Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- BCAAs
branched‐chain amino acids
- PEA
palmitoylethanolamide
- RCTs
randomized controlled trials
- TCM
traditional Chinese medicine
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes c |
| CB1 receptors | Hydroxy‐3‐methyl‐glutaryl‐CoA reductase |
| CB2 receptors | Transporters d |
| Nuclear hormone receptors b | P‐glycoprotein |
| Pregnane X receptors |
| LIGANDS | |
|---|---|
| Cholesterol | Hyperforin |
| Curcumin | Lovastatin |
| Epigallocatechin‐3‐gallate | PEA, N‐palmitoylethanolamine |
| Genistein |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a,b,c,d).
Introduction
The term ‘nutraceutical’, a hybrid term introduced in 1989 to designate the link between ‘nutrition’ and ‘pharmaceutical agents’, has actually no universally accepted definition (Aronson, 2017, Table 1). The broad canopy of ‘nutraceutical’ covers a wide range of different, naturally occurring, products, which are advocated to influence human health positively and so a variety of functional foods, fortified foods and dietary supplements have found their place here (Palthur et al., 2010; Schmitt and Ferro, 2013; Drake et al., 2017).
Table 1.
Some definitions in the nutraceutical field
| Definiendum | Definition | Comment |
|---|---|---|
| Active ingredient (active principle) | A phytochemical contained in herbal drug (or extract) mostly responsible of its pharmacological activity. |
Historical examples include the alkaloids morphine (from poppy opium) and atropine (from Atropa belladonna leaves). In cases where it is not possible to identify the active ingredients, the whole herbal medicine may be considered as one active ingredient.a |
| Dietary supplement | A substance added to the diet, often taken as a pharmaceutical formulation, to treat or prevent a deficiency.b | Dietary supplements include vitamins, amino acids, proteins, minerals, fibre and plant extracts. The arbitrary inclusion in the dietary supplement category of herbal medicinal products has been criticized.c Authentic supplements to the diet (i.e. vitamins, minerals), have nutritional value. Herbal extracts may contain pharmacologically active ingredients and should be regulated as medicinesc |
| Herbal drug | Part of the plant (e.g. roots, stems, leaves, bark, fruits and exudates) used for pharmaceutical/nutraceutical purpose. | Crude drugs may be obtained from wild or cultivated plants. Quality specifications for crude drugs are given in Pharmacopoeias.d They constitute the starting material for preparation of herbal preparations, such as herbal extracts. |
| Herbal extract | Preparation of herbal drugs which contains all the constituents which are soluble in the solvent used in making the extract.d | Extracts are very complicated mixture of phytochemicals of which the pharmacologically active compound(s), named active principle(s) or active ingredient(s), often constitutes only a small part.d To ensure a reliable dosage, the content of key pharmacologically active constituent(s) should be determined or a HPLC fingerprint provided (extract standardization). |
| Herbal medicine | Herbal medicines include herbs, herbal materials, herbal preparations and finished herbal products that contain as active ingredients parts of plants, or other plant materials, or combinations.a | Medicines containing plant‐derived pure compounds are not considered to be herbal medicines |
| Fortified food | Foodstuffs to which compounds of therapeutic or preventive efficacy have been added.b | Examples include bread with added folic acid to prevent neural tube defects, salt with added iodide to prevent hypothyroidism, milk derivatives containing phytosterols to decrease blood cholesterol levels.b |
| Functional food | No satisfactory definition.b | Health Canada has defined a functional food as one that ‘is similar in appearance to, or may be, a conventional food that is consumed as part of a usual diet, and is demonstrated to have physiological benefits and/or reduce the risk of chronic disease beyond basic nutritional functions’.b |
| Nutraceutical | No satisfactory definition.b | The original definition of the term was ‘a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease’. A recent analysis of the literature revealed the existence of 25 definitions, with the majority of them relating nutraceuticals to food, food components, or nutrients providing health benefits behind their nutritional value.e |
World Health Organization (WHO): http://www.who.int/medicines/areas/traditional/definitions/en/
Aronson (2017)
Marcus (2016)
Samuelsson (1999)
Palthur et al. (2010)
Nutraceuticals contribute to high rates of polypharmacy, particularly among multi‐morbid older people (Brown, 2016; Pitkala et al., 2016). If we only consider dietary supplements, it has been reported that American adults, with the exclusion of pregnant women, have used one or more dietary supplements at least once during the preceding month (Dickinson and MacKay, 2014; Borchers et al., 2016). The main reasons for taking them are to enhance overall health and wellness, to fill dietary nutrient gaps, to stimulate immune health and to boost energy, bone and heart health (Brown, 2016). It has been estimated that herbal dietary supplements account for approximately 20–25% of dietary supplements sales in the USA (Borchers et al., 2016; Brown, 2016), with the top best‐sellers shown in Table 2 (Smith et al., 2016).
Table 2.
Key information on the 25 best‐selling herbal dietary supplements in the US mainstream multi‐outlet channel in 2015
| Rank | Common/Latin Name | Retail sales a | % Changes 2014 | Part of the plant generally used | Key constituent(s) | Condition frequently treated | Clinical efficacyb (Authors conclusions, reference) |
|---|---|---|---|---|---|---|---|
| 1 |
Horehoundc
Marrubium vulgare |
114,9 | 8.5 | Leaves and flowering tops | Diterpenes (e.g. marrubiin; flavonoids) | Catarrh associated with cooling; Dyspepsia symptoms such as swelling or flatulence | No systematic review/meta‐analyses available |
| 2 |
Cranberry Vaccinium macrocarpon |
65,7 | 16.0 | Fruits | Proanthocyanidins | Urinary tract infection (prevention) | ‘Given the large number of dropouts/withdrawals from studies (mainly attributed to the acceptability of consuming cranberry products particularly juice, over long periods), and the evidence that the benefit for preventing UTI is small, cranberry juice cannot currently be recommended for the prevention of UTIs’ (Jepson et al., 2012) |
| 3 |
Echinacea
Echinacea spp |
60.1 | 7.4 | Roots, aerial parts | Alkylamides, polysaccharides, caffeic acid derivatives | Common cold (immunostimulant) | ‘Echinacea products have not here been shown to provide benefits for treating colds, although, it is possible there is a weak benefit from some Echinacea products: the results of individual prophylaxis trials consistently show positive (if non‐significant) trends, although potential effects are of questionable clinical relevance’ (Karsch Völk et al., 2014) |
| 4 |
Garcinia Garcinia cambogia |
54.8 | −23.3 | Pericarp of the fruit | Hydroxycitric acid | Weight loss | ‘Garcinia extracts can cause short‐term weight loss. The magnitude of the effect is small, and the clinical relevance is uncertain’ (Onakpoya et al., 2011a) |
| 5 |
Green tea Camellia sinensis |
48.9 | −23.4 | Leaves | Caffeine, polyphenols (e.g. epigallocatechin and epigallocatechin‐3‐gallate) | Prevention of cardiovascular diseases and cancer; weight loss |
Cardiovascular ‘The limited evidence suggests that tea has favourable effects on cardiovascular risk factors, but due to the small number of trials contributing to each analysis the results should be treated with some caution’ (Hartley et al., 2013). Cancer ‘There is insufficient and conflicting evidence to give any firm recommendations regarding green tea consumption for cancer prevention (Boehm et al., 2009)’. Weight loss ‘Green tea preparations appear to induce a small, statistically non‐significant weight loss in overweight or obese adults’ (Jurgens, 2012) |
| 6 |
Black cohosh Cimicifuga racemosa |
42.9 | −5.1 | Rhizome | Triterpenes (e.g. actein, cimicifugoside, 27‐deoxyactein) | Menopausal symptoms | ‘There is currently insufficient evidence to support the use of black cohosh for menopausal symptoms’ (Leach and Moore, 2012). |
| 7 |
Flax seed/oil Linum usitatissimum |
36.3 | −1.4 | Seeds | α‐Linolenic acid, lignans, fibre, | Improvement of cardiovascular health | ‘The present meta‐analysis suggests that consumption of flaxseed may lower blood pressure slightly. The beneficial potential of flaxseed to reduce blood pressure (especially diastolic blood pressure) may be greater when it is consumed as a whole seed and for a duration of >12 wk’ (Khalesi et al., 2015). |
| 8 |
Ginger Zingiber officinale |
25.6 | 21.8 | Rhizome | Gingerols | Prevention of nausea and vomiting | ‘For mild symptoms of nausea and emesis of pregnancy, ginger…..is associated with greater benefit than placebo’ (McParlin et al., 2016). |
| 9 |
Valerian Valeriana officinalis |
25.3 | 4.0 | Roots | Iridoids, valepotriates, sesquiterpenes | Insomnia | ‘There is insufficient evidence to support the use of herbal medicine [including valerian] for insomnia’ (Leach and Page, 2015) |
| 10 | Bioflavonoid complex | 24.6 | 24.4 | d | Hesperidin, rutin, naringin, quercitin and others. | To support optimal health | e |
