Abstract
The consumption of tea (Camellia sinensis) has been correlated with a low incidence of chronic pathologies, such as cardiovascular disease and cancer, in which oxidative stress plays a critical role. Tea catechins and theaflavins are, respectively, the bioactive phytochemicals responsible for the antioxidant activity of green tea (GT) and black tea (BT). In addition to their redox properties, tea catechins and theaflavins could have also pharmacological activities, such as the ability to lower glucose, lipid and uric acid (UA) levels. These activities are mediated by pharmacological mechanisms such as enzymatic inhibition and interaction with transporters. Epigallocatechin gallate is the most active compound at inhibiting the enzymes involved in cholesterol and UA metabolism (hydroxy‐3‐methyl‐glutaryl‐CoA reductase and xanthine oxidase respectively) and affecting glucose transporters. The structural features of catechins that significantly contribute to their pharmacological effect are the presence/absence of the galloyl moiety and the number and positions of the hydroxyl groups on the rings. Although the inhibitory effects on α‐glucosidase, maltase, amylase and lipase, multidrug resistance 1, organic anion transporters and proton‐coupled folate transport occur at higher concentrations than those apparent in the circulation, these effects could be relevant in the gut. In conclusion, despite the urgent need for further research in humans, the regular consumption of moderate quantities of GT and BT can effectively modulate their antioxidant capacity, mainly in people subjected to oxidative stress, and could improve the metabolism of glucose, lipid and UA.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- BT
black tea
- BTE
black tea extract
- CYP450
cytochrome p450
- DNMT
DNA‐methyltransferase
- EC
epicatechin
- ECG
epicatechin gallate
- EGC
epigallocatechin
- EGCG
epigallocatechin gallate
- GLUT1
sodium‐independent glucose transporter
- GST
glutathione S‐Transferase
- GT
green tea
- GTE
green tea extract
- HMGR
hydroxy‐3‐methyl‐glutaryl‐CoA reductase
- IsoP
isoprostanes
- MDR1
multidrug resistance 1
- NEAC
non enzymatic antioxidant capacity
- Nrf2
nuclear factor‐erythroid 2‐related factor 2
- OAT
organic anion transporters
- OCT
organic cation transporters
- OSRRF
oxidative stress‐related risk factors
- OT
oolong tea
- PCFT
proton‐coupled folate transport
- RNase A
ribonuclease A
- SGLT1
sodium‐dependent glucose transporter
- TrxR
thioredoxin reductase
- UA
uric acid
- XO
xanthine oxidase
Tables of Links
| TARGETS | ||
|---|---|---|
| Enzymes a | Glucosidase | Transporters b |
| Amylase | Glutathione reductase | GLUT1 |
| COX‐1 | HMGR | MDR1 |
| Cytochrome p450 1 | Lipase | OAT |
| Cytochrome p450 2 | Maltase | OCT1 |
| Cytochrome p450 3 | Xanthine oxidase | OCT3 |
| DNA methyltransferase | PCFT | |
| DNA polymerase | SGLT1 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
The consumption of tea (Camellia sinensis) has been correlated with low incidence of chronic pathologies, such as cardiovascular disease and cancer (Tang et al., 2015). However, although the molecules involved in this effect have been shown to have anti‐inflammatory and antioxidant effects, and to improve endothelial function, no clear‐cut conclusion has been reached on their mechanism of action. The health benefits ascribed to the consumption of teas are thought to be associated with their high content of bioactive ingredients such as polyphenols. The latter are secondary plant metabolites and include the subclasses of flavonoids, flavones, flavonols, flavanols, isoflavones, flavanones and anthocyanidins (Del Rio et al., 2013). Within the polyphenols, the tea flavanols, catechins and theaflavins, have been identified as the bioactive phytochemicals of green tea (GT) and black tea (BT) respectively, and shown to be responsible for their antioxidant activity (Serafini et al., 2011). The antioxidant properties of GT and BT in humans were discovered in 1996 (Serafini et al. 1996), where in healthy subjects, the non enzymatic antioxidant capacity (NEAC) of plasma was shown to significantly increase after the ingestion of 300 mL of either BT or GT (Serafini et al. 1996). However, when GT and BT were consumed with milk, the antioxidant activity was drastically reduced or totally inhibited (Serafini et al., 1996).
Apart from their antioxidant activity, tea flavanols could also have other activities of pharmacological interest, such as the ability to lower glucose (Liu et al., 2013; Zheng et al., 2013), lipid (Zheng et al., 2011; Hartley et al., 2013; Onakpoya et al., 2014) and uric acid (UA) (Peluso et al., 2015a) concentrations. These activities could be mediated by their effects on various enzymes and transporters (Peluso et al., 2015b). One of the most important mechanisms of food–drug interactions has been suggested to be mediated by effects on transporters (Shang et al., 2014; Werba et al., 2015). With the increasing interest in the health promoting properties of tea, in this review we have evaluated the role of teas in modulating oxidative stress in humans and the mechanisms involved in the pharmacological effects of tea flavanols.
Flavanols in teas and their pharmacokinetics in humans
GT and BT are the two types of tea mostly consumed throughout the world, and they contain different phytochemicals endowed with biological activities, such as flavanols (Serafini et al., 2011). The processing or harvesting times of the leaves of C. sinensis leads to the different composition of flavonoids between GT and BT (Serafini et al., 2011). In the case of GT, the leaves are steamed quickly after harvesting. Home tea preparation has also an impact on the flavonoid content of tea; brews of 5 min at temperatures of 100°C result in infusions with greater antioxidant capacity than teas with a shorter brewing time (2 min) at lower temperatures (60–80°C) (Sharpe et al., 2016).
The major active flavonoids in GT are epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC) and epigallocatechin gallate (EGCG), as displayed in Table 1. The structure of EGCG includes a benzenediol ring joined to a tetrahydropyran moiety, a pyrogallol ring and a galloyl group (with the pyrogallol ring) (Figure 1) (Serafini et al., 2011). ECG lacks a hydroxyl group on the pyrogallol ring; while in EGC, the galloyl group is replaced by a hydrogen atom (Figure 1). These flavonoids are present in lower amounts in BT where they are in part converted, during the enzymatic fermentation process driven by polyphenol oxidase, to complex condensation products, such as theaflavins (Figure 1) and thearubigins (Serafini et al., 2011; Stodt et al., 2014). The latter are known to be heterogeneous polymers, but their formation and characterization have yet to be elucidated, whereas the former possess a characteristic benzotropolone moiety that is produced by condensation between a catechol‐type ring of EC and a pyrogallol‐type ring of EGC (Tanaka et al., 2009).
Table 1.
Flavanol content of GT, BT and OT
| GT infusion mg ·100 mL‐1 ± SD | BT infusion mg ·100 mL‐1 ± SD | OT infusion mg ·100 mL‐1 ± SD | References | |
|---|---|---|---|---|
| EC | 7.93 ± 13.74 | 3.94 ± 4.27 | 2.7 ± 3.77 | Arts et al., 2000; Begona Barroso and Werken van de, 1999; Bronner and Beecher, 1998; Ding et al., 1992; Ding et al., 1999; Khokhar and Magnusdottir, 2002; Kilmartin and Chyong, 2003; Kuhr and Engelhardt, 1991; Lee and Ong, 2000; Liang et al., 2003; Lin et al., 1996; Lin et al., 1998; Long et al., 2001; Luximon‐Ramma et al., 2005; Pascual‐Teresa et al., 2000; Pelillo et al., 2002; Price and Spitzer, 1993; Rechner et al., 2002; Stewart et al., 2005; Wang et al., 2000; Zhang et al., 1997 |
| ECG | 7.50 ± 10.19 | 7.34 ± 7.10 | 4.99 ± 6.55 | |
| EGC | 19.68 ± 25.11 | 7.19 ± 10.87 | 9.65 ± 14.16 | |
| EGCG | 27.16 ± 39.91 | 9.12 ± 12.67 | 17.89 ± 27.41 | |
| TF | – | 3.27 ± 3.31 | – | Liang et al., 2003; Stewart et al., 2005; Ding et al. 1992 |
| TF3G | – | 1.58 ± 2.97 | – | |
| TF3′G | – | 4.08 ± 4.08 | – | |
| TFDG | – | 3.52 ± 3.89 | – |
TF, theaflavin; TF3G, theaflavin 3‐O‐gallate; TF3′G, theaflavin 3′‐O‐gallate; TFDG, theaflavin 3,3′‐O‐digallate. (Source: http://phenol‐explorer.eu/, values calculated by combining the data from the publications listed).
Figure 1.
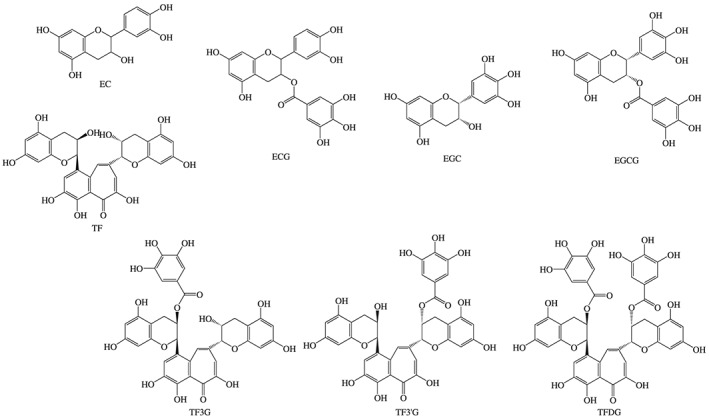
Chemical structures of bioactive ingredients in green and black tea. TF, theaflavins; TF3G, theaflavin 3‐O‐gallate; TF3′G, theaflavin 3′‐O‐gallate; TFDG, theaflavin 3,3′‐O‐digallate.
