thyroid glands from 6 patients contained foci of papillary carcinoma ranging from <0.1 up to 1.1 cm, with most (11 of 12) tumors being microscopic (measuring 1.0 cm or less by definition). Tumors were present in both thyroid lobes in 4 patients. One patient had a minimally invasive follicular carcinoma measuring 1.2 cm.
Clinical Treatment and Patient Outcome
A Sistrunk procedure was performed in all patients. Total thyroidectomy was performed in 16 patients in addition to the Sistrunk procedure (12 as a second procedure and 4 concurrently). Additional tumors were identified in 7 of the 16 patients having undergone total thyroidectomy. These were papillary carcinomas in 6 patients and a follicular carcinoma in 1 patient as noted above.
Neck dissection of the central and/or lateral compartments was performed in 5 patients. Lymph node metastases were identified in 3 patients (Fig. 3). The involved lymph nodes were located adjacent to the cyst in 2 patients and involved central and lateral neck lymph nodes in one patient. Of the 3 patients with lymph node metastases, 1 had multiple foci of papillary carcinoma involving both lobes of the thyroid while 2 had normal thyroid glands, uninvolved by papillary carcinoma. The TGDC associated tumor in 2 of these patients showed extension into surrounding soft tissues.
The mean MACIS score was 4.76 (range 3.28–6.92). The majority (82 %) of patients had scores <6, while 4 patients were in the 6–6.99 score group. Applying AJCC staging criteria as currently defined for the thyroid gland, 21 patients were stage group I, and 1 patient was stage group II. However, if tumor involvement of soft tissue outside of the cyst was considered equivalent to extrathyroidal extension as in the thyroid gland proper (pT3), 15 patients were staged group I, and 7 patients were staged group III. All group III patients were 45 years or older. Postoperative radioactive iodine therapy was administered in 13 patients.
Follow up information was available for all patients with a mean duration of 3.8 years (range 0.4–10.8 years). One patient with a 1.0 cm TGDC carcinoma with soft tissue extension and bilateral microscopic papillary carcinomas of the thyroid gland recurred with cervical lymph node metastasis 57 months after initial treatment. All patients were otherwise free of disease. No tumor associated mortality was identified.
Discussion
While TGDCs are among the most common pathologic lesion affecting the neck, carcinomas arising from TGDCs are rare. Due to their rarity, there have been few large series of TGDCCa reported in the literature. Prior studies that have included at least 5 or more cases are summarized in Table 2 [6–15, 21, 22, 25, 26, 28–35]. The reported incidence of malignancy in TGDCs ranges from <1 to 19.6 % [5–11, 13–16], however in the present community practice based series, 3.2 % of TGDCs contained an associated carcinoma. As these cases were identified among all TGDCs reviewed during a consecutive 10 year period without referral or academic center bias, this observed rate may be more reflective of the true incidence of TGDCCa.
