Abstract
Averrhoa bilimbi L. belongs to family Oxalidaceae. Traditionally, people use this plant (root, bark, leaves and fruits) for treating several illnesses include itches, boils, syphilis, whooping cough, hypertension, fever and inflammation. The aim of the study was to evaluate the nitric oxide (NO) scavenging activity and GC–MS analysis of A. bilimbi L. fruit extract. Averrhoa bilimbi L. fruits were collected for the preliminary phytochemical analysis, antioxidant scavenging activity and biologically important compounds were identified by GC–MS analysis. The preliminary phytochemicals, GC–MS, total phenolic content and NO scavenging activity of the plant were analysed. In the present investigation, the A. bilimbi L. fruit extract has major phytochemicals. Among the 151 compounds identified in GC–MS, 15 compounds are found to have diverse biological activity. We also observed that the A. bilimbi L. fruit extract has high level of total phenolic compounds at a concentration of 209.25 GAE mg/g. Presence of phenolic compound apparently explains the antioxidant activity of the plant. Antioxidant activity of A. bilimbi L. fruit extract is proven from its high level of NO scavenging activity of potent IC50 value of 108.10. From the above study, it is apparent that the A. bilimbi L. fruit extract is a rich source of phytochemicals (natural products) with biological activity. The GC–MS report on this fruit proves that natural products have pharmacologically and biologically active compounds. A high phenolic content is observed in our study. A. bilimbi L. fruit extract is also found to have NO scavenging activity in our study.
Keywords: Averrhoa bilimbi L., GC–MS, Nitric oxide, Phenol, Phytochemical, Antioxidant
Introduction
Everyday 50,000 premature deaths are caused due to infectious diseases (Singh et al. 1992; Robin et al. 1998). In accordance with the World Health Organization (WHO) 2014 diseases like malaria, dengue, leishmaniasis, Lyme disease, tuberculosis, schistosomiasis, and yellow fever, carried by mosquitoes, flies, ticks, water snails and air infect one billion people and more than one million people will die. Pathogens and diseases become drug resistant and the best alternate approach are plants to eliminate diseases and therapeutic complications (Fabricant and Farnsworth 2001). From time immemorial plants are used as medicine to treat diseases. Before the discovery of allopathy humans depended on Ayurveda and homeopathy medicine which are completely based on plants and herbs. These herbs and plant materials act as medicine to cure diseases (Nostro et al. 2000). Tribal people depend on the rich diversity of forest to overcome the health care needs. Forests have excellent vegetation (flora) with high quality of medicinal value (Kadhirvel et al. 2010). Phytochemicals are the non-nutrient compounds with beneficial health effects leading to pharmacological importance and are used in medication (Nisa et al. 2011). Fruits play major role in human diet due to their bioactive compounds, natural sugars and organic acids with relatively high antioxidant activity (Rechkemmer 2001) and are a rich source of vitamins (A, B6, C, E, niacin, and thiamine) dietary fibre and minerals (Wargovich 2000). Averrhoa bilimbi L. is a long-lived green plant which gives edible fruits, belonging to the family Oxalidaceae–Oxalis and grows 16–33ft (5–10 m) in height, with short trunk dividing into number of upright branches. It is found throughout Malaysia, Indonesia, Myanmar, Bangladesh, Srilanka and common in Southeast Asian countries (Rahman et al. 2014). In India it is available mostly in Kerala regions, particularly the Kani tribal traditional healers in Thodu hills region (Kerala) use the raw leaves and fruits of A. bilimbi L. plant for ailments in circulatory system (Xavier et al. 2014) and the local name is Irumban puli or Pulingi. The parts like bark, leaves, seeds, flowers, fruits, roots and the entire A. bilimbi L. plant is used as alternative medicine to treat numerous diseases majorly as anti-diabetic agent (Kumar et al. 2013). Traditionally it is used in medication to cure cough, cold, boils, itches, syphilis, whooping cough, rheumatism and hypertension (Sabiha et al. 2012). A. bilimbi L. shows antimicrobial activity against gram positive and gram negative bacteria (Karon et al. 2011), antifungal activity (Nazmul et al. 2011), cytotoxic activity (Das et al. 2011), anti-diabetic activity (Pushparaj et al. 2000) and the leaves of A. bilimbi L. could increase the serum insulin level (Patel et al. 2012) in diabetes mellitus. Administration of A. bilimbi L. fruit (toxicity studies) extract 1 g/kg bw did not affect the mice (Savithri et al. 2009). However, in spite of having of such a great traditional medicinal use, the knowledge on its phytochemical is limited. Few studies of phytochemicals on the A. bilimbi L. fruit extract have shown contradictory data on the presence of alkaloids, tannins, glycosides, saponins and steroids (Sabiha et al. 2012). This study is therefore designed to analyse the active phytochemicals present in the fruits of A. bilimbi L. by biochemical tests and GC–MS which may be useful for exploring its ethno-pharmacological significance and to validate scientifically its medicinal properties. Several previous studies have shown the free radical scavenging activity of A. bilimbi L. fruit extract through DPPH scavenging activity (Asna and Noriham 2014; Sabiha et al. 2012). Oxidative stress has major action in human anatomy, physiology and diseases like cardiovascular diseases, diabetes, inflammatory conditions, ageing and cancer (Joyce 1987). Nitric oxide (NO) plays a major role in several in vivo diseases like neuronal signalling, smooth muscle relaxation, regulation of cell-mediated toxicity and inhibition of platelet aggregation (Hagerman et al. 1998). Surplus NO is reported to direct DNA fragmentation, cell damage and neuronal cell death. NO will not affect the DNA and proteins directly but NO is very unstable in aerobic condition and produces NO2, N3O4, N2O4 intermediates which are genotoxic, affecting the DNA repair proteins and also deaminate DNA bases (Umamaheswari and Chatterjee 2008). Hence it is essential to reduce the levels of NO in human body. Besides to the reactive oxygen species (ROS) NO was found to be elevated in inflammation (Baygutalp et al. 2015), colon cancer (Erdman et al. 2009) and pathological conditions like gastrointestinal disorders (Cho 2001). The traditional usage of the fruits of A. bilimbi L. for anti-inflammation, anti-diabetic and anti-hypertensive was highlighted in a report on Malaysian medicinal plants (Harun et al. 2015). A. bilimbi L. is a rich source of vitamin C, A, B1 and 100 g of edible portion was found to have moisture, 94.2–94.7 g; fibre, 0.6 g; ash, 0.31–0.40; protein, 0.61 g; calcium, 3.4 g; iron, 1.01 mg; riboflavin, 0.32 mg; thiamine, 0.010 mg; ascorbic acid, 15.5 mg (Zakaria et al. 2007). Ascorbic acid has been used as standard drug for the estimation of nitric oxide scavenging activity (singh et al. 2012). As the A. bilimbi L. fruit extract has also shown a good antioxidant potential against DPPH (Chauhan and Kapfo 2013), we made an attempt to analyse its in vitro NO scavenging activity.
Materials and methods
Chemicals and reagents
All the chemicals used for this experiment were analytical grade purchased from Hi-Media, and SD Fine Chemicals.
Selection and authentication of fruit
Averrhoa bilimbi L. fruit samples were collected from Palakkad district, Kerala, during February to March (2015). The fruits of A. bilimbi L. are used as a source of food and medicine by tribes and settler communities of the local people. The authentication of the fruit was done by the Botanical Survey of India (BSI) Coimbatore, Tamilnadu, India. The authentication number given by the BSI is BSI/SRC/5/23/2015/Tech.
