Abstract
Purpose
Although Sjögren’s syndrome (SS) is the most common disease causing xerostomia, autoimmune thyroid diseases can also affect the salivary glands. The aim of our study was to estimate the prevalence of thyroid diseases (TD) in subjects with symptoms of xerostomia and evaluate the efficacy of salivary gland scintigraphy (SGS) in the detection of TD in patients with SS and without SS.
Methods
We retrospectively reviewed the SGS findings of 173 subjects (men:women, 29:144) with symptoms of xerostomia. Ejection fractions (EF) in the parotid and submandibular glands were calculated. Thyroid disease was diagnosed on the basis of the results of the visual assessment of tracer uptake in the thyroid gland on SGS images as well as serological thyroid function tests.
Results
Based on the American-European Criteria, 94 patients were diagnosed with SS. Hashimoto’s thyroiditis was diagnosed in 63 patients, subacute thyroiditis in 23, subclinical hypothyroidism in five, and Graves’ disease in one. There were significant differences in the EF values of the parotid and submandibular glands between patients with TD and those with undetermined diagnoses.
Conclusions
More than half of patients with xerostomia exhibited TD. Thyroid assessment by SGS is feasible, and SGS appears to be useful for the patients with xerostomia caused by TD. SGS may be the first imaging modality capable of evaluating both salivary gland function and thyroid gland status in patients with xerostomia. This strategy would make the requirement for additional workup for thyroid disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s13139-016-0455-4) contains supplementary material, which is available to authorized users.
Keywords: Salivary gland scintigraphy, Sjögren’s syndrome, Thyroid disease, Xerostomia
Introduction
The salivary glands are responsible for the production and secretion of saliva, which protects and maintains the integrity and lubrication of the oral mucous membrane. Saliva has direct antifungal, antibacterial, and antiviral activities and a buffering capacity for the maintenance of optimal oral pH and tooth integrity [1]. Dysfunction of the salivary glands is a principal feature of xerostomia, a condition which can cause impairments in the sense of taste, speech difficulties, an alteration in eating habits, increased susceptibility to tooth decay, and opportunistic infections of the oral cavity [2].
Xerostomia is a multifactorial condition most commonly caused by an adverse drug reaction. Sjögren’s syndrome (SS), sarcoidosis, amyloidosis, human immunodeficiency virus (HIV), Epstein–Barr virus, hepatitis C virus infections, diabetes mellitus, chronic renal failure, primary biliary cirrhosis, chronic pancreatitis, radiotherapy, chemotherapy, and chronic graft-versus-host disease are other possible etiologies [3]. Sjögren’s syndrome, a chronic autoimmune disorder that affects the exocrine glands, is the most common disease causing xerostomia [4, 5]. Although the disease primarily affects the salivary and lacrimal glands in the early stages, it can subsequently involve other organs or systems of the body, such as the thyroid gland, lungs, kidneys, and the circulatory and central nervous systems [6].
The thyroid gland, in particular, is histologically similar to the lacrimal and salivary glands. Therefore, the coexistence of thyroid diseases (TD) in patients with SS and vice versa has often been reported [7–9]. The presence of common pathophysiological operating mechanisms between SS and TD is strongly supported by the shared genetic and immunopathological characteristics such as common periepithelial lymphocytic infiltration, oligoclonal B-cell expansion, and human leukocyte antigen (HLA) [7]. One-third of patients with TD may have symptoms of xerostomia and approximately 10 % of patients with anti-nuclear antibody (ANA)-positive autoimmune thyroiditis fulfill the criteria for the diagnosis of SS [5, 10]. In a previous study, patients with TD who also had xerostomia were reported to exhibit significantly poorer salivary gland function than patients with TD without xerostomia and healthy controls [1]. In addition, almost 60 % of patients who present with xerostomia and fibromyalgia-related symptoms exhibit serum thyroid autoantibodies [11].
Salivary gland scintigraphy (SGS) with technetium (Tc)-99 m pertechnetate has been widely used in evaluating the function of the salivary glands in patients with xerostomia [12]. This imaging modality plays a substantial role not only in the diagnosis of diseases that affect the salivary glands, it also aids in determining their functional status. SGS, a non-invasive imaging technique, which can be performed easily and quickly, is associated with low radiation exposure, does not interfere with the normal physiology of the body, and is well tolerated by patients [13]. Thyroid scintigraphy with Tc-99 m pertechnetate has been widely used for the assessment of thyroid function in patients with TD [14]. As SGS employs the same radiotracer as thyroid scintigraphy, the former can be used for the assessment of TD in cases where the thyroid gland remains in the imaging field of view [15]. Several studies have used SGS for the assessment of patients with SS or TD [12–16]. However, to our knowledge, no study to date has reported the scintigraphy findings of the thyroid gland in patients with xerostomia using SGS.
The aim of the present study was to estimate the prevalence of TD in patients with symptoms of xerostomia, and evaluate the efficacy of SGS in the assessment of TD in these patients.