| 11 |
Green coffeef
Coffea Arabica |
23.4 | 40.7 | Seeds | Caffeine, chlorogenic acids, diterpenes, lipids | Weight loss | ‘the results …..are promising, but the studies are all of poor methodological quality’ (Onakpoya et al., 2011b) |
| 12 |
Yohimbe Pausinystalia johimbe |
21.8 | 9.1 | Bark | Indole alkaloids (e.g. yohimbine) | body weight reduction, erectile dysfunction | No recent systematic review/meta‐analyses published |
| 13 |
Ivy Hedera helix |
18.6 | 129.4 | Leaf | Sterols, saponins, flavonoids, alkaloids | Respiratory diseases | ‘Although all studies report that ivy extracts are effective to reduce symptoms of upper respiratory tract infections, there is no convincing evidence due to serious methodological flaws and lack of placebo controls’ (Holzinger and Chenot, 2011) |
| 14 |
Aloe vera (Aloe gel) Aloe vera |
17.1 | 1.5 | Mucilaginous tissue from the leaves | Polysaccharides, aloins | Dermatological conditions |
Phlebitis ‘There is no strong evidence for preventing or treating infusion phlebitis with external application of Aloe vera.’ (Zheng et al., 2014) Acute and chronic wounds ‘There is currently an absence of high quality clinical trial evidence to support the use of Aloe vera topical agents or Aloe vera dressings as treatments for acute and chronic wounds’ (Dat et al., 2012). Psoriasis ‘Results on the effectiveness of Aloe vera are contradictory; our analysis reveals the presence of methodological gaps preventing to reach final conclusions’ (Miroddi et al., 2015) |
| 15 |
Saw palmetto Serenoa repens |
16.8 | −6.4 | Fruits | Fatty acids, sterols | Benign prostatic hyperplasia | ‘Serenoa repens, at double and triple doses, did not improve urinary flow measures or prostate size in men with lower urinary tract symptoms consistent with benign prostatic hyperplasia’ (Tacklind et al., 2012) |
| 16 |
Milk thistle Silybum marianum |
16.8 | 2.6 | Fruits | A mixture of flavonolignans called silymarin | Liver diseases | ‘The clinical evidence of therapeutic effect of silymarin in toxic liver diseases is scarce... It is reasonable to employ silymarin as a supportive element in the therapy of Amanita phalloides poisoning but also (alcoholic and grade Child ‘A’) liver cirrhosis’ (Saller et al., 2008). |
| 17 |
Garlic Allium sativum |
16.5 | 8.4 | Bulb | Alliin, diallyl disulphide, ajoens | Hypercholesterolemia, Hypertension |
Hypercholesterolemia ‘Garlic reduces total cholesterol to a modest extent,without appreciable LDL lowering or HDL elevation’ (Reinhart et al., 2009). Hypertension: ‘Although evidence from this review suggests that garlic preparations may lower blood pressure in hypertensive individuals, the evidence is not strong’ (Rohner et al., 2015). |
| 18 | Plant sterols | 16.2 | 46.5 | NA | NA | Hypercholesterolemia, hypertriglyceridemia | ‘Results show that phytosterols exert a modest triclycerides‐lowering effect which is dependent on baseline concentrations’ (Demonty et al., 2013). |
| 19 |
Turmeric Curcuma longa |
15.7 | 117.7 | Rhizome | Curcuminoids |
Inflammatory/autoimmune diseases Dermatological conditions |
Arthritis ‘These RCTs provide scientific evidence that supports the efficacy of turmeric extract in the treatment of arthritis. However, the total number of RCTs included in the analysis, the total sample size, and the methodological quality of the primary studies were not sufficient to draw definitive conclusions’ (Daily et al., 2016) Dermatological conditions (acne, alopecia, atopic dermatitis, facial photoaging, oral lichen planus, pruritus, psoriasis, radiodermatitis, and vitiligo: ‘There is early evidence that turmeric/curcumin products and supplements, both oral and topical, may provide therapeutic benefits for skin health. However, currently published studies are limited’ (Vaughn et al., 2016) |
| 20 |
Cinnamon Cinnamomum spp |
14.6 | 2.2 | Bark | Volatile oil, the main component is cinnamaldehyde | Loss of appetite, dyspepsia, diabetes | No recent systematic reviews/meta‐analyses available |
Million US dollars in rounded figures (Sales are from Smith et al., 2016);
based on systematic reviews/meta‐analyses of clinical data;
herb coded as a primary substance in throat lozenges that may contain other herbs;
Bioflavonoids are extracted from Citrus fruits;
Citrus flavonoids are mainly promoted as antioxidants to promote and support optimal health. Many systematic reviews are available related to flavonoid intake and a number of conditions such as oxidative stress, immune functions, cancer prevention and decline of cognitive functions
from not‐roasted coffee beans;
NA = not applicable.
Because of this widespread use, it is incumbent on the scientific community to have access to rigorous and reliable information of the experimental and clinical pharmacology of such products. Therefore, based on a number of informative reviews published in this themed issue, this article aims to provide an overview on the pharmacological basis of nutraceutical action, including efficacy and safety, with a special focus on herbal dietary supplements.
Pharmacologically active ingredients in dietary supplements
Herbal dietary supplements contain herbal extracts, that is, complex mixtures of phytochemicals of which the pharmacologically active compound(s), named active ingredient(s) or active principle(s), often constitute only a small part (Samuelsson, 1999). Minor constituents of the herbal extract may, in an additive or synergistic way, enhance the pharmacological action of the main active ingredient(s). Synergistic interactions have been advocated to explain the efficacy of apparently low doses of active constituents in herbal extracts (Williamson, 2001). The chief herbal, pharmacologically active, ingredients include carbohydrates, lipids, polyphenols, terpenes, steroids/ols and alkaloids (Samuelsson, 1999; Capasso et al., 2003). Below, we report a brief overview on the pharmacology of some plant active ingredients. More comprehensive information regarding the pharmacological effects, the mode of action and the clinical pharmacology can be found in the accompanying review articles published in this themed issue (Bifari and Nisoli, 2017; Cicero et al., 2017; Currò et al., 2017; Goszcz et al., 2017; Kunnumakkara et al., 2017; Milani et al., 2017; Peluso and Serafini, 2017; Petrosino and Di Marzo, 2017; Smeriglio et al., 2017; Rietjens et al., 2017).
Polyphenols
Polyphenols, being omnipresent in all plant parts, represent a prominent portion of the human diet, contained within fruits, vegetables and beverages (Bravo, 1998; Manach et al., 2004; D'archivio et al., 2007). Consumption of diets rich in polyphenols, such as the Mediterranean diet, is believed to be a nutritional strategy to improve or to prevent chronic diseases such as metabolic syndrome and cancer (Amiot et al., 2016; Di Daniele et al., 2017). The main classes of pharmacologically relevant polyphenols include coumarins, chromones, xanthones, stilbenes and flavonoids (Table 3). Flavonoids are most extensively widespread among the plant polyphenolic compounds and include the subclasses of flavones, flavonols, flavanols, isoflavones, flavanones, anthocyanidins and proanthocyanidins (Table 4).
Table 3.
Examples of pharmacologically relevant classes of polyphenols
| Class | Basic structure | Occurrence | Comment |
|---|---|---|---|
| Coumarins |
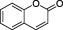
|
Melilotus officinalis (sweet clover), Aesculus hippocastanum (horsechestnut). Also widespread in the Apiaceae botanical family | This group include coumarins, furanocoumarins and psoralenes. A number of coumarins are reported to exert anticoagulant effects. Coagulation is impaired in cattle eating mouldy sweet clover, resulting in fatal haemorrhage (sweet clover disease). This discovery led to the introduction in therapy of dicoumarol as anticoagulant drug. Warfarin is chemically related to dicoumarol. Psoralens are used in photochemotherapy |
| Chromones |
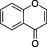
|
Ubiquitous in plants | Khellin, found in the fruit of Amni visnaga is a chromone formerly used as antispasmodic. Efforts to find better drugs led to the chemical development of sodium cromoglycate |
| Xanthones |
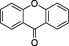
|
Many higher plants, fungi, ferns, lichens and bacteria | Xanthones are present in the pericarp of the fruit of the tropical evergreen tree purple mangosteen (Garcinia mangostana), a nutraceutical promoted for metabolic syndrome |
| Stilbenes |
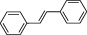
|
Present in low quantities in the human diet. Present in limited and heterogeneous group of plant families such as Vitaceae and Leguminosae | Resveratrol, found in the skin of grapes, is the most studied among the stilbenes. It has been suggested that oral supplementation with resveratrol exerts cardioprotective effects, but a recent meta‐analysis of available RCTs does not suggest any benefit of resveratrol supplementation on cardiovascular risk factorsa |
| Flavonoids |
|
Widespread in ferns and higher plants | Flavonoids contribute to the yellow colours of flowers and fruits where they are present as glycosides dissolved in the cell sap. More than 2000 flavonoids have been isolated so far and form part of human diet. The most common classes are flavanols, flavones, flavonols, flavanones, anthocyanidines, proanthocyanidins and isoflavonoids (Table 4) |
Table 4.