Extraction of fruit material
The fruits of the A. bilimbi L. were collected, air dried and made into fine powder by the mortar and pestle. Extraction from the fruits was done according to the method described by Singh et al. (2012). The powder (25 g) was used for the extraction with 250 ml of methanol (95% v/v) in a soxhlet apparatus. The remaining methanol was evaporated using rotary evaporator. The obtained thick semi-solid crude extract was stored at 2–4 °C for further use.
Phytochemical screening of A. bilimbi L. fruits
The A. bilimbi L. fruit (methanol) extract was analysed for the presence of alkaloid, carbohydrate, glycosides, phenols, flavonoids, saponins, steroids and tannins using the respective biochemical tests as follows.
Test for alkaloids
Two millilitre of 1% HCl was mixed with 0.1 gm of crude extract and heated slightly. After cooling Wagner’s reagent and Mayer’s reagent were added to it. The presence of buff-coloured precipitate indicated the presence of alkaloids (Sofowora 1993).
Test for carbohydrates
Benedict’s reagents was mixed with the 0.1 gm of crude extract and slightly boiled, appearance of reddish brown precipitate indicated the presence of the carbohydrates (Harborne 1973).
Test for flavonoids
The appearance of pink scarlet colour when 0.1 gm of crude extract was mixed with few drops of concentrated HCl and Mg pellets indicated the presence of flavonoids (Odebiyi and Sofowora 1978).
Test for phenols
Two millilitre of 2% ferric chloride was mixed with the 0.1 gm of crude extract and the presence of blue-green or black coloration indicated the presence of phenols (Yadav and Agarwala 2011).
Test for saponins
Saponin presence was detected by the frothing test. Briefly 0.1 gm of crude extract was mixed well in water and shaken, the appearance of foam indicated the preliminary evidence for the presence of saponins (Kumar et al. 2009).
Test for steroid (Liebermann test)
0.1 gm of crude extract was mixed with 2 ml H2SO4 and slowly added to 2 ml of acetic anhydride. The colour change from violet to green or blue indicated the presence of steroids (Edeoga et al. 2005).
Test for tannins
0.1gm of crude extract of A. bilimbi L. fruit was mixed in distilled water and filtered. Few drops of ferric chloride solution were added to the filtrate. The green or blue-green precipitate indicated the presence of tannins (Trease and Evans 2002).
GC–MS analysis on A. bilimbi L. fruit extract
Averrhoa bilimbi L. methanolic fruit extract was subjected to gas chromatography–mass spectroscopy (GC–MS) analysis. The Thermo GC-Trace Ultra VER: 5.0 (Bremen, Germany) and Mass Spectroscopy (MS) MS DSQ II electron ionization mode with ionization energy of 70 eV were used. The temperature of the column was set to 80–250 °C at 8 °C/min rate. Temperature of 280 and 290 °C were set for the GC injector and MS transfer, respectively. Helium was used as a carrier gas at a flow rate of 1.0 ml/min. The sample volume of 1 μl was used for analysis. By the retention time and mass fragmentation patterns, the major compounds present in the fruit extract were analysed. The National Institute of Standards and Technology (NIST) and Wiley 9.0 library was used (Sakthivel and Guruvayoorappan 2013) for the detection of compounds.
Estimation of phenols in A. bilimbi L. fruit extract
The total phenolic compounds were estimated using the Folin–Ciocalteu reagent (Slinkard and Singleton 1977). Briefly 0.1 ml of A. bilimbi L. fruit extract of different concentrations (50, 100, 150, 200, and 250 µg/ml) were mixed with 2 ml of 10% Folin–Ciocalteu reagent and 3 ml of 7% Na2 CO3 was added. This was incubated for 30 min at room temperature and the absorbance was measured using UV-spectrophotometer at 760 nm. Gallic acid was used as standard and all the results were performed in triplicates. The total phenol concentration is expressed in mg gallic acid equivalent (GAE).
Nitric oxide (NO) scavenging activity of A. bilimbi L. fruit extract
The nitric oxide (NO) scavenging activity of the A. bilimbi L. fruit extract was expressed in percentage inhibition (Vaijanathappa et al. 2008). Briefly 3 ml of 10 mM sodium nitroprusside (0.5 mM PBS pH 7.4) was mixed with 1 ml of A. bilimbi L. fruit extract at different concentrations (25, 50, 75, 100, 125, and 150 µg/ml) and incubated at 25 °C for 150 min. Then 0.5 ml of the reaction mixture was removed and 1 ml of sulfanilic acid reagent (0.33% in 20% glacial acetic acid) was added and again incubated for 5 min at 25 °C. After adding 1 ml of naphthyl ethylene diamine dichloride (0.1 w/v), the entire reaction mixture was allowed to stand for 30 min at room temperature. The absorbance was measured at 540 nm. Similar procedure was repeated for the standard ascorbic acid at different concentrations (25, 50, 75, 100, 125, 150 µg/ml). The same reaction mixture with the methanol served as control (without extract and standard)
where A 0 is the absorbance of the control and A 1 is the absorbance of the sample.
Results
Phytochemical analysis
Preliminary phytochemical tests revealed the presence of alkaloids, carbohydrates, phenols, flavonoids, saponins and tannins (Table 1). The presence of more phenols was observed in the preliminary screening. The test for sterols answered negative in our study.
Table 1.
The phytochemicals present in A. bilimbi L. fruit extract. It reveals the presence of alkaloids, carbohydrates, phenols, flavonoids, saponins, tannins. (++ indicates more amount) and the absence of steroids (−)
| S. no | Test | Results |
|---|---|---|
| 1 | Alkaloids | + |
| 2 | Carbohydrates | + |
| 3 | Phenols | ++ |
| 4 | Flavonoids | + |
| 5 | Saponins | + |
| 6 | Steroids | − |
| 7 | Tannins | + |
Phytochemical compounds identified by GC–MS analysis
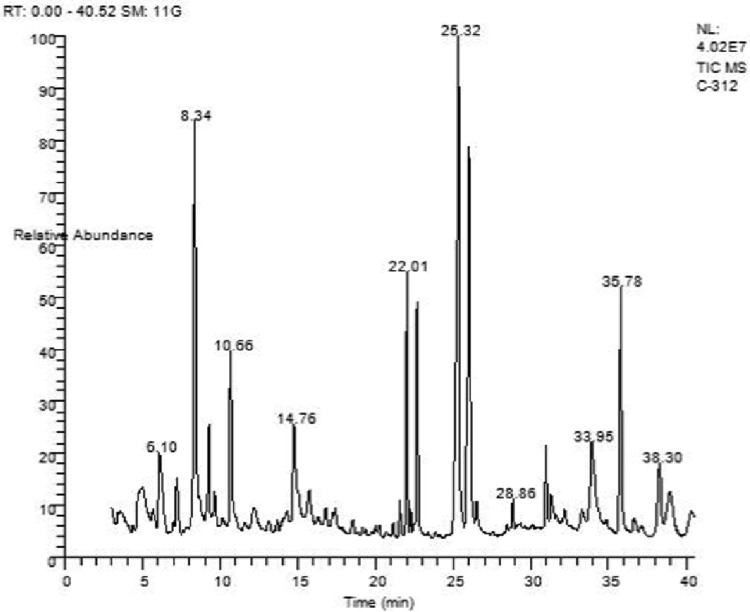
By comparing with the National Institute of Standards and Technology (NIST) and Wiley 9.0 library, the major compounds are identified and listed in Table 2. The GC–MS chromatogram is shown in Fig. 1. Among the 151 compounds identified, 15 compounds are found to have various biological activity which were reported from other studies as mentioned in Table 3. Furthermore, the GC–MS analysis reported the presence of various phenol, flavonoid, lipid, alkaloid and acid compounds which were shown in basic phytochemical screening test.
