Abstract
Human immune deficiency virus (HIV) is a leading cause of death. It attacks the immune system, thereby rendering the infected host susceptible to many HIV-associated infections, malignancies and neurocognitive disorders. The altered immune system affects the way the human host responds to disease, resulting in atypical presentation of these disorders. This presents a diagnostic challenge and the clinician must use all diagnostic avenues available to diagnose and manage these conditions. The advent of highly active antiretroviral therapy (HAART) has markedly reduced the mortality associated with HIV infection but has also brought in its wake problems associated with adverse effects or drug interaction and may even modulate some of the HIV-associated disorders to the detriment of the infected human host. Nuclear medicine techniques allow non-invasive visualisation of tissues in the body. By using this principle, pathophysiology in the body can be targeted and the treatment of diseases can be monitored. Being a functional imaging modality, it is able to detect diseases at the molecular level, and thus it has increased our understanding of the immunological changes in the infected host at different stages of the HIV infection. It also detects pathological changes much earlier than conventional imaging based on anatomical changes. This is important in the immunocompromised host as in some of the associated disorders a delay in diagnosis may have dire consequences. Nuclear medicine has played a huge role in the management of many HIV-associated disorders in the past and continues to help in the diagnosis, prognosis, staging, monitoring and assessing the response to treatment of many HIV-associated disorders. As our understanding of the molecular basis of disease increases nuclear medicine is poised to play an even greater role. In this review we highlight the functional basis of the clinicopathological correlation of HIV from a metabolic view and discuss how the use of nuclear medicine techniques, with particular emphasis of F-18 fluorodeoxyglucose, may have impact in the setting of HIV. We also provide an overview of the role of nuclear medicine techniques in the management of HIV-associated disorders.
Keywords: FDG PET, Nuclear medicine, HIV-associated malignancies, HIV-associated infections, HIV-associated neurocognitive disease, HAART
Introduction
Human immune deficiency virus (HIV) is a leading cause of death worldwide. As of December 2014, an estimated 36.9 million people were living with the infection; in 2014, 2 million were newly infected and 1.2 million died [1, 2]. HIV is a lentevirus that affects the human immune system [3]. It causes immune suppression that becomes more profound as the disease progresses. The immune suppression renders the infected patient susceptible to many opportunistic infections, malignant processes and disorders of other systems in the human body. The opportunistic diseases that develop in these immune suppressed patients may have different presentations compared to the same diseases in persons with an intact immune system. This may result in diagnostic challenges for the clinician. This situation makes it imperative to understand the use and limitations of all the diagnostic options (including nuclear medicine) available to the clinician to make a rapid and accurate diagnosis, staging, prognosis and assess response to therapy of HIV infection itself or the associated diseases.
The advent of highly active antiretroviral therapy (HAART) has had a remarkable impact on HIV presentation and management [4, 5]. It has changed what was once a progressive and almost fatal disease to a chronic infection. This has also had an impact on the way disease manifests in HIV patients, which further complicates an already challenging situation. On the one hand, HAART has caused a decline in incidence of AIDS-defining malignancies such as Kaposi sarcoma. On the other hand, there has been an increase in malignancy as the patients live longer and histology types have changed. The HAART itself is also not without problems. HAART is taken for long periods (usually for the rest of life) and the patients are exposed to various adverse effects. Also, drug interactions with treatment for other comorbid conditions frequently present in this population. In some HIV-associated disorders, the toxicity has been postulated to be a contributing factor of some of the disorders seen in HIV patients, such as HIV-associated neurocognitive deficits (HAND) [5].
Historical Perspective of Nuclear Medicine and HIV
Nuclear medicine has played a role in the management of HIV and AIDS in the past. Imaging with Gallium-67 (67Ga) citrate has long been known to detect some opportunistic infections, such as Pneumocystis pneumonia, when plain radiographs were normal. The use of thallium-201 (201Tl) and 67Ga citrate concurrently to distinguish non-Hodgkin’s lymphoma, Kaposi sarcoma and an infective process has been invaluable to clinicians as all these diseases could have similar clinical presentation but the management is very different and could be rapidly fatal if not rapidly and accurately diagnosed and managed appropriately [6]. These diseases usually involve sites where tissue diagnosis is difficult or even dangerous (due to underlying immune suppression and invasiveness of procedure for getting tissue) such as the brain or lung. Nuclear medicine was found helpful in making rapid and appropriate clinical decisions without subjecting these patients to invasive procedures. In many cases response to therapy could also be monitored.
Advances in Nuclear Medicine and HIV
Recent advances in nuclear medicine and the development of hybrid cameras with integrated functional and anatomical imaging has extended the role of nuclear medicine in HIV further. Positron emission tomography integrated with computed tomography (PET/CT) is able to correlate the anatomic and pathophysiological process of the immune system to provide a non-invasive way of assessing the immune status of HIV-infected patients. This has not only improved our understanding of the disease but also had an effect on managing the disease and the associated opportunistic infection or malignancies. Nuclear medicine techniques are also very helpful in providing alternative diagnosis by directing the site of biopsy. In malignancies, nuclear medicine is able to detect, stage, monitor therapy, assess for recurrence and provide prognosis of the malignancy. In infection, the role of nuclear medicine continues to expand and new nuclear medicine techniques are used [7]. Nuclear medicine procedures are in general sensitive but may suffer from lack of specificity. For example, in an HIV patient where a nuclear scan is used to evaluate the patient for malignancy, false-positive results may occur, since positive lymph nodes may also be the result of inflammation or a benign reaction due to HIV.
Pathophysiology and Natural History of HIV
HIV gains entry into the human host by crossing mucosal surfaces to gain access to the blood [8]. In the circulation, HIV binds to resting CD4 T-lymphocytes, causing them to home from the blood into the lymph nodes. In the lymph nodes, the infected CD4 lymphocytes are induced into apoptosis. The clinical manifestation of this pathological process is the development of a generalised peripheral and clinically discernible lymphadenopathy that may involve lymph nodes in unusual sites such as the epitroclear nodes in the early and midstage of the disease. These nodes eventually become clinically impalpable as they undergo involution due to atrophy in the latter stages of the disease. The activation of resting T-lymphocytes is an energy-dependent process, in which the lymphocytes switch to glycolysis and increase the glucose uptake by about 20-fold over 24 h [5]. This increase enables the process to be visualised by nuclear medicine imaging techniques such as 18F-fluorodeoxyglucose (FDG)-PET or FDG-PET/CT. This has helped in the interpretation of FDG-PET scans in the setting of HIV and has assisted in the management of HIV and other comorbid conditions (Table 1). In the tissues, HIV is taken up by macrophages and dendritic cells that express CD4. In the tissues, HIV virus may remain quiescent in sanctuary sites such as the brain or testes, escaping the immune defences mounted by the host. This usually occurs in the midstage phase of the disease after an initial acute phase where the patient now has no symptoms and the viral loads in the blood remain relatively stable. This stage is referred to as chronic infection or the patient is said to be a non-progressor. Eventually host immunity would fail as the peripheral CD4 lymphocyte count drops to a critical level and viral replication is no longer kept in check but rapidly increases. At this stage, the patient becomes symptomatic as immunity further drops and the patient is said to have developed acquired immune deficiency syndrome (AIDS), where certain AIDS-defining symptoms and diseases, such as AIDS-defining malignancies or infections or severe weight loss, manifest.
Table 1.
