Abstract
Polyphenols are widely regarded to have a wide range of health‐promoting qualities, including beneficial effects on cardiovascular disease. Historically, the benefits have been linked to their well‐recognized powerful antioxidant activity. However, the concept that the beneficial effects are attributable to direct antioxidant activity in vivo does not pay sufficient heed to the fact that polyphenols degrade rapidly, are poorly absorbed and rapidly metabolized, resulting in very low bioavailability. This review explores alternative mechanisms by which polyphenols, or their metabolites, exert biological activity via mechanisms that can be activated by physiologically relevant concentrations. Evidence is presented to support the action of phenolic derivatives on receptors and signalling pathways to induce adaptive responses that drive changes in endogenous antioxidant, antiplatelet, vasodilatory and anti‐inflammatory effects. The implications are that in vitro antioxidant measures as predictors of polyphenol protective activity in vivo hold little relevance and that closer attention needs to be paid to bioavailable metabolites to understand the mode of action of these diet‐derived components.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- A549
adenocarcinomic human alveolar basal epithelial cells
- CD62P
P‐selectin
- CSE
cigarette smoke extract
- EGCG
epigallocatechin‐3‐gallate
- eNOS
endothelial NOS
- ER‐α/β
oestrogen receptor‐α/β
- GR
glutathione reductase
- GSL
glutamyl‐cysteine ligase
- GST
GSH‐S‐transferase
- HO1
haem oxygenase 1
- ICAM‐1
intercellular adhesion molecule 1
- IκB‐α
inhibitor of κ‐B α
- Nrf2
nuclear factor E2‐related factor 2
- ˙OH
hydroxyl radical
- ONOO−
peroxynitrite
- ox‐LDL
oxidized LDL
- VCAM‐1
vascular cell adhesion molecule 1
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | eNOS |
| TNF‐α | ERK1 |
| GPCRs b | ERK2 |
| ETA receptors | GR, glutathione‐disulfide reductase |
| ETB receptors | GSK3β |
| Nuclear hormone receptors c | HO 1 |
| ER‐α | MAPK |
| ER‐β | PI3K |
| Enzymes d | Src |
| AKT serine/threonine kinase 2 | PKC |
| COX‐1 | PKA |
| COX‐2 |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a,b,c,d).
Introduction
Polyphenols have long been recognized to provide health benefits, but their reputation has been boosted recently following a number of encouraging clinical studies in a range of disease profiles that appear to confirm efficacy. Increased consumption of fruit and vegetables that are rich in polyphenolic compounds is known to be associated with health benefits related to cardiovascular function. For example, the Prevención con Dieta Mediterránea study found that risk of cardiovascular disease (CVD) was reduced by 46% in individuals with a diet rich in polyphenols (Tresserra‐Rimbau et al., 2014). Other published reports show that polyphenolics can improve endothelial function (Vita, 2005), inhibit abnormal platelet aggregation (Tangney and Rasmussen, 2013), reduce inflammation and improve plasma lipid profiles (Arranz et al., 2012), thereby offering protection to cardiovascular health at a number of levels. However, despite numerous studies conducted in the field, the mechanisms through which these compounds exert cardioprotective actions are not yet fully understood, and, as a result, the link between cardiovascular benefits of particular diets and their polyphenolic content is not strictly proven.
For decades, phenolic compounds have been recognized to have powerful free radical scavenging activities, determined by specific structural features, such as the number and position of hydroxyl and catechol units (Fraga et al., 2010; Castellano, 2012). The best known polyphenolic antioxidants are delphinidin, cyanidin, pelargonidin, peonidin, malvidin (anthocyanins), quercetin, keampferol, myricetin, morin, luteolin (flavonols), gallic acid, caffeic acid, syringic acid, protocatechuic acid (phenolic acids), catechin, epicatechin, epicatechin gallate, epigallocatechin gallate (flavanols), ellagic acid and curcumin (Figure 1) (Duthie et al., 2003; Kahkonen and Heinonen, 2003; Lianda et al., 2012). Many aspects of cardiovascular disease are associated with oxidative stress – the excessive production of pro‐oxidants and/or depression of counteractive endogenous antioxidant systems. Polyphenols are believed to be able to reduce the prevalence of various biomarkers of oxidative stress. It is the association between antioxidant properties of polyphenolic compounds with reduced risk of CVD that has dominated the literature in this area, although a direct causal link has always been assumed rather than conclusively proven (Jacob and Burri, 1996; Schneider et al., 1996; Wan et al., 2001; Acquaviva et al., 2002; Borbalan et al., 2003; Lugasi, 2003).
Figure 1.
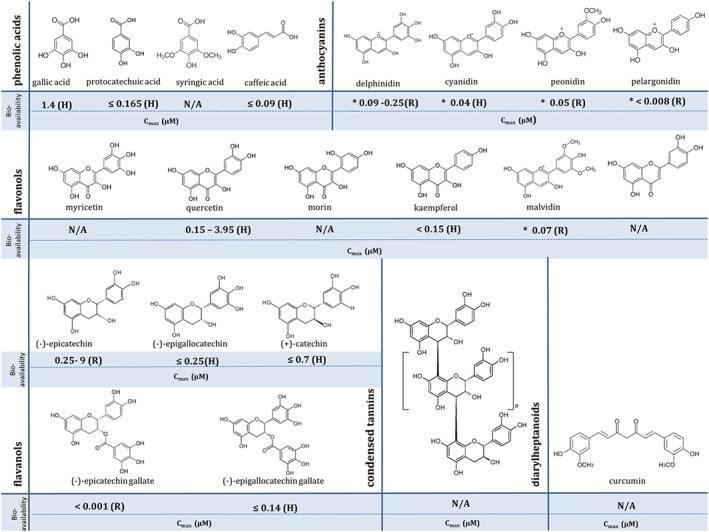
Chemical structures of polyphenols of high antioxidant potential, including their bioavailability in plasma, analysed without enzymic conjugation. Data were obtained from Phenol‐Explorer database (www.phenol‐explorer.eu). (H) – Cmax data obtained from human studies; (R) – Cmax data obtained from animal studies – rats; *presented values correspond to the following glycosides: delphinidin‐3‐O‐rutinoside, cyanidin‐3‐O‐glucoside, peonidin‐3‐O‐glucoside and malvidin‐3‐O‐glucoside; N/A‐ data not available.
The reason for doubt over the seemingly obvious link between strong antioxidant activity of polyphenols in vitro and reduction in oxidative stress in vivo is the very poor bioavailability of native polyphenols because of their extracellular decomposition, poor absorption and rapid metabolism: Bioavailable concentrations of polyphenolic compounds are simply too low to mediate direct antioxidant activity in vivo (i.e. to act as chemical scavengers of radicals). In addition, a considerable number of studies support the hypothesis that polyphenols can oxidize readily in beverages, tissue culture media and phosphate buffers, with the potential to cause paradoxical adverse effects in vivo through pro‐oxidative activity (Babich et al., 2008; Prochazkova et al., 2011), thus raising the spectre of counter‐intuitive toxicity with very high consumption of polyphenols (Martin and Appel, 2010). However, the pro‐oxidant activity of phenolic compounds may also prove to be beneficial at moderate concentrations because, by inducing a mild degree of oxidative stress, they can activate intracellular antioxidant defence mechanisms (Moskaug et al., 2005; Nabavi et al., 2016; Scapagnini et al., 2011). Moreover, it has become clear that the mechanism of action of polyphenols goes beyond the single action of the modulating of oxidative stress: Their role as mediators in cell–cell signalling, receptor activation and gene regulation in vivo adds interesting dimensions to their complexity and to their scope for preventative and therapeutic applications (Scalbert et al., 2005; Schewe et al., 2008). Thus, polyphenols are likely to contribute to cardioprotection, but their direct antioxidant effects might only play a very minor role, if any at all. The characteristics that are more likely to determine their in vivo efficacy are their stability under physiological conditions, their rate of absorption, metabolism and excretion, their metabolic products and the pharmacological targets (receptors, enzymes and nuclear factors) of either the original polyphenols or their metabolites, as opposed to their in vitro antioxidant potential.
Although increased consumption of polyphenol‐rich foods has been associated with reduced risk of many diseases, including cancer, diabetes and neurodegenerative disorders, this review will focus only on the role of polyphenols in cardiovascular health in light of these newly emerging trends.
CVD and oxidative stress
CVD is a global term used for the group of diseases affecting the heart and/or blood vessels and includes coronary artery disease, cerebrovascular disease, peripheral artery disease, congenital heart disease, hypertension, heart failure and stroke (Nicholson et al., 2008). The incidence rate of CVD has dramatically increased in the past three decades: In 2014, CVD, together with cancer, was the biggest cause of death in the UK, causing 28 and 29% of all deaths in women and men respectively. Coronary heart disease accounted for 45% of all CVD deaths, while 25% of deaths were stroke‐related (Jin et al., 2011). Coronary artery disease and ischaemic stroke, as well as peripheral artery disease, are underpinned by a common pathological process – atherosclerosis (Le Brocq et al., 2008). Atherosclerosis is a multi‐factorial, progressive disorder of medium‐sized and large conduit arteries, which is fuelled by deposition of modified lipids in the vessel wall (Falk, 2006; Megson et al., 2016). Age, smoking, hyperlipidaemia, hypertension and diabetes are risk factors for the disease. Inflammation, oxidative stress and endothelial dysfunction are strongly associated with the atherogenic process (Le Brocq et al., 2008; Loke et al., 2010), while endothelial cells, smooth muscle cells, neutrophils, macrophages and platelets are all potential sources and targets of oxidants (Park and Kim, 2012). Generation of oxidants occurs during physiological processes, such as cellular respiration and metabolism, and is strictly regulated by antioxidant defence mechanisms in healthy cells (Sies, 1997). However, prolonged exposure to stress (Bouayed et al., 2009), pollution (Lodovici and Bigagli, 2011), smoking and excessive drinking (Barreiro et al., 2010; Galicia‐Moreno and Gutiérrez‐Reyes, 2014), as well as ageing (Finkel and Holbrook, 2000), results in an imbalance of oxidative species (also known as reactive oxygen species; ROS) in excess of the endogenous defences – so‐called oxidative stress (Sies, 1997; Khurana et al., 2013).