Table 2.
Compounds identified by GC–MS in the A. bilimbi L. fruit extract
| S. no | Compound | Empirical formula | Empirical weight | Probability | Area % |
|---|---|---|---|---|---|
| 1 | N-Methoxy-N-methylacetamide | C4H9NO2 | 103 | 26.21 | 2.96 |
| 2 | Propane nitrile, 3-(methylthio)-(CAS) | C4H7NS | 101 | 12.73 | 2.96 |
| 3 | d-Mannitol | C6H14O6 | 182 | 7.33 | 2.96 |
| 4 | d-Glycero-d-manno-heptitol | C7H16O7 | 212 | 4 | 2.96 |
| 5 | Propionic acid, 2-mercapto-, allyl ester | C6H10O2S | 146 | 3.69 | 2.96 |
| 6 | Boronic acid, ethyl-, bis(2-mercaptoethyl ester) | C16H15BO2S2 | 194 | 3.69 | 2.96 |
| 7 | N1-Methyluracil | C5H6N2O2 | 126 | 13.48 | 3.26 |
| 8 | d-alanine, N-propargyloxycarbonyl-, isohexyl ester | C13H21NO4 | 255 | 7.91 | 3.26 |
| 9 | Uracil, 1-n-methyl | C5H6N2O2 | 126 | 6.06 | 3.26 |
| 10 | 1,5-Bis(dimethylpiperidyl)-2,2-dimethylpentane | C21H42N2 | 322 | 5.82 | 3.26 |
| 11 | d-alanine, N-propargyloxycarbonyl-, decyl ester | C17H29NO4 | 311 | 5.14 | 3.26 |
| 12 | 2,2-Diethyl-N-ethylpyrrolidine | C19H33NO4 | 155 | 4.74 | 3.26 |
| 13 | l-alanine, n-propargyloxycarbonyl-, dodecyl ester | C19H33NO4 | 339 | 3.63 | 3.26 |
| 14 | N-Cyano-3-oxobutanamide | C5H6N2O2 | 126 | 3.21 | 3.26 |
| 15 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | C6H8O4 | 144 | 90.5 | 2.05 |
| 16 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl(CAS) | C6H8O4 | 144 | 90.5 | 2.05 |
| 17 | (2R*, 3R*)-2-butyl-3-hydroxy-3-phenylpropionoic acid ethyl ester | C15H22O3 | 250 | 0.34 | 2.05 |
| 18 | 2-n-Propylthiane | C8H16S | 144 | 0.26 | 2.05 |
| 19 | 5-Hydroxymethylfurfural | C6H6O3 | 126 | 75.99 | 12.5 |
| 20 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- (CAS) | C6H6O3 | 126 | 75.99 | 12.5 |
| 21 | 5-(hydroxymethyl)-2-Furanecarboxaldehyde | C6H6O3 | 126 | 20.71 | 12.5 |
| 22 | 5-Hydroxymethyl-2-furaldehyde | C6H6O3 | 126 | 75.99 | 12.5 |
| 23 | Thienylethanal | C6H6OS | 126 | 1.4 | 12.5 |
| 24 | 5-(hydroxymethyl)-2-(dimethoxymethyl)Furan | C8H12O4 | 172 | 65.9 | 2.41 |
| 25 | Oxiraneethanol, á-(1-ethoxyethoxy)-, [2R-[2R@[r@(R@)]]] | C9H14O3 | 170 | 8.04 | 2.41 |
| 26 | 4-Methoxymethoxy-4-methyl-hex-2-ynal | C9H14O3 | 170 | 8.04 | 2.41 |
| 27 | 2-(methoxycarbonylmethylidene)-5-Hydroxymethyltetrahydrofuran | C8H12O4 | 172 | 1.85 | 2.41 |
| 28 | 4-Hydroxylamino-6-methylpyrimidin-2(1H)-one | C5H7N3O2 | 141 | 1.56 | 2.41 |
| 29 | Methyl 4-chloro-2,2-dimethyl-4-pentenoate | C8H13ClO2 | 176 | 1.1 | 2.41 |
| 30 | 3-(hydroxymethyl)-9-Oxabicyclo [3.3.1] nonan-3-ol | C9H16O3 | 172 | 0.82 | 2.41 |
| 31 | Methanal, (5-methyl-3-isoxazolyl)amino-, oxime | C5H7N3O2 | 141 | 0.72 | 2.41 |
| 32 | Ethyl 4-hydroxy-3-methylbut-2-enoate | C7H12O3 | 144 | 87.65 | 1.1 |
| 33 | (E)-ethyl-4-hydroxy-3-methylcrotonate | C7H12O3 | 144 | 2.38 | 1.1 |
| 34 | Acetic acid, 2-methylhex-3-yl ester | C9H18O2 | 158 | 0.53 | 1.1 |
| 35 | 2,4,4-Trimethyl-2-pentyl-3-oxa-zolidinyloxy | C11H22NO2 | 200 | 0.45 | 1.1 |
| 36 | 4,5-Dihydro-2-methyl-5-(nitrimino)-1H-tetrazole | C2H4N6O2 | 144 | 0.27 | 1.1 |
| 37 | 1-Naphthyl n-propyl carbamate | C14H15NO2 | 229 | 0.26 | 1.1 |
| 38 | Glutaric acid, 2,4-dichlorobenzyl hexadecyl ester | C28H44Cl2O4 | 514 | 0.25 | 1.1 |
| 39 | Glutaric acid, 3,4-difluorobenzyl nonyl ester | C21H30F2O4 | 384 | 0.25 | 1.1 |
| 40 | Glutaric acid, 3-heptyl hexyl ester | C18H34O4 | 314 | 0.22 | 1.1 |
| 41 | Glutaric acid, decyl 2,5-difluorobenzyl ester | C22H32F2O4 | 398 | 0.22 | 1.1 |
| 42 | (−)-Hygroline | C8H17NO | 143 | 19.21 | 5.12 |
| 43 | dl-Proline, 5-oxo-, methyl ester | C6H9NO3 | 143 | 8.17 | 5.12 |
| 44 | (Z)-2-Pentenal | C5H8O | 84 | 7.22 | 5.12 |
| 45 | Methyl pyroglutamate | C6H9NO3 | 143 | 6.38 | 5.12 |
| 46 | l-Proline, 5-oxo-, methyl ester (CAS | C6H9NO3 | 143 | 6.38 | 5.12 |
| 47 | dl-Proline, 5-oxo-, methyl ester | C6H9NO3 | 143 | 8.17 | 5.12 |
| 48 | (+)-Sedridine [2-(2-hydroxypropyl) piperdine] | C8H17NO | 143 | 6.13 | 5.12 |
| 49 | rac-5-oxopyrrolidine-2-carbonsaure-methylester | C6H9NO3 | 143 | 4.45 | 5.12 |
| 50 | l-Proline, 5-oxo-, methyl ester | C6H9NO3 | 143 | 6.38 | 5.12 |
| 51 | Cyclohexanone, 2,3,4-trihydroxy-6-methyl-, [2S-(2à,3á,4á,6à)] | C7H12O4 | 160 | 10.49 | 1.05 |
| 52 | Guanosine (CAS) | C10H13N5O5 | 283 | 7.83 | 1.05 |
| 53 | 2-Amino-9-(3,4-dihydroxy-5-hydroxymethy l-tetrahydro-furan-2-yl)-3,9-dihydro-puri | C10H13N5O5 | 283 | 7.22 | 1.05 |
| 54 | (2s,3r,4r,6r)-2,3,4-trihydroxy-6-methylcyclohexanon | C7H12O4 | 160 | 10.49 | 1.05 |
