Abstract
Background and Purpose
Butyrate has shown benefits in inflammatory bowel diseases. However, it is not often administered orally because of its rancid smell and unpleasant taste. The efficacy of a more palatable butyrate‐releasing derivative, N‐(1‐carbamoyl‐2‐phenylethyl) butyramide (FBA), was evaluated in a mouse model of colitis induced by dextran sodium sulphate (DSS).
Experimental Approach
Male 10 week‐old BALB/c mice received DSS (2.5%) in drinking water (for 5 days) followed by DSS‐free water for 7 days (DSS group). Oral FBA administration (42.5 mg·kg−1) was started 7 days before DSS as preventive (P‐FBA), or 2 days after DSS as therapeutic (T‐FBA); both treatments lasted 19 days. One DSS‐untreated group received only tap water (CON).
Key Results
FBA treatments reduced colitis symptoms and colon damage. P‐FBA and T‐FBA significantly decreased polymorphonuclear cell infiltration score compared with the DSS group. FBA reversed the imbalance between pro‐ and anti‐inflammatory cytokines (reducing inducible NOS protein expression, CCL2 and IL‐6 transcripts in colon and increasing TGFβ and IL‐10). Morever, P‐FBA and T‐FBA limited neutrophil recruitment (by expression and localization of the neutrophil granule protease Ly‐6G), restored deficiency of the butyrate transporter and improved intestinal epithelial integrity, preventing tight‐junction impairment (zonulin‐1 and occludin). FBA, similar to its parental compound sodium butyrate, inhibited histone deacetylase‐9 and restored H3 histone acetylation, exerting an anti‐inflammatory effect through NF‐κB inhibition and the up‐regulation of PPARγ.
Conclusions and Implications
FBA reduces inflammatory intestinal damage in mice indicating its potential as a postbiotic derivative without the problems associated with the oral administration of sodium butyrate.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- Anx‐A1 and Anxa1
annexin A1
- CON
control
- DSS
dextran sodium sulphate
- IBD
inflammatory bowel disease
- iNOS
inducible NOS
- Ly‐6G and Ly6g
lymphocyte antigen 6
- MPO
myeloperoxidase
- P‐B
sodium butyrate as preventive treatment
- P‐FBA
N‐(1‐carbamoyl‐2‐phenylethyl) butyramide as preventive treatment
- PMNs
polymorphonuclear cells
- SCFAs
short‐chain fatty acids
- T‐B
sodium butyrate as therapeutic treatment
- T‐FBA
FBA as therapeutic treatment
- TJP1
tight junction protein 1
- UC
ulcerative colitis
- ZO‐1
zonula occludens 1
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes d |
| TNFα | HDAC9 |
| GPCRs b | iNOS |
| FFA2 (GPR43) receptor | MPO |
| FPR1 | Transporters e |
| FPR2 | MCT1 (Slc16a1) |
| Nuclear hormone receptors c | |
| PPARγ |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
Host‐microbial homeostasis requires appropriate immune regulation within the gut mucosa, preventing uncontrolled immune responses against the beneficial commensal microbiota, which could potentially lead to inflammatory bowel diseases (IBDs), such as ulcerative colitis (UC) (Geuking et al., 2014). Several studies have indicated that products of bacterial metabolism, such as short‐chain fatty acids (SCFAs), can modulate the immune response of the host (McDermott and Huffnagle, 2014). In particular, butyrate produced by intestinal microbial fermentation of undigested resistant starches and dietary fibres is absorbed by the colonic cell and extensively metabolized, constituting the main source of energy. Many intestinal and extra‐intestinal effects are ascribed to butyrate (Canani et al., 2011), suggesting that it could have possible therapeutic effects in gastroenterology. To date, several studies have evaluated the effectiveness of butyrate in animal models of UC (Vieira et al., 2012; Mishiro et al., 2013). In humans, few studies have been performed probably due to the low compliance with the oral route of administration (because of its rancid taste) or by rectal enemas (this is cumbersome to the patient and may cause irritability due to its acid property). Moreover, rectal administration of butyrate or a mixture of SCFAs did not show beneficial effects and displayed only trends towards clinical amelioration (Scheppach et al., 1992; Steinhart et al., 1996; Breuer et al., 1997; Vernia et al., 2003; Hamer et al., 2010). The discrepancy in human studies using enemas may be due to differences in treatment duration, use of butyrate alone or a mixture of SCFAs in the enemas and use of several concentrations and volumes of these mixtures. Conversely, other studies have reported that fermentable dietary fibre supplementation, which resulted in increased fecal butyrate levels, was effective in maintaining remission in UC, revealing a significant improvement in clinical and inflammatory characteristics (Fernandez‐Banares et al., 1999; Hanai et al., 2004; Wedlake et al., 2014). The importance of butyrate supplementation has been demonstrated by the impaired butyrate metabolism in the inflamed, intestinal mucosa of patients affected by IBD (De Preter et al., 2012). In fact, data show that this deficiency in butyrate results from a reduction in butyrate uptake by the inflamed mucosa due to the down‐regulation of the monocarboxylate transporter (MCT)‐1 expressed on the apical membrane of intestinal epithelium (Thibault et al., 2007). In particular, the reduction in the intracellular availability of butyrate in colonic cells may decrease its protective effects against cancer in IBD patients (Thibault et al., 2010).
FFA2 is a G‐protein‐coupled receptor expressed in colonic epithelium, adipose tissue and immune cells (Bindels et al., 2013) and together with GPR109A are considered the main butyrate targets involved in the suppression of colonic inflammation and carcinogenesis (Singh et al., 2014). Moreover, butyrate modulates histone acetylation, as it is a histone deacetylase (HDAC) inhibitor, and alters the host's epigenome, leading to its epigenetic mechanism (Hamer et al., 2008; Canani et al., 2011).
On the basis of all its characteristics, butyrate can be considered to be a postbiotic, being a non‐viable bacterial metabolic product obtained from probiotic microorganisms that have biological activity in the host. The purpose of this study was to investigate the efficacy of a more palatable butyrate‐releasing compound, the N‐(1‐carbamoyl‐2‐phenyl‐ethyl) butyramide (FBA), in a dextran sodium sulphate (DSS)‐induced colitis model, as an innovative postbiotic derivative. Our hypothesis is that FBA similarly or better than its parental compound (sodium butyrate) is able to reduce colon inflammation and the symptoms of colitis by decreasing the recruitment of neutrophils and the production/release of pro‐inflammatory mediators in the colonic mucosa following DSS exposure. The mechanisms behind these effects could be related to the restoration of the butyrate transporter, PPARγ and tight junctions in colon tissue together with an inhibitory effect on the HDAC9/NF‐κB axis.
Methods
Induction of colitis and treatments
Experimental colitis was induced in 10 week‐old BALB/c AnNHsd male mice (25 ± 2 g) (Harlan‐Corezzano, Italy) by 2.5% wt:vol DSS (36–50 kDa, MP Biomedical) in drinking water ad libitum from day 7 until 12 followed by DSS‐free water from day 13 until day 19 (end of experimental protocol). Mice were randomly divided into the four following groups (n = 10 per group): (1) control mice (CON group); (2) mice receiving DSS (DSS group); (3) DSS‐fed mice receiving FBA as preventive therapy (P‐FBA); (4) DSS‐fed mice receiving FBA therapeutically (T‐FBA). Untreated CON and DSS groups received tap water by gavage as vehicle.