Examples of pharmacologically relevant classes of flavonoids
| Class | Basic structure | Typical rich food source | Example | Comment |
|---|---|---|---|---|
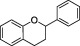
| Flavanolsa |
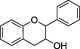
|
Soy flour, red wine, green tea, grape, wine, cocoa, apricot, beans | Catechin, epicatechin | The intake of flavanol‐rich foods (such as Cocoa flavonols) has been evaluated in relation to cardiovascular healthb |
| Flavanones |
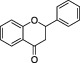
|
Citrus fruits (Tomás‐Barberán and Clifford, 2000) | Hesperetin, Naringenin | Flavanones and flavanones‐rich botanical extracts have been a subject of great interest for scientific research for as a possible emerging treatment for diabetes and its complications and cardiovascular protectionc |
| Flavones |
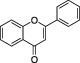
|
Green leafy spices, for example, Parsley | Apigenin, Luteolin | Flavones can contribute to plant tissue colour, if they occur in high concentrations or are complexed with metal ions. Flavones participate in taste. |
| Flavonols |
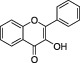
|
Nearly ubiquitous in foods, for example, quercetin. Main sources include yellow onion, curly kale and leek. | Kaempferol, Myricetin Quercetin | The most abundant flavonoid assumed with the diet is the flavonol quercetin |
| Isoflavonoids |
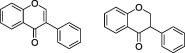
|
Soybeans, soy foods and legumes | Daidzein, Genistein | lsoflavonoids are a distinct class of flavonoids with estrogenic activity. They are found almost exclusively in legumes, particularly soybeans. Isoflavonoid‐containing preparations are promoted for alleviating menopausal symptoms |
| Proanthocyanidins |
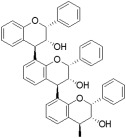
|
The foods with the highest levels of total proanthocyanidins are, in decreasing order, ground cinnamon, sorghum (sumac bran), dry grape seed, unsweetened baking chocolate, raw pinto beans, sorghum (high‐tannin whole grain), choke berries, red kidney beans, hazelnuts and pecan nutsd | Pycnogenol proanthocyanidin A‐2 (in hawthorn berry) | Also named condensed tannins, represents the most abundant plant‐derived polyphenols. Proanthocyanidins are responsible for the astringent taste of fruits. Potential areas of medical interest include the prevention of cardiovascular and metabolic disorders. |
| Anthocyanidins |
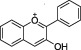
|
Red, purple and blue Berries (e.g. blackberry, strawberry, blueberry), aubergine, red wine |
Cyanidin, Delphinidin, peonidin | Anthocyanins produce the blue and red coloration of berry fruits, cherries and plums, eggplant, red cabbage and radishes. Fruit anthocyanins content usually increases as the fruit matures |
General information, including chemistry and occurrence, has been extracted from Peterson and Dwyer (1998); Samuelsson (1999); Beecher (2003); Manach et al. (2004); D'Archivio et al. (2007); Cui and Duke (2015);
Flavanols may exist both as monomer (catechins) and polymer (proanthocyanidins) form;
Chanet et al. (2012);
Green tea and black tea are rich sources of plant polyphenols, the most abundant being epigallocatechin‐3‐gallate and theaflavins. In this issue of the BJP, Peluso and Serafini cover recent findings on the antioxidant activity of tea polyphenols as well as more specific pharmacological mechanisms such as enzymatic inhibition and interaction with transporters. Indeed, catechins and theaflavins have been shown to inhibit a number of enzymes involved in lipid and glucose metabolism, including maltase, glucosidase, amylase, lipases as well as the enzyme involved in cholesterol synthesis, that is, hydroxyl‐3‐methyl‐glutaryl‐CoA reductase (Peluso and Serafini, 2017). Although caution is needed in extrapolating to clinical situations from in vitro experimental studies, and despite the need for further research, the authors conclude that the regular consumption of tea can modulate antioxidant capacity of body fluids and could improve glucose and lipid metabolism (Peluso and Serafini, 2017). The authors' conclusion is supported by a recent systematic review and meta‐analysis of observational studies, which revealed the association of tea consumption and decreased probability of developing the metabolic syndrome (Marventano et al., 2016). Nevertheless, further high‐quality clinical research is needed in this evolving area of nutritional pharmacology. Polyphenols in green tea and grapes are further considered by Santini and Novellino (2017) in the context of hypercholestrolaemia. The authors conclude that while positive effects may be suggested from in vitro and rodent studies, these do not extrapolate well to clinical evidence, with much higher doses being required, accompanied by safety concerns. Polyphenolic extracts from Annurca apples however have been endorsed by the FDA as safe and have potential to lower cholesterol. The authors provide an interesting example of how the beneficial properties of extracts can be significantly influenced by the species, with marked differences between closely related plants, in this case varieties of apples (Santini and Novellino, 2017).
The difficulties in interpreting the antioxidant effects of polyphenols are further explored by Goszcz et al. (2017) in the context of cardiovascular disease. Doubts exist as to whether the cardioprotective effects of these agents can be solely ascribed by the antioxidant properties demonstrated in vitro (Goszcz et al., 2017). This is because of the rapid degradation and poor absorption of the original chemical species in vivo. Indeed, in contrast, potential pro‐oxidant actions of breakdown products have been postulated. The authors present a broad overview of diverse direct cellular mechanisms, occurring at realistic concentrations and which may be invoked following diffusion of the polyphenol or its metabolite into the cell (Goszcz et al., 2017). Interactions with pathways of inflammation, platelet aggregation and also modulation of oestrogen signalling through genomic and non‐genomic mechanisms are discussed, leading to the conclusion that the dogma of polyphenols acting mainly as antioxidants needs to be critically re‐assessed
Anthocyanins
Anthocyanins (Greek anthos, flower and kyáneos, blue) are water‐soluble flavonoids responsible for the varied red‐orange to blue‐violet colour of fruits and flowers. The basic structural unit of anthocyanins, which are mainly found in nature as glycosides (anthocyanidins), is the flavylium ion (2‐phenylchromenylium) (Table 4). The main alimentary sources of anthocyanins include berries (blueberries, bilberries and strawberries), wine, grapes and red/purple vegetables (Wallace and Giusti, 2015; Vlachojannis et al., 2015; Smeriglio et al., 2016; Eghbaliferiz and Iranshahi, 2016). In this issue of the BJP, Lin et al. (2017) summarise the latest findings on the anti‐cancer activities of anthocyanins. Mechanisms believed to be relevant for their anti‐tumour action include antioxidant and anti‐inflammatory effects, inhibition of cell growth, induction of cell cycle arrest, stimulation of apoptosis (or autophagy) and anti‐invasion and anti‐metastatic actions (Lin et al., 2017). Clinically, there are conflicting results concerning the possible intake of anthocyanins and cancer prevention in humans (Lin et al., 2017). It is noteworthy that an Italian observational study reported that moderate wine consumption exerted protective effects on radiotherapy‐induced skin toxicity in breast cancer patients (Morganti et al., 2009). Consequently, an intervention trial aimed at evaluating the possible protective effects of anthocyanin‐containing dietary supplements on the inflammatory response to radiation and the resulting skin toxicity has been designed (Cerletti et al., 2017).
Proanthocyanidins
Proanthocyanidins, also called catechin tannins or condensed tannins, are the most abundant plant‐derived polyphenols widely available in fruits, vegetables, nuts, seeds, flowers and bark. Proanthocyanidins are oligomers or polymers, with flavanols being the building blocks (Table 4). As highlighted in this themed issue (Smeriglio et al., 2017), in addition to well‐established free radical scavenging and antioxidant properties, proanthocyanidins exert potentially relevant antimicrobial, anti‐tumour, anti‐inflammatory and cardioprotective actions. The potential beneficial pharmacological actions of proanthocyanidins have been attributed to their conjugated metabolites derived from gut microbiota (Bladé et al., 2016). A number of epidemiological studies have tried to correlate proanthocyanidin consumption with beneficial cardiovascular and metabolic effects (Bladé et al., 2016; Nassiri‐Asl and Hosseinzadeh, 2016; Akaberi and Hosseinzadeh, 2016). However, further studies are needed to firmly establish the potential benefits of increased proanthocyanidin intake to human health.
Phytoestrogens
Phytoestrogens represent a diverse group of naturally occurring polyphenols with structural similarity to 17β‐oestradiol, the primary female sex hormone. Main dietary phytoestrogens include isoflavones (e.g. genistein), prenylated flavonoids (e.g. 8‐prenylnaringenin), coumestans (e.g. coumestrol) and lignans (e.g. enterolactone). Dietary sources of phytoestrogens are nuts and oilseeds, soy products, cereals, breads and legumes (particularly soybeans) (Thompson et al., 2006). In this issue of the BJP, a wide overview of the potential health effects of dietary phytoestrogens with a specific focus on cardiovascular diseases, obesity and metabolic syndrome, menopausal health and cancer prevention, is provided by Rietjens et al., (2017).
Phytosterols
Phytosterols are plant‐derived, non‐nutritive steroid compounds structurally similar to cholesterol (Gylling and Simonen, 2015; Ogbe et al., 2015). A Western‐type diet contains about 200–500 mg cholesterol and up to 500 mg of phytosterols (Köhler et al., 2017). ‘Functional foods’ supplemented with phytosterols are widely promoted as dietary modifiers of serum lipids as they impair the intestinal absorption of cholesterol by competing with it for absorption into micelles in the gastrointestinal tract (Hunter and Hegele, 2017). In this issue of the BJP, Köhler et al. (2017) summarize, on the basis of animal and human studies, the current evidence about phytosterol‐containing functional foods and atherosclerosis. Starting from the premise that it is a misconception to accept as true that any treatment leading to a reduction in LDL cholesterol levels also leads to reduction of atherosclerosis, the authors conclude that ‘clear evidence that functional foods supplemented with phytosterols are safe and effective in the prevention of cardiovascular diseases is as yet unavailable and individual studies show that they may even be harmful’ (Köhler et al., 2017).
Overall, current evidence seems to cautiously support the recommendation of phytosterols as LDL cholesterol‐lowering agents (Gylling et al., 2014; Hunter and Hegele, 2017), but, because most trials have been of short duration, no data are available to date on cardiovascular endpoints (Silbernagel et al., 2015; Hunter and Hegele, 2017). Phytosterol supplements should be avoided in patients with sitosterolaemia, in which the excretion of dietary sterols is impaired (Hunter and Hegele, 2017).
