Abstract
Curcumin, a yellow pigment in the Indian spice Turmeric (Curcuma longa), which is chemically known as diferuloylmethane, was first isolated exactly two centuries ago in 1815 by two German Scientists, Vogel and Pelletier. However, according to the pubmed database, the first study on its biological activity as an antibacterial agent was published in 1949 in Nature and the first clinical trial was reported in The Lancet in 1937. Although the current database indicates almost 9000 publications on curcumin, until 1990 there were less than 100 papers published on this nutraceutical. At the molecular level, this multitargeted agent has been shown to exhibit anti‐inflammatory activity through the suppression of numerous cell signalling pathways including NF‐κB, STAT3, Nrf2, ROS and COX‐2. Numerous studies have indicated that curcumin is a highly potent antimicrobial agent and has been shown to be active against various chronic diseases including various types of cancers, diabetes, obesity, cardiovascular, pulmonary, neurological and autoimmune diseases. Furthermore, this compound has also been shown to be synergistic with other nutraceuticals such as resveratrol, piperine, catechins, quercetin and genistein. To date, over 100 different clinical trials have been completed with curcumin, which clearly show its safety, tolerability and its effectiveness against various chronic diseases in humans. However, more clinical trials in different populations are necessary to prove its potential against different chronic diseases in humans. This review's primary focus is on lessons learnt about curcumin from clinical trials.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviation
- MCP
monocyte chemoattractant protein
Tables of Links
| TARGETS | |
|---|---|
| Enzymes a | G protein‐coupled receptors b |
| 5‐LOX | CXCR4 |
| COX‐2 | MCP‐1 receptor (CCR2) |
| Cytosolic PLA2 | Nuclear hormone receptors c |
| DNMTs | AR |
| ERK | ER‐α |
| FAK | PPAR‐γ |
| HATs | Other protein targets d |
| HDACs | Bcl‐2 |
| iNOS | Bcl‐xL |
| JAK | IAP |
| JNK | TNF‐α |
| ODC | XIAP |
| p38 MAPK | Catalytic receptors e |
| PKA (Akt) | EGFR |
| PKC | ROS receptors |
| uPA |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
Despite the substantial advances in the treatment of complex, multigenic and chronic human diseases, their occurrence rate has increased significantly in recent times (Gupta et al., 2012). A number of mono‐targeted therapies, also referred to as ‘smart drugs’, have been designed over the past few years for the treatment of these chronic diseases. However, complex diseases like cardiovascular, metabolic, cancer and neurological diseases occur due to perturbations of multiple signalling pathways. Therefore, targeting a single pathway among many of the pathways involved is not likely to be effective for the prevention and treatment of these diseases (Bordoloi et al., 2016). Besides, high cost and adverse side effects are the other major disadvantages associated with these smart drugs. These limitations necessitate the urge to develop multi‐targeted, cost‐effective, readily available, non‐toxic and highly potent agents for the management of different human diseases (Gupta et al., 2012).
Among the numerous natural remedies, turmeric has gained considerable attention due to its profound medicinal values (Prasad et al., 2014a). This agent possesses antioxidant, anti‐inflammatory, anticancer, antigrowth, antiarthritic, antiatherosclerotic, antidepressant, antiaging, antidiabetic, antimicrobial, wound healing and memory‐enhancing activities (Aggarwal et al., 2013a). Moreover, it exerts chemopreventive, chemosensitization and radiosensitization effects as well (Goel and Aggarwal, 2010; Gupta et al., 2011a). In traditional Indian medicine, this spice has been also used to treat different ailments such as gynecological problems, gastric problems, hepatic disorders, infectious diseases, blood disorders, acne, psoriasis, dermatitis, rash and other chronic ailments (Gupta et al., 2013a). Diverse in vivo studies have also indicated its potential against pro‐inflammatory diseases, cancers, neurodegenerative diseases, depression, diabetes, obesity and atherosclerosis (Gupta et al., 2013c). Among the huge number of compounds isolated from turmeric (Tyagi et al., 2015), curcumin (a diferuloylmethane) was found to be the most widely studied compound as evinced by more than 9000 citations in the literature. It was first discovered by Vogel and Pelletier from the rhizomes of turmeric (Curcuma longa) (Prasad et al., 2014b). Structurally, it can exist in at least two tautomeric forms, keto and enol and they possess antioxidant, anti‐inflammatory, anticancer, antiviral, antibacterial and antidiabetic properties (Aggarwal et al., 2008; Goel et al., 2008; Gupta et al., 2010; Gupta et al., 2012; Aggarwal et al., 2013b; Rainey et al., 2015). These traits can possibly be attributed to the methoxy, hydroxyl, α, β‐unsaturated carbonyl moiety or diketone groups present in curcumin (Aggarwal et al., 2015). Besides its safety and tolerability, cost‐effectiveness is an added advantage of this compound (Shoba et al., 1998; Rasyid and Lelo, 1999; Rasyid et al., 2002; Lao et al., 2006; Tuntipopipat et al., 2006; Juan et al., 2007; Vareed et al., 2008; Shimouchi et al., 2009; Dominiak et al., 2010; Cuomo et al., 2011; Pungcharoenkul and Thongnopnua, 2011; Sasaki et al., 2011; DiSilvestro et al., 2012; Kusuhara et al., 2012; Sugawara et al., 2012; Vitaglione et al., 2012; Aggarwal et al., 2013a; Jager et al., 2014; Klickovic et al., 2014). Because of its amazing properties, curcumin is being marketed in several countries of the world in various forms (Prasad et al., 2014b).
However, the utility of curcumin is greatly hindered by its colour, lack of water solubility and low bioavailability (Anand et al., 2008). Prime factors contributing towards the low bioavailability of curcumin in both plasma and tissue might be associated with its poor absorption, rapid metabolism and rapid systemic elimination. Therefore, to enhance these, various approaches have been sought that include the use of adjuvants, liposomal curcumin, curcumin nanoparticles, curcumin phospholipid complexes, curcumin reformulated with various oils and with inhibitors of metabolism, conjugation of curcumin prodrugs and linking curcumin with polyethylene glycol (Anand et al., 2007; 2008; Goel et al., 2008; Nair et al., 2010). The use of structural analogues of curcumin and synthesis of ‘man‐made’ curcumin analogues also play a role in the enhancement of its bioavailability. For instance, the natural analogues of curcumin such as demethoxycurcumin and bidemethoxycurcumin were reported to have a similar biological activity to curcumin (Kocaadam and Şanlier, 2015). Furthermore, it has been proposed that the presence of an active methylene group and β‐diketone moiety causes curcumin to be unstable under physiological conditions together with its poor absorption and rapid metabolism. Supporting this proposal, more recently, different structural modifications were performed and many of the active methylene and carbonyl substituted curcumin derivatives/analogues were found to exert much improved antioxidant activity when compared with curcumin (Sahu et al., 2016). Thus, diverse synthetic derivatives of curcumin can be obtained with various chemical modifications including phenolic hydroxyl groups, acylation, alkylation, glycosylation and amino acylation to improve its bioavailability (Kocaadam and Şanlier, 2015).
