Abstract
In the past few years, increasing interest has been directed to bioactive peptides of animal and plant origin: in particular, researchers have focused their attention on their mechanisms of action and potential role in the prevention and treatment of cancer, cardiovascular and infective diseases. We have developed a search strategy to identify these studies in PubMed (January 1980 to May 2016); particularly those papers presenting comprehensive reviews or meta‐analyses, plus in vitro and in vivo studies and clinical trials on those bioactive peptides that affect cardiovascular diseases, immunity or cancer, or have antioxidant, anti‐inflammatory and antimicrobial effects. In this review we have mostly focused on evidence‐based healthy properties of bioactive peptides from different sources. Bioactive peptides derived from fish, milk, meat and plants have demonstrated significant antihypertensive and lipid‐lowering activity in clinical trials. Many bioactive peptides show selective cytotoxic activity against a wide range of cancer cell lines in vitro and in vivo, whereas others have immunomodulatory and antimicrobial effects. Furthermore, some peptides exert anti‐inflammatory and antioxidant activity, which could aid in the prevention of chronic diseases. However, clinical evidence is at an early stage, and there is a need for solid pharmacokinetic data and for standardized extraction procedures. Further studies on animals and randomized clinical trials are required to confirm these effects, and enable these peptides to be used as preventive or therapeutic treatments.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- APO
apolipoprotein
- BBI
Bowman‐Birk inhibitor
- Bcl
B cell lymphoma
- C33‐A
cervical carcinoma cell line
- CYPTA
cholesterol 7α‐hydroxylase
- eNOS
endothelial NOS
- HT
human colon adenocarcinoma cell line
- Sw480
human colon carcinoma cell line
- U87
U87‐MG human glioma cells
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes e |
| Bax | ACE |
| Bcl‐2 | Akt |
| NPC1L1 | Caspase 3 |
| TNF‐α | Caspase 2 |
| GPCRs b | Caspase 9 |
| D1 receptor | COX‐2 |
| NTS2 receptor | eNOS |
| Other ion channels c | FAK |
| Cx43 | GSK3β |
| Transporters d | HMG‐CoA reductase |
| PepT1 (PEPT1) | IKK‐α |
| IKK‐ß | |
| iNOS | |
| JNK | |
| PI3K |
| LIGANDS | |
|---|---|
| CCL2 (MCP‐1) | IL‐1β |
| CCL5 (RANTES) | IL‐2 |
| cGMP | IL‐5 |
| ICAM‐I | IL‐6 |
| IFN‐γ | VCAM‐I |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
There is an abundance of bioactive peptides contained in a wide range of food sources (products of plant, animal and marine origin) and generated by fermentation, enzymatic, chemical hydrolysis or gastrointestinal digestion processes from food proteins (Udenigwe and Aluko, 2012). In recent years, there has been a marked increase in the number of publications highlighting their potential effects on blood pressure (BP) and lipid metabolism, in addition to their anticancer, immunomodulatory, antimicrobial, analgesic, antioxidant and anti‐inflammatory activity. In particular, interest has been directed towards their effects on hypertension and dyslipidaemia, which are two of the most important risk factors for cardiovascular diseases and represent a major cause of mortality in developed countries. There is now much evidence that many non‐pharmacological treatments, including bioactive peptides, have the ability to regulate BP and lipids levels (Cicero and Colletti, 2015a,b).
Together with cardiovascular diseases, cancer is one of the main causes of death in developed countries (Siegel et al., 2013). Conventional chemotherapy remains one of the principle treatments, but it is usually associated with significant side effects, because it is often not selective for cancer cells (Holohan et al., 2013). The anticancer effects of peptides have been extensively explored (Boohaker et al., 2012; Gautam et al., 2014), and there is an increasing number of approved peptide‐based drugs (Vlieghe et al., 2010). These drugs have great potential because they exhibit antitumoral activity (Thundimadathil, 2012) by acting on multiple molecular pathways involved in carcinogenesis and are usually not genotoxic (Blanco‐Míguez et al., 2016).
Another important field for the application of bioactive peptides is the regulation of immunity and the prevention of infections. An important advantage in the use of bioactive peptides for reinforcing immunity is that they have a large spectrum of activity and have effects on both non‐specific and specific immunity (Yang et al., 2009; Wang et al., 2010; Cheung et al., 2015). Bioactive peptides have also been demonstrated to have analgesic, antioxidant and anti‐inflammatory activity both in vitro and in vivo (Chakrabarti et al., 2014).
In this context, the aim of this review is to analyse the results of the studies and clinical trials conducted with bioactive peptides, to evaluate their mechanism of action and their potential role in the prevention and treatment of a wide range of pathologies.
Methods
A search strategy was developed to identify trials in PubMed (January 1980 to May 2016).
Firstly, the authors assessed the major fields of study of bioactive peptides through the MeSH terms (major subject heading) ‘bioactive peptides’ and ‘review’ or ‘meta‐analysis’. Then the search was refined to evaluate clinical trials: the MeSH terms ‘bioactive peptides’ and ‘cardiovascular diseases’ or ‘hypertension’ or ‘dyslipidaemia’ or ‘immunity’ or ‘cancer’ or ‘analgesic’ or ‘antimicrobial’ or ‘antioxidant’ or ‘anti‐inflammatory’ were incorporated into an electronic search strategy. The bibliographies of all the studies and review articles identified were examined to obtain additional studies of interest. The authors reviewed all of the citations retrieved from the electronic search to identify potentially relevant articles for this review. They subsequently reviewed the potential trials to determine their eligibility. They selected papers reporting comprehensive reviews or meta‐analyses, or original in vitro and in vivo studies and clinical trials on bioactive peptides with an action on cardiovascular diseases, immunity and cancer or with antioxidant, anti‐inflammatory and antimicrobial activity. Studies were also selected based on the quality of the methodology, data completeness and extent of sampling.
Blood pressure lowering effect
According to the European guidelines for hypertension management (Zannad et al., 2012), the nutraceutical approach could be a good compromise for patients with borderline values of BP and an adjuvant in combination with antihypertensive drugs in the treatment of moderate hypertension (Sirtori et al., 2015; Borghi and Cicero, 2016). The use of nutraceuticals in the treatment or prevention of hypertension would result in a reduction in the typical side effects of conventional drugs (cough, skin rashes, hyperkalaemia, loss of taste, sleep apnoea, erectile dysfunction and angioedema) and hypothetically have economic savings on health expenditure due to a potential reduction in cardiovascular disease (Houston, 2013).
Over the past few years, researchers have investigated different types of bioactive peptides derived from heterogeneous sources, such as fish, milk, meat and plant derivatives, which have potential antihypertensive activity (Hartmann, Meisel, 2007; Bhat et al., 2015). The antihypertensive action of some bioactive peptides is now known to be due to a competitive and/or non‐competitive inhibition of ACE, which is responsible for the conversion of angiotensin I in angiotensin II. Angiotensin II is an octapeptide that increases the peripheral vascular resistance and the preload, inducing a hypertensive action. Furthermore, ACE determines the cleavage and inactivation of bradykinin, a vasodilator peptide. Other putative mechanisms of action are attributed to an increase in the activity of certain vasodilating agents including endothelial NOS (eNOS); the increased production of endothelial NO and the inhibition of renin, which converts angiotensinogen to angiotensin I, increases the substrate of ACE. Furthmore, bioactive peptides can act to reduce the activity of the sympathetic system, inducing vasodilatation (Aluko, 2015).
The clinical efficacy of antihypertensive bioactive peptides depends substantially on two factors: their resistance to degradation by gastrointestinal peptidases and their absorption into the blood stream. The absorption of these peptides may be carried by a transporter peptide [peptide transporter 1(PEPT1), for peptides with a maximum of three aminoacids], by pinocytosis (highly soluble peptides) or by the paracellular (through the aqueous transport) or transcellular routes (Rotimi, 2015). Based on these two factors and the amino acid sequence of the bioactive peptide, the clinical results in terms of BP reduction will be different.
The main bioactive peptides used in the treatment/prevention of hypertension, the source from which they were extracted and their mechanism of action are presented in Figure 1.
Figure 1.
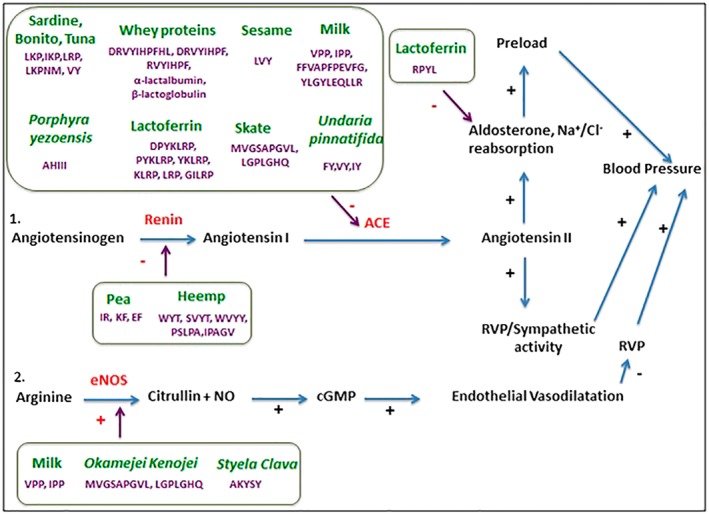
Main bioactive peptides that have been shown to lower blood pressure: proposed mechanisms of action. ACE, angiotensin converting enzyme; eNOS, endothelial NOS; RVP, renal venous pressure; EF, glutamate‐ phenylalanine; FY, phenylalanine‐ tyrosine; IKP, isoleucine‐lysine‐proline; IPP, isoleucine‐proline‐proline; IR, isoleucine‐arginine; IY, isoleucine‐tyrosine; KF, lysine‐phenylalanine; LKP, leucine‐lysine‐proline; LRP, leucine‐arginine‐proline; LVY, leucine‐valine‐tyrosine; VPP, valine‐proline‐proline; VY, valine‐tyrosine; WYT, tryptophan‐tyrosine‐threonine.
