Abstract
Nanotechnology is the engineering and manipulation of materials and devices with sizes in the nanometer range. Colloidal gold, iron oxide nanoparticles and quantum dot semiconductor nanocrystals are examples of nanoparticles, with sizes generally ranging from 1 to 20 nm. These nanotechnologies have been researched tremendously in the last decade and this has led to a new area of “nanomedicine” which is the application of nanotechnology to human health-care for diagnosis, monitoring, treatment, prediction and prevention of diseases. Recently progress has been made in overcoming some of the difficulties in the human use of nanomedicines. In the mid-1990s, Doxil was approved by the FDA, and now various nanoconstructs are on the market and in clinical trials. However, there are many obstacles in the human application of nanomaterials. For translation to clinical use, a detailed understanding is needed of the chemical and physical properties of particles and their pharmacokinetic behavior in the body, including their biodistribution, toxicity, and biocompatibility. In this review, we provide a broad introduction to nanomedicines and discuss the preclinical and clinical trials in which they have been evaluated.
Keywords: Nanomedicine, Nanotechnology, Human clinical trial, Iron oxide, Gold nanoparticle, Quantum dot
Introduction
For the past 30 years, the field of nanotechnology or making nanomaterials has grown explosively and intensive research has been conducted in the worldwide. This huge interest has led to the launch of various research initiatives worldwide such as the National Nanotechnology Initiative in the US, and the European COST Action 523. National Nanotechnology Initiative (started in 2001) has established general definitions. Nanotechnology is defined as manipulation of matter involved in the design, synthesis, characterization, and application of materials or devices with at least one dimension sized from typically 1 to 100 nm [1, 2]. Since its launch, nanotechnology has gained considerable attention from both academia and industry for its potential not only in material science and engineering applications, but also in biotechnology and medical applications. This great interest has led to the emergence of a new term “nanomedicine” that refers to the application of nanotechnology in medicine [3]. The application of nanotechnology to the field of medicine offers advanced technologies including early detection, imaging and treatment of cancers, medical analysis, drug manipulation, and multifunctionality. Eventually, advances in nanomedicine could offer many advantages including greatly improved management of patients with various diseases. However, nanomaterials also bring unique environmental and societal challenges, particularly in regard to the toxic nature of the materials, and adverse health effects, about which no or very little information is available. Hence, it is necessary to understand the precise pharmacokinetics and to continue the research effort [4].
This review is intended to serve as a broad introduction rather than an exhaustive review. We therefore focus on superparamagnetic iron oxide nanoparticles (SPIONs), gold nanoparticles (AuNPs), and quantum dots (QDs) which have been widely investigated in nanomedicine. We also discuss three nanoparticles that have either already advanced to clinical use or are undergoing in vivo/in vitro toxicity evaluation. Besides these three nanoparticles, there are a lot of other nanosubstances or nanomaterials that comprise two or three materials combined, such as liposomes, chitosan nanoparticles, and block copolymer nanoparticles.
Medical doctors and researchers who work in the field of medicine are familiar with biology, physiology, biochemistry and pathology, but nanotechnology could be a strange approach to these individuals. Nanotechnology could be compared and related to engineering, but the structure of biomolecules and drugs, and all biological processes, is three-dimensional and is in the nanometer range, and is therefore of the same order as the double helix ladder structure of DNA which is about 2 nm in size.
Superparamagnetic Iron Oxide Nanoparticles
SPIONs contain one or more superparamagnetic iron oxide cores composed of a mixture of γ-Fe2O3 (maghemite) and Fe3O4 (magnetite) and a biocompatible coating [5]. Two main classes of SPIONs based on materials are currently used for medical applications: (1) SPIO with a mean particle diameter of 50 – 100 nm (including the coating), and (2) ultra-small superparamagnetic iron oxide (USPIO) nanoparticles with a size below 50 nm (hydrodynamic size including the coating) [6]. Unlike gadolinium-based contrast agents which create a bright signal in MR images, SPIO and USPIO are usually called negative contrast agents because they can significantly enhance T2* relaxation, resulting in a signal void in T2*-weighted images [7].
Various techniques for chemical synthesis of SPIONs have been introduced, such as high-temperature decomposition, microemulsion, coprecipitation, sol–gel and forced hydrolysis techniques, and sonochemical and surfactant medicated/template synthesis [8]. Amongst these methods, coprecipitation of Fe2+/Fe3+ ions in a basic aqueous medium such as NaOH or NH4OH solutions is an easy and important method, but the nanoparticles are usually polydispersed and poorly crystallized. For these reasons, thermal decomposition methods, first applied to synthesize semiconductor nanocrystals, were introduced for the production of SPIONs with monodispersity and high crystallinity. Subsequently, the hydrophobic iron oxide nanoparticles can be coated with various materials such as dextran, alginate, protein, phospholipids, silica, or synthetic polymers, including polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), polyvinyl alcohol and polyethyleneimine, to provide good solubility and biocompatibility in vivo [9]. Our group has developed SPION coated with ATP and gluconic acid rather than the polymers mentioned above. The basic characteristic of this SPION is its rapid degradation in vitro and in vivo [10].
Interest in SPIONs has dramatically increased over the last decades. Due to their low toxicity, large magnetic moments and superparamagnetic properties, they have been investigated for many medical applications such as drug and gene delivery, magnetic separation (e.g. in rapid DNA sequencing), antitumor treatment with magnetic hyperthermia therapy, magnetotransfection, and stem cell tracking. Even though the fields of application of SPIONs are diverse, the following section briefly reviews the three main applications of SPIONs.
Applications
Antitumor Treatment with Magnetic Hyperthermia Therapy
Magnetic hyperthermia therapy (magnetic heating therapy) is a form of cancer treatment in which tumor cells are heated using targetable magnetic nanoparticles by an alternating magnetic field. The potential of hyperthermia therapy using SPIONs to damage and destroy localized cancer cells is being intensively investigated. However, hyperthermia has not yet been established in clinical routine due to limitations of the currently available techniques, lack of selectivity for targeting the tumor region and the difficulty in homogeneously distributing the heat within the tumor [11]. Delivery of adequate amounts of SPIONs into tumors is a prerequisite for successful hyperthermia. A number of investigators in proof-of-principle experiments injected the SPIONs (i.e. polymer hydrogel matrix-loaded SPIONs) directly into the tumor as, but this method is not favored owing to the obvious difficulties in translating it to the clinic. Although the favored route of administration is intravenous, the successful accumulation of SPIONs in tumors by this route is difficult [12]. The molecular mechanisms of hyperthermia are not clearly understood, but irreversible protein denaturation, DNA damage, and ultimately apoptosis (and other possible effects) may be triggered by the increase in temperature [13].