Molecular targets of curcumin
Curcumin can impact a diverse range of molecular targets and signalling pathways, which augment the efficacy of existing chemotherapeutic agents (Figure 1). It can interact with a huge number of different proteins such as nuclear factor E2‐related factor 2 (Nrf2), β‐catenin, NF‐κB, p38 MAPK, DNA (cytosine‐5)‐methyltransferase‐1, COX‐2, 5‐lipoxygenase, PGE2, FOXO3, inducible NOS, ROS, cyclin D1, VEGF, glutathione, cytosolic PLA2, p‐Tau (p‐τ) and TNF‐α. This ability of curcumin facilitiates selective modulation of multiple cell signalling pathways linked to different chronic diseases, which strongly suggest that it is a potent multi‐targeted polyphenol (Anand et al., 2008; Kunnumakkara et al., 2008; Ravindran et al., 2009; Goel and Aggarwal, 2010; Hasima and Aggarwal, 2012; Aggarwal et al., 2015; Rainey et al., 2015). The common molecular targets of curcumin include transcription factors, inflammatory mediators, protein kinases and enzymes like protein reductases and histone acetyltransferase (Goel et al., 2008; Yadav and Aggarwal, 2011; Gupta et al., 2011b; 2012). A plausible mechanism through which curcumin exerts its manifold effects might be via epigenetic regulation (Tuorkey, 2014). Many recent studies have reported curcumin as a potent epigenetic regulator in different diseases, such as neurological disorders, inflammation, diabetes and different cancer types. The epigenetic regulatory roles of curcumin primarily include inhibition of DNA methyltransferases, regulation of histone modifications via effects on histone acetyltransferases and histone deacetylases and regulation of micro RNAs (Reuter et al., 2011; Boyanapalli and Tony Kong, 2015; Remely et al., 2015). Curcumin also modulates various proteosomal pathways (Hasima and Aggarwal, 2014) and impairs glycogen metabolism through selective inhibition of phosphorylase kinase (Reddy and Aggarwal, 1994). Nonetheless, it has been shown to exhibit anti‐inflammatory effects by down‐regulating various cytokines, such as TNF‐α, IL‐1, IL‐6, IL‐8, IL‐12, monocyte chemoattractant protein (MCP)‐1 (also known as CCL2) and IL‐1β, and various inflammatory enzymes and transcription factors (Bharti et al., 2004; Davis et al., 2007; Aggarwal and Sung, 2009; Gupta et al., 2011a; 2014).
Figure 1.
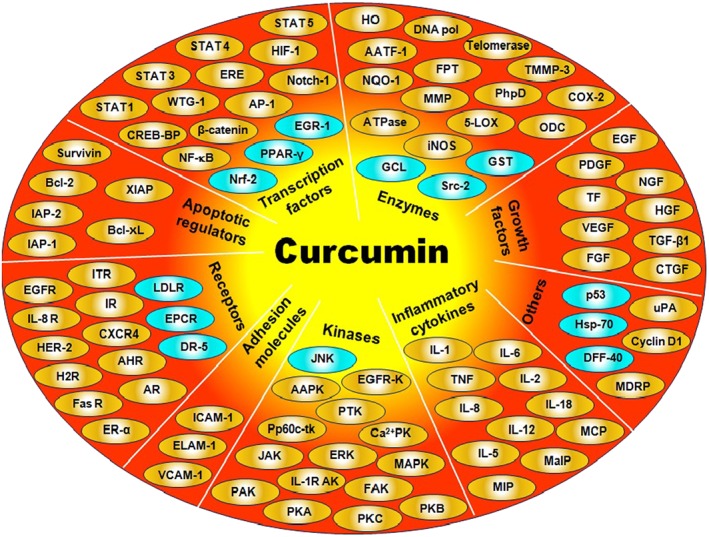
Molecular targets of curcumin. 5‐LOX, 5‐lipoxygenase; AAPK, autophosphorylation‐activated protein kinase; AATF‐1, arylamine N‐acetyltransferases‐1; AHR, aryl hydrocarbon receptor; AP‐1, activating protein‐1; AR, androgen receptor; Bcl‐2, beta‐cell lymphoma protein 2; Bcl‐xL, beta‐cell lymphoma extra large; Ca2+PK, Ca2+‐dependent protein kinase; CXCR4, chemokine (C‐X‐C motif) receptor 4; CREB‐BP, CREB‐binding protein; CTGF, connective tissue growth factor; DFF‐40, DNA fragmentation factor 40‐kd subunit; DR5, death receptor‐5; ELAM‐1, endothelial leukocyte adhesion molecule‐1; EPCR, endothelial protein C‐receptor; ERE, electrophile response element; ER‐α, estrogen receptor‐alpha; FAK, focal adhesion kinase; FPT, farnesyl protein transferase; FR, Fas receptor; GCL, glutamyl cysteine ligase; GST, gluthathione‐S‐transferase; H2R, histamine (2)‐receptor; HER‐2, human epidermal growth factor receptor‐2; HGF, hepatocyte growth factor; HIF‐1, hypoxia inducible factor‐1; HO, haem oxygenase 1; HSP‐70, heat‐shock protein 70; IAP‐1, inhibitory apoptosis protein‐1; ICAM‐1, intracellular adhesion molecule‐1; iNOS, inducible NOS; IR, integrin receptor; MaIP, macrophage inflammatory protein; MCP, monocyte chemoattractant protein; MDRP, multi‐drug resistance protein; MIP, migration inhibition protein; NGF, nerve growth factor; NQO‐1, NAD(P)H:quinoneoxidoreductase‐1; Nrf, nuclear factor 2‐related factor; ODC, ornithine decarboxylase; PAK, protamine kinase; PhpD, phospholipase D; Pp60c‐tk, pp60c‐src tyrosine kinase; PTK, protein tyrosine kinase; Src‐2, Src homology 2 domain‐containing tyrosine phosphatase 2; STAT, signal transducer and activator of transcription; TF, tissue factor; TMMP‐3, tissue inhibitor of metalloproteinase‐3; uPA, urokinase‐type plasminogen activator; VCAM‐1, vascular cell adhesion molecule‐1; WTG‐1, Wilms' tumour gene 1.
Numerous preclinical and clinical studies have shown the effectiveness of curcumin in the prevention and treatment of various human diseases; however, the main focus of this review is the lessons learnt from clinical trials.
Clinical studies with curcumin
Encouraging outcomes of preclinical studies have engendered ample clinical trials of curcumin to evaluate its safety and efficacy against a diverse range of human diseases (Figure 2; Tables 1 and 2). Approximately 120 clinical trials have been successfully carried out so far, involving more than 6000 human participants. In addition, there are several systematic reviews/meta‐analyses based on the clinical trials of curcumin for human data (Table 2).
Figure 2.
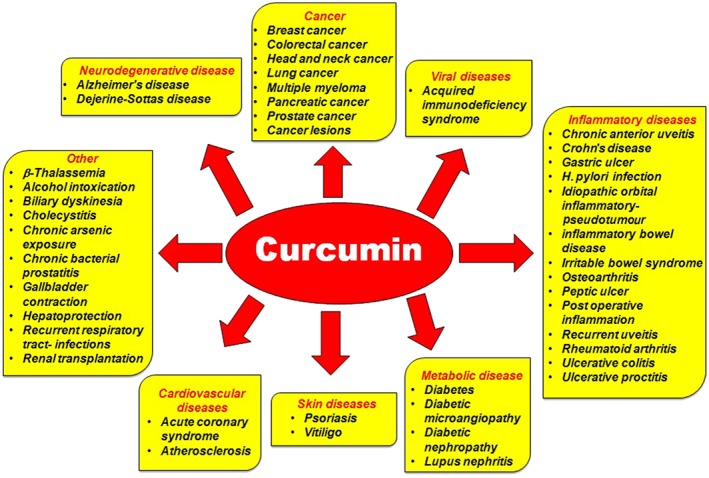
Activity of curcumin against different human diseases based on clinical findings.
Table 1.