Many functions of the endothelium are affected by ROS (Nicholson et al., 2008). The best recognized is endothelium‐dependent vasorelaxation, which is impaired by a loss of NO bioactivity and/or bioavailability (Hopkins, 2013). NO is a powerful vasodilator that also acts to prevent inflammatory cell activation and adhesion (Taniyama and Griendling, 2003). In the presence of the superoxide radical (O2 .−), endothelium‐derived NO rapidly reacts to form peroxynitrite (ONOO−), which acts as a powerful oxidant, and is also harmful to endothelial cells (Curtin et al., 2002). Prolonged exposure of endothelial cells to O2 .−, H2O2, ONOO− and/or oxidized LDL (ox‐LDL) induces apoptosis (Le Brocq et al., 2008), which leads to cell damage and loss, a key early event in atherogenesis. Atherosclerotic lesions start to develop under an intact but leaky, activated and dysfunctional endothelium (Falk, 2006). LDL ordinarily diffuses freely across the damaged endothelium in both directions. However, under oxidative stress, LDL undergoes peroxidation to ox‐LDL, becoming cytotoxic and pro‐inflammatory. Meanwhile, damaged or activated endothelial cells express adhesion molecules, primarily vascular cell adhesion molecule 1 (VCAM‐1), that bind monocytes and T cells prior to transmigration into the vessel wall (Gerhardt and Ley, 2015). The monocytes become activated and differentiate into macrophages, ultimately becoming engorged with ox‐LDL taken up via scavenger receptor‐mediated phagocytosis, forming so‐called fatty streaks in the vessel wall (Martinez‐Cayuela, 1995; Le Brocq et al., 2008; Hopkins, 2013). Lipid engorged macrophages (foam cells) ultimately undergo pro‐inflammatory necrotic cell death in situ, contributing to the formation of a soft and destabilizing lipid‐rich core within atherosclerotic plaques (Singh et al., 2002). Disease progression can terminate in plaque stabilization through smooth muscle cells secreting a collagen‐rich matrix containing fibroblasts to create a protective cap over the plaque. However, prolonged inflammation can lead to unstable plaques that are prone to rupture (Singh et al., 2002). Such ruptured plaques induce a rapid thrombotic response, leading to vessel occlusion and heart attacks, ischaemic strokes or peripheral ischaemia, depending on the site of the atherosclerotic lesion (Falk, 2006).
Oxidative stress represents a key feature of the progression of atherosclerosis, influencing both the oxidative modification of LDL and the dysfunction of the endothelium which are central to the aetiology of the disease. However, it is important to recognize that, while oxidative stress is a valid therapeutic target for prevention and treatment strategies, the role of inflammation should not be overlooked.
Polyphenols
Polyphenols are the most widespread class of plant secondary metabolites (Falk, 2006; Tsao, 2010; Castellano, 2012), where they are involved in defence against ultraviolet radiation, cold temperatures or drought, as well as contributing to the colour of leaves, berries and fruits. They also act as antifeedants and toxins that assist plants in their defence against herbivores, parasites and pathogens (Gould et al., 2009). Approximately 8000 phenolic structures have been identified so far, several hundred of which are found in edible plants (Perez‐Jimenez et al., 2010; Tsao, 2010) (Figure 2). Polyphenols are characterized by the presence of several phenolic groups (aromatic rings) (Manach et al., 2004). They are highly diverse and can be divided into several sub‐groups, depending on the number of phenol rings that they contain and of the structural elements that bind these rings to one another (Quideau et al., 2011) (Figure 2).
Figure 2.
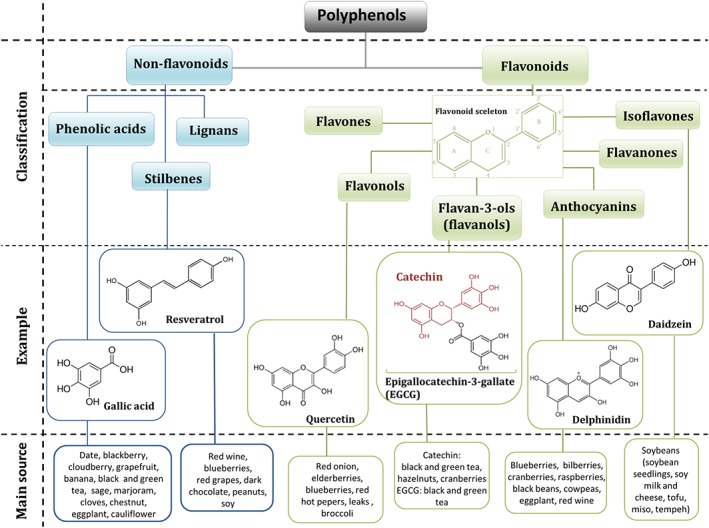
Polyphenol classification. Classes of polyphenols with examples that exhibit possible cardioprotective effects.
Polyphenols as antioxidants
Historically, polyphenol‐induced benefits have been largely attributed to the known ability of polyphenols to act as powerful antioxidants. Indeed, under in vitro conditions, phenolic compounds can readily donate an electron or H atom from an aromatic hydroxyl group to a free radical, thereby ‘neutralizing’ it. Direct antioxidant properties of polyphenols depend on the arrangement of functional groups in their core structure (Prochazkova et al., 2011). The antioxidant capabilities of polyphenols are complex: The hydroxylation patterns – such as the 3‐hydroxy group in flavanols, or electron deficiency in the case of anthocyanins – as well as the presence of catechol groups – are important in the antioxidant activities (Kahkonen and Heinonen, 2003; Hanif et al., 2008). The in vitro antioxidant activity of several polyphenols is comparable to that of vitamins C and E (Gardner et al., 1998; Prior and Cao, 2000). Polyphenols have been reported to scavenge ROS and reactive nitrogen species, including O2 .−, H2O2, hydroxyl radicals (.OH), hypochlorous acid and NO (Tsao, 2010). Moreover, they can act as direct radical scavengers of the peroxidation products of lipids, proteins, DNA and RNA (Quideau et al., 2011). Additionally, polyphenols can act as metal ion chelators, thereby reducing the rate of Fenton reactions and formation of highly damaging .OH (Fraga et al., 2010).
Despite their excellent antioxidant activity in vitro, the evidence in support of direct antioxidant activity of polyphenols in vivo is weak. For example, in the blood, plasma levels of unconjugated polyphenols rarely exceed 1 μM and, moreover, the products of polyphenol metabolism tend to have lower antioxidant capacity, because the radical‐scavenging −OH groups are blocked by methylation, sulfation and glucuronidation (Pollard et al., 2006). In the context of plasma, where highly abundant proteins, thiols, uric acid and vitamin C already constitute a formidable antioxidant barrier, any phenolic contribution is negligible (Turell et al., 2013). Therefore, the concept that the observed increases in antioxidant capacity of plasma after consumption of polyphenol‐rich foods is directly attributable to increased polyphenol load is implausible. It is much more likely that other readily absorbed dietary components ingested alongside polyphenols, such as vitamin C, vitamin E (Cao et al., 1998) or even fructose because of a recognized interaction with uric acid (Lotito and Frei, 2004), are responsible for the observed effect of fruit and vegetable rich diets on plasma antioxidant capacity.
Polyphenols as pro‐oxidants
Despite the fact that pro‐oxidant activities of polyphenols were reported nearly three decades ago (Tulyathan et al., 1989), more attention has been paid to their widely described antioxidant capacities. Spontaneous oxidation of a common phenolic metabolite, gallic acid, leads to generation of a variety of highly reactive species, including O2 .−, H2O2, quinones and semiquinones (Gil‐Longo and Gonzalez‐Vazquez, 2010). Epigallocatechin‐3‐gallate (EGCG), epicatechin‐3‐gallate (Severino et al., 2009; Lambert and Elias, 2010), quercetin (Lapidot et al., 2002), theaflavin (Babich et al., 2008) and a variety of plant extracts, including apple (Bellion et al., 2009), pomegranate (Weisburg et al., 2010), black and green tea extracts (Severino et al., 2009), as well as red wine (Elias et al., 2009) generate ROS, and H2O2 in particular. The extent of pro‐oxidant activity of polyphenols is dependent on the polyphenol in question, its concentration and the conditions of the environment (Babich et al., 2008; Bellion et al., 2009). The mechanism by which oxidation takes place remains equivocal, although reduction of iron and copper ions might help to promote Fenton chemistry and pro‐oxidant activities of polyphenols (Gil‐Longo and Gonzalez‐Vazquez, 2010; Martin and Appel, 2010).
The relevance of polyphenol oxidation under physiological conditions remains unclear, but the products of polyphenol auto‐oxidation have been shown to be toxic in human lung carcinoma cells (H460) (Leung et al., 2007). However, the concentrations used in these studies (50–80 μM) were supraphysiological, and it is reasonable to assume that physiological levels are non‐toxic. Indeed, the potential toxicity of auto‐oxidation products of polyphenols is likely to be the reason for the low absorption in the gut and the rapid conjugation and metabolism of absorbed polyphenols as a means of detoxification.
Polyphenols as xenobiotics (oral bioavailability, metabolism and clearance)
The way polyphenols are handled by the body is characteristic of that for xenobiotics – substances that are not normally found in vivo that can become toxic without appropriate metabolism and excretion (Croom, 2012; Cardona et al., 2013). Thus, regardless of the amount of ingested polyphenol‐rich food, the bioavailability of the native polyphenols tends to be maintained in the nM to low μM range (D'Archivio et al., 2007; Mazza, 2007).