Drug Delivery
The large surface area of SPIONs allows the possibility of conjugation with targeting moieties, drug molecules and imaging probes. Widder et al. [14] applied the first magnetic drug delivery systems, in which doxorubicin (DOX) and magnetite were encapsulated in albumin microspheres. After this initial report, intensive research focused on targeted drug delivery and imaging using magnetic nanoparticles. A major concern in obtaining an efficient drug effect is the targeting yield (or targeting capability) of the nanoparticle, that is its delivery into the tumor or target organ. In order to improve the targeting yield, it is essential to attach targeting molecules such as antibody, nucleotide, hormone or receptor ligand to the nanoparticle surface. Many targeting strategies have been tried for delivering the drug to the target site, including (1) site-specific drug release from the SPION, (2) targeted prodrug delivery, and (3) imaging-guided drug delivery (IGDD). For example, Yang et al. [15] investigated the pH-dependent release of doxorubicin from SPION-loaded amphiphilic triblock copolymer nanoparticles, and Hwu et al. [16] investigated the conjugation of paclitaxel to SPIONs through a phosphodiester bond and then the release of the paclitaxel molecules from the particles by the action of intracellular phosphodiesterase. In the area of IGDD, nanomaterials are increasingly being developed in forms combining the diagnostic and therapeutic concepts (nanotheranostics). Nanotheranostics make possible noninvasive biodistribution imaging and drug-release monitoring, and thus may predict therapeutic responses and facilitate therapeutic interventions [17]. Fan et al. [18] synthesized SPIO-conjugated, doxorubicin-loaded microbubbles for brain tumor drug delivery. Initially drug release was controlled by focused ultrasound, and then the released SPIO particles after destruction were deposited within the brain tumor using a magnetic transducer; the overall process was monitored by MRI.
Stem Cell Tracking
Stem cells are classified into totipotent, pluripotent, multipotent, oligopotent or unipotent, and they are characterized by their self-renewal ability through mitotic cell division, and they have the potential to differentiate into a variety of important tissues, such as bone, cartilage and adipose tissue [8]. Their unique properties have led to the rapidly emerging field of regenerative medicine. For stem cell therapy to be successful, the movement and survival of the implanted stem cells in the body need to be tracked to ensure that they reach their target tissue. MRI is commonly used in stem cell tracking studies because of its high spatial resolution.
The first clinical MRI tracking of therapeutic cells (immature dendritic cells) in humans was reported in 2005 [19]. However, the use of SPIONs for stem cell tracking is limited. The division, migration and differentiation of stem cells lead to weakening and distortion of the images generated. The low sensitivity (about 10−3 – 10−5 mol/L) and high cost of MRI, and the recent withdrawal of clinical formulations of SPIONs in the US, will probably limit clinical translation. The cytotoxicity of SPIONs to stem cells has been explored by several researchers. It has been discovered that the nanoparticles do not affect the growth, differentiation or viability of stem cells. However, there is evidence that the endocytic life cycle of stem cells can be affected by nanoparticles residing in endosomes. Lindvall et al. have demonstrated that the use of SPIONs to label human neural stem cell is feasible [20]. However, their results also support the above-mentioned disadvantages. Tracking of stem cells over the short and medium term is feasible, but SPIONs have been found in brain parenchyma cells in a long-term analysis (>5 months). Recently, the use of 19F-MRI for stem cell tracking as an alternative to SPIONs has been studied. The 19F-MRI signal (perfluorocarbon) intensity shows an excellent correlation with the number of 19F-labeled cells [21]. The first clinical application of 19F-MRI has recently been reported [22].
Molecular Imaging Tools
SPIONs for MRI instruments have relatively low sensitivity as previously mentioned. To overcome this limitation, coated SPIONs can be used as a platform to conjugate to the targeting moiety and the other imaging molecules such as fluorescence dyes for optical imaging and radioisotopes for gamma imaging that have more sensitivity [10, 23]. The other method for molecular imaging using SPIONs is to make nanoparticles including SPIONs or to make layers containing iron oxide [24]. These particles can be used in dual or triple imaging platforms.
SPIONs are one of the few clinically approved metal oxide nanoparticles and the first clinically approved as MRI contrast agents in nanodiagnostics. Nanodiagnostics is the term used for the application of nanotechnology in molecular diagnosis. Five types of SPIONs, ferumoxides (Feridex® in the US, Endorem™ in Europe; Berlex Laboratories, Wayne, NJ), ferucarbotran (Resovist®; Bayer Healthcare, Berlin, Germany), ferucarbotran C (Supravist™, SHU 555 C; Schering, Berlin, Germany), ferumoxtran-10 (Combidex; Advanced Magnetics, Cambridge, MA), and NC100150 (Clariscan®, Nycomed, Wayne, PA) have been designed and clinically tested as MRI contrast agents. However, ferucarbotran is currently available in only a few countries, and the other four agents have been removed from the market.
Recently, ferumoxytol (Feraheme® in the US, Rienso® in Europe; AMAG Pharmaceuticals, Waltham, MA) has been approved by the FDA (2009) as an iron replacement drug for the treatment of iron deficiency anemia in patients with chronic renal insufficiency. This is a second-generation USPIO with a carboxymethyl dextran coating. Of the USPIOs, ferumoxytol has the fastest r1 and r2 relaxivity, that allows improved lesion detection compared with other agents. VSOP-C184 (Ferropharm, Teltow, Germany) is another small iron oxide in early phases of evaluation as a potential blood pool agent. VSOP-C184 has been tested in human clinical trials up to phase II (2011). Gastrointestinal luminal contrast agents for oral administration have been developed, including AMI-121 (Ferumoxsil; Lumirem from Guerbet and Gastromark from Advanced Magnetics, Cambridge, MA) and OMP (Abdoscan, Nycomed, Wayne, PA).
Toxicity
SPIONs have attracted much attention not only due to their superparamagnetic properties but also their low toxicity in the human body. Biocompatibility is the first prerequisite for clinical translation, and SPIONs have long been believed to have low toxicity as they degrade into Fe2+ and Fe3+. In vivo studies have shown that SPIONs are relatively safe as they do not accumulate in the vital organs and are rapidly eliminated from the body. However, nanoparticles including SPIONs mediate reactive oxygen species (ROS) and inflammatory responses have been reported. Recent studies have shown that magnetite nanoparticles can seriously damage healthy cells via oxidative stress or by disrupting the existing cytoskeleton [25]. The production of ROS is a major reason for cell death (via DNA damage and lipid peroxidation). The oxidative stress caused by excessive production of ROS can affect the immune system and has been linked with various illnesses including cardiovascular diseases, inflammatory bowel disease, diabetes, Parkinson’s disease, rheumatoid arthritis, and atherosclerosis. Also, reduced cell viability has been reported as the most common toxic effect of iron oxide nanoparticles in many studies. Iron oxide nanoparticles coated with different substances have shown variable cell viability results.
Gold Nanoparticles
There has been great interest in the use of AuNPs in various biomedical applications (diagnosis and treatment of disease, and pharmaceutical drug delivery) because of their easy synthesis, easy surface functionalization, unique optical properties and biocompatibility. Colloidal gold of high quality and yield can be quickly made by the well-known citrate reduction method and the particle size can be controlled by adjusting the compound concentration ratio. Colloidal AuNPs can be made by the reduction of chloroauric acid (Au3+ in HAuCl4) to neutral gold (Au0) by sodium citrate [26]. It is popular and convenient; however, the stability and dispersity of the product are often limited. In recent years, a method of synthesis has been developed that controls the process by adjusting the reaction conditions to provide higher monodispersity and better size distribution. Therefore, various methods of synthesis of AuNPs have been reported and reviewed.