One of the richest sources of proteins and bioactive peptides is milk that contains a large number of peptides including the tripeptides valine‐proline‐proline (VPP) andisoleucine‐proline‐proline (IPP), and the polypeptides phenylalanine‐phenylalanine‐valine‐alanine‐proline‐phenylalanine‐proline‐glutamate‐valine‐phenylalanine‐glycine‐lysine (FFVAPFPEVFGK) and tyrosine‐leucine‐glycine‐tyrosine‐leucine‐glutamate‐glutamine‐leucine‐leucine‐arginine (YLGYLEQLLR) peptides; there are numerous clinical trials that have evaluated their effect on the major cardiovascular parameters (Cicero et al., 2013). In particular, the tripeptides VPP/IPP have been shown (at dosages between 5 and 100 mg·day−1) to have a variable clinical efficacy, more evident in Asian subjects (suggesting a possible genetic/population‐dependent effect, as already seen for some antihypertensive drugs); in a meta‐analysis by our group considering 18 randomized clinical trials, the pooled effect of peptides was a reduction of −3.73 mmHg (95% CI: −6.70, −1.76) for systolic BP and 1.97 mmHg (95%CI: −3.85, −0.64) for diastolic BP (Cicero et al., 2011a). More recent data show that these peptides could also positively modulate pulse wave velocity in mildly hypertensive subjects. No safety concerns were raised (Cicero et al., 2011b, 2016).
Whey proteins are also a rich source of bioactive peptides; they can be converted into peptides through different types of treatments (enzymatic hydrolysis by trypsin, alcalase and pepsin); in particular, the aspartate‐arginine‐valine‐tyrosine‐isoleucine‐histidine‐proline‐phenylalanine‐histidine‐leucine (DRVYIHPFHL), aspartate‐arginine‐valine‐tyrosine‐isoleucine‐histidine‐proline‐phenylalanine (DRVYIHPF) and arginine‐valine‐tyrosine‐isoleucine‐histidine‐proline‐phenylalanine (RVYIHPF) peptides (respectively a deca, octa and heptapeptide) have shown antihypertensive activity with an inhibitory action on the renin‐angiotensin‐aldosterone (RAS) system (Yadav et al., 2015). In general, whey and caseins proteins have significant antihypertensive effects both in normotensive/pre‐hypertensive and in obese subjects (Bhat et al., 2015; Nongonierma and FitzGerald, 2015). Moreover, numerous studies have reported that biological active peptides isolated from the whey of cow's milk can affect BP. Studies on animals and humans have shown that α‐lactalbumin and β‐lactoglobulin, which are obtained from enzymatically hydrolysed whey, are able to inhibit ACE, while lactorphins lower BP by normalizing endothelial function or by an opioid receptor‐dependent mechanism (Dong et al., 2013).
Several marine peptides with antihypertensive activity have been detected in species such as bonito, tuna and sardine (leucine‐lysine‐proline ‐ LKP, isoleucine‐lysine‐proline ‐ IKP, leucine‐arginine‐proline ‐ LRP), Okamejei kenojei (methionine‐valine‐glycine‐serine‐alanine‐proline‐glycine‐valine‐leucine – MVGSAPGVL, leucine‐glycine‐proline‐leucine‐glycine‐histidine‐glutamine – LGPLGHQ) and Styela clava (alanine‐histidyne‐isoleucine‐isoleucine‐ilesoleucine – AHIII); the presence of these bioactive peptides has led to an increase of endothelial NO levels and aorta vasodilatation in rats (Cheung et al., 2015).
Finally, numerous plant species provide a wide range of bioactive peptides and proteins. The intake of plant proteins (in particular, soy, oak, barley and pea proteins) seems to be associated with a mild but significant lowering of BP levels (Altorf‐van der Kuil et al., 2010; Malaguti et al., 2014; Nirupama, et al., 2015). In particular, those peptides extracted from cereals, such as oats and barley, isoleucine‐valine‐tyrosine (from wheat germ), isoleucine‐aspartate‐proline show a strong inhibitory action on ACE (Motoi and Kodama, 2003; Nirupama et al., 2015). However, it is not easy to discriminate between the effect of plant proteins and other associated dietary components on BP levels. For example, the isoflavones taken with soy could really be responsible for the soy‐related decrease in BP; a recent meta‐analysis has shown that in hypertensive patients the intake of soy isoflavones is associated with a decrease in SBP by – 5.94 mmHg (95% CI: −10.55, −1.34) (P = 0.01) and of DBP by −3.35 mmHg (95% CI: −6.52, −0.19) (P = 0.04) (Liu et al., 2012).
In conclusion, peptides derived from milk, whey, fish and plants have demonstrated a mild but significant antihypertensive effect in humans, based on the inhibition of the RAS system and/or an increase in endothelial NO levels. These data have been confirmed through further long‐term randomized clinical trials in normotensive and pre‐hypertensive patients.
Cholesterol‐lowering effect
Another important cardiovascular risk factor is represented by dyslipidaemia. The bioactive peptides with the most clinical evidence for inducing a reduction in cholesterolaemia are those derived from soy, lupine and milk proteins (Lammi et al., 2014; Butteiger et al., 2016).
A recent meta‐analysis included 35 studies that examined the effects of soy protein (in particular B‐conglycinin globulin) on lipid parameters, with treatments that varied from 4 months to 1 year. The results showed that soy proteins have a cholesterol‐lowering effect with a reduction in LDL‐cholesterol (LDL‐C) of 3% (−4.83 mg·L−1; 95% CI: −7.34, −2.31), in total cholesterol (TC) of 2% (−5.33 mg·L−1; 95% CI: −8.35, −2.30) and in triacylglycerol of 4% (−4.92 mg·L−1; 95% CI: −7.79, −2.04); moreover, a significant improvement in HDL‐cholesterol (HDL‐C) of 3% (1.40 mg·L−1; 95% CI: 0.58, 2.23) was observed. The LDL‐C reduction was greater in moderately hypercholesterolaemic patients (−7.47 mg·L−1; 95% CI: −11.79, −3.16) compared with healthy subjects (−2.96 mg·L−1; 95% CI: −5.28, −0.65) (Tokede et al., 2015).
In particular, among the bioactive peptides derived from soy, lunasin, a 43‐aminoacid peptide characterized by a RGD sequence followed by eight aspartate residues at its carboxyl‐end with high bioavailability and stability (Hernández‐Ledesma et al., 2013), showed a potential cholesterol‐reducing activity; however, it is important to emphasize that studies conducted with this peptide have hitherto involved animal models and cell lines (Lule et al., 2015). Tests are therefore necessary to assess its efficacy and safety in clinical practice.
The main mechanisms of action whereby soy and lupine peptides reduce blood cholesterol levels can be attributed to an up‐regulation of LDL receptors, the regulation of the sterol regulatory element‐binding protein 2 (SREBP2) pathway (via PI3K/Akt/GSK3β pathways), the inhibition of the hydroxymethylglutaril‐CoA (HMG‐CoA) reductase enzyme and an increase in the faecal excretion of bile salts (Marsh et al., 2011; Lammi et al., 2014).Therefore, the up‐regulation in the transcription of LDL receptors results in an enhanced catabolism or a reduced synthesis of intracellular cholesterol (Cho et al., 2007).
The proteins derived from lupine (50 mg·day−1) demonstrated LDL‐C lowering efficacy in a rat model; the conglutin γ (isolated from lupine) was also found to increase the number of LDL receptors in a hepatoma G2 cell line (Sirtori et al., 2012).
Another peptide with cholesterol‐lowering activity is derived from the hydrolyzate extracted of Mucuna pruriens; it interacts with micelle formation and the absorption of exogenous cholesterol (Herrera Chalé et al., 2016). Peptides from cowpea have also been demonstrated to inhibit cholesterol synthesis and its solubilization into micelles (Marques et al., 2015).
A bioactive peptide derived from milk with cholesterol‐lowering action is β‐lactotensin; at a dose of 100 mg·Kg−1 p.o., it significantly reduced serum cholesterol in mice and increased the excretion of bile acids in the faeces (Yamauchi et al., 2003). Its cholesterol‐lowering effect is probably due to its effects on neurotensin NTS2 and dopamine D1 receptors, which leads to an increased synthesis of bile acids from cholesterol, enhanced further by the direct action of β‐lactotensin on mRNA (induced by increasing levels of CYP7A)(Yoshikawa, 2015) (Figure 2).
Figure 2.
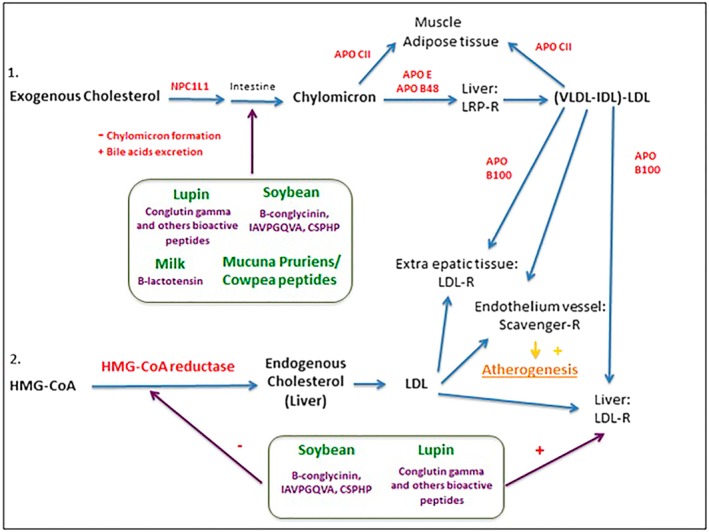
Main bioactive peptides that have been shown to have a beneficial effect on cholesterol metabolism: proposed mechanisms of action. APO, apolipoprotein; HMG‐CoA, hydroxymethylglutaril‐CoA; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LDL‐R, low‐density lipoprotein‐receptor; LRP‐R, low‐density lipoprotein receptor‐related protein – receptor; NPC1L1, Niemann‐pick C1‐like 1; VLDL, very low‐density lipoprotein.