Oolong tea (OT) also originates from C. sinensis and is produced using a shorter fermentation time than BT and contains fewer flavanols (35.72 mg · 100 mL‐1 infusion) than BT and GT (76.46 mg ·100 mL‐1 GT infusion, 82.6 mg ·100 mL‐1 BT infusion) (Table 1). In addition, a new group of polymeric oxidized flavanols have been isolated and identified from OT and are known as theasinensins (Weerawatanakorn et al., 2015). Theasinensins are quinone dimers of EGC and EGCG produced by these two catechin quinone monomers and have been suggested to contribute to biological activities of OT (Weerawatanakorn et al., 2015) despite there being no data available on their content and absorption.
At present the data published on the absorption of green tea phenolics vary considerably and are controversial (Manach et al., 2005). Analytical limitations have drastically biased the identification and characterization of flavan‐3‐ol catabolites, but also a lack of available pure standards for each specific catabolite has significantly reduced the quality of absorption studies. The liver and intestines play a major role in the first‐pass metabolism and absorption of catechins (Feng, 2006). After ingestion of GT, ECG is generally absent from plasma, whereas EGCG, EGC and EC are found in various forms, free or conjugated with glucuronide, sulfate or methyl groups (Table 2). Williamson et al. (2011), Clifford et al. (2013) and Del Rio et al. (2013) reviewed the pharmacokinetic data of the consumption of GT in humans (Table 2).
Table 2.
Pharmacokinetics of tea flavanols and their plasma metabolites in humans
| Ingested dose | Detected compound | Cmax | Tmax (h) | References | |
|---|---|---|---|---|---|
| GT | EGCG 63–328.5 mg | EGCG | 55–711 nM | 1.3–2.7 | http://phenol‐explorer.eu/, Williamson et al., 2011; Clifford et al., 2013; Del Rio et al., 2013 |
| GT | EGC 32–306 mg | EGC | 40–1791 nM | 1.3–2.2 | http://phenol‐explorer.eu/, Williamson et al., 2011; Clifford et al., 2013; Del Rio et al., 2013 |
| GT | EC 12–113 mg | EC | 29–655 nM | 1.4–1.8 | http://phenol‐explorer.eu/, Williamson et al., 2011; Clifford et al., 2013; Del Rio et al., 2013 |
| GT | flavanols 32–154 mg | Methyl‐EGC | 62–5300 nM | 2–2.3 | http://phenol‐explorer.eu/, Williamson et al., 2011; Clifford et al., 2013; Del Rio et al., 2013 |
| GT | flavanols 12–17 mg | Methyl‐EC‐sulfate | 14–90 nM | 1.3–1.7 | http://phenol‐explorer.eu/, Williamson et al., 2011; Clifford et al., 2013; Del Rio et al., 2013 |
| GT | Flavanols 648 μM in 500 mL | EC‐glucuronide | 29 nM | 1.7 | http://phenol‐explorer.eu/, Williamson et al., 2011; Clifford et al., 2013; Del Rio et al., 2013 |
| EC‐sulfates | 89 nM | 1.6 | |||
| EGC‐glucuronide | 126 nM | 2.2 | |||
| Met‐EGC‐glucuronide | 46 nM | 2.3 | |||
| TFs | 700 mg dissolved in 150 mL hot water: | Theaflavins | ≈1 μg·L−1 | 2 | http://phenol‐explorer.eu/, Mulder et al., 2001 |
| TF 123.9·700 mg−1 DW | |||||
| TF3G 222.6·700 mg−1 DW | |||||
| TF3'G 116.9·700 mg−1DW | |||||
| TFDG 219.8·700 mg−1DW |
Cmax, peak‐plasma concentrations; DW, dry weight; TF, theaflavin; TF3G, theaflavin 3‐O‐gallate; TF3′G, theaflavin 3′‐O‐gallate; TFDG, theaflavin 3,3′‐O‐digallate; Tmax, time at which the Cmax is observed.
Although the F values of bioavailability were not estimated in humans, after the consumption of a cup of GT containing 112 mg of EGCG, 51 mg of EGC and 15 mg of EC in 200 mL, the predicted peak‐plasma concentrations (Cmax) values (total free and sulfate/glucuronide conjugates) are 125, 181 and 76 nM, respectively, together with 94 nM methyl‐EGC and 51 nM methyl‐EC (Williamson et al., 2011); these Cmax values occurred 1.3–2.7 h after ingestion (Table 2). After the ingestion of 500 mL of GT, the reported t 1/2 of flavanols' conjugates ranged between 1 and 3.1 h (Clifford et al. (2013).
The serum metabolites of GT (GTM, sulfates/glucuronides of EC, EGC, ECG and EGCG: 32.47, 33.29, 0.13 and 0.40 μM respectively), prepared from rats given an infusion of GT, significantly inhibited the activity of the organic anion transporters (OAT) in CHO cells expressing OAT1 and in HEK293 cells expressing OAT3 (Peng et al., 2015).
Urine collected 0–24 h after GT ingestion contained flavan‐3‐ol metabolites similar to those detected in plasma (Del Rio et al., 2013). The percentage of metabolites excreted in urine range from 8.1 to 28.5% of the ingested GT flavanols (Del Rio et al., 2013).
Only limited data are available on the pharmacokinetic of theaflavins in humans: after the consumption of 700 mg theaflavins, equivalent to about 30 cups of black tea, the maximum concentration detected in blood plasma was around 1.0 μg·L−1 in a sample collected after 2 h and also the concentration in urine peaked after 2 h at 4.2 μg·L−1 (Mulder et al., 2001), as shown in Table 2.
In addition to the intestinal or hepatic metabolites, metabolites derived from colonic bacteria have been identified (Sang et al., 2008). In particular, the action of the microbiota results in their conversion to C‐6‐C‐5 phenylvalerolactones and phenylvaleric acids, which undergo side‐chain shortening to produce C‐6‐C‐1 phenolic and aromatic acids that enter the bloodstream and are excreted in urine in amounts equivalent to 36% of flavanol intake (Clifford et al., 2013). It has recently been observed that the microbial metabolite 5‐(3′,5′‐dihydroxyphenyl)‐γ‐valerolactone (EGC‐M5) and the 5‐(3′,5′‐dihydroxyphenyl)‐γ‐valerolactone‐3′‐O‐glucuronide (EGC‐M5‐glucuronide) significantly increase CD4+ activity (ATP level), having immunostimulatory activity, whereas EGC and EGCG decreased the CD4+ activity (Kim et al., 2016).
Most polyphenols present in tea undergo drastic modifications due to the action of human and microbial enzymes leading to a wide array of metabolites. Moreover, as the gut micro flora vary significantly among subjects, this could result in different microbial catabolism and, consequently, diverse biological effects.
Antioxidant activity
The structural features of GT catechins that significantly contribute to their antioxidant action are the presence/absence of the galloyl moiety and the number and positions of the hydroxyl groups on the rings. The latter determine their ability to interact with biological matter through hydrogen bonding, or electron and hydrogen transfer processes within their antioxidant activities. In fact, the antioxidant mechanism implies hydrogen atom transfer or single electron transfer reactions, or both (Lambert and Elias, 2010). Tea catechins are thought to display antioxidant activity, scavenging lipid alkoxyl and peroxyl radicals by acting as chain‐breaking antioxidants (Lambert and Elias, 2010). In vitro, a stoichiometric factor n of 4.16 ± 0.51 was obtained for EGCG, which is considered to be responsible for most of the antioxidant activity of GT (Khan et al., 2006). In contrast, a factor of 2.20 ± 0.26 was found for EGC, during the reaction with peroxyl radicals generated by thermolysis of the azo initiator 2,2′‐azobis(2,4‐dimethylvaleronitrile) (Valcic et al., 2000).
Despite the large body of evidence for the antioxidant effect of flavanols in vitro, the results from human trials are inconsistent and are related to ingested dose, measured biomarkers and the extent of oxidative stress in the subjects. As observed for healthy subjects (Serafini et al., 1996; van het Hof et al., 1997) the consumption of GT, but not BT, increased NEAC in subjects with risk factors. In particular, Bertipaglia de Santana et al. (2008) found increased of NEAC levels in hypercholesterolaemic subjects (n = 25) who consumed 500 mL of GT for 90 days. In contrast, the long‐term consumption of five cups (21 days) (Davies et al., 2003) or 900 mL (28 days) (Widlansky et al., 2005) of BT did not increase plasma NEAC either in mildly hypercholesterolaemic subjects (Davies et al., 2003) or in patients with coronary artery disease (Widlansky et al., 2005). In a randomized cross‐over study that investigated the dose–response effect of 500 mL of GT with different solid contents (1.4, 1.6, 1.8 and 2.0 g·L−1), a linear increase in NEAC was observed when the amount of tea solids present in GT was increased. This highlights the presence of a linear association between the amount of flavonoids ingested and the extent of the antioxidant response in humans (Pecorari et al., 2010). Only one human study has investigated the ability of a ready‐to‐drink OT to modulate plasma antioxidant status; it showed that ingestion of 500 mL of OT significantly increased plasma and urinary NEAC levels (Villaño et al. 2012).
