Abstract
Growing attention is now being given to the possible preventive/alternative ways to avoid illness onset. Changes in lifestyle and food habits are taking over from the conventional pharmaceutical‐based approach, especially for chronic pathologies. Nutraceuticals have been proposed as key tools for the prevention and cure of some pathological conditions. This is leading research to develop new formulations based on these pharma‐foods addressed in a specific way to prevent and cure health issues, which, in turn, will have an effect on therapy‐related costs sustained by any National Health Organization. According to existing regulations, nutraceuticals cannot be categorized as either food or drugs but, by definition, often inhabit a grey area in between the two, being assimilated into food supplements, notwithstanding the beneficial properties that they can provide for some pathological conditions. A nutraceuticals‐based approach for health management, in particular for some pathological conditions, has resulted in a worldwide growing ‘nutraceutical’ revolution. An outstanding example is the approach to the ‘metabolic syndrome’, which includes overweight, obesity and cardiovascular‐related diseases, causing a sort of cascade of chronic health conditions, which is becoming a norm in modern life. Hypercholesterolaemia is one of these. It represents an example of a pathology that can be linked to both a poor lifestyle and dietary habits. The nutraceutical approach to hypercholesterolaemia is described in the present review as a possible alternative to the conventional drug‐based therapy.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- CYP7A1
cholesterol 7 α‐hydroxylase also known as cholesterol 7‐α‐monooxygenase
- EFSA
European Food Safety Authority
- FDA
Food and Drugs Administration
- HepG2
hepato carcinoma cell lines
- HDL‐C
HDL cholesterol
- HMG‐CoA
hydroxymethylglutaryl CoA
- LDL‐C
LDL cholesterol
- OTC
over the counter products
- PCSK9
proprotein convertase subtilisin/kexin type 9
- SREBP
sterol regulatory element‐binding proteins
- TC
total cholesterol
Tables of Links
| LIGANDS |
|---|
| Cholesterol |
| HMG‐CoA |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Nutraceutical, is a term coined in 1989 by Stephen DeFelice, and combines the words ‘nutrient’ (a nourishing food or food component) with ‘pharmaceutical’ (a medical drug), and indicates that these products have a potential application in pathological conditions, and hence should be treated in a similar way to pharmaceuticals (Colonna et al., 2013). According to the original definition, they are ‘a food or part of a food that provides medical or health benefits, including the prevention and/or treatment of disease’. However, the term nutraceutical is often used in a misleading and overlapping way with regard to dietary supplements, functional foods, prebiotics, probiotics and/or herbal products. Table 1 presents the accepted definitions of nutraceutical used in the publication of results, together with the commonly accepted definitions of functional food, dietary supplements and phytochemicals (herbal products).
Table 1.
Definitions accepted for nutraceuticals and dietary/food supplements
| Nutraceutical | Food or part of food that provides medical or health benefits, including the prevention and/or treatment of a disease. | DeFelice 1995 |
| Nutraceutical | A diet supplement that delivers a concentrated form of a biologically active component of food in a nonfood matrix to enhance health. | Zeisel 1999 |
| Nutraceutical | Definition 1: a specially treated food, vitamin, mineral, herb etc, that you eat or drink in order to improve your health Definition 2: a foodstuff (as a fortified food or dietary supplement) that provides health benefits in addition to its basic nutritional. | Merriam‐Webster Online Dictionary 2014 |
| Dietary supplement | A product (other than tobacco) in the form of a capsule, powder, softgel or gelcap intended to supplement the diet to enhance health that bears or contains one or more of the following dietary ingredients: a vitamin, mineral, amino acid, or other botanical or dietary substance. | Dietary Supplement Health and Education Act (DSHEA), 1994; Merriam Webster Dictionary 2014. |
| Phytochemical | Substances found in edible fruit and vegetables that can be ingested daily (in quantities of g) by humans and that exhibit a potential to favourably modulate human metabolism to prevent cancer and other diseases (isoflavones, resveratrol, garlic allyl sulphides, tomato lycopene, onion quercetin, etc.). | Bloch and Thomson 1995 |
| Functional food | Nutrient consumed as part of a normal diet but delivering one or more active ingredients (that have physiological effects and may enhance health) within the food matrix. | Zeisel 1999 |
| Functional food | Any food or ingredient that has a positive impact on an individual's health, physical performance or state of mind, in addition to its nutritive value. | Hardy 2000 |
The current European regulations consider nutraceuticals as belonging to the same category as food supplements. The Directive 2002/46/EC on food supplements and novel foods, recently modified by the new European Parliament and Council Regulation (EU) 2015/2283, which defines new foods categories, completes the classification of food supplements, but it still does not mention the term ‘nutraceutical’. Article 3 (par. iv) of this regulation seems to be potentially appropriate to assess the identity of nutraceuticals, since they are extracts from vegetal or animal foodstuff matrices, and have also the added value of possessing beneficial properties for health. The Dietary Supplement Health and Education Act (DSHEA 1994) defines dietary supplements as a category of food. According to the Food and Drugs Administration (FDA) a ‘dietary supplement’ is a product intended to supplement one or more nutrients, with the aim of increasing their total daily intake (Zeisel 1999). A ‘functional food’ is instead defined as a food product to be taken as a part of the usual diet in order to have beneficial effects that go beyond basic nutritional needs. Functional foods can be enriched with ingredients (micronutrients) that are not usually present in a particular food, or contain a larger than usual amount of a specific nutrient.
Based on the definitions reported in Table 1, nutraceuticals, as per their definition, differ from dietary supplements and other food supplements notwithstanding the fact that the form they are administered as can be the same (e.g. pills, tablets, capsules or liquid). The main differences can be summarized as follows: (i) nutraceuticals do not only supplement the diet but also help and/or assist the prevention and/or treatment of some diseases and health issues; (ii) nutraceuticals are intended for use as a conventional food or as an item of meal or diet; (iii) nutraceuticals are active substances extracted from vegetal (as phytocomplexes) or animal origin food concentrated and administered in a suitable pharmaceutical form; (iv) scientific evidence has substantiated the effectiveness of a nutraceutical in the context of a specific health issue; (v) food supplements are formulations, often multi‐component, micro‐nutrient based, containing dietary ingredients, for example, vitamins, minerals, amino acids, or other botanical or dietary substances, which are not specifically addressed to prevent and/or cure an health issue; (vi) food supplements and herbal products should be used when necessary to add/supplement the body with one or more micro or macro nutrients for which there is the need of; and (vii) food supplements, pro/pre biotics and/or herbal products do not need to possess a clinically proven efficacy on a specific health condition, but can help to prevent illness or provide micro nutrients as an integral part of a diet.
The difference between food/dietary supplements and nutraceuticals is quite clear; nutraceuticals are closer to pharmaceuticals, since they must have clinically proven efficacy towards a specific pathological condition and, hence, a valid help in both disease prevention and therapy. They, when looked at as pharma‐foods, position themselves in the space ‘beyond the diet before the drugs’, as defined by Ettore Novellino in 2012, this quote being widely accepted as proper term of reference for nutraceuticals (Santini 2014). Nutraceuticals can help in fighting some of the major health challenges of the century, for example metabolic syndrome, obesity, cardiovascular diseases, osteoporosis, diabetes and, among these, hypercholesterolaemia, which is one of the main health risks. The aim of this review is to summarize the positive outcome and mechanism of action of some of the most well‐known lipid‐lowering nutraceuticals on hypercholesterolaemia.
Nutraceuticals and hypercholesterolaemia: the terms of reference
Hypercholesterolaemia is a pathological condition that is indicated by high levels of lipids, for example cholesterol, tryglicerides and/or fat phospholipids, in the blood. This can be also due to a prolonged increase in insulin levels as well as a high level of O‐GlcNAc (O‐linked β‐N‐acetylglucosamine) transferase, which can lead to dyslipidaemia. In developed countries, dyslipidaemias are often due to both poor dietary habits and lifestyle, and are linked to the so called ‘metabolic syndrome’, a worldwide epidemic disease mainly associated with the increased onset of health conditions and mortality. This type of dyslipidaemia is characterized by high triglyceride and low HDL cholesterol (HDL‐C) levels and/or is triggered by different cardio metabolic risk conditions, which include obesity, insulin resistance, hypertension, cardiovascular disease and type 2 diabetes.