| 55 | Xanthosine (CAS) | C10H12N4O6 | 284 | 5.67 | 1.05 |
| 56 | Guanosine (CAS) | C10H13N5O5 | 283 | 7.83 | 1.05 |
| 57 | à-d-Galactopyranoside, methyl 3,6-anhydro- (CAS) | C7H12O5 | 176 | 5.23 | 1.05 |
| 58 | 2-Deoxy-d-galactose | C6H12O5 | 164 | 4.83 | 1.05 |
| 59 | d-fructose, 1,3,6-trideoxy-3,6-epithio- (CAS) | C6H10O3S | 162 | 3.79 | 1.05 |
| 60 | 2-Cyclohexylpiperidine | C11H21N | 167 | 47.66 | 0.83 |
| 61 | à-Pyrrolidone, 5-[3-hydroxybutyl]- | C8H15NO2 | 157 | 47.66 | 0.83 |
| 62 | l-Serine, O-(phenylmethyl)- (CAS) | C10H13NO3 | 195 | 7.52 | 0.83 |
| 63 | 2-[p-chlorobenzyl]Piperidine | C12H16ClN | 209 | 3.2 | 0.83 |
| 64 | Formyl glutamine | C9H14N2O5 | 230 | 2.95 | 0.83 |
| 65 | 4-[Dichloromethyl]-2-[[2-[1-methyl-2-pyrrolidinyl]ethyl]a mino-6-trichloromethylpyrimidine | C13H17Cl5N4 | 404 | 2.26 | 0.83 |
| 66 | Tridecanedioic acid (CAS) | C13H24O4 | 244 | 1.92 | 0.83 |
| 67 | à-Methyl-l-sorboside | C7H14O6 | 194 | 88 | 3.97 |
| 68 | Methyl-à-d-fructopyranoside | C7H14O6 | 194 | 7.92 | 3.97 |
| 69 | 2-Methylacetophenone-dioxolane | C11H14O2 | 178 | 0.51 | 3.97 |
| 70 | d-glucose (CAS) | C6H12O6 | 180 | 0.2 | 3.97 |
| 71 | á-d-Glucopyranose, 4-O-á-d-galactopyranosyl | C12H22O11 | 342 | 0.19 | 3.97 |
| 72 | Isopropyl-á-d-thiogalactopyranoside | C9H18O5S | 238 | 0.15 | 3.97 |
| 73 | 4′-Methylphenyl-1C-sulfonyl-á-d-galactoside | C13H18O7S | 318 | 0.15 | 3.97 |
| 74 | Ethyl-1-thio-á-d-glucopyranoside | C8H16O5S | 224 | 0.14 | 3.97 |
| 75 | Galactopyranodise, 1-deoxy-1-undecylthio | C17H34O5S | 350 | 0.1 | 3.97 |
| 76 | 2-Octenoic acid, 4,5,7-trhydroxy | C8H14O5 | 190 | 9.79 | 1.26 |
| 77 | Desulphosinigrin | C10H17NO6S | 279 | 8.65 | 1.26 |
| 78 | 2-Octenoic acid, 4,5,7-trhydroxy | C8H14O5 | 190 | 8.32 | 1.26 |
| 79 | 2-d,2-pentadecyl-1,3-dioxepane | C20H39DO2 | 312 | 6.7 | 1.26 |
| 80 | 2-Acetylamino-3-hydroxy-propionic acid | C5H9NO4 | 147 | 5.4 | 1.26 |
| 81 | 2-acetylamino-3-hydroxy-propionic acid | C5H9NO4 | 147 | 4.56 | 1.26 |
| 82 | 2-Hydroxyhexadecyl butanoate | C20H40O3 | 328 | 3.58 | 1.26 |
| 83 | 2-[(N,N-Dimethylamino)methyl]-4-fluorophenol | C9H12FNO | 169 | 38.97 | 0.49 |
| 84 | 1-Isobutyl-7,7-dimethyl-hexahydro-isobenzofuran-3a-ol | C14H26O2 | 226 | 5.55 | 0.49 |
| 85 | Hydrazinecarboxamide, 2-(2-methylcyclohexylidene)- | C8H15N3O | 169 | 38.97 | 0.49 |
| 86 | 1,3-Diethyl-1,3,3a,5,6,6a-hexahydrocyclopenta[c]thiophen -4-one | C11H18OS | 198 | 3.92 | 0.49 |
| 87 | 2-Furoic acid, bromomethyldimethylsilyl ester | C8H11BrO3Si | 262 | 3.16 | 0.49 |
| 88 | 2-Furancarboxylic acid, tert-butyldimethylsilyl ester | C11H18O3Si | 226 | 2.35 | 0.49 |
| 89 | Hydrazinecarboxamide, 2-(2-methylcyclohexylidene)(CAS) | C8H15N3O | 169 | 4.25 | 0.49 |
| 90 | 3-Furoic acid, benzyldimethylsilyl ester | C14H16O3Si | 260 | 2.08 | 0.49 |
| 91 | 2-Furoic acid, (3-cyanopropyl)dimethylsilyl ester | C11H15NO3Si | 237 | 2.93 | 0.84 |
| 92 | Chimanine D | C12H11NO | 185 | 39.01 | 0.55 |
| 93 | Methyl 5-(N-Hydroxy)carboximidamido-2-thiophenecarboxylate | C7H8N2O3S | 200 | 29.88 | 0.55 |
| 94 | Octadecanoic acid, 2,3-dihydroxypropyl ester (CAS) | C21H42O4 | 358 | 6.81 | 0.55 |
| 95 | 2-[5-(2-Hydroxy-propyl)-tetrahydrofuran-2-yl]-propionic acid, t-butyl ester | C14H26O4 | 258 | 1.7 | 0.55 |
| 96 | à-D-Glucopyranoside | C20H34O9 | 418 | 1.17 | 0.55 |
| 97 | 1-allyl-2,3-5,6-tetra-o-acetyl-mannofura noside | C17H24O9 | 372 | 1.12 | 0.55 |
| 98 | Mannofuranoside, 1-allyl-2,3-5,6-tetra-O-acetyl | C17H24O9 | 372 | 1.12 | 0.55 |
| 99 | 9-Hexadecenoic acid, methyl ester, (Z)- (CAS) | C17H32O2 | 268 | 37.1 | 0.79 |
| 100 | Methyl hexadec-9-enoate | C17H32O2 | 268 | 26.94 | 0.79 |
| 101 | Pentadecanoic acid, 14-methyl-, methyl ester (CAS) | C17H34O2 | 270 | 13.97 | 5.54 |
| 102 | Hexadecanoic acid (CAS) | C16H32O2 | 270 | 54.59 | 5.54 |
| 103 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 652 | 14.07 | 5.22 |
| 104 | 9-Octadecenoic acid (Z)- (CAS) | C18H34O2 | 282 | 4.71 | 5.22 |
| 105 | Pentadecanoic acid | C15H30O2 | 242 | 3.8 | 5.22 |
| 106 | Elaidinsaeure methyl ester | C19H36O2 | 296 | 7.69 | 14.89 |
| 107 | Methylelaidate | C19H36O2 | 296 | 19.42 | 14.89 |
| 108 | cis-vaccenic acid | C18H34O2 | 282 | 16.52 | 12.28 |
| 109 | Oleic acid | C18H34O2 | 282 | 4.9 | 12.28 |
| 110 | Heptadecene-(8)-carbonic acid-(1) | C18H34O2 | 282 | 3.16 | 12.28 |
| 111 | Octadecanoic acid, 2-(2-hydroxyethoxy)ethyl ester | C22H44O4 | 372 | 4.87 | 0.57 |
| 112 | Octadecanoic acid (CAS) | C18H36O2 | 284 | 58.57 | 0.57 |
