Abstract
Purpose
Until now, there was no single standardized regional segmentation method of planar lung perfusion scan. We compared planar scan based two segmentation methods, which are frequently used in the Society of Nuclear Medicine, with reference to the lung perfusion single photon emission computed tomography (SPECT)/computed tomography (CT) derived values in lung cancer patients.
Methods
Fifty-five lung cancer patients (male:female, 37:18; age, 67.8 ± 10.7 years) were evaluated. The patients underwent planar scan and SPECT/CT after injection of technetium-99 m macroaggregated albumin (Tc-99 m-MAA). The % uptake and predicted postoperative percentage forced expiratory volume in 1 s (ppoFEV1%) derived from both posterior oblique (PO) and anterior posterior (AP) methods were compared with SPECT/CT derived parameters. Concordance analysis, paired comparison, reproducibility analysis and spearman correlation analysis were conducted.
Results
The % uptake derived from PO method showed higher concordance with SPECT/CT derived % uptake in every lobe compared to AP method. Both methods showed significantly different lobar distribution of % uptake compared to SPECT/CT. For the target region, ppoFEV1% measured from PO method showed higher concordance with SPECT/CT, but lower reproducibility compared to AP method. Preliminary data revealed that every method significantly correlated with actual postoperative FEV1%, with SPECT/CT showing the best correlation.
Conclusion
The PO method derived values showed better concordance with SPECT/CT compared to the AP method. Both PO and AP methods showed significantly different lobar distribution compared to SPECT/CT. In clinical practice such difference according to different methods and lobes should be considered for more accurate postoperative lung function prediction.
Keywords: Lung cancer, Lung function, Perfusion imaging, SPECT-CT
Introduction
For early stage lung cancer patients, surgical resection is considered the optimal treatment [1]. However, some patients have poor pulmonary reserve which may affect the outcome of the surgery. So predicting postoperative lung function is important to determine whether a patient can tolerate the surgery [2–5]. Currently predicted postoperative percentage forced expiratory volume in 1 s (FEV1) (ppoFEV1%) is the most firmly established parameter to estimate postoperative pulmonary status. It is well known that ppoFEV1% below 40 % is significantly correlated with postoperative mortality [6]. Also, recent study showed that predicted postoperative lung function was more strongly associated with long-term survival than preoperative lung function itself [7].
With the combined use of spirometry and planar lung perfusion scan, postoperative regional lung function can be assessed by dividing the lungs into lobar regions and determining the relative contribution of each region to overall ventilation and perfusion [4]. However, due to overlap of pulmonary regions and differences in individual patient lung anatomy, it has inherent limitations. Also, there is yet no single standardized method for region segmentation and quantification. Generally, nuclear medicine experts use a method which divides the lung into three equal regions and the fractional activity in each region is reported for regional lung perfusion [8–10]. However, some experts use an alternative method for segmentation which is thought to more closely correspond to the pulmonary anatomy [4].
The advent of single-photon emission tomography (SPECT)/computed tomography (CT) allows a similar approach to be undertaken in three dimensional space (3D), and when combined with each individual patient’s segmental anatomy, determined based on the CT scan, much more accurate assessment of lobar or segmental lung function can be derived [9–12].
As a preliminary result of our ongoing prospective study, we compared the two segmentation methods based on planar scan, which are frequently used in the Society of Nuclear Medicine, with reference to the SPECT/CT derived values in lung cancer patients.
Materials and Methods
Patients
We evaluated 55 lung cancer patients (male:female, 37:18; age, 67.8 ± 10.7 years) who visited our hospital and were referred to the department of nuclear medicine for planar lung scan and lung perfusion SPECT/CT from June 2015 to February 2016. Twenty-one patients underwent chemotherapy or radiation therapy. The remaining 34 patients underwent surgical resection. Among the surgical resection group, 31 underwent lobectomy, one underwent bilobectomy, and two underwent pneumonectomy. The current study was approved by the institutional review board and the need for written informed consent was waived.
