Abstract
Purpose
Following determination of the maximum standardized uptake values (SUVmax) of the mediastinal lymph nodes (SUV-LN) and of the primary tumor (SUV-T) on 18F-FDG PET/CT in patients with non-small-cell lung cancer (NSCLC), the aim of the study was to determine the value of the SUV-LN/SUV-T ratio in lymph node staging in comparison with that of SUV-LN.
Methods
We retrospectively reviewed a total of 289 mediastinal lymph node stations from 98 patients with NSCLC who were examined preoperatively for staging and subsequently underwent pathologic studies of the mediastinal lymph nodes. We determined SUV-LN and SUV-R for each lymph node station on 18F-FDG PET/CT and then classified each station into one of three groups based on SUV-T (low, medium and high SUV-T groups). Diagnostic performance was assessed based on receiver operating characteristic (ROC) curve analysis, and the optimal cut-off values that would best discriminate metastatic from benign lymph nodes were determined for each method.
Results
The average of SUV-R of malignant lymph nodes was significantly higher than that of benign lymph nodes (0.79 ± 0.45 vs. 0.36 ± 0.23, P < 0.0001). In the ROC curve analysis, the area under the curve (AUC) of SUV-R was significantly higher than that of SUV-LN in the low SUV-T group (0.885 vs. 0.810, P = 0.019). There were no significant differences between the AUCs of SUV-LN and of SUV-R in the medium and high SUV-T groups. The optimal cut-off value for SUV-R in the low SUV-T group was 0.71 (sensitivity 87.5 %, specificity 85.9 %).
Conclusions
The SUV-R performed well in distinguishing between metastatic and benign lymph nodes. In particular, SUV-R was found to have a better diagnostic performance than SUV-LN in the low SUV-T group.
Keywords: Non-small-cell lung cancer, 18F-FDG PET/CT, Mediastinal lymph node, SUV
Introduction
In patients with non-small-cell lung cancer (NSCLC), differentiating benign from metastatic mediastinal lymph nodes is decisive in determining further therapeutic strategies. Therefore, the importance of preoperative staging of these nodes cannot be over-emphasized [1, 2]. Surgery remains the standard treatment in patients with medically operable clinical stage I or II NSCLC when either there is no evidence of lymph node metastases or there are only hilar or intrapulmonary metastases. In the case of N2 lymph node metastasis, definitive concurrent chemoradiation therapy or induction chemotherapy with or without radiation therapy is recommended [2–5]. To evaluate the accuracy of preoperative staging in NSCLC, a widely and routinely used noninvasive diagnostic method is contrast-enhanced computed tomography (CT). However, numerous studies have now shown that this method of lymph node staging has limited reliability when compared with the evaluation of tumor size and the invasion of adjacent structures [6–8]. Currently, the combination of 18F-fluoro-2-deoxyglucose positron emission tomography and CT imaging (18F-FDG PET/CT) is widely used as a noninvasive diagnostic method. Unlike CT alone, PET/CT provides functional metabolic information along with anatomic information. The standardized uptake value (SUV) is a relative measure of FDG uptake. Recent studies have demonstrated that the diagnostic performance of 18F-FDG PET/CT is superior to that of contrast-enhanced CT in the preoperative staging of mediastinal lymph nodes [3, 9–11].
Although 18F-FDG PET/CT is one of the most accurate noninvasive diagnostic methods for mediastinal lymph node staging, it is limited in its ability to differentiate between benign and metastatic lymph nodes. Small lymph nodes, micrometastases or an adjacent primary mass could be the reason for false-negative results, whereas inflammation or granulation tissue could be the reason for false-positive results [12, 13]. Therefore, precise diagnostic criteria are needed for successful mediastinal lymph node staging in NSCLC using 18F-FDG PET/CT. To overcome these limitations, several kinds of semiquantitative and quantitative methods, in addition to visual assessment, have been proposed, including the maximum SUV (SUVmax) threshold, metabolic heterogeneity analysis, lymph node SUVmax (SUV-LN) to blood pool SUVmax ratio, and the SUV-LN to primary tumor SUVmax (SUV-T) ratio (SUV-R) [12–16]. Although SUV-R can easily be determined during the routine measurement of SUVmax, its diagnostic performance in mediastinal lymph node staging is controversial [13, 14, 16], possibly owing to differences in the number of patients studied, patient characteristics, scanner hardware, and approaches to reconstruction. To investigate the diagnostic performance of SUV-R more accurately, we grouped patients according to their SUV-T and compared SUV-R and SUV-LN in each group.
