Abstract
Primary lingual intestinal-type adenocarcinomas are extremely rare with only a few cases described. A case with immunohistochemical expression of Androgen Receptor (AR) which was treated solely by chemo-radiotherapy is reported herein. A 54-year-old male was referred with symptoms of fullness in his tongue. Clinical examination showed an asymmetry of the tongue with a hard mass palpable within the middle of the tongue. Biopsy showed intestinal-type adenocarcinoma. The tumour showed positive staining with cytokeratin 7, cytokeratin 20, CDX2, AR, β-catenin and was mismatch repair proteins (MMR) proficient. The molecular analysis did not show mutations in the KRAS, NRAS, BRAF and PIK3CA genes. The patient was treated with radiochemotherapy and is in remission 3.5 years after the diagnosis. This is the first case of intestinal-type tongue adenocarcinoma which showed AR expression and was treated solely with radical chemoradiotherapy.
Keywords: Tongue, Adenocarcinoma, Salivary gland, Intestinal type, Androgen receptor
Introduction
Primary tongue adenocarcinoma is a rare neoplasm, which mainly stems from the minor salivary glands with identical histotypes as in major salivary glands such as adenoid cystic carcinoma, mucoepidermoid carcinoma, carcinoma ex pleomorphic adenoma, etc. The rare types of carcinomas such as cribriform adenocarcinoma and hyalinising clear cell carcinoma, also involve the tongue with a non-random preference [1, 2]. Primary colonic type adenocarcinoma of the tongue is a recently reported neoplasm with only four cases described in the literature [3–5].
The authors present an additional case of lingual intestinal-type adenocarcinoma with immunohistochemical and molecular analysis.
To the best of our knowledge this is the first case of this extraordinarily rare tumour, showing immunohistochemical positive staining with Androgen Receptor (AR) and treated solely by chemo-radiotherapy.
Case Presentation
A 54 year old male was referred in 2012 with 6 weeks of symptoms of fullness in his tongue during eating. Clinical examination showed an asymmetry of the tongue, restriction of tongue movement and deviation to the left on protrusion with a hard mass palpable within the substance of the middle third of the tongue. The mucosa was normal.
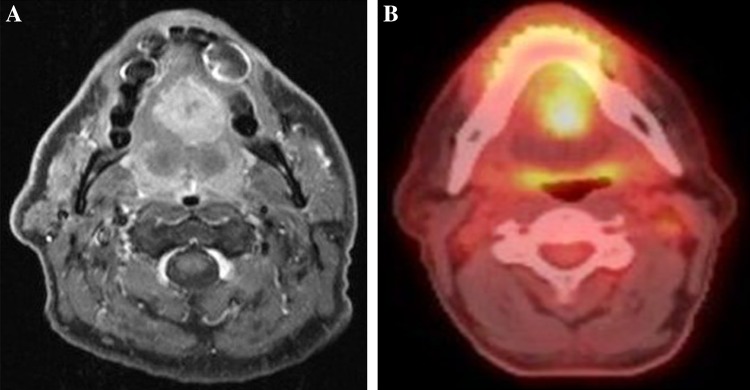
A biopsy was performed and histological examination revealed a moderately differentiated colonic-type adenocarcinoma. A MRI showed a 54 mm solid tumour, with no cystic component, extending between the genioglossus muscles within the tongue (Fig. 1a). The patient underwent PET–CT scan which showed a metabolically active tumour in the mid tongue (Fig. 1b) and a single cold 7 mm nodule in the left upper lobe of the lung.
Fig. 1.
a and b MRI post-gadolinium axial T1 fat suppressed image showing enhancing lesion in the mid tongue. Pre-treatment PET–CT scan showing a metabolically active tumour in the mid tongue, Standardized Uptake Value (SUV) max 10
The tumour was staged T4N0M0. No abnormality was seen in the sinonasal tract on MRI or on the PET scan. Surgery would have been mutilating.
Chemo-radiotherapy was offered. Colonoscopy and dental extractions prior to radical chemo-radiotherapy treatment were performed. Colonoscopy did not reveal any abnormality.
Two courses of Cisplatinum 60 mg/m2 day 1 and capecytabine 625 mg/m2 bd days 1–21 repeated 3 weekly were given prior to radiotherapy 65 Gy in 30 fractions using Intensity Modulated Radiotherapy (IMRT) with one further course of chemotherapy during radiotherapy. The 4th course was cancelled due to severe depression.