Planar Lung Scan
Planar scan was acquired using a SPECT/CT scanner (NM/CT670; GE Healthcare, USA) equipped with low-energy high-resolution collimators. Patient was stated in the supine position. Regional planar images over anterior, posterior, right lateral, left lateral, left posterior oblique, and right posterior oblique were obtained (0.6 million counts per each view) 3–5 min after the intravenous administration of Tc-99 m macroaggregated albumin (MAA) (dose, 185Mbq).
Lung Perfusion SPECT/CT
Immediately after the planar scan acquisition, SPECT/CT images were acquired using the same SPECT/CT scanner (NM/CT670; GE Healthcare). CT images were first obtained during end-inspiration using the following parameters: tube voltage of 120 kV, tube current of 40 mA with autoMa function, and matrix of 512 × 512. The CT images were reconstructed using adaptive statistical iterative reconstruction algorithm (ASiRTM; GE Healthcare) into 1.25-mm-thick slices. Then, SPECT images were acquired during free-breathing using the following parameters: energy peak of 140.5 KeV with 10 % window, step-and-shot mode acquisition 15 sec/frame (16 sec/step and 60 steps/detector) with 3° angular increment, and body contour scanning option. Extra-window for scatter correction was set at 120 KeV with 10 % window. SPECT images were reconstructed using an iterative ordered subset expectation maximization (OSEM) algorithm (two iterations and ten subsets) with CT-based attenuation correction, scatter correction, and resolution recovery on the vendor-supplied software (Volumetric MITM; GE Healthcare). Reconstructed images were set at matrix of 128 × 128 with slice thickness of 3.87 mm and zoom factor of 1.5.
Perfusion Parameters and Predicted Lung Function
- % Uptake of Planar scan: Two different methods for the region segmentation were used.
-
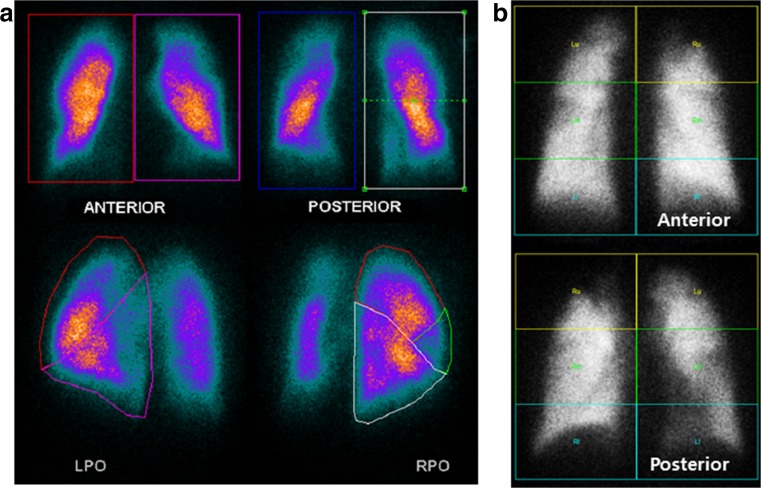
Segmentation from the Posterior Oblique (PO) View (PO method)Each lung was divided into lobar regions from the posterior oblique view image. Region of interest (ROI) for each lobe was segmented manually based on the well-known lobar division template [13]. For some patients, who had previous surgery such as lobectomy, given conventional anatomical image such as chest CT and chest X-ray was additionally considered for proper matching. The counts of each lobe were divided by the total counts over the ipsilateral lung measured from the same oblique projection. This fraction was then multiplied by the fractional contribution of the ipsilateral lung (obtained from the anterior and posterior projections geometric mean) to obtain a value for lobar % uptake to overall lung perfusion (Fig. 1a) [4].
-
Segmentation from the Anterior and Posterior (AP) View (AP method)Each lung was generally divided into three equal rectangular ROI on anterior and posterior views: top, middle, and bottom. The counts in each ROI were divided by the total counts over the ipsilateral lung measured from the anterior and posterior projections geometric mean [8]. The fractional contribution in the left bottom, right top, right middle, and right bottom matched to the % uptake of left lower lobe (LLL), right upper lobe (RUL), right middle lobe (RML), and right lower lobe (RLL), respectively. The sum of fractional contribution in the left top and middle was regarded as % uptake in the left upper lobe (LUL) (Fig. 1b).