Curcumin clinical trials in patients with various chronic diseases
| Disease | Curcumin dose | Pts (#) | Clinical outcome | References |
|---|---|---|---|---|
| Safety and tolerability | ||||
| Healthy volunteers | 2 gb , d | 10 | Safe and highly bioavailable | Shoba et al., 1998 |
| 20 mgd | 12 | Safe and induced gall‐bladder contraction | Rasyid and Lelo, 1999 | |
| 20, 40, 80 mgd | 12 | Safe and increased gall‐bladder contraction | Rasyid et al., 2002 | |
| 0.5 g; 2 daysc | 10 | Safe and no effect on iron absorption | Tuntipopipat et al., 2006 | |
| 500–12 000 mgd | 24 | Safe and well tolerated | Lao et al., 2006 | |
| 300 mg·day−1; 6 daysb | 12 | Safe | Juan et al., 2007 | |
| 10 and 12 gd | 12 | Safe and improved absorption | Vareed et al., 2008 | |
| 500 mgb , c , d | 8 | Safe and activated bowel motility | Shimouchi et al., 2009 | |
| 150 mg·day−1; 2 weeksb | 11 | Safe and well tolerated | Dominiak et al., 2010 | |
| 0.5–6 g·day−1; 7 days | 24 | Safe and decreased lipid levels | Pungcharoenkul and Thongnopnua, 2011 | |
| 30 mga , d | 14 | Safe and bioavailable | Sasaki et al., 2011 | |
| 3 × 209–376 mg·day−1 a | 9 | Safe and improved absorption | Cuomo et al., 2011 | |
| 80 mg·day−1; 4 weeks | 38 | Safe and have multiple health benefits | DiSilvestro et al., 2012 | |
| 150 mg·day−1; 8 weeks | 45 | Safe and improved BP and heart rate | Sugawara et al., 2012 | |
| 1 gd | 10 | Safe and bioavailable | Vitaglione et al., 2012 | |
| 2 gb , d | 8 | Safe and bioavailable | Kusuhara et al., 2012 | |
| 4 × 4 g; 2 daysb | 8 | Safe and highly bioavailable | Volak et al., 2013 | |
| 376 mga , d | 15 | Safe and bioavailable | Jager et al., 2014 | |
| 12 ga , d | 10 | Safe and well tolerated | Klickovic et al., 2014 | |
| Cancer | ||||
| BPH | 1 g·day−1; 24 weeksb | 61 | Reduced signs and symptoms | Ledda et al., 2012 |
| Breast | 6 g·day−1; 7 daysb | 14 | Safe and well tolerated | Bayet‐Robert et al., 2010 |
| Cancerous lesions | Ointment | 62 | Reduced lesion size and pain | Kuttan et al., 1987 |
| 0.5–1.2 g·day−1; 3 months | 25 | Well tolerated and efficacious | Cheng et al., 2001 | |
| Cervical | 500 mg·day−1; 30 days | 280 | Increased HPV clearance rate | Basu et al., 2013 |
| CML | 3 × 5 g; 6 weeksc | 50 | Reduced nitric oxide levels | Ghalaut et al., 2012 |
| Colorectal | 220 mg·day−1; 29 daysa | 15 | Inhibited basal and LPS‐induced PGE2 | Plummer et al., 2001 |
| 2.2 g·day−1 c; 4 months | 15 | Well tolerated | Sharma et al., 2001 | |
| 0.45, 3.6 g·day−1; 4 months | 15 | Well tolerated and efficacious | Sharma et al., 2004 | |
| 0.45, 1.8, 3.6 mg·day−1; 7 days | 12 | Inhibited inflammation and DNA damage | Garcea et al., 2005 | |
| 1.08 g·day−1; 10–30 days | 26 | Improved the general health | He et al., 2011 | |
| 2 or 4 g·day−1; 30 days | 44 | 40% reduction in ACF number | Carroll et al., 2011 | |
| 2.35 g·day−1; 14 days | 26 | High levels of curcumin were recovered | Irving et al., 2013 | |
| HNSCC | 2 gd | 39 | Decreased IKKβ kinase activity in saliva | Kim et al., 2011 |
| Pancreatic | 8 g·day−1; 8 weeks | 25 | Safe, well tolerated and efficacious | Dhillon et al., 2008 |
| 8 g·day−1; 4 weeksb | 17 | Showed partial response and stable disease | Epelbaum et al., 2010 | |
| 8 g·day−1; 14 days every 3 weeksb | 21 | Safe and well tolerated | Kanai et al., 2011 | |
| 0.2–0.4 g·day−1; 9 months | 16 | Safe and well tolerated | Kanai et al., 2013 | |
| Prostate | 100 mg·day−1; 6 monthsb | 85 | Reduced serum PSA levels | Ide et al., 2010 |
| 3 g·day−1; 3 months | 40 | No significant effect | Hejazi et al., 2016 | |
| Solid tumours | 3 × 100 mg·day−1;4 monthsa | 160 | Decreased side effects of chemotherapy | Belcaro et al., 2014 |
| 180 mg·day−1; 8 weeks | 80 | Improved quality of life | Panahi et al., 2014c | |
| Cardiovascular disease | ||||
| ACS | 15–60 mg·day−1; 2 years | 75 | Reduced total and LDL cholesterol | Alwi et al., 2008 |
| AMI | 4 g·day−1; 7 days | 121 | Inhibited MI associated with CABG | Wongcharoen et al., 2012 |
| CVH | 180 gc , d | 14 | Improves postprandial endothelial function | Nakayama et al., 2014 |
| Dyslipidemia | 1 g·day−1; 30 days | 30 | Decreased triglycerides level | Mohammadi et al., 2013 |
| Metabolic and CVH | 0.9 g·day−1; 24 weeksb | 56 | No effect | Soare et al., 2014 |
| MS | 1890 mg·day−1; 12 weeks | 65 | Lowered lipid level | Yang et al., 2014 |
| 1000 mg·day−1; 8 weeksb | 100 | Effective as adjunctive therapy | Panahi et al., 2014a | |
| Inflammatory diseases | ||||
| Bronchial asthma | 500 mg·day−1; 30 days | 77 | Decreased airway obstruction | Abidi et al., 2014 |
| CKD | 2 × 824 mg·day−1; 8 weeksb | 16 | Safe and well tolerated | Moreillon et al., 2013 |
| Crohn's disease | 1.1 and 1.6 g·day−1; 1 month | 5 | Efficacious | Holt et al., 2005 |
| FAP | 3 × 480 mg·day−1; 6 monthsb | 5 | Decreased number and size of adenomas | Cruz‐Correa et al., 2006 |
| Gastritis | 3 × 700 mg·day−1; 4 weeksc | 36 | No significant effect | Koosirirat et al., 2010 |
| Gingivitis | Mouthwash | 30 | Effective in mechanical periodontal therapy | Muglikar et al., 2013 |
| H. pylori infection | 2 × 30 mg·day−1; 7 days | 25 | Improved dyspeptic symptoms | Di Mario et al., 2007 |
| IBD | 1–4 g·day−1; 3 weeks | 11 | Significant decrease in relapse | Suskind et al., 2013 |
| Nephritis | 500 mg·day−1; 3 months | 24 | Decreased proteinuria, haematuria and BP | Khajehdehi et al., 2012 |
| OLP | 2000 mg·day−1; 7 weeks | 100 | Safe and well‐tolerated | Chainani‐Wu et al., 2007 |
| 6000 mg·day−1 | 20 | Safe, well‐tolerated and efficacious | Chainani‐Wu et al., 2012b | |
| 2.137 g·day−1; 30 months | 53 | Efficacious | Chainani‐Wu et al., 2012a | |
| Oral mucositis | 2 × 10 drops per daya; 21 days | 7 | Well‐tolerated and efficacious | Elad et al., 2013 |
| With honeyb , c | 60 | Inhibited oral mucositis | Francis and Williams, 2014 | |
| Osteoarthritis | 1 g·day−1 a | 100 | Safe and efficacious | Belcaro et al., 2010a |
| 200 mga | 50 | Efficacious | Belcaro et al., 2010b | |
| 1000 mg·day−1; 3 months | 44 | Served as adjuvant therapy | Pinsornsak and Niempoog, 2012 | |
| 1500 mg·day−1; 4 weeks | 185 | As effective as ibuprofen | Kuptniratsaikul et al., 2014 | |
| 1500 mg·day−1; 3 weeks | 40 | Safe and efficacious | Panahi et al., 2014b | |
| 180 mg·day−1; 8 weeksa | 45 | Efficacious | Nakagawa et al., 2014 | |
| 2 × 126 mg·day−1; 3 months | 22 | Significant improvement | Henrotin et al., 2014 | |
| Pancreatitis | 0.5 g·day−1; 6 weeksb | 20 | Reduced MDA and increased GSH | Durgaprasad et al., 2005 |
| Peptic ulcer | 3 g·day−1; 4–12 weeks | 45 | Alleviated abdominal pain and discomfort | Prucksunand et al., 2001 |