It is concluded that the mechanisms of action of lipid‐lowering peptides are clear thanks to the numerous studies performed in vitro and in animal models; they mainly act by inhibiting the production of endogenous cholesterol and promoting the faecal excretion of exogenous cholesterol. However, it is still necessary to evaluate their pharmacokinetics profiles, bioavailability and long‐term effective dosages in humans.
Anti‐inflammatory activity
Recent in vitro and in vivo studies have shown a potential anti‐inflammatory activity of several bioactive peptides derived mostly from bovine milk, eggs, soy and fish (Marcone et al., 2016); nevertheless, the mechanisms of action are known for only a few of these peptides. The available evidence suggests that the anti‐inflammatory activity of bioactive peptides is mainly due to the modulation of transcription factors, kinases (NF‐kB and MAPK) and/or cytosolic compounds (Figure 3). However, the mechanism through which these peptides penetrate the cell is unclear; it is not known if they act directly on the cell membrane or whether they interact with different receptors (Majumder et al., 2016).
Figure 3.
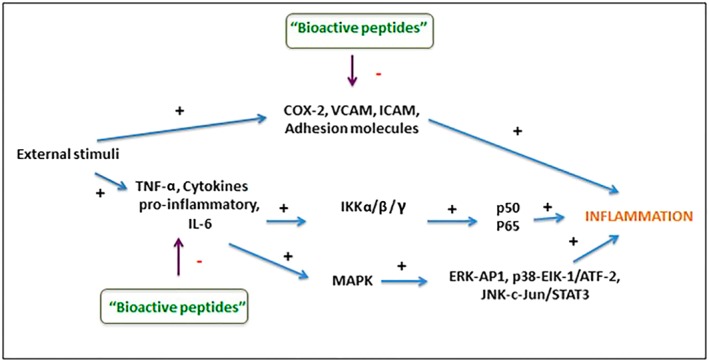
Anti‐inflammatory effects of bioactive peptides: main proposed mechanisms of action. ERK‐AP1, ERK‐activator protein 1; ICAM, intracellular adhesion molecule; IKK, IκB kinase; p38‐Elk‐1/ATF‐2, protein 38‐Elk‐1/activating transcription factor 2; VCAM, vascular cell adhesion molecule.
IPP and VPP peptides, derived from the bacterial fermentation of casein, show anti‐inflammatory activity; they prevent the formation of atherosclerotic plaque by inhibiting the pro‐inflammatory JNK‐MAPK pathway (Aihara et al., 2009). Another polypeptide (aspartate‐methionine‐proline‐isoleucine‐glutamine‐alanine‐phenylalanine‐leucine‐leucine‐tyrosine‐glutamine‐glutamate‐proline‐valine‐leucine‐glycine‐proline‐valine‐arginine – DMPIQAFLLYQEPVLGPVR) derived from β‐casein exerts an anti‐inflammatory action through the inhibition of NF‐kB pathway (Malinowski et al., 2014). In humans, the administration of proteins derived from milk reduces postprandial inflammation in obese and non‐diabetic subjects, as indicated by a reduction in the serum inflammatory biomarkers monocyte chemoattractant protein‐1 (MCP‐1, also known as CCL2) and chemokine (C‐C motif) ligand 5 (CCL5, also known as RANTES) (Holmer‐Jensen et al., 2011).
A tripeptide derived from ovotransferrin, which is present in the albumen of eggs, can reduce the expression of factors such as NF‐kB (which inhibits the nuclear translocation of p50 and p65) that mediate the transcription of adhesion molecules [vascular cell adhesion molecule 1 (VCAM1) and intracellular adhesion molecule 1 (ICAM‐1)] (Majumder et al., 2013).
Peptides derived from soy, beans and milk have also shown anti‐inflammatory effects on intestinal inflammation; among their mechanisms of action, these peptides may inhibit the expression of pro‐inflammatory cytokines and chemokines. In particular, ɣ‐glutamyl cysteine, a peptide isolated from various food sources including the edible beans, inhibits the phosphorylation of JNK and IkB, unlike valine‐proline‐tyrosine (VPY) that exerts its anti‐inflammatory action by inhibiting the secretion of IL‐8 and TNF‐α (Majumder et al., 2016).
Lunasin, has shown anti‐inflammatory properties, probably due to the inhibition of IL‐6 and IL‐1β production, activation of NF‐kB Akt‐mediated (interaction with αVβ3 integrin), COX‐2 and inducible NOS expression and PGE2 production (Dia et al., 2009; Hernandez‐Ledesma et al., 2009; Cam and de Mejia, 2012).
Although the anti‐inflammatory mechanisms of action of bioactive peptides are not completely clarified, they seem to be related to the modulation of transcription factors and the inhibition of the expression of pro‐inflammatory cytokines and chemokines. Data in humans are, however, at the preliminary stage.
Anticancer effect
Bioactive peptides have demonstrated their potential to exhibit cytotoxic activity in numerous cancer cell lines and, consequently, induce possible cancer‐selective activity that may be devoid of the side effects of conventional chemotherapy. Peptides may be utilized directly as cytotoxic agents through various mechanisms or may act as carriers of cytotoxic agents and radioisotopes by specifically targeting cancer cells (Thundimadathil, 2012). Their advantages include low intrinsic toxicity, high tissue penetration for their small size, cell diffusion and permeability (Mader and Hoskin, 2006; Otvos, 2008). They are also able to affect one or more specific molecular pathways involved in carcinogenesis, and they are not usually genotoxic (Blanco‐Míguez et al., 2016).
Their main mechanisms of action are inhibition of cell migration, inhibition of tumour angiogenesis, antioxidant activity, the inhibition of gene transcription/cell proliferation, the induction of apoptosis, and the disorganization of tubulin structure and cytotoxicity (Table 1) (Schweizer, 2009; Tyagi et al., 2015).
Table 1.
Main bioactive peptides with anticancer activity: mechanisms of action
| Source | Bioactive peptide | Mechanism of action | Effect | Model | Reference |
|---|---|---|---|---|---|
| Soybean | Lunasin | Cell proliferation and cancerous foci formation inhibition | Cytotoxic | 7,12‐dimethylbenz(a)anthracene (DMBA) and 3‐methylcholanthrene‐treated (MCA) fibroblast NIH/3 T3 cells | Hsieh et al., 2010 |
| Soybean | Lunasin | Direct binding with α5β1 integrin suppressing FAK/ERK/ NF‐κB signalling | Metastasis inhibition | Human colon cancer cells | Dia and de Mejia, 2011 |
| Soybean | Lunasin | Apoptosis through caspase‐3 activation | Cytotoxic | L1210 Leukaemia cells | de Mejia et al., 2010 |
| Soybean | Lunasin | Apoptosis through caspase‐3 activation | Cytotoxic | HT‐29 colon cancer cells | Dia and Mejia, 2010 |
| Soybean | Lunasin | Histone acetylation inhibition | Anticancer | Mice | Jeong et al., 2002 |
| Soybean | Lunasin | Inhibition of cell proliferation and arrest of cell cycle at S‐phase | Anticancer | Xenograft MDA‐MB‐231 or chemically induced breast cancer mice | Hsieh et al., 2010 |
| Soybean | Lunasin | Synergistic effect with IL‐2 cytokine in reducing tumour Volume | Reduce lymphoma volume | Xenograft Raji mice lymphoma model | Chang et al., 2014 |
| Soybean | Lunasin | Induction of the apoptotic mitochondrial pathway by modulating expression of Bcl‐2, Bax, nCLU,cytochrome c and caspase‐2, −3 and −9; Arrest cell cycle at G2/M phase; cytotoxic effects | Delay liver metastasis | Colon cancer KM12L4 cells directly injected into athymic mice | Dia and de Mejia, 2011 |
| Rice bran | Glu‐Gln‐Arg‐Pro‐Arg | Cancer growth inhibition | Cytostatic | Colon cancer cells (Caco‐2, HCT‐116), breast cancer cells (MCF‐7, MDA‐MB‐231), liver cancer cells (HepG‐2) | Kannan et al., 2010 |
| Soybean | Bowman‐Birk inhibitor | Tumour suppressor gene Cx43 and cell cycle arrest in G1/S phase induction | Cytostatic and cytotoxic | Human osteosarcoma cells and in M5067 ovarian sarcoma mouse model | Chen et al., 2005; Saito et al., 2007; Suzuki et al., 2005. |
| Soybean | Bowman‐Birk inhibitor | Apoptosis induction by crossing the membrane of the breast cancer cells and co‐localizing with the proteasome in cytoplasm and mainly in nucleus | Cytostatic and cytotoxic | MCF‐7 breast cancer cells | Souza Lda et al., 2014 |
| Soybean | Bowman‐Birk inhibitor | Unknown | Decrease in serum PSA levels | Humans with benign prostatic hyperplasia | Malkowicz et al., 2001 |
| Soybean | Bowman‐Birk inhibitor | Unknown | Clinical effect against oral leukoplakia and effects on potential biomarkers | Humans with oral leukoplakia | Armstrong et al., 2000 |
| Tepary bean | Lectins | Colony formation inhibition | Cytostatic and cytotoxic | C33‐A and Sw480 cell lines | Valadez‐Vega et al., 2011 |
| Mistletoe | Lectins | Delayed development of colon cancer | Immunomodulatory activity | Mice model | Ma et al., 2008 |
| Mistletoe | Lectins | Improvement of the survival | Immunomodulatory activity | Mice with leukaemia cells | Seifert et al., 2008 |
| Whey | Lactoferrin | Arrests cell cycle at the G1/S transition | Inhibit breast cancer MDA‐MB‐231 cells growth | Breast cancer MDA‐MB‐231 cells | Damiens et al., 1999 |
| Whey | Lactoferrin | Suppresses Akt signalling | Inhibit nasopharyngeal carcinoma cells growth | Nasopharyngeal carcinoma cells | Deng et al., 2013 |
| Whey | Lactoferrin | Increase in IL‐8 and activation of natural killer and CD8+ T‐cells | Anticancer activity | Mice model of head‐and‐neck squamous cell carcinoma | Varadhachary et al., 2004 |
| Whey | Lactoferrin | Unclear | Improvement of the chemotherapeutic effects of tamoxifen | 4 T1 breast cancer xenograft Balb/c mice model | Sun et al., 2012 |
| Whey | Lactoferricin | Induction of apoptosis, modulation of gene expression, prevention of angiogenesis and ability to arrest cell cycle | Cytotoxic | different types of cancer cell lines | de Mejía and Dia, 2010 |
| Whey | Lactoferricin | — | Inhibit spontaneous B16‐BL6 melanoma cells growth and to suppress L5178Y‐ML25 lymphoma metastases in liver and lung | Mice | Yoo et al., 1997 |