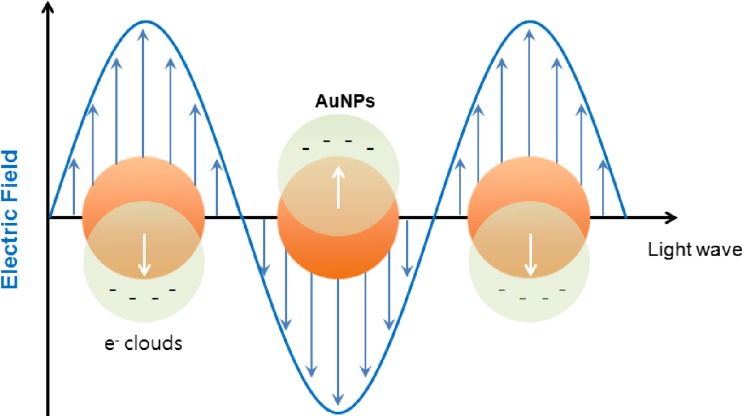
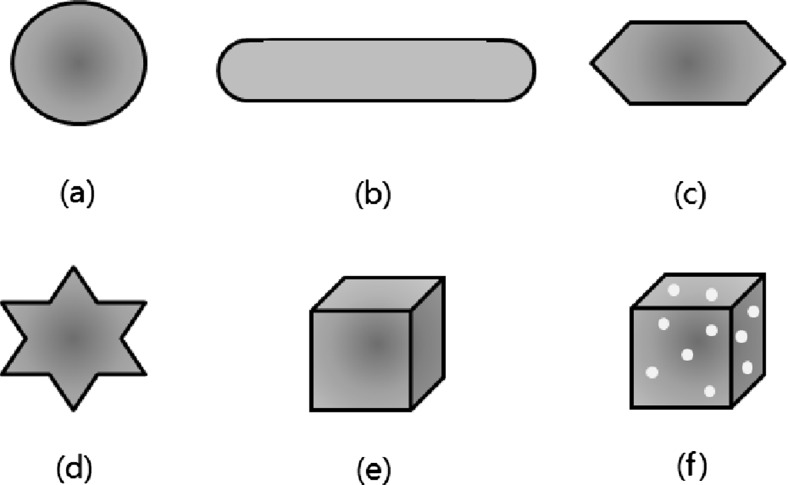
AuNPs have a specific optical property called surface plasmon resonance (SPR). Figure 1 shows a schematic illustration of localized SPR on AuNPs. Collective oscillation of electrons on the AuNP surface occurs when light of a specific wavelength (frequency) excites the AuNPs. This oscillation, the SPR, results in strong extinction of light (scattering and absorption). This strong absorption of the incident light can be measured using a UV-vis spectrometer. AuNPs show the SPR band to be around 520 nm in the visible region with a red-wine color. The SPR band is strongly dependent on the size of the AuNPs, shape, surface character and agglomeration state of the AuNPs. For example, the plasmon oscillation of 5-nm gold particles are strongly damped and their absorption becomes weak and broad, and completely disappears at less than 2 nm size [27]. The SPR band can easily be tuned from around 500 nm into the near-infrared (NIR) range by changing the particle shape. AuNPs of various shapes have been introduced, such as stars and nanorods that show electromagnetic field enhancement, and can be used in surface enhanced Raman spectroscopy (SERS) imaging. Various shapes including nanosphere, nanorod, nanopyramid, nanostar, nanocube and nanocage AuNPs are shown in Fig. 2 [28].
Fig. 1.
Schematic illustration of surface plasmon resonance in spherical gold nanoparticles
Fig. 2.
Various shapes of gold nanoparticles: a nanosphere, b nanorod, c nanopyramid, d nanostar, e nanocube, f nanocage
AuNPs are capped with various capping agents such as citrate, tannic acid, dodecanethiol, cetyltrimethylammonium bromide (CTAB), hydroxylamine hydrochloride, and water-soluble polymers such as thiolated PEG, PVP, and chitosan. These capping agents bind to the AuNP surface and prevent aggregation by repulsive or steric forces. Typically, gold salt is reduced by a reducing agent, which may also act as a capping agent. For example, citrate initially acts as a reducing agent and finally as a capping agent. Citrate is adsorbed on the surface of the nanoparticle surface weakly and so is readily displaced by other molecules including amine and thiol polymers, proteins and antibodies, and stable Au–S bonds are formed. However, citrate-stabilized AuNPs tend to aggregate upon stress such as repeated centrifugation, dilution and dialysis, and upon contact with biological media. Tannic acid is a multidentate capping ligand that can be displaced with many thiol-containing molecules. Although PVP binds strongly to the AuNP surface and provides greater stability than other small molecules, it is more difficult to exchange [29]. The following section briefly reviews some important applications of AuNPs.
Applications
X-ray Imaging and Computed Tomography
Current contrast agents used in X-ray imaging (barium sulfate and iodine) have serious limitations for medical imaging: short imaging times, the need for catheterization in many cases, occasional renal toxicity, severe adverse effects and poor contrast in large patients. The high atomic number (Z = 79) and electron density of gold leads to efficient absorption of X-ray radiation, superior to that using conventional iodine-based contrast agents. Additionally, AuNPs can offer a longer circulation time that allows a longer imaging time, targeting and cell tracking. In general, AuNPs are considered nontoxic. However, despite broad consensus on the low cytotoxicity of AuNPs in vitro, a few less-favorable reports in the literature have arouse attention. For example, Pernodet et al. [30] found that AuNPs alter the morphology of human dermal fibroblasts at a concentration of 0.4 mM (about 0.08 mg/mL). Damage to the cellular cytoskeleton could affect cell proliferation, motility and adhesion. In addition, Mironava et al. [31] found that AuNP-exposed cells undergo cytoskeletal filament disruption and apoptosis. Over the last decade, there has been significant progress in the design of functional AuNPs as X-ray contrast agents; however, much work is still needed before translation to the clinic [32].
Surface-enhanced Raman Spectroscopy Imaging
Raman spectroscopy is a laser-based technique used for the analysis of molecular vibrations, rotations and other processes. The Raman spectrum is a “fingerprint” of the molecule under investigation, giving information on bonds, conformations, and intermolecular interactions [33]. In spite of its advantages, its practical uses have been significantly limited because the Raman scattering signal of a molecule is intrinsically weaker as a result of the small scattering cross-section area (about 10−30/cm2) than that of other fluorescence dyes (about 10−16/cm2) [34]. The fraction of Raman-scattered photons is very small (about 1 in 107). Various enhancement methods have been developed to extend the detection limit. Among these, absorption of molecules on AuNPs or on particles of other metals (Ag, Co) with high curvature has been shown to enhance the intensity of the vibrational spectra by several orders of magnitude (up to about 10−16/cm2, i.e. similar to that of fluorescence dyes). This technique termed (SERS) enables the detection of single molecules. This phenomenon has been widely studied using AuNPs, and is thought to be due to an electromagnetic field close to the particle surface produced by localized SPR. The primary limitation of SERS imaging is deep tissue penetration. Like other optical modalities, absorption and scattering of photons is affected by depth. Another hurdle is that wide-field Raman scanners with real-time imaging capability are not yet available. The most important and difficult obstacle to overcome will be obtaining the FDA approval for systemic injection of SERS nanoparticles in humans [35].
Photoacoustic Imaging
Due to their strong absorption of light, the use of AuNPs as contrast agents for photoacoustic (thermoacoustic) imaging has been explored. Photoacoustic imaging is advantageous compared to other imaging techniques because it is a nonionizing noninvasive imaging method with higher spatial resolution due to the lower scattering, deep penetration and high contrast in vivo. Photoacoustic signals are generated via the following steps: the adsorbed light energy is converted to heat and simultaneously the temperature rises causing thermoelastic expansion which is translated to acoustic waves (or pressure), which are finally detected by an ultrasound transducer and translated into an image. This phenomenon is triggered by a short-pulse radiation source such as a pulsed laser. Photoacoustic imaging has been demonstrated successfully using gold nanorods (AuNRs), nanocages and nanoshells with high and tunable optical absorption cross sections [36]. Recently, Zhong et al. [37] investigated image-guided photoacoustic tumor therapy with folic acid-labeled AuNRs in mouse with human cancer cell xenografts. They found that the photoacoustic waves can generate a strong shock wave of up to 100 mPa and this pressure can kill cancer cells.