| Periodontitis | 2% gelc | 37 | Effective in scaling and root planing | Behal et al., 2011 |
| 1%·week−1; 3 weeksa | 23 | Mild to moderate beneficiary effect | Gottumukkala et al., 2013 | |
| 1%; 1, 3 and 6 monthsa | 20 | Inhibited growth of oral bacteria | Bhatia et al., 2014 | |
| 50 mg·cm−2; 6 monthsa | 60 | Reduced plaque and gingival index scores | Gottumukkala et al., 2014 | |
| Plaque | 2 × 0.1%; 21 daysc | 100 | Prevented plaque and gingivitis | Waghmare et al., 2011 |
| Prostatitis | 200 mg·day−1; 14 daysb | 284 | Improved efficacy of prulifloxacin | Cai et al., 2009 |
| Pulmonary complication | 500 mg; 4 weeks | 89 | Safe, well‐tolerated and efficacious | Panahi et al., 2015b |
| Rheumatoid arthritis | 1.2 g·day−1; 2 weeks | 18 | Reduced stiffness and joint swelling | Deodhar et al., 1980 |
| 2 × 500 mg·day−1; 8 weeksb | 45 | Reduced DAS and ACR scores | Chandran and Goel, 2012 | |
| Ulcerative colitis | 2 g·day−1; 6 months | 45 | Prevented disease relapse | Hanai et al., 2006 |
| 0.5 g·day−1; 1 year | 1 | Efficacious | Lahiff and Moss, 2011 | |
| 140 mg·day−1; 8 weeksa , b | 45 | Safe and efficacious | Singla et al., 2014 | |
| 3 g·day−1; 1 monthb | 50 | Effective, no adverse effects | Lang et al., 2015 | |
| Ulcerative proctitis | 1.1 g and 1.65 g·day−1; 1 month | 5 | Efficacious | Holt et al., 2005 |
| Uveitis | 1.125 g·day−1; 12 weeks | 53 | Efficacy equal to corticosteroid therapy | Lal et al., 1999 |
| 2 × 0.6 g·day−1; 12–18 months | 106 | Well tolerated and reduced eye discomfort | Allegri et al., 2010 | |
| Metabolic disease | ||||
| Diabetes | 5 g·day−1; 3 months | 1 | Decreased fasting blood sugar level | Srinivasan, 1972 |
| 600 mg·day−1; 8 weeks | 72 | Inhibited cytokines and oxidative stress | Usharani et al., 2008 | |
| 3 × 500 mg·day−1; 2 monthsc | 40 | Attenuated proteinuria, TGFβ and IL‐8 | Khajehdehi et al., 2011 | |
| 1.5 g·day−1; 3, 6 and 9 months | 240 | Safe, well tolerated and efficacious | Chuengsamarn et al., 2012 | |
| 300 mg·day−1; 3 months | 100 | Effective, decreased serum A‐FABP level | Na et al., 2014 | |
| 2 × 750 mg·day−1; 6 months | 240 | Lowered the atherogenic risks | Chuengsamarn et al., 2014 | |
| 500 mg·day−1; 15–30 days | – | Reduced albumin excretion, activated Nrf2 | Yang et al., 2015 | |
| 1 g·day−1; 4 weeksa | 25 | Decreased oedema score, improved response | Appendino et al., 2011 | |
| 500 mg·day−1; 4 weeksa | 38 | Efficacious | Steigerwalt et al., 2012 | |
| Obesity | 1 g·day−1; 30 days | 30 | Decreased oxidative stress | Sahebkar et al., 2013 |
| 1 g·day−1; 4 weeks | 30 | Improved immune response | Ganjali et al., 2014 | |
| 1 g·day−1; 30 days | 30 | Reduced anxiety | Esmaily et al., 2015 | |
| Neurological disease | ||||
| Alzheimer's disease | 2 and 4 g·day−1; 24 weeks | 33 | Patients' response yet to be published | Ringman et al., 2005 |
| 1 and 4 g·day−1; 6 months | 34 | Safe and increased vitamin E level | Baum et al., 2008 | |
| Depression | 500 mg·day−1; 5 weeks | 40 | Reduced symptoms | Bergman et al., 2013 |
| 1 g·day−1; 8 weeks | 56 | Reduced depression | Lopresti et al., 2014 | |
| 1000 mg·day−1; 6 weeks | 60 | Safe and efficacious | Sanmukhani et al., 2014 | |
| 10–1000 mg·day−1; 6 weeksb | 111 | Safe and efficacious | Panahi et al., 2015a | |
| 2 × 0.5 g·day−1; 8 weeks | 50 | Reduced IDS‐SR30 score | Lopresti et al., 2015 | |
| 2 × 1 g·day−1; 6 weeks | 108 | Reduced depression | Yu et al., 2015 | |
| Skin diseases | ||||
| Psoriasis | 2 × 1%·day−1; 4 weeks | 40 | Suppressed PhK activity | Heng et al., 2000 |
| 4.5 g·day−1; 16 weeksa | 12 | Showed response rate 16.7% | Kurd et al., 2008 | |
| 2 g·day−1; 12 weeks | 63 | Effective and decreased serum IL‐22 levels | Antiga et al., 2015 | |
| Radiation dermatitis | 6 g·day−1 throughout RT | 30 | Reduced severity of radiation | Ryan et al., 2013 |
| Vitiligo | 2× cream per day; 12 weeks | 10 | Improved degree of repigmentation | Asawanonda and Klahan, 2010 |
| Infectious diseases | ||||
| HIV | 2.5 g·day−1; 56 days | 40 | Well tolerated | James, 1996 |
| Tuberculosis | 6 g·day−1; 2–4 monthsa | 578 | Prevented hepatotoxicity | Adhvaryu et al., 2008 |
| Others | ||||
| Arsenic carcinogenicity | 2 × 500 mg·day−1; 3 monthsb | 286 | Reduced DNA damage | Biswas et al., 2010 |
| Cholecystectomy | 500 mg every 6 h | 50 | Improved post‐operative pain | Agarwal et al., 2011 |
| CRT | 480 mg·day−1; 1 monthb | 43 | Improved graft function, reduced rejection | Shoskes et al., 2005 |
| Déjérine‐Sottas | 50–75 mg·kg−1·day−1; 12 months | 1 | Improved patient's quality of life | Burns et al., 2009 |
| MGUS and SMM | 2 × 2 g·day−1; 3 months | 36 | Slowed the disease process | Golombick et al., 2012 |
| MGUS | 2 × 2 g·day−1; 3 months | 26 | Reduced paraprotein levels | Golombick et al., 2009 |
| Oxidative stress | 90 mgd | 10 | Reduced oxidative stress | Takahashi et al., 2014 |
| PMS | 2 capsules·day−1; 7 days | 70 | Attenuated severity of PMS symptoms | Khayat et al., 2015 |
| Pruritus | 1 g·day−1; 4 weeks | 96 | Safe, effective and anti‐inflammatory | Panahi et al., 2012a |
| Salivary pathogens | 1.5 g·L−1 | 13 | Not effective | Araujo et al., 2012 |
| Thalassemia | 3 × 500 mg·day−1; 12 months | 21 | Ameliorated oxidative damage | Kalpravidh et al., 2010 |
| VEF | 150 mg·day−1; 8 weeks | 32 | Improved endothelial function | Akazawa et al., 2012 |
Curcumin formulation.
Combination.
Turmeric.
Administered once.
AC, arsenic carcinogenicity; ACS, acute coronary syndrome; ACR, American College of Rheumatology; AMI, acute myocardial infarction; BPH, benign prostatic hyperplasia; CABG, coronary artery bypass graft; CBP, chronic bacterial prostatitis; CDAI, clinical disease activity index; CKD, chronic kidney disease; CML, chronic myeloid leukaemia; CP, chronic periodontitis; CRT, cadaveric renal transplantation; CVH, cardiovascular health; DM, diabetic microangiopathy; DR, diabetic retinopathy; DAS, disease activity score; FAP, familial adenomatous polyposis; GSH, glutathione; HC, hepatocellular carcinoma; HM, haematological malignancies; HNSCC, head and neck squamous cell carcinoma; IBD, inflammatory bowel disease; LN, lupus nephritis; MI, myocardial infarction; MDA, malonaldialdehyde; MDD, major depressive disorder; MGUS, monoclonal gammopathy of undetermined significance; MS, metabolic syndrome; OLP, oral lichen planus; PSA, prostate‐specific antigen; PMS, premenstrual syndrome; SMM, smoldering multiple myeloma; T2D, type 2 diabetes; THC, tetrahydrocurcuminoid; UC, ulcerative colitis; VEF, vascular endothelial function.
Table 2.