Correlation of immunological state in HIV and FDG uptake
| Author | Journal | Study | Objective | Comment or significant finding |
|---|---|---|---|---|
| Sharko et al. 1996 [9] | Proc Natl Acad Sci USA | Preclinical- Rhesus monkeys | Determined if FDG was able to identify activated lymphoid tissue and reflect extent of infection. | Infected animals were distinguishable from uninfectedcontrols. There was widespread lymphoid activation which correlated to area of viral replication. Significant abdominopelvic lymph node activation occurred particularly in terminal stages of disease. Fewer tissues had high FDG uptake in terminal animals compared with midstage animals. |
| Wallace et al. 2002 [10] | Virology | Preclinical- Rhesus monkeys | Determined if FDG reflected extent of infection. | Within a few days of primary infection a distinct pattern of lymphoid tissue activation was noted. At this earlystage of infection the axillary,cervical and mediastinal lymph nodes were activated. Increased FDG uptake preceded fulminant viral replication. |
| Scharko et al. 2003 [11] | Lancet | Clinical | Translational study of preclinical findings. | Distinct pattern of lymph node activation was observed. Head and neck activation in the acute stage, a generalised peripheral lymph node activation in the midstage and involvement of (central) abdominal lymph nodes in the final stage. |
| Iyengar et al. 2003 [12] | Lancet | Clinical | Investigated the ability of PET to measure magnitude of lymph node activation in patient recently infected and compared it to uninfected patients who received killed influenza vaccines. | Lymph node activation was more localised after vaccination with killed influenza virus compared with infected patients. Lymph nodeactivation in early infection (>18 months since seroconversion) was much more in cervical and axillary nodes compared with inguinal and iliac groups. Patient with chronic long term infection (stable viral load) had smallnumbers of persistently active disease. |
| Hardy G et al. 2004 [13] | HIV Med | Clinical | Provided evidence of thymic reconstitution after HAART initiation. | A clear correlation between FDG uptake in thymus and increase in number of CD4 cells. When there was no thymic uptake therewas no increase in peripheral CD4 cell count. |
| Brust et al. 2006. [14] | AIDS | Clinical | Evaluated biodistribution visually and quantitatively in both uninfected and infected patients. Infected patients were at different stages of disease and different states of viral suppression by HAART. | Uninfected and healthy infected patients with suppressed viral loads had little or no FDG nodal uptake. Vireamic patients on the contrary with early or advanced infection had increased FDG uptake in peripheral nodes. The biodistribution was similar for early and advanced–stage disease. Patients who discontinued HAART had negative baseline scans but developed nodal uptake and increase inviralloadaftertherapycessation. Splenic uptake was higher in actively replicating vireamic patients. |
| Liu et al. 2009 | Nucl Med Commun | Clinical | Evaluated the clinical significance of increased splenic uptake of FDG. | Splenic uptake is significantly greater than liver uptake in the actively replicating vireamic group. |
| Lucignani et al. 2009 [15] | Eur J Nucl Med Mol Imaging | Clinical | Determined whether infected patients can be differentiated on the basis of sites of viral replication and whether findings can be related to immunological variables and AIDS history status. | PET demonstrated different patterns of uptake. All infected patients who were on HAART showed normal FDG uptake whether they had suppressed or high viral load. In HAART-naive infected patients with high vireamia the scan showed multiple foci of increased FDG uptake in lymph nodes. FDG nodal uptake in the upper torso particularly the axillary correlated to vireamia levels when below 100,000 copies/ml. In the HAART-naive group with viral load greater than 100,000 copies/ml, FDG uptake was observed in the inguinal nodes. |
| Sathekge et al. 2010 [16] | Nucl Med Commun | Clinical | Determined distribution of nodal uptake and correlated uptake with immunological and virological factors in patients on HAART. | Predominant sites of lymph node involvement were the cervical and axillary regions, followed by the inguinal region. CD4 count was inversely correlated to the average SUV of the lymph nodes. The viral load was positively correlated with the averaged SUV of the involved lymph nodes. |
| Transkovic et al. 2011 [17] | AIDS | Clinical | Determined the correlation between thymic uptake and recovery of CD4. | Thymic tissue did not show any uptake in patients with poor recovery of CD4 after initiation of HAART. |
| Lilievere et al. 2012 [18] | J Acquire immune Defic Syndr | Clinical | Evaluated thymic activity with FDG uptake and precursors for thymic activity. | Thymic uptake was lower in HAART-treated patients compared with age-matched controls. Metabolic thymic activity correlated with indirect molecular and phenotypic markers of thymic output |
Laboratory Indicators for Monitoring HIV
As HIV infection progresses there is a decline in the circulating CD4 T-lymphocyte count, which is a measure of the host’s immunity. After immunological failure that manifests by a decline in the CD4 count, the viral load that has been held in check at a fixed level (balance viral replication and viral elimination) by the host begins to increase. The relative increase in viral replication results in an increase of the viral count in the blood and this can be measured by different methods. These measurements of CD4 count and viral load are used to determine stage of infection and guides HAART. The stage also determines the opportunistic disorders the patient is likely to encounter. This natural progression of disease has been interrupted and changed by HAART, bringing in its wake new diagnostic challenges for the clinician.
Imaging and HIV
Imaging has played a major role in the management of diseases that the HIV-infected person may present. A good history, clinical examination and appropriate laboratory tests are important to settle the definitive diagnosis. Anatomical imaging such as CT scan or magnetic resonance imaging (MRI) is important when opportunistic diseases cause anatomical changes like oedema, abscesses or space-occupying lesions. However, in some situations, such as early in HAND and most other disorders, the physiological changes usually precede anatomical changes and nuclear medicine may be helpful in diagnosing these disorders at a much early stage than anatomically based imaging modalities.
Metabolic Imaging for HIV
Nuclear medicine, by virtue of the fact that it is functional imaging, is able to provide non-invasive information on underlying tissue at the molecular level. The available radiopharmaceuticals have played and continue to play a vital role in the management of HIV infection and associated diseases. The development of new tracers and repurposing of already existing tracers will no doubt contribute immensely to the management of HIV and associated disorders. We will now review the current and present role of nuclear medicine in HIV and discuss some potential future uses of nuclear medicine techniques in patients with HIV infection.
FDG Uptake in Lymphoid Tissue in Relation to the Stage and Immunovirological State of HIV
Several investigators have examined the relation between FDG uptake in lymph nodes and the pattern of distribution of the lymph nodes, the magnitude of the uptake and the relationship with circulating CD4 T-lymphocytes and the viral load (Table 1).
In preclinical setting investigators used living rhesus monkeys that were infected with Simian immunodeficiency virus. The pattern of lymph nodes was noted and related to the stage of the infection (early, midstage or late). The intensity and number of lymph nodes activated at each stage was noted. The activated lymph nodes were confirmed to be the site of viral replication by in situ hybridisation studies. In summary, the findings of the preclinical studies were as follows:
There was widespread lymph tissue activation in monkeys affected with the virus when compared with the uninfected controls.
There was a clear pattern of distribution depending on the stage of the disease.
In the early stage there was activation of the axillary, cervical and mediastinal lymph nodes; in the midstage there was a generalised peripheral activation of lymph nodes; in the terminal stages of infection there was the activation of lymph nodes around the colon involving the mediastinal and the ileocecal nodes [5, 9, 10].
Fewer tissues had high FDG uptake at the terminal stage of the disease compared to the midstage, which indirectly supports tissue apoptosis after activation of lymphoid tissue.
At the site of FDG uptake it was also noted that the increased uptake preceded fulminant tissue activation [9, 10].
After preclinical studies, human studies were conducted to assess the distribution and activation of lymphoid tissue. Other investigators looked at the correlation of peripheral CD4 counts and viral load with FDG scan. It was evaluated whether there was a history of AIDS, or the effect of viral replication or suppression in various patient groups, including recently seroconverted patients, non-progressors (i.e. asymptomatic patients with stable viral loads), HAART naive HIV infected patients or those taking HAART or who had just discontinued HAART effected the uptake pattern on FDG-PET scan as a measure of lymphoid activation [11, 12, 14–16, 19].
In humans, the different pattern of distribution of lymph nodes at different stages of infection was found to be similar to the distribution described in preclinical studies (with lesser involvement of the mediastinal nodes in humans). In both preclinical and, particularly, clinical studies, the lymph nodes were found to be engaged in a predictable sequence, suggesting that a diffusible factor from host or viral origin is responsible for changes in lymph nodes. This represents a potential target for drugs against disease progression [11, 12]. One study investigated the ability of PET to measure the magnitude of lymph node activation in newly infected asymptomatic patients and chronic infected non-progressor, i.e. asymptomatic patients with stable viral load. FDG uptake in these HIV infected groups was compared to controls that were not infected with HIV but received licensed killed influenza vaccines. They found that in both early and chronic HIV disease, node activation was greater in the cervical and axillary chain of nodes than inguinal and iliac chain of lymph nodes [12]. Again in yet another study, the FDG signal was found to correlate with the viral load and correlate inversely with the CD4 count [16]. Other studies compared patients at different stages of immune suppression who were either on HAART or not or had recently stopped HAART. It was concluded that healthy infected HIV patients with suppressed viral loads had no FDG nodal uptake, whereas viremic patients with early or advanced disease had nodal uptake [16].
Splenic uptake, significantly higher than the liver, was also found in viremic patients but not in those with suppressed viral load [14, 19]. The splenic uptake may be due to massive stimulation of B cells in the spleen [5]. In another study, the level of viral replication in HAART-naive subjects determined the distribution of lymph node uptake on FDG-PET imaging. Patients with lower levels of replication showed uptake in the upper torso, whilst patients with higher levels (above 100,000 copies /ml) showed more inguinal uptake [15]. FDG-PET thus constitutes a non-invasive imaging marker for disease state and can be considered a marker of disease.
As a consequence, in the clinical setting, when performing FDG-PET on a patient with HIV for a condition that causes lymph node uptake the scan results must always be interpreted together with the immune and virological data. When patient parameters would favour high FDG uptake due to HIV disease one cannot reliably differentiate between nodal uptake due to HIV or to another underlying disease.