In addition, we decided to set up two further groups of DSS‐fed mice treated with its parental compound sodium butyrate (B): (5) DSS‐fed mice receiving sodium butyrate as preventive therapy (P‐B); (6) DSS‐fed mice receiving sodium butyrate therapeutically (T‐B); FBA and sodium butyrate were given daily. Sodium butyrate (B, 20 mg∙kg−1) or FBA (42.5 mg∙kg−1, the equimolecular dose of B) was administered by gavage, and the treatment started 7 days before (preventive) or 2 days after (therapeutic) DSS challenge, and continued throughout the experimental period. We used the parental compound in the evaluation of body weight, disease activity index (DAI), colon length, histopathological score, survival rate and mechanistic studies to confirm the similar profile of FBA as a butyrate‐releasing derivative. Mortality rate was assessed during the entire experimental time (from day 1 to day 19) and a Kaplan–Meier survival curve was calculated (Figure S1). All procedures involving animals and their care were conducted in accordance with international and national law and policies [EU Directive 2010/63/EU for animal experiments, ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015) and the Basel declaration including the 3R concept] and were approved by the Institutional Committee on the Ethics of Animal Experiments (CSV) of the University of Naples ‘Federico II’ and by the Ministero della Salute under protocol no. 0022569‐P‐20/12/2010. At day 19, following an overnight fast, animals were killed by an i.p. injection of a mixture of ketamine/xylazine followed by cervical dislocation.
Evaluation of experimental colitis
In all mice, their weight, presence of blood and gross stool consistency were determined daily as previously described (Dieleman et al., 1997). Each score was determined as follows: (1) change in weight (0: weight loss <1% compared with the starting weight, 1: weight loss between 1 and 5%, 2: weight loss between 6 and 15%, 4: weight loss >15%); (2) stool blood (0: negative, 2: positive, 4: gross bleeding); and (3) stool consistency (0: normal, 2: loose stools, 4: diarrhoea) as previously described (Cooper et al., 1993). Briefly, the disease activity index (DAI) was determined by combining the scores from these three categories and dividing that number by 3.
Histological analysis and scoring of colon sections
At day 19, the mice were killed, tissues were collected, and colon length was measured. Distal sections were stored in formalin 10% or O.C.T. for histological and immunofluorescent analyses. Following haematoxylin and eosin (H&E) staining, colon sections were analysed in a blinded manner for the evaluation of the histopathological score as previously described (Chang et al., 2014).
Real‐time PCR
Total RNA isolated from colon was extracted using TRIzol Reagent (Invitrogen), according to the manufacturer's instructions. cDNA was synthesized using a Maxima First Strand cDNA Synthesis Kit (Fermentas) from 2 μg total RNA. PCRs were performed with Bio‐Rad CFX96 Connect Real‐time PCR System and software (Bio‐Rad Laboratories). The primer sequences for target genes and PCR conditions are reported in Table S1.
Serum TNF‐α determination
At day 19, mice were killed and blood was collected by cardiac puncture. Sera were obtained by centrifugation at 1500 × g at 4°C for 15 min and stored at −70°C. TNF‐α levels (pg·mL−1) were measured by ELISA kits for mice from BD Pharmingen, according to the manufacturer's instructions.
Western blotting
Colon tissue was homogenized, and protein lysates were subjected to SDS‐PAGE as described previously (Simeoli et al., 2015). The filters were probed with primary antibody overnight. To evaluate NF‐κB activation and histone H3 acetylation, NF‐κB p50 (Cell Signaling Technology), Acetyl‐H3 and H3 (EMD Millipore) were measured in nuclear extracts. Inducible NOS (iNOS) (Cayman Chemicals) protein expression was evaluated in whole colon lysates. The blot was developed using enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ) and the immune complex visualized by Image Quant. The protein bands were scanned and densitometrically analysed with a model GS‐700 imaging densitometer (Bio‐Rad Laboratories). Western blots for lamin A and GAPDH (Sigma‐Aldrich) were performed to ensure equal sample loading in nuclear and whole lysates respectively.
Measurement of myeloperoxidase (MPO) activity
Proximal colonic tissues were homogenized as described previously (Bradley et al., 1982). The homogenates were assessed for myeloperoxidase (MPO) activity as described previously (Smith and Castro, 1978). MPO was expressed as U·mg−1 protein with one unit hydrolyzing 1 μmol H2O2·min−1.
Immunofluorescence analysis of lymphocyte antigen 6 (Ly‐6G) and annexin A1
Colon samples for immunofluorescence were embedded in O.C.T. (PelcoCryo‐Z‐T, Ted Pella Inc), and cryosectioned (10 μm thick). Tissue sections were then fixed in 4% paraformaldehyde for 10 min at room temperature (RT). To examine co‐localization of Anx‐A1 (annexin A1) with lymphocyte antigen 6 (Ly‐6G), sections were blocked and then incubated with a monoclonal antibody anti Ly‐6G FITC (BD Biosciences) and a rabbit anti‐AnxA1 antibody overnight at 4°C. Sections were then incubated (1 h at RT) with Alexa‐Fluor® 546 goat anti‐rabbit IgG (Invitrogen) for Anx‐A1. After incubation with secondary antibody, sections were incubated with DAPI to visualize nuclei. Fluorescence was visualized with an Olympus BX51 fluorescence microscope (Olympus) equipped with a DS‐QiMc monochromatic camera (Nikon) and X‐Cite® Series 120Q Xenon lamp. NIS‐Elements BR3.1 software (Nikon) was used for all analyses. Merge images were performed with ImageJ® software. Two negative controls were used: slides incubated with or without primary antibody. Images were recorded at identical gain settings and performed in duplicate in non‐serial distant sections. Four image fields were taken of each section.
Immunofluorescence analysis of occludin and zonula occludens (ZO)‐1
Colon segments were fixed in 10% formalin and embedded in paraffin. Then 7 μm sections were deparaffinized in decreasing ethanol concentrations, and antigens were unmasked. After antigen retrieval, sections were permeabilized in Tris‐buffered saline (TBS) plus 0.1% Triton X‐100. After non‐specific background blocking, sections were incubated with anti‐occludin or anti‐zonula occludens 1 (ZO‐1) (1:50 for occludin Santa Cruz Biotechnology and 1:100 for ZO‐1 Invitrogen). Sections were probed with secondary Alexa Fluor® 488 antibody (1:200, Invitrogen Corporation). Slides were visualized on a fluorescence microscope, and images were stored digitally with Leica software. Two negative controls were used: slides incubated with or without primary antibody. The quantitative measurements of immunofluorescence analysis for occludin and ZO‐1 were performed and revealed by Integrated Pixel Intensity using ImageJ® software.
Statistical analysis
All data are presented as means ± SEM. The statistical analyses were performed with the use of Graph‐Pad Prism (Graph‐Pad Software). For all the experimental data, we evaluated group differences with one‐way ANOVA followed by Bonferroni multiple comparison test. For the analysis of the body weight changes during the entire experimental period, we used two‐way ANOVA followed by Tukey's multiple comparison test, setting as variables the treatment and the time. Survival study was analysed using the Kaplan–Meier log‐rank test. Statistical significance was set at P < 0.05. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Reagents and diet
Prof. Antonio Calignano provided FBA (International application patent with publication number WO2009130735); its synthesis and characterization was as previously reported (Mattace Raso et al., 2013). FBA is stable in acids and alkalis and capable of releasing butyric acid in both the small and large bowel in a constant manner over time. Interestingly, FBA does not present the unpleasant odour of butyrate and, being tasteless, overcomes the poor palatability of butyrate that often reduces the therapy compliance. All chemicals, including sodium butyrate, were purchased from Sigma‐Aldrich (Saint Louis, MO, USA). The standard laboratory unpurified diet was purchased from Harlan Teklad. The 2018 Teklad Global Protein Rodent Diet contained the following: water, 120 g·kg−1; protein, 185 g·kg−1; fat, 55 g·kg−1; fibre, 45 g·kg−1; ash, 60 g·kg−1; minerals, 0.13 g·kg−1 (containing 50 mg ferrous Fe·kg−1 and 44 mg Mn·kg−1); and a vitamin mix, 0.52 g·kg−1 (containing 81 mg vitamin E·kg−1).