Systematic review/meta‐analyses based on clinical trials of curcumin for human data
| Disease | Publications analysed | Outcome | References |
|---|---|---|---|
| Skin health | PubMed and Embase till Aug 2015 | Benefits skin health | Vaughn et al., 2016 |
| Depressive disorder | Literature until Aug 2015 | Reduces depressive symptoms | Al‐Karawi et al., 2016 |
| Circulating TNF‐α | PubMed‐Medline, Scopus, Web of Science, Google Scholar till Sep 2015 | Lowers circulating TNF‐α | Sahebkar et al., 2016 |
| Painful conditions | Literature till Sep 2014 | Safe and effective | Sahebkar and Henrotin, 2016 |
| Musculoskeletal pain | CINAHL, Embase, CENTRAL, PubMed, Scopus, PsycINFO, Clinicaltrials.gov, unpublished studies | Analysis not completed | Gaffey et al., 2015 |
| IBD | Cochrane Library, Pubmed/Medline, PsychINFO, Scopus through Mar 2014 | Effective | Langhorst et al., 2015 |
| Dementia | Medline, Embase, Cochrane till Jul 2013 | Safe (Short term use) | Brondino et al., 2014 |
| Diabetes | Medline database in 2013 | Effective | Zhang et al., 2013 |
| Blood lipid levels | PubMed‐Medline, Scopus, Ovid‐AMED, Clinical trial registry, Cochrane through Sep 2012 | No effect | Sahebkar, 2014a |
| Malignant disorders | PubMed, Google J‐Gate | – | Ara et al., 2016 |
| Analgesic efficacy and safety | Scopus and Medline till Sep 2014 | Safe and effective | Sahebkar and Henrotin, 2016 |
| Circulating CRP levels | PubMed/Medline and Scopus | Reduces circulating CRP levels | Sahebkar, 2014b |
CENTRA, Cochrane Central Register of Controlled Trials; CRP, c‐reactive protein; IBD, inflammatory bowel disease
Safety and adequate daily intake (ADI) value of curcumin as well as its derivatives
In general the consumption of curcumin is considered to be safe. As per JECFA (The Joint FAO/WHO Expert Committee on Food Additives) and EFSA (European Food Safety Authority) reports, the ADI value of curcumin is 0–3 mg·kg−1 (Kocaadam and Şanlier, 2015). In addition, the safety and efficacy of curcumin was evaluated in several clinical trials involving healthy human subjects. For instance, in one such study in healthy human volunteers, the effect of curcumin combined with piperine was measured; this increased the bioavailabilty of curcumin by approximately 2000% without causing any adverse effects (Shoba et al., 1998). Furthermore, curcumin was found to exhibit positive cholekinetic effect as it induced a significant contraction of the human gall‐bladder (Rasyid and Lelo, 1999). At the dosage of 40 mg, curcumin evoked a 50% contraction of the gall bladder (Rasyid et al., 2002). A dose‐response study was undertaken to detect the maximum tolerated dose and safety of a single dose of standardized powder extract; uniformly‐milled curcumin was administered to healthy volunteers at doses ranging from 500 to 12 000 mg and it was found to be profoundly well tolerated (Lao et al., 2006). Concomitant administration of curcumin and talinolol reduced the bioavailability of talinolol possibly due to the low intraluminal curcumin concentration or an up‐regulation of further ATP‐binding cassette transporters in different tissues (Juan et al., 2007). Another study was attempted to evaluate the pharmacokinetics of a curcumin preparation in healthy human volunteers for up to 72 h following a single oral dose of curcumin. It was found to be absorbed after oral dosing in humans and was detected in plasma as glucuronide and sulfate conjugates (Vareed et al., 2008). Moreover, dietary turmeric was shown to activate bowel motility as well as carbohydrate colonic fermentation (Shimouchi et al., 2009). In addition, the ingestion of a capsule containing curcumin (30%), resveratrol (15%), EGCG (30%) and soybean extract (25%) was found to exert a protective effect against oxidative stress in normal healthy adults (Dominiak et al., 2010). Treatment with curcumin (500 mg·day−1) also markedly lowers serum cholesterol and triglyceride levels in healthy human subjects (Pungcharoenkul and Thongnopnua, 2011). The efficacy of curcumin dispersed with colloidal nano‐particles, known as Theracurmin was also investigated in terms of absorption and was compared with that of curcumin powder. However, the former showed a much higher bioavailability and thus may be of immense use with ample clinical benefits in humans even at a very low dose (Sasaki et al., 2011). Meriva, the lecithin formulation of a standardized curcuminoid mixture also exhibited a much improved absorption and plasma curcuminoid profile at significantly lower doses (Cuomo et al., 2011). Another trial in healthy middle aged people showed that treatment with curcumin caused a marked reduction in plasma triglyceride values, salivary amylase levels, plasma β amyloid protein concentrations, plasma sICAM readings, plasma alanine amino transferase activities and increased salivary radical scavenging capacities, plasma catalase activities, plasma myeloperoxidase without increasing C‐reactive protein (CRP) levels or plasma nitric oxide (DiSilvestro et al., 2012). Regular endurance exercise together with daily curcumin administration caused a marked reduction in left ventricular afterload (Sugawara et al., 2012). A formulation of curcumin in combination with a hydrophilic carrier, cellulosic derivatives and natural antioxidants was shown to enhance the bioavailability of curcumin in blood (Jager et al., 2014). On the other hand, another study indicated that the short term use of a piperine‐enhanced curcuminoid preparation is ineffective at producing a clinically significant interaction involving CYP3A, CYP2C9 or the paracetamol conjugation enzymes (Volak et al., 2013). Also in another clinical trial, oral curcumin administration was linked with poor bioavailability and was shown not to increase haemoxygenase 1 (HO‐1) in peripheral blood mononuclear cells (Klickovic et al., 2014).
Curcumin for cancer
Cancer is one of the prime health concerns today, affecting people of all ages worldwide. The first clinical trial on curcumin was done by Kuttan and colleagues in 1987 by enrolling 62 patients with external cancerous lesions to investigate its potential against cancer. An ethanolic extract of turmeric and an ointment of curcumin caused significant symptomatic relief in these patients along with a reduction in itching and smell. In 70% of the patients, dry lesions were observed and in a few cases, a reduction in lesion size and pain was observed (Kuttan et al., 1987). Henceforth, numerous clinical trials have been carried out using curcumin and its ability to affect multiple targets has enabled it to exert notable activities against different cancer types in human clinical trials (Gupta et al., 2012).
Cervical cancer
Cervical cancer is the second most common form of malignancy in women worldwide. Curcumin exhibits potent effects against this cancer in vitro, in vivo and in clinical settings. From an initial study, a dose of 500–12 000 mg·day−1 of curcumin was found to be safe, well tolerated and have chemopreventive properties against cervical cancer (Cheng et al., 2001). In another study, when HPV‐positive cervical neoplasia patients were treated with Basant polyherbal vaginal cream (containing extracts of curcumin, reetha, amla and Aloe vera), HPV clearance rate was found to be significantly high with no adverse side effects (Basu et al., 2013). These studies showed curcumin to be a safe and efficacious compound for the prevention and treatment of cervical cancer.
Colon cancer
Colon cancer ranks third among the most commonly occurring cancers in the world. Despite significant advances in cancer therapy, mortality from colon cancer persists at the same level, highlighting the necessity of improved therapies (Nautiyal et al., 2011). The efficacy of oral curcumin (2 g or 4 g daily for 30 days) in the prevention of colorectal neoplasia was evaluated in a nonrandomized, open‐label clinical trial enrolling 44 patients. The results showed a marked reduction in ACF number with 4 g dose of curcumin, which was possibly associated with its increased bioavailabity (fivefold) in plasma (Carroll et al., 2011). A dose‐response study was designed to investigate the pharmacology of curcumin in humans with doses ranging from 0.45–3.6 g·day−1 up to 4 months. A dose of 3.6 g curcumin per day caused 62 and 57% decrease in inducible PGE2 production in blood samples taken 1 h after dosing on days 1 and 29, respectively with no dose limiting toxicities (Sharma et al., 2004). Similarly in another pilot dose‐response study with curcuma extract in advanced colorectal cancer, the production of basal and LPS‐mediated PGE2 was significantly reduced in a dose‐dependent manner (Plummer et al., 2001). Administration of curcumin caused a reduction in M(1)G levels in malignant colorectal tissue, whereas COX‐2 protein levels in malignant colorectal tissue remained unaltered (Garcea et al., 2005). Furthermore, curcumin treatment has a significant impact on improving the general health of colorectal cancer patients by enhancing expression of p53 molecules in tumour cells and consequently promoting the apoptosis of tumour cells (He et al., 2011). In colorectal mucosa, pharmacologically active concentrations of curcumin were achieved after administration of curcumin C3 complex (Irving et al., 2013).