At 3 months after the end of radiotherapy CT scan and MRI showed virtually no response in the primary tumour but a PET–CT scan was encouraging at 4 months (Fig. 2).
Fig. 2.
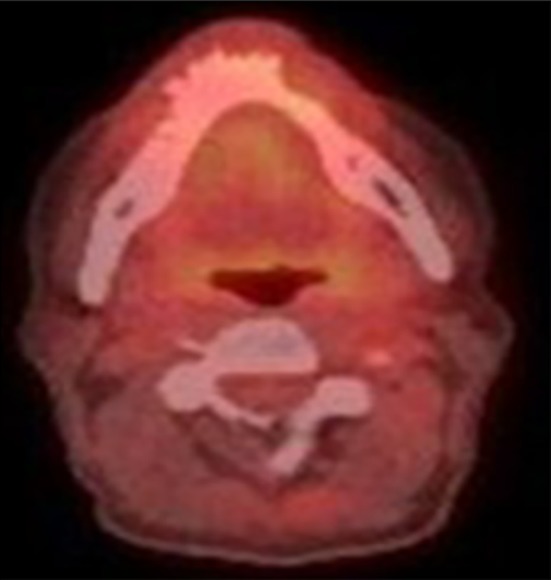
Post-treatment PET–CT scan showing complete metabolic response to treatment
Six months after the end of treatment a chest X-ray showed a number of small lung metastases. These were initially managed with observation after they were assessed as being too numerous for local ablative treatment. Biopsy was not considered necessary. A year later they became symptomatic and he underwent palliative chemotherapy with Carboplatin and Capecitabine (1,000 mg/m2 bd days 1–14) four 3-weekly cycles stopping because of good response and fatigue preventing him form working. 10 months later he restarted this regime after return of his symptoms. Raltitrexed (6 mg iv 3 weekly) was substituted for Capecitabine after one course because of a cardiac ischaemic episode. After completing five courses he is again in remission working full-time 3.5 years after diagnosis and 2.5 years after developing lung metastases.
Methods
Immunohistochemistry
Serial sections at 3 μm were cut from Formalin Fixed Paraffin Embedded (FFPE) tissue blocks and mounted onto adhesive slides (Surgipath Snowcoat) and dried at 65 °C for 15 min. Each series of sections were stained on Bond Max Automated Immunohistochemistry System Leica XL Stainer using Novolink Polymer Detection Systems. All antigen retrieval methods were performed on board. Each run included a negative control, where the primary antibody was omitted.
Immunostaining for Cytokeratin 7 (CK7), Cytokeratin 20 (CK20), CDX2, β-catenin, Thyroid Transcription Factor 1 (TTF1), p63, Androgen Receptor (AR) and Prostate Specific Antigen (PSA) was performed. Microsatellite instability status was determined by immunostaining for mismatch repair proteins hMLH1, hMSH2, hMSH6 and hPMS2 [6, 7].
DNA Analysis
3 × 10 μm unstained FFPE tissue sections were used for DNA extraction. The neoplastic nuclei content and cellularity were recorded and an assessment made as to whether macrodissection was required to enrich the tumour cells prior to DNA extraction of the sample.
Genomic DNA was isolated from the three FFPE tissue sections using the QIAamp® DNA FFPE tissue kit (Qiagen, Crawley, UK), according to the manufacturers instructions. DNA was quantified using the high sensitivity Qubit® 2.0 assay kit and fluorometer (Life Technologies, Paisley, UK).
The DNA was then subjected to sequencing using a TruSeq Custom Amplicon (TSCA) panel (Illumina, San Diego, CA, USA) that targets specific hotspot regions for KRAS (exon 2–4), NRAS (exon 2–4), PIK3CA (exon 10 and 21) and BRAF (exon 15) and all the coding exons of TP53 (exons 2–11) including flanking intronic regions. The sensitivity of the assay is >95 % and the specificity is >98 %, with a detection limit of 3 % allele frequency. The TSCA panel is optimised to work with 30 μL of genomic DNA at >6 ng/μL (180 ng in total) to obtain amplicon sizes of 80–150 bp. Sequencing was performed on the MiSeq platform and analysed using Illumina VariantStudio 2.2 (Illumina, San Diego, CA, USA).