-
-
% Uptake of SPECT/CT
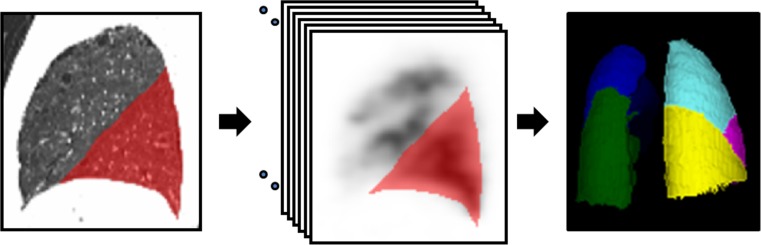
We used dosimetry software (Dosimetry ToolkitTM; GE Healthcare) for the quantitation of % uptake in each lobe. Multiple single-slice ROIs for each lobe were manually drawn in the saggital CT images using the fissures as the anatomical reference. These ROIs were also reflected in the SPECT images. The volume of interest of each lobe was generated by merging multiple single-slice ROIs (Fig. 2). The counts of each lobe were divided by the total counts of all lobes to calculate % uptake of each lobe.
After obtaining lobar % uptake from each method, target region % uptake was defined as sum of % uptake of surgically resected lobes. Finally for each method the ppoFEV1% of target region was calculated as follows:Through entire image analysis, two experienced nuclear medicine physicians analyzed each method independently.
Fig. 1.
Planar lung scan based segmentation methods. (a) PO method: each lung was divided into lobar regions from the posterior oblique view image. The counts of each lobe were divided by the total counts over the ipsilateral lung measured from the same oblique projection. This fraction was then multiplied by the fractional contribution of the ipsilateral lung, obtained from the anterior and posterior projections, (b) AP method: three equal rectangular regions of interest (ROI) on anterior and posterior views were drawn: top, middle, and bottom. The counts in each ROI were divided by the total counts over the ipsilateral lung measured from the anterior and posterior projections
Fig. 2.
Demonstration of how we measured lobar % uptake in the SPECT/CT. ROIs for each lobe were manually drawn in the saggital CT images using the fissures as the anatomical reference. These ROIs were also reflected in the multiple slices of SPECT images. The volume of interest of each lobe was generated by merging multiple single-slice ROIs
Statistical Analysis
Statistical software (MedCalc version 12.4.0.0; MedCalc, Mariakerke, Belgium) was used for the analysis throughout the study. To determine the concordance between the planar scan and SPECT/CT derived lobar % uptake, concordance correlation coefficient (CCC) was used. The difference between the planar scan and SPECT/CT derived % uptake in each lobe was analyzed using paired t test. Agreement between planar scan and SPECT/CT derived ppoFEV1% was analyzed using CCC and Bland-Altman method [14]. Intra-class correlation coefficient (ICC) was used to evaluate reproducibility of each method. Preliminary result of nine patients to analyze correlation between the ppoFEV1% from each method and actual postoperative FEV1% was done using spearman correlation coefficient. A P value of less than 0.05 was considered to indicate a significant difference.