The possibility of implementing a non‐pharmacological nutraceutical based treatment for hypercholesterolaemia is getting increasing attention, and is considered to be an important preventive action to take when hypercholesterolaemia is mild or moderate, representing a low and/or moderate risk factor (Volpe and Sotis 2015), and when there is no evidence of organ damage. A nutraceutical approach, which can or cannot include statins, has been suggested by the international guidelines for the treatment of dyslipidaemia (Reiner et al., 2011), and could help to confront the hypercholesterolaemia and, at the same time, any possible unwanted side effects (Afilalo et al., 2008). Figure 1 shows the roles of the key players in this scenario: safety and proven efficacy based on scientific evidence must substantiate the clinical use of an alternative therapy based on nutraceuticals, given that: (i) safety and the mechanism of action are clearly assessed; (ii) efficacy is assessed; and (iii) no unwanted side effects are observed. Nevertheless, not all nutraceuticals with lipid‐lowering effect are clearly endorsed by the guidelines. As an example, the guidelines for the use of plant sterols/stanols are controversial (Weingärtner et al., 2014). Another relevant aspect is the dose to be administered to obtain efficacy and not cause any unwanted side effect or toxicity. If in fact it is true that ‘All things are poison and nothing is without poison; only the dose makes a thing not a poison’, as stated by Paracelsus, even a drug like digitalis from Digitalis purpurea, which is used to increase cardiac contractility (being a positive ionotrope) and as an antiarrhythmic agent to control the heart rate, can become an health threat depending on the dose. The same considerations hold true for supplements and nutraceuticals, and care should be paid to this aspect when using them as preventive or therapeutic tools.
Figure 1.
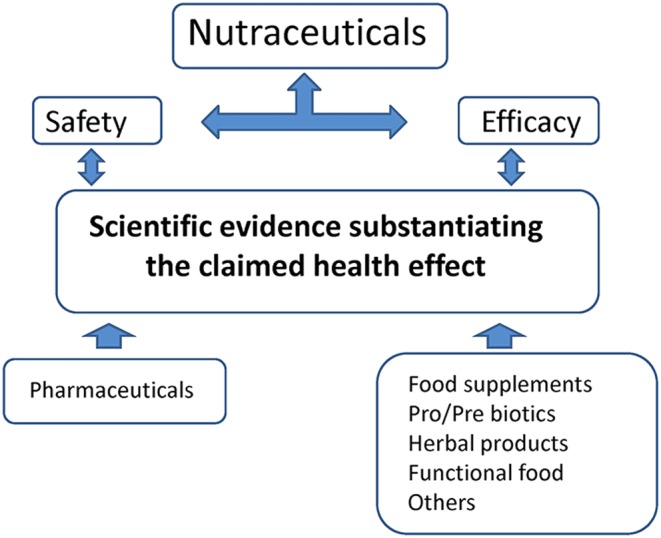
The key players that must be substantiated before the clinical use of nutraceuticals in therapy.
Figure 2 defines the schedule that should be followed in the identification of a potential health condition target and then the subsequent steps for the identification and development of a correct, safe and effective nutraceutical. Many different steps are involved, each requiring different professional expertise. The first step is the identification of the epidemiological target, followed by the identification and fractioning of the starting food matrix. The study of bioactivity and safety are a must, as well as identifying the metabolic profile of the phytocomplex, if the starting material is a foodstuff of vegetal origin. The study of the mechanism of action is then followed by (i) the establishment of the proper formulation and (ii) the fine tuning to develop the correct pharmaceutical form for the particular use and dosage; the bioavailability of the nutraceutical being developed should be considered; clinical trials are performed to assess its efficacy.
Figure 2.
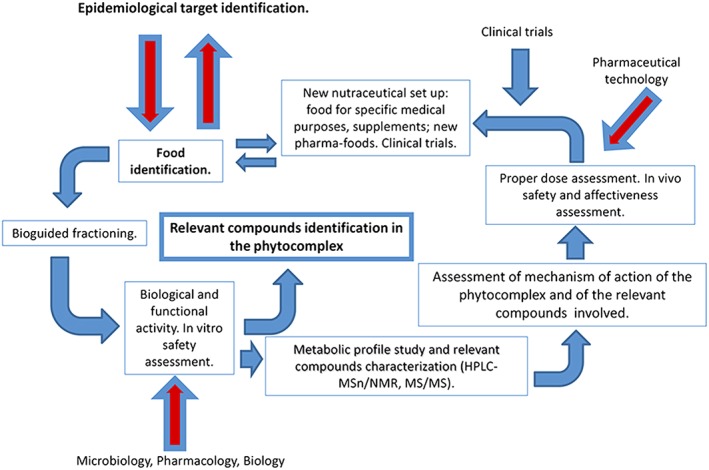
Nutraceutical development pathway.
Figure 3 summarizes the possible approaches to hypercholesterolaemia. If it has already reached an advanced stage and organ damage is apparent then it is primarily treated with pharmaceuticals. This being the case, statins are at the forefront of strategies to manage it even if these drugs are not always well tolerated; quite high discontinuation rates have been observed in patients starting statin therapy (Vinogradova et al., 2015). Furthermore, many patients are considered statin‐resistant because they fail to achieve an adequate reduction in LDL cholesterol (LDL‐C) levels or are subjected to adverse effects, for example myopathy and increased activity of liver enzymes. The resistance to statins has been associated with many different causes, including polymorphisms in 3‐hydroxy‐3‐methylglutaryl CoA reductase, P‐glycoprotein, cholesterol 7 α‐hydroxylase (CYP7A1), apolipoprotein E, proprotein convertase subtilisin/kexin type 9 (PCSK9), LDL receptor, lipoprotein A and cholesteryl ester transfer protein (Reiner 2014).
Figure 3.
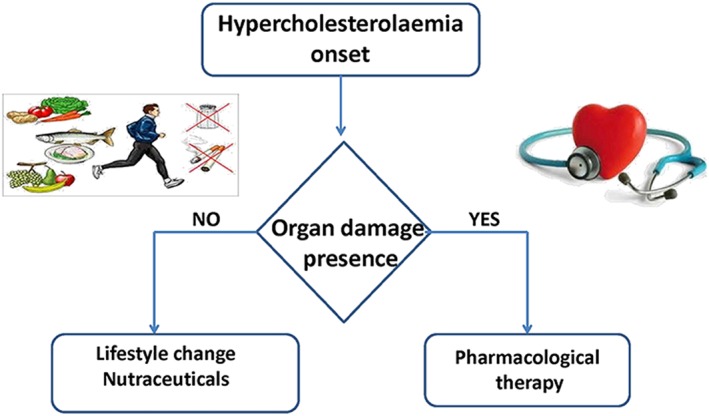
Hypercholesterolaemia: possible alternative approaches.
For people showing an intolerance to statin therapy, and that qualify for pharmacological treatment due to their obvious, high cardiovascular risk, the most appropriate alternatives are cholesterol absorption inhibitors or the new PCSK9 inhibitors. They have been shown to act by decreasing the degradation of LDL receptors, increasing the recirculation of the receptor to the surface of the hepatocytes and, hence, lowering LDL‐C levels in the bloodstream (Brendan et al., 2015).
Nevertheless, based on their ability to lower LDL‐C with no adverse side effects, the use of nutraceuticals should be considered: (i) in individuals with high cholesterol levels at intermediate or low global cardiovascular risk, who do not qualify for pharmacotherapy; and (ii) as a support to pharmacological therapy in high and very high risk patients who fail to achieve LDL‐C goals with statins or are statin intolerant. Due to the possible resistance connected to the statin therapy, the interest both from pharmaceutical research and from patients requesting supplementary and/or alternative treatments is growing. Some functional foods and nutraceuticals have been shown to be effective, safe, with high bioavailability and, at the same time, are well tolerated in patients, as documented by clinical trials showing a reduction in the total cholesterol (TC) level and an effect on both HDL‐C and LDL‐C respectively.
A large number of nutraceuticals claimed to have lipid‐lowering activity are currently available both in pharmacies and as over the counter products (OTC) even though, in most cases, their lipid‐lowering activity has not been completely assessed and has not been adequately correlated with a concrete reduction in cholesterol level and absence of risk due to their use.
For subjects with a history of statin intolerance, drugs like ezetimibe, which has proven efficacy and safety, are an important alternative to statins. Moreover, a PCSK9 antibody molecular biology‐based treatment represents a challenging opportunity for statin‐intolerant subjects (Chait and Eckel 2016). These pharmacological options for lowering lipids need endpoint studies and these are now been undertaken. While for drugs, it is clear that rigid endpoints studies need to be performed prior to market circulation, for diet/food supplements and nutraceuticals this is not the case. This makes the evidence for the efficacy and safety of the latter weaker, and also emphasizes the need for clinical data substantiating any claim related to cholesterol‐lowering efficacy.
In the following, some alternative approaches to statins for the prevention and treatment of hypercholesterolaemia are described.