In HIV-infected patients treated with HAART metabolic uptake in the thymus has been shown to be a non-invasive marker of thymic output. Patients without thymic uptake showed a poor CD4 recovery after initiation of HAART [13, 17, 18]. In adults and particularly when infected with HIV virus, the thymus is inactive due to involution of the thymus and the destruction of CD4 T-lymphocytes by the virus. The FDG activity observed in thymus of some HIV adult patients following the initiation of HAART was found to correlate with the regeneration of peripheral CD4 T-lymphocyte and T-lymphocyte from the emigrants from thymus as a result of thymopoiesis. Thymopoeisis can be assessed by quantification of recent thymic emigrants, T-cell receptor excision levels and T-cell receptor repertoire diversity [5, 20]. Thymopoiesis is an energy-dependent process that results in the restoration of the depleted T cells in a manner similar to resumption of erythropoiesis by fat marrow following severe anaemia. The restoration is incomplete and may not result in the restoration of the full repertoire of T cells lost particularly in chronic infection. The utilisation of glucose is visualised as thymic FDG uptake. In the clinical setting this thymic uptake can be erroneously interpreted as anterior mediastinal disease.
Nuclear Medicine and Malignancies Associated with HIV
HIV infection, and other disorders associated with cell-mediated immunity predispose the patients to carcinogenesis, and malignancy occurs more frequently in this circumstance [21]. In immune-compromised patients, a common feature is the tendency for malignancies to occur at a younger age, involving unexpected sites (Fig. 1), with higher tumour grades and at follow-up often an unusual clinical course can be found when compared to individuals with intact immune systems [22, 23]. In HIV infection, malignancies can be classified as AIDS-defining cancers (ADCs) that include cervical cancer, Kaposi sarcoma and non-Hodgkin’s lymphoma [22, 24]. These cancers have declined significantly since the advent of HAART. On the other hand, non-AIDS-defining cancers (NADCs) such as anal cancer, hepatocellular carcinoma, lung cancer (Fig. 2) and colorectal cancer have emerged as a major fraction of overall cancer burden [25, 26]. It has been found that the CD4 count is strongly correlated with the risk of death in both ADC and NADC [26].
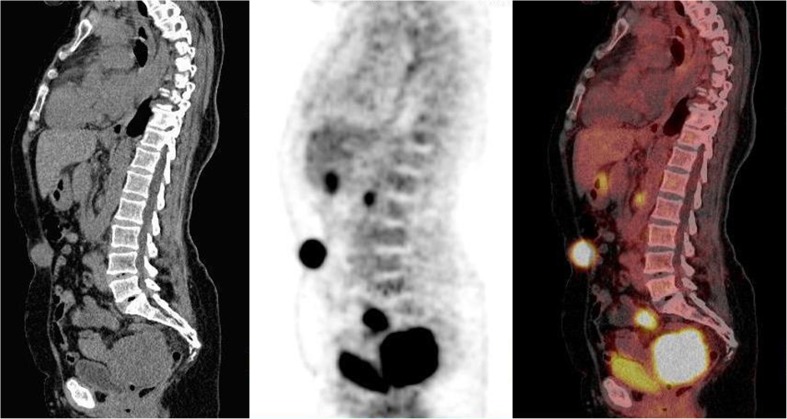
Fig. 1.
Sagittal CT, FDG-PET and fused images of a 37-year-old woman with HIV seropositive serum. She was referred for restaging of cervical adenocarcinoma. CT was non-conclusive about recurrence or residual disease. PET demonstrated metabolically active disease in the pelvis and the presence of a pelvic lymph node. In addition, metabolically active disease noted in the umbilical region due to uptake in the umbilicus associated to peritoneal carcinomatois (atypical finding). This finding was confirmed by biopsy. A follow-up scan 6 months later (not shown) revealed a rapidly progressive disease
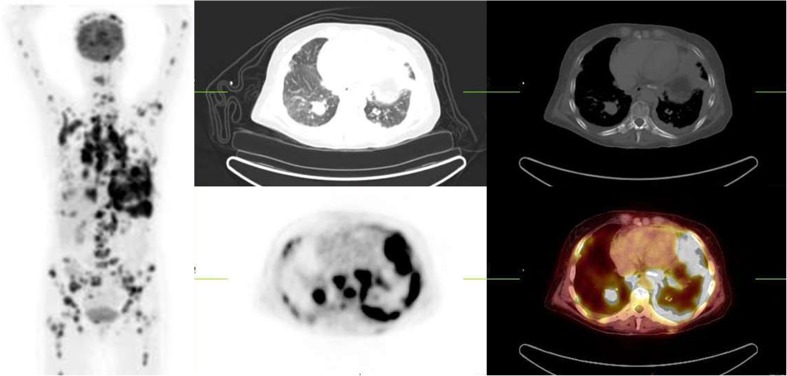
Fig. 2.
Maximum intensity projection, transverse CT (lung and soft tissue setting), FDG-PET and fused images of a 32-year-old HIV-positive man with lung cancer for staging. Chest CT demonstrated an irregular mass in the right lung. At the time of the scan his CD4 was 69/ml but viral load was not available. Intense FDG uptake is noted in lungs, mediastinal and hilar lymph nodes and diffusely in the skeleton consistent with widespread metastasis
Non-AIDS-Defining Cancers
NADC are a very heterogeneous group of cancers of increasing importance [5]. In HIV the outcome of these cancer types is poor with rapid progression, high rate of relapse and poor response to treatment [24]. Factors that influence the development of NADC include HIV infection, chronic immune suppression and coinfection with other oncogenes such as hepatitis C for hepatocellular carcinoma or human papilloma virus for anal cancer [23, 27, 28]. Two factors associated with the prevention of both ADC and NADC are CD4 lymphocyte count of more than 500/ml and an undetectable viral load [29, 30]. The role of FDG PET is similar to that in uninfected patients [5].
Hodgkin’s Lymphoma
Hodgkin’s lymphoma is an NADC with increased incidence in HIV patients and an incidence of approximately 9–18 % in that population [31, 32]. The histological pattern differs from individuals without HIV with mixed cellularity being the most common histology amongst HIV patients in contrast to nodular sclerosis that is the commonest among individuals without HIV. Lymphocyte depletion occurs more commonly in the setting of HIV and has the worse prognosis of all the subtypes of Hodgkin’s lymphoma. In the setting of HIV, extranodal disease occurs more commonly and is associated with Epstein–Barr virus. FDG-PET is known to show uptake in all subtypes [33].
AIDS-Defining Cancers
Cervical Cancer
Cervical cancer is an ADC and is also a human papillomavirus (HPV)-related malignancy [28]. It is the commonest malignancy in developing countries and the incidence of HIV amongst patients with cervical cancer is 20–25 % [30]. In HIV infection cervical cancer patients present 10–15 years earlier than in non-HIV-infected patients and they also present with more advanced disease. Persistent oncogenic HPV infection increases the risk for development of cervical cancer. HIV infection and low CD4 counts are major risk factors for cervical cancer. Trends in HIV-associated cervical cancer have changed in the HAART era. HAART prolongs survival amongst women with HIV but does not protect them from developing cervical cancer [34]. The increasing availability of HAART is likely to lead to an increase in the incidence of cervical cancer [5]. Natural progression of the HPV infection may be related to immune dysfunction as well as HIV or HPV synergistic mechanism. When HAART is used, cervical cancer treatments are affected by concomitant drug toxicity that could potentially limit the benefit of either HAART or the concomitant chemo-radiotherapy that is the standard treatment for locally advanced cervical cancer. FDG-PET has been shown to be effective in the initial staging, detection of early metastasis and predicting prognosis in cervical cancer. FDG-PET demonstrates abnormal uptake in virtually all patients with active cervical cancer (Fig. 1). Three-dimensional (3D) volumetric analysis of tumours with PET has been shown to be of more clinical significance than clinical stage [35, 36]. Pre-treatment FDG-PET lymph node status, cervical tumour (SUV) max and tumour volume combined on a nomogram have been shown to be a good model for cervical cancer recurrence, disease-free survival, overall survival, and to support decision-making [37]. The use of FDG for cervical cancer in HIV patients is similar to that for non-HIV patients. Further analysis of data from this subgroup is needed as pelvic and extra pelvic reactive HIV lymph nodes may impact therapy decisions.