Materials and Methods
Patients
A total of 179 patients with symptoms of xerostomia were referred to the Department of Nuclear Medicine for SGS evaluation for suspected SS between January 2012 and December 2013. Drug-induced xerostomia was clinically ruled out in all patients. Patients with a history of radiation therapy (n = 1) or surgery for head and neck tumors (n = 3), including thyroid cancer, were excluded. Additionally, two patients were excluded because their SGS images did not include the thyroid gland in the field of view. Finally, 173 patients (women, 144; men, 29; mean age, 53.3 ± 13.3 years) were enrolled in this study, which was approved by the ethics committee of the hospital.
Diagnosis of Thyroid Disease
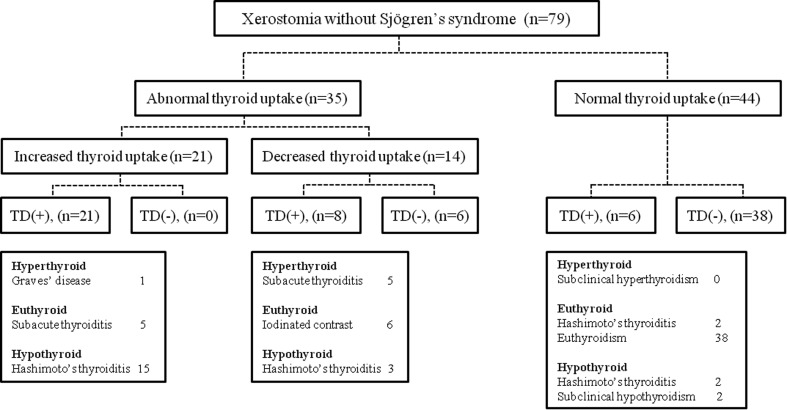
Patients were clinically diagnosed with TD on the basis of their scintigraphy patterns as well as available medical history and physical and biochemical data. As diagnosis of TD using SGS has already been reported in previous studies [14, 15, 17], we evaluated the scintigraphy patterns of the thyroid gland on SGS images. The intensity and size of the tracer uptake in the thyroid gland were categorized as increased, normal, or decreased. Biochemical data, including the levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), free thyroxine (fT4), and TSH receptor (TSH-R) and thyroid peroxidase (TPO) antibodies, were obtained through laboratory tests.
An accurate differential diagnosis was made in accordance with the reviews and guidelines for scintigraphic evaluation of TD [18–21]. Table 1 presents the differential diagnoses of TD based on certain imaging findings. Graves’ disease was diagnosed when the patient was biochemically hyperthyroid with high levels of thyroid stimulating autoantibodies and increased thyroid uptake. Recovery phase of subacute thyroiditis was diagnosed when the patient showed increased thyroid uptake on the scan and, on the other hand, demonstrated biochemically euthyroid status. Acute phase of subacute thyroiditis was diagnosed when the patient showed decreased thyroid uptake on the scan and demonstrated biochemically hyperthyroid status. Hashimoto’s thyroiditis was diagnosed when the patient demonstrated biochemically euthyroid status with positive anti-thyroid autoantibodies or hypothyroid except subclinical hypothyroidism. Subclinical hypothyroidism was defined as an increase in the TSH levels beyond the upper limit of the reference range, with normal fT4 levels and thyroid uptake. Subclinical hyperthyroidism was defined as a decrease in the serum TSH levels below the reference range, with normal serum fT4 and T3 levels and normal thyroid uptake [22].
Table 1.
Differential diagnoses of thyroid disease based on imaging findings
| Thyroid function | Uptake pattern on scintigraphy images | |||
|---|---|---|---|---|
| Increased (n = 53) | Normal (n = 91) | Decreased (n = 29) | ||
| Hyperthyroid | Graves’ disease (n = 1) | Subclinical hyperthyroidism (n = 0) | Subacute thyroiditis, acute phase (n = 6) | |
| Euthyroid | aAuto-Ab (+) | Subacute thyroiditis, recovery phase (n = 18) | Hashimoto’s thyroiditis (n = 3) | High-iodine diet Iodinated contrast (n = 7) |
| aAuto-Ab (−) | Euthyroidism (n = 74) | |||
| Hypothyroid | Hashimoto’s thyroiditis (n = 34) | Hashimoto’s thyroiditis (n = 9) Subclinical hypothyroidism (n = 5) |
Hashimoto’s thyroiditis (n = 16) | |
aAuto-Ab, autoantibodies including thyroid-stimulating hormone receptor and thyroid peroxidase antibodies
The serum TSH levels (reference range, 0.3–4.0 μIU/mL) were measured using a commercial immunoradiometric assay kit (TSH 1 RIA, B·R·A·H·M·S, Hennigsdorf, Germany). The serum T3 and fT4 levels (reference ranges, 0.6–1.9 ng/mL and 0.8–1.8 ng/dL, respectively) were measured using radioimmunoassay kits (RIA-gnost® T3, Cisbio, Bedford, MA, USA and FT4 RIA Kit, Beckman Coulter, Brea, CA, USA, respectively). The TSH-R antibody levels (reference range, 0–1.0 IU/L) were measured using a radio-receptor assay kit (TRAK human RIA, B·R·A·H·M·S, Hennigsdorf, Germany) and the TPO antibody levels (reference range, 0–60 U/mL) were measured using a radioimmunoassay kit (anti-TPOn RIA, B·R·A·H·M·S, Hennigsdorf, Germany).