Red yeast rice
Extracts of red yeast rice fermented by Monascus purpureus (responsible for the reddish colour of cooked fermented rice), produce fungal secondary metabolites; they contain sterols, isoflavones, mono unsaturated fatty acids and monacolin K, also known as lovastatin. Red yeast rice also contains other monacolin‐related substances whose mechanism of action has been correlated to their ability to inhibit 3‐hydroxy‐3‐methylglutaryl CoA reductase, which may either have lipid‐lowering effects or potentiate the monacolin K effects (Gerards et al., 2015). The mechanism of action is known; monacolin K (lovastatin) acts as an inhibitor of the hydroxymethylglutaryl‐CoA (HMG‐CoA) reductase. With respect to statins, red yeast rice may be less likely to deplete mevalonate metabolites distal to HMG‐CoA reductase, such as ubiquinone and GTP‐binding regulatory proteins, which are believed to mediate the adverse effects of statins on muscle (Lachenmeier et al., 2012).
In human studies, the bioaivalability values for both lovastatin and its active metabolite, lovastatin acid, were higher in volunteers receiving red yeast rice capsules or powder than in those receiving lovastatin in the same pharmaceutical form. This has been correlated to a higher dissolution rate and lower degree of crystallinity in red yeast rice containing products (Chen et al., 2013).
In 2016, a study analysed in a systematic way, 10 randomized controlled trials involving 905 Chinese subjects with dyslipidaemia: the use of red yeast rice (monacolin K) and simvastatin were compared, and no statistically significant difference in any of the outcomes examined was observed, suggesting that the use of red yeast rice as an alternative to simvastatin is not supported on current evidence (Ong and Aziz 2016).
Notwithstanding the efficacy of red yeast rice nutraceuticals, it is evident that in commercially available products, composition uniformity, purity, labelling and safety often cannot be guaranteed (Klimek et al., 2009). Another concern regards the possible presence in some red yeast rice preparations of citrinin, a secondary toxic metabolite of microfungine origin responsible for hepatic and kidney injuries (Avula et al., 2014). The limit values for this contaminant have been set by the European Commission in 2014 (European Commission 2014). Citrinin, a nephrotoxic mycotoxin that has been proven to be genotoxic at high concentrations in cultured human lymphocytes, has been identified in red yeast rice samples and represents a threat for the consumers' health even if acute toxicity is quite a rare event (Pascual‐Ahuir et al., 2014).
A cause and effect relationship has been established by European Food Safety Authority (EFSA) between the consumption of monacolin K from red yeast rice and maintenance of normal blood LDL‐C levels, given that daily dietary intake level is at least 10 mg.day‐1 of monacolin K from fermented red yeast rice based preparations (Agostoni et al., 2011a). This value has been determined based on the average values reported in two double‐blind, randomized, placebo‐controlled trials, which observed a reduction in TC and LDL‐C of 16 and 22%, respectively, induced by administering a dose of around 7.5 mg.day‐1 of the active principle for 12 weeks (Heber et al., 1999), and 20% and 26% for TC and LDL‐C, respectively, by administering a dose of about 11.4 mg of active principle for 8 weeks (Lin et al., 2005). The FDA admitted that red yeast rice (powder) that contains more than 0.4% monacolin K (lovastatin) can reduce cholesterol, while the United States District Court of Utah outlined that red yeast rice products when containing significant amounts of lovastatin, for example >0.4%, should not be considered as dietary supplements but should be treated like drugs and be subjected to their regulations.
Possible unwanted side effects, mainly due to lovastatin, have been reported, for example myopathy and abnormal liver function. The incidence of kidney damage was evaluated in 2895 subjects, liver damage in 2895 patients and muscle symptoms in 105 patients. Liver abnormalities and kidney injury were assessed in 14 and 8 studies respectively (Gerards et al., 2015).
A recent warning report issued by the German Federal Institute for Drugs and Medical Devices, on the consumption of red yeast rice cholesterol lowering products outlines safety requirements as set by the European Regulation for use of food supplements with high doses of active compounds (European Parliament Directive 2002/46/EC 2002). In particular, the content of monacolin K, should be considered a drug at levels higher than 5 mg.day‐1 as a results of existing scientific data, which support a pharmacological effect at this dose, as recently observed by the german expert panel of the Bundesamt für Verbraucherschutz und Lebensmittelsicherheit und das Bundesinstitut für Arzneimittel und Medizinprodukte (see BVL/BfArM 2016). The exact amount of monacolin K in red yeast rice has been found to be highly variable, ranging from 3 to 30 mg.day‐1 depending on the brand (report by Avis Du Conseil Superieur De La Sante 2016). This report follows the opinion of the Autorité française de Sécurité sanitaire de l'Alimentation, de l'Environnement et du Travail (ANSES 2014), which seems to suggest that high levels of monacolin K in red yeast rice‐containing products available as over the counter food supplements, should be treated with caution due to their variable monacolin K content.
Berberine
Berberine is an alkaloid drug that can be found in plants belonging to the Berberis species; it is usually found in the roots, rhizomes, stems and bark isolated from the Chinese herb Coptis chinensis. Using HepG2 human hepatoma cells, it has been demonstrated that berberine extracts inhibit cholesterol synthesis in a similar manner to the AMP‐activated protein kinase (AMPK) activator 5‐aminoimidazole‐4‐carboxamide 1‐β‐ribofuranoside. Activation of AMPK has recently been confirmed by measuring the phosphorylation of acetyl‐CoA carboxylase, a substrate of AMPK, correlated with a subsequent increase in fatty acids oxidation (Brusq et al., 2006).
The mechanism of action seems to involve a decrease in plasma cholesterol in hypercholesterolaemic patients, mediated by increasing the hepatic LDL receptor expression. This effect can be attributed to the inhibition of the transcription of the mRNA encoding the proprotein convertase subtilisin/kexine type 9, which helps the transition of the hepatic LDL‐C receptor from the cell surface towards the lysosomes. This can increase the half‐life of the receptor, which captures LDL‐C from the bloodstream and sends it to the bile for its elimination. Based on this mechanism, experimental results on hamsters fed berberine, 50 mg kg−1 twice a day, indicated a reduction in TC up to 27%, a LDL‐C lowering of 0.4%, a TG lowering of 0.22%, while no change in HDL‐C was observed with reference to baseline (Brusq et al., 2006). A recent randomized single‐blind study by parallel group, focused on the use of a nutraceutical based protocol (500 mg berberine plus red yeast rice and other components vs. placebo) involving 80 elderly dyslipidaemic patients intolerant to statins with high total cholesterolaemia and high LDL‐C (Marazzi et al., 2011). A statistically significant reduction in total cholesterolaemia (20%) and LDL‐C (31%) was obtained with the nutraceutical, suggesting that berberine‐based nutraceuticals may be used to obtain acceptable plasma LDL‐C levels. Berberine‐based nutraceuticals showed both high safety and tolerance, even though there is a lack of studies on the possible matrix‐related unwanted side effects and on contaminants presence in the vegetal matrix. The effects have also been shown to be independent of dose: 500 mg day−1 in combination with red yeast rice and other nutraceuticals induced a reduction of approximately 20% of the LDL‐C and 25% of triglycerides in patients with either pure hypercholesterolaemia, mixed dyslipidaemia, type 2 diabetis or hepatopathy (Cicero et al., 2015).
The potential therapeutic uses of berberine as a nutraceutical is, however, limited by its low oral bioavailability, attributed to an unclear mechanism of intestinal metabolism, poor absorption and intestinal first‐pass effect (Liu et al., 2016). Many studies have been conducted to explore the possibility to increase bioavailability by reducing the intestinal first‐pass metabolism with the use of permeation enhancers (Fan et al., 2013), P‐glycoprotein inhibitors and microparticle delivery systems (Zhu et al., 2013). No serious side effects associated with the use of berberine have been reported according to a recent survey, even though these might include constipation, diarrhoea, abdominal distension and bitter taste in the mouth (Dong et al., 2013). Moreover, possible berberine‐drug interactions should be taken into account, considering that this compound can decrease CYP2D6, CYP2D9 and CYP3A4 activities in healthy subjects (Mannarino et al., 2014). Finally, it is evident that there is a need for quality, larger controlled trials with a better methodological approach using standardized preparations to further quantify the therapeutic effect of berberine (Jiarong et al., 2015). According to the EFSA, the claim that berberine extracts lower cholesterol has not been properly assessed, and this compound can be a source of concern, as outlined by a report from EFSA Scientific Cooperation group (ESCO 2012). Similarly, the FDA has not yet approved berberine for any prescription or OTC drug use.