Table 2.
Literature summary of thyroglossal duct cyst (remnant) carcinoma
| Author | N | M | F | Age mean (years) | Size mean (cm) | PTC | FTC | Thyroidectomy | TG PTC | LN mets | Local Recur-rence | Distant mets | DOD | FU, mean (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jaques [21] | 18 | 9 | 9 | 37.5 | 3.3 | 18 | 0 | 4 | 1 | 3 | 0 | 0 | 0 | 66.6 |
| Page [30] | 6 | 2 | 4 | 30.5 | 3.2 | 6 | 0 | 3 | 0 | 1 | 1 | 0 | 0 | 60.2 |
| Livolsi [9] | 7 | 4 | 3 | 39.3 | 2.3 | 7 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 83.1 |
| Fernandez [11] | 10 | 1 | 9 | 38.4 | 2.1 | 9 | 1 | 5 | 2 | 4 | 0 | 0 | 0 | 96.7 |
| Pacheco-Ojeda [29] | 5 | 0 | 5 | 34.0 | 4.3 | 5 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 60.0 |
| Heshmati [13] | 12 | 6 | 6 | 40.0 | 1.9 | 12 | 0 | 9 | 3 | 3 | 0 | 0 | 0 | 156 |
| O’Connell [32] | 8 | 5 | 3 | 44.6 | 4.3 | 7 | 1 | 3 | 2 | 1 | 1 | 0 | 0 | 150 |
| Doshi [10] | 14 | 6 | 8 | 39.3 | 2.5 | 14 | 0 | 10 | 5 | 4 | 2 | 1 | 0 | 54.7 |
| Patel [15] | 5 | 2 | 3 | 45.0 | 2.0 | 4 | 1 | 4b | 0 | 0 | 1 | 0 | 0 | 111 |
| Miccoli [28] | 18 | 4 | 14 | 38.2 | nr | 16 | 2a | 18 | 6 | 3 | 2 | 0 | 0 | 85 |
| Luna-Ortiz [25] | 5 | 2 | 3 | 49.0 | 2.3 | 5 | 0 | 4 | 1 | 1 | 1 | 1 | 1 | 75 |
| Plaza [12] | 5 | 0 | 5 | 28.6 | 2.8 | 5 | 0 | 5 | 1 | 1 | 0 | 0 | 0 | 124 |
| Hartl [26] | 18 | 5 | 13 | 41.5 | 1.5 | 18 | 0 | 16c | 9 | 12 | 0 | 0 | 0 | 132 |
| Forest [14] | 9 | 3 | 6 | 43.2 | 1.3 | 9 | 0 | 8 | 4 | 2 | 2 | 0 | 0 | 80.4 |
| Dzodic [31] | 12 | 3 | 9 | 40.6 | nr | 11 | 1 | 12 | 2 | 6 | 1 | 1 | 0 | 140 |
| Olimpia Cid [6] | 22 | 4 | 18 | 36.9 | nr | 22 | 0 | 14 | 7 | 4 | 0 | 0 | 0 | 96 |
| Chrisoulidou [34] | 6 | 2 | 4 | 39.3 | 0.7 | 6 | 0 | 6 | 5 | 1 | 1 | 0 | 0 | 46 |
| Pellegriti [22] | 26 | 6 | 20 | 41.2 | 1.0 | 23 | 3 | 26 | 16 | 15 | 6 | 0 | 0 | 74.2 |
| Choi [35] | 10 | 2 | 8 | 54.0 | 1.9 | 10 | 0 | 6 | 2 | 2 | 2 | 1 | 1 | 28.5 |
| Rossi [7] | 9 | 4 | 5 | 46.0 | 2.8 | 9 | 0 | 9 | 5 | 2 | 0 | 0 | 0 | nr |
| Wei [8] | 18 | 5 | 13 | 34.2 | 1.3 | 18 | 0 | 10 | 5 | 4 | 1 | 0 | 0 | 72 |
| Chala [33] | 6 | 1 | 5 | 48.8 | 4.5 | 6 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | nr |
| Current series | 22 | 5 | 17 | 40.0 | 1.4 | 22 | 0 | 16 | 7 | 4 | 0 | 0 | 0 | 45.4 |
| Totals | 271 | 81 | 190 | 40.4 | 2.4 | 262 | 9 | 197 | 86 | 76 | 21 | 4 | 2 | 87.5 |
N number, M male, F female, PTC papillary thyroid carcinoma, FTC follicular thyroid carcinoma, TG thyroid gland, LN mets lymph node metastasis, Distant mets distant metastasis, DOD dead of disease, nr not reported, FU follow-up
a1 follicular carcinoma and 1 “oxyphilic” carcinoma
b3 isthmusectomy, 1 lobectomy
c15 thyroidectomy, 1 isthmusectomy
Unlike nonneoplastic TGDCs, which show no gender predilection [2–4], TGDCCa affect women more frequently than men with a 3:1 female to male ratio observed in the present series, or 2.3:1 overall when including the literature (Table 2). The mean age at presentation is approximately 40 years [2, 3, 15, 17, 18, 20, 22, 23, 30, 35]. Pediatric patients were uncommonly affected by carcinoma, with only 2 in this clinical series.