Results
FBA similarly to butyrate reduces disease activity index and colon tissue damage
The oral treatment with P‐FBA and T‐FBA significantly reduced the development of colitis evaluated by DAI at day 12 and 18 compared with the DSS group (Figure 1A). A similar effect was observed even when DSS‐mice were treated with butyrate as a preventive or therapeutic therapy.
Figure 1.
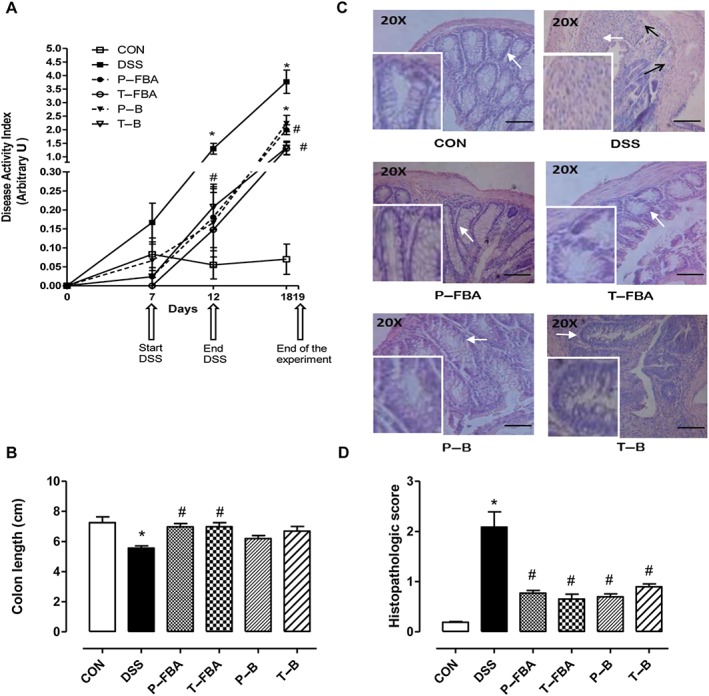
Effects of FBA and butyrate on DSS‐induced colitis. (A) Disease activity index (DAI) on days 0, 7, 12 and 18. (B) Colon length expressed in cm. (C) Distal colons were stained with H&E. Scale bar: 100 μm. White arrows indicate areas shown in the inset squares (objective 40×). Black arrows indicate infiltrated cells in the submucosa. (D) Histopathological scores were determined in a blinded fashion. Colons were excised at day 19. Data are means ± SEM, n = 8. * Significant versus CON; # versus DSS.
The beneficial effects of FBA were more than those of butyrate, and were also evident in the excised colon samples, which showed an improvement in the tissue shortening induced by DSS (Figure 1B). Control colon sections stained with H&E showed an intact epithelium, well‐defined crypt length, no oedema or neutrophil infiltration in mucosa and submucosa, and no ulcers or erosions (Figure 1C). In contrast, colon tissue from the DSS group showed severe inflammatory lesions throughout the mucosa and loss of crypt architecture. Both FBA and butyrate were able to protect colonic mucosa structure, ameliorating the mucosal integrity and crypt structure and improving the epithelial surface compared with DSS group (Figure 1C). The beneficial effects mediated by FBA and butyrate either as a preventive or therapeutic schedule were also confirmed by evaluation of the histopathological score of distal colon sections stained with H&E (Figure 1D).
The mortality rate and body weight were also monitored throughout the experimental period (Figure S1 and Figure S2).
Effects of FBA on neutrophil infiltration in colonic mucosa
We determined the expression and localization of the neutrophil granule protease Ly‐6G in colon tissue by fluorescence microscopy (Figure 2A). To verify if the protective effect of FBA was also associated with modulation of pro‐resolving factors, such as annexin A1, double‐staining immunofluorescence was performed; this revealed high Anx‐A1 levels in neutrophils, confirmed by a marked co‐localization with Ly‐6G in the colons of mice with active disease. Both FBA schedules were able to counteract neutrophil infiltration induced by DSS‐challenge reducing the Ly‐6G and Anx‐A1 staining (Figure 2A). This effect was also quantitatively demonstrated since polymorphonuclear cells (PMNs) infiltrating score was lower in the P‐FBA and T‐FBA colonic mucosa compared with that from the DSS group (Figure 2B). Similarly, FBA, either as a preventive or therapeutic treatment, inhibited the DSS‐mediated effects reducing Anxa1 mRNA transcripts (Figure 2C). Furthermore, in both FBA‐treated groups, the activity of MPO, a lysosomal haemoprotein found in the azurophilic granules in neutrophils (Figure 2D), and Ly6g mRNA levels (Figure 2E) were significantly less than in the control DSS group.
Figure 2.
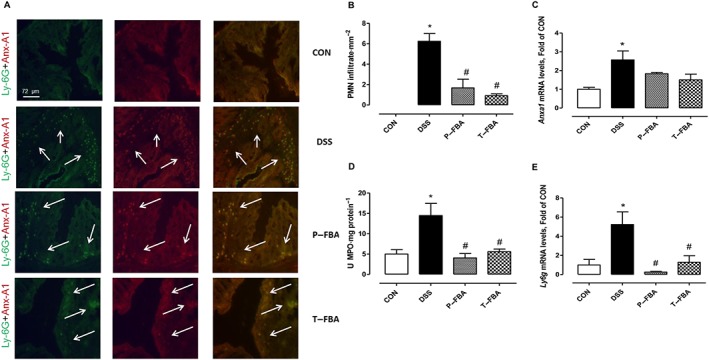
FBA reduces PMN infiltration in colonic mucosa. (A) Double‐staining immunofluorescence detection of Anx‐A1 and Ly‐6G (Ly‐6G + Anx‐A1 yellow staining) in DSS compared with control and FBA groups. White arrows indicate PMN infiltration. (B) PMN infiltration score was obtained by counting Ly‐6G+ cells in four random mucosal and submucosal views of three different sections from the descending colon and was expressed as number of cells·mm−2. (C) Real‐time PCR of Anxa1 is shown. (D) MPO activity and Ly6g mRNA levels (E) are also reported. Real‐time data are presented as means ± SEM, n = 8. * Significant versus CON; # versus DSS. Immunofluorescence stainings are representative of three slides for each group, magnification 200×.
Effect of FBA on neutrophil markers and FFA2 expression in colonic mucosa
Butyrate can influence the chemotaxis of immune cells through FFA2 receptor (Bindels et al., 2013). Therefore, we analysed mRNA levels of this receptor in colon. As depicted in Figure 3A, FBA, especially when used as therapeutic, was able to increase significantly mRNA levels for free fatty acids receptor‐2 (Ffar2, which encode for FFA2) compared with the control and DSS groups. In contrast, FBA, either as a preventive or therapeutic treatment, counteracted the DSS‐mediated effects reducing formyl peptide receptor 1 (Fpr1) and Fpr2 mRNA levels (Figure 3B–C). These data highlight the involvement of FFA2 in neutrophil recruitment during inflammatory conditions in the colon and confirm that FBA is able to reduce PMN infiltration compared with the DSS group.