Plant sterols/stanols
The first mechanism of action proposed for plant sterols/stanols was their possible competition with intestinal cholesterol for incorporation into micelles. In recent years, the focus has been on the role of sterol transporters localized in the enterocyte membranes as a means to reduce intestinal cholesterol absorption. More recently, the existence of a direct secretion of cholesterol from the circulation into the intestinal lumen has been described. Inhibition of intestinal cholesterol absorption can lower the concentration of LDL‐C and other ApoB100‐containing lipoprotein fractions. However, the cholesterol absorption process is known to involve many steps, the final being the esterification by acyl‐CoA cholesterol acyltransferase‐2 in the enterocytes, and the subsequent incorporation into chylomicrons, which involves the microsomial triglyceride transfer protein, to be finally released into the lymph (Smet et al., 2012). Recently, the European Atherosclerosis Society Consensus Panel on Phytosterols appraised evidence relevant to a beneficial effect in lowering cardiovascular risk of plant sterols and/or plant stanols, when used as a food supplement to reduce plasma LDL‐C levels. In particular, plant sterols/stanols taken at a dose of 2 g day‐1 can inhibit the absorption of cholesterol and lower LDL‐C levels by 8 to 10% and reduce plasma triglycerides by 6–9% in hypertriglyceridaemic patients, and evoke no unwanted side effects in long‐term human studies (Gylling et al., 2014). At this dose, the plant sterol/stanol‐mediated LDL‐C lowering effect is additive to that of statins in dyslipidaemic subjects, mediating an effect equivalent to doubling the dose of statin. Plant sterols/stanols can qualify as a potential nutraceutical source in subjects with moderate to high cholesterol levels who do not qualify for pharmacotherapy, and also as a support to pharmacological therapy in moderate/high risk patients who fail to achieve LDL‐C targets on statins or are statin intolerant. It must be noted, however, that there is lack of information, since no randomized controlled clinical trial data are available to establish any clinical benefit from the use of these substances. A major concern is their possible negative interaction with fat‐soluble vitamins absorption, as this has been reported for plant sterols based food supplements. Nevertheless, observational studies seem to suggest an independent association between plasma levels of plant sterols and the risk of coronary heart disease. In vivo studies in animals fed high doses of plant sterols seem to show an opposite effect, namely an amelioration of the development of new plaques and the onset of lipid lesions (Rocha et al., 2011). Many different factors can affect the efficacy and safety of plant sterols both in free and esterified forms. It has been reported that plant sterols, at doses in the range 1–3 g day‐1 as part of a healthy diet and lifestyle, are useful dietary components for lowering LDL‐C. At the same time, a negative effect represented by the reduction in the absorption of plants sterols induced by carotenoid‐rich should be taken into account (Berger et al., 2004).
Experimental studies and clinical trials have lead to speculation that plant sterols might be atherogenic, raising concerns and safety issues on the use of plant sterols in to prevent adverse cardiovascular events. The use of plant sterols for this reason has become controversial, and the need for shared guidelines for the use of these substances for medical purposes has been raised (Weingärtner et al., 2014). Notwithstanding the possible negative implications, the use of plant sterols as cholesterol‐reducing substances has been explored and positive outcomes have been reported. A comparative meta analysis on eight eligible clinical trials (on a total of 263 subjects, lasting 4 and 6 weeks, in the year range 1992–2013) resulted in it being concluded that plant sterol doses in the range 1.0 to 3.0 g day‐1, administered with meals, decreased LDL‐C concentrations on average by 120 mg·L−1 (−0.31 mmol·L−1) compared with a placebo (Shaghaghi et al., 2013).
Phytosterols dose–response relationship for their cholesterol lowering effect has been evaluated in several meta‐analyses by calculating averages for different dose ranges or by applying continuous dose–response functions. Both approaches have advantages and disadvantages. A recent meta‐analysis based on a total of 124 studies revealed that an average phytosterol dose in the range 0.2–9.0 g day‐1 (with phytosterol intakes in the range 0.6–3.3 g day‐1) resulted in a gradual reduction in the LDL‐C cholesterol level by 6–12%. The same study also reported that the LDL‐C lowering effect of both plant sterols and stanols continues to increase up to intakes of approximately 3 g day‐1 to get an average reduction effect up to 12% (Rouyanne et al., 2014).
The effective use of plant sterols/stanols as food supplements or ingredients in functional foods is, however, quite limited due to their low solubility in fats and insolubility in water. The availability is consequently moderate to low, suggesting that in order to enhance the lowering effect on cholesterol absorption there is need for research on how to maximize their availability and solubility in mixed bile salt micelles within the small intestine (e.g. emulsification, microemulsion and esterification).
Dietary fibres
The term ‘dietary fibre’ indicates in general plant substances resistant to digestion in the human small intestine. Soluble fibres include psyllium, pectin, flaxseed, β glucans and guar gum. Fibres like cellulose, lignins and wheat bran are insoluble. It has been observed that diets high in total dietary fibre are associated with a reduced cholesterol level in the bloodstream and reduced onset of cardiovascular disease (Liu et al., 2006).
At the same time, the role of dietary fibre on mineral bioavailability has been extensively studied outlining that while in vitro studies show mineral binding properties, both animal and human studies did not assess negative effects on mineral absorption, and in some cases reported absorption enhancing properties. A recent report outlines how dietary fibres could have negative effects on mineral absorption in the gastrointestinal tract due to mineral binding or physical entrapment, even though colonic fermentation of dietary fibres can release the bound minerals and facilitate their absorption (Baye et al., 2015).
In general, the health benefits of dietary fibres have been associated with: (i) their ability to act as bile acid sequestrants; (ii) their ability to up‐regulate LDL receptors; and (iii) the increase in LDL‐C clearance. They can inhibit the synthesis of hepatic fatty acids and, as secondary effects, reduce the absorption of macronutrients and improve intestinal motility.
The proposed mechanism of action for soluble fibres has been associated with the binding of bile acids during the intraluminal formation of micelles, and this seems connected to a physical entrapment rather than chemical binding (Anderson and Tietyen‐Clark 1986). The effect could stimulate the increase of bile acid synthesis, as well as a reduction in the hepatic cholesterol content, an up‐regulation of LDL‐C receptors and an increased LDL‐C clearance (Brown et al., 1999).
Another mechanism has also been reported to justify the existing difference in the effects of soluble and insoluble fibres on LDL‐C. Soluble fibres, while contributing to an increase in intraluminal viscosity and the binding of bile acids, can also entrap cholesterol in the small intestine and reduce, by slowing it, the absorption of macronutrients. While, the non‐soluble fibres did not show any effect on LDL‐C, the dietary fibres caused a reduction in TC, of about 17 mg·L−1 g−1 of intake, and a reduction in LDL‐C reduction of about 22 mg·L−1 g−1 intake (Kris‐Etherton et al., 1988).
However, according to a recent EFSA opinion, no cause and effect relationship between the consumption of dietary fibre and blood cholesterol concentration has been established (Agostoni et al., 2011b). The FDA suggestion is that the addition of soluble fibre to a diet, that is low in saturated fat and cholesterol, may help to reduce the risk of cardiovascular diseases under precise conditions. In particular, dietary intake levels associated with reduced risk of cardiovascular diseases should include ≥3 g day−1 of [β]‐glucan soluble fibre from either whole oats or barley, or a combination of whole oats and barley and/or an amount ≥ 7 g day−1 of soluble fibre from psyllium seed husk (Health claims 2008). A pooled analysis of 10 prospective cohort studies revealed a 12% reduction in the risk for coronary events and 19% for coronary deaths, for each 10 g day−1 increment in dietary fibre (Bazzano et al., 2003; Pereira et al., 2004). Controlled studies have demonstrated a limited, even if statistically significant, reduction in LDL‐C by approximately 5–7% when ingesting soluble fibre with food. A meta‐analysis of 67 trial studies showed that ingestion of soluble fibre, 2–10 g day−1, was associated with a small but significant, 7%, reduction in LDL‐C. A daily intake of 5 to 10 g of soluble fibre as a therapeutic option to enhance a reduction in LDL‐C has been suggested, even if the palatability of foods containing fibre can be a limiting factor for patients along with a possible increase in flatulence as a result of colonic fermentation (NCEP 2001).
β‐Glucans
The β‐glucans are a group of β‐D‐glucose polysaccharides that occur naturally in the cell walls of cereals, yeast and fungi. They have a linear backbone with 1–3 β‐glycosidic bonds but differ with respect to solubility, viscosity, branching structure and properties, causing diverse physiological effects. There has been recently a growing interest in barley as a therapeutic food due to its high content of β‐glucan, a viscous soluble fibre recognized for its cholesterol‐lowering properties (Ho et al., 2016). A systematic review and meta‐analysis of randomized controlled trials investigating the cholesterol‐lowering potential of barley β‐glucan on LDL‐C, non‐HDL‐C and apolipoprotein B for cardiovascular disease risk reduction indicated that an average dose of 6.5 to 6.9 g day−1 of barley β‐glucan for 4 weeks significantly reduced LDL‐C (average −0.25 mmol·L−1), non‐HDL‐C (−0.31 mmol·L−1), with no significant changes to apoB levels compared with control diets. In 2006, the FDA (2006) endorsed a health claim on the relationship between β‐glucan soluble fibre from whole oat sources and reduced risk of coronary heart disease by adding barley as an additional source of β‐glucan soluble fibre.