| Casein | β‐Casomorphin 7 and β‐Casomorphin 5 | Arrest cell cycle | Cytostatic | Breast cancer cells | Hatzoglou et al., 1996 |
| Casein | β‐Casomorphin 7 and β‐Casomorphin 5 | Induce apoptosis | Cytotoxic | Intestinal tumour HT‐29 and AZ‐97 cells | Perego et al., 2012 |
| Whey | α‐lactalbumin | Activation of apoptosis in tumour cells, but spares healthy cells | Anticancer activity | 40 different lymphoma and carcinoma cell lines | Fast et al., 2005 |
| Egg | Egg yolk hydrolyzates | Antioxidant activity | Inhibit tumour cells proliferation of colorectal cancer | Rats | Azuma et al., 2000 |
| Egg | Lysozyme | Indirect action mediated by the induction of host responses. | Reduce the formation of lung metastases of B16 melanoma | Mice bearing B16 melanoma | Sava, 1989 |
| Egg | Ovomucin | Slight activation of the immune system. | Inhibit tumour growth | Mice | Watanabe et al., 1998 |
| Egg | Lysozyme | Immunostimulator | Improve the effectiveness of chemotherapy treatments on primary tumor growth and on lung metastasis formation and particularly on the postsurgical survival time | Mice bearing advanced mammary carcinomas and treated with 5‐fluorouracil | Sava et al., 1995 |
| Marine sponge | Hemiasterlin | Spindle microtubule dynamics inhibition (Hemiasterlin A produced abnormal mitotic spindles), arrest in mitotic metaphase | Cytotoxic | MCF‐7 human mammary carcinoma cells | Anderson et al., 1997 |
| Dolabella auricularia | Dolastatin 10 | Tubulin polymerization inhibition and growth of L1210 murine leukaemia cells inhibition | Cytostatic | Murine leukaemia cells in culture | Bai et al., 1990 |
| Red Sea Moses sole | Pardaxin | Induction of c‐FOS | Cytotoxic | Cancer cell lines | Ting et al., 2014 |
| Oyster | Hydrolysates | Unclear | Cytostatic | BALB/c mice (sarcoma‐S180) | Wang et al., 2010 |
| Tuna dark muscle | Hydrolysates | Unclear | Cytostatic | Human breast cancer cell line MCF‐7 | Kuo‐Chiang Hsu et al., 2011 |
| Giant squid | Esperase hydrolysate | Radical scavenging and metal chelating capacity | Cytotoxic | MCF‐7 (human breast carcinoma) and glioma cell lines | Alemán et al., 2011 |
| Bullacta exarata | BEPT II and BEPT II‐1 | Induction of apoptosis | Cytotoxic | Prostate cancer cells | Ma et al., 2013 |
| Arca subcrenata Lischke | Polypeptide P2 | Proliferation inhibition of HeLa and HT‐29 cell lines | Cytotoxic | Sarcoma S‐180‐bearing mice | Hu et al., 2012 |
| Reniochalina stalagmitis | Reniochalistatins A‐E | Unclear | Cytotoxic | Several tumour cell lines (RPMI‐8226, MGC‐803, HL‐60, HepG2 and HeLa) | Zhan et al., 2014 |
| Aplidium albicans | Aplidine | Cell proliferation inhibition, apoptosis induction and cell cycle arrest | Anticancer | Breast, melanoma and lung cancer cells in humans | García‐Fernández et al., 2002 |
Bioactive peptides with potential cytotoxic activity can be derived from plants, milk, eggs and marine organisms. Among the peptides of plant origin, particular interest has been directed towards lunasin that has exerted anti‐neoplastic effects in breast, skin, colon, prostate, leukaemia and lymphoma cell lines with different mechanisms of action (Hernandez‐Ledesma and Hsieh, 2015). In mouse fibroblast, NIH 3 T3 and human breast Michigan cancer foundation‐7 (MCF‐7) cells, lunasin suppresses their transformation, induced by chemical carcinogens, by inhibiting histone acetylation in the presence of the histone deacetylase inhibitor sodium butyrate (Jeong et al., 2002; Hsieh et al., 2010; Jeong et al., 2010); in L1210 leukaemic cells, it has shown a cytotoxic effect, inducing cell cycle arrest in G2/M phase and apoptosis through the activation of caspase‐3 (de Mejia et al., 2010). Moreover, in the human colon adenocarcinoma cell line, HT‐29, lunasin induced apoptosis by the activation of caspase‐3 through the intrinsic apoptotic pathway, as indicated by the induction of B cell lymphoma 2 (Bcl‐2)‐associated X (Bax) protein and a reduction in Bcl‐2 protein levels (Dia and Mejia, 2010); it is also able to inhibit the metastasis of human colon cancer cells by direct binding with α5β1 integrin, suppressing focal adhesion kinase (FAK)/ERK/NF‐κB signalling and to potentiate the effect of oxaliplatin in preventing the outgrowth of metastasis (Dia and de Mejia, 2011).
In vivo studies have confirmed the anti‐carcinogenic effects of lunasin. For example, in mice models lunasin has been shown to act against chemically‐induced breast cancer (Hsieh et al., 2010), to reduce lymphoma volume (Chang et al., 2014) and to delay liver metastasis of colon cancer KM12L4 cells (Dia and de Mejia, 2011).
Besides lunasin, a pentapeptide Glutammate‐Glycine‐Arginine‐Proline‐Arginine, extracted from rice bran, demonstrated to cause the 84% inhibition of the growth of colon cancer cells (Caco‐2 and human colorectal adenocarcinoma cell line, HCT‐116), 80% of the growth of breast cancer cells (MCF‐7, MDA‐MB‐231) and 84% of that of liver cancer cells (HepG‐2) at a dose of 600–700 µg·mL−1 (Kannan et al., 2010).
Bioactive peptides are also contained in legume seeds, which are a source of protease inhibitors, for example the Bowman‐Birk inhibitor (BBI). BBI has a well‐characterized ability to inhibit trypsin and chymotrypsin activities and seems to have a preventive effect against prostate, breast and colon cancers (Park et al., 2005a,b) and to be an effective suppressor of carcinogenesis. Its mechanism of action is based on its ability to induce the tumour suppressor gene connexin 43(Cx43) and cell cycle arrest in the G1/S phase, as demonstrated in human osteosarcoma cells and in the M5067 ovarian sarcoma mouse model (Chen et al., 2005). Moreover, BBI demonstrated a cytostatic and cytotoxic effect in MCF‐7 breast cancer cells by means of apoptosis; it is able to cross the membrane of the breast cancer cells and co‐localizes with the proteasome in cytoplasm and mainly in the nucleus (Souza Lda et al., 2014).
The use of BBI concentrate in clinical trials as a New Investigational Drug was approved by the Food and Drug Administration (FDA). Human trials in patients with benign prostatic hyperplasia (Malkowicz et al., 2001) or oral leukoplakia (Armstrong et al., 2000) have also shown its safety and tolerability over a prolonged period of time.
Another group of plant‐derived bioactive proteins is represented by lectins, which are able to recognize specific carbohydrate moieties displayed by malignant cells or tissues. For example, tepary bean (Phaseolus acutifolius) lectins have demonstrated antiproliferative and cytotoxic effects on the cervical carcinoma cell line, C33‐A, and human colon carcinoma cell line, Sw480, by the inhibition of colony formation (Valadez‐Vega et al., 2011). Lectins derived from mistletoe have been studied in a mouse model and demonstrated an immunomodulatory effect that delayed the development of colon cancer (Ma et al., 2008) and improved the survival of mice with leukaemia cells (Seifert et al., 2008).
The most studied peptides of animal origin derived from milk, eggs and marine species.
Milk‐derived peptides showed interesting chemopreventive effects. In particular, lactoferrin, a whey protein, has been demonstrated, in vitro, to inhibit the growth of breast cancer (MDA‐MB‐231) and nasopharyngeal carcinoma cells by, respectively, arresting the cell cycle at the G1/S transition and suppressing Akt signalling (Damiens et al., 1999; Deng et al., 2013). Studies in mouse model in vivo confirmed its anticancer activity against head‐and‐neck squamous cell carcinoma (Varadhachary et al., 2004) and demonstrated an improvement in the chemotherapeutic effects of tamoxifen in 4 T1 breast cancer (Sun et al., 2012).
Lactoferricin has been demonstrated to act against different types of cancer cell lines through the induction of apoptosis, the modulation of gene expression, the prevention of angiogenesis and its ability to arrest the cell cycle (de Mejía and Dia, 2010). Studies in mice in vivo demonstrated that it is able to inhibit spontaneous melanoma cells growth and to suppress lymphoma metastasis in the liver and lung (Yoo et al., 1997).
Casein‐derived peptides, β‐casomorphin 7 and β‐casomorphin 5, are able to arrest the cell cycle in breast cancer cells (Hatzoglou et al., 1996) and to induce apoptosis in intestinal tumour HT‐29 and AZ‐97 cells (Perego et al., 2012). Moreover, a whey protein, α‐lactalbumin, showed chemopreventive properties in vitro through the activation of apoptosis (Fast et al., 2005).
Interesting results have been reported with egg‐derived peptides, in particular, hydrolysates from egg yolk protein, lysozyme and ovomucin. Studies in rats showed that hydrolysates from egg yolk protein are able to inhibit the proliferation of tumour cells in colorectal cancer, probably through its antioxidant activity, while lysozyme reduces the formation of lung metastases of B16 melanoma; also, ovomucin has been demonstrated to inhibit tumour growth (Azuma et al., 2000; Sava, 1989; Watanabe et al., 1998). Moreover, lysozyme has been reported to improve the effectiveness of chemotherapy treatments (Sava et al., 1995).