Absorption
Polyphenols can be found in wide range of fruit and vegetables. In some plants, their concentration can be as high as 750 mg·100 g−1 of fruit (Manach et al., 2004; Bohn, 2014). Berries, whole‐grain cereals, cacao, tea, coffee and red wine are common rich dietary sources of polyphenols. Depending on diet, gender and other socio‐economic factors, the total daily intake of polyphenols is around 1 g a day (Scalbert and Williamson, 2000; Grosso et al., 2014; Mullie, 2014).
It has been estimated that only 1–10% of total polyphenol intake is found in plasma and urine samples (Duthie et al., 2003; D'Archivio et al., 2007; Nicholson et al., 2008). Bioavailability of polyphenols is first determined by their rate, site and means of absorption. Moreover, direct interaction between polyphenols and other compounds and food components, such as proteins, carbohydrates, fibre, fat and alcohol, can also affect their absorption (D'Archivio et al., 2007). Maximal concentrations are usually reached within 0.5–2 h after ingestion, falling to baseline levels within 8–12 h (Beattie et al., 2005). Just as with drugs, attainment of steady‐state conditions requires regular, frequent, repeated ingestion (dosing). Most polyphenols (except flavanols) are present in food as glycosides – esters or polymers of very complex structures and high molecular weight, limiting their absorption in their native form (Manach et al., 2004). For instance, the molecular weight of proanthocyanidins, oligomers of catechin, epicatechin and their gallic acid esters, ranges from 500 to 20 000 g·mol−1 (Sepúlveda et al., 2011). Most polyphenols remain relatively stable at the low pH experienced in the stomach and resist acid hydrolysis, therefore facilitating their transit to the small intestine intact. Only anthocyanin glycosides are absorbed in both the stomach and small intestine without modification, but the rate of absorption is limited by the type of sugar moiety attached (Mazza, 2007; Hassimotto et al., 2008; Wiczkowski et al., 2010). The remaining intact polyphenols that reach the small intestine undergo hydrolysis catalysed by lactase phloridizin hydrolase present in the brush‐border of the epithelial cells in the small intestine. The released aglycones (lacking their sugar moiety) are then capable of entering the epithelial cells by passive diffusion because of their increased lipophilicity (Del Rio et al., 2013). However, only some glycosides are hydrolysed in the small intestine. Those polyphenols linked to a rhamnose, arabinose and xylose moieties, as well as compounds with more complex structures (e.g. tannins) reach the colon, where they are hydrolyzed by the microflora before absorption can occur (Manach et al., 2004). The colonic microbiota are responsible for extensive breakdown of complex polyphenols, leading to the release of low molecular weight phenolic metabolites, such as phenolic acids and urolithins, that only now are available for absorption. However, the rate of absorption in the colon is lower than that in the small intestine. Unabsorbed polyphenols are excreted from the body in faeces (Scalbert and Williamson, 2000).
In addition to the poor absorption of polyphenols, it is important to recognize that some are intrinsically prone to decomposition in aqueous medium. Anthocyanins, for instance, are known to be very unstable in tissue culture medium. Compared to other anthocyanins, delphinidin has the lowest stability in tissue culture medium, with substantial degradation to gallic acid and aldehyde being found as early as 30 min in solution (Kay et al., 2009; Woodward et al., 2009). Pelargonidin is the most stable anthocyanin (Kern et al., 2007). Degradation of anthocyanins, as well as the other polyphenols, can be accelerated by light, pH and temperature, as well as by the composition of accompanying substances (enzymes, proteins and other flavonoids) and the redox environment (He and Giusti, 2010; Fang, 2014). For example, the stability of resveratrol is affected by light and alkaline pH (Trela and Waterhouse, 1996). Similarly, artemetin (another flavonoid), is characterized by low stability at room temperature (Weathers and Towler, 2012). Moreover, cocoa flavanols [(−)‐epicatechin, (+)‐catechin] and their dimers are highly unstable in simulated intestinal fluid or alkaline pH (Zhu et al., 2002). Ultimately, the identity of the phenolic compounds that persist in the lumen of the gut is an important consideration in determining the nature and bioactivity of the phenolic derivatives that eventually reach the bloodstream.
Metabolism and excretion
Bioavailable polyphenols can be found in plasma in both their native intact form and the glucuronidated and/or methylated forms, with sulfo‐conjugates being less common (Matsumoto et al., 2006). Extensive conjugation occurs on first pass through the liver. This metabolic detoxification process is common to many xenobiotics, acting to prevent potential toxic effects, and is followed by urinary elimination because of the increased hydrophilicity of the conjugates (D'Archivio et al., 2007). These conjugation mechanisms, together with low stability of many polyphenols under physiological conditions, contribute to the very low concentrations of aglycones found in the blood, even in individuals on a polyphenol‐rich diet. All forms of polyphenols, however, are rapidly excreted from the body. The maximal urine concentration of polyphenols is often attained within 2–4 h after ingestion (Jin et al., 2011).
Antioxidant therapies in CVD
It is well recognized that diets rich in fruit and vegetables promote health and attenuate or delay the onset of CVD (López‐Sepúlveda et al., 2011). The cardio‐protective effects of such dietary interventions have been associated with a wide variety of chemical constituents of fruit and vegetables, many of which are considered to be powerful antioxidants, such as vitamins A, C and E, (Beckman et al., 2001; Otero et al., 2005; Hozawa et al., 2007). Given that oxidative stress is a key feature of atherogenesis, antioxidant therapy is a potential option (Nicholson et al., 2008). However, some intervention trials have failed to find a correlation between antioxidant vitamin consumption and reduced CVD (Lonn et al., 2005). Therefore, interest has been directed towards other bioactive compounds found in fruit and vegetables, namely, polyphenols (Duthie et al., 2003) that might mediate the benefits independent of the abundant antioxidants. Epidemiological studies have also shown that polyphenols found in berries (Ellingsen et al., 2008; Li et al., 2015), chocolate (Jumar and Schmieder, 2016; Larsson et al., 2016), coffee (Grosso et al., 2016) and red wine (Cosmi et al., 2015) are associated with slower CVD progression. However, given their low bioavailability, the assumption that the observed health beneficial effects are driven by their direct antioxidant activity (chemical antagonism) seems very unlikely. Nevertheless, further studies have reported that properties other than antioxidant activity might underpin the benefits of polyphenols in the setting of CVD. The ability of native polyphenols and/or their metabolites to interact with enzymes, transcription factors (Aggarwal et al., 2006; Kode et al., 2008) and receptors (Chalopin et al., 2010; Grossini et al., 2015) strongly suggests that they might act as signalling molecules and be able to express their beneficial effects at a molecular level (Fraga et al., 2010; Loke et al., 2010).
Polyphenols as pharmacological agents
Collectively, studies have demonstrated that dietary polyphenols are biologically active substances, with therapeutic effects in cells and/or tissues. Phenolic compounds provide a wide spectrum of bioactivities: Aside from their broadly described free radical scavenging properties, the existence of both hydrophobic and hydrophilic domains within polyphenols enables them to potentially interact with, and diffuse through biological membranes, and to bind to receptors and enzymes to exert intracellular signalling effects (Bennick, 2002).
Indirect antioxidant activity
One of the hypotheses that has emerged to explain the antioxidant effects exerted by polyphenols is that they act as mild toxins and stimulate a general xenobiotic and/or antioxidant response in the target cells, activating many defence genes. Phenolic compounds can activate the nuclear factor E2‐related factor 2 (Nrf2)/antioxidant responsive element pathway, thereby leading to induction to detoxifying enzymes, such as glutathione (GSH)‐S‐transferase (GST), NADPH: quinone oxidoreductase 1, haem oxygenase 1 (HO1) and glutamyl‐cysteine ligase (GSL) (Figure 3) (Johnson et al., 2008; Nabavi et al., 2016; Scapagnini et al., 2011). Similar findings have been found for the green tea polyphenol, EGCG: Concentrations of 20–100 μM have been shown to induce HO1 expression in rat neurons (H‐19‐7), possibly via activation of transcription factor Nrf2 (Romeo et al., 2009). Moreover, curcumin (5–15 μM; the major component of the spice turmeric) has been reported to reduce hemin‐induced oxidative stress in primary cultures of cerebellar granule neurons in rats, as well as to increase intracellular GSH, expression of HO1, GSH reductase (GR), GST and superoxide dismutase, all of which might be mediated by Nrf2 activation (Gonzalez‐Reyes et al., 2013). Artemetin (10 pM and 1 μM) protected endothelial cells against H2O2‐induced oxidative stress by increasing GSH synthesis (Grossini et al., 2015). Kode et al. (2008) reported similar findings in human primary small airway epithelial and human alveolar epithelial (A549) cells. Resveratrol (10 μM) reduced cigarette smoke extract (CSE)‐induced ROS production in small airway epithelial and A549 cells by restoring CSE‐depleted GSH levels and up‐regulating GSL via activation of Nrf2 (Kode et al., 2008).
Figure 3.
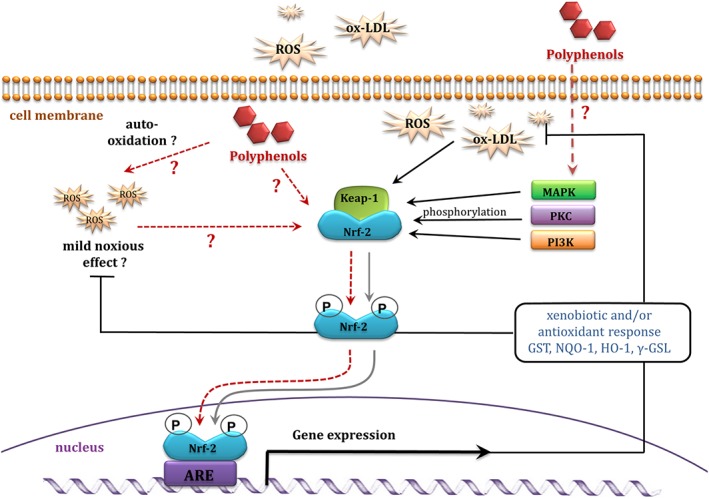
Indirect antioxidant activity of polyphenols mediated via gene expression – a proposed mechanism. ARE, antioxidant responsive element.