Non-Hodgkin’s Lymphoma (NHL)
NHL is particularly aggressive in the setting of HIV infection. The incidence of B cell lymphoma is dramatically increased in HIV-infected patients [31]. Before the advent of HAART, high-grade immunoblastic NHL was the commonest histological type, followed by Burkitt’s lymphoma and intermediate grade diffuse large B cell lymphoma (DLBCL). There has been a drastic decrease in immunoblastic lymphoma but an increase in DLBCL and Burkitt’s lymphoma since the introduction of HAART [32, 38]. Risk factors for NHL in the setting of HIV are low CD4 count and high viral load [39, 40]. The risk is significantly increased if the viral load exceeds 100,000 copies /ml and CD4 lymphocyte count drops below 50/ml [40]. Non-Hodgkin’s lymphomas may occur at any level of CD4 count but Burkitt’s lymphomas are more frequent in CD4 counts >200/ml, whilst primary central nervous system (CNS) lymphomas are seen almost exclusively in patients with CD4 counts <50/ml [41–43].
FDG-PET/CT is useful in diagnosing, staging, restaging and monitoring response to therapy in lymphomas in general and in the setting of HIV. The most common subtypes of NHLs diagnosed are usually DLBCLs (Fig. 3) and Burkitt’s lymphoma, which are frequently high grade and involve extranodal disease (in contrast to endemic Burkitt’s).
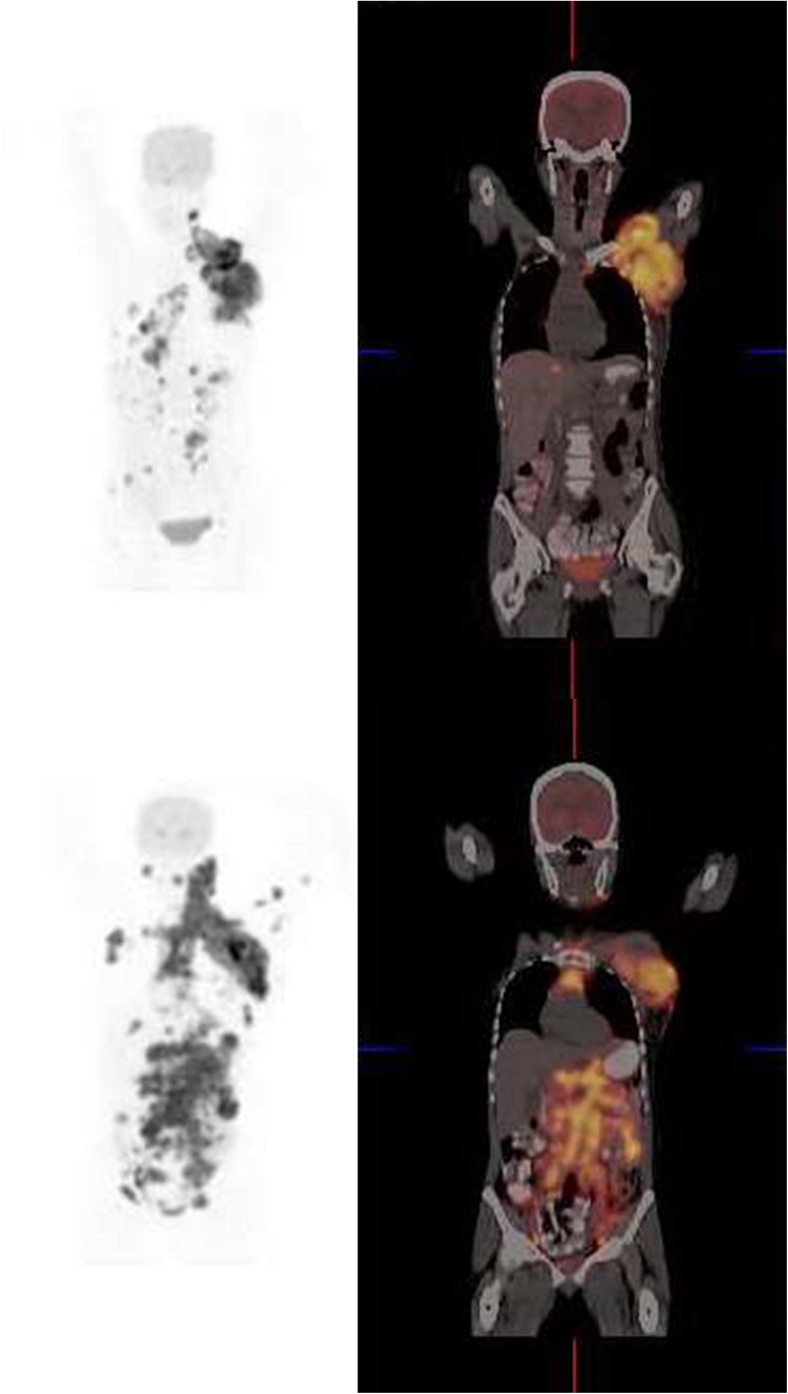
Fig. 3.
Coronal FDG-PET and fusion images of a 41-year-old woman with HIV infection and diffuse large B-cell lymphoma diagnosed by biopsy of a large mass in the left axilla. She has been on HAART for 17 months. Her CD4 count was 354 and her viral load less than 50 copies per ml. The scan shows FDG-avid lymph nodes in the left axilla, hilar, para-aortic, abdominal and pelvic nodes. There is also pelvic bone involvement. Lymphadenopathy is due to DLBL and not reactive lymph nodes of HIV when a scan is interpreted along with immunological parameters. On follow-up there was rapid progression of disease
In interpretation of scans in patients with lymphoma, the CD4 count and viral load and patient history must be carefully considered as reactive nodes of HIV may result in false-positive findings when patients are being evaluated by FDG-PET. A negative FDG-PET scan for lymphoma has a good prognosis. FDG-PET in the context of HIV may help to guide treatment management and to prevent long-term toxicity as a result of drug interaction. It has been used as a daily clinical routine tool in lymphomas and replaced 67Ga citrate completely (which was used a lot in the past) and CT only as the modality of choice [43, 44].
CNS Lymphoma Verses Opportunistic Infection
Less common types of lymphoma occurring in the setting of HIV include primary CNS lymphoma, primary effusion lymphoma and plasmablastic lymphoma [45–47]. One diagnostic dilemma frequently encountered by clinicians when treating HIV patients with CD4 counts less than 50/ml is the patient with the distinction of CNS lymphoma from other non-malignant conditions, especially cerebral toxoplasmosis. The treatment is very different and outcome disastrous if misdiagnosed. Neither MRI nor CT can reliably make this distinction in HIV patients [5]. In the past, nuclear medicine used agents like 201Tl to help in this scenario where 201Tl would not be taken up by infection but would be taken up by lymphoma. FDG-PET/CT has been studied in this scenario and it was found that the SUV was higher in malignant lesions than in infectious lesions [46, 48–50]. FDG-PET is also useful in the evaluation of CNS lesions and for guiding biopsy.
Kaposi Sarcoma
This is an ADC associated with human herpes virus 8 (HHV-8) [51]. HHV-8 is the cause of all HIV-associated and non-HIV-associated Kaposi sarcoma [52]. Kaposi sarcoma is the most common malignancy observed in HIV patients [53, 54]. It occurs much earlier than other malignancies. The introduction of HAART has resulted in a marked reduction in the incidence of Kaposi sarcoma in HIV [55]. HIV Kaposi sarcoma is more aggressive than the non-HIV-associated variant and lesions often have a different distribution. Visceral involvement is seen in 50 % of cases and is frequently fatal [56]. FDG-PET was found to be effective in detecting clinically occult Kaposi sarcoma lesions that were difficult to detect with traditional imaging techniques in more advanced Kaposi sarcoma [57, 58].
Multicentric Castleman Disease
This is a rare lymphoproliferative disorder that is also associated with HHV-8. In the setting of HIV it is highly aggressive and frequently lethal [59]. FDG-PET is able to detect abnormal uptake more frequently than CT and can be used to identify appropriate lesion location for biopsy, staging and monitoring lymphoproliferation in HIV associated Castleman disease [60].
Fever of Unknown Origin (FUO)
HIV-associated FUO is defined as fever 38.3 °C or higher on several occasions in a patient with a confirmed positive serology for HIV, with the duration of fever for 4 weeks or more for outpatients or 3 days or more for hospitalised patients [61, 62]. There are several reasons why a patient with HIV can have a fever. FUO may be due to HIV itself, and it may occur in 40–90 % of patients with primary HIV infection. Opportunistic infection, malignancies and febrile drug reactions may also cause fever [63–65]. Sexually transmitted infections, alcohol abuse and illicit drug use, which are more frequent in HIV patients, are also causes of fever [65]. The use of imaging can reduce time to initiation of treatment in FUO by localising infection or malignancy, thus identifying correct site of biopsy or sampling procedure. FDG-PET was found to have an overall sensitivity of 92 % and specificity of 94 % in detecting the cause of FUO, unexplained weight loss or confusion [50]. In a number of studies, soft-tissue abnormalities of the chest were visualised before conventional imaging [50, 66]. These soft-tissue abnormalities include both infections such as Mycobacteria sp., Streptococcus sp. and Pseudomonas sp. and malignancies such as lymphoma and Kaposi sarcomas. In a number of cases, these abnormalities were detected on FDG-PET before the chest radiograph detected these lesions. This is due to the fact that physiological changes underlying these pathological processes precede the anatomical changes. Studies have shown that high viral loads do not decrease the usefulness of FDG-PET/CT in the evaluation of HIV-associated FUO [67]. FDG-PET/CT has emerged as a valuable tool for diagnosis of the cause of FUO (Fig. 4) [66–68].