Most patients present with a mobile midline neck mass, clinically indistinguishable from benign TGDC [2, 13, 17, 19–21]. The finding of a fixed mass has been emphasized as a possible indicator of malignancy [3, 20, 36], but was present in only 2 patients in this series. TGDCCa are thus difficult to identify preoperatively, with the majority diagnosed after pathologic evaluation of the excised specimen.
FNA of TGDCCa has a reported true positive rate of 53 % and a false negative rate of 47 % [37–40]. This is consistent with the findings of the present series, which yielded a suspicious or definitive diagnosis of malignancy in 5 of 12 (42 %) patients in which preoperative fine needle aspiration was performed. This lack of efficacy has been attributed largely to poor cellular yield secondary to the cystic nature of the lesion and the often relatively small size of the malignant component [37–43].
A diagnosis of malignancy within a TGDC is similarly difficult to establish based on preoperative radiographic studies. While the presence of a solid component, mural nodule, or calcifications within an otherwise cystic midline neck mass by CT and MRI have been suggested as features useful for recognition of a carcinoma within a TGDC [44–49], this particular radiographic appearance was documented in only 9 patients in this study.
Histologically, the vast majority of TGDC associated malignancies are PTCs. Follicular carcinoma, squamous cell carcinoma, and various other histologic types of thyroid epithelial neoplasms have been described but are considered extremely rare [2, 3, 15, 17–20, 22, 23]. TGDC PTCs are morphologically identical to those arising in the thyroid gland and can be recognized by their papillary and or/follicular architecture, the presence of classical PTC nuclear features, and frequently infiltrative pattern of growth [22]. Soft tissue involvement of pericystic soft tissues by tumor is a common finding, observed in 59 % of cases in the present study.
TGDCCa are thought to represent primary neoplasms arising from ectopic thyroid gland tissue commonly found in TGDCs [7, 9, 17–21]. There is, however, theoretic consideration that these tumors may represent metastases from a primary carcinoma of the thyroid gland [50]. While the presence of a concurrent papillary carcinoma of the thyroid gland has been reported in 17–83 % of patients with TGDCCa who have undergone thyroidectomy [6–14, 21, 22, 25, 26, 28–32, 34, 35], the thyroid gland tumors are microscopic in most instances, and the synchronous neoplasms considered as multifocal independent primary carcinomas [12, 16, 20, 22].
Given the midline cervical location of TGDCCa, separating this lesion from a PTC of the thyroid gland involving the pyramidal lobe or metastatic to the Delphian lymph node is an important consideration [8, 16]. Distinction between these lesions can be problematic as evidenced in a recent study in which only 4 of 28 cancers initially classified as TGDCCa could be confirmed as such following detailed pathologic evaluation [16]. The presence of nonneoplastic thyroid parenchyma surrounding a PTC would favor a pyramidal lobe primary, while the finding of a dense lymphoid stroma associated with the tumor would support a Delphian node metastasis, though these histologic features should always be interpreted in the context of the clinical and radiographic findings.
A diagnosis of a primary TGDC carcinoma requires the histologic demonstration of an associated thyroglossal duct remnant lined by respiratory epithelium, squamous epithelium, or a combination of both. Identification of ectopic thyroid gland tissue further confirms the diagnosis [4, 9]. Some also recommend documentation of a normal thyroid gland that is free of malignancy prior to classifying a tumor unequivocally as a TGDC carcinoma [16, 18, 51]. This recommendation is difficult to satisfy in practice, however, as not all patients will undergo total thyroidectomy. In addition, synchronous thyroid gland and TGDC malignancies may represent independent primary tumors and do not necessarily indicate metastatic spread from the former to the latter site [16, 18, 20, 24]. All tumors in the present study were associated with cystic remnants of the thyroglossal duct identified histologically along with foci of ectopic thyroid gland tissue, supporting classification of these cases as primary TGDCCa.