Similarly, the EFSA in 2011 published an opinion following a request from the European Commission, which approved the substantiation of the claim related to β glucans from oat and barley as able to maintain normal blood cholesterol concentrations as well as the claim related to a reduction in the post‐prandial glycaemic response (EFSA Scientific Opinion 2011).
Polyphenols in green tea and grapes
Polyphenols are a family of substances of vegetal origin whose main feature is the presence of multiple phenolic groups with an antioxidant effect. Polyphenols are present in tea (the highest amount being found in green tea) as well as in many of the food in a typical Mediterranean diet, for example olives, olive oil, grapes, wine, fruits and vegetables. While evidence from existing studies indicates that phytates and polyphenols have an inhibitory effect on the absorption of large minerals, polyphenols have been postulated to be able to inhibit HMG‐CoA reductase, as well as acetyl‐CoA acetyltransferase 2 and microsomal triacylglycerol transport protein, justifying their hypocholesterolaemic effect (Amiot et al., 2016; Pang et al., 2016).
Red grape juice polyphenols have been shown to reduce circulating levels of LDL‐C and to increase LDL‐C receptor activity. The effect of these polyphenols on intracellular cholesterol homeostasis, human hepatocarcinoma HepG2 and promyelocytic leukaemia cell lines has been studied. Cell lines incubated in serum‐free medium, with or without LDL‐C, in the presence or absence of red grape juice, revealed that in the presence of LDL‐C, red grape juice increased both the activity and cell surface expression of the LDL‐C receptor. In a similar way, wine polyphenols, for example resveratrol and quercertin, have been shown to increase LDL‐C receptor binding activity and gene expression, suggesting that red wine polyphenols are able to regulate major pathways involved in lipoprotein metabolism (Dàvalos et al., 2006). In the same study, it was observed that in cells exposed to LDL‐C, red grape juice increased the levels of the active form of sterol regulatory element‐binding protein‐1 and mRNA expression of the LDL‐C receptor and hydroxyl methyl glutaryl‐CoA reductase.
The green tea cathechins as well as cathechins present, in different amounts, in all tea varieties, have a recognized cholesterol‐lowering activity, which has been attributed to the up‐regulation of the hepatic LDL‐C receptor (Bursill et al., 2007). Catechins belong to the chemical family of the flavanols, which constitute the main components of soluble solids in green tea being mainly constituted of epigallocatechin gallate, epigallocatechin, epicatechin gallate, which contribute to about 50% of the total, and epicatechin (Henning et al., 2005). In an in vivo study conducted in hypercholesterolaemic rats, it was observed that a nutraceutical based on an ethanolic extract of green tea, when administered for 8 weeks, reduced the TC level, by up to 15.4%. the LDL‐C by about 21.5%, and triglycerides by about 12.9%, when compared to the effects of a placebo (Yousaf et al., 2014). The mechanism of action was confirmed to be related to sterol regulatory element‐binding proteins (SREBP 2) and LDL‐receptor activation (Kuhn et al., 2004) and also to the CYP7A1 activation (Jiao et al., 2010).
While epidemiological studies associate polyphenol‐based food supplements with a decreased risk of developing hypercholesterolaemia, intervention studies, in general, do not confirm these beneficial effects, probably due to different doses, possible interaction with the food matrix, and mainly their moderate bioavailability (Bohn 2014). Endogenous factors, namely microbiota and digestive enzymes can affect the bioaccessibility, uptake and metabolism of polyphenols. Dietary fibre, minerals and protein‐rich meals are likely to decrease polyphenol bioaccessibility, while digestible carbohydrates, lipids and hydrophobic polyphenols, may enhance polyphenol availability. Following epithelial uptake, polyphenols may reduce phase II metabolism and excretion, enhancing bioavailability.
A recent study (on rats) examined the toxicity of tea polyphenols and showed the toxicity level is low, but also indicated that attention should given to their dosage, which can be a risk in concentrated products based on green tea extracts (GenLiang et al., 2015).
The oral bioavailability of the green tea catechins (e.g. epigallocatechin gallate) is in general low, resulting in systemic catechin levels in humans many fold less than the concentration determined in in vitro systems (Sherry Chow et al., 2005).
The availability increases with consumption on an empty stomach, and this has been correlated to catechin degradation in the lumen. On the other hand, green tea catechins are stable in acidic conditions and are more stable when pH values increase (>6.5). Risks associated with their consumption are, however, not completely absent. Among the hazards to be considered for nutraceuticals based on green tea extracts, are the possible contamination with ochratoxin A. This secondary metabolite is produced by naturally occurring mycotoxins, and it is a widespread food contaminant; its maximum safe level in foodstuff is set by the European Commission; it has been found in grapes, as well as in green tea, cereals and grains (Santini et al., 2015).
Flavonoids
There is, in general, controversy about flavonoid‐based food supplements/nutraceuticals with reference to their cholesterol‐lowering effects. This is due to factors like absorption and bioavailability. Some studies suggest that no effect or threat on health are present, while in vitro and in vivo data give contradictory results regarding safety, as shown in earlier clinical studies with β‐carotene supplements, which seem to stress the need for caution when using flavonoid supplements (Harnly 2016). One criticism, in particular, is whether the concentrations in one nutraceutical or food supplement based on these substances can really exceed the dose received from a daily diet that includes fruit and vegetables. The absorption of diet flavonoids is considered to be limited since food flavonoids are bound to glycosides. Consequently, it has been observed that only the aglycones pass freely into the blood stream from the gut wall, since no enzymes are secreted in the gut that can possibly cleave the glycosidic bonds. However, this opinion has been partially overwritten by studies on specific flavonoids, which have a bioavailability higher than previously believed (Kroft 2016). Therefore, the potential health benefits of individual flavonoids, as well as any potential harmful attributes, need to be characterized and completely assessed. As an example of efficacy, a citrus bioflavonoid, namely naringenin, which has be shown to be effective in reducing Acyl‐CoA cholesterol acyltransferase activity, caused a marked decrease in plasma cholesterol levels in rats. This could be a possible explanation for the mechanism of action, which it is still to be completely cleared. In a similar way, guineensine, isolated from one pepper variety (Piper longum), inhibited acyl‐CoA cholesterol acyltransferase activity in a dose‐dependent manner with an IC50 value of 3.12 μM. Hence, the efficacy of guineensine as a cholesterol‐lowering efficient nutraceutical should be further explored as (Lee et al., 2004).
Strangely, Mollace and colleagues, observed that a nutraceutical based on a bergamot polyphenols extract reduced LDL‐C (>30%) and TG levels (>40%) (Mollace et al., 2011). These results, however, are contradictory with those reported previously, which failed to demonstrate any effect in subjects treated with two different polyphenols (namely hesperidin and naringin) compared with a group on placebo (Demonty et al., 2010). From the available data, it appears some bioflavonoids have the ability to lower cholesterol levels, but at the same time also indicate the lack of information on their bioavailability, unwanted side effects and presence of contaminants in the original vegetal matrix. Studies are needed to assess these aspects related to the safe use of bioflavonoids as nutraceuticals.
Apple polyphenolic extract
The hypothesis that polyphenolic compounds, for example quercetin, (−)‐epicatechin, (+)‐catechin, procyanidins, anthocyanins, dihydrochalcones (phloridzin) and phenolic acids, play a key role in the healthy properties of apples has been associated with an important effect on cholesterol metabolism (Alonso‐Salces et al., 2004).