Fig. 4.
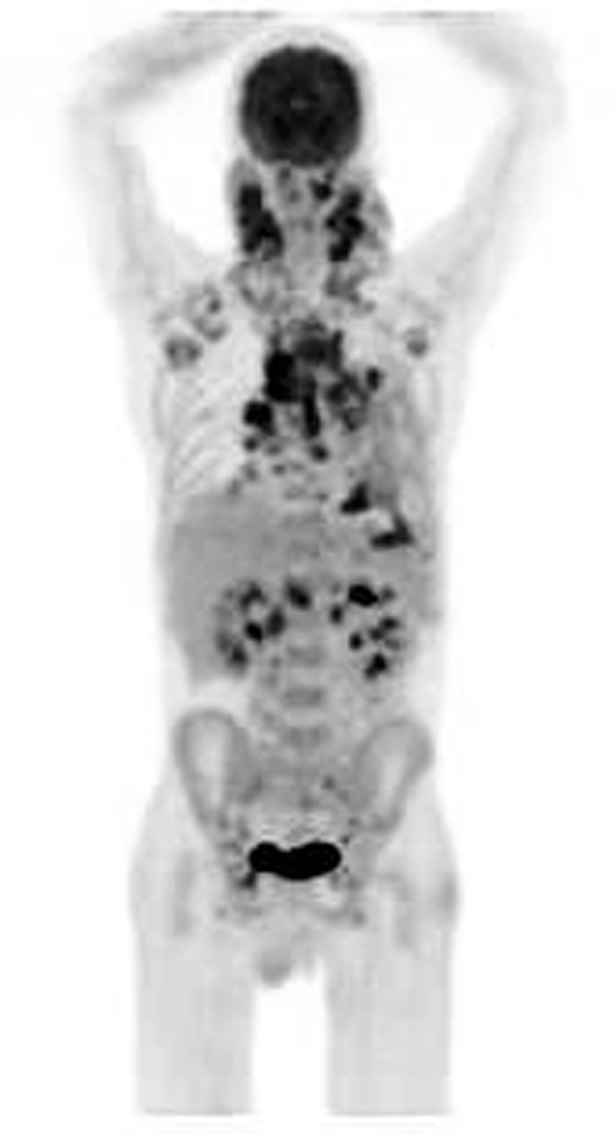
Maximum intensity projection image of a 28-year-old man with HIV infection presenting with fever of unknown origin and lymphadenopathy. He had a history of TB over 10 year ago and was negative for TB at the time of the scan. His CD4 count was 102 and viral load 189,000 per ml. Other investigations were unremarkable. Scan shows FDG-avid lymphadenopathy involving the cervical, axillary, mediastinal, hilar, abdominal and pelvic lymph nodes . There is also intense splenic uptake more than the liver. This is consistent with reactive HIV lymphadenopathy and splenic uptake
HIV-Associated Opportunistic Infections
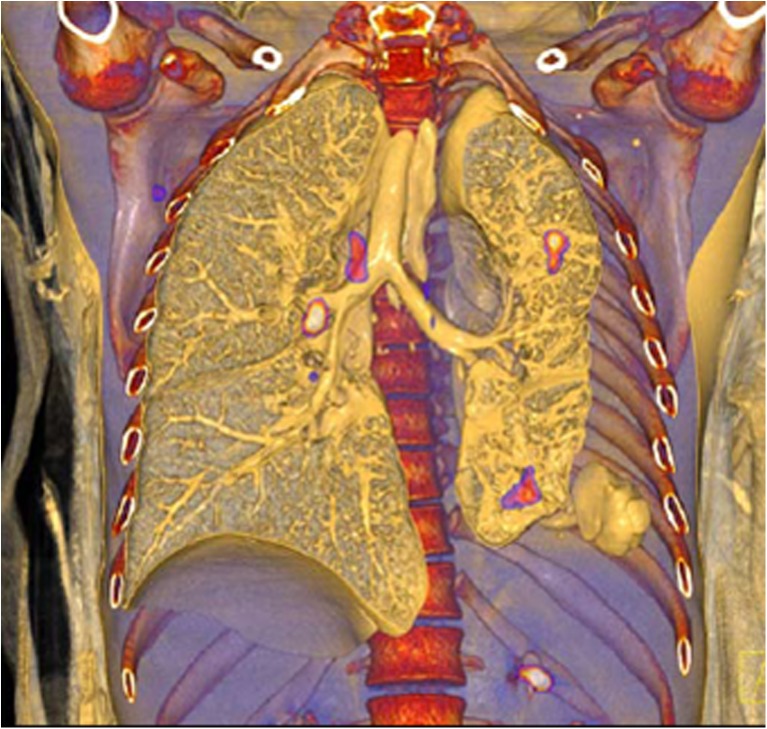
There are several opportunistic infections that occur in HIV. The most common infection encountered is tuberculosis (TB) [5]. The clinical presentation depends on the extent of immune suppression [69]. TB is known to avidly take up FDG, however the challenge of distinguishing reactive HIV nodes from TB has already been addressed in this review and in literature [5, 69, 70]. Interpretation of the scan should be made in light of viral load and CD4 count, together with the pattern. Figure 5 shows active TB complicating HIV. The relationship of the lesion to the bronchi and lung parenchyma is well appreciated on this 3D volume rendered PET/CT image.
Fig. 5.
Metabolically active tuberculosis with complicating HIV. The relationship of the lesions to the bronchi and the lung parenchyma are delineated on this volume-rendered FDG-PET/CT image
Fungal infections are also one of the common opportunistic infections encountered in HIV. Fungal infections are known to bind FDG in both children and adults [71]. The interpretation of the scan again suffers from the non-specific uptake that reactive HIV nodes may cause and as in all infection a good clinical history, physical examinations and laboratory finding may assist. In most opportunistic infections, FDG-PET may be helpful in detecting occult lesions that are not clinically apparent to properly stage infection. It may also help to guide duration of therapy in situations where the exact duration of treatment may not be standardised, such as extrapulmonary tuberculosis or some fungal infection [69, 71].
White blood cell (WBC) scintigraphy is considered the “gold standard” for imaging some infections in nuclear medicine, particularly in bone and soft tissue [72]. It is likely that this technique would be as effective in localising infection in HIV patients as in HIV-seronegative patients. There is an increased risk in transmission of HIV or other infection such as hepatitis C virus, hence other imaging techniques such as FDG-PET may be preferred to WBC scintigraphy.
There has been a rekindled interest of gallium-based radioisotopes particularly with the PET-based radioisotope gallium-68 [73]. 67Ga, the equivalent SPECT tracer to 68Ga, was used in the past in different aspects of HIV, including distinguishing infections from malignancy, diagnosing chest infections localising infections and assessing extent of mycobacterial, Pneumocystis, toxoplasmosis and cocciodiodomycosis infections [6, 74]. 68Ga has shown some promise a tracer in investigating infectious disease both as its citrate salt and when labelled with other complexes. Triacetylfusarinine C and 1,4,7-triazacyclononane-1,4,7-triacetic acid ubiquicidin have been shown to be useful in infection imaging in preclinical studies [73, 75]. 68Ga citrate is being evaluated to see whether it will be able to perform as well as its SPECT counterpart and has already shown promising results in bone infections [76]. 68Ga-PET is likely to play an important role in the management of infections in association with HIV in the future.