Results
Concordance of % Uptake Between Planar Scan and SPECT/CT
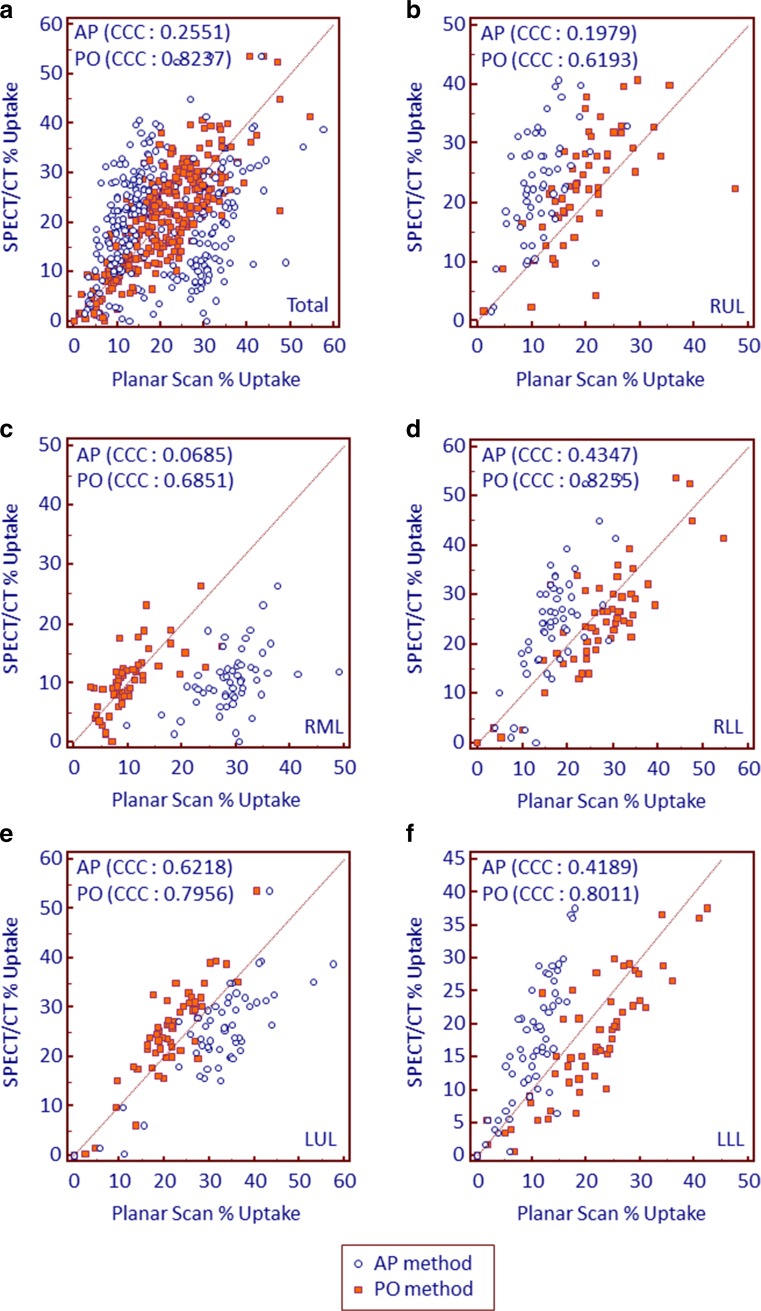
PO method showed higher concordance with SPECT/CT in every lobe compared to the AP method (CCC of PO vs AP = 0.8237 vs 0.2551, 0.6193 vs 0.1979, 0.6851 vs 0.0685, 0.8255 vs 0.4346, 0.7956 vs 0.6218, and 0.8011 vs 0.4189, for total lobes, RUL, RML, RLL, LUL, and LLL, respectively) (Fig. 3). By paired t test, PO method showed significantly higher % uptake compared to the SPECT/CT in the RLL (26.9 ± 10.6 vs 24.2 ± 11.3, p = 0.002) and LLL (20.7 ± 9.3 vs 17.2 ± 9.3, p < 0.001), but lower % uptake in the RUL (19.7 ± 7.8 vs 23.3 ± 8.9, p < 0.001) and LUL (21.3 ± 7.5 vs 24.7 ± 9.8, p < 0.001). AP method showed significantly higher % uptake in the RML (29.0 ± 6.1 vs 10.5 ± 5.2, p < 0.001) and LUL (32.2 ± 10.0 vs 24.7 ± 9.8, p < 0.001) compared to the SPECT/CT, but lower % uptake in the RUL (11.5 ± 4.5 vs 23.3 ± 8.9, p < 0.001), RLL (16.9 ± 5.8 vs 24.2 ± 11.3, p < 0.001), and LLL (10.4 ± 10.6 vs 17.2 ± 9.3, p < 0.001) (Table 1).
Fig. 3.