Apples varieties, however, differ widely in their polyphenolic composition: an in vitro study showed that the Annurca apple, the only cultivar native to the Campania region in Southern Italy, listed as a Protected Geographical Indication product [Commission Regulation (EC) No. 417/2006], has the highest polyphenolic concentration. The same study outlined how its polyphenols are unequivocally more effective on cholesterol metabolism, compared with the other more common commercially available cultivars, for example Red Delicious, Granny Smith, Fuji and Golden Delicious (Tenore et al., 2013). Polyphenols occur as a mix of dimers (mostly procyanidin B2), trimers (e.g. procyanidin C1), tetramers, pentamers and higher molecular weight oligomers. In the Annurca variety, the polyphenolic fraction is the richest in procyanidins, revealing an uncommon polyphenolic profile, in particular dimers and oligomers with 6 < n < 8, compared with the other above mentioned apple varieties. This corresponded to the highest hypolipidaemic effect in vitro. In vitro test on human HepG2 using the above mentioned apple cultivars polyphenolic extracts (at a concentration of 1 μM) indicated Annurca as the leader in increasing TC levels in the medium (5.5 times compared with the other varieties), indicating an important inhibition of cell cholesterol uptake. Using the same cell model system, the Annurca polyphenolic extract showed a higher capacity to increase apo‐AI levels (twofold), and to decrease LDL‐C concentrations (48%), in medium containing HepG2 cells (Tenore et al., 2014). Among all the procyanidin oligomers, the dimers (procyanidin B2) are the most effective in decreasing LDL‐C (60%), and increasing Apo‐AI (85.7%), while the oligomers 6 < n < 8 are mainly responsible for increasing (almost nine times the control) the TC, in the cell culture medium. These observations are consistent with previous data on HepG2 cells treated with catechins extracted from green tea, which are able to induce a substantial (30%) lowering of cell cholesterol content, and activation of SREBP‐1 transcription factor, which caused a marked up‐regulation of LDL receptors (Christina and Paul 2006).
In a similar way to Annurca catechins, the statins, the first‐line lipid‐lowering drug therapy, indirectly increase the SREBP‐2 expression, which in turn leads to the over‐expression of LDL‐receptors, the event mostly responsible for the effective lowering of plasma LDL‐C (DeBose‐Boyd et al., 2001). Therefore, it can be reasonably hypothesized that dimeric procyanidins from apples as well as the ones from green tea, have a statin‐like LDL‐C lowering effect. However, in contrast to statins, which do not substantially increase the HDL‐C plasma level (values on average below 10%), the apple dimeric procyanidins are fully able to increase the apo‐AI protein concentration in the cell medium (71.4%) in a HepG2 cell culture. The unique distribution of the polyphenolic fraction in the Annurca apple (28.5% procianidin B2 and 10.9% of oligomers with 6 < n < 8) can be associated with the high potency at lowering the LDL‐C and in increasing the Apo‐AI. Both these fractions seem to be responsible, with different mechanisms of action, for the plasma cholesterol‐lowering effect. Dimers are readily absorbed in the gut, quickly reaching the liver where they act indirectly by increasing the number of LDL receptors (Yasuda et al., 2011); the oligomers, on the other hand, which are confined to the gut, act locally by a mechanism similar to β‐cyclodextrin, trapping the cholesterol molecules and this way reducing their absorption (Leifert and Abeywardena 2008). Statins are known to inhibit the biosynthesis of cholesterol in the liver, an effect sensed by the SREBP pathway, which in turn induces the over‐expression of the LDL receptor (DeBose‐Boyd et al., 2001). Based on this, it can be reasonably hypothesized that dimeric procyanidins (procyanidins B2) present in Annurca apples may exert a statin‐like LDL‐C lowering effect. However, in contrast to statins, which do not substantially increase the plasma HDL‐C levels (<10%), the apple dimeric procyanidins are fully able to increase the apo‐AI protein concentration (85.7%) in the cell medium of a HepG2 cell culture.
To verify these properties of the Annurca apple in vivo, a 12‐week clinical trial on humans has been carried out. Recent interesting results indicate that Annurca apples (200 g day−1) fed to patients with mild hypercholesterolaemia (in a range between 2.10 and 2.50 g·L−1) showed the highest hypocholesterolaemic effect, documented by a TC reduction of 8.3%, when compared with a same quantity (g day−1) of Granny Smith and Red Delicious varieties, which resulted in a TC reduction of 4.4% and 3.1% respectively. Fuji and Golden Delicious obtained a TC reduction level of 2.0% and 1.3% respectively. The Annurca apple also reduced the LDL‐C by 14.5%, and increased HDL‐C by 15.2% (Tenore et al., 2016). Based on these findings, a nutraceutical product named AppleMets®, containing only the Annurca polyphenolic extract, has been prepared in our laboratories and tested on a large cohort of mildly hypercholesteroleamic healthy subjects in a clinical trial. Preliminary results indicate that administering two 400 mg capsules day−1, taken with the two main daily meals, resulted, on average, in a TC reduction of 25%, an increase in HDL‐C levels of 49.2%, a reduction in LDL‐C 37.5%, and caused no alteration in either triglyceride or sugar levels compared to baseline, taking into account that an intake of procyanidins below 1000 mg day−1 is generally considered to be safe in humans, does not cause any significant haematological, clinical, chemical, histopathological or urinary effects [Commission Regulation (EC) No. 258/1997].
Based on the previous findings, the intake of apples alone cannot guarantee a preventive effect on adverse cardiovascular events; however, this nutraceutical polyphenolic extract could have a significant effect on health. This is further outlined by the ratio of LDL‐C/HDL‐C in the plasma samples of patients treated with this nutraceutical, which decreased from 6.26 to 2.30, indicating a reduction in cardiovascular risk, which is considered relevant if this value is around 3 (Millán et al., 2009).
In the United States, a Granny Smith apple polyphenolic extract (Applephenon®), generally recognized as safe by the FDA, has entered the market as a nutraceutical aimed at reducing the serum cholesterol level, and at preventing obesity. The hypocholesterolaemic effect of this product has been documented by a randomized human placebo‐controlled intervention study that used different dosages. With the maximum dose administered (1500 mg day‐1), a modest reduction in TC (5%) and LDL‐C (8%), and increase in HDL‐C increase (5%) was obtained (Nagasako‐Akazome et al., 2005). The quite large difference in the cholesterol‐lowering effect of Granny Smith‐ and Annurca apple‐based nutraceuticals can be attributed to the different polyphenolic composition of the two varieties. The Annurca apple‐based, polyphenolic extract nutraceutical reduces the TC and enables a correct healthy lipidic balance to be reached in the bloodstream in favour of the ‘good’ cholesterol. Also, in contrast to the hydroxymethyl‐glutaryl‐CoA reductase inhibitors, the nutraceutical made from apple polyphenolic extract, has not been found to have any adverse effects, such as myopathy and diabetes.
Conclusions
Many food supplements which claim to have a cholesterol‐lowering effect are nowadays available on the market as OTC products and are often wrongly labelled as nutraceuticals, giving confusing information and indicating the need for a shared regulation for these last. However, any possible beneficial effect of nutraceuticals on hypercholesterolaemia, either as a supporting and/or therapeutic tool, should be always considered with regard to possible unwanted side effects, safety and bioavailability, especially in non‐statin tolerant patients eligible for an alternative approach to their health condition. In mild and moderate hypercholesterolaemia, a nutraceutical preventive approach would be a highly desired option.
Based on the alternative approaches described for hypercholesterolaemia, it can be concluded that red yeast rice, berberine, dietary fibre, phytosterols, polyphenols and Annurca apple extracts show promising clinical results and are likely to have a beneficial effect. Nevertheless, the potential of red yeast rice as a cholesterol lowering agent is due mainly to lovastatin (monacolin K), and the exact dose administered in the commercially available products is controversial. Berberine has a limited oral bioavailability and some unwanted adverse effects have been reported. The Annurca apple phytocomplex nutraceutical could conceivably be very effective in hypercholesterolaemia, especially in statin non‐tolerant subjects. Notwithstanding this, a pharmacological approach, which adopts non‐statin drugs, for example ezetimibe and PCSK9 antibody treatment, represents a solid and scientifically substantiated approach to dyslipidaemic statin non‐tolerant subjects. Further studies are needed to completely assess the potential of nutraceuticals for the prevention and treatment of hypercholesterolaemia, but they represent powerful tools to be taken into account to combat this health issue, which is considered among the highest of health risk factors.
Author contributions
All Authors contribute equally to the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Santini, A. , and Novellino, E. (2017) Nutraceuticals in hypercholesterolaemia: an overview. British Journal of Pharmacology, 174: 1450–1463. doi: 10.1111/bph.13636.
References
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ (2008). Statins for secondary prevention in elderly patients. J Am Coll Cardiol 51: 37–45. [DOI] [PubMed] [Google Scholar]
- Agostoni C, Bresson JL, Fairweather‐Tait S, Flynn A, Golly I, Korhonen H et al. (2011a). Scientific opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 9: 2304–2320. [Google Scholar]
- Agostoni C, Bresson JL, Fairweather‐Tait S, Flynn A, Golly I, Korhonen H et al. (2011b). Scientific opinion on the substantiation of health claims related to beta‐glucans from oats and barley and maintenance of normal blood LDL‐cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post‐prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J 9: 2207. [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso‐Salces RM, Ndjoko K, Queiroz EF, Ioset JR, Hostettmann K, Berrueta LA et al. (2004). On‐line characterisation of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection. J Chromatogr A 1046: 89–100. [PubMed] [Google Scholar]
- Amiot MJ, Riva C, Vinet A (2016). Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 17: 573–586. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Tietyen‐Clark J (1986). Dietary fiber: hyperlipidemia, hypertension, and coronary heart disease. Am J Gastroenterol 81: 907–919. [PubMed] [Google Scholar]
- ANSES (2014). Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the risks associated with the presence of “red yeast rice” in food supplements. Request No. 2012‐SA‐0228, 34 pp.