Photothermal Therapy
The superior light absorption and scattering properties (1,000 times higher than organic dyes) of AuNPs and their ability to convert light into thermal energy by SPR led to the early use of AuNPs in photothermal cancer therapies [38]. When AuNPs absorb a radiation from a heat source such as NIR light, the excited electrons (collective coherent oscillation) return to their ground state, and energy is released during this rapid relaxation in the form of heat resulting in a simultaneous increase in temperature that can destroy cancer cells. As mentioned above, this phenomenon can be used to provide contrast for photoacoustic imaging or photothermal therapy (PTT). Photothermal heating only occurs around the AuNPs, and local temperatures can easily reach tens to thousands of degrees kelvin, potentially reducing the negative side effects of cancer therapies [39]. The temperature rise is primarily dependent upon the shape and concentration of the AuNPs, incubation time, laser fluence (power per unit area) and the laser exposure time. For effective photothermal ablation of deep tumors, NIR light (650 – 1,100 nm) is required because this light can penetrate several centimeters with minimal absorption by hemoglobin and other molecules in living tissues.
In recent years, there have been great advances in the use of PTT as a result of the development of AuNRs. However, the overexpression of heat shock proteins as a defense mechanism against heat stress has also been observed in some cancers. This implies that cancer cells are more resistant than expected to heat-based therapies, so the effects of thermal therapy are not universal in all types of cancer. Recently, three groups have reported new nanoparticles as platforms for PTT. Li et al. [40] reported a new delivery and photothermal ablation system based on macrophages laden with AuNRs (macrophages carrying 7-nm AuNRs as Trojan horses). After intratumoral injection, the AuNR-laden macrophages showed an excellent photothermal ablation effect in the whole tumor area and a lower rate of tumor recurrence was observed than with free AuNRs. Piao et al. [41] investigated porous and hollow AuNPs coated with RBC membrane. RBC-AuNPs exhibited significantly enhanced in vivo blood retention and circulation and greatly enhanced tumor uptake when administered systemically, and mice that received PPT cancer treatment showed 100 % survival over 45 days. Rengan et al. [42] investigated the use of biodegradable gold-coated liposome nanoparticles (lipid–gold hybrid material) for photothermal therapy of cancer. They found that combination treatment of mice with liposomal AuNPs and laser resulted in a 4.63-fold reduction in tumor bioluminescence signal in comparison with controls.
In Vitro Diagnostics
AuNPs have interesting applications in the molecular diagnostic field. Early detection is important to reduce the impact of diseases and to improve survival rates. Binding AuNPs with target analytes leads to color change by aggregation and this is the basis of sensing, a principle introduced by Leuvering et al. [43]. The first study of the colorimetric sensing of DNA was reported by Mirkin et al. [44]. Fluorescence resonance energy transfer (FRET) is the mechanism by which the excitation energy of the donor is transferred to the acceptor. On the basis of this mechanism, an AuNP-based FRET technique can be used to monitor DNA hybridization and DNA cleavage by nucleases. DNA detection sensitivity associated with AuNP aggregation has been improved by using hyper-Raleigh scattering and a differential light-scattering spectroscopy. In recent years construction of immunosensors using AuNPs has received significant attention.
FDA-Approved AuNPs
Recombinant human tumor necrosis factor-alpha (rhTNF-α) is an anticancer agent. TNF-α became available in recombinant form in 1984 and remarkable antitumor effects were observed in experimental animals. However, phase I and II human clinical trials revealed high systemic toxicity including respiratory failure and coagulopathies. Aurimune (CYT-6091; CytImmune Sciences, Rockville, MD) is a 27-nm colloidal AuNP coated with thiolated PEG and conjugated with rhTNF-α. PEGylation can prevent immediate uptake by the reticuloendothelial system (RES), and reduce the severe toxicity of bare rhTNF-α. In preclinical studies, a dramatic effect on tumor regression was seen in TNF-sensitive tumor models. Aurimune has been tested phase I dose escalation clinical trials and is now in phase II clinical trials to treat patients with non-small-cell lung cancer. Aurimune is well tolerated at doses up to 600 μg/m2 of TNF, levels three times higher than the published maximum tolerated dose for native TNF [45].
AuroShell® (Nanospectra Biosciences Inc., Houston, TX) was approved by the FDA in 2012. AuroShell comprises gold nanoshells consisting of a silica core of 120 nm diameter with 10-nm gold shells with a coating of PEG5000 (155 nm diameter). This has been used in clinical trials to treat head and neck cancers using PTT. AuroShell particles are administered intravenously and accumulate in the tumor through the enhanced permeability and retention effect. The AuroShell particles are specifically designed to absorb 808-nm NIR laser light and convert the laser light into heat. This therapy is called AuroLase® therapy. AuroShell is currently being used to treat locally recurrent breast cancer. The Verigene® system (Nanosphere, Northbrook, IL) detects infectious pathogens and drug resistance markers in the blood within about 3 h.
Toxicity
Most investigations have demonstrated that AuNPs are nontoxic; however, findings are contradictory, for example showing the toxicity of SPIONs. The predominant mechanism of AuNPs toxicity involves ROS and the production of oxidative stress. After intracellular uptake, nanoparticles are confined in vesicular structures, endosomes, and finally accumulate in lysosomes. The acidic lysosomal pH (pH about 4.5) breaks the nanoparticles leading to the release of the relatively toxic ions (e.g. Ag+, Cd2+, Fe2+/Fe3+, Au+/Au3+ ions). Nanoparticles are required to be targeted to a particular tissue or organ and this can lead to an imbalance in its homeostasis as a result of high concentrations in a localized area. This toxic ion overload can cause aberrant cellular responses including increased ROS levels, cytotoxicity, DNA damage, oxidative stress, epigenetic events and inflammatory processes.
As mentioned previously, generally the toxicity of nanomaterials including AuNPs is thought to be associated with their size, shape, chemical composition, charge, and agglomeration state [46–48]. Size and charge can influence cellular uptake, distribution, and elimination of AuNPs. Chemical composition (degradability) of the surface and core can influence the interaction with biomolecules and is an important factor in acute and long-term toxicity. The relationship between size and toxicity of AuNPs has been much discussed. For example, Sonavane et al. [49] found that AuNPs (15 and 50 nm) are able to pass the blood–brain barrier because of the size similarity with cell components and proteins. In contrast to large particles, small particles (<50 nm) distribute in almost all tissues within 24 h after intravenous injection, and irreversibly bind to DNA causing genotoxicity [50]. Pan et al. [51] found that 1.4-nm gold nanospheres trigger necrosis by strong oxidative stress and mitochondrial damage in all the cell lines they examined, but they found no evidence of cellular damage caused by 15-nm gold nanospheres attached to the identical constituents.
With regard to shape, anisotropic AuNPs (e.g. nanorods) have been reported to be more susceptible to oxidation than isotropic AuNPs due to their highly exposed surface areas and defects [52]. However, Tarantola et al. [53] found by monitoring epithelial cells incubated with CTAB-coated gold spheres and rods that spherical nanoparticles are more toxic and easily internalized than rod-shaped nanoparticles. Fortunately, these toxic properties are generally controlled by the nature of the surface functionalizing molecular layer. With regard to the effect of charge, Goodman et al. [54] found that cationic gold nanospheres are more toxic at certain doses than negatively charged spheres. This can be explained by interaction with the negatively charged cell membrane and the resultant membrane disruption.