Activation of antioxidant defence mechanisms can occur at two different levels: Firstly, free radicals produced by pro‐oxidant anthocyanins might activate protein kinases (e.g. PI3K and PKC), that subsequently up‐regulate transcription factor Nrf‐2; Secondly, the compounds themselves might act as signalling molecules, interacting with protein kinases, thus inducing intracellular signalling cascades (Figure 3).
Vasodilation
Anthocyanin glycosides found in elderberry extract can be incorporated into the plasma membrane and to a lesser extent, the cytosol of endothelial cells (Youdim et al., 2000). Structural similarities of certain anthocyanins to oestrogen (17‐β‐oestradiol) are responsible for their binding affinity to the oestrogen receptors α and/or β (ER‐α/β) (Figure 4) (Fraga et al., 2010; Hidalgo et al., 2012; Zhang et al., 2013; Grossini et al., 2015). Delphinidin aglycone (17 μM) can induce endothelium‐dependent vasodilatation in aorta in mice through activation of ER‐α, leading to increased endothelial NOS (eNOS) activity and increased synthesis of the anti‐atherogenic vasodilatory mediator, NO. The PI3K/Akt and Src/ERK1/2 signalling pathways have both been implicated in delphinidin‐mediated vasodilatation in endothelial cells (Figure 5) (Marino et al., 2006; Chalopin et al., 2010; Lopez‐Sepulveda et al., 2011). In support of the relevance of this potential mode of action of polyphenols is the fact that physiologically relevant concentrations can invoke this activity: For example, isoflavones (genistein and daidzein) exhibit oestrogenic activity at ~0.1 μM (Kuiper et al., 1998). However, the aglycone form of delphinidin that has been found to mimic 17‐β‐oestradiol activity is unlikely to be found in plasma because of its rapid degradation, which undermines the concept, at least for delphinidin.
Figure 4.
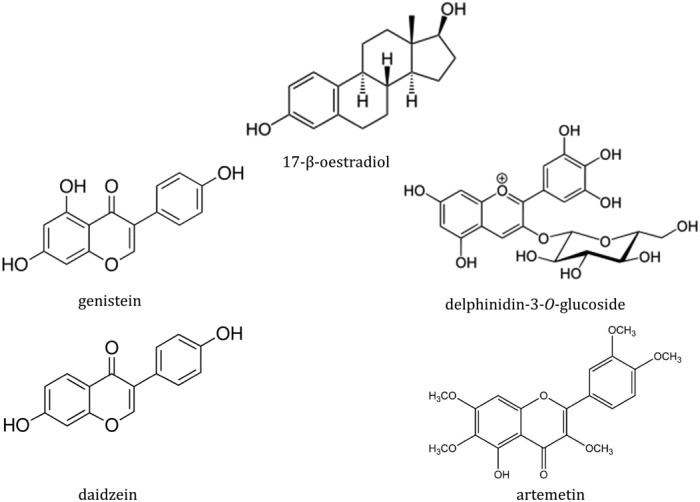
17‐β‐oestradiol and phytoestrogens.
Figure 5.
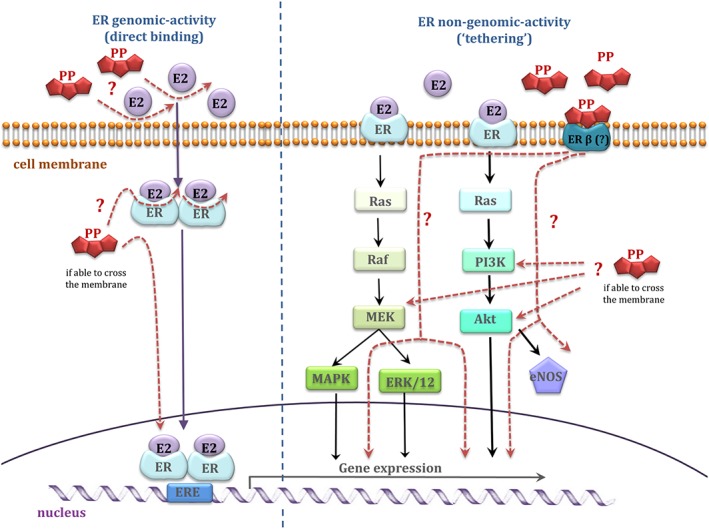
Oestrogen receptor and polyphenols – a proposed mechanism of action, taking into account two main ERs regulatory actions; classical (direct) and tethered pathway.
Hidalgo et al. (2012) found that delphinidin‐3‐O‐glucoside (the form which is more stable than the aglycone), pelargonidin‐3‐O‐glucoside, gallic acid and genistein have higher affinity to ER‐β than ER‐α (Kuiper et al., 1998; Hidalgo et al., 2012). Moreover, differential tissue distribution of the ER‐α (uterus, ovary, testis, skin and gut) and ER‐β receptors (fetal ovaries, testes, adrenals and spleen) suggests that these compounds may be tissue selective (Brandenberger et al., 1997).
Interestingly, Grossini et al. (2015) demonstrated that artemetin (the main phenolic component of herbs Artemisia absinthium and Achillea millefolium) at physiologically relevant concentrations (10 pM–100 μM) increased eNOS‐dependent NO production in porcine aortic endothelial cells through involvement of oestrogen receptors and activation of PKA, ERK1/2 and the Akt pathway. Similar effects were observed for genistein (1 μM) (Grossini et al., 2015). However, the relative instability of artemetin was not taken into consideration in these studies. Artemetin was found to be poorly extracted from Artemisia annua plant and was highly unstable in a tea infusion at room temperature (Weathers and Towler, 2012). There was no information given on its stability under physiologically relevant conditions.
The need for utilizing metabolites and degradation products of potentially bioactive polyphenols in in vitro studies is increasing. Zhang et al. (2013) have shown that S‐(−)equol (10 nM–250 nM), a metabolite of isoflavone daidzein, mimics the effects of its parent compound and activates the PI3K/Akt pathway through ER‐β receptors, increasing Nrf2 expression, an important factor in maintaining vascular redox homeostasis (Zhang et al., 2013). Alternatively, polyphenols can promote vasodilation through inhibition of the release of the vasoconstricting peptide, endothelin‐1 (ET‐1). Delphinidin and, to a lesser extent, cyanidin aglycone (50–100 μM) inhibited ET‐1 synthesis in HUVECs, with a simultaneous increase in eNOS expression (Lazze et al., 2006; Matsumoto et al., 2005). Pretreatment with delphinidin‐3‐O‐rutinoside (10 μM) exerted an inhibitory effect on ET‐1 induced contraction in bovine ciliary smooth muscle. Moreover, delphinidin‐3‐O‐rutinoside promoted vasodilation via stimulation of ETB receptors leading to NO production and activation of the cGMP pathway (Matsumoto et al., 2005). In addition, delphinidin glycosides inhibited the activity of other vasoconstrictor systems, such as angiotensin converting enzyme, but the concentrations required to cause this effect were unlikely to be achieved in vivo (IC50 ~ 65 μM) (Hidalgo et al., 2012).
Anti‐platelet activity
Phenolic compounds exhibited a range of inhibitory effects on platelet activation, related signal transduction pathways, enhancement of NO production and inhibition of receptors such as those for thromboxane A2 (TXA2) (Figure 6).
Figure 6.
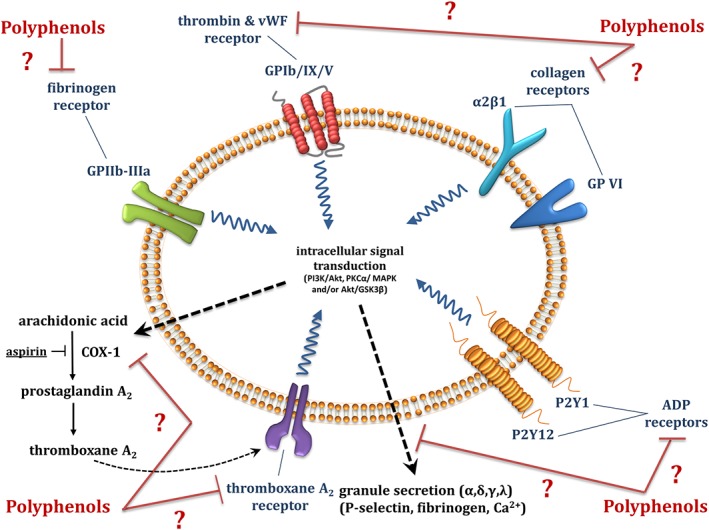
Proposed anti‐platelet mechanism of action of polyphenols.
An in vitro study by Yang et al. (2012) demonstrated that delphinidin‐3‐O‐glucoside inhibited platelet aggregation in platelet‐rich plasma and purified platelets from humans and mice, induced by collagen, thrombin, thrombin receptor activating peptide and ADP. Substantial effects were observed at supraphysiological concentrations (50 μM), but concentrations as low as 0.5 μM also had a modest but significant effect. Similar concentrations were found to significantly inhibit thrombus formation under high and low shear. However, only 5 and 50 μM delphinidin‐3‐O‐glucoside significantly inhibited expression of platelet activation markers, such as P‐selectin, CD63 and the CD40 ligand in purified platelets (Yang et al., 2012). Furthermore, 5 μM delphinidin was the threshold concentration for inhibition of fibrinogen binding (Yang et al., 2012). Moreover, quercetin (25–100 μM) inhibited collagen‐induced fibrinogen binding to its receptor, perhaps by causing conformational changes to the GPIIb IIIa receptor, thus reducing the affinity of fibrinogen for the receptor binding site (Oh et al., 2012). Similar findings have been reported for nobiletin (6.25–200 μM), a flavonoid found in citrus fruit (Vaiyapuri et al., 2015).