Carotenoids
Carotenoids are lipid‐soluble pigments widespread in the vegetable kingdom and are found in high concentrations in marine organisms such as algae and microorganisms. Chemically, they are isoprenoids and are classified into carotenes (e.g. β‐carotene and lycopene) and xanthophylls (e.g. lutein, fucoxanthin and zeaxanthin). Beside provitamin A activity, carotenoids are potentially important as antioxidants and in disease prevention (Namitha and Negi, 2010). In this themed issue of the BJP, Milani et al. (2017) review the biochemical and pharmacological properties of the main carotenoids, their proposed mode of action and the clinical areas of interest including cancer prevention. A number of carotenoids have been shown to inhibit tumour cell growth, possibly via inhibition of angiogenesis, stimulation of apoptosis and scavenging free radicals (Milani et al., 2017). Recent systematic review/meta‐analyses of epidemiological studies have shown that (i) there is an inverse correlation between blood carotenoid levels and lung cancer risk (Abar et al., 2016); (ii) there is an inverse relationship between α‐carotene intake and risk of non‐Hodgkin lymphoma (Chen et al., 2016); (iii) higher lycopene consumption or circulating concentration is associated with a lower risk of prostate cancer (Chen et al., 2015); and (iv) ingestion of tomatoes rich in carotenoids may have a small effect on the prevention of prostate cancer (Chen et al., 2013). Overall, the potential of carotenoids in cancer prevention seems promising, although further and more rigorous studies are needed to confirm these associations.
Curcumin is one of the best studied carotenoids. It is a yellow hydrophobic polyphenol extracted from the rhizomes of Curcuma longa (turmeric), a perennial herbaceous plant of the ginger family (Zingiberaceae), which has been used for years in Ayurvedic medicine to treat a number of diseases such as dyspepsia, infections and liver diseases (Deguchi, 2015; Sreedhar et al., 2016; Mazzanti and Di Giacomo, 2016). In this issue of the BJP, the clinical potential of curcumin for treating a number of diseases including metabolic diseases such as diabetes and inflammatory diseases has been reviewed (Kunnumakkara et al., 2017). Recent systematic reviews and meta‐analyses have provided promising, albeit preliminary, evidence of efficacy to treat joint arthritis (Daily et al., 2016), skin disease (Vaughn et al., 2016), depressive disorders (Al‐Karawi et al., 2016), inflammatory bowel disease (Langhorst et al., 2015) and as an analgesic (Sahebkar and Henrotin, 2016). However, the quality of the primary study, the total sample size, the lack of long‐term efficacy and other methodological caveats make it impossible to draw definitive conclusions. More rigorous and larger studies are needed to fully exploit the potential of curcumin for clinical application.
Palmitoylethanolamide
Palmitoylethanolamide (PEA) is a naturally occurring fatty acid amide isolated for the first time about 60 years ago, when it was believed to be the active anti‐inflammatory ingredient of lipid fractions from egg yolk, peanut oil and soybean lecithin (Skaper et al., 2015). Subsequently, it has been identified in a number of food sources, including human breast milk, common beans, garden peas, tomatoes, corn and peanuts. PEA is marketed as a food component for special medical purposes for a number of indications including pain and inflammation. In this issue of the BJP, Petrosino and Di Marzo (2017) have reviewed the pharmacology of PEA, with particular emphasis on neurodegenerative disorders, pain perception and inflammatory diseases. New PEA formulations (e.g. with small particle size) and their effect in combination with other plant‐derived ingredients such as flavonoid luteolin and stilbenes are also highlighted (Petrosino and Di Marzo, 2017).
Amino acids and peptides
Branched‐chain amino acids (BCAAs)
BCAAs are essential amino acids with aliphatic side‐chains, such as isoleucine, leucine and valine. They are extensively utilized in protein synthesis, but their fate is dependent on the metabolic state of the organism, and they can be routed towards oxidation in a catabolic state, as shown by Bifari and Nisoli (2017) in their review in this BJP issue. There is increasing evidence that they can act as nutrient sensors, particularly leucine (Wolfson et al., 2016), and changes in levels of BCAAs can modulate the levels of insulin, glucagon and adipokines. Against this background, supplementation has been proposed as being beneficial in catabolic states, to promote protein synthesis (Shimomura et al., 2006) and also to regulate metabolic homeostasis. The authors review the evidence concerning the efficacy and safety of BCAA supplementation, addressing appropriate dosing and safety margins in human trials to date. They conclude that supplementation may be of benefit in catabolic states to normalize protein and amino acid homeostasis, but the value of supplementation in conditions such as obesity where BCAAs can modulate both catabolism and anabolism is less well supported. Bifari and Nisoli (2017) provide a thorough overview of clinical and preclinical data, including epidemiological data to support their conclusions.
Bioactive peptides
Bioactive peptides are specific protein fragments derived through enzymic hydrolysis of food proteins. Bioactive peptides are inactive within the sequence of the parent protein molecule. However, they can be released after gastrointestinal digestion, fermentation and hydrolysis by proteolytic enzymes (Udenigwe and Aluko, 2012; Bhat et al., 2015). These peptides originate from foodstuffs containing milk, dairy products, eggs (Park and Nam, 2015), soybean, oat, wheat (Maestri et al., 2016), fish and algae (Ruiz‐Ruiz et al., 2017) foodstuffs. A number of bioactive peptides derived from food sources have been incorporated in fortified foods or dietary supplements and are commercially promoted to reduce the risk of chronic diseases such as hypertension, hypercholesterolemia and obesity (Hayes and Tiwari, 2015).
In this BJP themed issue, Cicero et al. (2017) have reviewed the experimental and clinical data and the potential role of bioactive peptides in the prevention of chronic diseases, with a special focus on the cardiovascular system and on cancer prevention. The review of the literature revealed that, in spite of the promising experimental pharmacological studies, clinical evidence is at an early stage (Cicero et al., 2017). Preliminary evidence in humans is related mainly to cardiovascular effects. A systematic review of the literature reported that there was limited but consistent evidence that consumption of fermented‐milk products containing bioactive peptides improved arterial stiffness (Pase et al., 2011). A subsequent review published in the British Journal of Clinical Pharmacology concluded that that ‘while many studies have described promising health promoting effects of milk‐derived peptides in cardiovascular disease, further studies and, in particular, more in vivo research with a focus on toxicity, will be required before their application’ (Marcone et al., 2017).
Drugs in specific conditions
Anti‐ageing compounds
Ageing represents the greatest risk factor for nearly every major cause of morbidity and mortality (Fontana et al., 2010). Despite this, in the past, research has focused on individual life‐threatening age‐related disorders rather than on the complex molecular pathways leading to ageing (Fontana et al., 2010; Fontana and Partridge, 2015). In more recent years, robust preclinical evidence has demonstrated that life extension is, in most cases, accompanied by delayed or reduced morbidity, from cardiovascular disease, neurodegeneration and cancers among others (Fontana et al., 2010; Colman et al., 2014; Vaiserman and Marotta, 2016). The organisms most often used to detect age‐related effects of genetic, pharmacological and/or dietary interventions are the nematode Caenorhabditis elegans (lifespan of ~2 weeks), the fruit fly, Drosophila melanogaster (lives for 2 months) and the mouse Mus musculus (lives for ~2 years).
A number of nutraceuticals have shown promising efficacy as anti‐ageing agents (Longo et al., 2015, Vaiserman and Marotta, 2016). In this issue, Shen et al. (2017) have reviewed the plant ingredients and nutraceuticals from traditional Chinese medicine (TCM), which have been reported to have anti‐ageing effects in the past two decades. The main pharmacological mechanisms discussed include regulation of telomere and telomerase, sirtuins, nutrient and energy sensing pathways, including the TOT‐S6K pathway, and free radical scavenging effects. While the experimental results on TCM nutraceuticals are of potential interest, it should be highlighted that clinical research is at a very early stage and efficacy and/or safety data of many TCM ingredients are mostly based on poor‐quality research (Shen et al., 2017). Therefore, any extrapolation to clinical applications must be made with caution.
Functional and inflammatory bowel disorders
Nutraceutical treatment for intestinal disorders involves the use of fibre, herbal medicinal products, probiotics, prebiotics and synbiotics (Ford et al., 2014; Holtmann and Talley, 2015; Langhorst et al., 2015; Somani et al., 2015; Leiby and Vazirani, 2016). Probiotics are live microorganisms that may confer a health benefit to the host. The mode of action of probiotics includes strengthening of barrier function, changing immune responses and modulation of neurotransmitter release (Sanchez et al., 2017). Prebiotics are fermented ingredients that can change the composition/activity of the gut microbiota, thus conferring health benefits to the host (Valcheva and Dieleman, 2016). Prebiotics are carbohydrate compounds, primarily oligosaccharides, resistant to digestion, which reach the colon where they are fermented by the gut microflora (Slavin, 2013). Combinations of pro‐ and prebiotics presumed to have synergistic effects are called synbiotics. Curro et al. (2017) focused on recent advances in the understanding of the pharmacological mechanisms of these dietary supplements for the possible treatment of functional and inflammatory bowel disorders, with special reference to irritable bowel syndrome and inflammatory bowel disease. This review is complemented by a further original article demonstrating, in mouse models, the use of butyrate in a derivative form to deliver an unpalatable agent to the inflamed colon (Simeoli et al., 2017). Varied formulation strategies have been exploited to enhance delivery of nutraceuticals in a number of settings including nano‐particles.
Hypercholesterolemia
Many nutraceuticals have been evaluated for potential lipid‐lowering properties. Those with the most promising evidence of efficacy include soy protein, green tea, plant sterols, probiotic yoghurt, marine‐derived ω‐3 fatty acids and red yeast rice (Hunter and Hegele, 2017). Lowering of cholesterol and triglycerides is an area where a number of nutraceuticals have received endorsement by the FDA or European Food Safety Authority. This is discussed in detail by Santini and Novellino (2017). This field provides an example of where the benefits derived from a foodstuff are actually brought about by an active ingredient, which is also marketed as a drug. Lovastatin is the active ingredient of red yeast rice, known to inhibit hydroxymethylglutaryl‐CoA reductase and hence limit cholesterol synthesis. However, in contrast to the purified drug, red yeast rice may also contain toxins, exposure to which has been limited by the European Commission in 2014.