Many bioactive peptides are derived from different marine species, for example, from ascidians, molluscs and sponges. Among the bioactive peptides identified in these species, dolastatins, mainly dolastatin 10 and 15, isolated from Dollabella auricularia, and hemiasterlin exert a cytotoxic action by blocking tubulin polymerization, arresting the cell cycle and triggering apoptosis (Anderson et al., 1997; Bai et al., 1990). Pardaxin, a 33‐aminoacid peptide, induced apoptosis by targeting the endoplasmatic reticulum and activating c‐FOS (Ting et al., 2014).
Many marine peptides induce antiproliferative activity against different cancer types; in vitro studies showed that peptides from tuna dark muscle had an antiproliferative effect on human breast cancer cells (Hsu et al., 2011), while peptides from squid gelatin had cytotoxic activity on human breast carcinoma (MCF‐7) and glioma cell lines (Alemán et al., 2011). Other peptides, for example, BEPT II e BEPT II‐1 from Bullacta exarata have also demonstrated apoptotic activity towards prostate cancer cells (Ma et al., 2013). Furthermore, the peptide reniochalistatin E from the sponge Reniochalina stalagmites showed a cytotoxic activity towards several tumour cell lines (Zhan et al., 2014).
These results have been confirmed in vivo. The polypeptide P2 of Arca subcrenata has been demonstrated to have an antiproliferative action on HeLa and HT‐29 cells in S‐180 tumour‐bearing mice (Hu et al., 2012) and peptides extracted from oyster (Crassostrea gigas) showed an antiproliferative effect in mice against sarcoma‐S180 (Wang et al., 2010).
Aplidine, a cyclodepsipeptide isolated from the tunicate Aplidium albicans, has also been tested in phase I clinical trials in humans and was shown to have anticancer activity against different cancer cell lines, such as breast, melanoma and lung cancer cells, induced by inhibiting cell proliferation, apoptosis induction and cell cycle arrest; phase I clinical trials confirmed its efficacy in humans, and phase II are ongoing (García‐Fernández et al., 2002).
In summary, many studies have demonstrated the cytotoxic and anti‐tumoural activity of bioactive peptides in different tumoral cell lines (but not in non tumoral ones). Nevertheless, again, the translation of the numerous preclinical data to the clinical setting has to be thoroughly investigated before any kind of optimistic conclusion can be reached.
Immunomodulatory activity
Bioactive peptides of animal origin may also improve immune responses, as observed in several studies conducted in vivo and in vitro (Table 2).
Table 2.
Main bioactive peptides with demonstrated activities on the immune system
| Source | Bioactive peptide | Mechanism of action | Effect | Model | Reference |
|---|---|---|---|---|---|
| Bovine milk | s1‐casein | Mitogenic activity, immunoglobulin production enhancement | Humoral immunostimulator | Cell cultures | Hata et al., 1998 |
| Fish | Fish protein hydrolysate | Phagocytic activity enhancement and number of IgA‐secreting cells increase | Gut‐associated non‐specific immunity modulator | Mice | Duarte et al., 2006 |
| Oyster (Crassostrea gigas) | Hydrolysates | Activity of natural killer cells, spleen proliferation of lymphocytes and phagocytic rate of macrophages enhancement | Immunostimulator | Sarcoma S180‐bearing mice | Wang et al., 2010 |
| Atlantic cod (Gadus morhua) | Medium size (3000 d > Mw > 500 d) peptides | Oxidative burst reactions promotion in leucocytes | Immunostimulator | Head kidney leucocytes from Atlantic salmon | Gildberg et al., 1996 |
| Chum Salmon (Oncorhynchus keta) | Oligopeptide preparation | Enhancement of lymphocyte proliferation induced by the mitogen concanavalin A, number of plaque‐forming cells, natural killer cell activity, percentage of CD4+T helper (Th) cells in spleen and the secretion of Th1 (IL‐2, IFN‐γ) and Th2 (IL‐5, IL‐6) type cell cytokines. | Immunostimulator | Female mice | Yang et al., 2009 |
Peptides from casein and whey proteins possess immunostimulatory activity. Phosphopeptides derived from αS1‐casein as well as β‐casein are reported to stimulate phagocytes, the production of IgG in lymphocytes and the proliferation of T‐lymphocytes (Lahov and Regelson, 1996; Hata et al., 1998). Peptides derived from fish have shown strong immunomodulatory effects in animals, that may be due to enhanced macrophage activity and lymphocyte proliferation, natural killer cell activity and cytokine regulation (Yang et al., 2009; Cheung et al., 2015);for example, these peptides modulate gut‐associated non‐specific immunity by enhancing phagocytic activity and the number of IgA‐secreting cells in the mouse small intestine lamina propria (Duarte et al., 2006). Among the marine hydrolysates, the one from Atlantic cod promotes the oxidative burst of leukocytes and enhances the bactericidal power of phagocytes (Gildberg et al., 1996), while the one from Chum Salmon (Oncorhynchus keta) increases the lymphocyte proliferation induced by the mitogen concanavalin A, the number of plaque‐forming cells, natural killer cell activity, the percentage of CD4+ T helper cells in spleen and the secretion of cytokines in mice (Yang et al., 2009). Oyster hydrolysates are able to enhance the activity of natural killer cells, the spleen proliferation of lymphocytes and the phagocytic rate of macrophages in mice (Wang et al., 2010). Immunomodulatory peptides derived from tryptic hydrolysates of rice and soybean proteins stimulate the production of superoxide anions (ROS), which trigger non‐specific immune defence systems (Kitts and Weiler, 2003).
In conclusion, bioactive peptides have an immunostimulant effect on both non‐specific and specific immunity in vitro and in animal models, but these effects need to be confirmed in humans.
Other biological activities
Bioactive peptides from many sources such as wheat gliadin, pea, soy proteins (Wang et al., 2007; Pownall et al., 2010; Malaguti et al., 2014) and also egg yolk proteins, porcine myofibrillar proteins and aquatic by‐products have a protective effect against oxidative damage (Pihlanto, 2006), acting as free radical scavengers and metal ion chelators against enzymatic and non‐enzymatic peroxidation of lipids and essential fatty acids. Among them, lunasin has been demonstrated to scavenge both peroxyl and superoxide radicals, confirming its antioxidant properties in vitro. Moreover, it has been shown to protect cell viability and antioxidant defences of human Caco‐2 cells treated with hydrogen peroxide and tert‐butylhydroperoxide (Garcia‐Nebot et al., 2014). Carnosine and anserine, which are the most abundant antioxidant peptides in meat, are reported to have a role in the prevention of stress‐related diseases (Hipkiss and Brownson, 2000). Other antioxidant peptides have been identified in marine organisms such as oysters, shrimps, squid and blue mussels (Harada et al., 2010).
In addition to antioxidant activity, some bioactive peptides possess (in experimental models) analgesic activity, thanks to their affinity with opiate receptors. Among them, α‐lactorphin and β‐lactorphin are opioid peptides found in α‐lactalbumin and β‐lactoglobulin; they are liberated during the in vitro proteolysis of bovine whey proteins and have shown pharmacological activity at μmolar concentrations (Pihlanto‐Leppälä, 2000). Bioactive peptides behaving like opioid receptor ligands can also be found in wheat gluten, rice albumin, in possible constituents of bovine meat, such as serum albumin or haemoglobin, and even in vegetables like spinach (Teschemacher, 2003).
Another interesting field of action of bioactive peptides is related to their antimicrobial effects. For example, antimicrobial peptides can affect the activity of bacteria, viruses and fungi; they are also well tolerated and do not induce pathogen resistance (Wang et al., 2010). The best investigated antimicrobial peptide is the fragment 17‐41 of lactoferrin, more commonly known as lactoferricin; its bactericidal activity is due to its binding to the lipid A part of bacterial lipopolysaccharides, with an associated increase in membrane permeability. It is active not only against bacteria but also against fungi, protozoa and viruses (Orsi, 2004).
Moreover, four peptides derived from bovine meat (GFHI, DFHING, FHG and GLSDGEWQ) have also shown antimicrobial activity against Gram‐positive and Gram‐negative bacteria, for example Bacillus cereus, Listeria monocytogenes , Staphylococcus aureus , Salmonella typhimurium, Escherichia coli and Pseudomonas aeruginosa (Jang et al., 2008).
Discussion
The current progress in bioactive peptides is an exciting and growing research field. Their potential should not be surprising, because amino acid sequences are responsible for the control, and direct all aspects, of cellular function and coordinate most intercellular communications (Craik et al., 2013). However, as yet, the evidence available on bioactive peptides has several limitations.
Firstly, the mechanistic processes that regulate the putative activities of individual peptides should be more thoroughly investigated. Many peptides appear to act through more than a single mechanism of action and, therefore, to possess pleiotropic activities. Nevertheless, often it is difficult to attribute the mechanism of action to a single peptide, because a complex of the protein or peptides as a whole is studied. Individual components of the complex peptide‐rich hydrolysates should be characterized to find out their actions and the specific receptors and signalling pathways involved in mediating some of their beneficial effects. Then, extraction procedures should be standardized. A solution could be made by applying in vitro activity‐guided fractionation, where analytical separation of protein‐digested fractions is combined with in vivo evaluation of specific biological activity, to identify the peptide responsible for the effect (Sato et al., 2013).
Secondly, solid pharmacokinetic data is needed to determine proper dosage and frequency of administration, analysing the variability in intake and biological effects (Rutherfurd‐Markwick, 2012; Yoshikawa, 2015). In order to get consistent pharmacokinetic data, it is important to study biopharmaceutical aspects of these peptides; the particle size, the mode of administration (fed or fasted phase, with or without water) and the dosage form (suspension, powder, micelle, emulsion, etc.) are only some examples of how the bioavailability of the same peptide can differ because of these variables.
The chemical structure of the peptide must be also considered: processes such as digestion can modify the peptide's bioavailability and activity turning an apparent bioactive peptide into an inactive one and vice versa; moreover, the active peptide could be degraded during digestion, not be absorbed or reach the target tissues at a concentration sufficient to exert its effect (Malaguti et al., 2014). Therefore, some peptides may require protection from gastrointestinal enzymes when administered orally using non‐conventional dosage forms.