Several studies have shown that AuNPs can cause nephrotoxicity, hemolysis, neurotoxicity and genotoxicity. Sereemaspun et al. [55] were the first to report that AuNPs can penetrate renal cells, and this implies the possibility nephrotoxicity. Uchiyama et al. [56] found in rats that injection with AuNP-IgG or citrate-AuNP did not cause hemorrhage, hemolysis or thrombus formation, but suppressed leukocyte adhesion that can lead to inflammation. Klien and Godnić-Cvar reviewed studies on the genotoxicity of metal nanoparticles including AuNPs [57]. Recently, Jung et al. [58] found that intracellular AuNPs increase the excitability of hippocampal CA1 neurons by generating more action potentials and this might lead to disturbances in neuronal functions and hyperexcitability. Sousa et al. [59] found that polyelectrolyte multilayer coated AuNPs 7 days after injection are mainly present in the hippocampus, thalamus, hypothalamus, and the cerebral cortex.
Quantum Dots
Before 1993, QDs were normally prepared in aqueous solution with stabilizing agents. QDs from this method were of low quality (poor fluorescence quantum yields and large size variations). In 1993, Murray et al. [60] developed a high-temperature organometallic method for producing high-quality CdSe QDs. Since then, a tremendous research effort has resulted in the production of high-optical quality QDs for applications in biology and medical fields. QDs have become important fluorescent probes for bioimaging research. QD nanocrystals offer several unique advantages compared with other organic fluorophores, such as emission tunablility from visible to NIR wavelengths by controlling the composition and size of the crystals, large absorption coefficient across a wide spectral range, very high quantum yield, and photostability. Furthermore, their large surface area to volume ratio permits the attachment of a large quantity of target moieties or drugs and their superior stability enabling longer investigation times are the main advantages of QDs compared with other fluorescent agents [61].
QDs are light-emitting colloidal semiconductor nanocrystals with a core–shell structure and a diameter typically ranging from 2 to 10 nm. They contain a small finite number of conduction band electrons and valence band holes, or excitons. QDs fluorescence occurs when an excited electron moves from the conduction band to its valence band, and emits a photon with a longer wavelength than first absorbed. The wavelength difference between the maxima of the excitation and emission spectra is known as Stokes shift. The band gap energy depend on the size of the QDs: larger QDs have smaller band gaps and emit red light, while smaller QDs emit short wavelength blue light (quantum confinement). In smaller QDs, the electrons and holes begin to “squeeze”, and this quantum confinement causes the energy state to shift to a higher level [62]. Therefore, the color and emission wavelength of QDs are determined by their size and composition. The shell layer is made of a semiconductor material with a higher band gap than that of the core, such as ZnS (core–shell, alloying, and doping). The shell protects the core from oxidation, increases photostability and improves its quantum yield up to 95 % [63] when compared with that of the core alone, which is usually less than 10 %. New synthetic techniques such as alloying, core–shell, and doping developed in recent years will likely play key roles in the future developments in tuning the band gap.
Figure 3 shows the internal structures of QDs. The core of QDs is usually composed of elements of groups II–VI such as CdSe, CdS and CdTe, groups III–V such as InP or InAs, groups IV–VI such as PbSe, or I/III/VI compounds such as CuInS2, and the shell is usually composed of ZnS [64, 65]. Although Cd-based QDs have received much attention, they are potentially toxic to humans and the environment. Other materials, such as binary III–V (InP) and ternary I/III/VI QD compounds have been considered as replacements for Cd-based materials. The syntheses and optical properties of ternary I/III/VI compounds, including CuInS2 (CIS), CuInSe2 (CISe), CuGaS2 (CGS), AgInS2 (AIS), and AgGaS2 (AGS) have been extensively examined [66]. In particular, CuInS2 exhibits low toxicity, tunable emissions in the visible to the NIR region, high absorption coefficients, and large Stokes shift. For these characteristics, I/III/VI compounds are considered to be alternative low-toxicity materials for bioimaging and solid-state lighting. Therefore, synthesis of high-quality CuInS2-based QDs is of great interest.
Fig. 3.
Schematic representations of the internal structure of quantum dots
Even though significant progress has been made in simplifying the synthesis routes, improving quantum yields, controlling the size and shape, and elucidating the mechanisms of formation of QDs, chemical synthesis strategies usually rely on harsh reaction conditions and toxic organic solvents. The major challenge with organic-phase QDs is their nonbiocompatibility, which restricts their direct applications in biological and medical research. To address this issue, great effort has been directed towards water solubilization and biofunctionalization of chemically synthesized QDs [67]. Recently, carbon-based QDs such as carbon dot and graphene carbon dot have received attention because of their excellent biocompatibility and tunable physicochemical properties.
QDs have been used in cell-labeling studies, biosensing (e.g. immunoassay), imaging studies to view native processes in living animals, and numerous diagnostic applications including cancer management, blood flow investigation, and virus detection. It has also been suggested that QDs could be used to deliver targeted therapy in the treatment of tumors.
Applications
Lymphatic System Imaging
Mapping of sentinel lymph nodes (SLNs) is important for assessing the stage of cancer and for planning efficient treatment and for evaluating prognosis. In breast cancer patients, the axillary lymph nodes are most important lymphatic drainage system and metastatic route. SLN mapping can be performed with a variety of imaging modalities, but optical imaging tool has been widely investigated because of its sufficient spatial resolution in real-time during surgery (image-guided surgery). Among the many fluorophores, QDs are a very attractive SLN mapping material because of their size-tunable emission wavelength ranging from visible to NIR, sharp and nearly symmetrical emission peak, high quantum yields (sensitivity), good penetration depth, photostability and resistance to photobleaching.
Ever since lymph node mapping using NIR QDs was first reported by Kim et al. [68] in the nude mouse and pig, the use of QDs for imaging the vasculature of several organs such as the lung, esophagus and gastrointestinal (GI) tract has been evaluated in animal models. Wang et al. [69] reviewed the use of QDs in axillary lymph node imaging in animal models. Although there are great advantages in the use of QDs, some serious limitations such as their inherent toxicity and nonbiocompatibility make translation to human applications difficult. Erogbogbo et al. [70] synthesized surface functionalized silica-coated CdSe QDs of various particle sizes and found that they showed no toxicity in axillary lymph nodes. Pons et al. [71] compared the toxicity of CdTeSe/CdZnS with that of CuInS2/ZnS during SLN detection. Lymph nodes clearly showed a greater inflammatory response to the cadmium-based QDs in terms of increased lymph node weight and number of inflammatory sites. However, CuInS2/ZnS QDs did not show any features of toxicity under the same conditions.
Cancer Imaging
The great significance of cancer in human life has made in vivo imaging to determine the location and distribution of tumors a high research priority. Early diagnosis of cancer with QDs has been the main focus of significant research effort. QDs play an increasingly important role in tumor imaging, especially NIR (700 – 900 nm) imaging. Antibodies are commonly used to target tumors. Active targeting of cancer antigens with antibody–QD conjugates is an effective strategy for tumor detection.There have been many studies of cancer-specific monoclonal antibodies targeting, for example, carbohydrate antigen 125 for ovarian cancer, epidermal growth factor receptor (EGF-R) for brain cancer, human epidermal growth factor receptor 2 (HER-2) for breast cancer, prostate-specific antigen (PSA) for prostate cancer, and anti-claudin 4 for pancreatic cancer [72]. Recently, NIR QDs combined with magnetic iron oxide for multimodality probes with improved sensitivity, accuracy and targeting efficacy have been introduced. Ding et al. [73] synthesized PEG-coated Mn-doped core-shell CuInS2@ZnS and evaluated its use for dual modality fluorescence and MRI tumor imaging.