Results
Histopathology
Neoplasm was an invasive moderately differentiated colonic-type adenocarcinoma with tubulo-glandular growth pattern showing pseudo stratification and loss of polarity of nuclei. The mitoses were frequent. The cells showed moderate nuclear pleomorphism with elongated (cigar shaped) nuclei. The chromatin was homogeneous and generally the nucleoli were inconspicuous. Intraglandular necrosis was focally present. Neural invasion was detected, but no lymphovascular invasion was seen. No frank mucinous differentiation was observed. The overlying squamous epithelium was not included (Fig. 3a, b).
Fig. 3.
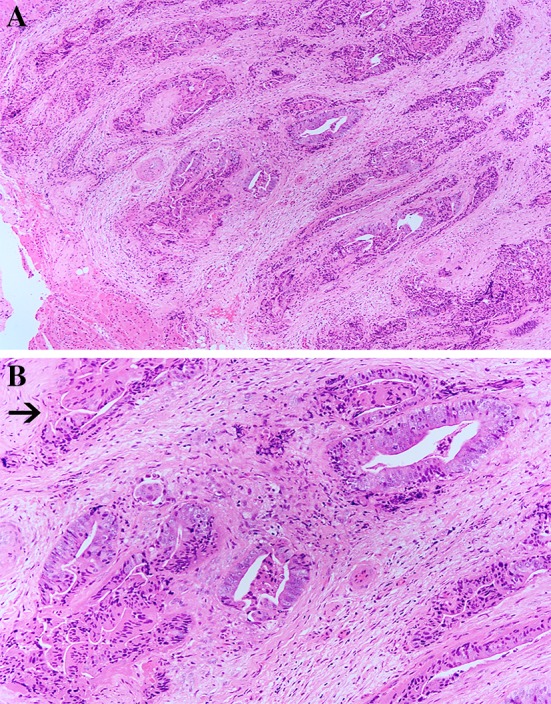
a and b Moderately differentiated intestinal-type adenocarcinoma with glands and tubular structures. The glands are lined by cells with elongated nuclei. The arrow indicates nerve invasion (original magnification ×40; Haematoxylin and Eosin)
Immunohistochemistry
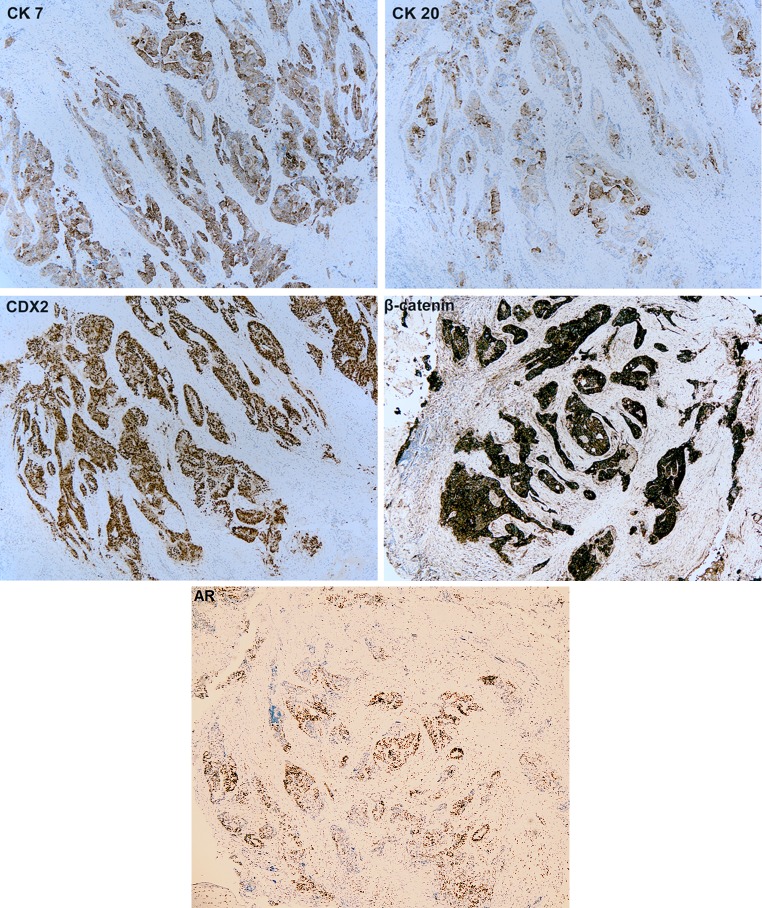
The tumour showed strong and diffuse positive cytoplasmic staining with CK7 and focal, moderate cytoplasmic positivity with CK20. There was strong and diffuse nuclear and membrane staining with CDX2 and AR and β-catenin, respectively (Fig. 4). Also, a positive nuclear staining for hMLH1, hMSH2, hMSH6, and hPMS-2 mismatch repair proteins was observed. TTF1, p63 and PSA were negative.