It is important to highlight the fact that the specific duodenal peptide transporter responsible for their bioavailability absorbs the major part of bioactive peptides too; therefore, any mechanism of competition for the same intestinal transporter should be investigated.
Finally, another aspect to consider in humans is inter‐individual variability (concomitant therapy, age, sex, diseases, etc.) that can greatly influence the final effectiveness of the nutraceutical, as shown for lactotripeptides (genetic/population‐dependent effect on Asian subjects) (Cicero et al., 2011a).
In general, the main limitation in evaluating the clinical effects of these peptides is their short half‐life (less than 2 h) and their low plasma concentrations (pmol·mL−1); consequently, it is not easy to measure their bioavailability after oral intake (Iwai et al., 2005; Ichikawa et al., 2010) but also not impossible if the appropriate methodology is applied (Shigemura et al., 2009).
Although many studies conducted in vivo have demonstrated the effectiveness of bioactive peptides, most of them are at an early stage, especially as regards clinical data. Currently, the best evidence in humans is related to the BP and lipid‐lowering peptides that confirm their efficacy and their optimal tolerability and safety; antihypertensive peptides have also been demonstrated to modulate the pulse wave velocity in humans, which is a reliable prognostic parameter for cardiovascular morbidity and mortality (Cicero et al., 2011b, 2016). However, even if they seem to be safe, the presence of immunogenic proteins and peptides within the protein hydrolysates could induce or exacerbate allergic reactions (Franck et al., 2002).
Anti‐inflammatory peptides have been shown to lower inflammation parameters in humans, but the data are poor and their mechanism of action needs to be clarified. The immunomodulatory, antioxidant, analgesic and possible microbial activity of many of the peptides have been evaluated in numerous studies. In addition, the effects of numerous of these peptides have been investigated on a variety of cancer cell lines (but not in non‐tumoral ones) and in animal models. However, once again, a limited number of phase I clinical trials has been performed and more studies are needed to confirm their efficacy, safety and tolerability in humans.
In conclusion, so far the results obtained from in vitro and in vivo studies of bioactive peptides are encouraging and have shown they have potential as treatments of numerous diseases or risk factors. However, studies on humans are needed to better understand the pharmacokinetic profiles of these compounds and to test them for potential immunogenicity. Therefore, further middle/long‐term randomized clinical trials will be necessary to confirm the effects of these peptides in disease‐preventing/health promoting activities and their potential therapeutic usefulness, with the objective of using them to effectively control the growing burden of chronic illnesses with minimal side effects.
Conflict of interest
The authors declare no conflicts of interest.
Cicero, A. F. G. , Fogacci, F. , and Colletti, A. (2017) Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. British Journal of Pharmacology, 174: 1378–1394. doi: 10.1111/bph.13608.
References
- Aihara K, Ishii H, Yoshida M (2009). Casein‐derived tripeptide, Val‐Pro‐Pro (VPP), modulates monocyte adhesion to vascular endothelium. J Atheroscler Thromb 16: 594–603. [DOI] [PubMed] [Google Scholar]
- Alemán A, Pérez‐Santín E, Bordenave‐Juchereau S, Arnaudin I, Gómez‐Guillén MC, Montero P (2011). Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res Int 44: 1044–1051. [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Other ion channels. Br J Pharmacol 172: 5942–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altorf‐van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G et al. (2010). Dietary protein and blood pressure: a systematic review. PLoS One 5: e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluko RE (2015). Antihypertensive peptides from food proteins. Annu. Rev Food Sci Technol 6: 235–262. [DOI] [PubMed] [Google Scholar]
- Anderson HJ, Coleman JE, Andersen RJ, Roberge M (1997). Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother Pharmacol 39: 223–226. [DOI] [PubMed] [Google Scholar]
- Armstrong WB, Kennedy AR, Wan XS, Atiba J, McLaren E et al. (2000). Single‐dose administration of Bowman‐Birk inhibitor concentrate in patients with oral leukoplakia. Cancer Epidemiol Biomarkers Prev 9: 43–47. [PubMed] [Google Scholar]
- Azuma N, Suda H, Iwasaki H, Yamagata N, Saeki T, Kanamoto R et al. (2000). Antitumorigenic effects of several food proteins in a rat model with colon cancer and their reverse correlation with plasma bile acid concentration. J Nutr Sci Vitaminol 46: 91–96. [DOI] [PubMed] [Google Scholar]
- Bai R, Pettit GR, Hamel E (1990). Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol 39: 1941–1949. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Kumar S, Bhat HF (2015). Bioactive peptides of animal origin: a review. J Food Sci Technol 52: 5377–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco‐Míguez A, Gutiérrez‐Jácome A, Pérez‐Pérez M, Pérez‐Rodríguez G, Catalán‐García S, Fdez‐Riverola F et al. (2016). From amino acid sequence to bioactivity: scientific evidence on antitumor peptides. Protein Sci . doi:10.1002/pro.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR (2012). The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem 19: 3794–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi C, Cicero AF (2016). Nutraceuticals with clinically detectable blood pressure lowering effect: a review of available randomized clinical trials and their meta‐analyses. Br J Clin Pharmacol. doi:10.1111/bcp.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butteiger DN, Hibberd AA, McGraw NJ, Napawan N, Hall‐Porter JM, Krul ES (2016). Soy protein compared with milk protein in a western diet increases gut microbial diversity and reduces serum lipids in golden Syrian hamsters. J Nutr 146: 697–705. [DOI] [PubMed] [Google Scholar]
- Cam A, de Mejia EG (2012). RGD‐peptide lunasin inhibits Akt‐mediated NF‐κB activation in human macrophages through interaction with the αVβ3 integrin. Mol Nutr Food Res 56: 1569–1581. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Jahandideh F, Wu J (2014). Food‐derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int 2014: 608979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Lewis D, Tung CY, Han L, Henriquez SMP, Voiles L et al. (2014). Soypeptide lunasin in cytokine immunotherapy for lymphoma. Cancer Immunol Immunother 63: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Huang SC, Lin‐Shiau SY, Lin JK (2005). Bowman‐Birk inhibitor abates proteasome function and suppresses the proliferation of MCF7 breast cancer cells through accumulation of MAP kinase phosphatase‐1. Carcinogenesis 26: 1296–1306. [DOI] [PubMed] [Google Scholar]
- Cheung RC, Ng TB, Wong JH (2015). Marine peptides: bioactivities and applications. Mar Drugs 13: 4006–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SJ, Juillerat MA, Lee CH (2007). Cholesterol lowering mechanism of soybean protein hydrolysate. J Agric Food Chem 55: 10599–10604. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Colletti A (2015a). Nutraceuticals and blood pressure control: results from clinical trials and meta‐analyses. High Blood Press Cardiovasc Prev 22: 203–213. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Colletti A (2015b). Combinations of phytomedicines with different lipid lowering activity for dyslipidemia management: the available clinical data. Phytomedicine Nov 10. pii: S0944‐7113(15)00330‐X. doi:10.1016/j.phymed.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Gerocarni B, Laghi L, Borghi C (2011a). Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta‐analysis of current available clinical trials. J Hum Hypertens 25: 425–436. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Rosticci M, Gerocarni B, Bacchelli S, Veronesi M, Strocchi E et al. (2011b). Lactotripeptides effect on office and 24‐h ambulatory blood pressure, blood pressure stress response, pulse wave velocity and cardiac output in patients with high‐normal blood pressure or first‐degree hypertension: a randomized double‐blind clinical trial. Hypertens Res 34: 1035–1040. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Aubin F, Azais‐Braesco V, Borghi C (2013). Do the lactotripeptides isoleucine–proline–proline and valine–proline–proline reduce systolic blood pressure in European subjects? A meta‐analysis of randomized controlled trials. Am J Hypertens 26: 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero AF, Colletti A, Rosticci M, Cagnati M, Urso R, Giovannini M et al. (2016). Effect of lactotripeptides (isoleucine‐proline‐proline/valine‐proline‐proline) on blood pressure and arterial stiffness changes in subjects with suboptimal blood pressure control and metabolic syndrome: A double‐blind, randomized, crossover clinical trial. Metab Syndr Relat Disord 14: 161–166. [DOI] [PubMed] [Google Scholar]
- Craik DJ, Fairlie DP, Liras S, Price D (2013). The future of peptide‐based drugs. Chem Biol Drug Des 81: 136–147. [DOI] [PubMed] [Google Scholar]
- Damiens E, Yazidi I, Mazurier J, Duthile I, Spik G, Boilly‐Marer Y (1999). Lactoferrin inhibits G1 cyclin‐dependent kinases during growth arrest of human breast carcinoma cells. J Cell Biochem 74: 486–498. [PubMed] [Google Scholar]
- de Mejía EG, Dia VP (2010). The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metast Rev 29: 511–528. [DOI] [PubMed] [Google Scholar]
- de Mejia EG, Wang W, Dia VP (2010). Lunasin, with an arginine‐glycine‐aspartic acid motif, causes apoptosis to L1210 leukemia cells by activation of caspase‐3. Mol Nutr Food Res 54: 406–414. [DOI] [PubMed] [Google Scholar]