Figure 3.
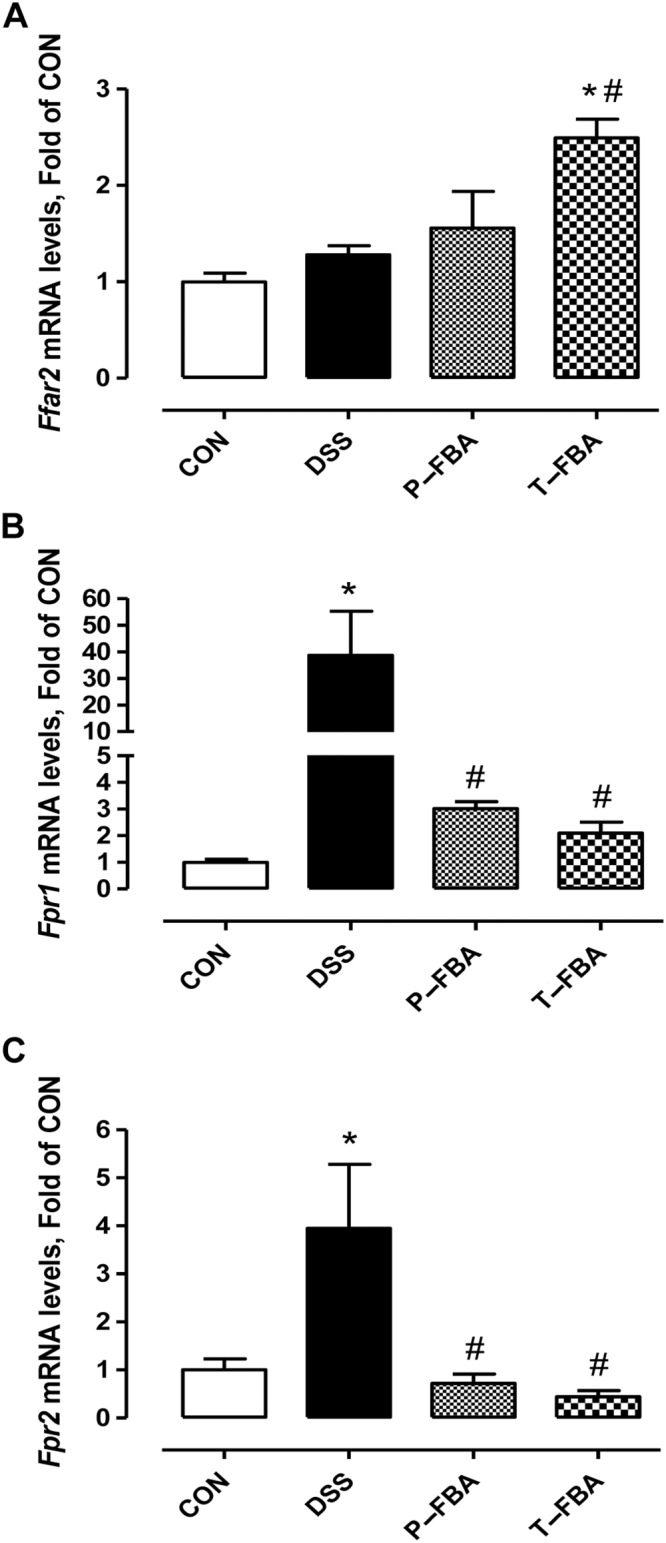
Effect of FBA on the expression of FFA2 receptors (Ffar2) and neutrophil markers in colonic mucosa. Transcriptional levels of Ffar2 (A), Fpr1 (B) and Fpr2 (C) were evaluated following treatment with FBA (preventive and therapeutic). Real‐time PCR data are presented as means ± SEM, n = 8. * Significant versus CON; # versus DSS.
FBA modulates pro‐ and anti‐inflammatory mediators in the colon and serum
The protein expression of iNOS, which is responsible for NO production, and mRNA levels of Ccl2 gene, which encode monocyte chemoatractant protein (MCP)‐1 (also known as CCL2), were greater in the colon of DSS‐challenged mice than those of the control group (Figure 4A–B). Similarly, TNF and IL‐6 mRNA levels were greater in the DSS group than in controls (Figure 4C–D). Conversely, the transcriptional levels of the anti‐inflammatory cytokines IL‐10 and TGFβ were impaired by DSS (Figure 4E–F). FBA treatments, similarly reduced the levels of pro‐inflammatory iNOS and Ccl2, TNF and IL‐6 and at least in part restored the IL‐10 and TGFβ transcripts in colon tissue compared with the DSS group. Moreover, we also analysed the TNF‐α levels in serum (n = 5, each group) to confirm the systemic anti‐inflammatory properties of FBA and butyrate (CON = 1.67 ± 0.34 pg·mL−1 and DSS = 28.74 ± 4.57, P < 0.05 vs CON; P‐FBA = 15.22 ± 3.85 and T‐FBA = 12.89 ± 2.76, P < 0.05 vs DSS respectively).
Figure 4.
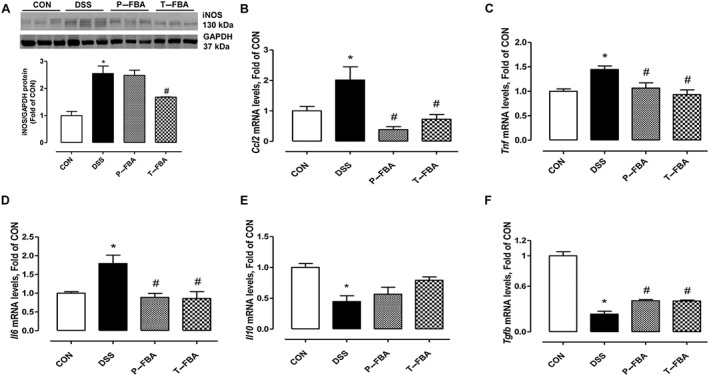
FBA reduces pro‐inflammatory mediators and increases anti‐inflammatory markers in colon tissue. (A) Western blot showing iNOS protein expression. GAPDH blot was used as equal loading control. mRNA transcriptional levels of Ccl2 (B), TNF (C) and IL‐6 (D) are also shown. Real‐time PCR of IL‐10 (E) and TGFβ (F) was performed in colon from CON and DSS mice treated or not with FBA. Data are presented as means ± SEM, n = 8. * Significant versus CON; # versus DSS.
FBA preserves MCT‐1 transporter and epigenetically counteracts inflammation induced by DSS
DSS‐challenged mice showed lower mRNA levels of the monocarboxylic acid carrier, the solute transporter family Slc16a1, than the control group (Figure 5A). This reduction was preserved only by therapeutic treatments with FBA or butyrate. To determine the mechanisms involved in the butyrate effect, the modulation of several transcription factors and histone acetylation were analysed. DSS challenge significantly reduced the PPARγ mRNA (Figure 5B) and increased nuclear NF‐κB p50 protein (Figure 5D) compared with the control group. These effects were counteracted by preventive and therapeutic treatments with FBA. We also assessed the ability of FBA to normalize the transcriptional levels of a specific member of class IIA HDAC involved in the pathogenesis of colitis in mice (de Zoeten et al., 2010), HDAC9, which was impaired in DSS animals. As shown in Figure 5C, FBA, such as sodium butyrate, counteracted the DSS‐induced up‐regulation of HDAC9 mRNA levels. However, FBA when used as a therapeutic was able to significantly increase histone H3 acetylation confirming HDAC inhibition (Figure 5E). Interestingly, FBA and butyrate did not modify histone 3 (H3) acetylation when used as a preventive treatment.