| 113 | Tricosane | C23H48 | 324 | 11.2 | 0.83 |
| 114 | Eicosane | C20H42 | 282 | 7.46 | 0.83 |
| 115 | Pentacosane | C25H52 | 352 | 7.17 | 0.83 |
| 116 | Heneicosane | C21H44 | 296 | 6.33 | 0.83 |
| 117 | Hexatriacontane | C36H74 | 506 | 6.09 | 0.83 |
| 118 | Nonadecane | C19H40 | 268 | 5.38 | 0.83 |
| 119 | Docosane | C22H46 | 310 | 5.38 | 0.83 |
| 120 | Pentatriacontane | C35H72 | 492 | 5.17 | 0.83 |
| 121 | Triacontane | C30H62 | 422 | 4.36 | 0.83 |
| 122 | 1-Heptacosanol | C27H56O | 396 | 6.62 | 1.94 |
| 123 | n-Tetracosanol-1 | C24H50O | 354 | 5.6 | 1.94 |
| 124 | 1-Heneicosanol | C21H44O | 312 | 4.94 | 1.94 |
| 125 | Z-12-Pentacosene | C25H50 | 350 | 4.75 | 1.94 |
| 126 | 9-Hexacosene | C26H52 | 364 | 3.54 | 1.94 |
| 127 | 10-Heneicosene (c,t) | C21H42 | 294 | 3.27 | 1.94 |
| 128 | 9-Tricosene, (Z)- | C23H46 | 322 | 3.27 | 1.94 |
| 129 | n-Nonadecanol-1 | C19H40O | 284 | 2.89 | 1.94 |
| 130 | 1-Heneicosyl formate | C22H44O2 | 340 | 2.27 | 1.94 |
| 131 | Octacosane (CAS) | C28H58 | 394 | 5.4 | 1.38 |
| 132 | 9-Octadecenamide | C18H35NO | 281 | 18.5 | 0.51 |
| 133 | cis-13-Eicosenoic acid | C20H38O2 | 309 | 17.07 | 0.51 |
| 134 | 2-Hexadecanol | C16H34O | 242 | 8.48 | 0.74 |
| 135 | 17-Pentatriacontene | C35H70 | 490 | 5.81 | 0.74 |
| 136 | cis-10-nonadecenoic acid | C19H36O2 | 296 | 3.73 | 0.74 |
| 137 | cis-11-Eicosenoic acid | C20H38O2 | 310 | 3.44 | 0.74 |
| 138 | Erucic acid | C22H42O2 | 338 | 3.04 | 0.74 |
| 139 | 1-[(4′á)-3′-Ethylenedioxy-18′-norkaur-15′-en-17′-yl]pyrroli dine | C25H39NO2 | 385 | 51.45 | 4.63 |
| 140 | 3á-(Peroxymethyl)-5-vinyl-A,B-bisnor-5á-cholestane | C28H48O2 | 416 | 15.13 | 4.63 |
| 141 | 2-Allyl-6-(1,1-dimethylpropyl)-3-n-pentadecylphenol | C29H50O | 414 | 9.17 | 4.63 |
| 142 | (2S,3S)-2,3-Isopropylidenedioxy-4-tosyloxybutan-1-yl tetrahydropyran ether | C19H28O7S | 400 | 6.1 | 4.63 |
| 143 | 3-O-(trimethylsilyl)-5,7,4′-tri-O-methylkaempferol | C21H24O6SI | 400 | 1.4 | 4.63 |
| 144 | N,N-Diethyl-1,3-dihydro-1-oxo-3,3-diphenyl-5-isobenzo-f urancarboxamide | C25H23NO3 | 385 | 0.59 | 4.63 |
| 145 | 13-Docosenamide | C22H43NO | 337 | 60.05 | 6.91 |
| 146 | Squalene | C30H50 | 410 | 10.15 | 0.63 |
| 147 | trans-Geranylgeraniol | C20H34O | 290 | 4.62 | 0.63 |
| 148 | Methyl trisporate C | C19H28O4 | 320 | 6.92 | 2.03 |
| 149 | 2-Cyclohexene-1-carboxylic acid | C19H28O4 | 3200 | 6.92 | 2.03 |
| 150 | Thalmiculimine | C37H38N2O7 | 622 | 3.1 | 2.03 |
| 151 | Bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propyl] maleate | C38H56O6 | 608 | 2.43 | 2.03 |
Fig. 1.
GC–MS chromatogram of A. bilimbi L. fruit extract performed in the THERMO GC—TRACE ULTRA VER: 5.0, THERMO MS DSQ II machine. Non-polar column DB 5-MS capillary standard, helium gas as a carrier, with an injection volume of 1 μl was used
Table 3.
Major compounds identified by GC–MS in the A. bilimbi L. fruit extract, reported to have biological activity and cited in PUBMED
| S. no | Compound | Activity |
|---|---|---|
| 1 | Hexadecanoic acid, ethyl ester | Antioxidant, hypocholesterolemic nematicide, pesticide, anti-androgenic flavour, hemolytic, 5-alpha reductase inhibitor (Kumar et al. 2010) |
| 2 | Squalene | Chemo-preventive against colon cancer (Rao and Harold 1998) |
| 3 | Erucic acid | X-linked adrenoleukodystrophy (Rizzo et al. 1989) |
| 4 | Oleic acid | Reduce blood pressure (Teres et al. 2008) |
| 5 | Chimanine D | Antileishmanial (Fournet et al. 1993) |
| 6 | Boronic acid | Potential pharmaceutical agent (selective reduction of aldehydes, enzyme inhibitors, asymmetric synthesis of amino acids) (Yang 2003) |
| 7 | 5-Hydroxymethyl furfural | Against sickle cell anaemia (Lin et al. 2008) |
| 8 | Mannitol | Used for acute traumatic brain injury (Wakai et al. 2013) |
| 9 | Desulphosinigrin | Antibacterial (Sabreen et al. 2015) |
| 10 | Methyl Pyroglutamate | Antibiotic preparation. Smith 1997 in the book Alkaloids: Chemical and Biological Perspectives Chapter 4: Pyroglutamate as a Chiral Template for the Synthesis of Alkaloids |
Total phenolic content
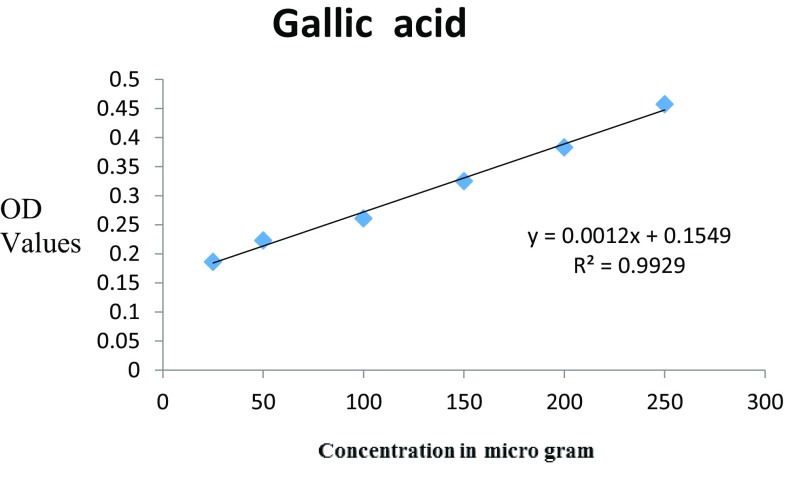
The total phenolic content of the A. bilimbi L. fruit extract was expressed as gallic acid equivalent in milligram per gram (GAE mg/g) of methanolic fruit extract. The optical density values and its straight line equation (y = mx + c) of standard gallic acid is shown in Fig. 2. The total phenolic content for 250 µg/ml is found to be 209.25 GAE mg/g.