In contrast, delphinidin, petunidin and malvidin glycosides (50 μM) failed to inhibit collagen‐induced platelet aggregation in human whole blood samples (Garcia‐Alonso et al., 2004). The discrepancy, however, might be a result of some differences in methodology used, such as different pre‐incubation times with the target polyphenol. Another anthocyanin, cyanidin‐3‐O‐glucoside (5 and 50 μM), reduced platelet aggregation in healthy and hypercholesterolaemic patients by inhibition of platelet α‐, δ‐ and γ‐ granule secretion, as evaluated by P‐selectin, the chemokines CCL5 and CXCL4, β‐thromboglobulin, TGF‐β1, 5‐HT, ATP and CD63 release. The mechanism of action proposed by Song et al. (2014) is activation of the PI3K/Akt signalling pathway (Figure 6) (Song et al., 2014). Meanwhile, resveratrol inhibited collagen‐induced platelet aggregation by stimulating platelet NO production and reducing platelet ROS production, when present at physiologically relevant concentrations (0.1–1 μM) (Messina et al., 2015). Simple phenolic acids, such as gallic acid, also inhibit platelet aggregation and activation via inhibition of the phosphorylation of PKCα/p38 MAPK and Akt/GSK3β (Chang et al., 2012). Similar findings were obtained for hippuric acid, the predominant metabolite of some phenolic acids and polyphenols, which reduced platelet aggregation and P‐selectin/CD62P expression at 1–2 mM. Hippuric acid, however, is actively excreted from the body with maximal plasma concentrations of ~200–300 μM (Santhakumar et al., 2015). Meanwhile, pelargonidin aglycone, but not the glucoside adduct, inhibited thrombin‐induced fibrin polymerization and platelet aggregation and elicited anticoagulant effects in mice. As shown previously, the concentrations used were not attainable physiologically and were ≥10 μM (Ku et al., 2016). Another in vitro study by Macakova et al. (2012) reported that differences in chemical structure of several 4‐methylcoumarin analogues (absence of hydroxyl group, or altered hydroxyl group at position at C‐5, and different substitutions at C‐3) determined their anti‐platelet function. The 5,7‐dihydroxy‐4‐methylcoumarins, especially those with a lipophilic side chain at C‐3, had anti‐platelet activity that was similar to that of acetylsalicylic acid (aspirin) in arachidonic acid‐induced platelet aggregation. Interestingly, only synthetic coumarins, but not the native equivalents (no substitutions on C‐5 and C‐3), inhibited platelet aggregation. The most effective was synthetic 3‐ethoxycarbonylethyl‐5,7‐dihydroxy‐4‐methylcoumarin at 10 μM (Macakova et al., 2012). Because the effect of this compound was specific to aggregation induced by arachidonic acid, the mechanism of action proposed was inhibition of COX 1 and competitive antagonism at TXA2 receptors (Macakova et al., 2012).
Polyphenols can, therefore, inhibit platelet aggregation induced by a range of agonists (Figure 6). However, the inhibition almost exclusively requires concentrations that are supra‐physiological.
Anti‐inflammatory mechanisms
Atherosclerosis is an inflammatory disease. Chronic inflammation plays a crucial role in development and progression of CVD. Phenolic compounds exert anti‐inflammatory activities by altering the recruitment of inflammatory cells by decreasing production of pro‐inflammatory molecules such as TNF‐α, IL‐6 and C‐reactive protein, and inhibiting the production of adhesion molecules [VCAM‐1 and intercellular adhesion molecule 1 (ICAM‐1)] by the endothelium, thereby suppressing cellular migration of monocytes into the subendothelial space (Tangney and Rasmussen, 2013). Polyphenols are likely to promote their anti‐inflammatory properties by modulating transcriptional networks and/or signalling cascades that modulate gene expression, leading to inhibition of inflammatory mediators (Figure 7).
Figure 7.
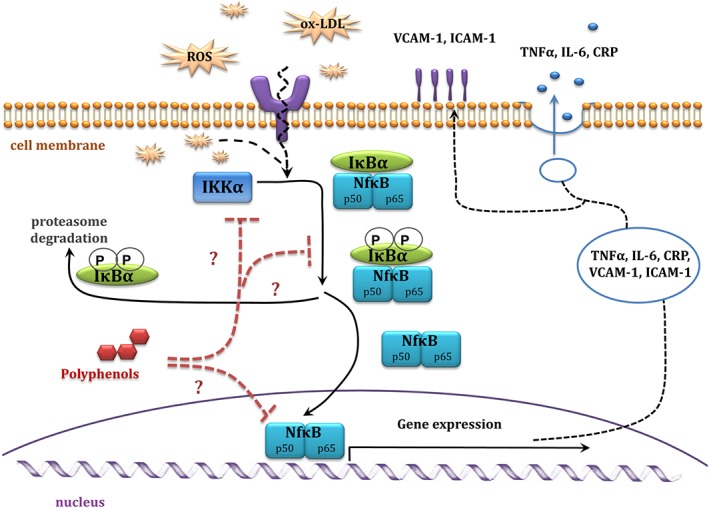
Diagram of NfκB pathways, and proposed anti‐inflammatory mechanism of action of polyphenols. CRP, C‐reactive protein; IKK, IκB kinase.
There is a significant reduction in secretion of the adhesion molecules [ICAM‐1 and VCAM‐1] and of the chemokine CCL2, when endothelial cells were pretreated with gallic acid (10–100 μM), but concentrations likely to be attainable in vivo (1 μM) were less effective in this regard (Hidalgo et al., 2012). Similarly, the anthocyanins, delphinidin‐ and cyanidin‐3‐O‐glucosides (0.1–50 μg·mL−1; 200 nM–100 μM) inhibited LPS‐induced VCAM‐1 expression in porcine iliac artery endothelial cells (Zhu et al., 2013). Moreover, delphinidin aglycone (50–200 μM) decreased ox‐LDL induced expression of adhesion molecules (ICAM‐1 and P‐selectin) in a human endothelial hybrid cell line, as well as decreasing adhesion of monocytes to endothelial cells by reducing intracellular ROS, p38MAPK expression, inhibitor of κ‐B α (IκB‐α) degradation and NF‐κB transcription activity (Chen et al., 2011). Another in vitro study showed that quercetin (125 μM) partly suppressed leptin‐induced TNF‐α secretion and significantly inhibited leptin‐induced NF‐κB expression in HUVECs (Indra et al., 2013). Moreover, epicatechin (1–100 μg·mL−1; approximately 3–340 μM), suppressed production of the pro‐inflammatory cytokines, IL‐6 and IL‐8, with a simultaneous increase in expression of the anti‐inflammatory cytokine IL‐10 in whole blood cultures (Al‐Hanbali et al., 2009). Additionally, apigenin (30 μM) and kaempferol (30 μM), but not resveratrol (50 μM), suppressed expression of LPS‐induced IL‐1. All of these polyphenols, and resveratrol in particular, effectively decreased LPS‐induced expression of TNF‐α in J774 macrophages (Kowalski et al., 2005).
A wide range of phenolic compounds (daidzein, genistein, kaempferol, pelarginidin, naringenin and isorhamnetin; all at 100 μM) also reduced LPS‐induced NF‐κB formation in J774 cells (macrophages) (Hamalainen et al., 2007). Similarly, epicatechin decreased the amount of NF‐κB in cytoplasmic fractions (Al‐Hanbali et al., 2009).
Various flavonoids can suppress expression of other pro‐inflammatory mediators (Hou et al., 2005, López‐Posadas et al., 2010). COX 2, an important player in inflammatory responses, was inhibited by two anthocyanins, delphinidin and cyanidin (25–100 μM), in LPS‐activated RAW264 cells (Hou et al., 2005). Other tested compounds such as pelargonidin, malvidin and peonidin did not show any inhibitory effects (Hou et al., 2005). Similarly, kaempferol (25 and 100 μM) suppressed COX‐2 and TNF‐α gene expression in LPS‐treated RAW264.7 cells (Kim et al., 2015). Activation of the transcription factor NF‐κB plays a crucial role in inflammation, mainly due to its induction of pro‐inflammatory genes, thereby regulating the immune response (Tak and Firestein, 2001). Therefore, direct inhibition of NF‐κB by polyphenols is recognized to be a fundamental mechanism underpinning their anti‐inflammatory activity (Figure 7). A synthetic resveratrol derivative (propynyl resveratrol; 5–10 μM) suppressed the activity of NF‐κB, most likely by interfering with its DNA binding ability, possibly via its association with the IκB‐α site of NF‐κB (Banaganapalli et al., 2013). Interestingly, native resveratrol was not as effective as its synthetic derivative, either because of the lower stability of resveratrol or through modified structure:function relations (Banaganapalli et al., 2013). Likewise, curcumin analogues inhibited NF‐κB activation and gene regulation via inhibition of IκB kinase and Akt activation. In the same study, curcumin (50 μM) blocked phosphorylation of IκBα and p65, leading to suppression of events necessary for NF‐κB gene expression, mainly degradation of IκB‐α and nuclear translocation of p65 (Aggarwal et al., 2006).
Another anti‐inflammatory mechanism that can be potentially targeted by polyphenols is the MAPK pathway. MAPKs are involved in the production of pro‐inflammatory agents (IL‐6, TNF‐α, CCL2 and iNOS) and downstream signalling events that lead to inflammation and apoptosis (Thalhamer et al., 2008). Malvidin (50 μM), an anthocyanin found in red wine, inhibited LPS‐induced MAPK signalling in RAW 264.7 macrophages, with simultaneous enhancement of MAPK phosphatase‐1 (MKP‐1; the protein that down‐regulates the activity of all three branches of MAPKs) (Bognar et al., 2013). Similar findings were identified for another anthocyanin, delphinidin: Pretreatment with delphinidin aglycone (100 μM) suppressed phosphorylation of JNK1/2, ERK1/2 and p38 kinases (three branches of MAPKs). Meanwhile, the glycoside, delphinidin‐3‐sambubioside (50–200 μM), only successfully suppressed the ERK1/2 phosphorylation with little effect on the phosphorylation of JNK1/2 and p38 in LPS‐induced RAW 264.7 (Sogo et al., 2015).