The picture is even more complicated if we select intervention studies looking at isoprostanes (IsoP), a reliable marker of oxidative stress. The consumption of BT, GT, green tea extract (GTE) or catechins did not change IsoP levels either in healthy subjects or in disease patients (Table 3). Of the 17 interventions, from 13 studies, only one reported a decrease in plasma and serum IsoP after 4 weeks consumption of BT (500 mL·day−1) in 12 healthy volunteers, whereas no effect was observed after acute drinking of a single dose (Wolfram et al., 2002). The consumption of GT (Müller et al., 2010) or catechins (Loke et al., 2008) did not change the levels of IsoP in an acute intervention study, despite the increase in NEAC and/or markers of absorption of polyphenols (Table 3). Similarly, Braga et al. (2012) found increased levels of NEAC after 2 days of GTE consumption in pancreatic cancer patients, whereas IsoP levels were unchanged. No changes in IsoP levels were observed after the repeated consumption of GT, GTE or catechins (7–112 days) in either healthy (Table 3) or hypertensive subjects (Hodgson et al., 2002a), even when consumed in association with onions (O'Reilly et al., 2001) or lutein (Li et al., 2010). Moreover, there is a clear discrepancy between effects on antioxidant capacity and on oxidative stress markers, as demonstrated in Table 3. The effect of teas on plasma NEAC was described in a meta‐analysis by Lettieri‐Barbato et al. (2013), who investigated the antioxidant effect of plant food ingestion in humans. The main result from the 17 interventions showed that tea consumption induced a similar increase in plasma NEAC after both acute and chronic ingestion (Lettieri‐Barbato et al., 2013) and that GT had a stronger antioxidant effect than BT (Lettieri‐Barbato et al., 2013). When participants were divided into healthy subjects and subjects exposed to oxidative stress‐related risk factors (OSRRF), in the beverage category including teas, an effect on plasma NEAC was clearly detected in the OSRRF category, whereas no changes in plasma NEAC were observed in healthy subjects (Lettieri‐Barbato et al., 2013). Biomarkers of oxidative stress such as isoprostanes increase significantly only when an ongoing oxidative stress is present, following that, antioxidant modulation might occur only if levels are significantly high; unfortunately, there are no data available on the physiological level of isoprostanes in healthy humans. Moreover, NEAC represents the overall molecular antioxidant defences of plasma and, despite being regulated endogenously, might be more liable to increase after the consumption of tea or plant food supplements compared with the reducing markers of oxidative stress. In intervention studies, it is highly recommended that different markers of oxidative stress, antioxidant status and redox enzymes should be measured in order to have a complete picture of the phenomenon and define the results according to the different aspects of the redox mechanism. Overall, it is possible that the antioxidant activity of teas is strictly associated with the presence of chronic oxidative stress, when an increase in antioxidant activity from dietary sources is required to improve the antioxidant defences. In this regard, more evidence is needed to identify differences in the level of markers of oxidative stress and antioxidants status between healthy and pre‐pathological conditions.
Table 3.
Human intervention studies with BT, GT, EC and EGCG: effect on absorption of IsoP, NEAC and polyphenols
| Subjects | Dose day−1 (n° days) | IsoP | NEAC | PC§ | Reference | |
|---|---|---|---|---|---|---|
| BT | 10 (mildly dyslipidaemic) | 10 g in1250 mL (28) | ↔ | – | – | Hodgson et al., 2002b |
| BT | 12 | 500 mL (1) | ↔ | – | – | Wolfram et al., 2002 |
| BT | 12 | 500 mL (28) | ↓ | – | – | Wolfram et al., 2002 |
| BT | 13 (hypertensive) | 10 g in 1000 mL (7) | ↔ | – | – | Hodgson et al., 2002a |
| BT | 15 (mildly dyslipidaemic) | Five cups (21) | ↔ | ↔ | – | Davies et al., 2003 |
| BT | 22 (mildly dyslipidaemic) | 10 g in 1250 mL (28) | ↔ | – | – | Hodgson et al., 2002a |
| BT | 66 (coronary artery disease) | 900 mL (28) | ↔ | ↔ | – | Widlansky et al., 2005 |
| BT + Onions | 32 | 300 mL + 150 g of onion cake (14) | ↔ | – | – | O'Reilly et al., 2001 |
| GT | 13 (hypertensive) | 10 g in 1000 mL (7) | ↔ | – | – | Hodgson et al., 2002a |
| GT | 22 | 6.3 g in 700 mL (14) | ↔ | – | ↑ | Hirano‐Ohmori et al., 2005 |
| GT | 33 | 600 mL (1) | ↔ | ↑ | ↑ | Müller et al., 2010 |
| GTE | 20 | 3 g (28) | ↔ | – | – | Freese et al., 1999 |
| GTE | 36 (pancreatic cancer) | 1000 mg (2) | ↔ | ↑ | – | Braga et al., 2012 |
| GTE | 9 | 844 mg (catechins) (14) | ↔ | – | – | Donovan et al., 2005 |
| GTE + lutein | 40 | 200 + 12 mg lutein (112 days) | ↔ | – | ↔ | Li et al., 2010 |
| EC | 12 | 200 mg (1) | ↔ | – | ↑ | Loke et al., 2008 |
| EGCG | 12 | 200 mg (1) | ↔ | – | ↑ | Loke et al., 2008 |
IsoP, isoprostanes; NEAC, non‐enzymatic antioxidant capacity; PC, polyphenols concentration, §reported as the plasma or urinary concentrations of a single cathechin, total catechins or total phenols or their metabolites; ↔, unchanged; ↑, increased; ↓, decreased.
Enzymatic inhibition
In vitro GTE, black tea extract (BTE), catechins and theaflavins have been shown to inhibit various enzymes involved in glucose and lipid metabolism, such as amylase, maltase, glucosidase and lipase and the enzyme involved in cholesterol synthesis hydroxy‐3‐methyl‐glutaryl‐CoA reductase (HMGR). As shown in Table 4, the IC50 values range between 10−8 and 10−5 M. In the majority of the cases, GTE, BTE, catechins and theaflavins inhibit the enzymes in a non‐competitive manner with respect to substrate concentration. EGCG potently inhibits the in vitro activity of HMGR (Ki in the nanomolar range) by competitively binding to the co‐factor site of the reductase (Cuccioloni et al., 2011). In contrast, EGCG interacts with Val21, Glu188 and Glu220 of lipase, inducing conformational alterations and decreasing the enzyme's catalytic activity (Wu et al., 2013). The galloyl moiety seems to be involved in the inhibitory effect on pancreatic lipase, because theaflavins and catechins without galloyl moieties did not inhibit this enzyme (Ikeda et al., 2005; Kobayashi et al., 2009).
Table 4.
Inhibitory effect in vitro of tea flavanols on selected enzymes
| Enzyme | IC50 or Ki | References | |
|---|---|---|---|
| GTE | α‐glucosidase, maltase or | 2.82 μg·mL−1 | Forester et al., 2012; Nguyen et al., 2012; Simsek et al., 2015; Yang and Kong, 2016. |
| BTE | amylase | 2.25 μg·mL−1 | |
| EGCG | 10−5 M | ||
| BTE | Lipase | 0.9–1.3 μg·mL−1 | Grove et al., 2012; Kobayashi et al., 2009; Wang et al., 2014; Yuda et al., 2012. |
| TFDG, EGCG | 10−6 M | ||
| EGCG | HMGR | 10−8 M | Cuccioloni et al., 2011. |
| BTE | XO | 5.8% | Aucamp et al., 1997; Lin et al., 2000; Dew et al., 2005. |
| TFDG | 10− 6–10−5 M | ||
| EC, EGC | 10−5 M | ||
| ECG | 10−6 M | ||
| EGCG | 10−7 M | ||
| EGC, GCG and EGCG | GST | 10−6 M | Boušová et al., 2012. |
| GTE | TrxR | 256 μg·mL−1 | Wang et al., 2008; Du et al., 2009. |
| EGCG, ECG | 10−5 M | ||
| TF3G, TF3′G and TFDG | 10−5 M | ||
| EGCG | COX‐1 | 10−6 M | Lee et al., 2013. |
| EGC and ECG | DNA‐pol | 10−4 M | Mizushina et al., 2005. |
| EGCG | 10−6 M | ||
| ECG > Met‐ | DNMT | 10− 6–10−5 M | Fang et al., 2003; Rajavelu et al., 2011. |
| EGCG > EGC > Di‐Me‐ | |||
| EGCG > EC | |||
| EGCG | 10− 7–10−6 M | ||
| TFDG | 10−5 M | ||
| GTE | RNase A | 10−4 M GAE | Ghosh et al., 2004. |
| EGCG | 10−5 M |
TF, theaflavin; TF3G, theaflavin 3‐O‐gallate; TF3′G, theaflavin 3′‐O‐gallate; TFDG, theaflavin 3,3′‐O‐digallate; DNA‐pol, DNA‐polymerase; GAE, gallic acid equivalents.
However, the results in humans are contrasting as highlighted by different meta‐analyses on human interventions with GT, BT or catechins. In particular, Zheng et al. (2013) and Liu et al. (2013) found decreased glucose levels, whereas no significant effects on glucose were observed in a recent meta‐analysis (Khalesi et al., 2014; Li et al., 2016). Similarly, a different meta‐analysis reported a reduction in cholesterol levels (Zheng et al., 2011; Hartley et al., 2013; Khalesi et al., 2014; Onakpoya et al., 2014), but this was not confirmed by Li et al. (2016) and Zhao et al. (2015).