Diagnosis of Sjögren’s Syndrome
Patients were clinically diagnosed with SS on the basis of their medical history as well as the results of physical and laboratory examinations, including labial salivary gland biopsy [23]. The functional status of the lacrimal and salivary glands was analyzed by the Schirmer-I and break-up time (BUT) tests, Oxford staining scores, SGS, and laboratory tests including anti-SSA/Ro and anti-SSB/La antibody assays. Keratoconjunctivitis sicca (KCS) was diagnosed when the results of the Schirmer-I (<10 mm/5 min), BUT (<10 s), and/or fluorescein tests were positive. The labial salivary glands were biopsied according to the standard method. A small ellipsoid incision was made in the lower lip mucosa, and a small sample of the submucosal tissue and several minor salivary glands were removed. The biopsy samples were processed per routine procedure using hematoxylin and eosin stains, examined by standard light microscopy, and assigned positive, negative, or non-diagnostic results [24]. The anti-SSA/Ro and anti-SSB/La antibody concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (CHORUS SS-A and CHORUS SS-B, respectively; DIESSE, Monteriggioni, Italy).
Salivary Gland Scintigraphy
Salivary gland scintigraphy images were acquired using a dual-head gamma camera (Infinia II; GE Healthcare, Milwaukee, WI, USA) equipped with a low-energy, high-resolution, parallel-hole collimator. The field of view of the SGS images of all patients covered the major salivary glands as well as the thyroid gland. Static images with 600,000 counts were acquired in the anterior view 20 min after the intravenous administration of 370 megabecquerel (MBq) Tc-99 m pertechnetate. Following this, static images in the right and left lateral planes were acquired with the same acquisition time as the anterior images. Salivary secretion was then stimulated with a sialagogue. Static images were acquired 10 min after sialagogue administration, with the same acquisition time as the initial static images (Fig. 1). The static approach was chosen because dynamic SGS images are susceptible to artifacts caused by the movement of the patient during salivary gland stimulation. The images were digitally recorded in 128 × 128 matrices. The energy window around the 140 keV photo-peak of Tc-99 m was 15 %.
Fig. 1.
Representative static images. Static images in the anterior (a) and the right and left lateral planes (b and c) obtained 20 min after tracer administration. Static images in the same planes (d, e, and f) acquired 10 min after sialagogue administration
Visual Analysis
Two nuclear medicine physicians, blinded to the results of the clinical evaluation, visually analyzed the SGS images. The image reading was subjective, and grayscale/color bars were not used. Disagreements in their interpretation were resolved by a third nuclear medicine physician, who independently evaluated the images and helped establish the final interpretation. A ‘normal’ finding was defined as a prominent Tc-99 m pertechnetate uptake and prompt excretion of the tracer upon sialagogue stimulation at both the parotid and submandibular glands. An ‘abnormal’ finding was defined as a decreased uptake of the tracer and/or decreased excretion of the tracer upon sialagogue stimulation, at the parotid or submandibular glands [25].
Semi-quantitative Analysis for Ejection Fraction of the Salivary Glands
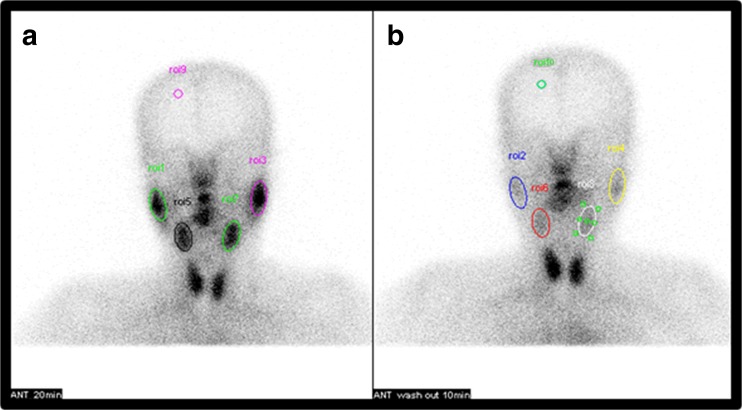
Ovoid regions of interest (ROIs) were drawn over the right and left parotid (RP and LP) and submandibular (RS and LS) glands, as well as the background (BG), on the anterior static images obtained before and after sialagogue administration (Fig. 2). The BG ROI was placed over the ipsilateral frontal region [12, 15, 26]. When a gland could not be visualized, the ROIs were drawn over the location of the parotid and submental area. In order to minimize the effect of size and increase reproducibility, we used the mean count per pixel value for each ROI. The ejection fraction (EF) expressed as a percentage for each salivary gland was calculated using the equation below. The BG counts were subtracted from the counts obtained for each gland, and the counts were all corrected for the decay of Tc-99 m pertechnetate.