Finding a drug specific for a disease of complex aetiology, such as CVD, is challenging. However, many natural products are characterized by weak binding affinities for any given target, thereby increasing the likelihood of binding to many targets at lower affinity, with a combined effect that is sufficient to provide an overall health benefit (Wang et al., 2016).
Conclusions
That nutritional polyphenols can have cardioprotective activity in vivo and are important health‐promoting components of our diet is not in doubt. However, the fundamental mechanisms that underpin the protective activity are much less clear, a situation that is not helped by the multitude of in vitro data produced using inappropriate concentrations of native polyphenols that have poor bioavailability and are rapidly metabolized to simple phenols, aldehydes and salicylates. In particular, the measurement of direct antioxidant capacity of extracts or even pure compounds in vitro as a predictor of in vivo antioxidant activity is unjustified, given the low bioavailability and rapid metabolism of the component polyphenols. Instead, there is a far more complex picture emerging of the mechanisms involved in the cardioprotective effects of dietary polyphenols that involves pharmacological activity at receptor, cell signalling and gene expression levels. Crucially, the concentrations required to activate these pathways are often orders of magnitude lower than those necessary for direct antioxidant activity, and many of the products of polyphenol metabolism are as active as the parent compounds.
There is a need to return to first principles in pharmacology to gain a full understanding of the activity of polyphenols in cardioprotection. In particular, not enough attention has been paid to stability, absorption, distribution and metabolism of polyphenols in order to inform the design of experiments to test the mechanism(s) of action in vitro. This need extends to in vivo pharmacokinetic and pharmacodynamic experiments as a forerunner to mechanistic studies in order to determine the concentrations of polyphenols and/or their metabolites to be tested in cell culture. Critically, acute exposure to a high concentration of a phenolic compound is not an acceptable surrogate for the more realistic chronic exposure to a low concentration that might happen in vivo. The mechanisms revealed by each approach are likely to be very different, even if the eventual outcome is the same (e.g. antioxidant protection).
Understanding the pharmacological mechanism by which polyphenols bring about cardiovascular effects is critical not only to inform dietary advice but also to help design drugs and nutraceuticals with better bioavailability that target the same pathways. Diosmin is an example of a semi‐synthetic drug, based on the citrus polyphenol, hesperidin (Tong et al., 2013), that is used widely in Europe and USA, primarily in venous insufficiency (Amato, 1994, Maksimovic et al., 2008). Interestingly, the mode of action of diosmin is through venous contraction and not through any antioxidant effect. This example serves to highlight that polyphenols have great pharmacological potential, but a universal shift in emphasis away from the direct antioxidant notion for polyphenol activity is the first vital step to a full appreciation of polyphenol activity in vivo.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
K.G. is funded by Highlands and Islands Enterprise.
Goszcz, K. , Duthie, G. G. , Stewart, D. , Leslie, S. J. , and Megson, I. L. (2017) Bioactive polyphenols and cardiovascular disease: chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response?. British Journal of Pharmacology, 174: 1209–1225. doi: 10.1111/bph.13708.
References
- Acquaviva R, Russo A, Campisi A, Sorrenti V, Di Giacomo C, Barcellona ML et al. (2002). Antioxidant activity and protective effect on DNA cleavage of resveratrol. J Food Sci 67: 137–141. [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB (2006). Curcumin (diferuloylmethane) down‐regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of I kappa B alpha kinase and Akt activation. Mol Pharmacol 69: 195–206. [DOI] [PubMed] [Google Scholar]
- Al‐Hanbali M, Ali D, Bustami M, Abdel‐Malek S, Al‐Hanbali R, Alhussainy T et al. (2009). Epicatechin suppresses IL‐6, IL‐8 and enhances IL‐10 production with NF‐kappa B nuclear translocation in whole blood stimulated system. Neuroendocrinol Lett 30: 131–138. [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5734–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to pharmacology 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato C (1994). Advantage of a micronized flavonoidic fraction (Daflon 500 mg) in comparison with a nonmicronized diosmin. Angiology 45: 531–536. [PubMed] [Google Scholar]
- Arranz S, Chiva‐Blanch G, Valderas‐Martínez P, Medina‐Remón A, Lamuela‐Raventós RM, Estruch R (2012). Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 4: 759–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich H, Gottesman RT, Liebling EJ, Schuck AG (2008). Theaflavin‐3‐gallate and theaflavin‐3′‐gallate, polyphenols in black tea with prooxidant properties. Basic Clin Pharmacol Toxicol 103: 66–74. [DOI] [PubMed] [Google Scholar]
- Banaganapalli B, Mulakayala C, Gowsia D, Mulakayala N, Pulaganti M, Shaik NA et al. (2013). Synthesis and biological activity of new resveratrol derivative and molecular docking: dynamics studies on NFkB. Appl Biochem Biotechnol 171: 1639–1657. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin‐Corral J, Sanchez F et al. (2010). Cigarette smoke‐induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med 182: 477–488. [DOI] [PubMed] [Google Scholar]
- Beattie J, Crozier A, Duthie GG (2005). Potential health benefits of berries. Curr Nutr Food Sci 1: 71–86. [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Creager MA (2001). Ascorbate restores endothelium‐dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 103: 1618–1623. [DOI] [PubMed] [Google Scholar]
- Bellion P, Olk M, Will F, Dietrich H, Baum M, Eisenbrand G et al. (2009). Formation of hydrogen peroxide in cell culture media by apple polyphenols and its effect on antioxidant biomarkers in the colon cell line HT‐29. Mol Nutr Food Res 53: 1226–1236. [DOI] [PubMed] [Google Scholar]
- Bennick A (2002). Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med 13: 184–196. [DOI] [PubMed] [Google Scholar]
- Bognar E, Sarszegi Z, Szabo A, Debreceni B, Kalman N, Tucsek Z et al. (2013). Antioxidant and anti‐inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS One 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn T (2014). Dietary factors affecting polyphenol bioavailability. Nutr Rev 72: 429–452. [DOI] [PubMed] [Google Scholar]
- Borbalan AMA, Zorro L, Guillen DA, Barroso CG (2003). Study of the polyphenol content of red and white grape varieties by liquid chromatography‐mass spectrometry and its relationship to antioxidant power. J Chromatogr A 1012: 31–38. [DOI] [PubMed] [Google Scholar]
- Bouayed J, Rammal H, Soulimani R (2009). Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev 2: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB (1997). Tissue distribution of estrogen receptors alpha (ER‐alpha) and beta (ER‐beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metabol 82: 3509–3512. [DOI] [PubMed] [Google Scholar]
- Cao G, Booth SL, Sadowski JA, Prior RL (1998). Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr 68: 1081–1087. [DOI] [PubMed] [Google Scholar]
- Cardona F, Andrés‐Lacueva C, Tulipani S, Tinahones FJ, Queipo‐Ortuño MI (2013). Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24: 1415–1422. [DOI] [PubMed] [Google Scholar]
- Castellano G (2012). Classification of phenolic compounds by chemical structural indicators and its relation to antioxidant properties of Posidonia oceanica (L.) Delile MATCH Communications in Mathematical and in Computer. Chemistry 67: 231–250. [Google Scholar]
- Chalopin M, Tesse A, Martinez MC, Rognan D, Arnal JF, Andriantsitohaina R (2010). Estrogen receptor alpha as a key target of red wine polyphenols action on the endothelium. PLoS One 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SS, Lee VSY, Tseng YL, Chang KC, Chen KB, Chen YL et al. (2012). Gallic acid attenuates platelet activation and platelet‐leukocyte aggregation: involving pathways of Akt and GSK3 beta. Evid Based Complement Alternat Med 2012: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu JD et al. (2011). Inhibitory effect of delphinidin on monocyte‐endothelial cell adhesion induced by oxidized low‐density lipoprotein via ROS/p38MAPK/NF‐kappa B pathway. Cell Biochem Biophys 61: 337–348. [DOI] [PubMed] [Google Scholar]
- Cosmi F, Di Giulio P, Masson S, Finzi A, Marfisi RM, Cosmi D et al. (2015). Regular wine consumption in chronic heart failure impact on outcomes, quality of life, and circulating biomarkers. Circ Heart Fail 8: 428–437. [DOI] [PubMed] [Google Scholar]
- Croom E (2012). Metabolism of xenobiotics of human environments. Prog Mol Biol Transl Sci 112: 31–88. [DOI] [PubMed] [Google Scholar]
- Curtin JF, Donovan M, Cotter TG (2002). Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods 265: 49–72. [DOI] [PubMed] [Google Scholar]
- D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R (2007). Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita 43: 348–361. [PubMed] [Google Scholar]
- Del Rio D, Rodriguez‐Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A (2013). Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18: 1818–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie GG, Gardner PT, Kyle JA (2003). Plant polyphenols: are they the new magic bullet? Proc Nutr Soc 62: 599–603. [DOI] [PubMed] [Google Scholar]
- Elias RJ, Andersen ML, Skibsted LH, Waterhouse AL (2009). Key factors affecting radical formation in wine studied by spin trapping and EPR spectroscopy. Am J Enol Vitic 60: 471–476. [Google Scholar]
- Ellingsen I, Hjerkinn EA, Sejeflot I, Arnesen H, Tonstad S (2008). Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. Br J Nutr 99: 674–681. [DOI] [PubMed] [Google Scholar]
- Falk E (2006). Pathogenesis of atherosclerosis. J Am Coll Cardiol 47: 7–12. [DOI] [PubMed] [Google Scholar]
- Fang J (2014). Bioavailability of anthocyanins. Drug Metab Rev 46: 508–520. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Galleano M, Verstraeten SV, Oteiza PI (2010). Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med 31: 435–445. [DOI] [PubMed] [Google Scholar]