As shown in Table 4, theaflavins and catechins inhibit xanthine oxidase (XO) activity and UA production in vitro: theaflavin 3,3′‐O‐digallate (10−6 M) and EGCG (10−7 M) act as competitive inhibitors (Aucamp et al., 1997; Lin et al., 2000). Despite the effect of theaflavins and catechins as XO inhibitors, in a meta‐analysis reviewing human intervention studies that measured UA after tea products, no significant differences were observed between BT, GT and GTE (Peluso et al., 2015a). However, it must be taken into account that many studies had UA as secondary outpoint and did not consider the fact that the normal range of UA differs in males and females. Only the study of Bahorun et al. (2010) had UA as primary outpoint and BT as a treatment and reported data for men and women separately, stratifying them according to baseline levels. It showed a decrease of UA only in subjects with high baseline levels and in men with baseline UA concentrations above 80 mg·L−1, with the latter lowered after washout (BT 73 ± 17 mg·L−1 and water 80 ± 20 mg·L−1) suggesting an inhibitory effect on XO that persists after discontinuation of consumption (Bahorun et al., 2010). In accordance with the hypothesis of a persistent inhibitory effect on XO, in an uncontrolled trial, GTE (164 mg tea catechins) decreased UA after 7 days of washout, subsequent to 7 days of supplementation (Kimura et al., 2002). Panza et al. (2008) reported a decrease in UA levels after 7 days of ingestion of GT (600 mL) and an inhibition of the exercise‐induced activation of XO. Moreover, in an uncontrolled trial, decreases in UA after 9 weeks of GT (100 mg·day−1 of total catechins) and increases in UA with catechin‐enriched GT (400 mg·day−1 of total catechins) were observed, but these effects were not statistically significant (Sone et al., 2011). Furthermore, treatments of 2 weeks with GT (1.5 g, three times a day with total catechins 183 mg·g−1: 823.5 mg·day−1) (Gomikawa et al., 2008) or 16 weeks with GTE (200 mg·day−1) plus lutein (12 mg·day−1) (Li et al., 2010) were unable to change the UA concentration. Therefore, longer or higher consumptions of tea catechins do not seem to be associated with a greater effect, in contrast to the results of Kimura et al. (2002).
As shown in Table 4, catechins and theaflavins inhibit glutathione S‐transferase (GST) and thioredoxin reductase (TrxR) with IC50 values between 10−6 and 10−5 M (Table 4). However, catechins are also able to stimulate the transcription of antioxidant enzymes, including SOD, catalase, glutathione peroxidase, glutathione reductase and GST, through the nuclear factor‐erythroid 2‐related factor 2 (Nrf2)/antioxidant responsive elements pathway (Na and Surh, 2008). In particular, it has been suggested that some derivatives of catechins can oxidize highly reactive cysteine thiol groups of kelch‐like ECH‐associated protein‐1, resulting in disulfide bond formation and Nfr2 release (Na and Surh, 2008). In mice, a repeated (5 days) non‐lethal toxic dose (55 or 75 mg·kg−1) of EGCG decreased the expression of Nrf2 in the cytosol and increased it in the nucleus. As a result, mRNA expression and activities and/or protein levels of Nrf2‐target genes including GST and TrxR were increased (Wang et al., 2015).
Regarding the reported inhibition of COX‐1 activity in platelets (Lee et al., 2013; Table 4), this effect is not supported by the results of a human study conducted by Hirano‐Ohmori et al. (2005). After the consumption of seven cups of GT a day for 2 weeks by healthy subjects, no significant changes in the aggregation of platelets were observed, despite a significant decrease in the serum low density lipoproteins (MDA‐LDL).
Some catechins inhibited mammalian DNA‐polymerase, DNA‐methyltransferase (DNMT) and ribonuclease A (RNase A) (Table 4), with EGCG being the strongest inhibitor with IC50 values ranging between 10−7 M (DNMT9) and 10−5 M (RNase A). Among these enzymes, DNMT is involved in the hypermethylation of the promoter regions, which is an important mechanism for silencing the expression of many significant genes in cancer (Yiannakopoulou, 2015). However, data from meta‐analyses provided contrasting results and indicated that the associations differ according to sex, ethnicity, cancer and tea types (Zeng et al., 2014; Ma et al., 2015; Zhu et al., 2015; Huang et al., 2016; Zhou et al., 2016).
Flavanols are substrates of cytochrome p450 (CYP450) and are well‐known to interfere with the pharmacokinetics of drugs in humans (Shang et al., 2014; Werba et al., 2015). However, only one case report has documented the interaction between GT and an immunosuppressant (tacrolimus), a substrate for CYP3A4. This case involved a 58‐year‐old male kidney transplant recipient, genotyped as ‘poor metabolizer’ and treated with a low dose of tacrolimus (i.e. 1 mg · 24 h‐1). After GT ingestion, an increase in tacrolimus levels was observed, and a positive dechallenge of tea was performed (Vischini et al., 2011). In healthy volunteers, who received a cocktail of CYP450 metabolic probe drugs, including caffeine, dextromethorphan, losartan and buspirone for assessing the activity of CYP1A2, CYP2D6, CYP2C9 and CYP3A4, respectively, after 4 weeks of EGCG (800 mg) consumption, only a significant increase in the concentration of buspirone was found, suggesting a reduction in CYP3A4 activity (Chow et al., 2006). In contrast, despite GTE (844 mg · day‐1 for 14 days) inducing an increase in EGCG in plasma (1.3 ± 1.8 μM 2 h after treatment), no effect on CYP3A4 activity was found when alprazolam was used as the probe drug in healthy subjects (Donovan et al., 2004). Therefore, the interaction of tea catechins with CYP450 depends on the substrate present.
Interaction with transporters
Catechins interact with transporters of the phase III drug detoxifying system, mainly the multidrug resistance 1 (MDR1), OAT and organic cation (OCT) transporters (Table 5). These transporters are characterized by low substrate specificity, and mediate the uptake of numerous drugs and xenobiotics into cells (Ayrton and Morgan, 2001). They are also involved in the absorption of flavonoids in the gastrointestinal tract and their subsequent tissue distribution (Passamonti et al., 2009), as well as the extrusion of catechins (Vaidyanathan and Walle, 2003).
Table 5.
Inhibitory effect in vitro of tea flavanols on selected transporters
| Transporter | IC50 or tested concentration | References | |
|---|---|---|---|
| GT | MDR1 | 1% (v.v−1) | Kitagawa et al., 2004; Knop et al., 2015; Mei et al., 2004; Qian et al., 2005; Wang et al., 2002. |
| GT polyphenols | 40 μg·mL−1 | ||
| EGCG | 10− 5–10−6 M; 10 μg·mL−1 | ||
| GT | OAT | 0.39–2.6% (v.v−1) | Fuchikami et al., 2006; Knop et al., 2015; Misaka et al., 2014; Roth et al., 2011; Zhang et al., 2013. |
| EGCG | 10− 6–10−4 M | ||
| ECG | 10−5 M | ||
| GT | OCT1/OCT2 | 1.4–7.0% (v.v−1) | Jaiyen et al., 2015; Knop et al., 2015. |
| GTE | 1–3 mg·mL−1 | ||
| EGC | 10−3–10−4 M | ||
| EGCG | 10−4 M | ||
| EGCG | PCFT | 10−6 M (competitive) | Kissei et al., 2014. |
| ECG | GLUT1 /SGLT1 | 10−7–10−3 M (competitive) | Johnston et al., 2005; Kobayashi et al., 2000; Naftalin et al., 2003. |
| EGCG | 10−7–10−3 M (competitive) |
GLUT, sodium‐independent glucose transporter; SGLT, sodium‐dependent glucose transporter.
It is important to note that OAT (Sekine et al., 2006; Wang et al., 2010; Hu et al., 2012) plays an important role in the renal excretion of urate. Therefore, the concentration of uric acid can be affected by the consumption of tea not only by its inhibition of XO (Table 4) but also its interaction with OAT (Table 5).
A recent review of the experimental studies in humans and/or clinical observations about interactions between GT and cardiovascular drugs only yielded data for simvastatin and nadolol (Werba et al., 2015). The authors suggested that these effects could be due to the inhibition of MDR1 and OAT exerted by GT catechins. Accordingly, the in vitro IC50 of tea flavanols on transporters, shown in Table 5, was lower for MDR1 and OAT than for OCT. Jaiyen et al. (2015) suggested that the consumption of GT could not interfere with cationic drugs secreted via renal OCT2 in humans because he found the interaction of GTE and ECG with OCT2 to be weak and reversible.
Kitagawa et al. (2004) reported that the effect of EGCG on MDR1 was more significant than that of verapamil (a well‐known substrate for this transporter). The interaction of EGCG with MDR1 is at the level of the ATP‐binding site (Wang et al., 2002), in particular the ATP‐binding site of the carboxyl‐terminal nucleotide binding domain (Qian et al., 2005), and it has been suggested that GT polyphenols and EGCG can reverse multidrug resistance through modulation of the ATPase activity of MDR1 (Mei et al., 2004). Moreover, it has been suggested that the absorption of methotrexate can be reduced if it is consumed with GT, due to competitive inhibition of the proton‐coupled folate transporter (PCFT) (Kissei et al., 2014) (Table 5). However, while there are no data on this type of food and drug interaction, in an open‐labelled randomized crossover study in healthy volunteers, it has been reported that GTE and BTE (0.3 g extract . 250 mL‐1) decrease the bioavailability of the vitamin folic acid (0.4 and 5 mg), reducing the Cmax of serum folate by 30–40% (Alemdaroglu et al., 2008).
The glucose uptake pathways include sodium‐independent (GLUT1) and sodium‐dependent (SGLT1) transporters. Johnston et al. (2005) expressed both transporters in Caco‐2 cells and showed that GT polyphenols (100 μM) decrease glucose uptake both in sodium‐containing and sodium‐free medium. It has been suggested that the antidiabetogenic effects of GT are, at least in part, due to the inhibition of the glucose transporter GLUT1 (Naftalin et al., 2003). ECG and EGCG have high affinities for GLUT1 and competitively inhibit the uptake of glucose (ECG 0.14 μM; EGCG 0.9 μM) (Table 5). In particular, EGCG competitively inhibits the binding of glucose onto the external face of the carrier (Naftalin et al., 2003). In contrast, the ungallated catechins, EC and EGC have only weak effects on glucose transport (Naftalin et al., 2003). Similar results, but at higher concentrations compared with GLUT‐1 inhibition, were obtained on SGLT1‐mediated glucose transport that was competitively inhibited by ECG (390 μM) and EGCG (1 mM), whereas the inhibitory effects of EC and EGC were not significant (Kobayashi et al., 2000) (Table 5). These data imply that a galloyl ester group may be important for blocking glucose uptake.