- Deng M, Zhang W, Tang H, Ye Q, Liao Q, Zhou Y et al. (2013). Lactotransferrin acts as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT through multiple mechanisms. Oncogene 32: 4273–4283. [DOI] [PubMed] [Google Scholar]
- Dia VP, de Mejia EG (2011). Lunasin potentiates the effect of oxaliplatin preventing outgrowth of colon cancer metastasis, binds to α5β1 integrin and suppresses FAK/ERK/NF‐κB signaling. Cancer Lett 313: 167–180. [DOI] [PubMed] [Google Scholar]
- Dia VP, Mejia EG (2010). Lunasin promotes apoptosis in human colon cancer cells by mitochondrial pathway activation and induction of nuclear clusterin expression. Cancer Lett 295: 44–53. [DOI] [PubMed] [Google Scholar]
- Dia VP, Wang W, Oh VL, de Lumen BO, de Meja EG (2009). Isolation, purification and characterization of lunasin from defatted soybean flour and in vitro evaluation of its antinflammatory activity. Food Chem 114: 108–115. [Google Scholar]
- Dong JY, Szeto IM, Makinen K, Gao Q, Wang J, Qin LQ et al. (2013). Effect of probiotic fermented milk on blood pressure: a meta‐analysis of randomised controlled trials. Br J Nutr 110: 1188–1194. [DOI] [PubMed] [Google Scholar]
- Duarte J, Vinderola G, Ritz B, Perdigon G, Matar C (2006). Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 211: 341–350. [DOI] [PubMed] [Google Scholar]
- Fast J, Mossberg AK, Nilsson H, Svanborg C, Akke M, Linse S (2005). Compact oleic acid in HAMLET. FEBS Lett 579: 6095–6100. [DOI] [PubMed] [Google Scholar]
- Franck P, Moneret Vautrin DA, Dousset B, Kanny G, Nabet P, Guénard‐Bilbaut L et al. (2002). The allergenicity of soybean‐based products is modified by food technologies. Int Arch Allergy Immunol 128: 212–219. [DOI] [PubMed] [Google Scholar]
- García‐Fernández LF, Losada A, Alcaide V, Alvarez AM, Cuadrado A, González L et al. (2002). Aplidine induces the mitochondrial apoptotic pathway via oxidative stress‐mediated JNK and p38 activation and protein kinase C delta. Oncogene 21: 7533–7544. [DOI] [PubMed] [Google Scholar]
- Garcia‐Nebot MJ, Recio I, Hernandez‐Ledesma B (2014). Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco‐2 cells. Food Chem Toxicol 65: 155–161. [DOI] [PubMed] [Google Scholar]
- Gautam A, Kapoor P, Chaudhary K, Kumar R, Raghava GP (2014). Tumor homing peptides as molecular probes for cancer therapeutics, diagnostics and theranostics. Curr Med Chem 21: 2367–2391. [DOI] [PubMed] [Google Scholar]
- Gildberg A, Bøgwald J, Johansen A, Stenberg E (1996). Isolation of acid peptide fractions from a fish protein hydrolysate with strong stimulatory effect on Atlantic salmon (Salmo salar) head kidney leucocytes. Comp Biochem Physiol B Biochem Mol Biol 114: 97–101. [Google Scholar]
- Harada K, Maeda T, Hasegawa Y, Tokunaga T, Tamura Y, Koizumi T (2010). Antioxidant activity of fish sauces including puffer (Lagocephalus wheeleri) fish sauce measured by the oxygen radical absorbance capacity method. Mol Med Rep 3: 663–668. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Meisel H (2007). Food‐derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol 18: 163–169. [DOI] [PubMed] [Google Scholar]
- Hata I, Higashiyama S, Otani H (1998). Identification of a phosphopeptide in bovine alpha s1‐casein digest as a factor influencing proliferation and immunoglobulin production in lymphocyte cultures. J Dairy Res 65: 569–578. [DOI] [PubMed] [Google Scholar]
- Hatzoglou A, Bakogeorgou E, Hatzoglou CM, Martin P, Castanas E (1996). Antiproliferative and receptor binding properties of α‐ and β‐casomorphins in the T47D human breast cancer cell line. Eur J Pharmacol 310: 217–223. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Ledesma B, Hsieh CC (2015). Chemopreventive role of food‐derived proteins and peptides: a review. Crit Rev Food Sci Nutr. doi:10.1080/10408398.2015.1057632. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Ledesma B, Hsieh CC, de Lumen BO (2009). Antioxidant and anti‐inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem Biophys Res Commun 390: 803–808. [DOI] [PubMed] [Google Scholar]
- Hernández‐Ledesma B, Hsieh CC, de Lumen BO (2013). Chemopreventive properties of peptide lunasin: a review. Protein Pept Lett 20: 424–432. [PubMed] [Google Scholar]
- Herrera Chalé F, Ruiz Ruiz JC, Betancur Ancona D, Acevedo Fernández JJ, Segura Campos MR (2016). The hypolipidemic effect and antithrombotic activity of Mucuna pruriens protein hydrolysates. Food Funct 7: 434–444. [DOI] [PubMed] [Google Scholar]
- Hipkiss AR, Brownson C (2000). A possible new role for the anti‐ageing peptide carnosine. Cell Mol Life Sci 7: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer‐Jensen J, Karhu T, Mortensen LS, Pedersen SB, Herzig KH, Hermansen K (2011). Differential effects of dietary protein sources on postprandial low‐grade inflammation after a single high fat meal in obese non‐diabetic subjects. Nutr J 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2013). Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13: 714–726. [DOI] [PubMed] [Google Scholar]
- Houston MC (2013). The role of nutrition, nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Altern Ther Health Med 19 (Suppl. 1): 32–49. [PubMed] [Google Scholar]
- Hsieh CC, Hernández‐Ledesma B, de Lumen BO (2010). Soybean peptide lunasin suppresses in vitro and in vivo 7,12‐dimethylbenz[a]anthracene‐induced tumorigenesis. J Food Sci 75: H311–H316. [DOI] [PubMed] [Google Scholar]
- Hsu KC, Li‐Chan ECY, Jao CL (2011). Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF‐7. Food Chem 126: 617–622. [Google Scholar]
- Hu X, Song L, Huang L, Zheng Q, Yu R (2012). Antitumor effect of a polypeptide fraction from Arca subcrenata in vitro and in vivo. Mar Drugs 10: 2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Morifuji M, Ohara H, Matsumoto H, Takeuchi Y, Sato K (2010). Hydroxyproline‐containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int J Food Sci Nutr 61: 52–60. [DOI] [PubMed] [Google Scholar]
- Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y et al. (2005). Identification of food‐derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem 53: 6531–6536. [DOI] [PubMed] [Google Scholar]
- Jang A, Jo C, Kang KS, Lee M (2008). Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin‐converting enzyme (ACE) inhibitory peptides. Food Chem 107: 327–336. [Google Scholar]
- Jeong HJ, Jeong JB, Hsieh CC, Hernàndez‐Ledesma B, de Lumen BO (2010). Lunasin is prevalent in barley and is bioavailable and bioactive in in vivo and in vitro studies. Nutr Cancer 62: 1113–1119. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Lam Y, de Lumen BO (2002). Barley lunasin suppresses ras‐induced colony formation and inhibits acetylation in mammalian cells. J Agric Food Chem 50: 5903–5908. [DOI] [PubMed] [Google Scholar]
- Kannan A, Hettiarachchy NS, Lay JO, Liyanage R (2010). Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from ricebran. Peptides 31: 1629–1634. [DOI] [PubMed] [Google Scholar]
- Kitts DD, Weiler K (2003). Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des 9: 1309–1323. [DOI] [PubMed] [Google Scholar]
- Lahov E, Regelson W (1996). Antibacterial and immunostimulating casein‐derived substances from milk: casecidin, isracidin peptides. Food Chem Toxicol 34: 131–145. [DOI] [PubMed] [Google Scholar]
- Lammi C, Zanoni C, Scigliuolo GM, D'Amato A, Arnoldi A (2014). Lupin peptides lower low‐density lipoprotein (LDL) cholesterol through an up‐regulation of the LDL receptor/sterol regulatory element binding protein 2 (SREBP2) pathway at HepG2 cell line. J Agric Food Chem 62: 7151–7159. [DOI] [PubMed] [Google Scholar]
- Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG et al. (2012). Effect of soy isoflavones on blood pressure: a meta‐analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 22: 463–470. [DOI] [PubMed] [Google Scholar]
- Lule VK, Garg S, Pophaly SD, Hitesh, Tomar SK (2015). Potential health benefits of lunasin: a multifaceted soy‐derived bioactive peptide. J Food Sci 80: R485–R494. [DOI] [PubMed] [Google Scholar]
- Ma J, Huang F, Lin H, Wang X (2013). Isolation and purification of a peptide from Bullacta exarata and its impaction of apoptosis on prostate cancer cell. Mar Drugs 11: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YH, Cheng WZ, Gong F, Ma AL, Yu QW, Zhang JY et al. (2008). Active Chinese mistletoe lectin‐55 enhances colon cancer surveillance through regulating innate and adaptive immune responses. World J Gastroenterol 14: 5274–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader JS, Hoskin DW (2006). Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs 15: 933–946. [DOI] [PubMed] [Google Scholar]
- Majumder K, Chakrabarti S, Morton JS, Panahi S, Kaufman S, Davidge ST et al. (2013). Egg‐derived tri‐peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS One 8: e82829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder K, Mine Y, Wu J (2016). The potential of food protein‐derived anti‐inflammatory peptides against various chronic inflammatory diseases. J Sci Food Agric 96: 2303–2311. [DOI] [PubMed] [Google Scholar]
- Malaguti M, Dinelli G, Leoncini E, Bregola V, Bosi S, Cicero AF et al. (2014). Bioactive peptides in cereals and legumes: agronomical, biochemical and clinical aspects. Int J Mol Sci 15: 21120–21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski J, Klempt M, Clawin‐Rädecker I, Lorenzen PC, Meisel H (2014). Identification of a NFκB inhibitory peptide from tryptic β‐casein hydrolysate. Food Chem 165: 129–133. [DOI] [PubMed] [Google Scholar]
- Malkowicz SB, McKenna WG, Vaughn DJ, Wan XS, Propert KJ, Rockwell K et al. (2001). Effects of Bowman‐Birk inhibitorconcentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate 48: 16–28. [DOI] [PubMed] [Google Scholar]