Since TGDC malignancies are rarely recognized preoperatively, most TGDCCa are surgically managed initially as benign TGDCs. Excision via the Sistrunk procedure, which entails removal of the cyst along with the central portion of the hyoid bone as well as a core of tissue between the hyoid bone and the foramen cecum, is preferred and has been shown to be a significant predictor of overall survival [15]. Following adequate excision of the lesion, there are divergent opinions regarding further clinical management of patients with TGDC malignancies [23, 24]. While prior studies have concluded that the Sistrunk procedure alone represents adequate therapy for TGDCCa [18, 19, 21], others advocate the need for total thyroidectomy in all patients based on data reporting a potentially high risk of unsuspected thyroid gland involvement by a synchronous intrathyroidal papillary thyroid carcinoma [5, 13, 22, 24, 28, 31]. Thyroidectomy also allows for the option of radio ablative iodine therapy and more effective use of serum thyroglobulin levels for clinical surveillance. Among patients who had a thyroidectomy in the present study, there was an overall low incidence (38 %) of concurrent papillary thyroid gland carcinoma. However the majority (67 %) of these synchronous tumors were identified in patients 45 years of age or older. This suggests that while total thyroidectomy may not be indicated in all patients with TGDC papillary carcinomas, older patients may benefit from this procedure.
Regional lymph node metastases from TGDCCa have been described in 13–67 % of cases [6–14, 16, 21, 22, 25, 26, 28–35], with 14 % of this cohort observed to have cervical lymph node involvement at the time of initial presentation. Prophylactic lymph node dissection is not recommended on a routine basis, being reserved only for patients with clinically positive nodes [15, 25].
Risk group stratification has been suggested as a means of guiding therapeutic management of patients with TGDCCa [10, 12, 14, 15, 20, 24, 30, 52]. Patients are categorized as low or high risk based on various prognostic factors. The risk factors considered differ slightly among investigators, but in general use clinicopathologic parameters similar to those employed in the American Thyroid Association risk stratification system for differentiated carcinoma of the thyroid gland [53]. Patients <45 years of age with small tumors (cutoffs ranging from <1.0 up to 4.0 cm), exhibiting classical histology, no extracapsular spread, no vascular invasion, negative margins, no nodal or distant metastases, and a normal thyroid gland by imaging studies are defined as low risk and adequately treated by Sistrunk procedure alone. Patients not meeting these criteria are categorized as high risk and treated more aggressively with the addition of total thyroidectomy with or without lymph node dissection, and consideration of RAI therapy.
Given the various therapeutic options, utilization of a staging system would also be of value in determining appropriate treatment of TGDCCa. At present there is no stage classification system designed specifically for these tumors. The present study applied current AJCC (2010) staging criteria for cancer of the thyroid gland to carcinomas arising in thyroglossal duct cysts. In staging these tumors, involvement of adipose tissue, large nerves, perivascular tissue, and skeletal muscle was specifically considered equivalent to extrathyroidal extension in the thyroid gland and prompted assignment of the tumor to a pT3 category. This is based on the premise that the thyroglossal duct and ectopic thyroid gland tissue represent developmental extensions of the thyroid gland and thus tumors arising from these embryologic remnants would be subject to the same anatomic considerations as those originating in the thyroid gland proper. Studies have shown that the thyroid gland does not have a well-defined, fibrous connective tissue capsule [54]. Thus, even the definition of “extrathyroidal extension” for primary thyroid gland tumors is challenging. Further, skeletal muscle is commonly present over the isthmus or within the central neck (musculus levator glandulae thyroidae of Soemmerring). While there is still considerable interobserver variability about the detection of extrathyroidal extension [55], it seems that most pathologists use histologic features of tumor involving fat, skeletal muscle, nerve, and thick-walled vessels as major criteria to represent microscopic extrathyroidal extension [55], and thus similar criteria were employed here. In the present series, the majority of cases were staged group I (68 %) with the remainder, when using soft tissue extension criteria, group III (32 %). All group III patients were 45 years of age or older. It is important to remember that microscopic (minimal) soft tissue extension, while moving a tumor to the pT3 primary group, does not alter the overall group in patients <45 years. Thus, patient age is the most significant stage prognosticator. The one patient in this study who developed recurrent disease was classified stage group III. Clinical outcomes in this study were in keeping with these stage groupings as all patients were alive and without evidence of disease at last follow up (mean 3.8 years). Thus, based on data in this series and in the literature, patients <45 years of age with soft tissue (“extrathyroidal”) extension can be conservatively managed with a Sistrunk procedure only. Patients ≥45 years of age with soft tissue (“extrathyroidal”) extension should be classified as having a Group III tumor, and thus managed with follow-up completion thyroidectomy, appropriate for pT3 category tumors.