Fig. 2.
Representative regions of interest. Ovoid regions of interest (ROIs) are drawn over the parotid and submandibular glands as well as the background (BG) on anterior static images obtained before (a) and after (b) sialagogue stimulation. The BG ROI is placed over the ipsilateral frontal region
The EF of the RP gland was calculated as:
Statistical Analysis
All parameters are expressed as the mean ± standard deviation (SD). Analysis of the mean values and comparison of data between patients with SS and those with TD were performed using the Mann–Whitney test and Student’s t-test. Non-parametric data comparisons were performed using the chi-square test. All statistical analyses were performed using Medcalc version 15.4 (Medcalc Software). All p-values were two-sided, and statistical significance was accepted at p < 0.05.
Results
Table 2 presents the characteristics of patients included in this study. Forty-one patients (23.7 %) had abnormal tracer uptake in the thyroid gland alone, 41 patients (23.7 %) had abnormal tracer uptake in the salivary gland alone, and 41 patients (23.7 %) had abnormal tracer uptake in both the thyroid gland and salivary gland. Overall, 92 patients (53.2 %) were diagnosed with TD at the initial examination for xerostomia. While 57 patients (32.9 %) exhibited both TD and SS, 35 (20.2 %) exhibited TD alone. In addition to the major symptoms of xerostomia, 65 patients presented with additional symptoms. Of the 65 patients, six complained of two or more additional symptoms. The most frequent symptom was arthralgia (n = 29), followed by discoloration of the fingers, symptoms associated with Raynaud’s phenomenon (n = 10), depression (n = 9), oral ulcers (n = 9), fatigue (n = 4), neuropathic symptoms (n = 3), skin rashes (n = 2), myalgia (n = 2), anxiety (n = 1), photosensitivity (n = 1), and genital ulcers (n = 1).
Table 2.
Patient characteristics
| Characteristics | No. of patients (%) |
|---|---|
| Mean age, years | 53.3 ± 13.3 |
| Sex | |
| Women | 144 (83.2) |
| Men | 29 (16.8) |
| Thyroid uptake on SGS | |
| Normal | 91 (52.6) |
| Increased | 53 (30.6) |
| Decreased | 29 (16.8) |
| Salivary uptake on SGS | |
| Normal | 91 (52.6) |
| Abnormal | 82 (47.4) |
| Thyroid disease | 92 (53.2) |
| Hashimoto’s thyroiditis | 62 (35.8) |
| Subacute thyroiditis | 24 (13.9) |
| Subclinical hypothyroidism | 5 (2.9) |
| Graves’ disease | 1 (0.6) |
| Sjögren’s syndrome | 94 (54.3) |
SGS salivary gland scintigraphy
Scintigraphy Findings of the Thyroid Gland and Diagnosis of Thyroid Diseases
According to the visual assessment of the SGS images, 53 patients (30.6 %) exhibited diffusely increased tracer uptake in the thyroid gland, while 29 (16.8 %) exhibited diffusely decreased uptake (Fig. 3). Of the 53 patients with increased thyroid uptake, 34 (n = 34/173; 19.7 %) and 18 (n = 18/173; 10.4 %) exhibited Hashimoto’s and subacute thyroiditis, respectively, and one patient (n = 1/173; 0.6 %) was diagnosed with Graves’ disease. Of the 29 patients with decreased thyroid uptake, 16 (n = 16/173; 9.2 %) and six (n = 6/173, 3.5 %) exhibited Hashimoto’s and subacute thyroiditis, respectively, and seven patients (n = 7/173; 4.0 %) exhibited normal levels of thyroid hormone and antibodies. Of the 91 patients with normal thyroid uptake, 12 (n = 12/173; 6.9 %) and five (n = 5/173; 2.9 %) exhibited Hashimoto’s thyroiditis and subclinical hypothyroidism, respectively, and 74 (n = 74/173; 42.8 %) exhibited normal levels of thyroid hormone. Figures 4 and 5 present the prevalence of various thyroid diseases in relation to thyroid uptake among patients with or without SS in this study. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of SGS for the detection of TD were 81.5, 91.4, 91.5, and 81.3 %, respectively.
Fig. 3.
Prevalence of thyroid diseases in relation to thyroid uptake
Fig. 4.
Prevalence of thyroid diseases in relation to thyroid uptake in patients with Sjögren’s syndrome
Fig. 5.