Clinical efficacy
As it is the case for prescribed medicines, the evidence obtained from high‐quality randomized controlled trials (RCTs) represents the gold standard for assessing nutraceutical clinical efficacy (Visioli, 2012). In recent years, a number of systematic reviews and meta‐analyses have provided researchers and healthcare professionals with updated conclusions (Izzo et al., 2016). Unfortunately, although the reporting quality of primary studies has improved over the last several decades, the methodological quality is often still rated as unsatisfactory making it difficult to draw definitive conclusions (Pferschy‐Wenzig and Bauer, 2015). Frequent shortcomings include inadequate sample size, short trial duration and dose variability among the trials. Furthermore, a specific issue related to herbal dietary supplements is the failure in reporting detailed information on the product itself, such as the part of the plant used, the Latin name of the plant, extraction solvent/type of extraction and phytochemical characterization of the herbal extract (standardization) (Izzo et al., 2016; Sut et al., 2016). This information represents a pivotal requirement for alignment within systematic reviews (Pferschy‐Wenzig and Bauer, 2015; Izzo et al., 2016). Combining clinical data from different preparations – even if derived from the same plant – would be like comparing apples and pears (Pferschy‐Wenzig and Bauer, 2015).
The clinical evidence has to be evaluated according to each individual nutraceutical and a priori generalisations such as ‘nutraceuticals work’ or ‘nutraceuticals are no more than placebo’ cannot be scientifically accepted. The ‘weight of evidence’ and the ‘direction of evidence’ should be carefully monitored (Ernst et al., 2008). The ‘weight of evidence’ is based on a combination of three largely independent factors, that is, the level of evidence (the highest level being systematic review/meta‐analyses), the methodological quality of the trials and the sample size (i.e. the number of studies and patients). The ‘direction of evidence’ refers to the collective positive or negative outcome of the studies (e.g. positive, uncertain or negative results) (Ernst et al., 2008). The clinical significance of the results (i.e. the robustness of the effect) should be taken also into account and indeed considered in relation to the effect size of conventional medicines. In relation to the clinical efficacy, herbal dietary supplements can be tentatively and cautiously categorized into six groups.
The first group, which represents a minority, comprises herbal dietary supplements whose evidence of clinical efficacy has been revealed by systematic reviews and meta‐analyses published by the Cochrane library and/or by authoritative medical Journals (i.e. ‘weight of evidence’: satisfactory; ‘direction of evidence’: toward positive effects). Examples include horse chestnut (Aesculus hippocastanum) for chronic venous insufficiency (Pittler and Ernst, 2012) or ginger (Zingiber officinale) for mild symptoms of nausea in pregnant women (McParlin et al., 2016). Conversely, the second group of herbal dietary supplements includes products, which have been (and are) extensively used for specific conditions, but robust clinical evidence does not support their use (i.e. ‘weight of evidence’: satisfactory; ‘direction of evidence’: toward negative effects). This is the case of Echinacea (Echinacea spp) for the common cold (Karsch‐Volk et al., 2014) or valerian (Valeriana officinalis) for insomnia (Leach and Page, 2015). The third group includes remedies, which have shown a small effect but of uncertain clinical significance [e.g. garcinia (Garcinia cambogia) and green tea (Camellia sinensis), both promoted for weight reduction (Onakpoya et al., 2011a; Jurgens et al., 2012)]. The fourth group is formed by herbal supplements, which have provided contradictory results [e.g. aloe vera (Aloe vera) for psoriasis (Miroddi et al., 2015)]. The fifth group includes a large number of herbal dietary supplements, which have shown encouraging clinical data, but the overall effect is far from being compelling, and more rigorous studies are needed to fully support their use [e.g. Agnus castus (Vitex agnus castus) for premenstrual syndrome (van Die et al., 2013), garlic (Allium sativum) as an antihypertensive (Rohner et al., 2015); see Izzo et al., 2016 for further examples). The last group, which includes the vast majority (Marcus, 2016), is formed by herbal dietary supplements that have been not evaluated in RCTs. Examples include yohimbe (Pausinystalia johimbe) for erectile dysfunction or horehound (Marrubium vulgare for respiratory diseases), just to mention two of the best‐seller herbal dietary supplements, listed in Table 2.
In conclusion, generalisation about the efficacy of herbal remedies cannot be made, and judgements must be expressed on a case‐by‐case basis. In most instances, claims of effectiveness rely on poor‐quality trials and definitive conclusions cannot be drawn. It is noteworthy that the vast majority of herbal dietary supplements, including some best‐selling products, have not been evaluated in RCTs.
Adverse events and drug interactions
Many lay people believe that nutraceuticals are harmless because they are natural. The concept that “natural” means “safe” is obviously misleading, if we just consider that most potent poisons or toxins are naturally occurring molecules. A classical example is the death of the Greek philosopher Socrates, who was given a liquid preparation of hemlock plant (Conium maculatum), after being sentenced to death for corruption of young men and impiety. The hemlock plant contains a group of toxic piperidine alkaloids, of which the representative members include coniine (LD50 in the mouse: 7–12.1 mg·kg−1; Lee et al., 2008] and the more toxic γ‐coniceine (Reynolds, 2005).
The safety of herbal dietary supplements has become a relevant issue for healthcare regulatory authorities, based on the serious events reported in the literature (Shaw et al., 2012). For example, although the incidence is difficult to estimate, green tea (Thea sinensis) extracts, ginseng (Panax ginseng), black cohosh (Cimicifuga racemosa) and Chinese herbs have been associated with drug‐induced liver injury (Navarro et al., 2017). Despite this alarming premise, recent careful analyses of the literature have shown that adverse events due to herbal dietary supplements are relatively infrequent (Di Lorenzo et al., 2015; Lee et al., 2016; Lude et al., 2016), if assessed for causality. A systematic review published in the British Journal of Clinical Pharmacology concluded that there are numerous published reports of adverse events relating to the use of herbal dietary supplements, but after critical assessment of the causality, the number is strongly reduced (Di Lorenzo et al., 2015). The top herbal supplements most commonly involved in adverse drug reactions are soybean (Glycine max, 19.3%, mainly allergic reactions), liquorice (Glycyrrhiza glabra, 12.2%, with hypokalaemia and hypertension being the most frequent adverse events), green tea (Camellia sinensis, 8.7%, mainly acute hepatitis) and ginkgo (Ginkgo biloba, 8.5%, adverse reactions usually associated with coagulation difficulties) (Di Lorenzo et al., 2015).
Another relevant safety issue related to the use of dietary supplements is the possibility of drug interactions with prescribed drugs (Izzo and Ernst, 2009; Izzo, 2012; Posadzki et al., 2013; Mouly et al., 2016). As with interactions between synthetic drugs, dietary supplements‐prescribed drug interactions can have both a pharmacokinetic and pharmacodynamic basis (Izzo et al., 2002; Chen et al., 2012; Sprouse and Van Breemen, 2016; Choi et al., 2016). A systematic review of the literature identified 882 dietary supplements–drug interactions, with St. John's Wort, ginkgo, magnesium, calcium and iron having the greatest number of documented cases (Tsai et al., 2012). Warfarin, insulin, aspirin, digoxin and ticlopidine were the most common conventional medicines involved in dietary supplement–drug interactions. Probably the best documented interaction is the decreased blood cyclosporin concentration (associated in some cases to rejection episodes) observed in patients who have also taken St John's wort (Hypericum perforatum) (Colombo et al., 2014; Izzo et al., 2016). St John's Wort contains hyperforin, which, via activation of the pregnane X receptors (Moore et al., 2000), inhibits cytochrome P450 enzymes and P‐glycoprotein, both involved in cyclosporine absorption, metabolism and elimination (Zhou et al., 2004; Kober et al., 2008).
In conclusion, although herbal dietary supplements are generally safer than prescribed drugs, the possibility of adverse effects should be considered and the risk–benefit ratio has to be carefully assessed individually, for each nutraceutical.
Adulteration with synthetic drugs
Concerns related to the use of dietary supplements may derive not only from intrinsic pharmacological/toxicological properties but also by the inadequate control of quality. Safety issues include misidentification of the plant, contamination (presence of microorganisms, pesticides, radioactivity and heavy metals) and adulteration (Yau et al., 2015). Adulteration is believed to be the most significant safety concern posed by dietary supplements (Brown, 2016). Deliberate adulteration of dietary supplements with undeclared prescription and over‐the‐counter drugs, in order to obtain and/or intensify a therapeutic claim, is relatively common and can have a negative impact on consumer safety (Yau et al., 2015; Khazan et al., 2014; Skalicka‐Wozniak et al., 2016). The USA FDA reported 572 cases of adulteration from 2007 to 2014, mainly in products claimed to enhance sexual performance (238 entries) and for weight loss (228 entries). Synthetic drugs used as dopants included PDE5 inhibitors, sibutramine, fenfluramine and rimonabant (Da Justa Neves and Caldas, 2015). Similar figures have been reported by the European Union Rapid Alert System for Food and Feed (Da Justa Neves and Caldas, 2015). Adulterated dietary supplements have been associated with serious adverse effects on humans such as stroke, acute liver injury, kidney failure, pulmonary emboli and heart palpitations (Da Justa Neves and Caldas, 2015), and deaths have been reported. PDE5 inhibitors, sibutramine and fenfluramine, found in dietary supplements have led people to be hospitalized (Calahan et al., 2016). Finally, undeclared synthetic drugs may cause adverse effects not only by themselves but also via interaction with other prescribed (synthetic) drugs in consumers unaware of their presence (Calahan et al., 2016).