Head and neck cancer
Curcumin has also been found to have potential against head and neck cancer, which generally arises in the paranasal sinuses, nasal cavity, oral cavity, pharynx and larynx. An investigation was carried out by Kim et al. to determine the potential anti‐inflammatory effect of curcumin in HNSCC patients. Curcumin was found to suppress inflammatory cytokines such as IL‐6, IL‐8, granulocyte macrophage colony stimulating factor and TNF‐α as well as IKKβ kinase in the saliva of patients. They also suggested that IKKβ kinase could be a plausible biomarker for the detection of the effect of curcumin in head and neck cancer as curcumin inhibited IKKβ kinase activity in the saliva of HNSCC patients, and this effect was strongly correlated with the reduced expression of a number of cytokines (Kim et al., 2011).
Pancreatic cancer
Pancreatic cancer is one of the most lethal human cancers and the conventional treatment approaches have had little impact on the course of this aggressive neoplasm (Li et al., 2004). However, new therapeutic strategies based on curcumin seem to hold great promise. Studies have shown that oral curcumin is safe and well‐tolerated, and despite its limited absorption has clinical biological effects in pancreatic cancer patients. Its intake causes the down‐regulation of NF‐κB, COX‐2 and phosphorylated STAT3 in peripheral blood mononuclear cells from patients with pancreatic cancer (Dhillon et al., 2008). However, a study conducted by Epelbaum et al. to investigate the activity and feasibility of gemcitabine in combination with curcumin in advanced pancreatic cancer patients, suggested that the dose of 8 g curcumin per day is inadvisable and can be reduced by combining it with systemic gemcitabine (Epelbaum et al., 2010). Furthermore, the safety and feasibility of combination therapy using curcumin and gemcitabine was evaluated in a different study; this contradicted the previous report and suggested 8 g oral curcumin daily combined with gemcitabine‐based chemotherapy is extremely safe and practicable enough for pancreatic cancer patients (Kanai et al., 2011). Another group explored the safety of repeated administration of Theracurmin® in those pancreatic or biliary tract cancer patients who failed to respond to standard chemotherapy. Theracurmin® was administered orally, with standard gemcitabine‐based chemotherapy, starting with a dose containing 200 mg of curcumin (Level 1) and then increasing the dose to 400 mg of curcumin (Level 2). With this regime, peak plasma curcumin levels at Level 1 was found to be 324 ng·mL−1 and, at Level 2, 440 ng·mL−1. No adverse side reactions were observed and three patients continued the treatment for nine months (Kanai et al., 2013).
Other cancers
Curcumin exhibited potential against various other cancers as well in clinical settings. In an attempt to evaluate the clinical efficacy of curcuminoid therapy, a bioavailable‐boosted formulation was given to patients with solid tumours of different cancers such as colorectal, gastric, breast, sarcoma, lymphoma, prostate, bladder, oesophagus, ovary, testicles and hepatocellular carcinoma. It was observed that its supplementation suppressed systemic inflammation and significantly improved the quality of life of these patients (Panahi et al., 2014c). In a phase I clinical trial of curcumin in patients with high‐risk or pre‐malignant lesions of bladder cancer, oral leucoplakia, intestinal metaplasia of the stomach, uterine cervical intraepithelial neoplasm and Bowen's disease, the curcumin treatment was found to improve the histology of precancerous lesions (Cheng et al., 2001). In another study, a lecithinized delivery system of curcumin (Meriva®, Indena S.p.A. ‐ Viale Ortles, Milano, Italy) was shown to alleviate the adverse side effects associated with the chemo‐ and radiotherapy of different tumours, such as colon, liver, kidney, lung and stomach (Belcaro et al., 2014). Another clinical trial found that a dose of 6 g·day−1 of curcumin for seven consecutive days in every 3 weeks in combination with a standard dose of docetaxel was safe, tolerable and highly effective against breast cancer (Bayet‐Robert et al., 2010). The administration of curcumin to paediatric patients with relapsed brain tumours undergoing chemotherapy increased their response compared with the institutional controls (Wolff et al., 2012). Curcumin was also shown to possess a potent chemosensitizing effect in a study conducted with 50 chronic myeloid leukaemia patients, where the patients receiving both imatinib and curcumin showed better prognosis with reduced nitric oxide levels than the patients receiving imatinib alone (Ghalaut et al., 2012).
Curcumin for cardiovascular diseases
Cardiovascular diseases, which include acute coronary syndrome, acute myocardial infarction and dyslipidaemia, are the number one cause of mortality worldwide. There are many drugs approved for the treatment of this disease but they are not devoid of severe side effects. Therefore, the effect of curcumin has been studied in patients with this disease.
Acute coronary syndrome
Acute coronary syndrome (ACS) is used to define any group of clinical symptoms compatible with acute myocardial ischaemia (Kumar and Cannon, 2009). In a randomized controlled trial with 75 ACS patients, curcumin was evaluated for its effects on lipid levels. Curcumin was administered to the patients at increasing doses three times a day (low dose 15 mg, moderate dose 30 mg and high dose 60 mg). The findings revealed that curcumin effectively reduced the total cholesterol and low‐density lipoprotein cholesterol levels in the patients at low doses when compared with the higher doses (Alwi et al., 2008).
Acute myocardial infarction
Curcuminoid was found to reduce the myocardial infarction associated with coronary artery bypass grafting (CABG) significantly. Wongcharoen et al. evaluated the effects of curcuminoids on the frequency of acute myocardial infarction after CABG. A total of 121 patients were enrolled for this trial. The curcuminoid group exhibited lower levels of post‐operative C‐reactive protein (CRP), malondialdehyde and N‐terminal pro‐B‐type natriuretic peptide levels. These antioxidant and anti‐inflammatory effects might contribute to the cardioprotective effects of the curcuminoids (Wongcharoen et al., 2012).
Dyslipidaemia
Dyslipidaemia is a well‐established modifiable cardiovascular risk factor. Treatment of this disease is usual for the prevention of cardiovascular diseases (Cicero and Colletti, 2015). The hypolipidaemic activity of curcumin was examined in a randomized, double‐blind, placebo‐controlled, crossover trial. Supplementation of curcuminoid resulted in a decrease in the concentrations of serum triglycerides without causing any marked impact on the lipid profile, body mass index and body fat (Mohammadi et al., 2013).
Metabolic and cardiovascular health
Although dietary supplements have extensive health benefits, Soare et al. observed that a combination of dietary supplements had no cardiovascular or metabolic effects in non‐obese relatively healthy individuals. In their study, 24 weeks of dietary supplementation did not influence arterial stiffness or endothelial function, or alter body fat measurements, blood pressure, plasma lipids, glucose, insulin, insulin‐like growth factor‐1 (IGF1) and markers of inflammation and oxidative stress in non‐obese individuals (Soare et al., 2014). In contrast, it has been found that the consumption of curry spices rich in antioxidative compounds like curcumin and eugenol, improves postprandial endothelial function in healthy male subjects, which is beneficial for cardiovascular health. The participants who ate curry had an increased flow‐mediated vasodilatation response. Moreover, the presence of spices in the curry did not significantly change the systemic and forearm haemodynamics, or any biochemical parameters (Nakayama et al., 2014).
Regular consumption of curcumin is probably an alternative way of modifying cholesterol‐related parameters, as evidenced by a study that measured the effect of curcumin extract on weight, glucose and lipid profiles in patients with metabolic syndrome. At 12 weeks after intake of the curcumin extract, there was an elevation in the high‐density lipoprotein cholesterol level, whereas the level of low‐density lipoprotein cholesterol was decreased significantly (Yang et al., 2014). In another study conducted with 32 participants, curcumin was shown to increase the vascular endothelial function in postmenopausal women, which in turn decreases the risk of cardiovascular diseases (Akazawa et al., 2012).