Scatter plots with line of equality representing concordance between planar scan and SPECT/CT derived % uptake in (a) total lobes, (b) right upper lobe, (c) right middle lobe, (d) right lower lobe, (e) left upper lobe, and (f) left lower lobe. Each method is color coded
Table 1.
Difference of % uptake between planar scan and SPECT/CT in each lobe
| Region | SPECT/CT % uptake |
PO method % uptake |
p | AP method % uptake |
p |
|---|---|---|---|---|---|
| Total | 20.0 ± 10.6 | 19.8 ± 9.8 | 0.634 | 20.0 ± 11.1 | 0.980 |
| Lobe | |||||
| RUL | 23.3 ± 8.9 | 19.7 ± 7.8 | <0.001* | 11.5 ± 4.5 | <0.001* |
| RML | 10.5 ± 5.2 | 10.4 ± 5.2 | 0.917 | 29.0 ± 6.1 | <0.001* |
| RLL | 24.2 ± 11.3 | 26.9 ± 10.6 | 0.002* | 16.9 ± 5.8 | <0.001* |
| LUL | 24.7 ± 9.8 | 21.3 ± 7.5 | <0.001* | 32.2 ± 10.0 | <0.001* |
| LLL | 17.2 ± 9.3 | 20.7 ± 9.3 | <0.001* | 10.4 ± 4.3 | <0.001* |
Data are mean ± standard deviation. SPECT/CT, single photon emission computed tomography/computed tomography; PO, posterior oblique; AP, anterior posterior; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe. * p < 0.05
Concordance of ppoFEV1% Between Planar Scan and SPECT/CT
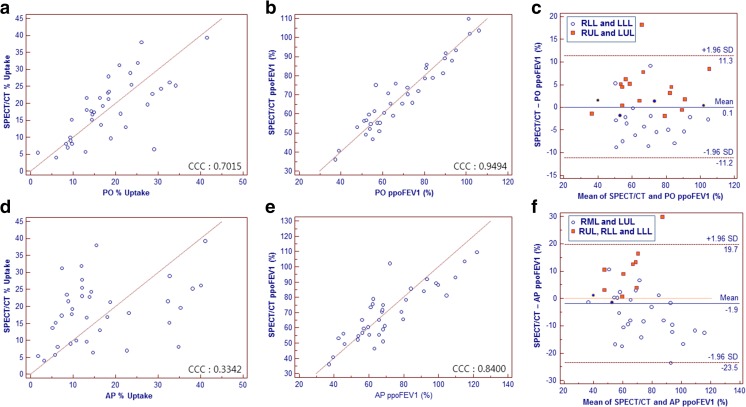
Interobserver reproducibility measuring % uptake and ppoFEV1% of target region showed ICC for % uptake 0.9637, 0.9191, and 0.9976 and for ppoFEV1% 0.9941, 0.9868, and 0.9995 in SPECT/CT, PO method and AP method respectively.
PO method showed higher concordance with SPECT/CT compared to the AP method for both % uptake (CCC, 0.7015 vs 0.3342) and ppoFEV1% (CCC, 0.9494 vs 0.8400) (Fig. 4a, b, d, e). The Bland-Altman plot showed limits of agreement between SPECT/CT and planar scan derived ppoFEV1% for PO method, determined as 0.1 ± 11.2 % (Fig. 4c), and for AP method, determined as −1.9 ± 21.6 % (Fig. 4f).
Fig. 4.