Fig. 2.
The total phenolic content of A. bilimbi L. fruit extract for 250 µg/ml is 209.25 GAE mg/g
Nitric oxide (NO) scavenging activity
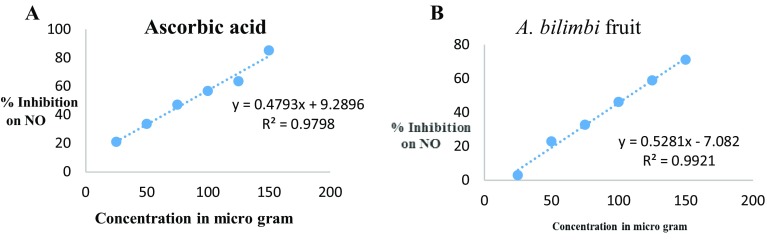
The A. bilimbi L. fruit extract showed an increased nitric oxide scavenging activity with increase of concentration of the extract. Ascorbic acid is used as a standard for determining the IC50 value. Decreased OD values were observed when the concentration of fruit extract increased. The percentage of inhibition is shown in Table 4 and the regression curve for the standard ascorbic acid and A. bilimbi L. extract is shown in Fig. 3, respectively. The IC50 value of A. bilimbi L. fruit extract and standard ascorbic acid was found to be 108.10 and 85.01 which is shown in Table 4.
Table 4.
Percentage inhibition of A. bilimbi L. fruit extract on nitric oxide and its comparison with that of standard ascorbic acid. The IC50 of ascorbic acid is 85.01 and IC50 of A. bilimbi L. extract is 108.10
| S. no | Concentration (µg) | Ascorbic acid % inhibition on nitric oxide | A. bilimbi L. fruit extract % inhibition on nitric oxide |
|---|---|---|---|
| 1 | 25 | 21.10 ± 0.84 | 2.95 ± 0.88 |
| 2 | 50 | 33.67 ± 0.87 | 22.9 ± 1.90 |
| 3 | 75 | 47.15 ± 1.89 | 32.70 ± 1.60 |
| 4 | 100 | 56.74 ± 1.22 | 46.23 ± 1.56 |
| 5 | 125 | 63.55 ± 4.67 | 58.84 ± 1.84 |
| 6 | 150 | 85.14 ± 1.17 | 71.09 ± 2.67 |
| IC50 | 85.01 | 108.10 |
Fig. 3.
A Percentage inhibition of standard (ascorbic acid) at different concentrations on NO B Percentage inhibition of A. bilimbi L. fruit extract at different concentrations on NO
Discussion
Traditionally 6000 plants are used in Indian folk and herbal medication and 3000 plants are in documented medicine used against diseases (Rajshekharan 2002). Their medicinal value is due to the presence of phytochemicals. Phytochemicals are also called as natural products, plant constituents, and secondary metabolites which have medicinal properties to which they belong and the mechanism of action was not known up to the extent. These phytochemicals have great potentialities in drug discovery for various diseases (Justin et al. 2014). The phytochemicals like alkaloid, carbohydrate, glycosides, phenols, flavonoids, saponins, steroids, and tannins compounds are remedy to cure diseases and fight against different kinds of pathogens, as medicine (Hassan et al. 2004). In the current investigation, we have revealed the presence of phytochemicals (alkaloids, carbohydrate, phenols, flavonoids, saponins and tannins) in the A. bilimbi L. fruit extract. Our result on phytochemical presence is consistent with an earlier study (Hasanuzzaman et al. 2013). Moreover, there may be a region-wise difference in the presence of phytochemicals in any plant. Gas chromatographic–mass spectrometry (GC–MS) is a ubiquitous analytical technique of choice in toxicology, environmental research, food science and forensic research. A. bilimbi L. fruit extract was separated by GC and the compounds were identified by the MS by the NIST and Wiley 9.0 libraries. GC–MS analysis revealed the presence of major biologically active compounds (4H-pyran-4-one, 2,3-dihydro3,5-dihydroxy-6-methyl, hexadecanoic acid, squalene, erucic acid, oleic acid, chimanine D, boronic acid, 5-hydroxymethyl furfural, 2-deoxy-d-galactose, mannitol, desulphosinigrin, methyl pyroglutamate) having medicinal important as given in Table 3. We have not identified any steroid compounds in GC–MS report which correlates well with the results of phytochemical screening. Total phenolic compounds present in the A. bilimbi L. fruit extract were determined by Folin–Ciocalteu method. We have also observed a high level of total phenolic compounds in the A. bilimbi L. fruit extract at a concentration of 209.25 GAE mg/g. Presence of phenolic compound apparently explains the antioxidant nature of the plant (Awika et al. 2003) due to its hydroxyl group which have the scavenging activity (Hatano et al. 1989). More and more phenolic compounds are used in foods to improve the nutritional quality (Kahkonen et al. 1999). The presence of benzenoid ring (hydrophobic) and hydrogen bonding in phenolic hydroxyl groups will help in interacting with the proteins, accounting for its potent nature to act as antioxidants (Parr and Bolwel 2002). Free radicals possess high reactive nature; they attack nearest stable molecules like lipids, proteins, DNA and carbohydrates by sneaking their electrons (Patil et al. 2013). Different forms of free radicals are reactive oxygen species (ROS) and reactive nitrogen species (RNS). Antioxidants are the molecules that scavenge free radicals. They safeguard the cell components from the free radicals (Shenoy and Shirwaikar 2002) by scavenging the free radicals by scavenging the ROS and RNS (Rozina et al. 2012). NO is one of the abundant free radicals categorized under RNS. It is a highly reactive nitrogen species formed during inflammations, capable of damaging proteins, lipids and DNA (Valko et al. 2007). Synthetic antioxidants like butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT) and tertiary butyl hydroquinone are used in food supplements. They are used to treat numerous human diseases, but these compounds have toxic effects (Kombo 2000). Plants are the natural sources for the antioxidants they possess high quantity and quality of antioxidants which can scavenge the free radicals (Wang et al. 1996). In the present investigation, the antioxidant activity for A. bilimbi L. fruit extract is proven from its high level of NO scavenging activity similar to the standard ascorbic acid used in our study. The IC50 value of nitric oxide is 85.01, whereas the IC50 value of ascorbic acid is 108.10. Furthermore, boronic acid identified in our GC–MS analysis was reported to have nitric oxide scavenging activity (Yang et al. 2003). This may be one of the reasons for the significant nitric oxide scavenging activity observed in this study for A. bilimbi L. fruit extract.