This study aimed to determine the value of SUV-R for mediastinal lymph node staging in NSCLC in comparison with that of SUV-LN. In addition, we determined the optimal thresholds of each of these parameters for such staging.
Materials and Methods
Patients
In this retrospective study, we reviewed the records of 98 patients with NSCLC (adenocarcinoma, squamous cell carcinoma, and adenosquamous carcinoma) who underwent preoperative 18F-FDG PET/CT for staging and subsequent pathologic studies of mediastinal lymph nodes at our institution between May 2014 and October 2015. The study group comprised 65 men and 33 women with a mean age of 68.1 ± 9.1 years. Pathologic results from a total of 289 mediastinal lymph node stations were obtained by lymphadenectomy and/or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) within 2 months of the 18F-FDG PET/CT scan. Of the 98 patients, 34 underwent lymphadenectomy only, 51 underwent EBUS-TBNA only, and 13 underwent EBUS-TBNA followed by lymphadenectomy. Patients with a history of lung cancer or who had undergone neoadjuvant chemotherapy or radiotherapy for NSCLC were excluded from the analysis. The study protocol for this retrospective study was reviewed and approved by the institutional review board at our institution.
18F-FDG PET/CT Imaging
18F-FDG PET/CT images were obtained using a Gemini TF PET/CT scanner (Philips Medical Systems, Cleveland, OH), with an axial field of view of 18 cm and a spatial resolution of 4.4 mm. All patients had fasted for at least 6 h prior to the scanning. 18F-FDG PET/CT was performed 60 min after intravenous injection of 370 to 555 MBq of 18F-FDG, depending on body weight. The PET scan was obtained from the skull base to the thigh for 1 min per bed position, and was followed by a low-dose CT scan (50 mA, 120 kV, 512 × 512 matrix) for attenuation correction. Maximum intensity projection, cross-sectional views, and fusion images were reviewed.
Image Analysis
For semiquantitative analysis, all images were reviewed on a dedicated workstation (Extended Brilliance Workspace 4.0, with a PET/CT viewer for automated image registration; Philips, Amsterdam, The Netherlands). Lymph node stations were assigned according to the Lung Cancer Staging manual of the American Joint Committee on Cancer (7th edition). We measured SUV-T and SUV-LN within a designated region of interest. SUVmax was the highest SUV of the pixels within the region of interest. If there was more than one mass in the lung, the mass with the highest SUVmax was considered the primary lung tumor. We also assumed that the lymph node with the highest SUVmax had the highest probability of malignancy, and we measured these nodes for each nodal station. Each nodal station was classified as either positive or negative based on the pathologic findings, and the SUV-R was determined in each case, as follows:
Data Analysis
We classified the primary lung tumors into low, medium and high SUV-T groups because we assumed SUV-LN and SUV-R would vary according with SUV-T. In particular, we assumed that a higher SUV-T might have a lower SUV-R, and a lower SUV-T might have a higher SUV-R. SUV-T were divided into three groups based on their tertile distribution. Nodal stations with an SUV-T of ≤5, >5 – 8 and >8 were allocated to the low, medium and high SUV-T groups, respectively. From 13 patients, 17 nodal stations were evaluated by both lymphadenectomy and EBUS-TBNA. Of these nodal stations, the results for two were discordant, and in these cases the pathologic result on lymphadenectomy was selected for analysis.