HIV-Associated Neurocognitive Deficit (HAND) and HIV-Associated Dementia (HAD)
This is a subcortical dementia found in HIV patients which was previously called AIDS dementia complex [5, 21, 77]. It is characterised by disturbances in cognition, motor performance and movement. Many of these symptoms can be caused by other conditions common to HIV and these other conditions are usually treatable [77, 78]. It is, therefore, imperative that these conditions are excluded before a diagnosis of HAND is made. The introduction of HAART has reduced the incidence of HAND from 7 to 1 % [79]. The severity of the neurological deficit in patients with HAND also appears to have been attenuated after introduction of HAART; however, the prevalence of HAND continues at high rates. Prevalence has been estimated as 33 % for asymptomatic neurological impairment, 12 % for minor neurocognitive disorder and 2 % for HIV-associated dementia [80]. Conditions to be excluded before a diagnosis of HAND is made include CNS opportunistic infections, neurosyphilis, substance abuse, delirium, toxic-metabolic disorder, psychiatric disease and age-related dementia. A well-documented cognitive decline and exclusion of the confounding conditions must be performed before a definite diagnosis is settled. There is no biomarker for HAND [77]. MRI and CT are the main imaging modalities used in evaluating neurological disease in HIV patients. In HAND functional imaging may play an important role because functional abnormalities precede structural atrophy, ventricular dilatation or focal CNS lesions. Two major patterns of FDG brain uptake in patients with HAND are recognised: (1) subcortical hypermetabolism that usually occurs early in disease and appears to be a disease specific marker for early CNS involvement in HAND [81–83]; (2) a non-specific pattern that correlates with age and cerebral dysfunction. As the disease progresses there is reduced cerebral uptake in the cortical and subcortical structures. Some researchers demonstrated that as HIV infection progressed the relative uptake in the striatum and parietal lobe also increased [84]. Some authors also found a significant relation between frontal lobe metabolism and severity of dementia, whilst others found that frontomesial hypometabolism was associated with deteriorating motor function [85]. Despite these findings, FDG cannot be recommended for the diagnosis of HAND and more specific tracers are needed [77].
Other Radiopharmaceuticals and Hand
Some pathogenic similarities exist between HAND and other neurodegenerative disease, so that other tracers have been used to study HAND. 11C Pittsburgh compound (PIB) and 11C PK11195 compounds, which play a significant role in assessing abnormal protein accumulation in the brain and neuro-inflammation, were investigated; however, these were unable to help in the diagnosis of HAND [84–87]. SPECT tracers such as technetium–99m–hexamethylpropylene amine oxime and iodine-labelled ligands were also not found to be useful [88–91]. A search is underway for an appropriate ligand to image HAND and other neurological changes associated with HIV.
Lipodystrophy
This is a complication of patients treated with HAART and may be present in up to 80 % of this population [92]. Lipodystrophy is a chronic progressive syndrome of abdominal obesity and/or peripheral fat loss, and occurs together with hyperlipidaemia. Hyperinsulinaemia, increased C-peptide concentration, insulin resistance and impaired glucose tolerance are frequently observed. FDG-PET has been studied as a tool for monitoring lipodystrophy and to help inform clinicians when a particular regime of HAART should be modified to prevent the metabolic complications of lipodystrophy. The conclusions of these studies were that FDG-PET was able to detect lipodystrophy in HIV patients [93–95]. The potential to use FDG-PET to monitor patients on HAART for lipodystrophy with the aim of reducing insulin resistance must be investigated further [96].
Cardiovascular Disease and Arterial Inflammation
People living with HIV have a higher risk for stroke and myocardial infarction than the general population [91]. The risk is due to the chronic low-grade inflammation associated with host response to HIV. FDG-PET has been investigated as a non-invasive, sensitive, specific and reproducible biological marker for early atheroma in metabolically active, rupture prone atherosclerotic plaques [97–100]. The studies had favourable results. FDG-PET/CT imaging also demonstrated significant arterial inflammation of the carotid artery in HIV patients with low Framingham coronary heart risk scores [5, 101–103]. Further research is needed to validate FDG-PET in for assessing atherosclerosis in HIV patients.
Conclusions and Future Perspectives
Nuclear medicine has had important applications in the management of HIV and HIV-associated diseases in the past and even has greater significance in recent times. The role of nuclear medicine is likely to increase as we unravel the molecular basis underlying HIV infection and associated diseases. In some HIV-associated diseases the role of nuclear medicine is well established, in others there are a number of studies confirming its usefulness but further studies are needed to recommend its use routinely in clinical practice. In neuro-HIV for example, plans are well advanced in developing a radioimmunotherapeutic agent to kill HIV-infected cells in the nervous system. This will help us understand the pathology in HAND and probably lead to the development of new therapeutic agents [77]. In the different HIV-associated manifestations, newer and more specific tracers that are able image and possibly treat disease are likely to be developed, or old radiotracers may be repurposed to manage these diseases. Nuclear medicine as a molecular imaging modality is a very powerful diagnostic tool in the management of the global pandemic of HIV infection.
Compliance with Ethical Standards
Funding
None.
Conflict of Interest
Alfred O. Ankrah, Andor W.J.M. Glaudemans, Hans C Klein, Rudi A.J.O. Dierckx and Mike M. Sathekge declare that they have no conflict of interest.
Ethical Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and the national regulations and also with the principles of the 1964 Declaration of Helsinki and its later amendments as required for a review article.
References
- 1.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016. doi:10.1016/S1473-3099(15)00485-5 [DOI] [PubMed]
- 2.UNAIDS. Global Statistics. UNAIDS, 2015 http://www.unaids.org/ en/resources/campaigns/HowAIDSchangedeverything/factsheet. Accessed 1 Mar 2016.
- 3.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre D, Speranza F, Martegani R. Impact of highly active antiretroviral therapy on organ-specific manifestations of HIV-1 infection. HIV Med. 2005;6:66–78. doi: 10.1111/j.1468-1293.2005.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sathekge M, Maes A, Vande WC. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med. 2013;43:349–66. doi: 10.1053/j.semnuclmed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Dayem HM, Bag R, DiFabrizio L, Aras T, Turoglu HT, Kempf JS, et al. Evaluation of sequential thallium and gallium scans of the chest in AIDS patients. J Nucl Med. 1996;37:1662–7. [PubMed] [Google Scholar]
- 7.Signore A, Glaudemans AW, Rouzet GF. Imaging infection and inflammation. Biomed Res Int. 2015;2015:615150. doi: 10.1155/2015/615150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn TC. Global burden of HIV pandemic. Lancet. 1996;348:99–106. doi: 10.1016/S0140-6736(96)01029-X. [DOI] [PubMed] [Google Scholar]
- 9.Scharko AM, Perlam SB, Hinds PW, 2nd, Hanson JM, Uno H, Pauza CD. Whole body positron emission tomography imaging of simian immunodeficiency virus-infected rhesus macaques. Proc Natl Acad Sci U S A. 1996;93:6425–30. doi: 10.1073/pnas.93.13.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace M, Pyzalski R, Horejsh D, Brown C, Diavani M, Lu Y, et al. Whole body positron emission tomography imaging of activated lymphoid tissues during acute simian-human immunodeficiency virus 89.6PD infection in rhesus macaques. Virology. 2000;274:255–61. doi: 10.1006/viro.2000.0479. [DOI] [PubMed] [Google Scholar]
- 11.Scharko AM, Perlman SB, Pyzalski RW, Graziano FM, Sosman J, Pauza CD. Whole-body positron emission tomography in patients with HIV-1 infection. Lancet. 2003;362:959–61. doi: 10.1016/S0140-6736(03)14366-8. [DOI] [PubMed] [Google Scholar]
- 12.Iyengar S, Chin B, Margolick JB, Sabundayo BP, Schwartz DH. Anatomical loci of HIV-associated immune activation and association with viraemia. Lancet. 2003;362:945–50. doi: 10.1016/S0140-6736(03)14363-2. [DOI] [PubMed] [Google Scholar]