Prevalence of thyroid diseases in relation to thyroid uptake in patients without Sjögren’s syndrome
Scintigraphy Findings of the Salivary Glands and Diagnosis of Sjögren’s Syndrome
The visual assessment of the SGS images revealed abnormal findings in the salivary glands of 82 patients (47.4 %), of whom, 73 were diagnosed with SS according to the American-European Criteria [23]. The remaining nine patients exhibited decreased tracer uptake in one of the salivary glands; sialadenitis was confirmed upon histopathological examination in each case. In patients with normal uptake in the salivary glands (n = 91/173; 52.6 %), 21 were clinically diagnosed as having SS. The sensitivity, specificity, PPV, and NPV of SGS for the diagnosis of SS were 77.7, 88.6, 89.0, and 76.9 %, respectively.
Among patients included in this study, 94 (54.4 %) exhibited SS. The most frequent underlying autoimmune disease was systemic lupus erythematosus (SLE; n = 7), followed by rheumatoid arthritis (n = 5), interstitial lung disease (n = 2), systemic sclerosis (n = 2), autoimmune hepatitis (n = 2), and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (n = 1). Among patients with SS, while 47 exhibited abnormal findings in the thyroid gland on SGS images (increased uptake, 32; decreased uptake, 15), 57 were diagnosed with TD. Among all patients included in this study, 131 complained of ocular symptoms of SS, 56 exhibited ocular signs, 59 were positive for anti-SSA/Ro and/or anti-SSB/La antibodies, and 45 demonstrated positive pathologic findings in the labial biopsy.
Ejection Fraction
Table 3 presents the comparison of EF between patients with TD and those with an undetermined diagnosis (i.e., patients who were not diagnosed with either SS or TD), and patients with SS and TD and those with SS without TD. There were significant differences in the EF values of the parotid and submandibular glands between patients with TD and those with an undetermined diagnosis (p < 0.05; Fig. 6). However, the differences in EF values between patients with SS with and without TD were not statistically significant (p > 0.05).
Table 3.
Comparison of ejection fraction between different subgroups of patients
| Patients with TD (n = 92) | Patients with an undetermined diagnosis (n = 44) | p-value | Patients with SS with TD (n = 57) | Patients with SS without TD (n = 37) | p-value | |
|---|---|---|---|---|---|---|
| Ejection fraction | ||||||
| RP, mean % ± SD | 49.2 ± 18.0 | 55.5 ± 14.5 | 0.0498 | 43.0 ± 19.7 | 46.7 ± 20.8 | 0.3941 |
| LP, mean % ± SD | 48.2 ± 18.4 | 59.0 ± 12.4 | 0.0001 | 42.6 ± 19.7 | 46.3 ± 19.1 | 0.3746 |
| RS, mean % ± SD | 36.8 ± 17.8 | 47.0 ± 12.3 | 0.0002 | 29.7 ± 17.5 | 30.1 ± 18.2 | 0.9219 |
| LS, mean % ± SD | 37.3 ± 16.6 | 47.7 ± 14.4 | 0.0007 | 31.2 ± 16.4 | 28.9 ± 19.1 | 0.5393 |
SS Sjögren’s syndrome, TD thyroid disease, RP right parotid gland, LP left parotid gland, RS right submandibular gland, LS left submandibular gland, SD standard deviation
Fig. 6.
Box plot of ejection fraction of each subgroup. There are significant differences in the ejection fraction (EF) values of the right and left parotid (RP and LP) and submandibular (RS and LS) glands between patients with thyroid disease (TD) and those with an undetermined diagnosis (p < 0.05)
Discussion
Although SS, a chronic autoimmune disorder affecting the exocrine glands, is the most common causal disease of xerostomia [4, 5], patients with TD with xerostomia have also been reported to exhibit relatively poor salivary function [1]. In the present study, 53.2 % of the patients with xerostomia were diagnosed with TD, of whom, 62.0 % also exhibited SS, while 38.0 % had TD alone. In other words, 44.3 % of patients had TD without SS. Of patients with TD, 30.4 % had Hashimoto’s thyroiditis, 10.1 % had subacute thyroiditis, 2.5 % had subclinical hypothyroidism, and 1.3 % had Graves’ disease. Milic et al. reported a prevalence of Hashimoto’s thyroiditis of 25.4 % in subjects with symptoms of xerostomia without SS (n = 59) [27]; however, the authors did not evaluate other TD, such as subacute thyroiditis or subclinical hypothyroidism, and diagnosed using only laboratory tests. To the best of our knowledge, the present study is the first to report the scintigraphy findings of the thyroid gland in patients with xerostomia using SGS.