Potential adverse effects
The Dietary Supplement Information Expert Committee (DSI EC) have systematically reviewed the safety information for GT products and indicated that the consumption of GTE could induce liver damage (Sarma et al., 2008). In fact, there is an increasing number of case reports of hepatoxicity associated with the intake of GT dietary supplements (Schönthal, 2011, Stickel et al., 2011, Teschke et al., 2012, Mazzanti et al., 2015). The patients showed clinical symptoms of different severity, ranging from a mild increase in serum aminotransferase levels to fulminant hepatitis requiring a liver transplant (Di Lorenzo et al., 2015). The types of preparation responsible for these adverse effects were plant food supplements based on GTE, among these were a hydroalcoholic extract and an aqueous extract of GT consumed as tea or in capsules (Di Lorenzo et al., 2015). The dose of the tea supplement ingested ranged between 320 mg·day−1 catechins (710 mg·day−1 polyphenols) for the decaffeinated extract and 1 g·day−1 catechins for the micronized powder (Mazzanti et al., 2015). For patients who consumed the GTEs as infusions, the ingested dose ranged from two cups to 3 L·day−1, corresponding to about 186 and 1395 mg polyphenols ·day−1 (Mazzanti et al., 2015). The components most frequently indicated as responsible for hepatotoxicity are catechins and in particular EGCG supplements (Bunchorntavakul and Reddy, 2013, Di Lorenzo et al., 2015).
Discussion and conclusion
In recent years, the attention of the scientific community has been focused on understanding the mechanisms of action of tea flavanols, due to evidence that the consumption of tea has beneficial effects on health (Serafini et al., 2011). In addition to conventional antioxidant properties (Serafini et al., 1996; Lettieri‐Barbato et al., 2013; Table 3), there is evidence from in vitro experiments that antioxidants in tea may act by pharmacological mechanisms, such as inhibiting various enzymes and interacting with transporters (Tables 4 and 5). In this context, some considerations should be taken into account. Firstly, the biological effect of flavanols depends on their absorption, which tends to be low in humans (Table 2). Secondly, once ingested, they are extensively metabolized into molecules with different chemical structures and activity compared with the ones originally present in the teas. Therefore, differences in microbiota (van Duynhoven et al., 2014) and genetic polymorphism of metabolizing enzymes (Hursel et al., 2014) could play a role in the inter‐individual variability in the response to treatment. This implies that we must exercise caution when speculating about the effects of a cup of tea from in vitro data and results obtained in animals. Furthermore, the poor of absorption of flavonoids and the extensive metabolic activity they undergo during absorption lead to very low plasma concentrations and to the presence in the blood stream of a wide variety of known and lesser‐known metabolites (Del Rio et al., 2013). Also most of the in vitro evidence for the beneficial effects of flavonoids has been obtained with pure compounds, which are present at low concentrations in humans (Table 2). However, EGCG at concentrations similar to its Cmax (10−8–10−7 M, Table 2) after the consumption of a cup of GT, can effectively inhibit the enzymes involved in cholesterol and UA metabolism (HMGR: IC50 10−8 M; XO: IC50 10−7 M, Table 4) and the glucose transporters (IC50 10−7 M, Table 5). The structural features of catechins that significantly contribute to their pharmacological effect are the presence/absence of the galloyl moiety and the number and positions of the hydroxyl groups on the rings. This also accounts for the higher antioxidant activity of GT than BT, both in vitro (Serafini et al., 1996) and in human intervention studies (Lettieri‐Barbato et al., 2013).
At a pharmacological level, although the inhibitory effect on α‐glucosidase, maltase, amylase and lipase, as well as on MDR1, OAT and PCFT, occurs at higher concentrations (IC50 10−6–10−5 M, Tables 4 and 5) compared to circulating levels (Table 2), these effects could be relevant in the gut. In particular, in humans, the GTE‐induced decrease in the digestion and absorption of carbohydrates (Lochocka et al., 2015) and lipids (Lisowska et al., 2015) have been confirmed by the starch 13C breath test and the 13C‐labelled mixed triglyceride breath test.
It has been suggested that the food–drug interactions with cardiovascular drugs could be due to the inhibitory effects of GT catechins on MDR1 and OAT (Werba et al., 2015) and that their ability to reduce the bioavailability of the vitamin folic acid could be due to competitive inhibition of PCFT (Alemdaroglu et al., 2008). Furthermore, as flavanols are substrates of CYP450, they interfere with the pharmacokinetics of many drugs in humans (Vischini et al., 2011; Shang et al., 2014; Werba et al., 2015). Their extensive hepatic metabolism could also account for the case reports of hepatoxicity associated with an intake of GTE in humans. However, in a recent systematic review, it was found that liver‐related adverse events were only reported in four out of the 34 trials examined (Isomura et al., 2016). A meta‐analysis of these four trials gave a summary odds ratio for liver‐related adverse events in subjects who received green tea intervention versus placebo of 2.1 (Isomura et al., 2016) and it was concluded that liver‐related adverse events after the consumption of GTE are likely to be rare.
The antioxidant effect of tea ingestion requires more evidence to unravel the mechanism of action and the ingredients involved. Despite there being no convincing evidence from long‐term intervention studies in humans, tea flavanols are still considered to be the major candidates involved in the biological activity of teas. Possible mechanisms of action, such as the induction of an endogenous redox pathway or direct effects of polyphenol metabolites, should be elucidated so that the molecules responsible for the effect can isolated and clear‐cut evidence can be obtained from long‐term intervention studies.
In conclusion, despite the urgent need for further research in humans, the regular consumption of moderate quantitities of GT and BT can effectively modulate the antioxidant capacity of individuals, mainly of people experiencing conditions of oxidative stress, and could improve glucose, lipid and UA metabolism.
Author contributions
M.S. drafted the aspect related to antioxidant activity, planned and critically reviewed the manuscript. I.P. drafted the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Claudio Andrew Gobbi for English review of the manuscript.
Peluso, I. , and Serafini, M. (2017) Antioxidants from black and green tea: from dietary modulation of oxidative stress to pharmacological mechanisms. British Journal of Pharmacology, 174: 1195–1208. doi: 10.1111/bph.13649.
References
- Alemdaroglu NC, Dietz U, Wolffram S, Spahn‐Langguth H, Langguth P (2008). Influence of green and black tea on folic acid pharmacokinetics in healthy volunteers: potential risk of diminished folic acid bioavailability. Biopharm Drug Dispos 29: 335–348. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts IC, van De Putte B, Hollman PC (2000). Catechin contents of foods commonly consumed in the Netherlands. 2. Tea, wine, fruit juices and chocolate milk. J Agric Food Chem 48: 1752–1757. [DOI] [PubMed] [Google Scholar]
- Aucamp J, Gaspar A, Hara Y, Apostolides Z (1997). Inhibition of xanthine oxidase by catechins from tea (Camellia sinensis). Anticancer Res 17 (6D): 4381–4385. [PubMed] [Google Scholar]
- Ayrton A, Morgan P (2001). Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 31: 469–497. [DOI] [PubMed] [Google Scholar]
- Bahorun T, Luximon‐Ramma A, Gunness TK, Sookar D, Bhoyroo S, Jugessur R et al. (2010). Black tea reduces uric acid and C‐reactive protein levels in humans susceptible to cardiovascular diseases. Toxicology 278: 68–74. [DOI] [PubMed] [Google Scholar]
- Begona Barroso M, Werken van de G (1999). Determination of green and black tea composition by capillary electrophoresis. J High Resolut Chrom 22: 225–230. [Google Scholar]
- Bertipaglia de Santana M, Mandarino MG, Cardoso JR, Dichi I, Dichi JB, Camargo AE et al. (2008). Association between soy and green tea (Camellia sinensis) diminishes hypercholesterolemia and increases total plasma antioxidant potential in dyslipidemic subjects. Nutrition 24: 562–568. [DOI] [PubMed] [Google Scholar]
- Boušová I, Hájek J, Dršata J, Skálová L (2012). Naturally occurring flavonoids as inhibitors of purified cytosolic glutathione S‐transferase. Xenobiotica 42: 872–879. [DOI] [PubMed] [Google Scholar]
- Braga M, Bissolati M, Rocchetti S, Beneduce A, Pecorelli N, Di Carlo V (2012). Oral preoperative antioxidants in pancreatic surgery: a double‐blind, randomized, clinical trial. Nutrition 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Bronner WE, Beecher GR (1998). Method for determining the content of catechins in tea infusions by high‐performance liquid chromatography. J Chromatogr A 805: 137–142. [DOI] [PubMed] [Google Scholar]
- Bunchorntavakul C, Reddy KR (2013). Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther 37: 3–17. [DOI] [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, Cordova CA, Chew WM et al. (2006). Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomarkers Prev 15: 2473–2476. [DOI] [PubMed] [Google Scholar]