- Galicia‐Moreno M, Gutiérrez‐Reyes G (2014). The role of oxidative stress in the development of alcoholic liver disease. Rev Gastroenterol Méx 79: 135–144. [DOI] [PubMed] [Google Scholar]
- Garcia‐Alonso M, Rimbach G, Rivas‐Gonzalo JC, De Pascual‐Teresa S (2004). Antioxidant and cellular activities of anthocyanins and their corresponding vitisins A – studies in platelets, monocytes, and human endothelial cells. J Agric Food Chem 52: 3378–3384. [DOI] [PubMed] [Google Scholar]
- Gardner PT, McPhail DB, Duthie GG (1998). Electron spin resonance spectroscopic assessment of the antioxidant potential of teas in aqueous and organic media. J Sci Food Agric 76: 257–262. [Google Scholar]
- Gerhardt T, Ley K (2015). Monocyte trafficking across the vessel wall. Cardiovasc Res 107: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐Longo J, Gonzalez‐Vazquez C (2010). Vascular pro‐oxidant effects secondary to the autoxidation of gallic acid in rat aorta. J Nutr Biochem 21: 304–309. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Reyes S, Guzman‐Beltran S, Medina‐Campos ON, Pedraza‐Chaverri J (2013). Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin‐induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid Med Cell Longev 2013: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K, Davies KM, Winefield C. (eds) (2009). Anthocyanins: Biosynthesis, Functions, and Applications. Springer‐Verlag: New York. [Google Scholar]
- Grossini E, Marotta P, Farruggio S, Sigaudo L, Qoqaiche F, Raina G et al. (2015). Effects of artemetin on nitric oxide release and protection against peroxidative injuries in porcine coronary artery endothelial cells. Phytother Res 29: 1339–1348. [DOI] [PubMed] [Google Scholar]
- Grosso G, Stepaniak U, Polak M, Micek A, Topor‐Madry R, Stefler D et al. (2016). Coffee consumption and risk of hypertension in the Polish arm of the HAPIEE cohort study. Eur J Clin Nutr 70: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G, Stepaniak U, Topor‐Madry R, Szafraniec K, Pajak A (2014). Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 30: 1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007). Anti‐inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT‐1 and NF‐kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF‐kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif S, Shamim U, Ullah MF, Azmi AS, Bhat SH, Hadi SM (2008). The anthocyanidin delphinidin mobilizes endogenous copper ions from human lymphocytes leading to oxidative degradation of cellular DNA. Toxicology 249: 19–25. [DOI] [PubMed] [Google Scholar]
- Hassimotto NM, Genovese MI, Lajolo FM (2008). Absorption and metabolism of cyanidin‐3‐glucoside and cyanidin‐3‐rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr Res (New York, NY) 28: 198–207. [DOI] [PubMed] [Google Scholar]
- He JA, Giusti MM (2010). Anthocyanins: natural colorants with health‐promoting properties. Annu Rev Food Sci Technol 1: 163–187. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Martin‐Santamaria S, Recio I, Sanchez‐Moreno C, de Pascual‐Teresa B, Rimbach G et al. (2012). Potential anti‐inflammatory, anti‐adhesive, anti/estrogenic, and angiotensin‐converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr 7: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins PN (2013). Molecular biology of atherosclerosis. Physiol Rev 93: 1317–1542. [DOI] [PubMed] [Google Scholar]
- Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M (2005). Anthocyanidins inhibit cyclooxygenase‐2 expression in LPS‐evoked macrophages: structure–activity relationship and molecular mechanisms involved. Biochem Pharmacol 70: 417–425. [DOI] [PubMed] [Google Scholar]
- Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee DH (2007). Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the coronary artery risk development in young adults (CARDIA)/young adult longitudinal trends in antioxidants (YALTA) study. Clin Chem 53: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra MR, Karyono S, Ratnawati R, Malik SG (2013). Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NF kappa B activation in leptin‐induced human umbilical vein endothelial cells (HUVECs). BMC Res Notes 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob RA, Burri BJ (1996). Oxidative damage and defense. Am J Clin Nutr 63: 985–990. [DOI] [PubMed] [Google Scholar]
- Jin Y, Alimbetov D, George T, Gordon MH, Lovegrove JA (2011). A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur J Clin Nutr 65: 849–856. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR et al. (2008). The Nrf2‐ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci 1147: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumar A, Schmieder RE (2016). Cocoa flavanol cardiovascular effects beyond blood pressure reduction. J Clin Hypertens 18: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkonen MP, Heinonen M (2003). Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem 51: 628–633. [DOI] [PubMed] [Google Scholar]
- Kay CD, Kroon PA, Cassidy A (2009). The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol Nutr Food Res 53: 92–101. [DOI] [PubMed] [Google Scholar]
- Kern M, Fridrich D, Reichert J, Skrbek S, Nussher A, Hofem S et al. (2007). Limited stability in cell culture medium and hydrogen peroxide formation affect the growth inhibitory properties of delphinidin and its degradation product gallic acid. Mol Nutr Food Res 51: 1163–1172. [DOI] [PubMed] [Google Scholar]
- Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC (2013). Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 5: 3779–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park JG, Lee J, Yang WS, Park GW, Kim HG et al. (2015). The dietary flavonoid kaempferol mediates anti‐inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm 2015: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I (2008). Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke‐mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: 478–488. [DOI] [PubMed] [Google Scholar]
- Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T (2005). Effect of apigenin, kaempferol and resveratrol on the expression of interleukin‐1beta and tumor necrosis factor‐alpha genes in J774.2 macrophages. Pharmacol Rep 57: 390–394. [PubMed] [Google Scholar]
- Ku SK, Yoon EK, Lee W, Kwon S, Lee T, Bae JS (2016). Antithrombotic and antiplatelet activities of pelargonidin in vivo and in vitro. Arch Pharm Res 39: 398–408. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT et al. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139: 4252–4263. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ (2010). The antioxidant and pro‐oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Walker MD, Kanner J (2002). Antioxidant and prooxidant effects of phenolics on pancreatic beta‐cells in vitro. J Agric Food Chem 50: 7220–7225. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Akesson A, Gigante B, Wolk A (2016). Chocolate consumption and risk of myocardial infarction: a prospective study and meta‐analysis. Heart 102: 1017–1022. [DOI] [PubMed] [Google Scholar]
- Lazze MC, Pizzala R, Perucca P, Cazzalini O, Savio M, Forti L et al. (2006). Anthocyanidins decrease endothelin‐1 production and increase endothelial nitric oxide synthase in human endothelial cells. Mol Nutr Food Res 50: 44–51. [DOI] [PubMed] [Google Scholar]
- Le Brocq M, Leslie SJ, Milliken P, Megson IL (2008). Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal 10: 1631–1674. [DOI] [PubMed] [Google Scholar]
- Leung HWC, Lin CJ, Hour MJ, Yang WH, Wang MY, Lee HZ (2007). Kaempferol induces apoptosis in human lung non‐small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem Toxicol 45: 2005–2013. [DOI] [PubMed] [Google Scholar]
- Li SH, Zhao P, Tian HB, Chen LH, Cui LQ (2015). Effect of grape polyphenols on blood pressure: a meta‐analysis of randomized controlled trials. PLoS One 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianda RLP, Sant'Ana LDO, Echevarria A, Castro RN (2012). Antioxidant activity and phenolic composition of Brazilian honeys and their extracts. J Braz Chem Soc 23: 618–627. [Google Scholar]
- Lodovici M, Bigagli E (2011). Oxidative stress and air pollution exposure. J Toxicol 2011: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M et al. (2010). Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E‐knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol 30: 749–757. [DOI] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JMO et al. (2005). Effects of long‐term supplementation on and cancer vitamin E cardiovascular events – a randomized controlled trial. JAMA 293: 1338–1347. [DOI] [PubMed] [Google Scholar]
- López‐Posadas R, Ballester I, Mascaraque C, Suárez MD, Zarzuelo A, Martínez‐Augustin O et al. (2010). Flavonoids exert distinct modulatory actions on cyclooxygenase 2 and NF‐kB in an intestinal epithelial cell line (IEC18). Br J Pharmacol 160: 1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Sepúlveda R, Gómez‐Guzmán M, Zarzuelo MJ, Romero M, Sanchez M, Quintela AM et al. (2011). Red wine polyphenols prevent endothelial dysfunction induced by endothelin‐1 in rat aorta: role of NADPH oxidase. Clin Sci (London, England : 1979) 120: 321–333. [DOI] [PubMed] [Google Scholar]
- Lotito SB, Frei B (2004). The increase in human plasma antioxidant capacity after apple consumption is due to the metabolic effect of fructose on urate, not apple‐derived antioxidant flavonoids. Free Radic Biol Med 37: 251–258. [DOI] [PubMed] [Google Scholar]
- Lugasi A (2003). Polyphenol content and antioxidant properties of beer. Acta Alimentaria 32: 181–192. [Google Scholar]
- Macakova K, Rehakova Z, Mladenka P, Karlickova J, Filipsky T, Riha M et al. (2012). In vitro platelet antiaggregatory properties of 4‐methylcoumarins. Biochimie 94: 2681–2686. [DOI] [PubMed] [Google Scholar]
- Maksimovic ZV, Maksimovic M, Jadranin D, Kuzmanovic I, Andonovic O (2008). Medicamentous treatment of chronic venous insufficiency using semisynthetic diosmin‐a prospective study. Acta Chir Iugosl 55: 53–59. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004). Polyphenols: food sources and bioavailability. Am J Clin Nutr 79: 727–747. [DOI] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P (2006). Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Appel CL (2010). Polyphenols as dietary supplements: adouble‐edged sword. Nutr Diet Suppl 2: 1–12. [Google Scholar]