Curcumin for inflammatory diseases
The effect of curcumin on different inflammatory diseases in humans, such as bronchial asthma, uveitis, periodontitis and inflammatory bowel diseases, has also been studied in detail.
Biliary diseases
The first clinical trial of curcumin in human diseases was done by Oppenheimer in 1937 to examine the effects of ‘curcumen’ or ‘curcunat’ (contains 0.1 to 0.25 g sodium curcumin and 0.1 g calcium cholate) on human biliary diseases. Healthy persons were subjected to an i.v. injection of 5% sodium curcumin solution, which resulted in rapid emptying of the gallbladder. Notably, one patient showed a complete cure throughout a long period of observation (Oppenheimer, 1937). In another study, Cholagogum F Nattermann (dried extracts from Schöllkraut and Curcuma) treatment caused an effective reduction in biliary dyskinesia (Niederau and Gopfert, 1999).
Bronchial asthma
Curcumin has also found to be highly effective against bronchial asthma. Abidi et al. (2014) investigated the effectiveness of curcumin as an add‐on therapy in patients with bronchial asthma. Administration of curcumin capsules improved the mean forced expiratory volume 1 s (FEV1) values, which signifies an improvement in the airway obstruction. Moreover, improved haematological parameters were also obtained (Abidi et al., 2014).
Chronic anterior uveitis
Uveitis is a major cause of vision loss worldwide. Chronic anterior uveitis (CAU) includes a heterogeneous group of diseases, of which some are idiopathic in origin (McCluskey et al., 2000). As curcumin has shown to be effective as a treatment of diverse inflammatory conditions, a few clinical trials were attempted to evaluate its efficacy against CAU of different aetiologies. The oral administration of curcumin to CAU patients improved their health and a follow‐up after 3 years indicated a 55% recurrence rate (Lal et al., 1999). Another group investigated the efficacy of oral phospholipidic curcumin on recurrent CAU of different aetiologies. The findings claimed that phospholipidic curcumin reduced the symptoms and signs of eye discomfort efficiently after a few weeks treatment in the majority of the patients (Allegri et al., 2010).
Chronic cutaneous complications
Chronic cutaneous complications are one of the major and frequent complaints of patients exposed to sulphur mustard (SM). A trial conducted by Panahi et al. investigated the effect of curcumin on serum inflammatory biomarkers such as IL‐8 and hs‐CRP and their association with the severity of a chronic cutaneous complication called pruritus. The results implied that curcumin is highly effective at lessening the inflammation in patients with chronic SM‐induced cutaneous complications, which might account for its ability to ameliorate pruritus and improve the quality of life of these patients (Panahi et al., 2012b).
Chronic periodontitis
Curcumin, being a well‐known anti‐inflammatory agent, can be used to develop an effective preventive and treatment approach for chronic periodontitis. A comparative study was conducted to measure the therapeutic efficacy of chlorhexidine (CHX) chips and indigenous curcumin‐based collagen as adjuncts to scaling and root planing in the management of chronic periodontitis through nonsurgical procedures. At the end of a 6 month study period, a decrease in plaque and gingival index scores and improved microbiological parameters, probing pocket depth and clinical attachment levels were observed in both CHX chips and curcumin‐based collagen‐treated patients (Gottumukkala et al., 2014), indicating their beneficial therapeutic effects in the nonsurgical treatment of periodontal disease. Another study carried out by the same group of investigators indicated that 1% curcumin irrigation when used as an adjunct to scaling and root planing had a mild to moderate beneficiary effect (Gottumukkala et al., 2013). In addition, 1% curcumin solution was found to cause a better resolution of inflammatory symptoms, in cases of chronic periodontitis (Suhag et al., 2007). Thus, based on the results of other experiments, a local drug‐delivery system comprising 2% whole turmeric gel, which exerts high activity, can be used as an adjunct to scaling and root planing in the treatment of periodontal pockets (Behal et al., 2011).
Gingivitis
Gingivitis is one of the most common inflammatory periodontal diseases that affect more than 80% of the world's population (Pulikkotil and Nath, 2015). Curcumin therapy holds high potential as a treatment of gingivitis. As an anti‐inflammatory, curcumin mouthwash was found to be almost as good as CHX and hence it may act as an efficacious adjunct to mechanical periodontal therapy (Muglikar et al., 2013). Similarly, the anti‐inflammatory potential of topical curcumin was found to be comparable with that of CHX‐MTZ and higher than CHX in affecting the levels of IL‐1β and CCL28 (Pulikkotil and Nath, 2015). Besides curcumin, in another clinical study turmeric mouthwash was found to be useful as an adjunct to mechanical plaque control methods in the prevention of plaque and gingivitis (Waghmare et al., 2011).
Oral mucositis
Oral mucositis is a commonly occurring problem in cancer therapy. Several in vivo studies have shown that curcumin can avert oral mucositis. In clinical settings as well, a pilot study was undertaken to measure the tolerability and efficacy of a curcumin mouthwash against oral mucositis in paediatric patients receiving doxorubicin‐based chemotherapy. Curcumin mouthwash resulted in decreased inflammatory scores, and the study documented no adverse reactions in the patients (Elad et al., 2013).
Oral lichen planus (OLP)
Oral lichen planus (OLP) is a chronic, mucocutaneous, immunological disease. Curcuminoids were assessed for their efficacy against OLP and found to be well tolerated (Chainani‐Wu et al., 2007). Another study performed by the same group suggested curcuminoids at doses of 6000 mg·day−1 in three divided doses to be well tolerated and might be of use in regulating the signs and symptoms of OLP (Chainani‐Wu et al., 2012b). Furthermore, in another controlled trial conducted with 53 patients, administration of 6000 mg·day−1 curcuminoids reduced the symptoms of OLP in 60% of the patients (Chainani‐Wu et al., 2012a).
Chronic pulmonary complications
Pulmonary complications are major and frequent chronic problems of SM intoxication. Curcuminoids were found to suppress systemic inflammation in patients with chronic pulmonary complications induced by SM. This anti‐inflammatory effect of curcuminoids was found be mediated through the modulation of inflammatory mediators such as IL‐6, IL‐8, TNF‐α, TGFβ, substance P, hs‐CRP, CGRP and MCP‐1. Curcuminoids were also found to be safe and well tolerated throughout the trial (Panahi et al., 2015b).
Chronic kidney disease
Chronic kidney disease (CKD) is characterized by reduced kidney function, enhanced inflammation and decreased antioxidants. To evaluate the effect of curcumin against CKD in humans, a study was conducted with 16 patients. A herbal supplement composed of C. longa and Boswellia serrata or placebo was given to non‐dialysis CKD patients and plasma levels of IL‐6, TNF‐α, glutathione peroxidase and serum CRP were measured. Curcumin was found to be safe and well tolerated and helped to reduce the levels of the inflammatory cytokine IL‐6 (Moreillon et al., 2013).
Gastritis
Gastritis is caused by the production of an array of inflammatory cytokines induced by Helicobacter pylori infection in the stomach. A study conducted among H. pylori‐infected gastritis patients by Koosirirat and colleagues evaluated the effect of curcumin on the production of IL‐8, IL‐1β, TNF‐α and COX‐2 in gastric mucosa. However, curcumin was ineffective at decreasing the production of these cytokines, which indicates it has a limited effect on H. pylori‐induced inflammatory cytokine production. Nevertheless, other studies have reported that the symptoms of these patients with gastritis were ameliorated by the curcumin treatment (Koosirirat et al., 2010).
Inflammatory bowel disease
Inflammatory bowel disease (IBD), which includes Crohn's disease and ulcerative colitis (UC), is a type of chronic and relapsing disorder characterized by inflammation of the gastrointestinal tract (Aguas et al., 2016). Although, mortality due to IBD is not very high, it still presents a major healthcare burden. It damages the patient's quality of life to a considerable extent due to its onset in early adulthood and chronicity (Simian et al., 2016). It enhances the risk of colorectal cancer and possibly is also associated with leukaemia and lymphoma (Wheat et al., 2016).