TGDC PTCs are associated with an excellent prognosis. They are generally indolent tumors with a small potential for locoregional recurrence [8, 10, 14, 15, 22, 25, 28, 30–32, 34, 35]. While no patients in this cohort had an adverse outcome, there have been rare reported cases of distant metastases and disease related mortality [10, 25, 31, 35].
Conclusion
Carcinomas associated with TGDCs are rare. The majority of these neoplasms are PTCs, likely arising from cyst associated ectopic thyroid gland remnants and representing primary tumors of cyst origin rather than metastatic disease from the thyroid gland. Patients are typically female in the 4th decade of life. Clinical presentation overlaps with that of benign TGDCs and diagnosis is usually established following surgical excision. The tumors are generally small, though not uncommonly involving adjacent soft tissues. Microcarcinomas, 1 cm or less in size in patients <45 years, are best managed conservatively by Sistrunk procedure alone. Complete thyroidectomy is recommended for tumors larger than 1 cm and in patients 45 years or older, as older patients have a higher incidence of concurrent papillary carcinoma of the thyroid gland and typically present with higher stage disease. Although there is no accepted stage classification system for papillary carcinoma developing in TGDCs, staging of these tumors based on AJCC (2010) criteria for thyroid gland malignancies, with specific consideration of soft tissue extension as equivalent to extrathyroidal extension, is advocated in order to guide treatment decisions and standardize management of these rare tumors.
Acknowledgments
Presented at the 104th Annual Meeting of the United States and Canadian Academy of Pathology, Seattle, WA, March 14–16, 2016.
Compliance with Ethical Standards
Conflict of interest
There are no conflicts of interest. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group.
References
- 1.Chou J, Walters A, Hage R, et al. Thyroglossal duct cysts: anatomy, embryology and treatment. Surg Radiol Anat. 2013;35:875–881. doi: 10.1007/s00276-013-1115-3. [DOI] [PubMed] [Google Scholar]
- 2.Allard RH. The thyroglossal cyst. Head Neck Surg. 1982;5:134–146. doi: 10.1002/hed.2890050209. [DOI] [PubMed] [Google Scholar]
- 3.Mondin V, Ferlito A, Muzzi E, et al. Thyroglossal duct cyst: personal experience and literature review. Auris Nasus Larynx. 2008;35:11–25. doi: 10.1016/j.anl.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Thompson LD, Herrera HB, Lau SK. A clinicopathologic series of 685 thyroglossal duct remnant cysts. Head Neck Pathol. 2016 doi: 10.1007/s12105-016-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturniolo G, Moleti M, Violi MA, et al. Prevalence of thyroglossal duct cyst carcinoma in adults having surgery for thyroglossal duct cysts. Thyroid. 2012;22:1191–1192. doi: 10.1089/thy.2012.0182. [DOI] [PubMed] [Google Scholar]
- 6.Olimpia CM, Carvalho MA, Zagalo C, et al. Papillary thyroid carcinoma of thyroglossal duct cyst: a retrospective analysis. Rev Laryngol Otol Rhinol (Bord) 2012;133:213–216. [PubMed] [Google Scholar]
- 7.Rossi ED, Martini M, Straccia P, et al. Thyroglossal duct cyst cancer most likely arises from a thyroid gland remnant. Virchows Arch. 2014;465:67–72. doi: 10.1007/s00428-014-1583-9. [DOI] [PubMed] [Google Scholar]
- 8.Wei S, LiVolsi VA, Baloch ZW. Pathology of thyroglossal duct: an institutional experience. Endocr Pathol. 2015;26:75–79. doi: 10.1007/s12022-015-9354-y. [DOI] [PubMed] [Google Scholar]