In Vivo Imaging Using Self-Illuminating QDs
QDs can undergo the FRET phenomenon, which is a popular tool in the development of biosensors and detection assays. FRET is the nonradiative transfer of energy between two light-sensitive molecules (from a donor to an acceptor) through near-field dipole–dipole coupling [74]. For this energy transfer between a donor and an acceptor to occur, a typical distance of 2 – 8 nm (known as the Forster distance) is needed so that there is overlap between the emission wavelength of the donor and the absorption wavelength of the acceptor. There are numerous examples of FRET-based QD biosensors. Medintz et al. [75] describe a QDs FRET sensing system for maltose based on Escherichia coli maltose binding protein (MBP) on the QDs and β-cyclodextrin QSY9 dark quencher bound to the MBP binding site. If maltose displaces the β-cyclodextrin QSY9, the QDs photoluminescence increases.
In general, QDs emit photons following sustained excitation from an external light source. However, autofluorescence can occur under external illumination with short-wavelength light, but the QDs also absorb and scatter the light, creating background interference. To solve this problem, a new sensing concept was introduced in which excitation occurs without external excitation.Bioluminescence resonance energy transfer (BRET) has been developed. This is a naturally occurring phenomenon in which a light-emitting protein (e.g. Renilla reniformis luciferase) acts as the donor and a fluorescent protein in close proximity acts as the acceptor [75]. So et al. [76] developed such a system that comprises the bioluminescent protein Renilla luciferase as the donor conjugated to QDs as the acceptor. When the QD conjugates are exposed to the luciferase substrate, the energy released in the oxidation of the substrate is transferred to the QDs through BRET, thus generating light that is emitted from the QDs. Compared with Luc8 (a variant of R. reniformis luciferase), the QD–Luc8 conjugate was found to have greatly enhanced sensitivity in small-animal imaging because of its longer emission wavelength, with a signal-to-background ratio of >103 for 5 pmol of conjugate. BRET QDs have been used for in vitro sensing of protease and nucleic acids and for in vivo lymphatic imaging. However, the applications of QDs made of heavy metals have largely been limited to translational research because of unfavorable in vivo pharmacokinetics after systemic injection and their intrinsic toxicity.
QDs can act as donors in FRET and as acceptors in BRET. Chemiluminescence resonance energy transfer (CRET) was introduced by Huang et al. [77]. CRET is resonance energy transfer between chemiluminescent donors and QDs as acceptors, and is similar to BRET. CdTe QD acceptors labeled with horseradish peroxidase (HRP) were reacted with luminol donors. Florescence was induced by luminol–H2O2 chemical reaction catalyzed by the HRP. However, few studies have so far been reported on the use of CRET.
Cellular Imaging
For the imaging of the behavior of single molecules in live cells to elucidate cellular events requires a high specificity and super-resolution imaging tool. The common fluorescent dyes suffer from the drawback of short fluorescent life-times. The assessment of crosstalk between cellular signaling needs long-term monitoring. QDs are suitable for long-term imaging applications due to their high photostability. However, imaging of live cells with QDs faces problems associated with the large size of QDs. Although it is relatively simple to target the extracellular surface, the delivery of monodispersed QDs into the cytoplasm is still challenging, and QDs often tend to aggregate or to become internalized by endosomes. Pioneering work byWu et al. [78] indicated the potential of QDs in cellular imaging. They showed that streptavidin-labeled QDs can be used to visualize actin filaments, microtubules, cytoskeleton fibers and cell surface HER-2 receptors. QD bioconjugates have since been used extensively in cellular imaging studies.
Detection of Circulating Tumor Cells
To initiate the metastatic process, cells detach from primary tumor sites, enter the circulation, settle at distant sites and develop secondary tumors. Conventional cancer detection systems involve the use of radiation or magnetic resonance that may harm patients. These modalities can also produce accurate results only in advanced cancers, and not in early stages. However, the detection of circulating tumor cells (CTCs) in the bloodstream can effectively contribute to establishing the prognosis in both early and advanced stages of cancer. The extremely low concentration of CTCs in blood samples limits detection sensitivity, and existing assays are usually very costly and require specialized equipment [79]. Recent advances for the isolation of CTCs combine multifunctional magnetic beads and their detection by QD–antibody conjugates. Zhang et al. [80] and Costa et al. [81] developed a microfluidic device to detect tumor cells that comprises a microbead-based array of AuNPs conjugated with multiple enzymes and labelled QDs to detect the signal from tumor cells amplified by RT-PCR. Due to the two signal amplification approaches, the system was able to detect as few as one HT29 cell in 1 ml of blood.
For clinical imaging and drug delivery applications in humans, the major concern about the potential toxicity of QDs needs to be overcome before their potential for healthcare and medical research applications can be fully realized. The toxic effects of QDs depend on many factors such as their composition, surface coating material, size, concentration, the interacting system and the duration of the interaction. Their high heavy metal content (Pb, Cd, Cr, Cu, Zn, Ni, As and Hg) makes QDs extremely toxic to living creatures [82]. Heavy metals cause developmental abnormalities and serious health issues, primarily affecting the liver and kidneys, and they can cross the blood–brain barrier, accumulate in adipose tissue, and remain in the body for up to 10 years. Carcinogenic effects of heavy metals may become apparent on long exposure. In addition, the excitation process results in the release of destructive reactive and free radical species. However, this cytotoxicity can generally be overcome by modifying the QDs with proteins or DNA or adding a protective hydrophilic coating. For example, although Cd core QDs are very toxic and kill cells, adequate coatings such as PEG or silica can serve as good barriers preventing dissolution of the core in biological media.
Concluding Remarks
In the main sections, we reviewed general synthetic methods, fields of application at the preclinical and clinical levels, the types of nanomaterials approved by the FDA, and the toxicity of SPIONs, AuNPs and QDs, which are widely used.
Nanomaterial research particularly the development of engineered nanoparticles will lead to great benefits and opportunities in medicine, for example significant improvements in diagnostic tools and therapeutic drugs. The application of these materials will also eventually lead to improved human health and quality of life. With the rapid expansion of nanomedicine, many new nanoparticle systems will be developed in the near future. However, the risk of human exposure to nanoparticles has not been well studied and therefore risk prediction is important. Thus, the physicochemical and biological properties of nanomaterials, and their impact on the human body and environment have to be completely characterized and understood.
A number of cancer nanodrugs are currently in clinical trials. Information on the phase and status of clinical trials is available from the US National Institutes of Health Clinical Trials web site (http://clinicaltrials.gov/ct2/home). The information provided on this site helps in the modification of present nanomedicinal systems and the development of new concepts.
In terms of molecular imaging and therapeutic medicine based on nanomaterials, we have to consider the outcomes of the application of nanotechnology. Nanomaterials can be used to acquire images with more sensitivity, which means fewer nanoparticles may be injected in the in vivo setting. Imaging increases the efficiency of lesion targeting and plays a role in establishing efficient therapeutic strategies. The know-how concerning molecular imaging in personalized medicine should be combined with nanomedicine to create an important axis to move from personalized to lesionalized medicine.
Acknowledgments
This work was supported by the Radiation Technology R&D program (2012M2A2A7014020, and 2015M2A2A6A04044884) through the National Research Foundation of Korea and the Mid-Carrier Researcher Program (2011–028581) funded by the Ministry of Science, ICT and Future Planning, respectively.