Fig. 4.
Immunohistochemistry shows intense and diffuse positive cytoplasmic staining with CK 7 and nuclear staining with CDX2 and AR while CK 20 is patchy and moderate. Lesional cells manifest strong positive staining for β-catenin
Molecular Analysis
The sample was representative of the tumour population containing approximately 30 % neoplastic nuclei with intermediate cellularity. Macrodissection was not performed. No mutations were detected in the regions analysed in the KRAS, NRAS, BRAF and PIK3CA genes.
A missense mutation was detected in exon 6 of the TP53 gene; c.581T >A p.(Leu194His).
Discussion
Primary intestinal-type adenocarcinomas of the head and neck mucosa are rare and the majority of cases have been reported in the sinonasal tract [8].
Intestinal-type lingual adenocarcinoma is an extremely rare disease with few case reported in the literature [3–5].
We report a primary colonic-type adenocarcinoma of the tongue in a patient with no previous history of intestinal adenocarcinoma. As in previous reports [3–5], the neoplasm occurred in the absence of a primary large intestine adenocarcinoma.
Despite the histopathological characteristics of intestinal adenocarcinoma of the tumour, and diffuse positive nuclear immunostaining with CDX2, we concluded that the lesion was primary, because lingual metastatic intestinal adenocarcinomas are usually preceded by colonic manifestations [9–11].
Furthermore, pancolonoscopy did not show any malignancy and follow-up after 3 years did not identify the presence of a primary colonic adenocarcinoma. Immunohistochemistry showed patchy and moderate positivity with CK 20, while CK7 was intense and diffuse. This finding supports the possible primary salivary gland origin of this neoplasm.
The expression of CK7 is variable in the previous reported cases of intestinal type tongue adenocarcinoma. The cases described by Slova et al. and McDaniel et al. showed a positive staining while in cases reported by Bell et al. the staining was entirely negative in one case and focally positive in another case.
The positive staining with AR, which is a characteristic of salivary duct carcinoma, has not been previously reported in colonic-type tongue adenocarcinoma and, therefore, we cannot state whether it is a casual finding or an immunohistochemical marker of this neoplasm.
The occurrence of intestinal-type adenocarcinoma (ITAC) in head and neck mucosa is well known and clinicians and pathologists are familiar with ITAC in the sinonasal tract. The histogeneis of this rare histotype of the sinonasal tract is unclear. The most plausible hypothesis is an origin from a transformed duct epithelium [12–17]. Transformation of the non-neoplastic respiratory epithelium of the sinonasal tract to an intestinal phenotype, adjacent to adenocarcinoma, has been reported [7, 18–21].
In the light of a report of an adenocarcinoma in a foregut duplication cyst of the floor of the mouth [22], an origin from gastrointestinal elements might represent a possible histogenesis for intestinal type tongue adenocarcinoma. However, even though we were not dealing with a complete resection specimen, a cystic component was not found in our case.
An additional hypothesis for histogenesis could be malignant transformation of a choristoma containing gastrointestinal tissue [23].
Comparative genomic hybridization (CGH) analysis showed different chromosomal abnormalities in ITACs which may cause genetic alteration such as PTEN and APC loss with consequent alteration of Wnt signaling pathway. Other genes which might be invoved are E-cadherin, TP53, DCC, DPC4, SMAD2, SMAD4, p14, p16, MOS and MYCC, LGALS4 and CLU [24].
Szablewski et al. [25] found that a subset of ITACs present KRAS mutations and frequently express EGF. They also hypothesised that KRAS mutations may predict the prognosis in ITACs.
A study showed that the MET signaling pathway is involved in the ITAC cancerogenesis [26].