Mammalian receptors as target of plant‐ and food‐derived compounds
Plant‐derived ingredients represent an important tool for the discovery and characterization of receptor types as well as for their deorphanization. Historical examples of plant compounds known to bind mammalian receptors selectively include the alkaloids nicotine (from Nicotiana tabacum) and morphine (from the opium poppy, Papaver somniferum). In this issue of the BJP, Jürg Gertsch provides an evolutionary perspective on the connection between dietary components and the endocannabinoid system (i.e. cannabinoid receptors, endocannabinoids and enzyme involved in the biosynthesis and degradation of endocannabinoids). Dietary phytochemicals that may modulate the activity of the endocannabinoid system include the CB2 agonists β‐caryophyllene (widespread in edible plants and spices) and 3,3′‐ diindolmethane (contained in Brassicaceae vegetables), the CB1 antagonist falcarinol (fatty alcohol found in carrots, parsley and celery), the endocannabinoid re‐uptake/enzymatic degradation inhibitors guineensine (from black pepper) and β‐amyrin (a pentacyclic triterpene widespread in vegetables, including in the cuticular wax of tomato, eggplant and white cabbage) (Naumoska and Vovk, 2015; Gertsch, 2017). Although the in vivo experimental evidence is limited to few such compounds (e.g. β‐caryophyllene, guineensine), it is possible that activation of CB2 receptors by phytonutraceuticals may provide a dietary mechanism to counteract inflammation and, conversely, CB1 blockade may have favourable effects on the metabolic syndrome (Gertsch, 2017).
Miscellaneous
The 15 review articles, which characterize the topic and represent the core of this themed issue, are complemented by two research papers that further stress the importance of nutraceutical research. Specifically, Simeoli et al. (2017) have explored the use of different formulations releasing butyrate in colitis, and Maione et al. (2017) have evaluated diterpenoid components (carnesol and carnosic acid) from Salvia officinalis extracts for their anti‐nociceptive properties, mediated via eicosanoid pathways.
Conclusions
All information summarized in this Editorial and in the accompanying articles published in this Themed Issue have elucidated the common problems and future challenges related to the experimental and clinical pharmacology of nutraceuticals. In view of the enormous commercial success of such products, it is imperative to have reliable information on the experimental (mode of action) and clinical (efficacy and safety) pharmacology, with research conducted with the same care and rigour as in any other medical area. Currently, the evidence of efficacy of herbal dietary supplements is mixed. Given the suboptimal quality of many trials, further rigorous research is needed to determine the real beneficial effect of many nutraceuticals. Also, vigilance by healthcare professionals is needed in relation to safety, including drug interaction and deliberate adulteration with synthetic drugs. Specific and crucial issues related to herbal nutraceuticals include plant misidentification, lack of standardization of the extracts, failure to report the extract type and solvent used and confusion among the part of the plant used. Lastly, due to lack of rigorous regulation, the need for the manufacturer of the nutraceutical to prove efficacy, safety and quality of a marketed product is less strongly enforced than in the pharmaceutical sector. Therefore, many available products might be ineffective (Izzo et al., 2016; Hunter and Hegele, 2017). Hopefully, this collection of articles and the present Editorial will strengthen our knowledge of the nutraceutical world and stimulate more rigorous research in this expanding area of pharmacology.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful to Professor Ferdinando Fiorino (Department of Pharmacy, University of Naples Federico II) for his help with the chemical structures.
Andrew, R. , and Izzo, A. A. (2017) Principles of pharmacological research of nutraceuticals. British Journal of Pharmacology, 174: 1177–1194. doi: 10.1111/bph.13779.
References
- Abar L, Vieira AR, Aune D, Stevens C, Vingeliene S, Navarro Rosenblatt DA et al. (2016). Blood concentrations of carotenoids and retinol and lung cancer risk: an update of the WCRF‐AICR systematic review of published prospective studies. Cancer Med 5: 2069–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaberi M, Hosseinzadeh H (2016). Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res 30: 540–556. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Karawi D, Al Mamoori DA, Tayyar Y (2016). The role of curcumin administration in patients with major depressive disorder: mini meta‐analysis of clinical trials. Phytother Res 30: 175–183. [DOI] [PubMed] [Google Scholar]
- Amiot MJ, Riva C, Vinet A (2016). Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 17: 573–586. [DOI] [PubMed] [Google Scholar]
- Aronson JK (2017). Defining ‘nutraceuticals’: neither nutritious nor pharmaceutical. Br J Clin Pharmacol 83: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher GR (2003). Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 133: 3248S–3254S. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Kumar S, Bhat HF (2015). Bioactive peptides of animal origin: a review. J Food Sci Technol 52: 5377–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Nisoli E (2017). Branched‐chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol 174: 1366–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladé C, Aragonès G, Arola‐Arnal A, Muguerza B, Bravo FI, Salvadó MJ et al. (2016). Proanthocyanidins in health and disease. Biofactors 42: 5–12. [DOI] [PubMed] [Google Scholar]
- Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S et al. (2009). Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev 3: CD005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers AT, Keen CL, Gershwin ME (2016). The basis of structure/function claims of nutraceuticals. Clin Rev Allergy Immunol 51: 370–382. [DOI] [PubMed] [Google Scholar]
- Bravo L (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56: 317–333. [DOI] [PubMed] [Google Scholar]
- Brown AC (2016). An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol https://doi.org/10.1016/j.fct.2016.11.001. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Calahan J, Howard D, Almalki AJ, Gupta MP, Calderón AI (2016). Chemical adulterants in herbal medicinal products: a review. Planta Med 82: 505–515. [DOI] [PubMed] [Google Scholar]
- Capasso F, Gaginella TS, Grandolini G, Izzo AA (2003). Phytotherapy. A Quick Reference to Herbal Medicine; Springer‐Verlag Berlin Heidelberg, Germany. [Google Scholar]
- Cerletti C, De Curtis A, Bracone F, Digesù C, Morganti AG, Iacoviello L et al. (2017). Dietary anthocyanins and health: data from FLORA and ATHENA EU projects. Br J Clin Pharmacol 83: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet A, Milenkovic D, Manach C, Mazur A, Morand C (2012). Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem 60: 8809–8822. [DOI] [PubMed] [Google Scholar]
- Chen F, Hu J, Liu P, Li J, Wei Z, Liu P (2016). Carotenoid intake and risk of non‐Hodgkin lymphoma: a systematic review and dose‐response meta‐analysis of observational studies. Ann Hematol https://doi.org/10.1007/s00277‐016‐2898‐1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Chen J, Song Y, Zhang L (2013). Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta‐analysis of prospective studies. J Nutr Sci Vitaminol (Tokyo) 59: 213–223. [DOI] [PubMed] [Google Scholar]
- Chen P, Zhang W, Wang X, Zhao K, Negi DS, Zhuo L et al. (2015). Lycopene and risk of prostate cancer: a systematic review and meta‐analysis. Medicine (Baltimore) 94: e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Sneed KB, Pan SY, Cao C, Kanwar JR, Chew H et al. (2012). Herb‐drug interactions and mechanistic and clinical considerations. Curr Drug Metab 13: 640–651. [DOI] [PubMed] [Google Scholar]
- Choi JG, Eom SM, Kim J, Kim SH, Huh E, Kim H et al. (2016). A comprehensive review of recent studies on herb‐drug interaction: a focus on pharmacodynamic interaction. J Altern Complement Med 22: 262–279. [DOI] [PubMed] [Google Scholar]
- Cicero AFG, Fogacci F, Colletti A (2017). Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br J Pharmacol 174: 1378–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM (2014). Caloric restriction reduces age‐related and all‐cause mortality in rhesus monkeys. Nat Commun 5: 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo D, Lunardon L, Bellia G (2014). Cyclosporine and herbal supplement interactions. J Toxicol 2014: 145325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui PH, Duke CC (2015). Chemical classification and chemistry of phytotherapeutics constituents In: Ramzan I. (ed.). Phytotherapiee: Efficacy, Safety, and Regulation. John Wiley & Sons: New Jersey, USA. [Google Scholar]
- Currò D, Ianiro G, Pecere S, Bibbò S, Cammarota G (2017). Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br J Pharmacol 174: 1426–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Justa Neves DB, Caldas ED (2015). Dietary supplements: international legal framework and adulteration profiles, and characteristics of products on the Brazilian clandestine market. Regul Toxicol Pharmacol 73: 93–104. [DOI] [PubMed] [Google Scholar]
- Daily JW, Yang M, Park S (2016). Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta‐analysis of randomized clinical trials. J Med Food 19: 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R (2007). Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita 43: 348–361. [PubMed] [Google Scholar]
- Dat AD, Poon F, Pham KB, Doust J (2012). Aloe vera for treating acute and chronic wounds. Cochrane Database Syst Rev 2: CD008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi A (2015). Curcumin targets in inflammation and cancer. Endocr Metab Immune Disord Drug Targets 15: 88–96. [DOI] [PubMed] [Google Scholar]
- Demonty I, Ras RT, van der Knaap HC, Meijer L, Zock PL, Geleijnse JM et al. (2013). The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur J Nutr 52: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Daniele N, Noce A, Vidiri MF, Moriconi E, Marrone G, Annicchiarico‐Petruzzelli M et al. (2017). Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 8: 8947–8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo C, Ceschi A, Kupferschmidt H, Lüde S, De Souza NE, Dos Santos A et al. (2015). Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol 79: 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, MacKay D (2014). Health habits and other characteristics of dietary supplement users: a review. Nutr J 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB (2005). Proanthocyanidins–a final frontier in flavonoid research? New Phytol 165: 9–28. [DOI] [PubMed] [Google Scholar]