- Avis Du Conseil Superieur De La Sante N° 9312 (2016). Compléments alimentaires à base de «levure de riz rouge». 20 pp.
- Avula B, Cohen PA, Wang Y, Sagi S, Feng W, Zweigenbaum J et al. (2014). Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography‐accurate QToF mass spectrometry: chemometrics application. J Pharm Biomed Anal 100: 243–253. [DOI] [PubMed] [Google Scholar]
- Baye K, Guyot JP, Mouquet‐Rivier C (2015). The unresolved role of dietary fibers on mineral absorption. Crit Rev Food Sci Nutr In press. doi:10.1080/10408398.2014.953030. [DOI] [PubMed] [Google Scholar]
- Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK (2003). Dietary fiber intake and reduced risk of coronary heart disease in US men and women the National Health and Nutrition Examination Survey I epidemiologic follow‐up study. Arch Intern Med 163: 1897–1904. [DOI] [PubMed] [Google Scholar]
- Berger A, Jones PJH, Abumweis SS (2004). Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis 3: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch A, Thomson CA (1995). Position of The American Dietetic Association (phytochemicals and functional foods). J Am Diet Assoc 95: 493–496. [DOI] [PubMed] [Google Scholar]
- Bohn T (2014). Dietary factors affecting polyphenols bioavailability. Nutr Rev 72: 429–452. [DOI] [PubMed] [Google Scholar]
- Brendan ME, Smith RJ, Hiatt WR (2015). Reducing LDL with PCSK9 Inhibitors. The clinical benefit of lipid drugs. New Engl J Med 373: 1588–1591. [DOI] [PubMed] [Google Scholar]
- Brown L, Rosner B, Willett WW, Sacks FM (1999). Cholesterol‐lowering effects of dietary fiber: a meta‐analysis. Am J Clin Nutr 69: 30–42. [DOI] [PubMed] [Google Scholar]
- Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y et al. (2006). Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res 47: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Bursill CA, Abbey M, Roach PD (2007). A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol‐fed rabbit. Atherosclerosis 193: 86–93. [DOI] [PubMed] [Google Scholar]
- BVL/BfArM . Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL) und das Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) (2016). http://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Zulassung/ZulRelThemen/abgrenzung/stellungnahmen/201601.pdf;jsessionid=CBC18D6FB04E23CAB8BAE1E1B5BA240C.1_cid340?__blob=publicationFile&v=4. (accessed 7/6/2016).
- Chait A, Eckel RH (2016). Lipids, Lipoproteins, and Cardiovascular Disease: Clinical Pharmacology Now and in the Future. J Clin Endocrinol Metab 101: 804–814. [DOI] [PubMed] [Google Scholar]
- Chen CH, Yang JC, Uang YS, Lin CJ (2013). Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int J Pharm 444: 18–24. [DOI] [PubMed] [Google Scholar]
- Christina AB, Paul DR (2006). Modulation of cholesterol metabolism by the green tea polyphenol (−)‐epigallocatechin gallate in cultured human liver (HepG2) cells. J Agr Food Chem 54: 1621–1626. [DOI] [PubMed] [Google Scholar]
- Cicero FG, Parini A, Rosticci M (2015). Nutraceuticals and cholesterol‐lowering action. IJC Metab Endocr 6: 1–4. [Google Scholar]
- Colonna S, Fulk G, Marangoni F (2013). The food for the health In: The health foods, Springer edn. Milan: Italy, pp. 211–220. [Google Scholar]
- Dàvalos A, Fernàndez‐Hernando C, Cerrato F, Martìnez‐Botas J, Gòmez‐Coronado D, Gòmez‐Cordovès Lasunciò MA (2006). Red grape juice polyphenols alter cholesterol homeostasis and increase LDL‐receptor activity in human cells in vitro. J Nutr 136: 1766–1773. [DOI] [PubMed] [Google Scholar]
- DeBose‐Boyd RA, Ou J, Goldstein JL, Brown MS (2001). Expression of sterol regulatory element‐binding protein 1c (SREBP‐1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc Natl Acad Sci U S A 98: 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice SL (1995). The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol 6: 59–61. [Google Scholar]
- Demonty I, Lin Y, Zebregs YE, Vermeer MA, van der Knaap HC, Jäkel M et al. (2010). The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr 140: 1615–1620. [DOI] [PubMed] [Google Scholar]
- Directive 2002/46/EC (2002). European parliament and of the council on the approximation of the laws of the member states relating to food supplements. Official J L 183: 51–57. [Google Scholar]
- Dong H, Zhao Y, Fuer L (2013). The effects of berberine on blood lipids: a systematic review and meta‐analysis of randomized controlled trials. Planta Med 79: 437–446. [DOI] [PubMed] [Google Scholar]
- DSHEA (1994). United States Food and Drug Administration (FDA). Dietary Supplement Health and Education Act (DSHEA). U.S. Department of Health and Human Services. United States. Public Law 103–417. Available at FDA website: http://www.fda.gov; https://ods.od.nih.gov/About/DSHEA_Wording.aspx (accessed 7/6/2016).
- EFSA Scientific Opinion (2011). EFSA J 9: 2207–2228. [Google Scholar]
- ESCO (2012). European Food Safety Authority scientific cooperation report. Compendium of botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements. EFSA J 10: 2663–2723. [Google Scholar]
- European Commission (2014). Amending Regulation (EC) No 1881/2006 as regards maximum levels of the contaminant citrinin in food supplements based on rice fermented with red yeast. Monascus purpureus. L67/3–67/4.
- European Parliament and Council Regulation (EU) 2015/2283 (2015). Regulation of the 25 November 2015 on novel foods, amending regulation (EU) no 1169/2011 of the European Parliament and of the Council and replacing the regulation (EC) no 258/97 of the European Parliament and of the Council, and the Commission Regulation (EC) No 1852/2001.
- Fan D, Wu X, Dong W, Sun W, Li J, Tang X (2013). Enhancement by sodium caprate and sodium deoxycholate of the gastrointestinal absorption of berberine chloride in rats. Drug Dev Ind Pharm 39: 1447–1456. [DOI] [PubMed] [Google Scholar]
- FDA (2006). Federal Register: May 22, 2006 (volume 71, number 98). Internet: http:// www.fda.gov/ohrms/dockets/98fr/06‐4703.htm.
- GenLiang Y, Li J, Guang Ling Y (2015). Experimental study on acute toxicity of tea polyphenols. J Food Saf Qual 6: 3730–3733. [Google Scholar]
- Gerards M, Terlou RJ, Yu H, Koks CHW, Gerdes VEA (2015). Traditional Chinese lipid‐lowering agent red yeast rice results in significant LDL reduction but safety is uncertain ‐ A systematic review and meta‐analysis. Atherosclerosis 2: 415–423. [DOI] [PubMed] [Google Scholar]
- Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W et al. (2014). Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. (2014). Atherosclerosis 232: 346–360. [DOI] [PubMed] [Google Scholar]
- Hardy G (2000). Nutraceuticals and functional foods: introduction and meaning. Nutrition 16: 688–689. [DOI] [PubMed] [Google Scholar]
- Harnly J (2016). Importance of accurate measurements in nutrition research: dietary flavonoids as a case study. Adv Nutr 7: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health claims : soluble fiber from certain foods and risk of coronary heart disease (CHD); 2008. [21 CFR sect. 101.81].