Thyroid diseases can affect the salivary function through a number of mechanisms. As patients with TD exhibit periepithelial lymphocytic infiltration and oligoclonal B-cell expansion, similar to patients with SS, the extrathyroidal manifestation of TD can directly affect the salivary function [7]. Additionally, TD can also affect the salivary function when patients with TD develop SS. Previous studies have reported that patients with TD exhibit relatively high concentrations of anti-nuclear antibodies and are at a high risk for developing systemic autoimmune diseases such as SS [9, 10]. In another study, half of the 176 patients with TD were reported as fulfilling the criteria for the diagnosis of SS [28]. The results of the present study were comparable to those of previous studies in the fact that we found significant differences in the salivary function between patients with TD and those with an undetermined diagnosis. Among the latter patients, the mean EF of the RP gland was lower compared with that of the LP gland (55.5 vs. 59.0 %); however, the difference was barely not significant (p = 0.0498), possibly because patients in this group had sialadenitis in the RP gland and exhibited asymmetric EF values, i.e., 5−22 %.
Conversely, SS can subsequently involve the thyroid gland [6]. However, in the present study, the presence of TD in patients with SS did not affect the salivary function. The results of previous hormonal and immunological studies revealed a high prevalence (36–45 %) of TD in patients with primary SS (pSS) [29–31]. However, since these hormonal and immunological examinations were limited in terms of the detection of seronegative thyroiditis, the prevalence might have been underestimated [32]. Since the present study involved a scintigraphic assessment as well as laboratory tests, the sensitivity of diagnosis of TD was relatively high; therefore, our reported prevalence of TD in patients with pSS (53.3 %) could be reasonable.
The coexistence of SS with TD indicates the importance of the contribution of endocrine signals in the pathogenesis and clinical expression of pSS, suggesting the role of hormonal therapy as a potential therapeutic strategy in these patients [5]. In addition, patients with SS and TD have an increased risk of lymphoproliferative disorders, especially non-Hodgkin’s lymphoma of the mucosa-associated lymphoid tissue (MALT) type [33]. However, Caramaschi et al. reported that patients affected by both pSS and TD exhibit a milder clinical phenotype of pSS, thus presenting a relatively low risk for the development of lymphoma [34].
In some cases, it can be difficult to clearly establish whether the salivary and ocular involvement of SS are an extrathyroidal manifestation of TD or, conversely, an extra-exocrine manifestation of SS [8]. Our results (Supplementary data), which indicate no significant difference in the frequency of clinical features between patients with SS without and with TD, support the former premise. Owing to similarity in clinical findings, TD could be misdiagnosed as SS and vice versa. Additionally, we found no significant difference in the prevalence of TD between patients with pSS and those with xerostomia without SS (53.3 vs. 43.0 %; p = 0.2638). The prevalence of TD appears high enough to warrant that investigations be undertaken to rule out TD in patients with xerostomia without SS. Therefore, symptoms of xerostomia should always be seriously considered as possible signs of TD. Periodic screening for thyroid function in adult women with SS, even in the absence of typical symptoms of TD, as well as for the possible coexistence of SS in women with TD, could be useful [33]. In fact, SGS, which is a non-invasive, safe, and convenient diagnostic imaging technique, can be used for the assessment of the salivary gland morphology and function. In addition, SGS will lead to performance of further thyroid evaluation in patients with abnormal thyroid uptake.
Our study has several limitations, including its retrospective design and inherent selection biases. Although the results of the thyroid scan and thyroid function test facilitate diagnosis of thyroid disease, additional findings from ultrasonography, histopathological investigations, and follow-up thyroid scan might help to diagnose TD. The retrospective design of the current study may be a limitation because we only reviewed the medical records. With the best information that we could obtain from the source, we categorized the thyroid status of each patient on the basis of previously published diagnostic criteria [21] and articles [18–20]. Although the patients were probable to be misdiagnosed with different thyroid disease, it was certain that the patients had TD. In order to minimize the proportion of misdiagnosing, a prospective large study is needed.
In the present study, the salivary gland function was evaluated on the basis of a parameter calculated using simple methods. We only calculated EF as a quantitative measure of the salivary gland function. The reason for the selection of such simple evaluation methods was their routine use in our department. Since this study involved a retrospective review of medical records and SGS data, images of the syringe before and after tracer administration could not be obtained; therefore, we were unable to calculate the absolute Tc-99 m uptake of the salivary and thyroid glands. Further studies are necessary to define a standard protocol with high diagnostic accuracy for the quantitative evaluation of the salivary gland function and thyroid uptake.
Conclusion
In the present study, more than half of the patients with xerostomia presented with TD; specifically, 60.6 % of patients with SS and 44.3 % of patients with xerostomia without SS were diagnosed with TD. The symptoms of xerostomia should be considered seriously as possible signs of TD. Thyroid assessment by SGS is feasible, and SGS appears to be useful for the evaluation of TD in patients with xerostomia caused by TD. Therefore, during the interpretation of SGS findings in patients with xerostomia, tracer uptake in the thyroid gland should not be overlooked. This strategy would make the requirement for additional work-up for thyroid disease in these patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 14 kb)
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
Compliance with Ethical Standards
Conflict of Interest
Ji-hoon Jung, Chang-Hee Lee, Seung Hyun Son, Ju Hye Jeong, Shin Young Jeong, Sang-Woo Lee, Jaetae Lee, Byeong-Cheol Ahn declare that they have no conflict of interest.