- Drake PM, Szeto TH, Paul MJ, Teh AY, Ma JK (2017). Recombinant biologic products versus nutraceuticals from plants – a regulatory choice? Br J Clin Pharmacol 83: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbaliferiz S, Iranshahi M (2016). Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: updated review of mechanisms and catalyzing metals. Phytother Res 30: 1379–1391. [DOI] [PubMed] [Google Scholar]
- Ernst E, Pittler MS, Wider B, Boddy K (2008). Oxford Handbook of Complementary Medicine. Oxford University Press: New York, USA. [Google Scholar]
- Fontana L, Partridge L, Longo VD (2010). Extending healthy life span – from yeast to humans. Science 328: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR et al. (2014). Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta‐analysis. Am J Gastroenterol 109: 1547–1561. [DOI] [PubMed] [Google Scholar]
- Gertsch J (2017). Cannabimimetic phytochemicals in the diet – an evolutionary link to food selection and metabolic stress adaptation? Br J Pharmacol 174: 1464–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goszcz K, Duthie GG, Stewart D, Leslie SJ, Megson IL (2017). Bioactive polyphenols and cardiovascular disease: chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br J Pharmacol 174: 1209–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D et al. (2004). Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr 134: 613–617. [DOI] [PubMed] [Google Scholar]
- Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W et al. (2014). Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 232: 346–360. [DOI] [PubMed] [Google Scholar]
- Gylling H, Simonen P (2015). Phytosterols, phytostanols, and lipoprotein metabolism. Forum Nutr 7: 7965–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley L, Flowers N, Holmes J, Clarke A, Stranges S, Hooper L et al. (2013). Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 6: CD009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M, Tiwari BK (2015). Bioactive carbohydrates and peptides in foods: an overview of sources, downstream processing steps and associated bioactivities. Int J Mol Sci 16: 22485–22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger F, Chenot JF (2011). Systematic review of clinical trials assessing the effectiveness of ivy leaf (Hedera helix) for acute upper respiratory tract infections. Evid Based Complement Alternat Med 2011: 382789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann G, Talley NJ (2015). Herbal medicines for the treatment of functional and inflammatory bowel disorders. Clin Gastroenterol Hepatol 13: 422–432. [DOI] [PubMed] [Google Scholar]
- Hunter PM, Hegele RA (2017). Functional foods and dietary supplements for the management of dyslipidaemia. Nat Rev Endocrinol https://doi.org/10.1038/nrendo.2016.210. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Izzo AA (2012). Interactions between herbs and conventional drugs: overview of the clinical data. Med Princ Pract 21: 404–428. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Ernst E (2009). Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69: 1777–1798. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R (2002). Herbal medicine: the dangers of drug interaction. Trends Pharmacol Sci 23: 358–359. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Hoon‐Kim S, Radhakrishnan R, Williamson EM (2016). A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother Res 30: 691–700. [DOI] [PubMed] [Google Scholar]
- Jepson RG, Williams G, Craig JC (2012). Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 10: CD001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E (2012). Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst Rev 12: CD008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsch‐Völk M, Barrett B, Kiefer D, Bauer R, Ardjomand‐Woelkart K, Linde K (2014). Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2: CD000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalesi S, Irwin C, Schubert M (2015). Flaxseed consumption may reduce blood pressure: a systematic review and meta‐analysis of controlled trials. J Nutr 145: 758–765. [DOI] [PubMed] [Google Scholar]
- Khazan M, Hedayati M, Kobarfard F, Askari S, Azizi F (2014). Identification and determination of synthetic pharmaceuticals as adulterants in eight common herbal weight loss supplements. Iran Red Crescent Med J 16: e15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober M, Pohl K, Efferth T (2008). Molecular mechanisms underlying St. John's wort drug interactions. Curr Drug Metab 9: 1027–1037. [DOI] [PubMed] [Google Scholar]
- Köhler J, Teupser D, Elsässer A, Weingärtner O (2017). Plant sterol enriched functional food and atherosclerosis. Br J Pharmacol 174: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S et al. (2017). Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 174: 1325–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ et al. (2015). Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis 9: 86–106. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Moore V (2012). Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev 9: CD007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MJ, Page AT (2015). Herbal medicine for insomnia: A systematic review and meta‐analysis. Sleep Med Rev 24: 1–12. [DOI] [PubMed] [Google Scholar]
- Lee ST, Green BT, Welch KD, Pfister JA, Panter KE (2008). Stereoselective potencies and relative toxicities of coniine enantiomers. Chem Res Toxicol 21: 2061–2064. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jun SA, Hong SS, Ahn YC, Lee DS, Son CG (2016). Systematic review of adverse effects from herbal drugs reported in randomized controlled trials. Phytother Res 30: 1412–1419. [DOI] [PubMed] [Google Scholar]
- Leiby A, Vazirani M (2016). Complementary, integrative, and holistic medicine: integrative approaches to pediatric irritable bowel syndrome. Pediatr Rev 37: e10–e15. [DOI] [PubMed] [Google Scholar]
- Lin X, Zhang I, Li A, Manson JE, Sesso HD, Wang L, Liu S (2016). Cocoa Flavanol Intake and Biomarkers for Cardiometabolic Health: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials. J Nutr 146: 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B‐W, Gong C‐C, Song H‐F, Cui Y‐Y (2017). Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol 174: 1226–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown‐Borg HM, Caruso C et al. (2015). Interventions to slow aging in humans: are we ready? Aging Cell 14: 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüde S, Vecchio S, Sinno‐Tellier S, Dopter A, Mustonen H, Vucinic S et al. (2016). Adverse effects of plant food supplements and plants consumed as food: results from the poisons centres‐based PlantLIBRA study. Phytother Res 30: 988–996. [DOI] [PubMed] [Google Scholar]
- Maestri E, Marmiroli M, Marmiroli N (2016). Bioactive peptides in plant‐derived foodstuffs. J Proteomics 147: 140–155. [DOI] [PubMed] [Google Scholar]
- Maione F, Cantone V, Pace S, Chini MG, Bisio A, Romussi G et al. (2017). Anti‐inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br J Pharmacol 174: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004). Polyphenols: food sources and bioavailability. Am J Clin Nutr 79: 727–747. [DOI] [PubMed] [Google Scholar]
- Marcone S, Belton O, Fitzgerald DJ (2017). Milk‐derived bioactive peptides and their health promoting effects: a potential role in atherosclerosis. Br J Clin Pharmacol 83: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DM (2016). Dietary supplements: what's in a name? What's in the bottle? Drug Test Anal 8: 410–412. [DOI] [PubMed] [Google Scholar]
- Marventano S, Salomone F, Godos J, Pluchinotta F, Del Rio D, Mistretta A et al. (2016). Coffee and tea consumption in relation with non‐alcoholic fatty liver and metabolic syndrome: a systematic review and meta‐analysis of observational studies. Clin Nutr 35: 1269–1281. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Giacomo S (2016). Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules 21: E1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McParlin C, O'Donnell A, Robson SC, Beyer F, Moloney E, Bryant A et al. (2016). Treatments for hyperemesis gravidarum and nausea and vomiting in pregnancy: a systematic review. JAMA 316: 1392–1401. [DOI] [PubMed] [Google Scholar]
- Milani A, Basirnejad M, Shahbazi S, Bolhassani A (2017). Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 174: 1290–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroddi M, Navarra M, Calapai F, Mancari F, Giofrè SV, Gangemi S et al. (2015). Review of clinical pharmacology of Aloe vera L. in the treatment of psoriasis. Phytother Res 29: 648–655. [DOI] [PubMed] [Google Scholar]
- Morganti AG, Digesù C, Panunzi S, De Gaetano A, Macchia G, Deodato F et al. (2009). Radioprotective effect of moderate wine consumption in patients with breast carcinoma. Int J Radiat Oncol Biol Phys 74: 1501–1505. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit‐Singh CJ, Willson TM et al. (2000). St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci 97: 7500–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly S, Lloret‐Linares C, Sellier PO, Sene D, Bergmann JF (2016). Is the clinical relevance of drug‐food and drug‐herb interactions limited to grapefruit juice and Saint‐John's Wort? Pharmacol Res https://doi.org/10.1016/j.phrs.2016.09.038 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Namitha KK, Negi PS (2010). Chemistry and biotechnology of carotenoids. Crit Rev Food 50: 728–760. [DOI] [PubMed] [Google Scholar]
- Nassiri‐Asl M, Hosseinzadeh H (2016). Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: an update. Phytother Res 30: 1392–1403. [DOI] [PubMed] [Google Scholar]
- Naumoska K, Vovk I (2015). Analysis of triterpenoids and phytosterols in vegetables by thin‐layer chromatography coupled to tandem mass spectrometry. J Chromatogr A 1381: 229–238. [DOI] [PubMed] [Google Scholar]
- Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH (2017). Liver injury from herbal and dietary supplements. Hepatology 65: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbe RJ, Ochalefu DO, Mafulul SG, Olaniru OB (2015). A review on dietary phytosterols: their occurrence, metabolism and health benefits. Asian J Plant Sci Res 5: 10–21. [Google Scholar]