- Heber D, Yip I, Ashley JM, Elashoff DA, Go VLW (1999). Cholesterol lowering effects of a proprietary Chinese red‐yeast‐rice dietary supplemement. Am J Clin Nutr 69: 231–236. [DOI] [PubMed] [Google Scholar]
- Henning SM, Niu TY, Liu Y, Lee NH, Hara Y, Thames GD et al. (2005). Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J Nutr Biochem 16: 610–616. [DOI] [PubMed] [Google Scholar]
- Ho HVT, Sievenpiper JL, Zurbau A, Mejia SB, Jovanovski E, Au‐Yeung F et al. (2016). A systematic review and meta‐analysis of randomized controlled trials of the effect of barley β‐glucan on LDL‐C, non‐HDL‐C and apoB for cardiovascular disease risk reduction. Eur J Clin Nutr . doi:10.1038/ejcn.2016.89. [DOI] [PubMed] [Google Scholar]
- Jiao R, Zhang Z, Yu H, Huang Y, Chen ZY (2010). Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and upregulation of CYP7A1. J Nutr Biochem 21: 1134–1139. [DOI] [PubMed] [Google Scholar]
- Jiarong L, Yanyun Z, Feixia D, Ziyou Y, Wenjie Z, Jinping F et al. (2015). Meta‐analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol 161: 69–81. [DOI] [PubMed] [Google Scholar]
- Klimek M, Wang S, Ogunkanmi A (2009). Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm Therapeut 34: 313–327. [PMC free article] [PubMed] [Google Scholar]
- Kris‐Etherton PM, Krummel D, Russell ME, Dreon D, Mackey S, Borchers J et al. (1988). The effect of diet on plasma lipids, lipoproteins, and coronary heart disease. J Am Diet Assoc 88: 1373–1400. [PubMed] [Google Scholar]
- Kroft KD (2016). Dietary flavonoids: antioxidants or not? Arch Biochem Biophys 595: 120–124. [DOI] [PubMed] [Google Scholar]
- Kuhn DJ, Burns AC, Kazi A, Dou QP (2004). Direct inhibition of the ubiquitin‐proteasome pathway by ester bond‐containing green tea polyphenols is associated with increased expression of sterol regulatory element‐binding protein 2 and LDL receptor. Biochim Biophys Acta 1682: 1–10. [DOI] [PubMed] [Google Scholar]
- Lachenmeier DW, Monakhova YB, Kuballa T, Loebell‐Behrends S, Maixner S, Kohl‐Himmelseher M et al. (2012). Regulatory evaluation of red yeast rice (Monascus spp) food supplements sold via internet. Dtsch Lebensmitt Rundsch 108: 357–360. [Google Scholar]
- Lee SW, Rho MC, Nam JY, Lim EH, Kwon OE, Kim YH et al. (2004). Guineensine, an Acyl‐CoA: cholesterol acyltransferase inhibitor, from the fruits of Piper longum . Planta Med 70: 678–679. [DOI] [PubMed] [Google Scholar]
- Leifert WR, Abeywardena MY (2008). Grape see and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5‐lipoxigenase activity. Nutr Res 28: 842–850. [DOI] [PubMed] [Google Scholar]
- Lin CC, Li TC, Lai MM (2005). Went rice in subjects with hyperlipidemia. Eur J Endocrinol 153: 679–686. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fonnebo V (2006). Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta‐analysis of randomized controlled trials. Chin Med 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CS, Zheng YR, Zhang YF, Long XY (2016). Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 109: 274–282. [DOI] [PubMed] [Google Scholar]
- Mannarino MR, Ministrini S, Pirro M (2014). Nutraceuticals for the treatment of hypercholesterolemia. Eur J Intern Med 7: 592–599. [DOI] [PubMed] [Google Scholar]
- Marazzi M, Cacciotti L, Pelliccia F, Iaia L, Volterrani M, Caminiti G et al. (2011). Long‐term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv Ther 28: 1105–1113. [DOI] [PubMed] [Google Scholar]
- Merriam‐Webster Online Dictionary (2014). Merriam‐Webster Inc., P.O. Box 281, Springfield, MA 01102, United States.
- Millán J, Pintó X, Muñoz A, Zuniga M, Rubies‐Prat J, Paillardo LF et al. (2009). Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 5: 757–765. [PMC free article] [PubMed] [Google Scholar]
- Mollace V, Sacco I, Janda E, Malara C, Ventrice D, Colica C et al. (2011). Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia 82: 309–316. [DOI] [PubMed] [Google Scholar]
- Nagasako‐Akazome Y, Kanda T, Ikeda M, Shimasaki H (2005). Serum cholesterol‐lowering effect of apple polyphenols in healthy subjects. J Oleo Sci 54: 143–151. [Google Scholar]
- NCEP (2001). Executive summary of the third report of the national cholesterol education program (ncep), expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- Ong YC, Aziz Z (2016). Systematic review of red yeast rice compared with simvastatin in dyslipidemia. J Clin Pharm Ther 41: 170–179. [DOI] [PubMed] [Google Scholar]
- Pang J, Zhang Z, Zheng T, Bassig BA, Mao C, Liu X et al. (2016). Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta‐analysis. Int J Cardiol 202: 967–974. [DOI] [PubMed] [Google Scholar]
- Pascual‐Ahuir A, Vanacloig‐Pedros E, Proft M (2014). Toxicity mechanisms of the food contaminant citrinin: application of a quantitative yeast model. Nutrients 6: 2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL et al. (2004). Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med 164: 370–376. [DOI] [PubMed] [Google Scholar]
- Reiner Z (2014). Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis 24: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O et al. (2011). European Association for Cardiovascular Prevention and Rehabilitation. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 32: 1769–1818. [DOI] [PubMed] [Google Scholar]
- Rocha M, Banuls C, Bellod L, Jover A, Victor VM, Hernandez‐Mijares A (2011). A review on the role of phytosterols: new insights into cardiovascular risk. Curr Pharm Des 17: 4061–4075. [DOI] [PubMed] [Google Scholar]
- Rouyanne TR, Geleijnse JM, Trautwein EA (2014). LDL‐cholesterol‐lowering effect of plant sterols and stanols across different dose ranges: a meta‐analysis of randomised controlled studies. Br J Nutr 112: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini A (2014). Nutraceuticals: An Healthy Bet for the Future. J Food Res 3: 1–2. [Google Scholar]
- Santini A, Raiola A, Meca G, Ritieni A (2015). Aflatoxins, ochratoxins, trichotecenes, patulin, fumonisins and beauvericin in finished products for human consumption. J Clin Toxicol 5: 265–277. [Google Scholar]
- Shaghaghi MA, Abumweis SS, Jones PJ (2013). Cholesterol‐lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta‐analysis. J Acad Nutr Diet 113: 1494–1503. [DOI] [PubMed] [Google Scholar]
- Sherry Chow HH, Iman AH, Donna RV, Crowell JA, Ranger‐Moore J, Chew WM et al. (2005). Effects of dosing condition on the oral bioavailability of green tea catechins after single‐dose administration of polyphenon e in healthy individuals. Clin Cancer Res 11: 4627–4633. [DOI] [PubMed] [Google Scholar]
- Smet ED, Mensink RP, Plat J (2012). Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol Nutr Food Res 56: 1058–1072. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl. Acids Res. 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenore GC, Campiglia P, Stiuso P, Ritieni A, Novellino E (2013). Nutraceutical potential of polyphenolic fractions from Annurca apple (M. pumila Miller cv Annurca). Food Chem 140: 614–622. [DOI] [PubMed] [Google Scholar]
- Tenore GC, Calabrese G, Stiuso P, Ritieni A, Giannetti D, Novellino E (2014). Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: a source of nutraceuticals potentially indicated for metabolic syndrome. Food Res Int 63: 252–257. [Google Scholar]
- Tenore GC, Caruso D, Buonomo G, D'Urso E, D'Avino M, Campiglia P et al. (2016). Annurca (M. pumila Miller cv Annurca) apple as a functional food for the contribution to a healthy balance of plasma cholesterol levels: results of a randomised clinical trial. J Food Sci Agric In press. doi:10.1002/jsfa.8016. [DOI] [PubMed] [Google Scholar]
- Vinogradova Y, Coupland C, Brindle P, Hippisley‐Cox J (2015). Patients who discontinued statin treatment: a protocol for cohort study using primary care data. BMJ Open 5: e008701. doi:10.1136/bmjopen-2015-008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe R, Sotis G (2015). Nutraceuticals: definition and epidemiological rationale for their use in clinical practice. High Blood Pres Cardiovasc Prev 22: 199–201. [DOI] [PubMed] [Google Scholar]
- Weingärtner O, Baber R, Teupser D (2014). Plant sterols in food: no consensus in guidelines. Biochem Biophys Res Commun 446: 811–813. [DOI] [PubMed] [Google Scholar]
- Yasuda A, Natsume M, Osakabe N, Kawahata K, Koga J (2011). Cacao polyphenols influence the regulation of apolipoprotein in HepG2 and Caco2 cells. J Agric Food Chem 59: 1470–1476. [DOI] [PubMed] [Google Scholar]
- Yousaf S, Butt MS, Suleria HAR, Iqbal MJ (2014). The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct 5: 545–556. [DOI] [PubMed] [Google Scholar]
- Zeisel SH (1999). Regulation of “nutraceuticals”. Science 285: 1853–1855. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Tang D, Feng L, Zheng ZG, Wang RS, Wu AG et al. (2013). Development of self‐microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev Ind Pharm 39: 499–506. [DOI] [PubMed] [Google Scholar]