Statistical Analysis
Statistical analyses were performed using MedCalc software version 12.3.0 (MedCalc, Mariakerke, Belgium). Differences were considered statistically significant when P values were less than 0.05. The significance of differences between benign and malignant lymph nodes was tested using Student’s t test for SUV-LN and SUV-R. One-way analysis of variance was used to compare SUV-LN and SUV-R in each of the three groups. Receiver operating characteristic (ROC) curve analysis was used to compare the diagnostic performance of the two variables SUV-LN and SUV-R, and to determine their optimal cut-off values for maximum sensitivity and specificity in detecting metastatic lymph nodes.
Results
Clinical Data
Of the 98 patients with NSCLC, metastatic involvement of mediastinal lymph nodes was detected in 49. Of 298 lymph node stations pathologically confirmed by EBUS-TBNA or lymphadenectomy, 213 were benign and 76 were malignant. The characteristics of the patients and lymph node stations are shown in Tables 1 and 2.
Table 1.
Characteristics of patients
| Characteristic | Value |
|---|---|
| Age | 68.1 ± 9.1 |
| Sex | |
| Male | 65 |
| Female | 33 |
| Pathologic N stage | |
| N0 | 49 |
| N1 | 8 |
| N2 | 31 |
| N3 | 10 |
| Histologic type of primary tumor | |
| Adenocarcinoma | 55 |
| Squamous cell carcinoma | 42 |
| Adenosquamous carcinoma | 1 |
The values are number of patients, except age in years, mean ± standard deviation
Table 2.
Characterization of mediastinal lymph node stations by confirmed methods
| Mediastinal lymph node station | Location | Benign (N) | Malignant (N) | EBUS-TBNA (N) | Lymphadenectomy (N) | Both (N) |
|---|---|---|---|---|---|---|
| 2 | Upper paratracheal | 16 | 7 | 8 | 15 | – |
| 4 | Lower paratracheal | 44 | 26 | 44 | 26 | 4 |
| 5 | Subaortic | 10 | 4 | 1 | 13 | – |
| 6 | Paraaortic | 7 | – | – | 7 | – |
| 7 | Subcarinal | 63 | 23 | 45 | 41 | 3 |
| 8 | Paraesophageal | 2 | – | 1 | 1 | – |
| 9 | Pulmonary ligament | 12 | 1 | – | 13 | – |
| 10 | Hilar | 28 | 2 | 3 | 27 | – |
| 11 | Interlobar | 41 | 9 | 12 | 38 | 2 |
| 12 | Lobar | 1 | 2 | 1 | 2 | – |
EBUS-TBNA Endobronchial ultrasound-guided transbronchial needle aspiration
18F-FDG Uptake on PET/CT
On preoperative 18F-FDG PET/CT, the mean SUVmax of malignant lymph nodes was significantly higher than that of benign lymph nodes (5.12 ± 2.70 vs. 1.92 ± 0.73, P < 0.001). The average SUV-R in patients with malignant lymph nodes was significantly higher than in those with benign lymph nodes (0.79 ± 0.45 vs. 0.36 ± 0.23, P < 0.001; Table 3). The mean SUV-T of squamous cell carcinoma was significantly higher than that of adenocarcinoma (8.89 ± 3.79 vs. 6.68 ± 4.68, P < 0.001). There was no significant difference in mean SUV-LN between patients with adenocarcinoma (5.11 ± 2.85) and those with squamous cell carcinoma (5.13 ± 2.44) or in mean SUV-R between patients with adenocarcinoma (0.82 ± 0.48) and those with squamous cell carcinoma (0.72 ± 0.39; Table 4).
Table 3.
Characterization of lymph node stations on 18F-FDG PET/CT
| Benign lymph nodes | Metastatic lymph nodes | P value | |
|---|---|---|---|
| Number | 213 | 76 | |
| SUV-LN, mean ± standard deviation | 1.92 ± 0.73 | 5.12 ± 2.70 | <0.001 |
| SUV-R, mean ± standard deviation | 0.36 ± 0.23 | 0.79 ± 0.45 | <0.001 |
Table 4.