- Onakpoya I, Hung SK, Perry R, Wider B, Ernst E (2011a). The use of garcinia extract (hydroxycitric acid) as a weight loss supplement: a systematic review and meta‐analysis of randomised clinical trials. J Obes 2011: 509038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I, Terry R, Ernst E (2011b). The use of green coffee extract as a weight loss supplement: a systematic review and meta‐analysis of randomised clinical trials. Gastroenterol Res Pract 2011: 382852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palthur MP, Palthur SSS, Chitta SR (2010). Nutraceuticals: a conceptual definition. Int J Pharmacy Pharm Sci 2: 19–27. [Google Scholar]
- Park YW, Nam MS (2015). Bioactive peptides in milk and dairy products: a review. Korean J Food Sci Anim Resour 35: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Grima NA, Sarris J (2011). The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr 93: 446–454. [DOI] [PubMed] [Google Scholar]
- Peluso I, Serafini M (2017). Antioxidants from black and green tea: from dietary modulation of oxidative stress to pharmacological mechanisms. Br J Pharmacol 174: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Dwyer J (1998). Taxonomic classification helps identify flavonoid‐containing foods on a semiquantitative food frequency questionnaire. J Am Diet Assoc 98: 677–682. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V (2017). The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol 174: 1349–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pferschy‐Wenzig E, Bauer R (2015). The relevance of pharmacognosy in pharmacological research on herbal medicinal products. Epilepsy Behav 52: 344–362. [DOI] [PubMed] [Google Scholar]
- Pitkälä KH, Suominen MH, Bell JS, Strandberg TE (2016). Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Med 48: 586–602. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E (2012). Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev 11: CD003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadzki P, Watson L, Ernst E (2013). Herb‐drug interactions: an overview of systematic reviews. Br J Clin Pharmacol 75: 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart KM, Talati R, White CM, Coleman CI (2009). The impact of garlic on lipid parameters: a systematic review and meta‐analysis. Nutr Res Rev 22: 39–48. [DOI] [PubMed] [Google Scholar]
- Reynolds T (2005). Hemlock alkaloids from Socrates to poison aloes. Phytochemistry 66: 1399–1406. [DOI] [PubMed] [Google Scholar]
- Rietjens IMCM, Louisse J, Beekmann K (2017). The potential health effects of dietary phytoestrogens. Br J Pharmacol 174: 1263–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner A, Ried K, Sobenin IA, Bucher HC, Nordmann AJ (2015). A systematic review and meta‐analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens 28: 414–423. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Ruiz FM, Mancera‐Andrade EI, Iqbal HM (2017). Marine‐derived bioactive peptides for biomedical sectors – a review. Protein Pept Lett 24: 109–117. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Henrotin Y (2016). Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta‐analysis of randomized controlled trials. Pain Med 17: 1192–1202. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM et al. (2015). Lipid and blood pressure meta‐analysis collaboration group. Lack of efficacy of resveratrol on C‐reactive protein and selected cardiovascular risk factors‐Results from a systematic review and meta‐analysis of randomized controlled trials. Int J Cardiol 189: 47–55. [DOI] [PubMed] [Google Scholar]
- Saller R, Brignoli R, Melzer J, Meier R (2008). An updated systematic review with meta‐analysis for the clinical evidence of silymarin. Forsch Komplementmed 15: 9–20. [DOI] [PubMed] [Google Scholar]
- Samuelsson G (1999). Drugs of Natural Origin: A Textbook of Pharmacognosy, 4th revised edn. Apotekarsocieten, Swedish Pharmacological Press: Stockholm, Sweden. [Google Scholar]
- Sánchez B, Delgado S, Blanco‐Míguez A, Lourenço A, Gueimonde M, Margolles A (2017). Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61: 1600240. [DOI] [PubMed] [Google Scholar]
- Santini A, Novellino E (2017). Nutraceuticals in hypercholesterolaemia: an overview. Br J Pharmacol 174: 1450–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Ferro A (2013). Nutraceuticals: is there good science behind the hype? Br J Clin Pharmacol 75: 585–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D, Graeme L, Pierre D, Elizabeth W, Kelvin C (2012). Pharmacovigilance of herbal medicine. J Ethnopharmacol 140: 513–518. [DOI] [PubMed] [Google Scholar]
- Shen C‐Y, Jiang J‐G, Yang L, Wang D‐W, Zhu W (2017). Anti‐aging active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and implications for drug discovery. Br J Pharmacol 174: 1395–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N et al. (2006). Nutraceutical effects of branched‐chain amino acids on skeletal muscle. J Nutr 136: 529S–532S. [DOI] [PubMed] [Google Scholar]
- Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R et al. (2017). An orally administered butyrate‐releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium‐induced murine colitis. Br J Pharmacol 174: 1484–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirerol JA, Rodríguez ML, Mena S, Asensi MA, Estrela JM, Ortega AL (2016). Role of natural stilbenes in the prevention of cancer. Oxid Med Cell Longev 2016: 3128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalicka‐Woźniak K, Georgiev MI, Orhan IE (2016). Adulteration of herbal sexual enhancers and slimmers: the wish for better sexual well‐being and perfect body can be risky. Food Chem Toxicol S0278‐6915(16)30199‐5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Barbierato M, Zusso M, Bruschetta G, Impellizzeri D (2015). N‐palmitoylethanolamine and neuroinflammation: a novel therapeutic strategy of resolution. Mol Neurobiol 52: 1034–1042. [DOI] [PubMed] [Google Scholar]
- Slavin J (2013). Fiber and prebiotics: mechanisms and health benefits. Forum Nutr 5: 1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeriglio A, Barreca D, Bellocco E, Trombetta D (2016). Chemistry, pharmacology and health benefits of anthocyanins. Phytother Res 30: 1265–1286. [DOI] [PubMed] [Google Scholar]
- Smeriglio A, Barreca D, Bellocco E, Trombetta D (2017). Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol 174: 1244–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Kawa K, Eckl V, Johnson J (2016). Sales of herbal dietary supplements in US increased 7.5% in 2015. HerbalGram 111: 67–73. [Google Scholar]
- Somani SJ, Modi KP, Majumdar AS, Sadarani BN (2015). Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother Res 29: 339–350. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse AA, van Breemen RB (2016). Pharmacokinetic Interactions between drugs and botanical dietary supplements. Drug Metab Dispos 44: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedhar R, Arumugam S, Thandavarayan RA, Karuppagounder V, Watanabe K (2016). Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discov Today 21: 843–849. [DOI] [PubMed] [Google Scholar]
- Sut S, Baldan V, Faggian M, Peron G, Dall Acqua S (2016). Nutraceuticals, a new challenge for medicinal chemistry. Curr Med Chem 23: 3198–3223. [DOI] [PubMed] [Google Scholar]
- Silbernagel G, Baumgartner I, März W (2015. V). Cardiovascular safety of plant sterol and stanol consumption. J AOAC Int 98:739–41. [DOI] [PubMed] [Google Scholar]
- Tacklind J, Macdonald R, Rutks I, Stanke JU, Wilt TJ (2012). Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 12: CD001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N (2006). Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer 54: 184–201. [DOI] [PubMed] [Google Scholar]
- Tomás‐Barberán FA, Clifford MN (2000). Flavanones, chalcones and dihydrochalcones – nature, occurrence and dietary burden. J Sci Food Agric 80: 1073–1080. [Google Scholar]
- Tsai HH, Lin HW, Simon Pickard A, Tsai HY, Mahady GB (2012). Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: a systematic literature review. Int J Clin Pract 66: 1056–1078. [DOI] [PubMed] [Google Scholar]
- Udenigwe CC, Aluko RE (2012). Food protein‐derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77: R11–R24. [DOI] [PubMed] [Google Scholar]
- Vaiserman AM, Marotta F (2016). Longevity‐promoting pharmaceuticals: is it a time for implementation? Trends Pharmacol Sci 37: 331–333. [DOI] [PubMed] [Google Scholar]
- Valcheva R, Dieleman LA (2016). Prebiotics: definition and protective mechanisms. Best Pract Res Clin Gastroenterol 30: 27–37. [DOI] [PubMed] [Google Scholar]
- van Die MD, Burger HG, Teede HJ, Bone KM (2013). Vitex agnus‐castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta Med 79: 562–575. [DOI] [PubMed] [Google Scholar]
- Vaughn AR, Branum A, Sivamani RK (2016). Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res 30: 1243–1264. [DOI] [PubMed] [Google Scholar]
- Visioli F (2012). Can experimental pharmacology be always applied to human nutrition? Int J Food Sci Nutr 63 (Suppl 1): 10–13. [DOI] [PubMed] [Google Scholar]
- Vlachojannis J, Erne P, Zimmermann B, Chrubasik‐Hausmann S (2016). The impact of cocoa flavanols on cardiovascular health. Phytother Res 30: 1641–1657. [DOI] [PubMed] [Google Scholar]
- Vlachojannis C, Zimmermann BF, Chrubasik‐Hausmann S (2015). Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother Res 29: 561–565. [DOI] [PubMed] [Google Scholar]
- Wallace TC, Giusti MM (2015). Anthocyanins. Adv Nutr 6: 620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EM (2001). Synergy and other interactions in phytomedicines. Phytomedicine 8: 401–409. [DOI] [PubMed] [Google Scholar]
- Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR et al. (2016). Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W‐P, How GH, Koh H‐L (2015). Quality control and quality assurance of phytomedicines: key considerations, methods and analytic challenges In: Ramzan I. (ed). Phytotherapies: efficacy, safety and regulation. John Wiley & Sons: New Jersey, USA. [Google Scholar]
- Zheng GH, Yang L, Chen HY, Chu JF, Mei L (2014). Aloe vera for prevention and treatment of infusion phlebitis. Cochrane Database Syst Rev 6: CD009162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lim LY, Chowbay B (2004). Herbal modulation of P‐glycoprotein. Drug Metab Rev 36: 57–104. [DOI] [PubMed] [Google Scholar]