Figure 5.
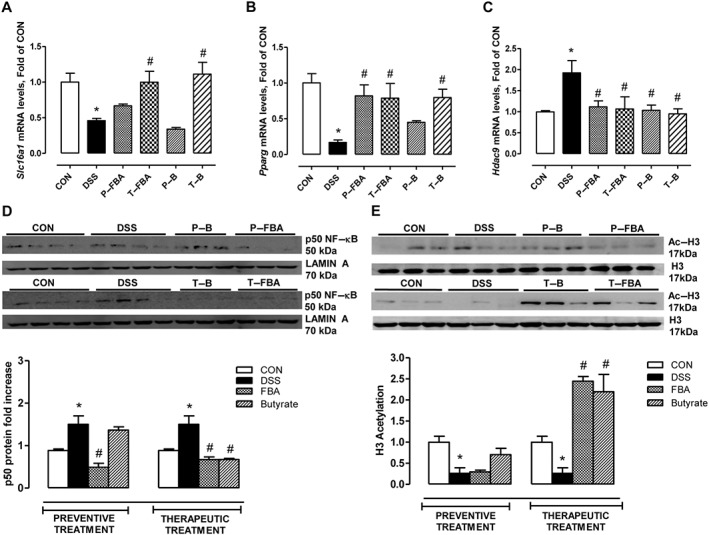
Mechanisms of anti‐inflammatory action mediated by FBA and sodium butyrate in DSS‐induced colon damage. The mRNA expressions of (A) Slc16a1, (B) Pparg and (C) Hdac9 are shown. Real‐time PCR data are presented as means ± SEM, n = 8. (D) Western blot showing p50 NF‐κB expression in nuclear extract is also presented. Both butyrate‐based compounds reduced Hdac9 transcriptional levels (C) and induced histone H3 acetylation (E). * Significant versus CON; # versus DSS.
Effects of FBA on intestinal barrier integrity and tight‐junction expression
To evaluate barrier integrity, we determined the distribution of two tight junction proteins (TJPs), occludin and ZO‐1, in distal colon. Staining for occludin (Figure 6A) and ZO‐1 (Figure 6C) in colonic mucosa of DSS‐fed mice displayed less intensity than the control group, as confirmed by the integrated pixel intensity analysis for occludin and ZO‐1 (Figure 6B and D respectively). However, compared with the DSS group, P‐FBA and T‐FBA significantly restored the distribution of occludin and ZO‐1 throughout the colonic mucosa, with a continuous staining pattern. These effects were highlighted by the evaluation of mRNA transcriptional levels for both proteins in colon sections (Figure S3). Furthermore, both FBA‐based treatments more than P‐B and T‐B were able to preserve not only the TJPs distribution but even the architecture of colons in DSS‐fed mice, which was similar to that of control mice.
Figure 6.
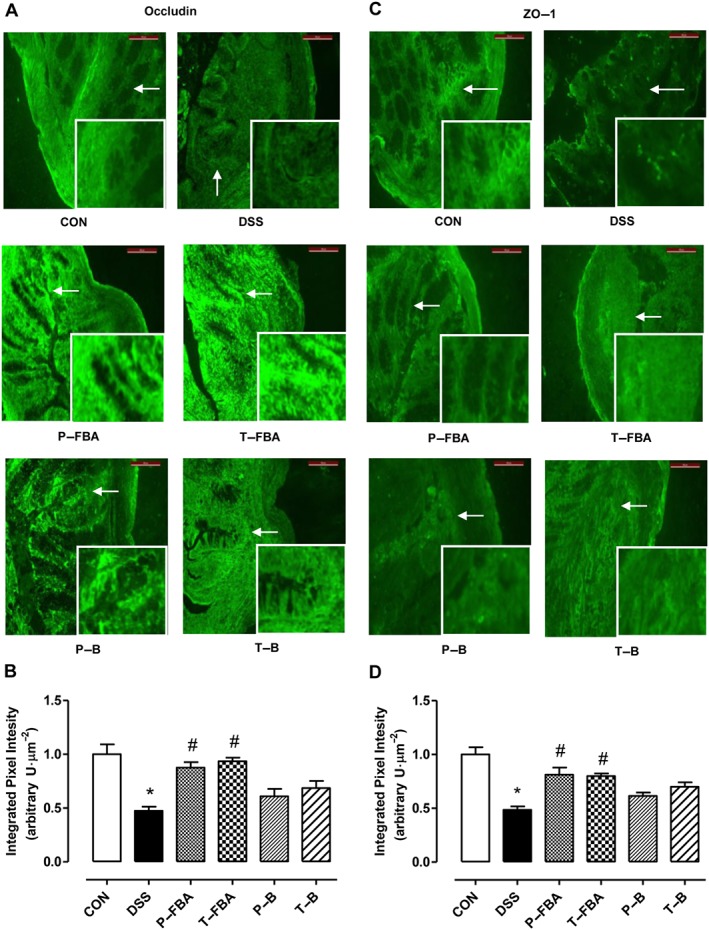
FBA‐based compounds restore TJP‐barrier function improving intestinal permeability. Immunofluorescence staining for occludin (A) and ZO‐1 (C) in colons of DSS‐fed mice treated or not with FBA or butyrate before or after DSS consumption is shown. Scale bar: 100 μm. White arrows indicate areas shown in the inset squares (objective 40×). Quantification of occludin (B) and ZO‐1 (D) expression is reported as integrated pixel intensity (in arbitrary units) per area. Data are presented as means ± SEM, n = 4. * Significant versus CON; # versus DSS.
Discussion
In this study, we demonstrated that FBA, a butyrate‐releasing derivative, protects mice from DSS‐induced colon injury, by reducing inflammation and restoring epithelial barrier integrity. The effects of SCFA, in particular butyrate, on intestinal diseases and their role on colonic functions are well‐known (Hamer et al., 2008; Hamer et al., 2009; Canani et al., 2011). However, despite the wide spectrum of possible indications, the major limits of butyrate in clinical practice are its unpleasant taste and odour, when administered orally, or discomfort, by rectal preparations. Even if dietary fibre intake, leading to SCFA production, has shown benefits in IBD (Hanai et al., 2004; Wedlake et al., 2014), other data had demonstrated an inverse association between intake of fruits and vegetables and risk of IBD (Amre et al., 2007), and more recently dietary intake and risk of developing IBD have been reviewed (Hou et al., 2011; Ananthakrishnan et al., 2013). Very recently, the rational identification of diet‐derived postbiotics in restoring intestinal microbiota composition and function has been reviewed (Klemashevich et al., 2014). For all these reasons, the use of postbiotics, such as butyrate, may potentially be alternative to the use of live probiotic organisms or dietary fibre intake as prebiotics. In fact, the beneficial effects of oral and topical administration of sodium butyrate on different models of DSS‐induced colitis in mice have already been demonstrated (Vieira et al., 2012; Mishiro et al., 2013). Recently, we investigated the role of butyrate and FBA in pain behaviour, identifying different and converging non‐genomic and genomic mechanisms of action, which cooperate in nociception maintenance (Russo et al., 2016). Notably, we found both compounds had a marked effect on inflammatory visceral pain probably due to a physiological effect of butyrate in the gut and to an elevation in the number of its transporters (i.e. MCT1) involved in its absorption.