We carried out the molecular analysis of a group of genes which present most prevalent mutations in colon adenocarcinoma. Our aim was to detect a possible mutation in these genes which could play a role in terms of target therapy.
We did not find any alteration in the KRAS, BRAF and PIK3CA genes which are relatively common to intestinal adenocarcinoma. However, Yom et al. [7] only detected a single KRAS mutation in one of the six sinonasal colonic-type adenocarcinoma they reported.
The tumour described herein was MMR proficient and expressed MLH1, hMSH2, hMSH6 and hPMS-2 proteins. Similar findings have been described in sinonasal tract colonic-adenocarcinomas [7].
No mutation was detected in NRAS, BRAF and PIK3CA genes. A mutation in TP53 gene was found, which is non specific and common to different types of malignancies. Nevertheless, due to the limited number of lingual intestinal-type adenocarcinomas analysed for molecular alterations, any definitive conclusions with regard to their molecular profile would be premature.
In summary, we report a rare case of intestinal-type tongue adenocarcinoma, possibly originating from malignant transformation of minor salivary duct epithelium, with lung metastasis. This is the first case where the patient was solely treated with radical chemo-radiotherapy. The patient is alive after 3.5 years from the initial diagnosis, having responded to palliative chemotherapy chosen because of the intestinal phenotype of the tumour.
Acknowledgments
The Authors are grateful to Dr Lisa Thompson of the Royal Marsden Hospital, London-United Kingdom and Dr Ihezue Chukwumobi of the Queen Alexandra Hospital, Portsmouth-United Kingdom for their valuable help in performing molecular analysis and elaborating images, respectively.
Compliance with ethical standards
Conflict of interest
No conflict of interest to disclose.
References
- 1.Michal M, Skálová A, Simpson RH, et al. Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology. 1999;35(6):495–501. doi: 10.1046/j.1365-2559.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 2.Balakrishnan R, Nayak DR, Pillai S, Rao L. Hyalinizing clear cell carcinoma of the base of the tongue. J Laryngol Otol. 2002;116(10):851–853. doi: 10.1258/00222150260293718. [DOI] [PubMed] [Google Scholar]
- 3.Bell D, Kupferman ME, Williams MD, Rashid A, El-Naggar AK. Primary colonic-type adenocarcinoma of the base of the tongue: a previously unreported phenotype. Hum Pathol. 2009;40:1798–1802. doi: 10.1016/j.humpath.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slova D, Mondolfi AP, Moisini I, et al. Colonic-type adenocarcinoma of the base of the tongue: a case report of a rare neoplasm. Head Neck Pathol. 2012;6:250–254. doi: 10.1007/s12105-011-0301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDaniel AS, Burgin SJ, Bradford CR, McHugh JB. Pathology quiz case 2. Diagnosis: primary colonic-type adenocarcinoma of the tongue. JAMA Otolaryngol Head Neck Surg. 2013;139(6):653–654. doi: 10.1001/jamaoto.2013.3240a. [DOI] [PubMed] [Google Scholar]
- 6.McKinney CD, Mills SE, Franquemont DW. Sinonasal intestinal-type adenocarcinoma: immunohistochemical profile and comparison with colonic adenocarcinoma. Mod Pathol. 1995;8:421–426. [PubMed] [Google Scholar]
- 7.Yom SS, Rashid A, Rosenthal DI, et al. Genetic analysis of sinonasal adenocarcinoma phenotypes: distinct alterations of histogenetic significance. Mod Pathol. 2005;18:315–319. doi: 10.1038/modpathol.3800315. [DOI] [PubMed] [Google Scholar]
- 8.Wenig BM, editor. Atlas of head and neck pathology. 2. Philadelphia: Saunders-Elsevier; 2008. pp. 121–123. [Google Scholar]
- 9.Piattelli A. Lingual metastasis from a carcinoma of the colon. A case report and review of the literature. Acta Stomatol Belg. 1990;87:257–264. [PubMed] [Google Scholar]