Metabolic characterization of adenocarcinoma and squamous cell carcinoma
| Adenocarcinoma (n = 55) | Squamous cell carcinoma (n = 42) | P value | |
|---|---|---|---|
| SUV-T | 6.68 ± 4.68 | 8.89 ± 3.79 | <0.001 |
| SUV-LN | 5.11 ± 2.85 | 5.13 ± 2.44 | NS |
| SUV-R | 0.82 ± 0.48 | 0.72 ± 0.39 | NS |
The data presented are means ± standard deviation
NS not significant
The mean SUV-T was 7.63 ± 4.43. Of the 289 lymph node stations, 102 were in the low SUV-T group, 101 were in the medium SUV-T group, and 86 were in the high SUV-T group. There were significant differences in SUV-LN and SUV-R between benign and metastatic lymph nodes in all three groups (P < 0.001) (Table 5). SUV-LN of metastatic lymph nodes differed significantly among the three SUV-T groups, but SUV-LN of benign lymph nodes did not differ significantly. There were significant differences in SUV-R among the three SUV-T groups for both benign and metastatic lymph nodes (Fig. 1).
Table 5.
SUV-LN and SUV-R for the three SUV-T groups
| Metastatic | Benign | P value | |
|---|---|---|---|
| Number of lymph nodes | |||
| Low SUV-T | 24 | 78 | |
| Medium SUV-T | 25 | 76 | |
| High SUV-T | 27 | 59 | |
| SUV-LN, mean ± standard deviation | |||
| Low SUV-T | 3.71 ± 0.56 | 1.86 ± 0.31 | <0.001 |
| Medium SUV-T | 5.00 ± 0.56 | 1.85 ± 0.32 | <0.001 |
| High SUV-T | 6.47 ± 0.54 | 2.08 ± 0.36 | <0.001 |
| SUV-R, mean ± standard deviation | |||
| Low SUV-T | 1.13 ± 0.10 | 0.55 ± 0.05 | <0.001 |
| Medium SUV-T | 0.76 ± 0.10 | 0.30 ± 0.05 | <0.001 |
| High SUV-T | 0.51 ± 0.09 | 0.17 ± 0.06 | <0.001 |
Low SUV-T SUV-T ≤5, Medium SUV-T SUV-T >5 – 8, High SUV-T SUV-T >8
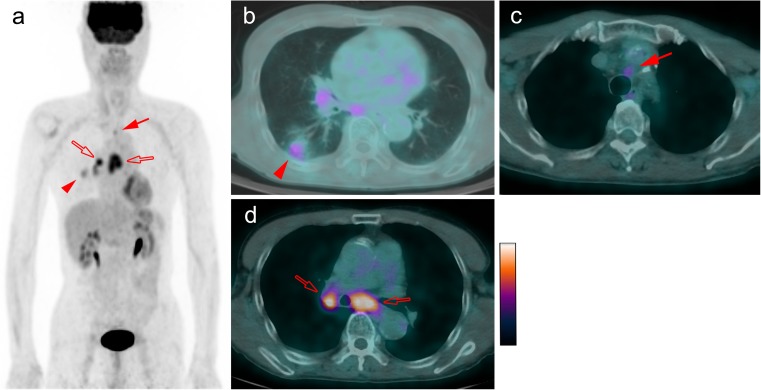
Fig. 1.