- Marcone S, Belton O, Fitzgerald DJ (2016). Milk‐derived bioactive peptides and their health promoting effects: a potential role in atherosclerosis. Br J Clin Pharmacol . doi:10.1111/bcp.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MR, Soares Freitas RA, Corrêa Carlos AC, Siguemoto ÉS, Fontanari GG, Arêas JA (2015). Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem 168: 288–293. [DOI] [PubMed] [Google Scholar]
- Marsh TG, Straub RK, Villalobos F, Hong MY (2011). Soy protein supports cardiovascular health by downregulating hydroxymethylglutaryl‐coenzyme A reductase and sterol regulatory element‐binding protein‐2 and increasing antioxidant enzyme activity in rats with dextran sodium sulfate‐induced mild systemic inflammation. Nutr Res 31: 922–928. [DOI] [PubMed] [Google Scholar]
- Motoi H, Kodama T (2003). Isolation and characterization of angiotensin I‐converting enzyme inhibitory peptides from wheat gliadin hydrolysate. Nahrung 47: 354–358. [DOI] [PubMed] [Google Scholar]
- Nirupama G, Mohammad B, Hossain DKR, Nigel PB (2015). A review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules 20: 10884–10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma AB, FitzGerald RJ (2015). Bioactive properties of milk proteins in humans: a review. Peptides 73: 20–34. [DOI] [PubMed] [Google Scholar]
- Orsi N (2004). The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17: 189–196. [DOI] [PubMed] [Google Scholar]
- Otvos L Jr (2008). Peptide‐based drug design: here and now. Methods Mol Biol 494: 1–8. [DOI] [PubMed] [Google Scholar]
- Park JH, Jeong HJ, de Lumen BO (2005a). Contents and bioactivities of lunasin, bowman‐birk inhibitor, and isoflavones in soybean seed. J Agric Food Chem 53: 7686–7690. [DOI] [PubMed] [Google Scholar]
- Park VV, Koo MS, Kasymova TD, Kwon DY (2005b). Isolation and identification of peptides from soy 11S‐globulin with hypocholesterolemic activity. Chem Nat Comp 41: 710–714. [Google Scholar]
- Perego S, Cosentino S, Fiorilli A, Tettamanti G, Ferraretto A (2012). Casein phosphopeptides modulate proliferation and apoptosis in HT‐29 cell line through their interaction with voltage‐operated L‐type calcium channels. J Nutr Biochem 23: 808–816. [DOI] [PubMed] [Google Scholar]
- Pihlanto A (2006). Antioxidative peptides derived from milk proteins. Int Dairy J 16: 1306–1314. [Google Scholar]
- Pihlanto‐Leppälä A (2000). Bioactive peptides derived from bovine whey proteins: opioid and ace‐inhibitory peptides. Trends Food Sci Technol 11: 347–356. [Google Scholar]
- Pownall TL, Udenigwe CC, Aluko RE (2010). Amino acid composition and antioxidant properties of pea seed (Pisum sativumL.) enzymatic protein hydrolysate fractions. J Agric Food Chem 58: 4712–4718. [DOI] [PubMed] [Google Scholar]
- Rotimi EA (2015). Antihypertensive Peptides from Food Proteins. Annu Rev Food Sci Technol 6: 235–262. [DOI] [PubMed] [Google Scholar]
- Rutherfurd‐Markwick KJ (2012). Food proteins as a source of bioactive peptides with diverse functions. Br J Nutr 108 (Suppl. 2): S149–S157. [DOI] [PubMed] [Google Scholar]
- Saito T, Sato H, Virgona N, Hagiwara H, Kashiwagi K, Suzuki K et al. (2007). Negative growth control of osteosarcoma cell by Bowman‐Birk protease inhibitor from soybean; involvement of connexin 43. Cancer Lett 253: 249–257. [DOI] [PubMed] [Google Scholar]
- Sato K, Egashira Y, Ono S, Mochizuki S, Shimmura Y, Suzuki Y et al. (2013). Identification of a hepatoprotective peptide in wheat gluten hydrolysate against D‐galactosamine‐induced acute hepatitis in rats. J Agric Food Chem 61: 6304–6310. [DOI] [PubMed] [Google Scholar]
- Sava G (1989). Reduction of B16 melanoma metastases by oral administration of egg‐whitelysozyme. Cancer Chemother Pharmacol 25: 221–222. [DOI] [PubMed] [Google Scholar]
- Sava G, Pacor S, Dasic G, Bergamo A (1995). Lysozyme stimulates lymphocyte response to ConA and IL‐2 and potentiates 5‐fluorouracil action on advanced carcinomas. Anticancer Res 15: 1883–1888. [PubMed] [Google Scholar]
- Schweizer F (2009). Cationic amphiphilic peptides with cancer‐selective toxicity. Eur J Pharmacol 625: 190–194. [DOI] [PubMed] [Google Scholar]
- Seifert G, Jesse P, Laengler A, Reindl T, Lüth M, Lobitz S et al. (2008). Molecular mechanisms of mistletoe plant extract‐induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro . Cancer Lett 264: 218–228. [DOI] [PubMed] [Google Scholar]
- Shigemura Y, Iwai K, Morimatsu F, Iwamoto T, Mori T, Oda C et al. (2009). Effect of prolyl‐hydroxyproline (Pro–Hyp), a food‐derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem 57: 444–449. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A (2013). Cancer statistics, 2013. CA Cancer J Clin 63: 11–30. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Arnoldi A, Cicero AF (2015). Nutraceuticals for blood pressure control. Ann Med 47: 447–456. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Triolo M, Bosisio R, Bondioli A, Calabresi L, De Vergori V et al. (2012). Hypocholesterolaemic effects of lupin protein and pea protein/fibre combinations in moderately hypercholesterolaemic individuals. Br J Nutr 107: 1176–1183. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza Lda C, Camargo R, Demasi M, Santana JM, de Sá CM, de Freitas SM (2014). Effects of an anticarcinogenic Bowman‐Birk protease inhibitor on purified 20S proteasome and MCF‐7 breast cancer cells. PLoS One 9: e86600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Jiang R, Przepiorski A, Reddy S, Palmano KP, Krissansen GW (2012). Iron‐saturated bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal‐like breast cancer in mice. BMC Cancer 12: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yano T, Sadzuka Y, Sugiyama T, Seki T, Asano R (2005). Restoration of connexin 43 by Bowman‐Birk protease inhibitor in M5076 bearing mice. Oncol Rep 13: 1247–1250. [PubMed] [Google Scholar]
- Teschemacher H (2003). Opioid receptor ligands derived from food proteins. Curr Pharm Des 9: 1331–1344. [DOI] [PubMed] [Google Scholar]
- Thundimadathil J (2012). Cancer treatment using peptides: current therapies and future prospects. J Amino Acids 2012: 967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CH, Huang HN, Huang TC, Wu CJ, Chen JY (2014). The mechanisms by which pardaxin, a natural cationic antimicrobial peptide, targets the endoplasmic reticulum and induces c‐FOS. Biomaterials 35: 3627–3640. [DOI] [PubMed] [Google Scholar]
- Tokede OA, Onabanjo TA, Yansane A, Gaziano JM, Djoussé L (2015). Soya products and serum lipids: a meta‐analysis of randomised controlled trials. Br J Nutr 114: 831–843. [DOI] [PubMed] [Google Scholar]
- Tyagi A, Tuknait A, Anand P, Gupta S, Sharma M, Mathur D et al. (2015). CancerPPD: a database of anticancer peptides and proteins. Nucl Acids Res 43: D837–D843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenigwe CC, Aluko RE (2012). Food protein‐derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77: R11–R24. [DOI] [PubMed] [Google Scholar]
- Valadez‐Vega C, Alvarez‐Manilla G, Riverón‐Negrete L, García‐Carrancá A, Morales‐González JA, Zuñiga‐Pérez C et al. (2011). Detection of cytotoxic activity of lectin on human colon adenocarcinoma (Sw480) and epithelial cervical carcinoma (C33‐A). Molecules 16: 2107–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhachary A, Wolf JS, Petrak K, O'Malley BW Jr, Spadaro M, Curcio C et al. (2004). Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer 111: 398–403. [DOI] [PubMed] [Google Scholar]
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M (2010). Synthetic therapeutic peptides: science and market. Drug Discov Today 15: 40–56. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao M, Zhao Q, Jiang Y (2007). Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem 101: 1658–1663. [Google Scholar]
- Wang YK, He HL, Wang GF, Wu H, Zhou BC, Chen XL et al. (2010). Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar Drugs 8: 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Tsuge Y, Shimoyamada M, Ogama N, Ebina T (1998). Antitumor effects of pronase‐treated fragments, glycopeptides, from ovomucin in hen egg white in a double grafted tumor system. J Agric Food Chem 46: 3033–3038. [Google Scholar]
- Yadav JS, Yan S, Pilli S, Kumar L, Tyagi RD, Surampalli RY (2015). Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol Adv 33 (6 Pt 1): 756–774. [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Ohinata K, Yoshikawa M (2003). Beta‐lactotensin and neurotensin rapidly reduce serum cholesterol via NT2 receptor. Peptides 24: 1955–1961. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang Z, Pei X, Han H, Wang J, Wang L et al. (2009). Immunomodulatory effects of marine oligopeptide preparation from chum salmon (Oncorhynchus keta) in mice. Food Chem 113: 464–470. [Google Scholar]
- Yoo YC, Watanabe S, Watanabe R, Hata K, Shimazaki K, Azuma I (1997). Bovine lactoferrin and lactoferricin, a peptide derived from bovine lactoferrin, inhibit tumor metastasis in mice. Jpn J Cancer Res 88: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M (2015). Bioactive peptides derived from natural proteins with respect to diversity of their receptors and physiological effects. Peptides 72: 208–225. [DOI] [PubMed] [Google Scholar]
- Zannad F, Dallongeville J, Macfadyen RJ, Ruilope LM, Wilhelmsen L, De Backer G et al. (2012). Prevention of cardiovascular disease guided by total risk estimations‐‐challenges and opportunities for practical implementation: highlights of a cardiovascular clinical trialists (CVCT) workshop of the ESC working group on cardiovascular pharmacology and drug therapy. ESC working group on cardiovascular pharmacology and drug therapy. Eur J Prev Cardiol 19: 1454–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan KX, Jiao WH, Yang F, Li J, Wang SP, Li YS et al. (2014). Reniochalistatins A‐E, cyclic peptides from the marine sponge Reniochalina stalagmitis. J Nat Prod 77: 2678–2684. [DOI] [PubMed] [Google Scholar]