- 9.LiVolsi VA, Perzin KH, Savetsky L. Carcinoma arising in median ectopic thyroid (including thyroglossal duct tissue) Cancer. 1974;34:1303–1315. doi: 10.1002/1097-0142(197410)34:4<1303::AID-CNCR2820340442>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Doshi SV, Cruz RM, Hilsinger RL., Jr Thyroglossal duct carcinoma: a large case series. Ann Otol Rhinol Laryngol. 2001;110:734–738. doi: 10.1177/000348940111000807. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez JF, Ordonez NG, Schultz PN, et al. Thyroglossal duct carcinoma. Surgery. 1991;110:928–934. [PubMed] [Google Scholar]
- 12.Plaza CP, Lopez ME, Carrasco CE, et al. Management of well-differentiated thyroglossal remnant thyroid carcinoma: time to close the debate? Report of five new cases and proposal of a definitive algorithm for treatment. Ann Surg Oncol. 2006;13:745–752. doi: 10.1245/ASO.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Heshmati HM, Fatourechi V, van Heerden JA, et al. Thyroglossal duct carcinoma: report of 12 cases. Mayo Clin Proc. 1997;72:315–319. doi: 10.4065/72.4.315. [DOI] [PubMed] [Google Scholar]
- 14.Forest VI, Murali R, Clark JR. Thyroglossal duct cyst carcinoma: case series. J Otolaryngol Head Neck Surg. 2011;40:151–156. [PubMed] [Google Scholar]
- 15.Patel SG, Escrig M, Shaha AR, et al. Management of well-differentiated thyroid carcinoma presenting within a thyroglossal duct cyst. J Surg Oncol. 2002;79:134–139. doi: 10.1002/jso.10059. [DOI] [PubMed] [Google Scholar]
- 16.Zizic M, Faquin W, Stephen AE, et al. Upper neck papillary thyroid cancer (UPTC): a new proposed term for the composite of thyroglossal duct cyst-associated papillary thyroid cancer, pyramidal lobe papillary thyroid cancer, and Delphian node papillary thyroid cancer metastasis. Laryngoscope. 2016;126:1709–1714. doi: 10.1002/lary.25824. [DOI] [PubMed] [Google Scholar]
- 17.Weiss SD, Orlich CC. Primary papillary carcinoma of a thyroglossal duct cyst: report of a case and literature review. Br J Surg. 1991;78:87–89. doi: 10.1002/bjs.1800780127. [DOI] [PubMed] [Google Scholar]
- 18.Joseph TJ, Komorowski RA. Thyroglossal duct carcinoma. Hum Pathol. 1975;6:717–729. doi: 10.1016/S0046-8177(75)80080-3. [DOI] [PubMed] [Google Scholar]
- 19.Van Vuuren PA, Balm AJ, Gregor RT, et al. Carcinoma arising in thyroglossal remnants. Clin Otolaryngol Allied Sci. 1994;19:509–515. doi: 10.1111/j.1365-2273.1994.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 20.Carter Y, Yeutter N, Mazeh H. Thyroglossal duct remnant carcinoma: beyond the Sistrunk procedure. Surg Oncol. 2014;23:161–166. doi: 10.1016/j.suronc.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaques DA, Chambers RG, Oertel JE. Thyroglossal tract carcinoma. A review of the literature and addition of eighteen cases. Am J Surg. 1970;120:439–446. doi: 10.1016/S0002-9610(70)80003-4. [DOI] [PubMed] [Google Scholar]
- 22.Pellegriti G, Lumera G, Malandrino P, et al. Thyroid cancer in thyroglossal duct cysts requires a specific approach due to its unpredictable extension. J Clin Endocrinol Metab. 2013;98:458–465. doi: 10.1210/jc.2012-1952. [DOI] [PubMed] [Google Scholar]
- 23.Motamed M, McGlashan JA. Thyroglossal duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:106–109. doi: 10.1097/00020840-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Pribitkin EA, Friedman O. Papillary carcinoma in a thyroglossal duct remnant. Arch Otolaryngol Head Neck Surg. 2002;128:461–462. doi: 10.1001/archotol.128.4.461. [DOI] [PubMed] [Google Scholar]