Conclusion
From the above study, it is apparent that the A. bilimbi L. fruit extract is a rich source of phytochemicals (natural products) with biological activity. The GC–MS report on this fruit proves that natural products have pharmacologically and biologically active compounds. A high phenolic content is observed in our study. A. bilimbi L. fruit extract is also found to have NO scavenging activity in our study.
Acknowledgements
The authors are thankful to the Department of Biosciences and Technology, Karunya University and The South Indian Textile Research Association (SITRA), Coimbatore.
Compliance with ethical standards
Conflict of interest
The author declares that there is no conflict of interest.
Contributor Information
Jagadish Kumar Suluvoy, Email: jagadishbabu77@gmail.com.
V. M. Berlin Grace, Phone: +91-9894051175, Email: berlsdg@gmail.com, Email: berlin@karunya.edu
References
- Abdul Rahman ZS. Herb and Perubatan. Kuala Lumpur: Zebra publications; 2003. p. 2. [Google Scholar]
- Asna NA, Noriham A. Antioxidant activity and bioactive components of oxalidaceae fruit extracts. Malays J Anal Sci. 2014;18(1):116–126. [Google Scholar]
- Awika JM, RooneyL W, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51:57–62. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Baygutalp F, Baygutalp NK, Ozturk N, Ugur M, Baykal T, Seferoglu B, Askin S. evaluation of circulating nitric oxide levels in patients with complex regional pain syndrome type 1. Int J Med Pharm. 2015;3(2):33–40. [Google Scholar]
- Chauhan JB, Kapfo W. Effect of Traditional Sun-drying on Phenolic Antioxidants of Averrhoa bilimbi L. Int J Appl Biol Pharm Technol. 2013;4(2):26–34. [Google Scholar]
- Cho CH. Current roles of nitric oxide in gastrointestinal disorders. J Physiol-Paris. 2001;95:253–256. doi: 10.1016/S0928-4257(01)00034-1. [DOI] [PubMed] [Google Scholar]
- Chowdhury SS, Uddin MG, Nazia G, Hossain M, Hasan SR. In-vitro antioxidant and cytotoxic potential of hydromethanolic extract of Averrhoa bilimbi fruits. IJPSR. 2012;3(07):2263–2268. [Google Scholar]
- Das S, Sultana S. Antibacterial and cytotoxic activities of methanolic extracts of leaf and fruit parts of the plant Averrhoa bilimbi (Oxalidaceae) Am J Sci Ind Res. 2011;2:531–536. [Google Scholar]
- Edeoga HA, Okwu DE, Mbaebie BO. Phytochemical constituent of some Nigerian medicinal plants. Afr J Biotech. 2005;4(7):685–688. doi: 10.5897/AJB2005.000-3127. [DOI] [Google Scholar]
- Erdmana SE, Rao VP, Poutahidisa T, Rogersa AB, Taylora CL, Jackson EA, Ge Z, Lee CW, Schauera DB, Wogan GN, Tannenbaumc SR, Foxa JG. Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. PNAS. 2009;106(4):1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;1(109):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. eCAM. 2001;4(S1):37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet A, Barrios AA, Victoria M, Reynald H, Andre C, Jean B. 2-Substituted quinoline alkaloids as potential antileishmanial drugs. Antimicrob Agents Chemother. 1993;37:859–863. doi: 10.1128/AAC.37.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman AE, RiedK M, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. And Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Phytochemical methods: a guide to modern technique of plant analysis. London: Chapman & Hall; 1973. [Google Scholar]
- Harun NH, Septama AW, Jantan I. Immunomodulatory effects of selected Malaysian plants on the CD18/11a expression and phagocytosis activities of leukocytes. Asian Pac J Trop Biomed. 2014;5(1):48–53. doi: 10.1016/S2221-1691(15)30170-2. [DOI] [Google Scholar]
- Hasanuzzaman M, Ramjan AM, Marjan H, Sourov K, Mohammad S. Evaluation of total phenolic content, free radical scavenging activity and phytochemical screening of different extracts of Averrhoa bilimbi (fruits) Int Curr Pharm J. 2013;2(4):92–96. doi: 10.3329/icpj.v2i4.14058. [DOI] [Google Scholar]
- Hassan MM, Oyewale AO, Amupitan JO, Abduallahi MS, Okonkwo EM. Preliminary phytochemical and antibacterial investigation of crude extracts of the root bark of Detariummicrocarpum. J Chem Soc Nigeria. 2004;29:26–29. [Google Scholar]
- Hatano T, Edamatsu R, Mori A, Fujita Y, Yasukara T, Yoshida T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on 2,20-diphenylpicrylhydrazyl. Chem Pharm Bull. 1989;37:2016–2021. doi: 10.1248/cpb.37.2016. [DOI] [Google Scholar]
- Joyce DA. Oxygen radicals in disease. Adv Drug Reac Bull. 1987;127:476–479. doi: 10.1097/00012995-198712000-00001. [DOI] [Google Scholar]
- Kabera JN, Semana E, Mussa AR, He X. Plant Secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol. 2014;2:377–392. [Google Scholar]
- Kadhirvel K, Ramya S, Sudha TPS, Ravi AV, Rajasekaran C, Selvi RV, Jayakumararaj R. Ethnomedicinal survey on plants used by tribals in Chitteri hills. Environ Int J Sci Tech. 2010;5:35–46. [Google Scholar]
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agri Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kamboj VP. Herbal medicine. Curr Sci. 2000;78:35–39. [Google Scholar]
- Karon B, Ibrahim M, Ayeasha M, Huq MM, Chowdhury MMU, Hossain MA, Rashid MA. Preliminary antimicrobial, cytotoxic and chemical investigations of Averrhoa bilimbi linn And Zizyphus mauritiana Lam. Bangladesh Pharm J. 2011;14(2):127–131. [Google Scholar]
- Kumar A, Ilavarasn R, Jayachandran T, Decaraman M, Aravindhan P, Padmanaban N. Phytochemical investigation on tropical plant. Pakistan J Nutr. 2009;8(1):83–85. doi: 10.3923/pjn.2009.83.85. [DOI] [Google Scholar]
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC–MS study of Vitexnegundo. African J Biochem Res. 2010;4(7):191–195. [Google Scholar]
- Kumar AK, Gousia SK, Anupama M, Latha JNL. A review on phytochemical constituents and biological assays of Averrhoa bilimbi. Int J Pharm Pharm Sci Res. 2013;3(4):136–139. [Google Scholar]
- Lin AS, Qian K, Usami Y, Lin L, Itokawa H, Hsu C, Morris-Natschke SL, Lee KH. 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed Rehmanniae Radix. J Nat Med. 2008;62:164–167. doi: 10.1007/s11418-007-0206-z. [DOI] [PubMed] [Google Scholar]
- Nazmul MHM, Salmah I, Syahid A, Mahmood A. In-vitro screening of antifungal activity of plants in Malaysia. Biomed Res. 2011;22(1):28–30. [Google Scholar]
- Nisa S, Bibi Y, Waheed A, Zia M, Sarwar S, Ahmed S, Chaudhary FM. Evaluation of anticancer activity of Debregeasia salicifolia extract against estrogen receptor positive cell line. Afr J Biotech. 2011;10:990–995. [Google Scholar]