- Clifford MN, van der Hooft JJ, Crozier A (2013). Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr 98 (6 Suppl): 1619S–1630S. [DOI] [PubMed] [Google Scholar]
- Cuccioloni M, Mozzicafreddo M, Spina M, Tran CN, Falconi M, Eleuteri AM et al. (2011). Epigallocatechin‐3‐gallate potently inhibits the in vitro activity of hydroxy‐3‐methyl‐glutaryl‐CoA reductase. J Lipid Res 52: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Judd JT, Baer DJ, Clevidence BA, Paul DR, Edwards AJ et al. (2003). Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr 133: 3298S–3302S. [DOI] [PubMed] [Google Scholar]
- Del Rio D, Rodriguez‐Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (2013). Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18: 1818–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew TP, Day AJ, Morgan MR (2005). Xanthine oxidase activity in vitro: effects of food extracts and components. J Agric Food Chem 53: 6510–6515. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo C, Ceschi A, Kupferschmidt H, Lüde S, De Souza NE, Dos Santos A et al. (2015). Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol 79: 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Yang H, Xiao S (1999). Rapid, direct determination of polyphenols in tea by reversed‐phase column liquid chromatography. J Chromatogr A 849: 637–640. [DOI] [PubMed] [Google Scholar]
- Ding Z, Kuhr S, Engelhardt UH (1992). Influence of catechins and theaflavins on the astringent taste of black tea brews. Zeitschrift fuer Lebensmittel Untersuchung und Forschung 195: 108–111. [Google Scholar]
- Donovan JL, Chavin KD, Devane CL, Taylor RM, Wang JS, Ruan Y et al. (2004). Green tea (Camellia sinensis) extract does not alter cytochrome p450 3A4 or 2D6 activity in healthy volunteers. Drug Metab Dispos 32: 906–908. [DOI] [PubMed] [Google Scholar]
- Donovan JL, DeVane CL, Chavin KD, Oates JC, Njoku C, Patrick KS et al. (2005). Oral administration of a decaffeinated green tea (Camellia sinensis) extract did not alter urinary 8‐epi‐prostaglandin F(2 alpha), a biomarker for in‐vivo lipid peroxidation. J Pharm Pharmacol 57: 1365–1369. [DOI] [PubMed] [Google Scholar]
- Du Y, Wu Y, Cao X, Cui W, Zhang H, Tian W et al. (2009). Inhibition of mammalian thioredoxin reductase by black tea and its constituents: implications for anticancer actions. Biochimie 91: 434–444. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H et al. (2003). Tea polyphenol (−)‐epigallocatechin‐3‐gallate inhibits DNA methyltransferase and reactivates methylation‐silenced genes in cancer cell lines. Cancer Res 63: 7563–7570. [PubMed] [Google Scholar]
- Feng WY (2006). Metabolism of green tea catechins: an overview. Curr Drug Metab 7: 755–809. [DOI] [PubMed] [Google Scholar]
- Forester SC, Gu Y, Lambert JD (2012). Inhibition of starch digestion by the green tea polyphenol, (−)‐epigallocatechin‐3‐gallate. Mol Nutr Food Res 56: 1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese R, Basu S, Hietanen E, Nair J, Nakachi K, Bartsch H et al. (1999). Green tea extract decreases plasma malondialdehyde concentration but does not affect other indicators of oxidative stress, nitric oxide production, or hemostatic factors during a high‐linoleic acid diet in healthy females. Eur J Nutr 38: 149–157. [DOI] [PubMed] [Google Scholar]
- Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y (2006). Effects of herbal extracts on the function of human organic anion‐transporting polypeptide OATP‐B. Drug Metab Dispos 34: 577–582. [DOI] [PubMed] [Google Scholar]
- Ghosh KS, Maiti TK, Dasgupta S (2004). Green tea polyphenols as inhibitors of ribonuclease A. Biochem Biophys Res Commun 325: 807–811. [DOI] [PubMed] [Google Scholar]
- Gomikawa S, Ishikawa Y, Hayase W, Haratake Y, Hirano N, Matuura H et al. (2008). Effect of ground green tea drinking for 2 weeks on the susceptibility of plasma and LDL to the oxidation ex vivo in healthy volunteers. Kobe J Med Sci 54: E62–E72. [PubMed] [Google Scholar]
- Grove KA, Sae‐tan S, Kennett MJ, Lambert JD (2012). (−)‐Epigallocatechin‐3‐gallate inhibits pancreatic lipase and reduces body weight gain in high fat‐fed obese mice. Obesity (Silver Spring) 20: 2311–2313. [DOI] [PubMed] [Google Scholar]
- Hartley L, Flowers N, Holmes J, Clarke A, Stranges S, Hooper L et al. (2013) Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. Jun 18; 6:CD009934. [DOI] [PMC free article] [PubMed]
- Hirano‐Ohmori R, Takahashi R, Momiyama Y, Taniguchi H, Yonemura A, Tamai S et al. (2005). Green tea consumption and serum malondialdehyde‐modified LDL concentrations in healthy subjects. J Am Coll Nutr 24: 342–346. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Croft KD, Mori TA, Burke V, Beilin LJ, Puddey IB (2002a). Regular ingestion of tea does not inhibit in vivo lipid peroxidation in humans. J Nutr 132: 55–58. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Puddey IB, Burke V, Watts GF, Beilin LJ (2002b). Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci (Lond) 102: 195–201. [PubMed] [Google Scholar]
- Hu QH, Zhang X, Wang X, Jiao RQ, Kong LD (2012). Quercetin regulates organic ion transporter and uromodulin expression and improves renal function in hyperuricemic mice. Eur J Nutr 51: 593–606. [DOI] [PubMed] [Google Scholar]
- Huang YQ, Lu X, Min H, Wu QQ, Shi XT, Bian KQ et al. (2016). Green tea and liver cancer risk: a meta‐analysis of prospective cohort studies in Asian populations. Nutrition 32: 3–8. [DOI] [PubMed] [Google Scholar]
- Hursel R, Janssens PL, Bouwman FG, Mariman EC, Westerterp‐Plantenga MS (2014). The role of catechol‐O‐methyl transferase Val(108/158)Met polymorphism (rs4680) in the effect of green tea on resting energy expenditure and fat oxidation: a pilot study. PLoS One 9: e106220. doi:10.1371/journal.pone.0106220 .eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda I, Tsuda K, Suzuki Y, Kobayashi M, Unno T, Tomoyori H et al. (2005). Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J Nutr 135: 155–159. [DOI] [PubMed] [Google Scholar]
- Isomura T, Suzuki S, Origasa H, Hosono A, Suzuki M, Sawada T et al. (2016). Liver‐related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr . doi:10.1038/ejcn.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiyen C, Jutabha P, Anzai N, Lungkaphin A, Soodvilai S, Srimaroeng C (2015). Interaction of green tea catechins with renal organic cation transporter 2. Xenobiotica 17: 1–10. [DOI] [PubMed] [Google Scholar]
- Johnston K, Sharp P, Clifford M, Morgan L (2005). Dietary polyphenols decrease glucose uptake by human intestinal Caco‐2 cells. FEBS Lett 579: 1653–1657. [DOI] [PubMed] [Google Scholar]
- Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht‐Nasrabadi E, Khosravi‐Boroujeni H (2014). Green tea catechins and blood pressure: a systematic review and meta‐analysis of randomised controlled trials. Eur J Nutr 53: 1299–1311. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H (2006). Targeting multiple signaling pathways by green tea polyphenol (−)‐epigallocatechin‐3‐gallate. Cancer Res 66: 2500–2505. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Magnusdottir SG (2002). Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem 50: 565–570. [DOI] [PubMed] [Google Scholar]
- Kilmartin PA, Chyong FH (2003). Characterisation of polyphenols in green, oolong, and black teas, and in coffee, using cyclic voltammetry. Food Chem 82: 501–512. [DOI] [PubMed] [Google Scholar]
- Kim YH, Won YS, Yang X, Kumazoe M, Yamashita S, Hara A et al. (2016). Green tea catechin metabolites exert immunoregulatory effects on CD4(+) T cell and natural killer cell activities. J Agric Food Chem 64: 3591–3597. [DOI] [PubMed] [Google Scholar]
- Kimura M, Umegaki K, Kasuya Y, Sugisawa A, Higuchi M (2002). The relation between single/double or repeated tea catechin ingestions and plasma antioxidant activity in humans. Eur J Clin Nutr 56: 1186–1193. [DOI] [PubMed] [Google Scholar]
- Kissei M, Itoh T, Narawa T (2014). Effect of epigallocatechin gallate on drug transport mediated by the proton‐coupled folate transporter. Drug Metab Pharmacokinet 29: 367–372. [DOI] [PubMed] [Google Scholar]
- Kitagawa S, Nabekura T, Kamiyama S (2004). Inhibition of P‐glycoprotein function by tea catechins in KB‐C2 cells. J Pharm Pharmacol 56: 1001–1005. [DOI] [PubMed] [Google Scholar]
- Knop J, Misaka S, Singer K, Hoier E, Müller F, Glaeser H et al. (2015). Inhibitory effects of green tea and (−)‐epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2‐K and P‐Glycoprotein. PLoS One 10: e0139370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ichitani M, Suzuki Y, Unno T, Sugawara T, Yamahira T et al. (2009). Black‐tea polyphenols suppress postprandial hypertriacylglycerolemia by suppressing lymphatic transport of dietary fat in rats. J Agric Food Chem 57: 7131–7136. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K et al. (2000). Green tea polyphenols inhibit the sodium‐dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem 48: 5618–5623. [DOI] [PubMed] [Google Scholar]