Ethical Statement
The study protocol had been approved by the Ethics Committee of the Kyungpook National University Hospital (KNUH 2016-03-034-002). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. No informed consent was needed, because of the retrospective design of our study.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13139-016-0455-4) contains supplementary material, which is available to authorized users.
Contributor Information
Ji-hoon Jung, Phone: +82-53-420-5577, Email: hoon2510@nate.com.
Byeong-Cheol Ahn, Phone: 82-53-420-5583, Email: abc2000@knu.ac.kr.
References
- 1.Changlai SP, Chen WK, Chung C, Chiou SM. Objective evidence of decreased salivary function in patients with autoimmune thyroiditis (chronic thyroiditis, Hashimoto’s thyroiditis) Nucl Med Commun. 2002;23:1029–33. doi: 10.1097/00006231-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Garg AK, Kirsh ER. Xerostomia: recognition and management of hypofunction of the salivary glands. Compend Contin Educ Dent. 1995;16(574):6–84. [PubMed] [Google Scholar]
- 3.Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:28–46. doi: 10.1016/j.tripleo.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–6. [PubMed] [Google Scholar]
- 5.Mavragani CP, Fragoulis GE, Moutsopoulos HM. Endocrine alterations in primary Sjogren’s syndrome: an overview. J Autoimmun. 2012;39:354–8. doi: 10.1016/j.jaut.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Kang JH, Lin HC. Comorbidities in patients with primary Sjogren’s syndrome: a registry-based case-control study. J Rheumatol. 2010;37:1188–94. doi: 10.3899/jrheum.090942. [DOI] [PubMed] [Google Scholar]
- 7.Alfaris N, Curiel R, Tabbara S, Irwig MS. Autoimmune thyroid disease and Sjogren syndrome. J Clin Rheumatol. 2010;16:146–7. doi: 10.1097/RHU.0b013e3181d52a28. [DOI] [PubMed] [Google Scholar]
- 8.Robazzi TC, Adan LF. Autoimmune thyroid disease in patients with rheumatic diseases. Rev Bras Reumatol. 2012;52:417–30. doi: 10.1590/S0482-50042012000300011. [DOI] [PubMed] [Google Scholar]
- 9.Lu MC, Yin WY, Tsai TY, Koo M, Lai NS. Increased risk of primary Sjogren’s syndrome in female patients with thyroid disorders: a longitudinal population-based study in Taiwan. PLoS One. 2013;8:e77210. doi: 10.1371/journal.pone.0077210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, Moutsopoulos HM. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis. 2004;63:1159–61. doi: 10.1136/ard.2004.022624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavragani CP, Skopouli FN, Moutsopoulos HM. Increased prevalence of antibodies to thyroid peroxidase in dry eyes and mouth syndrome or sicca asthenia polyalgia syndrome. J Rheumatol. 2009;36:1626–30. doi: 10.3899/jrheum.081326. [DOI] [PubMed] [Google Scholar]
- 12.Kang JY, Jang SJ, Lee WW, Jang SJ, Lee YJ, Kim SE. Evaluation of salivary gland dysfunction using salivary gland scintigraphy in Sjogren’s syndrome patients and in thyroid cancer patients after radioactive iodine therapy. Nucl Med Mol Imaging. 2011;45:161–8. doi: 10.1007/s13139-011-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CB, Xi H, Zhou Q, Zhang LM. The diagnostic value of technetium 99m pertechnetate salivary gland scintigraphy in patients with certain salivary gland diseases. J Oral Maxillofac Surg. 2015;73:443–50. doi: 10.1016/j.joms.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Taura S, Murata Y, Aung W, Ishida R, Zhang L, Hossain M, et al. Decreased thyroid uptake of Tc-99m pertechnetate in patients with advanced-stage Sjogren syndrome: evaluation using salivary gland scintigraphy. Clin Nucl Med. 2002;27:265–9. doi: 10.1097/00003072-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Gune S, Yilmaz S, Karalezli A, Aktas A. Quantitative and visual evaluation of salivary and thyroid glands in patients with primary Sjogren’s syndrome using salivary gland scintigraphy: relationship with clinicopathological features of salivary, lacrimal and thyroid glands. Nucl Med Commun. 2010;31:666–72. [PubMed] [Google Scholar]
- 16.Tensing EK, Nordstrom DC, Solovieva S, Schauman KO, Sippo-Tujunen I, Helve T, et al. Salivary gland scintigraphy in Sjogren’s syndrome and patients with sicca symptoms but without Sjogren’s syndrome: the psychological profiles and predictors for salivary gland dysfunction. Ann Rheum Dis. 2003;62:964–8. doi: 10.1136/ard.62.10.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erhamamci S, Horasanli B, Aktas A. Assessment of the effect of interferon-beta1a therapy on thyroid and salivary gland functions in patients with multiple sclerosis using quantitative salivary gland scintigraphy. Mol Imaging Radionucl Ther. 2014;23:43–7. doi: 10.4274/mirt.53825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JR, Oates E. Radionuclide imaging of the thyroid gland: patterns, pearls, and pitfalls. Clin Nucl Med. 2004;29:181–93. doi: 10.1097/01.rlu.0000114530.12565.5b. [DOI] [PubMed] [Google Scholar]
- 19.Slatosky J, Shipton B, Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician. 2000;61:1047–52. [PubMed]
- 20.Wang PW, Chen HY, Li CH, Liu RT, Chien WY, Tung SC. Tc-99m pertechnetate trapping and thyroid function in Hashimoto’s thyroiditis. Clin Nucl Med. 1994;19:177–80. doi: 10.1097/00003072-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Harbert JC, Eckelman WC, Neumann RD. Nuclear medicine: diagnosis and therapy. Thieme Medical Publishers, Stuttgart; 1996.