- 25.Luna-Ortiz K, Hurtado-Lopez LM, Valderrama-Landaeta JL, et al. Thyroglossal duct cyst with papillary carcinoma: what must be done? Thyroid. 2004;14:363–366. doi: 10.1089/105072504774193195. [DOI] [PubMed] [Google Scholar]
- 26.Hartl DM, Al GA, Chami L, et al. High rate of multifocality and occult lymph node metastases in papillary thyroid carcinoma arising in thyroglossal duct cysts. Ann Surg Oncol. 2009;16:2595–2601. doi: 10.1245/s10434-009-0571-9. [DOI] [PubMed] [Google Scholar]
- 27.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7. Berlin: Springer; 2010. [Google Scholar]
- 28.Miccoli P, Minuto MN, Galleri D, et al. Extent of surgery in thyroglossal duct carcinoma: reflections on a series of eighteen cases. Thyroid. 2004;14:121–123. doi: 10.1089/105072504322880355. [DOI] [PubMed] [Google Scholar]
- 29.Pacheco-Ojeda L, Micheau C, Stafford N, et al. Papillary carcinoma in thyroglossal duct remnants. Eur Arch Otorhinolaryngol. 1991;248:268–270. doi: 10.1007/BF00176752. [DOI] [PubMed] [Google Scholar]
- 30.Page CP, Kemmerer WT, Haff RC, et al. Thyroid carcinomas arising in thyroglossal ducts. Ann Surg. 1974;180:799–803. doi: 10.1097/00000658-197411000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzodic R, Markovic I, Stanojevic B, et al. Surgical management of primary thyroid carcinoma arising in thyroglossal duct cyst: an experience of a single institution in Serbia. Endocr J. 2012;59:517–522. doi: 10.1507/endocrj.EJ12-0070. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell M, Grixti M, Harmer C. Thyroglossal duct carcinoma: presentation and management, including eight cases reports. Clin Oncol (R Coll Radiol) 1998;10:186–190. doi: 10.1016/S0936-6555(98)80066-6. [DOI] [PubMed] [Google Scholar]
- 33.Chala A, Alvarez A, Sanabria A, et al. Primary papillary carcinoma in thyroglossal cysts. Case reports and literature review. Acta Otorrinolaringol Esp. 2016;67:102–106. doi: 10.1016/j.otorri.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Chrisoulidou A, Iliadou P, Doumala E, et al. Thyroglossal duct cyst carcinomas: is there a need for thyroidectomy? Hormones Athens. 2013;12:522–528. doi: 10.14310/horm.2002.1440. [DOI] [PubMed] [Google Scholar]
- 35.Choi YM, Kim TY, Song DE, et al. Papillary thyroid carcinoma arising from a thyroglossal duct cyst: a single institution experience. Endocr J. 2013;60:665–670. doi: 10.1507/endocrj.EJ12-0366. [DOI] [PubMed] [Google Scholar]
- 36.Cignarelli M, Ambrosi A, Marino A, et al. Three cases of papillary carcinoma and three of adenoma in thyroglossal duct cysts: clinical-diagnostic comparison with benign thyroglossal duct cysts. J Endocrinol Invest. 2002;25:947–954. doi: 10.1007/BF03344066. [DOI] [PubMed] [Google Scholar]
- 37.Yang YJ, Haghir S, Wanamaker JR, et al. Diagnosis of papillary carcinoma in a thyroglossal duct cyst by fine-needle aspiration biopsy. Arch Pathol Lab Med. 2000;124:139–142. doi: 10.5858/2000-124-0139-DOPCIA. [DOI] [PubMed] [Google Scholar]
- 38.Chang TJ, Chang TC, Hsiao YL. Fine needle aspiration cytology of thyroglossal duct cyst: an analysis of 10 cases. Acta Cytol. 1999;43:321–322. doi: 10.1159/000331177. [DOI] [PubMed] [Google Scholar]
- 39.Jayaram G, Pathmanathan R. Papillary carcinoma arising in a thyroglossal duct cyst and diagnosed by fine needle aspiration cytology. Acta Cytol. 1999;43:532–534. [PubMed] [Google Scholar]