18F-FDG PET/CT images in an example patient with NSCLC. Maximum intensity projection (MIP) image (a) and axial fused image (b) show the FDG-avid lesion in lung right lower lobe (arrowhead) with SUV-T 2.8, which was adenocarcinoma. Mild FDG uptake is seen in the left upper paratracheal lymph node (arrows a c) with SUV-LN 2.4 and SUV-R 0.86, which was confirmed as metastatic. This shows the ability of SUV-R to differentiate between benign and malignant lymph nodes in patients with low SUV-T, while SUV-LN was false-negative. The FDG-avid subcarinal and right hilar lymph nodes (open arrows a, d) with SUV-LN 5.6 and SUV-R 2.0 were also confirmed as metastatic
ROC Curve Comparison
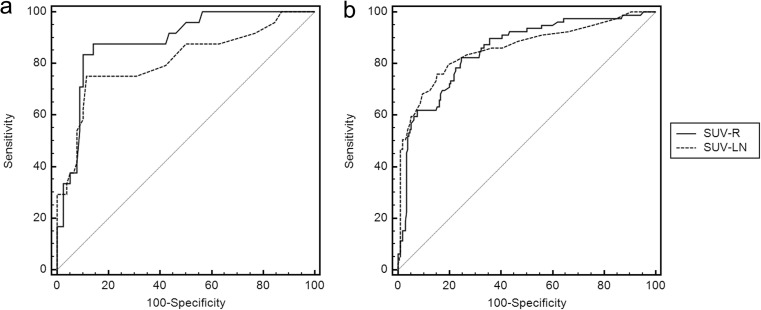
The diagnostic performances of SUV-LN and of SUV-R for each group were compared by ROC curve analysis. In the low SUV-T group, the areas under the curve (AUC) for SUV-R and SUV-LN were 0.885 and 0.810, respectively (P = 0.019). The AUCs for SUV-LN and SUV-R did not differ significantly in the medium SUV-T group (0.924 and 0.918, respectively; P = 0.31) or in the high SUV-T group (0.929 and 0.938, respectively; P = 0.47). The AUCs for SUV-LN and SUV-R did not differ significantly for all nodal stations without grouping (0.866 and 0.848, respectively; P = 0.19; Fig. 2). The optimal SUV-LN and SUV-R cut-off values were determined for each group. The optimal SUV-R cut-off value for the low SUV-T group was 0.71 (Table 6).
Fig. 2.
ROC curve analysis comparing the diagnostic performance of SUV-R and SUV-LN. a In the low SUV-T group, the AUC of SUV-R was significantly higher than that of SUV-LN (0.885 vs. 0.810, respectively; P = 0.019). b In all nodal stations without grouping, the AUCs of SUV-LN and SUV-R were not significantly different (0.866 vs. 0.848, respectively; P = 0.190)
Table 6.
Optimal SUV-LN and SUV-R cut-off values
| Group | SUV-LN | SUV-R | ||||
|---|---|---|---|---|---|---|
| Cut-off value | Sensitivity (%) | Specificity (%) | Cut-off value | Sensitivity (%) | Specificity (%) | |
| Low SUV-T | 2.3 | 87.5 | 85.9 | 0.71 | 87.5 | 85.9 |
| Middle SUV-T | 2.5 | 84.0 | 89.5 | 0.38 | 88.0 | 84.2 |
| High SUV-T | 3.5 | 81.5 | 100 | 0.26 | 88.9 | 94.9 |
Discussion
The results of this study indicate that SUV-LN and SUV-R, as determined on 18F-FDG PET/CT imaging, have good diagnostic performance for evaluating metastatic and benign lymph nodes. In the low SUV-T group, SUV-R had better diagnostic performance than SUV-LN. SUVmax is widely used as a semiquantitative diagnostic indicator of lymph node metastasis in lung cancer. For example, both Hellwig et al. [12] and Kumar et al. [17] found that SUVmax 2.5 was the optimal cut-off value for distinguishing metastatic from benign lymph nodes. In our study, despite having classified the SUV-R into three groups, we found generally similar cut-off values to those found previously.