Compliance with Ethical Standards
Conflicts of Interest
Eun-Mi Kim and Hwan-Jeong Jeong declare that they have no conflicts of interest.
Ethical Approval
This article does not describe any studies with human participants or animals performed by any of the authors.
This manuscript has not been published before and is not under consideration for publication anywhere else and has been approved by all the authors.
References
- 1.National Science and Technology Council Committee on Technology, The National Nanotechnology Initiative: research and development leading to a revolution in technology and industry, Office of Sciences and Technology Policy, Washington, DC. 2005. http://www.nano.gov/.
- 2.Wang R, Billone PS, Mullett WM. Nanomedicine in action: an overview of cancer nanomedicine on the market and in clinical trials. J Nanomater. 2013:629681.
- 3.Nanomedicine: grounds for optimism, and a call for papers. Lancet. 2003;362:673. [PubMed]
- 4.Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. Int J Nanomed. 2007;2:129–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer C, Whittaker MR, Bulmus V, Liu J, Davis TP. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010;2:23–30. doi: 10.1038/asiamat.2010.6. [DOI] [Google Scholar]
- 6.Di Marco M, Sadun C, Port M, Guilbert I, Couvreur P, Dubernet C. Physicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. Int J Nanomedicine. 2007;2:609–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Bronzino JD, Peterson DR. Biomedical signals, imaging, and informatics. In: Yuan C, Kerwin WS, Canton G, Wang J, Chen H, Balu N, editors. Magnetic resonance imaging of atherosclerosis. Boca Raton: CRC Press; 2015. pp. 16–33.
- 8.Bull E, Madani SY, Sheth R, Seifalian A, Green M, Seifalian AM. Stem cell tracking using iron oxide nanoparticles. Int J Nanomed. 2014;9:1641–53. doi: 10.2147/IJN.S48979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohapatra M, Anaud S. Synthesis and applications of nano-structured iron oxide/hydroxides – a review. Int J Eng Sci Technol. 2010;2:127–46. [Google Scholar]
- 10.Lee CM, Cheong SJ, Kim EM, Lim ST, Jeong YY, Sohn MH, et al. Nonpolymeric surface-coated iron oxide nanoparticles for in vivo molecular imaging: biodegradation, biocompatibility, and multiplatform. J Nucl Med. 2013;54:1974–80. doi: 10.2967/jnumed.113.122267. [DOI] [PubMed] [Google Scholar]
- 11.Hu R, Ma S, Li H, Ke X, Wang G, Wei D, et al. Effect of magnetic fluid hyperthermia on lung cancer nodules in a murine model. Oncol Lett. 2011;2:1161–4. doi: 10.3892/ol.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv. 2011;2:1001–14. doi: 10.4155/tde.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Xu CL, Liu CM. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089–100. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widder KJ, Morris RM, Poore G, Howard DP, Jr, Senyei AE. Tumor remission in Yosida sarcoma-bearing rats by selective targeting of magnetic albumin microspheres containing doxorubicin. Proc Natl Acad Sci U S A. 1981;78:579–81. doi: 10.1073/pnas.78.1.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Matson VZ, et al. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano. 2010;4:6805–17. doi: 10.1021/nn101670k. [DOI] [PubMed] [Google Scholar]
- 16.Hwu JR, Lin YS, Josephrajan T, Hsu MH, Cheng FY, Yeh CS, et al. Targeted paclitaxel by conjugation to iron oxide and gold nanoparticles. J Am Chem Soc. 2009;131:66–8. doi: 10.1021/ja804947u. [DOI] [PubMed] [Google Scholar]
- 17.Lammers T, Kiesling F, Hennink WE, Storm G. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm. 2010;7:1899–912. doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- 18.Fan CH, Ting CY, Lin HJ, Wang CH, Liu HL, Yen TC, et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–15. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- 19.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–13. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 20.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders – how to make it work. Nat Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 21.Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72:1696–701. [DOI] [PMC free article] [PubMed]
- 23.Zhou H, Hou X, Liu Y, Zhao T, Shang Q, Tang J, et al. Superstable magnetic nanoparticles in conjugation with near-infrared dye as a multimodal theranostic platform. ACS Appl Mater Interfaces. 2016;24:4424–33. doi: 10.1021/acsami.5b11308. [DOI] [PubMed] [Google Scholar]
- 24.Lee CM, Jang D, Kim J, Cheong SJ, Kim EM, Jeong MH, et al. Oleyl-chitosan nanoparticles based on a dual probe for optical/MR imaging in vivo. Bioconjug Chem. 2011;22:186–92. doi: 10.1021/bc100241a. [DOI] [PubMed] [Google Scholar]
- 25.Alarifi S, Ali D, Alkahtani S, Alhader MS. Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol Trace Elem Res. 2014;159:416–24. doi: 10.1007/s12011-014-9972-0. [DOI] [PubMed] [Google Scholar]
- 26.Frens G. Controlled nucleation for the regulation of particle size in monodisperse gold suspensions. Nat Phys Sci. 1972;241:20–2. doi: 10.1038/physci241020a0. [DOI] [Google Scholar]
- 27.Linka S, El-Sayeda MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem. 2000;l:409–53. doi: 10.1080/01442350050034180. [DOI] [Google Scholar]
- 28.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40:1647–71. doi: 10.1039/C0CS00018C. [DOI] [PubMed] [Google Scholar]
- 29.Alkilany AM, Yaseen AB, Kailani MH. Synthesis of monodispersed gold nanoparticles with exceptional colloidal stability with grafted polyethylene glycol-g-polyvinyl alcohol. J Nanomater. 2015;712359.
- 30.Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishnan A, Sokolov J, et al. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small. 2006;2:766–73. doi: 10.1002/smll.200500492. [DOI] [PubMed] [Google Scholar]
- 31.Mironava T, Hadjiargyrou M, Simon M, Jurukovski V, Rafailovich MH. Gold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure time. Nanotoxicology. 2010;4:120–37. doi: 10.3109/17435390903471463. [DOI] [PubMed] [Google Scholar]
- 32.Cole LE, Ross RD, Tilley JM, Vargo-Gogola T, Roeder RK. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomedicine. 2015;10:321–41. doi: 10.2217/nnm.14.171. [DOI] [PubMed] [Google Scholar]
- 33.Kumar C. Raman spectroscopy for nanomaterials characterization. In: Jeong DH, Kim G, Lee YS, Jun BH, editors. Immunoassays and imaging based on surface-enhanced Raman spectroscopy. New York: Springer; 2012. pp. 263.