- Nostro A, Germano MP, D’Angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 2000;30:379–384. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Odebiyi OO, Sofowora EA. Phytochemical screening of Nigerian medicinal plant II. Lloydia. 1978;41:234–246. [PubMed] [Google Scholar]
- Parr AJ, Bolwel JP. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agric. 2002;80:985–1012. doi: 10.1002/(SICI)1097-0010(20000515)80:7<985::AID-JSFA572>3.0.CO;2-7. [DOI] [Google Scholar]
- Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Jolly CI, Narayanan S. Free radical scavenging activity of acacia catechu and Rotulaaquatica: implications in cancer therapy. Indian Drugs. 2013;40:328–332. [Google Scholar]
- Pushparaj P, Tan CH, Tan BKH. Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol. 2000;72:69. doi: 10.1016/S0378-8741(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Rajshekharan PE. Herbal medicine. World Sci Employ News. 2002;3(4):21–27. [Google Scholar]
- Rahman MM, Habib MR, Hasan MA, Al Amin M, Saga A, Mannan A. Comparative assessment on in vitro antioxidant activities of ethanol extracts of Averrhoa bilimbi, Gymnema sylvestre and Capsicum frutescens. Pharmacognosy Res. 2014;6(1):36–41. doi: 10.4103/0974-8490.122915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VC, Newmark HL, Reddy BS. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19(2):287–290. doi: 10.1093/carcin/19.2.287. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G. Functional foods-nutrition of the future demarketingstrategy. Res Report Special Issue. 2001;1:12–15. [Google Scholar]
- Rizzo WB, Leshner RT, Odone A, Dammann AL, Craft DA, Jensen ME, Jennings SS, Davis S, Jaitly R, Sgro JA. Dietary erucic acid therapy for X-linked adrenoleukodystrophy. Neurology. 1989;39(11):1415–1422. doi: 10.1212/WNL.39.11.1415. [DOI] [PubMed] [Google Scholar]
- Robin EH, Anril W, Alexander M, Loeto M, Keith K. Nasopharyngeal carriage and antimicrobial resistance in isolates of Streptococcus pneumoniae and Haemophilus influenzae Type b in children under 5 years of age in Botswana. Int J Infect Dis. 1998;3(1):18–25. doi: 10.1016/S1201-9712(98)90090-X. [DOI] [PubMed] [Google Scholar]
- Rozina P, Sukalayan KK, Saha P. In vitro nitric oxide scavenging activity of methanol extracts of three Bangladeshi medicinal plants. The Pharma Innov. 2012;1(12):83–88. [Google Scholar]
- Sabreen AK, Lena FH, Hameed IH. Antibacterial activity of secondary metabolites isolated from Alternariaalternate. Afr J Biotechnol. 2015;14(43):2972–2994. doi: 10.5897/AJB2015.14906. [DOI] [Google Scholar]
- Sakthivel KM, Guruvayoorappan C. Acacia ferruginea inhibits tumor progression by regulating inflammatory mediators-(TNF-a, iNOS, COX-2, IL-1ß, IL-6, IFN-γ, IL-2, GM-CSF) and pro-angiogenic growth factor VEGF. Asian Pacific J Cancer Prev. 2013;14(6):3909–3919. doi: 10.7314/APJCP.2013.14.6.3909. [DOI] [PubMed] [Google Scholar]
- Savithri A, Subramoniam A, Nagarajan NS. Studies on the antihyperlipidemic properties of Averrhoa bilimbi Fruit in Rats. Planta Med. 2009;75(1):55–58. doi: 10.1055/s-0028-1088361. [DOI] [PubMed] [Google Scholar]
- Shenoy R, Shirwaikar A. Anti-inflammatory and free radical scavenging studies of Hyptissuaveolens (labiatae) Indian Drugs. 2002;39:574–577. [Google Scholar]
- Singh M, Chaudhry MA, Yadava JNS, Sanyal SC. The spectrum of antibiotic resistance in human and veterinary isolates of Escherichia coli collected from 1984–1986 in Northern India. J Antimicrob Chemother. 1992;29:159–168. doi: 10.1093/jac/29.2.159. [DOI] [PubMed] [Google Scholar]
- Singh D, Mishra M, Gupta M, Singh P, Gupta A, Nema R. Nitric oxide radical scavenging assay of bioactive compounds present in methanol extract of Centellaasiatica. Int Journal Pharm Pharm Sci Res. 2012;2(3):42–44. [Google Scholar]
- Singh DR, Singh S, Salim KM, Srivastava RC. Estimation of phytochemicals and antioxidant activity of underutilized fruits of Andaman Islands (India) Int J Food Sci Nutr. 2012;66:446–452. doi: 10.3109/09637486.2011.634788. [DOI] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- Smith MB. Pyroglutamate as a chiral template for the synthesis of alkaloids. Alkaloids Chem Biol Perspect. 1997;4(41):230–279. [Google Scholar]
- Sofowora A. Medicinal plants and traditional medicines in Africa. 5. New York: Wiley; 1993. pp. 97–145. [DOI] [PubMed] [Google Scholar]
- Teres S, Barcelo-Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE, EscribaP V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. PNAS. 2008;105(37):13811–13816. doi: 10.1073/pnas.0807500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trease GE, Evans WC (2002) Pharmacognosy. 15th Ed. Saunders Publishers, London. pp.42-44, 221-229, 246-249, 304-306, 331-332, 391-393
- Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Coccinia Grandis L. leaf extract. Afr J Trad Comp Alter Med. 2008;5:61–73. [PMC free article] [PubMed] [Google Scholar]
- Vaijanathappa J, Badami S, Bhojraj S. In vitro antioxidant activity of Enicostemma axillare. J Health Sci. 2008;54(5):524–528. doi: 10.1248/jhs.54.524. [DOI] [Google Scholar]
- Valko D, Leibfritz J, Moncol MTD, Cronin M, Telser MJ. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wakai A, Robert MA, Schierhout GI. Mannitol for acute traumatic brain injury (Review) The Cochrane Collaboration. 2013 [Google Scholar]
- Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44:701–705. doi: 10.1021/jf950579y. [DOI] [Google Scholar]
- Wargovich MJ. Anticancer properties of fruits and vegetables. HortScience. 2000;35:573–575. [Google Scholar]
- Xavier TF, Kannan M, Lija L, Auxillia A, Rose AK, Kumar SS. Ethnobotanical study of Kani tribes in Thodu hills of Kerala. South India J Ethnopharmacol. 2014;152:78–90. doi: 10.1016/j.jep.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Yadav RNS, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3(12):10–14. [Google Scholar]
- Yang W, Gao X, Wang B. Boronic acid compounds as Potential Pharmaceuticals Agents. Med Res Rev. 2003;23(3):346–368. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- Zakaria ZA, Zaiton H, Henie EFP, MatJais AM, Engku Zainuddin ENH. In vitro antibacterial activity of Averrhoa bilimbi L. leaves and fruits extracts. Int J Trop Med. 2007;2:96–100. [Google Scholar]