We also found that the SUV-R of malignant lymph nodes was significantly higher than that of benign lymph nodes. Consistent with our findings, Cerfolio et al. [14] found that the SUV-LN/SUV-T ratio in patients with NSCLC predicts mediastinal nodal pathology, and the ratio with the maximum sensitivity is 0.56 or greater (sensitivity 94 %, specificity 72 %). In agreement with that study, Cirak et al. [16] found that as SUV-R increases in patients with NSCLC, the possibility of detecting malignant lymph nodes may increase. In contrast to these results, Lee et al. [13] found that the difference in SUV-R between metastatic and benign lymph nodes is not significant, while the lymph node SUV to blood pool SUV ratio was significantly higher for malignant lymph nodes. Although the reasons for these different results are not clear, it is likely that they are related to the difference in the methods used to obtain the lymph nodes. Lee et al. obtained lymph nodes only by lymphadenectomy during surgery in patients in whom the clinical stage of the disease was relatively low and operability was sufficiently high, while Cerfolio et al. and Cirak et al. obtained biopsy specimens by mediastinoscopy for diagnostic purposes.
We classified lymph node stations into three groups assuming that the discriminating ability of SUV-LN and SUV-R vary depending on the SUV-T. Indeed, SUV-R had better diagnostic performance than SUV-LN in the low SUV-T group, which might have clinical implications. This group had a lower SUV-LN, which possibly increased the likelihood of a false-negative result, and in this situation the SUV-R value may be of help in making a clinical diagnosis. Another prospective advantage of using SUV-R, even though SUV measurements have been shown to vary by 15 – 20 % between centers because of variations in scanner hardware and reconstruction approaches [14, 18–22], is that it is relatively less vulnerable to such variations [14].
Size (short-axis), maximum Hounsfield units, coefficient of variation, and LN/blood pool SUV ratio were evaluated in the previous studies as criteria for diagnosing mediastinal lymph node metastasis. The generally accepted criterion for a normal sized mediastinal lymph node on CT is ≤1 cm [23]. The optimal cut-off values for maximum Hounsfield units on unenhanced CT, for the coefficient of variation for assessing the heterogeneity of FDG uptake, and for the LN/blood pool SUV ratio have been suggested to be 136, 0.20 [15], and 1.4 [13], respectively. Concerning other cancers, several studies have evaluated SUV-R in invasive breast cancer. The axillary lymph node to breast tumor ratio is a good diagnostic parameter [24] and is more predictive than visual analysis or the SUV of axillary lymph nodes [25]. In our study, adenocarcinoma had a lower SUV-T than squamous cell carcinoma. Consistent with this finding, previous studies have shown lower metabolism of adenocarcinoma. These findings are thought to be a result of the higher cellular proliferation index of squamous cell carcinoma [13, 26]. However, there were no significant differences in SUV-LN or SUV-R between adenocarcinoma and squamous cell carcinoma in this study.
Although mediastinoscopy has been considered the gold standard for staging mediastinal lymph nodes, accumulating evidence indicates that EBUS-TBNA and mediastinoscopy achieve compatible results for this purpose [27–31]. EBUS-TBNA might be preferable for histologic sampling of paratracheal and subcarinal mediastinal adenopathy because the diagnostic yield can surpass that of mediastinoscopy [29]. Recent studies have demonstrated that the sensitivity of EBUS-TBNA is about 90 % [3, 29, 32, 33]. Lymphadenectomy through surgery can confirm surgically resectable lymph nodes only at an operable stage. Therefore, in the present study, we used EBUS-TBNA in addition to lymphadenectomy through surgery to include nonresectable and nonoperable cases. In this way, we were able to minimize selection bias without prominently distinguishing the clinical stage.
Nevertheless, our study had some limitations. It was retrospective and may have shown a selection bias. Although EBUS-TBNA has good diagnostic performance, it can still result in false-negative findings. Also, it is possible that some FDG-avid lymph nodes could not be reached by either EBUS-TBNA or lymphadenectomy. Further studies are needed to confirm these results, with more data from a larger number of patients and from different centers.
Conclusions
18F-FDG PET/CT is a noninvasive and reliable diagnostic imaging modality, and SUV-LN in patients with NSCLC is known to be a helpful diagnostic parameter. We chose SUV-R to further determine its diagnostic accuracy in comparison with simply using SUV-LN and found that the SUV-R has good diagnostic performance for distinguishing metastatic from benign lymph nodes. In particular, SUV-R had better diagnostic performance than SUV-LN in the low SUV-T group with an optimal cut-off value of 0.71.