Scatter plots with line of equality representing target region concordance between SPECT/CT and (a) PO method % uptake, (b) PO method ppoFEV1%, (d) AP method % uptake, and (e) AP method ppoFEV1%. The Bland-Altman plot showing limits of agreement between SPECT/CT and planar scan derived ppoFEV1%, (c) PO method determined as 0.1 ± 11.2 %, and (f) AP method determined as −1.9 ± 21.6 %. Different lobar distribution is color coded. Blue coded dots are regions with pneumonectomy and regions, for the PO method, with right middle lobectomy
Preliminary Result of Correlation Between ppoFEV1% and Actual Postoperative FEV1%
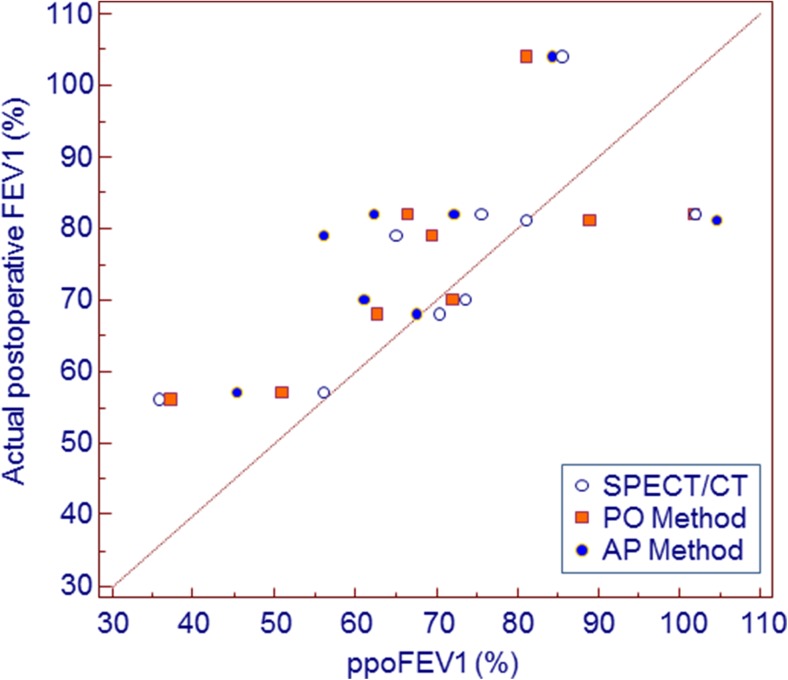
Our preliminary data regarding patients who have measured actual postoperative FEV1% showed significant correlation between each method and actual postoperative FEV1% (Fig. 5). Also, correlation coefficient gradually increased from AP method (Spearman coefficient, r = 0.7532) to PO method (r = 0.7784) and to SPECT/CT (r = 0.8953).
Fig. 5.
Scatter plots representing correlation between actual postoperative FEV1% and ppoFEV1% from each method. Each method is color coded
Discussion
Despite the long use of planar lung scan for the assessment of postoperative lung function, different methods are used for the segmentation of lung regions [4, 8, 15, 16]. There is no single standardized planar scan based segmentation method for the assessment of regional lung function after surgery [8]. In our study, we found that % uptake derived from PO method showed better concordance with SPECT/CT derived % uptake in every lobe compared to AP method. Both methods showed significantly different lobar distribution of % uptake compared to SPECT/CT. For the target region, ppoFEV1% derived from both PO method and AP method showed good agreement with SPECT/CT, while PO method showed better concordance. Preliminary data revealed that every method significantly correlated with actual postoperative FEV1%, with SPECT/CT showing the best correlation.
For lung cancer which is still the most common fatal malignancy, resection offers the best prospect of long-term survival and chance of cure [17, 18]. However, due to frequent coexistence of chronic pulmonary dysfunction, resection of the lung can result in more loss rather than gain [4, 5]. Also, recent study showed that, when categorizing the ppoFEV1% in 20-point intervals, survival curves were significantly different according to the category [7]. This study insists that not only lower ppoFEV1% limit of 40 % but also different levels of ppoFEV1% affect long-term mortality. Hence, postoperative lung function should be accurately predicted. AP method, which is widely used in the field of nuclear medicine, has its advantage in easy applicability and high reproducibility [8, 10]. In our study AP method also showed the highest reproducibility. AP method divides the lung into three equal ROI. However, anatomically in most case, RML has lower lung volume compared to the RUL and RLL. So dividing lung into three equal ROI, results in overestimation of RML % uptake and relative underestimation of % uptake in the RLL and RUL. In the same vein, since LUL and LLL has similar lung volume in most cases, assigning left top and middle lung zones to the LUL, results in overestimation of LUL and relative underestimation of LLL % uptake. Such lobar distribution was also shown in the recent study comparing planar scan with SPECT/CT [10]. To overcome such different anatomical distribution of AP method, some nuclear medicine departments use PO method [4]. In our study PO method, as it is thought to be more closely corresponding to the pulmonary anatomy, showed higher concordance with SPECT/CT derived % uptake than AP method. However, because attenuation is not corrected for the oblique image and segmentation is conducted in the posterior oblique view, perfusion in the posterior portion of the lung can be overestimated. Since lower lung lobes are most likely to make up higher proportion of posterior lung, LLL and RLL % uptake can be overestimated in the PO method. In contrary, LUL and RUL % uptake can be relatively underestimated. Also, segmentation in the PO method, although aided by Chest CT or Chest X-ray, is rather subjective, not objective [10]. For this reason the PO method showed somewhat lower reproducibility compared to the other two methods. In practice, such different distribution of uptake according to the methods and lobes should be considered for more accurate prediction of postoperative lung function.