- Martinez‐Cayuela M (1995). Oxygen free radicals and human disease. Biochimie 77: 147–161. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ichiyanagi T, Iida H, Ito K, Tsuda T, Hirayama M et al. (2006). Ingested delphinidin‐3‐rutinoside is primarily excreted to urine as the intact form and to bile as the methylated form in rats. J Agric Food Chem 54: 578–582. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kamm KE, Stull JT, Azuma H (2005). Delphinidin‐3‐rutino side relaxes the bovine ciliary smooth muscle through activation of ETB receptor and NO/cGMP pathway. Exp Eye Res 80: 313–322. [DOI] [PubMed] [Google Scholar]
- Mazza GJ (2007). Anthocyanins and heart health. Ann Ist Super Sanita 43: 369–374. [PubMed] [Google Scholar]
- Megson IL, Whitfield PD, Zabetakis I (2016). Lipids and cardiovascular disease: where does dietary intervention sit alongside statin therapy? Food Funct 7: 2603–2614. [DOI] [PubMed] [Google Scholar]
- Messina F, Guglielmini G, Curini M, Orsini S, Gresele P, Marcotullio MC (2015). Effect of substituted stilbenes on platelet function. Fitoterapia 105: 228–233. [DOI] [PubMed] [Google Scholar]
- Moskaug JO, Carlsen H, Myhrstad MC, Blomhoff R (2005). Polyphenols and glutathione synthesis regulation. Am J Clin Nutr 81: 277–283. [DOI] [PubMed] [Google Scholar]
- Mullie P (2014). Estimation of daily human intake of food flavonoids. Int J Food Sci Nutr 59: 291–298. [DOI] [PubMed] [Google Scholar]
- Nabavi SF, Barber AJ, Spagnuolo C, Russo GL, Daglia M, Nabavi SM et al. (2016). Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit Rev Clin Lab Sci 53: 293–312. [DOI] [PubMed] [Google Scholar]
- Nicholson SK, Tucker GA, Brameld JM (2008). Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc 67: 42–47. [DOI] [PubMed] [Google Scholar]
- Oh WJ, Endale M, Park S‐C, Cho JY, Rhee MH (2012). Dual roles of quercetin in platelets: phosphoinositide‐3‐Kinase and MAP kinases inhibition, and cAMP‐dependent vasodilator‐stimulated phosphoprotein stimulation. Evid Based Complement Alternat Med 2012: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero P, Bonet B, Herrera E, Rabano A (2005). Development of atherosclerosis in the diabetic BALB/c mice – prevention with vitamin E administration. Atherosclerosis 182: 259–265. [DOI] [PubMed] [Google Scholar]
- Park WH, Kim SH (2012). Involvement of reactive oxygen species and glutathione in gallic acid‐induced human umbilical vein endothelial cell death. Oncol Rep 28: 695–700. [DOI] [PubMed] [Google Scholar]
- Perez‐Jimenez J, Neveu V, Vos F, Scalbert A (2010). Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol‐Explorer database. Eur J Clin Nutr 64: 112–120. [DOI] [PubMed] [Google Scholar]
- Phenol‐Explorer . n.d. Database on polyphenol content in foods. Available at: www.phenol‐explorer.eu (accessed 8/11/2016)
- Pollard SE, Kuhnle GG, Vauzour D, Vafeiadou K, Tzounis X, Whiteman M et al. (2006). The reaction of flavonoid metabolites with peroxynitrite. Biochem Biophys Res Commun 350: 960–968. [DOI] [PubMed] [Google Scholar]
- Prior RL, Cao G (2000). Antioxidant phytochemicals in fruits and vegetables: diet and health implications. HortSci 35: 588–592. [Google Scholar]
- Prochazkova D, Bousova I, Wilhelmova N (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82: 513–523. [DOI] [PubMed] [Google Scholar]
- Quideau S, Deffieux D, Douat‐Casassus C, Pouysegu L (2011). Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 50: 586–621. [DOI] [PubMed] [Google Scholar]
- Romeo L, Intrieri M, D'Agata V, Mangano NG, Oriani G, Ontario ML et al. (2009). The major green tea polyphenol, (−)‐epigallocatechin‐3‐gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr 28: 492–499. [DOI] [PubMed] [Google Scholar]
- Santhakumar AB, Stanley R, Singh I (2015). The ex vivo antiplatelet activation potential of fruit phenolic metabolite hippuric acid. Food Funct 6: 2679–2683. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Johnson IT, Saltmarsh M (2005). Polyphenols: antioxidants and beyond. Am J Clin Nutr 81: 215–217. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Williamson G (2000). Dietary intake and bioavailability of polyphenols. J Nutr 130: 2073–2085. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G (2011). Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 44: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe T, Steffen Y, Sies H (2008). How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys 476: 102–106. [DOI] [PubMed] [Google Scholar]
- Schneider J, Kaffarnik H, Steinmetz A (1996). Alcohol, lipid metabolism and coronary heart disease. Herz 21: 217–226. [PubMed] [Google Scholar]
- Sepúlveda L, Ascacio A, Rodríguez‐Herrera R, Aguilera‐Carbó A, Aguilar CN (2011). Ellagic acid: biological properties and biotechnological development for production processes. Afr J Biotechnol 10: 4518–4523. [Google Scholar]
- Severino JF, Goodman BA, Kay CW, Stolze K, Tunega D, Reichenauer TG et al. (2009). Free radicals generated during oxidation of green tea polyphenols: electron paramagnetic resonance spectroscopy combined with density functional theory calculations. Free Radic Biol Med 46: 1076–1088. [DOI] [PubMed] [Google Scholar]
- Sies H (1997). Oxidative stress: oxidants and antioxidants. Exp Physiol 82: 291–295. [DOI] [PubMed] [Google Scholar]
- Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS (2002). Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol 7: 40–53. [PMC free article] [PubMed] [Google Scholar]
- Sogo T, Terahara N, Hisanaga A, Kumamoto T, Yamashiro T, Wu S et al. (2015). Anti‐inflammatory activity and molecular mechanism of delphinidin 3‐sambubioside, a Hibiscus anthocyanin. Biofactors 41: 58–65. [DOI] [PubMed] [Google Scholar]
- Song FL, Zhu YN, Shi ZY, Tian JJ, Deng XJ, Ren J et al. (2014). Plant food anthocyanins inhibit platelet granule secretion in hypercholesterolaemia: involving the signalling pathway of PI3K‐Akt. Thromb Haemost 112: 981–991. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: 1054–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS (2001). NF‐kappaB: a key role in inflammatory diseases. J Clin Invest 107: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney C, Rasmussen HE (2013). Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep 15: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y, Griendling KK (2003). Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Thalhamer T, McGrath MA, Harnett MM (2008). MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 47: 409–414. [DOI] [PubMed] [Google Scholar]
- Tong N, Zhang Z, Zhang W, Qiu Y, Gong Y, Yin L et al. (2013). Diosmin alleviates retinal edema by protecting the blood‐retinal barrier and reducing retinal vascular permeability during ischemia/reperfusion injury. PLoS One 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela BC, Waterhouse AL (1996). Resveratrol: isomeric molar absorptivities and stability. J Agric Food Chem 44: 1253–1257. [Google Scholar]
- Tresserra‐Rimbau A, Rimm EB, Medina‐Remón A, Martínez‐González MA, de la Torre R, Corella D et al. (2014). Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis 24: 639–647. [DOI] [PubMed] [Google Scholar]
- Tsao R (2010). Chemistry and biochemistry of dietary polyphenols. Nutrients 2: 1231–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulyathan V, Boulton RB, Singleton VL (1989). Oxygen uptake by gallic acid as a model for similar reactions in wines. J Agric Food Chem 37: 844–849. [Google Scholar]
- Turell L, Radi R, Alvarez B (2013). The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 65: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiyapuri S, Roweth H, Ali MS, Unsworth AJ, Stainer AR, Flora GD et al. (2015). Pharmacological actions of nobiletin in the modulation of platelet function. Br J Pharmacol 172: 4133–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita JA (2005). Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr 81: 292–297. [DOI] [PubMed] [Google Scholar]
- Wan Y, Vinson JA, Etherton TD, Proch J, Lazarus SA, Kris‐Etherton P (2001). Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am J Clin Nutr 74: 596–602. [DOI] [PubMed] [Google Scholar]
- Wang J, Guo Z, Fu Y, Wu Z, Huang C, Zheng C et al. (2016). Weak‐binding molecules are not drugs? – toward a systematic strategy for finding effective weak‐binding drugs. Brief Bioinform : 1–12. [DOI] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ (2012). The flavonoids casticin and artemetin are poorly extracted and are unstable in an Artemisia annua tea infusion. Planta Med 78: 1024–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg JH, Schuck AG, Silverman MS, Ovits‐Levy CG, Solodokin LJ, Zuckerbraun HL et al. (2010). Pomegranate extract, a prooxidant with antiproliferative and proapoptotic activities preferentially towards carcinoma cells. Anticancer Agents Med Chem 10: 634–643. [DOI] [PubMed] [Google Scholar]
- Wiczkowski W, Romaszko E, Piskula MK (2010). Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary‐relevant dose of anthocyanins in humans. J Agric Food Chem 58: 12130–12136. [DOI] [PubMed] [Google Scholar]
- Woodward G, Kroon P, Cassidy A, Kay C (2009). Anthocyanin stability and recovery: implications for the analysis of clinical and experimental samples. J Agric Food Chem 57: 5271–5278. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi ZY, Reheman A, Jin JW, Li CL, Wang YM et al. (2012). Plant food delphinidin‐3‐glucoside significantly inhibits platelet activation and thrombosis: novel protective roles against cardiovascular diseases. PLoS One 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim KA, Martin A, Joseph JA (2000). Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic Biol Med 29: 51–60. [DOI] [PubMed] [Google Scholar]
- Zhang T, Liang XY, Shi LY, Wang L, Chen JL, Kang C et al. (2013). Estrogen receptor and PI3K/Akt signaling pathway involvement in S‐(−) equol‐induced activation of Nrf2/ARE in endothelial cells. PLoS One 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QY, Holt RR, Lazarus SA, Ensunsa JL, Hammerstone JF, Schmitz HH et al. (2002). Stability of the flavan‐3‐ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J Agric Food Chem 50: 1700–1705. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T et al. (2013). Anti‐inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis 23: 843–849. [DOI] [PubMed] [Google Scholar]