- 40.Koybasioglu F, Simsek GG, Onal BU. Tall cell variant of papillary carcinoma arising from a thyroglossal cyst: report of a case with diagnosis by fine needle aspiration cytology. Acta Cytol. 2006;50:221–224. doi: 10.1159/000325937. [DOI] [PubMed] [Google Scholar]
- 41.Bardales RH, Suhrland MJ, Korourian S, et al. Cytologic findings in thyroglossal duct carcinoma. Am J Clin Pathol. 1996;106:615–619. doi: 10.1093/ajcp/106.5.615. [DOI] [PubMed] [Google Scholar]
- 42.Falconieri G, Della LD, Zanella M. Papillary thyroid carcinoma of the thyroglossal duct cyst: comparative cytohistologic and immunochemical study of 2 new cases and review of the literature. Int J Surg Pathol. 2001;9:65–71. doi: 10.1177/106689690100900114. [DOI] [PubMed] [Google Scholar]
- 43.Ranieri E, D’Andrea MR, Vecchione A. Fine needle aspiration cytology of squamous cell carcinoma arising in a thyroglossal duct cyst. A case report. Acta Cytol. 1996;40:747–750. doi: 10.1159/000333951. [DOI] [PubMed] [Google Scholar]
- 44.Branstetter BF, Weissman JL, Kennedy TL, et al. The CT appearance of thyroglossal duct carcinoma. AJNR Am J Neuroradiol. 2000;21:1547–1550. [PMC free article] [PubMed] [Google Scholar]
- 45.Glastonbury CM, Davidson HC, Haller JR, et al. The CT and MR imaging features of carcinoma arising in thyroglossal duct remnants. AJNR Am J Neuroradiol. 2000;21:770–774. [PMC free article] [PubMed] [Google Scholar]
- 46.Gaddikeri S, Vattoth S, Gaddikeri RS, et al. Congenital cystic neck masses: embryology and imaging appearances, with clinicopathological correlation. Curr Probl Diagn Radiol. 2014;43:55–67. doi: 10.1067/j.cpradiol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Shemen L, Sherman CH, Yurovitsky A. Imaging characteristics and findings in thyroglossal duct cyst cancer and concurrent thyroid cancer. BMJ Case Rep. 2016 doi: 10.1136/bcr-2016-215059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aculate NR, Jones HB, Bansal A, et al. Papillary carcinoma within a thyroglossal duct cyst: significance of a central solid component on ultrasound imaging. Br J Oral Maxillofac Surg. 2014;52:277–278. doi: 10.1016/j.bjoms.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Naphade PS, Bhagwat MR, Keraliya AR, et al. Photoclinic. Thyroglossal duct carcinoma. Arch Iran Med. 2015;18:203–204. [PubMed] [Google Scholar]
- 50.Nuttall FQ. Cystic metastases from papillary adenocarcinoma of the thyroid with comments concerning carcinoma associated with thyroglossal remnants. Am J Surg. 1965;109:500–505. doi: 10.1016/S0002-9610(65)80185-4. [DOI] [PubMed] [Google Scholar]
- 51.Widstrom A, Magnusson P, Hallberg O, et al. Adenocarcinoma originating in the thyroglossal duct. Ann Otol Rhinol Laryngol. 1976;85:286–290. doi: 10.1177/000348947608500215. [DOI] [PubMed] [Google Scholar]
- 52.Tradati N, DePaoli F, Benazzo M, et al. Papillary carcinoma in thyroglossal duct remnants: presentation of four cases and decision procedure for prophylactic thyroid gland dissection. Oncol Rep. 2000;7:1349–1353. doi: 10.3892/or.7.6.1349. [DOI] [PubMed] [Google Scholar]
- 53.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol. 2010;17:386–391. doi: 10.1245/s10434-009-0832-7. [DOI] [PubMed] [Google Scholar]
- 55.Su HK, Wenig BM, Haser GC, et al. Inter-observer variation in the pathologic identification of minimal extrathyroidal extension in papillary thyroid carcinoma. Thyroid. 2016;26:512–517. doi: 10.1089/thy.2015.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]