Here, we tested the efficacy of oral FBA in DSS colitis, which leads to a significant loss of body weight and is associated with diarrhoea, rectal bleeding and colon shortening (Yan et al., 2009). These macroscopic and pathological changes were counteracted by FBA, similarly to sodium butyrate, especially when used as a therapeutic protocol.
The role of the infiltration of immune cells in the inflammatory response during the development of colitis has already been assessed, as well as the efficacy of butyrate supplementation in limiting the recruitment of myeloid and lymphoid cells into the colonic mucosa (Tsou et al., 2007). According to these findings, FBA reduced DSS‐induced PMN infiltration in colonic mucosa, decreasing MPO activity and Ly6g mRNA levels. Ly‐6G, which only reacts in neutrophils, is a very useful marker to detect specifically cells of the neutrophil lineage (Tsou et al., 2007). Dense neutrophil infiltration and crypt abscess formation are pathological characteristics of the inflamed mucosa of UC patients (Raab et al., 1993). In fact, in Japan, granulocyte adsorption apheresis therapy had been reported to show a remarkable therapeutic effect in active UC patients (Shimoyama et al., 2001). Moreover, the faecal neutrophil‐derived biomarkers, calprotectin and lactoferrin, represent an ideal non‐invasive test for detecting intestinal inflammation (Sipponen, 2013). These findings strengthen the pivotal role of neutrophils in the pathogenesis of UC. Moreover, trans‐epithelial migration of PMNs from the microcirculation to the mucosa results in impaired barrier function and tissue destruction (Nusrat et al., 1997).
Among SCFAs, butyrate can influence chemotaxis of immune cells through FFA2 receptors, but this effect depends on the type of immune cells and butyrate concentration (Maslowski et al., 2009; Sina et al., 2009). Here, Ffar2 mRNA expression was increased by FBA alongside the reduction in Ly6g mRNA transcripts mediated by both FBA‐based treatments. These data confirm the involvement of the FFA2 receptor in neutrophil recruitment during inflammation and, at the same time, display the ability of FBA to reduce PMN infiltration. Therefore, we hypothesize that, when neutrophil recruitment is reduced by FBA treatments, the FFA2 receptor can be expressed on other cell populations, for example intestinal enteroendocrine L and epithelial cells, which are involved in intestinal barrier integrity.
We also assessed if the protective effect of FBA was associated with a modulation of pro‐resolving factors, such as annexin A1 and its receptors, FPR1 and FPR2. Annexin A1, a member of the superfamily of annexins, is a downstream mediator of glucocorticoids action (Gerke et al., 2005). In resting conditions, neutrophils, monocytes and macrophages constitutively contain high levels of annexin A1 in their cytoplasm (Perretti et al., 2000; Mulla et al., 2005), that are promptly secreted following cell activation (Perretti et al., 1996). Increased expression and secretion of annexin A1 has been reported to occur in inflamed mucosal tissues in rodents and humans (Vergnolle et al., 1995; Vergnolle et al., 2004). In particular, increased Anx‐A1 was observed in the intestinal epithelium and infiltrating leukocytes in the mucosa of UC patients compared with normal intestinal mucosa (Leoni et al., 2013). Anx‐A1 has also been reported to inhibit neutrophil influx and promote neutrophil apoptosis at the site of resolving inflammation (Perretti et al., 1996). In our experiments, a co‐localization between Ly‐6G and Anx‐A1 positive cells was obtained: FBA reduced Anx‐A1 levels as well as Ly‐6G+ cells. Moreover, both schemes of treatment with FBA reduced the transcriptional levels of Fpr1 and Fpr2. Therefore, in these settings, Anx‐A1 and its receptors seem to be a marker of neutrophil infiltration.
Our data clearly demonstrated that the preventive and therapeutic treatments with FBA corrected the imbalance between pro‐ and anti‐inflammatory mediators reducing iNOS protein expression and Ccl2, Tnf and Il6 transcripts in colon tissue; at the same time, both FBA protocols restored, at least in part, mRNA levels of TGF‐β and IL‐10. Consistently, previous data have shown that butyrate reduces the levels of several pro‐inflammatory mediators in colon lamina propria macrophages (Chang et al., 2014).
Butyrate regulates gene expression epigenetically by inhibiting HDAC, specifically class IIA and I (Steliou et al., 2012), and its anti‐inflammatory effects are related to this mechanism in many cell types (Chang et al., 2014). In particular, class IIA HDAC has been reported to suppress the expansion of regulatory T cells (Smith et al., 2013), and the inhibition of HDAC9 increases Treg function, reducing colitis in mice (Glauben et al., 2006). Due to its inhibitory effect on HDAC, butyrate can prevent NF‐κB activation in human colonic epithelial cells (CECs) (Segain et al., 2000). NF‐κB regulates many cellular genes involved in early immune inflammatory responses frequently dysregulated in IBDs (Schwab et al., 2007). Here, FBA reproduced the effect of butyrate, limiting the up‐regulation of the Hdac9 transcript induced by DSS challenge. Accordingly, FBA, especially when used as therapeutic treatment, inhibits NF‐κB activation and promotes histone H3 acetylation.
Moreover, we have demonstrated, in an in vivo model, that FBA restores Pparg transcription in colonic mucosa, confirming previous in vitro data demonstrating the involvement of PPARγ in the anti‐inflammatory activity of butyrate (Schwab et al., 2006). Genetic ablation of PPARγ resulted in increased susceptibility to experimental colitis in mice (Dubuquoy et al., 2006), and PPARγ protein expression is 60% lower in the inflamed colonic mucosa of UC patients than in controls (Dubuquoy et al., 2003). Previous data have shown that PPARγ can inhibit NF‐κB activation and cytokine expression in monocytes and CECs (Desreumaux et al., 2001).
Interestingly, we also observed a strong reduction in the MCT1 transporter in colonic mucosa of DSS mice, highlighting an impairment of butyrate uptake. MCT1 plays an important role in the absorption of butyrate by the colonocytes (Cuff et al., 2002), and previous data have shown that butyrate stimulates MCT1 promoter activity in Caco‐2, IEC‐6 and in rat intestinal mucosa (Borthakur et al., 2008, 2012). Both therapeutic FBA and butyrate preserved Slc16a1 down‐regulation, normalizing its transcriptional levels. These results may have translational potential, since down‐regulation of MCT1 in IBD patients could result in a deficiency in the uptake of butyrate (Thibault et al., 2010). We hypothesize that in our experimental conditions, the stronger effect of FBA could be related not only to the induction of MCT1 by butyrate released from FBA but also to the ability of undissociated FBA to interact and be carried by several members of the SLC family, such as those of phenylalanine.
Changes to the TJP in UC results in impaired barrier function, which may lead to increased uptake of luminal antigens and/or adjuvant that overcome the net suppressive tone of the mucosal immune system. SCFAs modulate key epithelial cell functions that help to maintain intestinal epithelial barrier integrity preventing injury (Peng et al., 2009). Analysis of the distribution and intensity of occludin and ZO‐1 staining and mRNA confirmed the beneficial effect elucidated by both butyrate‐based compounds. Gut integrity is essential to limit bacterial translocation and preserve mucosal immune homeostasis. In fact, damage‐ and pathogen‐associated molecular patterns determine a strong recruitment of immune cells in impaired mucosa causing subsequent inflammation.