- 34.Pieczonka NP, Aroca RF. Single molecule analysis by surfaced-enhanced Raman scattering. Chem Soc Rev. 2008;37:946–54. doi: 10.1039/b709739p. [DOI] [PubMed] [Google Scholar]
- 35.Andreou C, Kishore SA, Kircher MF. Surface-enhanced Raman spectroscopy: a new modality for cancer imaging. J Nucl Med. 2015;56:1295–9. doi: 10.2967/jnumed.115.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YS, Frey W, Kim S, Kruizinga P, Homan K, Emelianov S. Silica-coated gold nanorods as photoacoustic signal nanoamplifiers. Nano Lett. 2011;11:348–54. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong J, Wen L, Yang S, Xiang L, Chen Q, Xing D. Imaging-guided high-efficient photoacoustic tumor therapy with targeting gold nanorods. Nanomedicine. 2015;11:1499–509. doi: 10.1016/j.nano.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Hwang S, Nam J, Jung S, Song J, Doh H, Kim S. Gold nanoparticle-mediated photothermal therapy: current status and future perspective. Nanomedicine. 2014;9:2003–22. doi: 10.2217/nnm.14.147. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, El-Sayeda MA. Plasmonic photo-thermal therapy (PPTT) Alexandria J Med. 2011;47:1–9. doi: 10.1016/j.ajme.2011.01.001. [DOI] [Google Scholar]
- 40.Li Z, Huang H, Tang S, Li Y, Yu XF, Wang H, et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials. 2016;74:144–54. doi: 10.1016/j.biomaterials.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 41.Piao JG, Wang L, Gao F, You YZ, Xiong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8:10414–25. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 42.Rengan AK, Bukhari AB, Pradhan A, Malhotra R, Banerjee R, Srivastava R, et al. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015;15:842–8. doi: 10.1021/nl5045378. [DOI] [PubMed] [Google Scholar]
- 43.Leuvering JH, Thal PJ, van der Waart M, Schuurs AH. Sol particle immunoassay (SPIA) J Immunoassay. 1980;1:77–91. doi: 10.1080/01971528008055777. [DOI] [PubMed] [Google Scholar]
- 44.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–9. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 45.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr, Gannon WE, Walker M, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–49. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–9. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 47.Chen YS, Hung YC, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858–64. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 49.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf B Biointerfaces. 2008;66:274–80. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Kosaka N, McCann TE, Mitsunaga M, Choyke PL, Kobayashi H. Real-time optical imaging using quantum dot and related nanocrystals. Nanomedicine. 2010;5:765–76. doi: 10.2217/nnm.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, et al. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5:2067–76. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 52.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 53.Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, Rosman C, et al. Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology. 2011;5:254–68. doi: 10.3109/17435390.2010.528847. [DOI] [PubMed] [Google Scholar]
- 54.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 55.Sereemaspun A, Rojanathanes R, Wiwanitkit V. Effect of gold nanoparticle on renal cell: an implication for exposure risk. Ren Fail. 2008;30:323–5. doi: 10.1080/08860220701860914. [DOI] [PubMed] [Google Scholar]
- 56.Uchiyama MK, Deda DK, Rodrigues SF, Drewes CC, Bolonheis SM, Kiyohara PK, et al. In vivo and in vitro toxicity and anti-inflammatory properties of gold nanoparticle bioconjugates to the vascular system. Toxicol Sci. 2014;142:497–507. doi: 10.1093/toxsci/kfu202. [DOI] [PubMed] [Google Scholar]
- 57.Klien K, Godnić-Cvar J. Genotoxicity of metal nanoparticles: focus on in vivo studies. Arh Hig Rada Toksikol. 2012;63:133–45. doi: 10.2478/10004-1254-63-2012-2213. [DOI] [PubMed] [Google Scholar]
- 58.Jung S, Bang M, Kim BS, Lee S, Kotov NA, Kim B, et al. Intracellular gold nanoparticles increase neuronal excitability and aggravate seizure activity in the mouse brain. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sousa F, Mandal S, Garrovo C, Astolfo A, Bonifacio A, Latawiec D, et al. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study. Nanoscale. 2010;2:2826–34. doi: 10.1039/c0nr00345j. [DOI] [PubMed] [Google Scholar]
- 60.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115:8706–15. doi: 10.1021/ja00072a025. [DOI] [Google Scholar]
- 61.Chung Leland WK, Isaacs WB, Simons JW. Prostate cancer: biology, genetics, and the new therapeutics. In: Gao X, Xing Y, Chung Leland WK, Nie S, editors. Quantum dot nanotechnology for prostate cancer research. Totowa: Humana Press; 2007. pp. 231.
- 62.Rosenthal SJ, Chang JC, Kovtun O, McBride JR, Tomlinson ID. Biocompatible quantum dots for biological applications. Chem Biol. 2011;18:10–24. doi: 10.1016/j.chembiol.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McBride J, Treadway J, Feldman LC, Pennycook SJ, Rosenthal SJ. Structural basis for near unity quantum yield core/shell nanostructures. Nano Lett. 2006;6:1496–501. doi: 10.1021/nl060993k. [DOI] [PubMed] [Google Scholar]
- 64.Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–32. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Michalet X, Pinaud F, Bentolila L, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44. [DOI] [PMC free article] [PubMed]
- 66.Chuang PH, Lin CC, Liu RS. Emission-tunable CuInS2/ZnS quantum dots: structure, optical properties, and application in white light-emitting diodes with high color rendering index. ACS Appl Mater Interfaces. 2014;6:15379–87. doi: 10.1021/am503889z. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J, Yang Y, Zhang CY. Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem Rev. 2015;115:11669–717. doi: 10.1021/acs.chemrev.5b00049. [DOI] [PubMed] [Google Scholar]
- 68.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang LW, Peng CW, Chen C, Li Y. Quantum dots-based tissue and in vivo imaging in breast cancer researches: current status and future perspectives. Breast Cancer Res Treat. 2015;151:7–17. doi: 10.1007/s10549-015-3363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erogbogbo F, Yong KT, Roy I, Hu R, Law WC, Zhao W, et al. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS Nano. 2011;5:413–23. doi: 10.1021/nn1018945. [DOI] [PubMed] [Google Scholar]
- 71.Pons T, Pic E, Lequeux N, Cassette E, Bezdetnaya L, Guillemin F, et al. Cadmium-free CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano. 2010;4:2531–8. doi: 10.1021/nn901421v. [DOI] [PubMed] [Google Scholar]
- 72.Kierny MR, Cunningham TD, Kay BK. Detection of biomarkers using recombinant antibodies coupled to nanostructured platforms. Nano Rev. 2012;3:17240. doi: 10.3402/nano.v3i0.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding K, Jing L, Liu C, Hou Y, Gao M. Magnetically engineered Cd-free quantum dots as dual-modality probes for fluorescence/magnetic resonance imaging of tumors. Biomaterials. 2014;35:1608–17. doi: 10.1016/j.biomaterials.2013.10.078. [DOI] [PubMed] [Google Scholar]
- 74.Helms V. Fluorescence resonance energy transfer. Principles of computational cell biology. Weinheim: Wiley-VCH; 2008. [Google Scholar]
- 75.Medintz IL, Goldman ER, Lassman ME, Mauro JM. A fluorescence resonance energy transfer sensor based on maltose binding protein. Bioconjug Chem. 2003;14:909–18. doi: 10.1021/bc020062+. [DOI] [PubMed] [Google Scholar]
- 76.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24:339–43. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 77.Huang X, Li L, Qian H, Dong C, Ren J. A resonance energy transfer between chemiluminescent donors and luminescent quantum-dots as acceptors (CRET) Angew Chem Int Ed Engl. 2006;45:5140–3. doi: 10.1002/anie.200601196. [DOI] [PubMed] [Google Scholar]
- 78.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. [DOI] [PubMed]
- 79.Pantel K, Alix-Panabières C. Cell lines from circulating tumor cells. Oncoscience. 2015;2:815–6. doi: 10.18632/oncoscience.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Fu X, Hu J, Zhu Z. Microfluidic bead-based multienzyme-nanoparticle amplification for detection of circulating tumor cells in the blood using quantum dots labels. Anal Chim Acta. 2013;779:64–71. doi: 10.1016/j.aca.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 81.Costa C, Abal M, López-López R, Muinelo-Romay L. Biosensors for the detection of circulating tumour cells. Sensors (Basel) 2014;14:4856–75. doi: 10.3390/s140304856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. 2011;939161.