- Kuhr S, Engelhardt UH (1991). Determination of flavanols, theogallin. Zeitschrift fuer Lebensmittel Untersuchung und Forschung 192: 526–529. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ (2010). The antioxidant and pro‐oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Ong CN (2000). Comparative analysis of tea catechins and theaflavins by high‐performance liquid chromatography and capillary electrophoresis. J Chromatogr A 881: 439–447. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim YJ, Kim HH, Cho HJ, Ryu JH, Rhee MH et al. (2013). Inhibitory effects of epigallocatechin‐3‐gallate on microsomal cyclooxygenase‐1 activity in platelets. Biomol Ther (Seoul) 21: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri‐Barbato D, Tomei F, Sancini A, Morabito G, Serafini M (2013). Effect of plant foods and beverages on plasma non‐enzymatic antioxidant capacity in human subjects: a meta‐analysis. Br J Nutr 109: 1544–1556. [DOI] [PubMed] [Google Scholar]
- Li L, Chen CY, Aldini G, Johnson EJ, Rasmussen H, Yoshida Y et al. (2010). Supplementation with lutein or lutein plus green tea extracts does not change oxidative stress in adequately nourished older adults. J Nutr Biochem 21: 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang C, Huai Q, Guo F, Liu L, Feng R et al. (2016). Effects of tea or tea extract on metabolic profiles in patients with type 2 diabetes mellitus: a meta‐analysis of ten randomized controlled trials. Diabetes Metab Res Rev 32: 2–10. [DOI] [PubMed] [Google Scholar]
- Liang Y, Lu J, Zhang L, Wu S, Wu Y (2003). Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusion. Food Chem 80: 283–290. [Google Scholar]
- Lin JK, Chen PC, Ho CT, Lin‐Shiau SY (2000). Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL‐60 cells by theaflavin‐3,3′‐digallate, (−)‐epigallocatechin‐3‐gallate, and propyl gallate. J Agric Food Chem 48: 2736–2743. [DOI] [PubMed] [Google Scholar]
- Lin JK, Lin CL, Liang YC, Lin‐Shiau SY, Juan IM (1998). Survey of catechins, gallic acid, and methylxanthines in green, oolong, pu‐erh and black teas. J Agric Food Chem 46: 3635–3642. [Google Scholar]
- Lin Y, Juan I, Chen Y, Liang Y, Lin J (1996). Composition of polyphenols in fresh tea leaves and associations of their oxygen‐radical‐absorbing capacity with antiproliferative actions in fibroblast cells. J Agric Food Chem 44: 1387–1394. [Google Scholar]
- Lisowska A, Stawińska‐Witoszyńska B, Bajerska J, Krzyżanowska P, Walkowiak J (2015). Green tea influences intestinal assimilation of lipids in humans: a pilot study. Eur Rev Med Pharmacol Sci 19: 209–214. [PubMed] [Google Scholar]
- Liu K, Zhou R, Wang B, Chen K, Shi LY, Zhu JD et al. (2013). Effect of green tea on glucose control and insulin sensitivity: a meta‐analysis of 17 randomized controlled trials. Am J Clin Nutr 98: 340–348. [DOI] [PubMed] [Google Scholar]
- Lochocka K, Bajerska J, Glapa A, Fidler‐Witon E, Nowak JK, Szczapa T et al. (2015). Green tea extract decreases starch digestion and absorption from a test meal in humans: a randomized, placebo‐controlled crossover study. Sci Rep 5: 12015. doi:10.1038/srep12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD (2008). Pure dietary flavonoids quercetin and (−)‐epicatechin augment nitric oxide products and reduce endothelin‐1 acutely in healthy men. Am J Clin Nutr 88: 1018–1025. [DOI] [PubMed] [Google Scholar]
- Long H, Zhu Y, Huang T, Coury LA, Kissinger PT (2001). Identification and determination of polyphenols in tea by liquid chromatography with multichannel electrochemical detection. J Liq Chrom Relat Tech 24: 1105–1114. [Google Scholar]
- Luximon‐Ramma A, Bahorun T, Crozier A, Zbarsky V, Datla KP, Dexter DT et al. (2005). Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas. Food Res Int 38: 357–367. [Google Scholar]
- Ma S, Wang C, Bai J, Wang X, Li C (2015). Association of tea consumption and the risk of thyroid cancer: a meta‐analysis. Int J Clin Exp Med 8: 14345–14351. [PMC free article] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81 (1 Suppl): 230S–242S. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Sotto A, Vitalone A (2015). Hepatotoxicity of green tea: an update. Arch Toxicol 89: 1175–1191. [DOI] [PubMed] [Google Scholar]
- Mei Y, Qian F, Wei D, Liu J (2004). Reversal of cancer multidrug resistance by green tea polyphenols. J Pharm Pharmacol 56: 1307–1314. [DOI] [PubMed] [Google Scholar]
- Misaka S, Yatabe J, Müller F, Takano K, Kawabe K, Glaeser H et al. (2014). Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin Pharmacol Ther 95: 432–438. [DOI] [PubMed] [Google Scholar]
- Mizushina Y, Saito A, Tanaka A, Nakajima N, Kuriyama I, Takemura M et al. (2005). Structural analysis of catechin derivatives as mammalian DNA polymerase inhibitors. Biochem Biophys Res Commun 333: 101–109. [DOI] [PubMed] [Google Scholar]
- Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM (2001). Analysis of theaflavins in biological fluids using liquid chromatography‐electrospray mass spectrometry. Chromatogr B Biomed Sci Appl 760: 271–279. [DOI] [PubMed] [Google Scholar]
- Müller N, Ellinger S, Alteheld B, Ulrich‐Merzenich G, Berthold HK, Vetter H et al. (2010). Bolus ingestion of white and green tea increases the concentration of several flavan‐3‐ols in plasma, but does not affect markers of oxidative stress in healthy non‐smokers. Mol Nutr Food Res 54: 1636–1645. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ (2008). Modulation of Nrf2‐mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol 46: 1271–1278. [DOI] [PubMed] [Google Scholar]
- Naftalin RJ, Afzal I, Cunningham P, Halai M, Ross C, Salleh N et al. (2003). Interactions of androgens, green tea catechins and the antiandrogen flutamide with the externalglucose‐binding site of the human erythrocyte glucose transporter GLUT1. Br J Pharmacol 140: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TTH, Jung SH, Lee S, Ryu HJ, Kang HK, Moon YH et al. (2012). Inhibitory effects of epigallocatechin gallate and its glucoside on the human intestinal maltase inhibition. Biotechnol Bioproc Eng 17: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I, Spencer E, Heneghan C, Thompson M (2014). The effect of green tea on blood pressure and lipid profile: a systematic review and meta‐analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 24: 823–836. [DOI] [PubMed] [Google Scholar]
- O'Reilly JD, Mallet AI, McAnlis GT, Young IS, Halliwell B, Sanders TA et al. (2001). Consumption of flavonoids in onions and black tea: lack of effect on F2‐isoprostanes and autoantibodies to oxidized LDL in healthy humans. Am J Clin Nutr 73: 1040–1044. [DOI] [PubMed] [Google Scholar]
- Panza VS, Wazlawik E, Ricardo Schütz G, Comin L, Hecht KC, da Silva EL (2008). Consumption of green tea favorably affects oxidative stress markers in weight‐trained men. Nutrition 24: 433–442. [DOI] [PubMed] [Google Scholar]
- de Pascual‐Teresa S, Santos‐Buelga C, Rivas‐Gonzalo JC (2000). Quantitative analysis of flavan‐3‐ols in Spanish foodstuffs and beverages. J Agric Food Chem 48: 5331–5337. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Terdoslavich M, Franca R, Vanzo A, Tramer F, Braidot E et al. (2009). Bioavailability of flavonoids: a review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr Drug Metab 10: 369–394. [DOI] [PubMed] [Google Scholar]
- Pecorari M, Villaño D, Testa MF, Schmid M, Serafini M (2010). Biomarkers of antioxidant status following ingestion of green teas at different polyphenol concentrations and antioxidant capacity in human volunteers. Mol Nutr Food Res 54 (Suppl 2): S278–S283. [DOI] [PubMed] [Google Scholar]
- Pelillo M, Biguzzi B, Bendini A, Gallina Toschi T, Vanzini M et al. (2002). Preliminary investigation into development of HPLC with UV and MS‐electrospray detection for the analysis of tea catechins. Food Chem 78: 369–374. [Google Scholar]
- Peluso I, Palmery M, Vitalone A (2015b). Green tea and bone marrow transplantation: from antioxidant activity to enzymatic and multidrug‐resistance modulation. Crit Rev Food Sci Nutr . doi:10.1080/10408398.2013.826175. [DOI] [PubMed] [Google Scholar]
- Peluso I, Teichner A, Manafikhi H, Palmery M (2015a). Camellia sinensis in asymptomatic hyperuricaemia: a meta‐analysis of tea or tea extract effects on uric acid levels. Crit Rev Food Sci Nutr . doi:10.1080/10408398.2014.889653. [DOI] [PubMed] [Google Scholar]
- Peng YH, Sweet DH, Lin SP, Yu CP, Lee Chao PD, Hou YC (2015). Green tea inhibited the elimination of nephro‐cardiovascular toxins and deteriorated the renal function in rats with renal failure. Sci Rep 5: 16226. doi:10.1038/srep16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price WE, Spitzer JC (1993). Variations in the amounts of individual flavonols in a range of green teas. Food Chem 47: 271–276. [Google Scholar]
- Qian F, Wei D, Zhang Q, Yang S (2005). Modulation of P‐glycoprotein function and reversal of multidrug resistance by (−)‐epigallocatechin gallate in human cancer cells. Biomed Pharmacother 59: 64–69. [DOI] [PubMed] [Google Scholar]
- Rajavelu A, Tulyasheva Z, Jaiswal R, Jeltsch A, Kuhnert N (2011). The inhibition of the mammalian DNA methyltransferase 3a (Dnmt3a) by dietary black tea and coffee polyphenols. BMC Biochem 12: 16. doi:10.1186/1471-2091-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechner AR, Wagner E, Buren van L, Put van de F, Wiseman S, Rice‐Evans CA (2002). Black tea represents a major source of dietary phenolics among regular tea drinkers. Free Radic Res 36: 1127–1135. [DOI] [PubMed] [Google Scholar]
- Roth M, Timmermann BN, Hagenbuch B (2011). Interactions of green tea catechins with organic anion‐transporting polypeptides. Drug Metab Dispos 39: 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang S, Lee MJ, Yang I (2008). Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data‐dependent acquisition. Rapid Commun Mass Spectrom 22: 1567–1578. [DOI] [PubMed] [Google Scholar]
- Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB et al. (2008). Safety of green tea extracts : a systematic review by the US Pharmacopeia. Drug Saf 31: 469–484. [DOI] [PubMed] [Google Scholar]
- Schönthal AH (2011). Adverse effects of concentrated green tea extracts. Mol Nutr Food Res 55: 874–885. [DOI] [PubMed] [Google Scholar]
- Sekine T, Miyazaki H, Endou H (2006). Molecular physiology of renal organic anion transporters. Am J Physiol Renal Physiol 290: F251–F261. [DOI] [PubMed] [Google Scholar]
- Serafini M, Del Rio D, Yao DN, Bettuzzi S, Peluso I (2011). Chapter 12. Health benefits of tea In: Benzie IFF, Wachtel‐Galor S. (eds). Herbal Medicine: Biomolecular and Clinical Aspects, 2nd edn. CRC Press/Taylor & Francis: Boca Raton (FL). [Google Scholar]
- Serafini M, Ghiselli A, Ferro‐Luzzi A (1996). (1996) In vivo antioxidant effect of green and black tea in man. Eur J Clin Nutr 50: 28–32. [PubMed] [Google Scholar]
- Shang W, Lu W, Han M, Qiao J (2014). The interactions of anticancer agents with tea catechins: current evidence from preclinical studies. Anticancer Agents Med Chem 14: 1343–1350. [DOI] [PubMed] [Google Scholar]
- Sharpe E, Hua F, Schuckers S, Andreescu S, Bradley R (2016). Effects of brewing conditions on the antioxidant capacity of twenty‐four commercial green tea varieties. Food Chem 192: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M, Quezada‐Calvillo R, Ferruzzi MG, Nichols BL, Hamaker BR (2015). Dietary phenolic compounds selectively inhibit the individual subunits of maltase‐glucoamylase and sucrase‐isomaltase with the potential of modulating glucose release. J Agric Food Chem 63: 3873–3879. [DOI] [PubMed] [Google Scholar]
- Sone T, Kuriyama S, Nakaya N, Hozawa A, Shimazu T, Nomura K et al. (2011). Randomized controlled trial for an effect of catechin‐enriched green tea consumption on adiponectin and cardiovascular disease risk factors. Food Nutr Res 55. doi:10.3402/fnr.v55i0.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AJ, Mullen W, Crozier A (2005). On‐line high‐performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol Nutr Food Res 49: 52–60. [DOI] [PubMed] [Google Scholar]
- Stickel F, Kessebohm K, Weimann R, Seitz HK (2011). Review of liver injury associated with dietary supplements. Liver Int 31: 595–605. [DOI] [PubMed] [Google Scholar]
- Stodt UW, Blauth N, Niemann S, Stark J, Pawar V, Jayaraman S et al. (2014). Investigation of processes in black tea manufacture through model fermentation (oxidation) experiments. J Agric Food Chem 62: 7854–7861. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Matsuo Y, Kouno I (2009). Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int J Mol Sci 11: 14–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Zheng JS, Fang L, Jin Y, Cai W, Li D (2015). Tea consumption and mortality of all cancers, CVD and all causes: a meta‐analysis of eighteen prospective cohort studies. Br J Nutr 114: 673–683. [DOI] [PubMed] [Google Scholar]
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A (2012). Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int 32: 1543–1556. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan JB, Walle T (2003). Cellular uptake and efflux of the tea flavonoid (−)epicatechin‐3‐gallate in the human intestinal cell line Caco‐2. J Pharmacol Exp Ther 307: 745–752. [DOI] [PubMed] [Google Scholar]
- Valcic S, Burr JA, Timmermann BN, Liebler DC (2000). Antioxidant chemistry of green tea catechins. New oxidation products of (−)‐epigallocatechin gallate and (−)‐epigallocatechin from their reactions with peroxyl radicals. Chem Res Toxicol 13: 801–810. [DOI] [PubMed] [Google Scholar]
- van Duynhoven J, van der Hooft JJ, van Dorsten FA, Peters S, Foltz M, Gomez‐Roldan V et al. (2014). Rapid and sustained systemic circulation of conjugated gut microbial catabolites after single‐dose black tea extract consumption. J Proteome Res 13: 2668–2678. [DOI] [PubMed] [Google Scholar]
- van het Hof KH, de Boer HS, Wiseman SA, Lien N, Westrate JA, Tijburg LB (1997). Consumption of green or black tea does not increase resistance of low‐density lipoprotein to oxidation in humans. Am J Clin Nutr 66: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Villaño D, Lettieri‐Barbato D, Guadagni F, Schmid M, Serafini M (2012). Effect of acute consumption of oolong tea on antioxidant parameters in healthy individuals. Food Chem 132: 2102–2106. [DOI] [PubMed] [Google Scholar]
- Vischini G, Niscola P, Stefoni A, Farneti F (2011). Increased plasma levels of tacrolimus after ingestion of green tea. Am J Kidney Dis 58: 329. [DOI] [PubMed] [Google Scholar]
- Wang CP, Wang X, Zhang X, Shi YW, Liu L, Kong LD (2010). Morin improves urate excretion and kidney function through regulation of renal organic ion transporters in hyperuricemic mice. J Pharm Pharm Sci 13: 411–427. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang Y, Wan X, Yang CS, Zhang J (2015). Green tea polyphenol (−)‐epigallocatechin‐3‐gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol Appl Pharmacol 283: 65–74. [DOI] [PubMed] [Google Scholar]
- Wang EJ, Barecki‐Roach M, Johnson WW (2002). Elevation of P‐glycoprotein function by a catechin in green tea. Biochem Biophys Res Commun 297: 412–418. [DOI] [PubMed] [Google Scholar]
- Wang H, Helliwell K, You X (2000). Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem 68: 115–121. [Google Scholar]
- Wang S, Sun Z, Dong S, Liu Y, Liu Y (2014). Molecular interactions between (−)‐epigallocatechin gallate analogs and pancreatic lipase. PLoS One 9: e111143. doi:10.1371/journal.pone.0111143 .eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang H, Holmgren A, Tian W, Zhong L (2008). Inhibitory effect of green tea extract and (−)‐epigallocatechin‐3‐gallate on mammalian thioredoxin reductase and HeLa cell viability. Oncol Rep 20: 1479–1487. [PubMed] [Google Scholar]
- Weerawatanakorn M, Hungb WL, Panc MH, Lib S, Lid D, Wand X et al. (2015). Chemistry and health beneficial effects of oolong tea and theasinensins. Food Sci Human Wellness 4: 133–146. [Google Scholar]
- Werba JP, Misaka S, Giroli MG, Yamada S, Cavalca V, Kawabe K et al. (2015). Overview of green tea interaction with cardiovascular drugs. Curr Pharm Des 21: 1213–1219. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Duffy SJ, Hamburg NM, Gokce N, Warden BA, Wiseman S et al. (2005). Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic Biol Med 38: 499–506. [DOI] [PubMed] [Google Scholar]
- Williamson G, Dionisi F, Renouf M (2011). Flavanols from green tea and phenolic acids from coffee: critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol Nutr Food Res 55: 864–873. [DOI] [PubMed] [Google Scholar]
- Wolfram RM, Oguogho A, Efthimiou Y, Budinsky AC, Sinzinger H (2002). Effect of black tea on (iso‐)prostaglandins and platelet aggregation in healthy volunteers. Prostaglandins Leukot Essent Fatty Acids 66: 529–533. [DOI] [PubMed] [Google Scholar]
- Wu X, He W, Yao L, Zhang H, Liu Z, Wang W et al. (2013). Characterization of binding interactions of (−)‐epigallocatechin‐3‐gallate from green tea and lipase. J Agric Food Chem 61: 8829–8835. [DOI] [PubMed] [Google Scholar]
- Yang X, Kong F (2016). Evaluation of the in vitro α‐glucosidase inhibitory activity of green tea polyphenols and different tea types. J Sci Food Agric 96: 777–782. [DOI] [PubMed] [Google Scholar]
- Yiannakopoulou EC (2015). Targeting DNA methylation with green tea catechins. Pharmacology 95: 111–116. [DOI] [PubMed] [Google Scholar]
- Yuda N, Tanaka M, Suzuki M, Asano Y, Ochi H, Iwatsuki K (2012). Polyphenols extracted from black tea (Camellia sinensis) residue by hot‐compressed water and their inhibitory effect on pancreatic lipase in vitro. J Food Sci 77: H254–H261. [DOI] [PubMed] [Google Scholar]
- Zeng JL, Li ZH, Wang ZC, Zhang HL (2014). Green tea consumption and risk of pancreatic cancer: a meta‐analysis. Nutrients 6: 4640–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Chan PT, Luk YS, Ho WKK, Chen ZY (1997). Inhibitory effect of jasmine green tea epicatechin isomers on LDL‐oxidation. J Nutr Biochem 8: 334–340. [Google Scholar]
- Zhang Y, Hays A, Noblett A, Thapa M, Hua DH, Hagenbuch B (2013). Transport by OATP1B1 and OATP1B3 enhances the cytotoxicity of epigallocatechin 3‐O‐gallate and several quercetin derivatives. J Nat Prod 76: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Asimi S, Wu K, Zheng J, Li D (2015). Black tea consumption and serum cholesterol concentration: systematic review and meta‐analysis of randomized controlled trials. Clin Nutr 34: 612–619. [DOI] [PubMed] [Google Scholar]
- Zheng XX, Xu YL, Li SH, Hui R, Wu YJ, Huang XH (2013). Effects of green tea catechins with or without caffeine on glycemic control in adults: a meta‐analysis of randomized controlled trials. Am J Clin Nutr 97: 750–762. [DOI] [PubMed] [Google Scholar]
- Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH (2011). Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta‐analysis of 14 randomized controlled trials. Am J Clin Nutr 94: 601–610. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li H, Zhou JG, Ma Y, Wu T, Ma H (2016). Green tea, black tea consumption and risk of endometrial cancer: a systematic review and meta‐analysis. Arch Gynecol Obstet 293: 143–155. [DOI] [PubMed] [Google Scholar]
- Zhu G, Hua J, Wang Z, She F, Chen Y (2015). Tea consumption and risk of gallbladder cancer: a meta‐analysis of epidemiological studies. Mol Clin Oncol 3: 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]