- 13.Hardy G, Worrell S, Hayes P, Barnett CM, Glass D, Pido-Lopez J, et al. Evidence of thymic reconstitution after highly active antiretroviral therapy in HIV-1 infection. HIV Med. 2004;5:67–73. doi: 10.1111/j.1468-1293.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 14.Brust D, Polis M, Davey R, Hahn B, Bacharach S, Whatley M, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS. 2006;20:985–93. doi: 10.1097/01.aids.0000222070.52996.76. [DOI] [PubMed] [Google Scholar]
- 15.Lucignani G, Orunesu E, Cesari M, Marzo K, Pacei M, Bechi G, et al. FDG-PET imaging in HIV-infected subjects: relation with therapy and immunovirological variables. Eur J Nucl Med Mol Imaging. 2009;36:640–7. doi: 10.1007/s00259-008-1023-7. [DOI] [PubMed] [Google Scholar]
- 16.Sathekge M, Maes A, Kgomo M, Van de Wiele C. Fluorodeoxyglucose uptake by lymph nodes of HIV patients is inversely related to CD4 cell count. Nucl Med Commun. 2010;3:137–40. doi: 10.1097/MNM.0b013e3283331114. [DOI] [PubMed] [Google Scholar]
- 17.Tanaskovic S, Fernandez S, French MA, Price RI, Song S, Robins PD, et al. Thymic tissue is not evident on high-resolution computed tomography and [18F]fluoro-deoxy-glucose positron emission tomography scans of aviraemic HIV patients with poor recovery of CD4+ T cells. AIDS. 2011;25:1235–7. doi: 10.1097/QAD.0b013e3283474155. [DOI] [PubMed] [Google Scholar]
- 18.Lelièvre JD, Melica G, Itti E, Lacabaratz C, Rozlan S, Wiedemann A, et al. Initiation of c-ART in HIV-1 infected patients is associated with a decrease of the metabolic activity of the thymus evaluated using FDG-PET/computed tomography. J Acquir Immune Defic Syndr. 2012;61:56–63. doi: 10.1097/QAI.0b013e3182615b62. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. Clinical significance of diffusely increased splenic uptake on FDG-PET. Nucl Med Commun. 2009;30:763–9. doi: 10.1097/MNM.0b013e32832fa254. [DOI] [PubMed] [Google Scholar]
- 20.Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord transplantation. Blood. 2014;124:3201–11. doi: 10.1182/blood-2014-07-589176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003;32:527–33. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 22.Davison JM, Subramaniam RM, Surasi DS, Cooley T, Mercier G, Peller PJ. FDG PET/CT in patients with HIV. AJR Am J Roentgenol. 2011;197:284–94. doi: 10.2214/AJR.10.6332. [DOI] [PubMed] [Google Scholar]
- 23.Bedimo R. Non-AIDS-defining malignancies among HIV-infected patients in the highly active antiretroviral therapy era. Curr HIV/AIDS Rep. 2008;5:140–9. doi: 10.1007/s11904-008-0022-4. [DOI] [PubMed] [Google Scholar]
- 24.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet F, Chêne G. Evolving epidemiology of malignancies in HIV. Curr Opin Oncol. 2008;20:534–40. doi: 10.1097/CCO.0b013e32830a5080. [DOI] [PubMed] [Google Scholar]
- 26.Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–90. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 27.Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayor AM, Gómez MA, Ríos-Olivares E, Hunter-Mellado RF. AIDS-defining neoplasm prevalence in a cohort of HIV-infected patients, before and after highly active antiretroviral therapy. Ethn Dis. 2008;18(2 Suppl 2):S2–189–94. [PMC free article] [PubMed]
- 29.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 30.Moodley M, Mould S. Invasive cervical cancer and human immunodeficiency virus (HIV) infection in KwaZulu-Natal. S Afr J Obstet Gynaecol. 2005;25:706–10. doi: 10.1080/01443610500294599. [DOI] [PubMed] [Google Scholar]
- 31.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 32.Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Swiss HIV Cohort Study. Br J Cancer. 2010;103:416–22. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabkin CS, Yellin F. Cancer incidence in a population with a high prevalence of infection with human immunodeficiency virus type 1. J Natl Cancer Inst. 1994;86:1711–6. doi: 10.1093/jnci/86.22.1711. [DOI] [PubMed] [Google Scholar]
- 34.Adler DH. The impact of HAART on HPV-related cervical disease. Curr HIV Res. 2010;8:493–7. doi: 10.2174/157016210793499240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Einstein MH, Phaëton R. Issues in cervical cancer incidence and treatment in HIV. Curr Opin Oncol. 2010;22:449–55. doi: 10.1097/CCO.0b013e32833cff4f. [DOI] [PubMed] [Google Scholar]
- 36.Grigsby PW. Role of PET in gynecologic malignancy. Curr Opin Oncol. 2009;21:420–4. doi: 10.1097/CCO.0b013e32832ec63f. [DOI] [PubMed] [Google Scholar]
- 37.Kidd EA, El Naqa I, Siegel BA, Dehdashti F, Grigsby PW. FDG-PET-based prognostic nomograms for locally advanced cervical cancer. Gynecol Oncol. 2012;127:136–40. doi: 10.1016/j.ygyno.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106:128–35. doi: 10.1002/cncr.21562. [DOI] [PubMed] [Google Scholar]
- 39.Engels EA, Pfeiffer RM, Landgren O, Moore RD. Immunologic and virologic predictors of AIDS-related non-hodgkin lymphoma in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2010;54:78–84. doi: 10.1097/01.qai.0000371677.48743.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Clinical Epidemiology Group of the FHDH-ANRS CO4 cohort. Lancet Oncol. 2009;10:1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 41.Guech-Ongey M, Simard EP, Anderson WF, Engels EA, Bhatia K, Devesa SS, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116:5600–4. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabarre J, Raphael M, Lepage E, Martin A, Oksenhendler E, Xerri L, et al. Human immunodeficiency virus-related lymphoma: relation between clinical features and histologic subtypes. Am J Med. 2001;111:704–11. doi: 10.1016/S0002-9343(01)01020-8. [DOI] [PubMed] [Google Scholar]
- 43.Forsyth PA, DeAngelis LM. Biology and management of AIDS-associated primary CNS lymphomas. Hematol Oncol Clin North Am. 1996;10:1125–34. doi: 10.1016/S0889-8588(05)70388-9. [DOI] [PubMed] [Google Scholar]
- 44.Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med. 2006;47:1326–34. [PubMed] [Google Scholar]
- 45.Aoki Y, Tosato G. Neoplastic conditions in the context of HIV-1 infection. Curr HIV Res. 2004;2:343–9. doi: 10.2174/1570162043351002. [DOI] [PubMed] [Google Scholar]
- 46.Villringer K, Jäger H, Dichgans M, Ziegler S, Poppinger J, Herz M, et al. Differential diagnosis of CNS lesions in AIDS patients by FDG-PET. J Comput Assist Tomogr. 1995;19:532–6. doi: 10.1097/00004728-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Carbone A, Gloghini A. AIDS-related lymphomas: from pathogenesis to pathology. Br J Haematol. 2005;130:662–70. doi: 10.1111/j.1365-2141.2005.05613.x. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman JM, Waskin HA, Schifter T, Hanson MW, Gray L, Rosenfeld S, et al. FDG-PET in differentiating lymphoma from nonmalignant central nervous system lesions in patients with AIDS. J Nucl Med. 1993;34:567–75. [PubMed] [Google Scholar]
- 49.Heald AE, Hoffman JM, Bartlett JA, Waskin HA. Differentiation of central nervous system lesions in AIDS patients using positron emission tomography (PET) Int J STD AIDS. 1996;7:337–46. doi: 10.1258/0956462961918239. [DOI] [PubMed] [Google Scholar]
- 50.O’Doherty MJ, Barrington SF, Campbell M, Lowe J, Bradbeer CS. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997;38:1575–83. [PubMed] [Google Scholar]
- 51.Blum KA, Lozanski G, Byrd JC. Blood. 2004;104:3009–20. doi: 10.1182/blood-2004-02-0405. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz EJ, Dorfman RF, Kohler S. Human herpesvirus-8 latent nuclear antigen-1 expression in endemic Kaposi sarcoma: an immunohistochemical study of 16 cases. Am J Surg Pathol. 2003;27:1546–50. doi: 10.1097/00000478-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 54.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–24. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 55.Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:14–9. doi: 10.1097/01.qco.0000200295.30285.13. [DOI] [PubMed] [Google Scholar]
- 56.Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997;15:3085–92. doi: 10.1200/JCO.1997.15.9.3085. [DOI] [PubMed] [Google Scholar]
- 57.van de Luijtgaarden A, van der Ven A, Leenders W, Kaal S, Flucke U, Oyen W, et al. Imaging of HIV-associated Kaposi sarcoma; F-18-FDG-PET/CT and In-111-bevacizumabscintigraphy. J Acquir Immune Defic Syndr. 2010;54:444–6. doi: 10.1097/QAI.0b013e3181cdf61f. [DOI] [PubMed] [Google Scholar]
- 58.Morooka M, Ito K, Kubota K, Minamimoto R, Shida Y, Hasuo K, et al. Whole-body 18F-fluorodeoxyglucose positron emission tomography/computed tomography images before and after chemotherapy for Kaposi sarcoma and highly active antiretrovirus therapy. Jpn J Radiol. 2010;28:759–62. doi: 10.1007/s11604-010-0481-6. [DOI] [PubMed] [Google Scholar]