Compliance with Ethical Standards
Conflicts of Interest
Jaehyuk Cho, Jae Gol Choe, Kisoo Pahk, Sunju Choi, Hye Ryeong Kwon, Jae Seon Eo, Hyo Jung Seo, Chulhan Kim, and Sungeun Kim declare that they have no conflicts of interest.
Ethical Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the 1975 Declaration of Helsinki, as revised in 2000. Informed consent was waived by the IRB considering the retrospective nature of the analysis.
References
- 1.Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s – meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6. [DOI] [PubMed]
- 2.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Cancer Netw. 2015;13:515–24. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 3.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–50S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 4.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 5.Ost DE, Yeung SC, Tanoue LT, Gould MK. Clinical and organizational factors in the initial evaluation of patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e121S–41. [DOI] [PMC free article] [PubMed]
- 6.Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–9. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 7.An YS, Sun JS, Park KJ, Hwang SC, Park KJ, Sheen SS, et al. Diagnostic performance of (18)F-FDG PET/CT for lymph node staging in patients with operable non-small-cell lung cancer and inflammatory lung disease. Lung. 2008;186:327–36. doi: 10.1007/s00408-008-9109-3. [DOI] [PubMed] [Google Scholar]
- 8.Turkmen C, Sonmezoglu K, Toker A, Yilmazbayhan D, Dilege S, Halac M, et al. The additional value of FDG PET imaging for distinguishing N0 or N1 from N2 stage in preoperative staging of non-small cell lung cancer in region where the prevalence of inflammatory lung disease is high. Clin Nucl Med. 2007;32:607–12. doi: 10.1097/RLU.0b013e3180a1ac87. [DOI] [PubMed] [Google Scholar]
- 9.Chao F, Zhang H. PET/CT in the staging of the non-small-cell lung cancer. J Biomed Biotechnol. 2012;2012:783739. doi: 10.1155/2012/783739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinert HC, Hauser M, Allemann F, Engel H, Berthold T, von Schulthess GK, et al. Non-small cell lung cancer: nodal staging with FDG PET versus CT with correlative lymph node mapping and sampling. Radiology. 1997;202:441–6. doi: 10.1148/radiology.202.2.9015071. [DOI] [PubMed] [Google Scholar]
- 11.Wahl RL, Quint LE, Greenough RL, Meyer CR, White RI, Orringer MB. Staging of mediastinal non-small cell lung cancer with FDG PET, CT, and fusion images: preliminary prospective evaluation. Radiology. 1994;191:371–7. doi: 10.1148/radiology.191.2.8153308. [DOI] [PubMed] [Google Scholar]
- 12.Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GW, Schaefers HJ, et al. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J Nucl Med. 2007;48:1761–6. doi: 10.2967/jnumed.107.044362. [DOI] [PubMed] [Google Scholar]
- 13.Lee AY, Choi SJ, Jung KP, Park JS, Lee SM, Bae SK. Characteristics of metastatic mediastinal lymph nodes of non-small cell lung cancer on preoperative F-18 FDG PET/CT. Nucl Med Mol Imaging. 2014;48:41–6. doi: 10.1007/s13139-013-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerfolio RJ, Bryant AS. Ratio of the maximum standardized uptake value on FDG-PET of the mediastinal (N2) lymph nodes to the primary tumor may be a universal predictor of nodal malignancy in patients with nonsmall-cell lung cancer. Ann Thorac Surg. 2007;83:1826–9. doi: 10.1016/j.athoracsur.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Budiawan H, Cheon GJ, Im HJ, Lee SJ, Paeng JC, Kang KW, et al. Heterogeneity analysis of (18)F-FDG uptake in differentiating between metastatic and inflammatory lymph nodes in adenocarcinoma of the lung: comparison with other parameters and its application in a clinical setting. Nucl Med Mol Imaging. 2013;47:232–41. doi: 10.1007/s13139-013-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirak AK, Ceylan KC, Akpinar D, Kaya SO, Koparal H. Are ratio of lymph node to primary focus SUV-max and PET/CT 18FDG standard uptake value of lymph nodes meaningful in staging non-small cell lung cancer? Int J Hematol Oncol. 2011;21:217–22. doi: 10.4999/uhod.10107. [DOI] [Google Scholar]