- 10.Hatziotis JC, Constantinidou H, Papanayotou PH. Metastatic tumors of the oral soft tissues. Review of the literature and report of a case. Oral Surg Oral Med Oral Pathol. 1973;36:544–550. doi: 10.1016/0030-4220(73)90312-5. [DOI] [PubMed] [Google Scholar]
- 11.Davidson C. Chorioepithelion und Magenkrebs eine selten Verschmelzung zweier boesartiger Geschwulste. Chaite-Ann. 1905;29:426–437. [Google Scholar]
- 12.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Choi HR, Sturgis EM, Rashid A, et al. Sinonasal adenocarcinoma: evidence for histogenetic divergence of the enteric and nonenteric phenotypes. Hum Pathol. 2003;34:1101–1107. doi: 10.1053/j.humpath.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Donhuijsen K, Hattenberger S, Schroeder HG. Nasal sinus carcinoma after wood dust exposure. Morphological spectrum of 100 cases. Pathologe. 2004;25:14–20. doi: 10.1007/s00292-003-0668-z. [DOI] [PubMed] [Google Scholar]
- 15.Franquemont DW, Fechner RE, Mills SE. Histologic classification of sinonasal intestinal-type adenocarcinoma. Am J Surg Pathol. 1991;15:368–375. doi: 10.1097/00000478-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gnepp DR, Heffner DK. Mucosal origin of sinonasal tract adenomatous neoplasms. Mod Pathol. 1989;2:365–371. [PubMed] [Google Scholar]
- 17.Hopkin N, McNicoll W, Dalley VM, Shaw HJ. Cancer of the paranasal sinuses and nasal cavities. Part I. Clinical features. J Laryngol Otol. 1984;98:585–595. doi: 10.1017/S0022215100147140. [DOI] [PubMed] [Google Scholar]
- 18.Bashir AA, Robinson RA, Benda JA, Smith RB. Sinonasal adenocarcinoma: immunohistochemical marking and expression of oncoproteins. Head Neck. 2003;25:763–771. doi: 10.1002/hed.10285. [DOI] [PubMed] [Google Scholar]
- 19.Ariza M, Llorente JL, Alvarez-Marcas C, et al. Comparative genomic hybridization in primary sinonasal adenocarcinomas. Cancer. 2004;100:335–341. doi: 10.1002/cncr.11931. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy MT, Jordan RC, Berean KW, Perez-Ordoñez B. Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinoma. J Clin Pathol. 2004;57:932–937. doi: 10.1136/jcp.2004.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korinth D, Pacyna-Gengelbach M, Deutschmann N, et al. Chromosomal imbalances in wood dust-related adenocarcinomas of the inner nose and their associations with pathological parameters. J Pathol. 2005;207:207–215. doi: 10.1002/path.1819. [DOI] [PubMed] [Google Scholar]
- 22.Volchok J, Jaffer A, Cooper T, Al-Sabbagh A, Cavalli G. Adenocarcinoma arising in a lingual foregut duplication cyst. Arch Otolaryngol Head Neck Surg. 2007;133:717–719. doi: 10.1001/archotol.133.7.717. [DOI] [PubMed] [Google Scholar]
- 23.Gorlin RJ, Jirasek JE. Oral cysts containing gastric or intestinal mucosa: unusual embryological accident of heterotopia. Arch Otolaryngol. 1970;91:594–597. doi: 10.1001/archotol.1970.00770040824018. [DOI] [PubMed] [Google Scholar]
- 24.Hoeben A, van de Winkel L, Hoebers F, Kross K, Driessen C, Slootweg P, Tjan-Heijnen VC, van Herpen C. Intestinal-typr sinonasal adenocarcinomas: the road to molecular diagnosis and personalized treatment. Head Neck. 2016 doi: 10.1002/hed.24416. [DOI] [PubMed] [Google Scholar]
- 25.Szablewski V, Solassol J, Poizat F, Larrieux M, Crampette L, Mange A, Bascoul-Mollevi C, Costes V. EGFR expression and KRAS and BRAF mutational status in intestinal-type sinonasal adenocarcinoma. Int J Mol Sci. 2013;14:5170–5181. doi: 10.3390/ijms14035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Projetti F, Mesturoux L, Coulibaly B, Durand K, Chaunavel A, Leobon S, Gadeaud E, Caire F, Bessede JP, Labrousse F. Study of MET protein levels and MET gene copy number in 72 sinonasal intestinal-type adenocarcinomas. Head Neck. 2015;37(11):1563–1568. doi: 10.1002/hed.23795. [DOI] [PubMed] [Google Scholar]