- 59.Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406–12. [PubMed] [Google Scholar]
- 60.Barker R, Kazmi F, Stebbing J, Ngan S, Chinn R, Nelson M, et al. FDG-PET/CT imaging in the management of HIV-associated multicentric Castleman’s disease. Eur J Nucl Med Mol Imaging. 2009;36:648–52. doi: 10.1007/s00259-008-0998-4. [DOI] [PubMed] [Google Scholar]
- 61.Durack DT, Street AC. Fever of unknown origin--reexamined and redefined. Curr Clin Top Infect Dis. 1991;11:35–51. [PubMed] [Google Scholar]
- 62.Knockaert DC, Vanderschueren S, Blockmans D. Fever of unknown origin in adults: 40 years on. J Intern Med. 2003;253:263–75. doi: 10.1046/j.1365-2796.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- 63.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 64.De Munter P, Peetermans WE, Derdelinckx I, Vanderschueren S, Van Wijngaerden E. Fever in HIV-infected patients: less frequent but still complex. Acta Clin Belg. 2012;67:276–81. doi: 10.2143/ACB.67.4.2062672. [DOI] [PubMed] [Google Scholar]
- 65.Koopmans PP, Burger DM. Managing drug reactions to sulfonamides and other drugs in HIV infection: desensitization rather than rechallenge? Pharm World Sci. 1998;20:253–7. doi: 10.1023/A:1008617019897. [DOI] [PubMed] [Google Scholar]
- 66.Castaigne C, Tondeur M, de Wit S, Hildebrand M, Clumeck N, Dusart M. Clinical value of FDG-PET/CT for the diagnosis of human immunodeficiency virus-associated fever of unknown origin: a retrospective study. Nucl Med Commun. 2009;30:41–7. doi: 10.1097/MNM.0b013e328310b38d. [DOI] [PubMed] [Google Scholar]
- 67.Martin C, Castaigne C, Tondeur M, Flamen P, De Wit S. Role and interpretation of fluorodeoxyglucose-positron emission tomography/computed tomography in HIV-infected patients with fever of unknown origin: a prospective study. HIV Med. 2013;14:455–62. doi: 10.1111/hiv.12030. [DOI] [PubMed] [Google Scholar]
- 68.Glaudemans AW, Signore A. FDG-PET/CT in infections: the imaging method of choice? Eur J Nucl Med Mol Imaging. 2010;37:1986–91. doi: 10.1007/s00259-010-1587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ankrah AO, van de Werf TS, de Vries EF, Dierckx RA, Sathekge MM, Glaudemans AW. PET/CT imaging of Mycobacterium tuberculosis infection. Clin Trans Imaging. 2016;4:131–41. doi: 10.1007/s40336-016-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vorster M, Sathekge MM, Bomanji J. Advances in imaging of tuberculosis: the role of 18F-FDG PET and PET/CT. Curr Opin Pulm Med. 2014;20:287–93. doi: 10.1097/MCP.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 71.Ankrah AO, Sathekge MM, Dierckx RA, Glaudemans AW. Imaging fungal infections in children. Clin Transl Imaging. 2016;4:57–72. doi: 10.1007/s40336-015-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glaudemans AW, de Vries EF, Vermeulen LE, Slart RH, Dierckx RA, Signore A. A large retrospective single-centre study to define the best image acquisition protocols and interpretation criteria for white blood cell scintigraphy with 99mTc-HMPAO-labelled leucocytes in musculoskeletal infections. Eur J Nucl Med Mol Imaging. 2013;40:1760–9. doi: 10.1007/s00259-013-2481-0. [DOI] [PubMed] [Google Scholar]
- 73.Haas H, Petrik M, Decristoforo C. An iron-mimicking, Trojan horse-entering fungi—has the time come for molecular imaging of fungal infections? PLoS Pathog. 2015;11:e1004568. doi: 10.1371/journal.ppat.1004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beckerman C, Bitran J. Gallium-67 scanning in the clinical evaluation of human immunodeficiency virus infection: indication and limitations. Semin Nucl Med. 1988;18:273–86. doi: 10.1016/S0001-2998(88)80037-0. [DOI] [PubMed] [Google Scholar]
- 75.Ebenhan T, Zeevart JR, Venter JD, Govender T, Kruger GH, Jarvis NV, et al. Preclinical evaluation of 68Ga-labeled 1,4,7-triazacyclononane-1,4,7-triacetic acid ubiquicidin as a radioligand for PET infection imaging. J Nucl Med. 2014;55:308–14. doi: 10.2967/jnumed.113.128397. [DOI] [PubMed] [Google Scholar]
- 76.Kumar V, Boddeti DK. (68) Ga-radiopharmaceuticals for PET imaging of infection and inflammation. Recent Results Cancer Res. 2013;194:189–219. doi: 10.1007/978-3-642-27994-2_11. [DOI] [PubMed] [Google Scholar]
- 77.Sathekge M, McFarren A, Dadachova E. Role of nuclear medicine in neuroHIV: PET, SPECT, and beyond. Nucl Med Commun. 2014;35:792–6. doi: 10.1097/MNM.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–68. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 80.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24:1367–70. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- 81.Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med. 1996;37:1133–41. [PubMed] [Google Scholar]
- 82.Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, Dhawan V, et al. The metabolic pathology of the AIDS dementia complex. Ann Neurol. 1987;22:700–6. doi: 10.1002/ana.410220605. [DOI] [PubMed] [Google Scholar]
- 83.van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, Paz DK, et al. Cerebral metabolic dysfunction in AIDS: findings in a sample with and without dementia. J Neuropsychiatry Clin Neurosci. 1992;4:280–7. doi: 10.1176/jnp.4.3.280. [DOI] [PubMed] [Google Scholar]
- 84.Hinkin CH, van Gorp WG, Mandelkern MA, Gee M, Satz P, Holston S, et al. Cerebral metabolic change in patients with AIDS: report of a six-month follow-up using positron-emission tomography. J Neuropsychiatry Clin Neurosci. 1995;7:180–7. doi: 10.1176/jnp.7.2.180. [DOI] [PubMed] [Google Scholar]
- 85.von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol. 2000;57:1601–7. doi: 10.1001/archneur.57.11.1601. [DOI] [PubMed] [Google Scholar]
- 86.Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case–control study. Neurology. 2010;75:111–5. doi: 10.1212/WNL.0b013e3181e7b66e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69:72–7. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, et al. Imaging microglial activation in Huntington’s disease. Brain Res Bull. 2007;72:148–51. doi: 10.1016/j.brainresbull.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 89.Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord. 2007;22:1852–6. doi: 10.1002/mds.21552. [DOI] [PubMed] [Google Scholar]
- 90.Samuelsson K, Pirskanen-Matell R, Bremmer S, Hindmarsh T, Nilsson BY, Persson HE. The nervous system in early HIV infection: a prospective study through 7 years. Eur J Neurol. 2006;13:283–91. doi: 10.1111/j.1468-1331.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 91.Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, et al. Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm (Vienna) 2010;117:699–705. doi: 10.1007/s00702-010-0415-6. [DOI] [PubMed] [Google Scholar]
- 92.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 93.Behrens GM, Stoll M, Schmidt RE. Lipodystrophy syndrome in HIV infection: what is it, what causes it and how can it be managed? Drug Saf. 2000;23:57–76. doi: 10.2165/00002018-200023010-00004. [DOI] [PubMed] [Google Scholar]
- 94.Bleeker-Rovers CP, van der Ven AJ, Zomer B, de Geus-Oei LF, Smits P, Corstens FH, et al. F-18-fluorodeoxyglucose positron emission tomography for visualization of lipodystrophy in HIV-infected patients. AIDS. 2004;18:2430–2. [PubMed] [Google Scholar]
- 95.Sathekge M, Maes A, Kgomo M, Stolz A, Ankrah A, Van de Wiele C. Evaluation of glucose uptake by skeletal muscle tissue and subcutaneous fat in HIV-infected patients with and without lipodystrophy using FDG-PET. Nucl Med Commun. 2010;31:311–4. doi: 10.1097/MNM.0b013e3283359058. [DOI] [PubMed] [Google Scholar]
- 96.Warwick JM, Sathekge MM. PET/CT scanning with a high HIV/AIDS prevalence. Transfus Apher Sci. 2011;44:167–72. doi: 10.1016/j.transci.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 97.Kingsley LA, Cuervo-Rojas J, Muñoz A, Palella FJ, Post W, Witt MD, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22:1589–99. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoh CK. Clinical use of FDG PET. Nucl Med Biol. 2007;34:737–42. doi: 10.1016/j.nucmedbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Zhuang H, Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002;32(1):47–59. doi: 10.1053/snuc.2002.29278. [DOI] [PubMed] [Google Scholar]
- 100.Fox JJ, Strauss HW. One step closer to imaging vulnerable plaque in the coronary arteries. J Nucl Med. 2009;50:497–500. doi: 10.2967/jnumed.108.056325. [DOI] [PubMed] [Google Scholar]
- 101.Yarasheski KE, Laciny E, Overton ET, Reeds DN, Harrod M, Baldwin S, et al. 18FDG PET-CT imaging detects arterial inflammation and early atherosclerosis in HIV-infected adults with cardiovascular disease risk factors. J Inflamm (Lond) 2012;9:26. doi: 10.1186/1476-9255-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164–71. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]