In conclusion, the results of the FBA treatments confirm and improve the beneficial effects of butyrate at the intestinal level. The FBA counteracts the colon inflammation, neutrophil recruitment and alterations in intestinal permeability in the DSS‐induced colitis model. Indeed, our data indicate the potential clinical utility of FBA, a postbiotic derivative, as a preventive or therapeutic strategy for UC. Since this synthetic derivative of butyrate does not have the characteristic odour of rancid cheese, it may represent a viable alternative to butyrate, favouring better oral compliance and a greater effectiveness.
Author contributions
R.S., C.P., A.L., T.M.M. and A.S. performed research. R.S. and G.M.R. analysed data and wrote the manuscript. R. R. and R.B.C. reviewed the manuscript. A.C. and M.P. contributed to the study design, data analysis and review of the manuscript. R.M. designed research, analysed data and wrote the paper. All the authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Real‐time PCR primer sequences.
Figure S1 Preventive and therapeutic treatments with FBA increase survival rate in DSS‐fed mice. Data are presented as percent survival rate ± SEMs, n = 10. CON, control group; DSS, dextran sodium sulphate group; P‐B, DSS‐fed mice receiving preventive butyrate treatment; P‐FBA, DSS‐fed mice receiving preventive FBA treatment; T‐B, DSS‐fed mice receiving sodium butyrate as therapeutic treatment; T‐FBA, DSS‐fed mice receiving therapeutic FBA treatment. As depicted, in DSS group the survival rate at day 19 reached 57.1% compared to 100% shown in CON group. Both FBA‐based treatments displayed a comparable efficacy to P‐B at the end of the experimental protocol, with a mean survival time of 18 d. The same effect was not observed in DSS‐fed mice treated with sodium butyrate as therapeutic protocol.
Figure S2 Modification of body weight in CON and DSS mice that were untreated or administered FBA and butyrate as preventive or therapeutic protocol. Data are means ± SEMs, n = 8 (**P < 0.01 Vs CON). DSS, dextran sodium sulphate group; P‐B, DSS‐fed mice receiving preventive butyrate treatment; P‐FBA, DSS‐fed mice receiving preventive FBA treatment; T‐B, DSS‐fed mice receiving sodium butyrate as therapeutic treatment; T‐FBA, DSS‐fed mice receiving therapeutic FBA treatment.
Figure S3 FBA limits the impairment of tight‐junction transcriptional levels in colonic mucosa. Transcriptional levels of Ocln (which encode for occludin) and Tjp1 (tight junction protein 1, gene encoding for ZO‐1) were assessed in colon tissue. As shown, DSS challenge significantly reduced the mRNA levels of Ocln and Tjp1 compared to those revealed in control animals. Among treatments, preventive and therapeutic FBA were more effective than butyrate schemes in limiting tight‐junctions impairment. Data are presented as means ± SEMs, n = 8 (*P < 0.05 and ***P < 0.01 Vs CON; #P < 0.05 Vs DSS). CON, control group; DSS, dextran sodium sulphate group; P‐B, DSS‐fed mice receiving preventive butyrate treatment; P‐FBA, DSS‐fed mice receiving preventive FBA treatment; T‐B, DSS‐fed mice receiving sodium butyrate as therapeutic treatment; T‐FBA, DSS‐fed mice receiving therapeutic FBA treatment.
Supporting info item
Acknowledgements
We thank Antonio Baiano, Giovanni Esposito and Angelo Russo for animal care and assistance. This study was partially supported by grants from Agenzia Italiana del Farmaco, AIFA (FARM6FJ728 and MRAR08W002).
Simeoli, R. , Mattace Raso, G. , Pirozzi, C. , Lama, A. , Santoro, A. , Russo, R. , Montero‐Melendez, T. , Berni Canani, R. , Calignano, A. , Perretti, M. , and Meli, R. (2017) An orally administered butyrate‐releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium‐induced murine colitis. British Journal of Pharmacology, 174: 1484–1496. doi: 10.1111/bph.13637.
Contributor Information
Raffaele Simeoli, Email: raffaele.simeoli@kcl.ac.uk.
Rosaria Meli, Email: meli@unina.it.
References
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amre DK, D'Souza S, Morgan K, Seidman G, Lambrette P, Grimard G et al. (2007). Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. Am J Gastroenterol 102: 2016–2025. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR et al. (2013). A prospective study of long‐term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology 145: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels LB, Dewulf EM, Delzenne NM (2013). GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 34: 226–232. [DOI] [PubMed] [Google Scholar]
- Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK (2008). Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF‐kappaB pathway. J Cell Biochem 103: 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A, Priyamvada S, Kumar A, Natarajan AA, Gill RK, Alrefai WA et al. (2012). A novel nutrient sensing mechanism underlies substrate‐induced regulation of monocarboxylate transporter‐1. Am J Physiol Gastrointest Liver Physiol 303: G1126–G1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982). Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78: 206–209. [DOI] [PubMed] [Google Scholar]
- Breuer RI, Soergel KH, Lashner BA, Christ ML, Hanauer SB, Vanagunas A et al. (1997). Short chain fatty acid rectal irrigation for left‐sided ulcerative colitis: a randomised, placebo controlled trial. Gut 40: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A (2011). Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111: 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993). Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249. [PubMed] [Google Scholar]
- Cuff MA, Lambert DW, Shirazi‐Beechey SP (2002). Substrate‐induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol 539: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F et al. (2012). Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis 18: 1127–1136. [DOI] [PubMed] [Google Scholar]
- de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW (2010). Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology 138: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desreumaux P, Dubuquoy L, Nutten S, Peuchmaur M, Englaro W, Schoonjans K et al. (2001). Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator‐activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med 193: 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman LA, Pena AS, Meuwissen SG, van Rees EP (1997). Role of animal models for the pathogenesis and treatment of inflammatory bowel disease. Scand J Gastroenterol Suppl 223: 99–104. [PubMed] [Google Scholar]
- Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF et al. (2003). Impaired expression of peroxisome proliferator‐activated receptor gamma in ulcerative colitis. Gastroenterology 124: 1265–1276. [DOI] [PubMed] [Google Scholar]
- Dubuquoy L, Rousseaux C, Thuru X, Peyrin‐Biroulet L, Romano O, Chavatte P et al. (2006). PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 55: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Banares F, Hinojosa J, Sanchez‐Lombrana JL, Navarro E, Martinez‐Salmeron JF, Garcia‐Puges A et al. (1999). Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish group for the study of Crohn's disease and ulcerative colitis (GETECCU). Am J Gastroenterol 94: 427–433. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE (2005). Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6: 449–461. [DOI] [PubMed] [Google Scholar]
- Geuking MB, Koller Y, Rupp S, McCoy KD (2014). The interplay between the gut microbiota and the immune system. Gut microbes 5: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F et al. (2006). Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol 176: 5015–5022. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008). Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104–119. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A et al. (2009). Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 28: 88–93. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers DM, Vanhoutvin SA, Troost FJ, Rijkers G, de Bruine A et al. (2010). Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr 29: 738–744. [DOI] [PubMed] [Google Scholar]
- Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I et al. (2004). Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med 13: 643–647. [PubMed] [Google Scholar]