We found higher concordance between planar scan and SPECT/CT in measuring ppoFEV1% compared to the % uptake. Also, we found higher reproducibility in measuring ppoFEV1% compared to the % uptake in every method. Most of the patients in the study had FEV1% predicted value, which is defined as FEV1% of the patients divided by the average of FEV1% in the similar population group, lower than 100 % [19]. Hence, in most cases, multiplying such value attenuates the difference measured from the % uptake evaluation resulting in higher concordance and reproducibility. Although the difference was reduced, ppoFEV1% still showed the same tendency of different lobar distribution as % uptake, which is color coded in the Bland-Altman plot (Fig. 4c, f).
There are some limitations in this study. First, this is a preliminary study so comparison with actual postoperative FEV1% was conducted in only a small portion of patients. Although every method shows significant correlation with the actual postoperative FEV1% it should be validated in future study with more data including postoperative FEV1% and long-term outcome. Recent study showed that predicted diffusing capacity of the lung for carbon monoxide (DLCO) better correlated with the survival after lobectomy for lung cancer patients compared with FEV1 [20]. So predicted DLCO can also be evaluated in future study. In fact, the long-term prognostic factor after lung resection may be reassessed according to planar scan and SPECT/CT findings. Second, we obtained lung perfusion SPECT data during free-breathing and CT data during end-inspiration. Previous study insisted that respiratory gating for SPECT data acquisition is useful to reduce misregistration, when coregistrating SPECT/CT [12]. However, some patients with irregular breathing may need longer acquisition time. So study regarding the time and accuracy-effectiveness is needed. Lastly, in our study we could not include patients with severe pulmonary dysfunction. In fact, it is known that resection of severe emphysema may have beneficial effect in lung function [21]. So there might be controversy interpreting the result in such patients, but for these patients precise prediction of postoperative lung function is more important. A larger patient group including patients with severe pulmonary dysfunction is needed to further strengthen the clinical benefit of planar scan and SPECT/CT.
Conclusion
The PO method derived values showed better concordance with SPECT/CT derived values compared to the AP method. Both PO and AP methods showed significantly different lobar distribution compared to SPECT/CT. In clinical practice such difference according to different methods and lobes should be considered for more accurate postoperative lung function prediction. As an ongoing prospective study more data regarding actual postoperative lung function and long-term follow-up are needed to further validate the clinical benefit of planar lung scan and SPECT/CT.
Compliance with Ethical Standards
Conflict of Interest
Minseok Suh, Yeon-koo Kang, Seunggyun Ha, Yong-il Kim, Jin Chul Paeng, Gi Jeong Cheon, Young Tae Kim, Samina Park, Dong Soo Lee, E. Edmund Kim, and June-Key Chung declare that they have no conflicts of interest.
Ethical Statement
The original article was approved by an institutional review board (IRB No. 1605-040-760) and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The institutional review board waived the need to obtain informed consent from the patient. Details that might disclose the identity of the subject were omitted.