- 22.Ahn D, Sohn JH, Jeon JH. Hypothyroidism following hemithyroidectomy: incidence, risk factors, and clinical characteristics. J Clin Endocrinol Metab. 2016;101:1429–36. doi: 10.1210/jc.2015-3997. [DOI] [PubMed] [Google Scholar]
- 23.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamba R, Sweiss NJ, Langerman AJ, Taxy JB, Blair EA. The minor salivary gland biopsy as a diagnostic tool for Sjogren syndrome. Laryngoscope. 2009;119:1922–6. doi: 10.1002/lary.20292. [DOI] [PubMed] [Google Scholar]
- 25.Umehara I, Yamada I, Murata Y, Takahashi Y, Okada N, Shibuya H. Quantitative evaluation of salivary gland scintigraphy in Sjorgen’s syndrome. J Nucl Med. 1999;40:64–9. [PubMed] [Google Scholar]
- 26.Firat F, Cermik TF, Sarikaya A, Berkarda S. Effects of gender and age on the quantitative parameters of [99mTc]pertechnetate salivary gland scintigraphy in normal subjects. Nucl Med Commun. 2006;27:447–53. doi: 10.1097/00006231-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Milic VD, Radunovic G, Boricic I, Ognjanovic S, Petrovic R, Radak-Perovic M, et al. High prevalence of autoimmune thyroid disease in subjects with sicca symptoms without Sjogren’s syndrome. Rheumatology (Oxford) 2013;52:754–5. doi: 10.1093/rheumatology/kes423. [DOI] [PubMed] [Google Scholar]
- 28.Coll J, Anglada J, Tomas S, Reth P, Goday A, Millan M, et al. High prevalence of subclinical Sjogren’s syndrome features in patients with autoimmune thyroid disease. J Rheumatol. 1997;24:1719–24. [PubMed] [Google Scholar]
- 29.Perez B, Kraus A, Lopez G, Cifuentes M, Alarcon-Segovia D. Autoimmune thyroid disease in primary Sjogren’s syndrome. Am J Med. 1995;99:480–4. doi: 10.1016/S0002-9343(99)80223-X. [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Casals M, Garcia-Carrasco M, Cervera R, Gaya J, Halperin I, Ubieto I, et al. Thyroid disease in primary Sjogren syndrome. Study in a series of 160 patients. Medicine (Baltimore) 2000;79:103–8. doi: 10.1097/00005792-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Appenzeller S, Pallone AT, Natalin RA, Costallat LT. Prevalence of thyroid dysfunction in systemic lupus erythematosus. J Clin Rheumatol. 2009;15:117–9. doi: 10.1097/RHU.0b013e31819dbe4c. [DOI] [PubMed] [Google Scholar]
- 32.Grani G, Carbotta G, Nesca A, D’Alessandri M, Vitale M, Del Sordo M, et al. A comprehensive score to diagnose Hashimoto’s thyroiditis: a proposal. Endocrine. 2015;49:361–5. doi: 10.1007/s12020-014-0441-5. [DOI] [PubMed] [Google Scholar]
- 33.Jara LJ, Navarro C, Brito-Zeron Mdel P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M. Thyroid disease in Sjogren’s syndrome. Clin Rheumatol. 2007;26:1601–6. doi: 10.1007/s10067-007-0638-6. [DOI] [PubMed] [Google Scholar]
- 34.Caramaschi P, Biasi D, Caimmi C, Scambi C, Pieropan S, Barausse G, et al. The co-occurrence of Hashimoto thyroiditis in primary Sjogren’s syndrome defines a subset of patients with milder clinical phenotype. Rheumatol Int. 2013;33:1271–5. doi: 10.1007/s00296-012-2570-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)