- 17.Kumar A, Dutta R, Kannan U, Kumar R, Khilnani GC, Gupta SD. Evaluation of mediastinal lymph nodes using F-FDG PET-CT scan and its histopathologic correlation. Ann Thorac Med. 2011;6:11–6. doi: 10.4103/1817-1737.74270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol. 2009;11:118–22. doi: 10.1007/s11307-008-0177-9. [DOI] [PubMed] [Google Scholar]
- 19.Keyes JW., Jr SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–9. [PubMed] [Google Scholar]
- 20.Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392–404. doi: 10.1007/s00259-006-0224-1. [DOI] [PubMed] [Google Scholar]
- 21.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195:310–20. doi: 10.2214/AJR.10.4923. [DOI] [PubMed] [Google Scholar]
- 22.Iskender I, Kadioglu SZ, Kosar A, Atasalihi A, Kir A. Is there any maximum standardized uptake value variation among positron emission tomography scanners for mediastinal staging in non-small cell lung cancer? Interact Cardiovasc Thorac Surg. 2011;12:965–9. doi: 10.1510/icvts.2010.258103. [DOI] [PubMed] [Google Scholar]
- 23.Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 24.Futamura M, Asano T, Kobayashi K, Morimitsu K, Nawa M, Kanematsu M, et al. Prediction of macrometastasis in axillary lymph nodes of patients with invasive breast cancer and the utility of the SUV lymph node/tumor ratio using FDG-PET/CT. World J Surg Oncol. 2015;13:49. doi: 10.1186/s12957-014-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Byun BH, Noh WC, Lee SS, Kim HA, Kim EK, et al. Lymph node to primary tumor SUV ratio by 18F-FDG PET/CT and the prediction of axillary lymph node metastases in breast cancer. Clin Nucl Med. 2014;39:e249–53. doi: 10.1097/RLU.0b013e3182a75477. [DOI] [PubMed] [Google Scholar]
- 26.Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan D, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–8. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 27.Medford AR, Bennett JA, Free CM, Agrawal S. Mediastinal staging procedures in lung cancer: EBUS, TBNA and mediastinoscopy. Curr Opin Pulm Med. 2009;15:334–42. doi: 10.1097/MCP.0b013e32832b8a45. [DOI] [PubMed] [Google Scholar]
- 28.Clementsen PF, Skov BG, Vilmann P, Krasnik M. Endobronchial ultrasound-guided biopsy performed under optimal conditions in patients with known or suspected lung cancer may render mediastinoscopy unnecessary. J Bronchology Interv Pulmonol. 2014;21:21–5. doi: 10.1097/LBR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 29.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3:577–82. doi: 10.1097/JTO.0b013e3181753b5e. [DOI] [PubMed] [Google Scholar]
- 30.Navani N, Lawrence DR, Kolvekar S, Hayward M, McAsey D, Kocjan G, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012;186:255–60. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393–400.e1. doi: 10.1016/j.jtcvs.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 32.Rintoul RC, Tournoy KG, El Daly H, Carroll NR, Buttery RC, van Kralingen K, et al. EBUS-TBNA for the clarification of PET positive intra-thoracic lymph nodes – an international multi-centre experience. J Thorac Oncol. 2009;4:44–8. doi: 10.1097/JTO.0b013e3181914357. [DOI] [PubMed] [Google Scholar]
- 33.Herth FJ, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006;28:910–4. doi: 10.1183/09031936.06.00124905. [DOI] [PubMed] [Google Scholar]