References
- 1.Ettinger D, Bepler G, Bueno R, Chang A, Chang J, Chirieac L, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2006;4:548. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 2.Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med. 1994;150:947–55. doi: 10.1164/ajrccm.150.4.7921468. [DOI] [PubMed] [Google Scholar]
- 3.Bolliger CT, Jordan P, Solèr M, Stulz P, Grädel E, Skarvan K, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med. 1995;151:1472–80. doi: 10.1164/ajrccm.151.5.7735602. [DOI] [PubMed] [Google Scholar]
- 4.Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–10. doi: 10.1164/ajrccm/139.4.902. [DOI] [PubMed] [Google Scholar]
- 5.Boushy S, Helgason A, Billig D, Gyorky F. Clinical, physiologic, and morphologic examination of the lung in patients with bronchogenic carcinoma and the relation of the findings to postoperative deaths 1, 2. Am Rev Respir Dis. 1970;101:685–95. doi: 10.1164/arrd.1970.101.5.685. [DOI] [PubMed] [Google Scholar]
- 6.Vansteenkiste J, De Ruysscher D, Eberhardt W, Lim E, Senan S, Felip E, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45:660–4. doi: 10.1093/ejcts/ezt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker JA, Coleman RE, Grady E, Royal HD, Siegel BA, Stabin MG, et al. SNM practice guideline for lung scintigraphy 4.0. J Nucl Med Technol. 2012;40:57–65. doi: 10.2967/jnmt.111.101386. [DOI] [PubMed] [Google Scholar]
- 9.Roach PJ, Gradinscak DJ, Schembri GP, Bailey EA, Willowson KP, Bailey DL, editors. Spect/ct in v/q scanning. Semin Nucl Med; 2010;40:455–66. [DOI] [PubMed]
- 10.Toney LK, Wanner M, Miyaoka RS, Alessio AM, Wood DE, Vesselle H. Improved prediction of lobar perfusion contribution using technetium-99m–labeled macroaggregate of albumin single photon emission computed tomography/computed tomography with attenuation correction. J Thorac Cardiovasc Surg. 2014;148:2345–52. doi: 10.1016/j.jtcvs.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Gutte H, Mortensen J, Jensen CV, Johnbeck CB, von der Recke P, Petersen CL, et al. Detection of pulmonary embolism with combined ventilation–perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. J Nucl Med. 2009;50:1987–92. doi: 10.2967/jnumed.108.061606. [DOI] [PubMed] [Google Scholar]
- 12.Suga K, Kawakami Y, Zaki M, Yamashita T, Shimizu K, Matsunaga N. Clinical utility of co-registered respiratory-gated 99mTc-Technegas/MAA SPECT-CT images in the assessment of regional lung functional impairment in patients with lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1280–90. doi: 10.1007/s00259-004-1558-1. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen PE, Kirchner PT, Gerber FH. Oblique views in lung perfusion scanning: clinical utility and limitations. J Nucl Med. 1977;18(10):967–72. [PubMed] [Google Scholar]
- 14.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 15.Win T, Laroche CM, Groves AM, White C, Wells FC, Ritchie AJ, et al. Use of quantitative lung scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing lobectomy. Ann Thorac Surg. 2004;78:1215–8. doi: 10.1016/j.athoracsur.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Bolliger CT, Gückel C, Engel H, Stöhr S, Wyser CP, Schoetzau A, et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration. 2002;69:482–9. doi: 10.1159/000066474. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 18.Kirsh MM, Rotman H, Argenta L, Bove E, Cimmino V, Tashian J, et al. Carcinoma of the lung: results of treatment over ten years. Ann Thorac Surg. 1976;21:371–7. doi: 10.1016/S0003-4975(10)63881-7. [DOI] [PubMed] [Google Scholar]
- 19.Hole D, Watt G, Davey-Smith G, Hart C, Gillis C, Hawthorne V. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–5. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry MF, Yang C-FJ, Hartwig MG, Tong BC, Harpole DH, D’Amico TA, et al. Impact of pulmonary function measurements on long-term survival after lobectomy for stage I non-small cell lung cancer. Ann Thorac Surg. 2015;100:271–6. doi: 10.1016/j.athoracsur.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]