- Hou JK, Abraham B, El‐Serag H (2011). Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 106: 563–573. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemashevich C, Wu C, Howsmon D, Alaniz RC, Lee K, Jayaraman A (2014). Rational identification of diet‐derived postbiotics for improving intestinal microbiota function. Curr Opin Biotechnol 26: 85–90. [DOI] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J et al. (2013). Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest 123: 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D et al. (2009). Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O et al. (2013). Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One 8: e68626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AJ, Huffnagle GB (2014). The microbiome and regulation of mucosal immunity. Immunology 142: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishiro T, Kusunoki R, Otani A, Ansary MM, Tongu M, Harashima N et al. (2013). Butyric acid attenuates intestinal inflammation in murine DSS‐induced colitis model via milk fat globule‐EGF factor 8. Lab Invest 93: 834–843. [DOI] [PubMed] [Google Scholar]
- Mulla A, Leroux C, Solito E, Buckingham JC (2005). Correlation between the antiinflammatory protein annexin 1 (lipocortin 1) and serum cortisol in subjects with normal and dysregulated adrenal function. J Clin Endocrinol Metab 90: 557–562. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Parkos CA, Liang TW, Carnes DK, Madara JL (1997). Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology 113: 1489–1500. [DOI] [PubMed] [Google Scholar]
- Peng L, Li ZR, Green RS, Holzman IR, Lin J (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP‐activated protein kinase in Caco‐2 cell monolayers. J Nutr 139: 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ (1996). Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med 2: 1259–1262. [DOI] [PubMed] [Google Scholar]
- Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF et al. (2000). Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int 24: 163–174. [DOI] [PubMed] [Google Scholar]
- Raab Y, Gerdin B, Ahlstedt S, Hallgren R (1993). Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin‐8 in ulcerative colitis. Gut 34: 1203–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, De Caro C, Avagliano C, Cristiano C, La Rana G, Mattace Raso G et al. (2016). Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol Res 103: 279–291. [DOI] [PubMed] [Google Scholar]
- Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S et al. (1992). Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 103: 51–56. [DOI] [PubMed] [Google Scholar]
- Schwab M, Reynders V, Ulrich S, Zahn N, Stein J, Schroder O (2006). PPARgamma is a key target of butyrate‐induced caspase‐3 activation in the colorectal cancer cell line Caco‐2. Apoptosis 11: 1801–1811. [DOI] [PubMed] [Google Scholar]
- Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schroder O (2007). Involvement of different nuclear hormone receptors in butyrate‐mediated inhibition of inducible NF kappa B signalling. Mol Immunol 44: 3625–3632. [DOI] [PubMed] [Google Scholar]
- Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C et al. (2000). Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 47: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama T, Sawada K, Hiwatashi N, Sawada T, Matsueda K, Munakata A et al. (2001). Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher 16: 1–9. [DOI] [PubMed] [Google Scholar]
- Simeoli R, Mattace Raso G, Lama A, Pirozzi C, Santoro A, Di Guida F et al. (2015). Preventive and therapeutic effects of Lactobacillus paracasei B21060‐based synbiotic treatment on gut inflammation and barrier integrity in colitic mice. J Nutr 145: 1202–1210. [DOI] [PubMed] [Google Scholar]
- Sina C, Gavrilova O, Forster M, Till A, Derer S, Hildebrand F et al. (2009). G protein‐coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol 183: 7514–7522. [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H et al. (2014). Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen T (2013). Diagnostics and prognostics of inflammatory bowel disease with fecal neutrophil‐derived biomarkers calprotectin and lactoferrin. Dig Dis 31: 336–344. [DOI] [PubMed] [Google Scholar]
- Smith JW, Castro GA (1978). Relation of peroxidase activity in gut mucosa to inflammation. Am J Physiol 234: R72–R79. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM et al. (2013). The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart AH, Hiruki T, Brzezinski A, Baker JP (1996). Treatment of left‐sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther 10: 729–736. [DOI] [PubMed] [Google Scholar]
- Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV (2012). Butyrate histone deacetylase inhibitors. Biores Open Access 1: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C et al. (2007). Down‐regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133: 1916–1927. [DOI] [PubMed] [Google Scholar]
- Thibault R, Blachier F, Darcy‐Vrillon B, de Coppet P, Bourreille A, Segain JP (2010). Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis 16: 684–695. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP et al. (2007). Critical roles for CCR2 and MCP‐3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117: 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Comera C, Bueno L (1995). Annexin 1 is overexpressed and specifically secreted during experimentally induced colitis in rats. Eur J Biochem 232: 603–610. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Pages P, Guimbaud R, Chaussade S, Bueno L, Escourrou J et al. (2004). Annexin 1 is secreted in situ during ulcerative colitis in humans. Inflamm Bowel Dis 10: 584–592. [DOI] [PubMed] [Google Scholar]
- Vernia P, Annese V, Bresci G, d'Albasio G, D'Inca R, Giaccari S et al. (2003). Topical butyrate improves efficacy of 5‐ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest 33: 244–248. [DOI] [PubMed] [Google Scholar]
- Vieira EL, Leonel AJ, Sad AP, Beltrao NR, Costa TF, Ferreira TM et al. (2012). Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem 23: 430–436. [DOI] [PubMed] [Google Scholar]
- Wedlake L, Slack N, Andreyev HJ, Whelan K (2014). Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis 20: 576–586. [DOI] [PubMed] [Google Scholar]
- Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV et al. (2009). Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4: e6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Real‐time PCR primer sequences.
Figure S1 Preventive and therapeutic treatments with FBA increase survival rate in DSS‐fed mice. Data are presented as percent survival rate ± SEMs, n = 10. CON, control group; DSS, dextran sodium sulphate group; P‐B, DSS‐fed mice receiving preventive butyrate treatment; P‐FBA, DSS‐fed mice receiving preventive FBA treatment; T‐B, DSS‐fed mice receiving sodium butyrate as therapeutic treatment; T‐FBA, DSS‐fed mice receiving therapeutic FBA treatment. As depicted, in DSS group the survival rate at day 19 reached 57.1% compared to 100% shown in CON group. Both FBA‐based treatments displayed a comparable efficacy to P‐B at the end of the experimental protocol, with a mean survival time of 18 d. The same effect was not observed in DSS‐fed mice treated with sodium butyrate as therapeutic protocol.
Figure S2 Modification of body weight in CON and DSS mice that were untreated or administered FBA and butyrate as preventive or therapeutic protocol. Data are means ± SEMs, n = 8 (**P < 0.01 Vs CON). DSS, dextran sodium sulphate group; P‐B, DSS‐fed mice receiving preventive butyrate treatment; P‐FBA, DSS‐fed mice receiving preventive FBA treatment; T‐B, DSS‐fed mice receiving sodium butyrate as therapeutic treatment; T‐FBA, DSS‐fed mice receiving therapeutic FBA treatment.
Figure S3 FBA limits the impairment of tight‐junction transcriptional levels in colonic mucosa. Transcriptional levels of Ocln (which encode for occludin) and Tjp1 (tight junction protein 1, gene encoding for ZO‐1) were assessed in colon tissue. As shown, DSS challenge significantly reduced the mRNA levels of Ocln and Tjp1 compared to those revealed in control animals. Among treatments, preventive and therapeutic FBA were more effective than butyrate schemes in limiting tight‐junctions impairment. Data are presented as means ± SEMs, n = 8 (*P < 0.05 and ***P < 0.01 Vs CON; #P < 0.05 Vs DSS). CON, control group; DSS, dextran sodium sulphate group; P‐B, DSS‐fed mice receiving preventive butyrate treatment; P‐FBA, DSS‐fed mice receiving preventive FBA treatment; T‐B, DSS‐fed mice receiving sodium butyrate as therapeutic treatment; T‐FBA, DSS‐fed mice receiving therapeutic FBA treatment.
Supporting info item