Considering the well‐established anti‐inflammatory potential of curcumin, a pilot study was conducted to obtain a probable dosage of curcumin for children suffering from IBD. Curcumin was well tolerated, but a consistent increase in gassiness was reported in some patients. However, other patients showed an improvement in the symptoms of the disease (Suskind et al., 2013).
Crohn's disease
Crohn's disease is an immune‐mediated IBD, which has become increasingly prevalent throughout the past decade (Lauro et al., 2016; Manuc et al., 2016). A pilot study was conducted with Crohn's disease to determine the effect of curcumin, as an addition to the existing treatments, in decreasing inflammation. This was done by reducing the doses of the other concomitant anti‐inflammatory agents. Out of five patients, four showed lower Crohn's Disease Activity Index scores and sedimentation rates (Holt et al., 2005), indicating that curcumin has potential at ameliorating inflammatory Crohn's disease.
Ulcerative colitis (UC)
UC is a commonly occurring inflammatory disease and the usefulness of curcumin in the experimental models of UC has been well demonstrated. Its efficacy was investigated in a pilot study where it was evident that use of NCB‐02 (a standardized curcumin preparation) as an enema caused greater improvements in disease activity in distal UC patients (Singla et al., 2014). In another trial, curcumin improved both the clinical activity index and endoscopic index and in turn suppressed the morbidity linked with UC. Therefore, curcumin could be an important, safe and effective alternative treatment for maintaining remission in quiescent UC patients (Hanai et al., 2006; Lang et al., 2015).
Osteoarthritis (OA)
The management of osteoarthritis remains a challenge and hence a safe and efficient treatment modality is much in demand. Several in vitro studies have demonstrated the beneficial effects of curcumin on cartilage in OA. Hence, a handful of clinical trials were undertaken (Henrotin et al., 2014). Panahi et al. showed that treatment with curcuminoids (1500 mg·day−1 in three divided doses) of OA patients resulted in a reduction in pain and physical function scores but not the stiffness score OA index. Thus, curcuminoids present a safe and highly efficacious treatment choice for OA (Panahi et al., 2014b). Another study also reported the efficacy of curcumin in the treatment of knee OA patients as evinced through the decrease of a cartilage specific biomarker, Coll2‐1 (Henrotin et al., 2014). In addition, adjuvant therapy of curcumin with diclofenac has exhibited advantageous outcome in the treatment of primary knee OA (Pinsornsak and Niempoog, 2012). In addition, turmeric extract has also shown to be safe and effective in reducing the pain and improving the function of OA patients. In a study conducted with 367 patients, administration of Curcuma domestica extracts (1500 mg·day−1 for 4 weeks) resulted in improved osteoarthritis index, and its efficacy was found to be quite comparable with that of ibuprofen (Kuptniratsaikul et al., 2014).
To improve the efficacy of curcumin, different formulations have been used for the treatment of OA patients. Theracurmin® (manufactured by Theravalues Corporation, Kioicho, Tokyo, Japan) was used by the Nakagawa and group to evaluate its improved efficacy in the treatment of patients with knee OA. Theracurmin was shown to be effective against knee OA by lowering the knee pain visual analogue scale without causing any major toxic effects (Nakagawa et al., 2014). Another formulation Meriva, a complex of curcumin with soy phosphatidylcholine, has been found to be highly effective in the clinicomanagement of OA. The report also suggested the enhanced stability and improved absorption of curcumin taken in this form, as well as improvements in the clinical and biochemical end points in OA patients (Belcaro et al., 2010b; 2014).
Peptic ulcer
Peptic ulcer is a multifactorial disease, the complications of which remain a major challenge (Farzaei et al., 2015). There is much evidence suggesting that curcumin could play a pivotal role in the management of such ulcers. Henceforth, a phase II clinical trial to measure the effect of turmeric on the healing of peptic ulcers was performed. A few patients showed a complete absence of ulcers after the 8 weeks of treatment, whereas others did not have ulcers after 12 weeks (Prucksunand et al., 2001), indicating its great efficacy against this disease.
Rheumatoid arthritis (RA)
Curcumin has displayed potent antiarthritic effects. A pilot clinical study investigated the safety and efficacy of curcumin in active rheumatoid arthritis patients and it showed an improvement in overall DAS (disease activity score) and ACR (American College of Rheumatology) scores. The safety and superiority of curcumin treatment was well evidenced (Chandran and Goel, 2012). Moreover, the curcumin treatment was also found to reduce the stiffness and swelling in the joints of patients with RA (Hanai et al., 2006).
Curcumin for metabolic disease
Curcumin have also been shown to be very effective in the management of different metabolic diseases such as diabetes and obesity.
Diabetes
Diabetes is a cluster of metabolic diseases associated with high blood sugar levels. Several pilot studies have been carried out in human participants with curcumin to measure its effect on diabetes and associated metabolic conditions. The first study of this kind showed that curcumin could effectively lower the blood sugar levels in diabetic patients. Treatment with turmeric powder resulted in a decrease in fasting blood sugar from 1400 to 700 mg·L− 1 in a patient suffering from diabetes for 16 years (Srinivasan, 1972). The intake of curcuminoids exerted a favourable effect on endothelial dysfunction along with a reduction in cytokines and markers of oxidative stress (Usharani et al., 2008). Another trial advocated curcumin's potential in delaying the development of type 2 diabetes mellitus. It ameliorated beta cell functions, elevated HOMA‐β and reduced C‐peptide levels (Chuengsamarn et al., 2012). The same group also reported that the intake of curcumin could reduce atherogenic risks and amend the metabolic profiles of high‐risk populations (Chuengsamarn et al., 2014). Similarly, in overweight/obese type 2 diabetic patients, curcuminoids lower blood glucose levels (Na et al., 2014). Furthermore, Meriva was shown to be effective in the management of diabetic microangiopathy and retinopathy (Appendino et al., 2011; Steigerwalt et al., 2012). In a recent study, it was found that curcumin treatment improved the skeletal muscle atrophy in type 1 diabetic mice through inhibition of protein ubiquitination, inflammatory cytokines and oxidative stress (Ono et al., 2015). Another initial study indicated that a novel, chemically‐modified curcumin was able to normalize wound‐healing in diabetes I‐induced rats by reducing the excessive collagenase‐2 as well as MMP‐13/collagenase‐3 (Zhang et al., 2016).
Obesity
Obesity is a global health problem and is a condition where the excess fat accumulates and exerts a negative impact on health (Ganjali et al., 2014). Curcumin has proven its effectiveness in obese patients too. It reduces the symptoms of anxiety and depression associated with obesity (Esmaily et al., 2015). Curcumin modulates circulating levels of IL‐1β, IL‐4 and VEGF, thus exhibiting an immunomodulatory effect and also reduces oxidative stress in obese patients (Sahebkar et al., 2013; Ganjali et al., 2014).
Curcumin for neurological disease
The effect of curcumin was also studied in neurological disorders such as Alzheimer's disease and depression in humans.
Alzheimer's disease
Alzheimer's disease is a progressive neurodegenerative disorder, usually affecting people older than 65 years. A randomized, double‐blind, placebo‐controlled study enrolled 34 patients with Alzheimer's disease and randomly administered curcumin at two different doses (1 or 4 g) or placebo (4 g). The curcumin treatment resulted in elevated levels of vitamin E without causing any adverse reactions through the antioxidant effects of curcuma (Baum et al., 2008; Gupta et al., 2013b).
Depression
Depression is a disorder in which many dysregulated pathways have been identified. As curcumin is known to target many pathways, its effect on depression has also been studied and it was observed that treatment with curcumin altered the biomarkers of depression and also improved the mood of the patients (Lopresti et al., 2014; Lopresti et al., 2015). A study conducted by Sanmukhani et al. confirmed curcumin to be effective and safe for the treatment of patients with major depressive disorder without concurrent suicidal ideation or other psychotic disorders (Sanmukhani et al., 2014). In another randomized, double‐blind, placebo‐controlled study, it was observed that 4 to 8 weeks of treatment with curcumin was effective at improving several mood‐related symptoms in these patients (Lopresti et al., 2014). Subsequently, the same group demonstrated that curcumin supplementation affected several biomarkers such as thromboxane B2, substance P, aldosterone, cortisol, endothelin‐1 and leptin, which might be responsible for its antidepressant effect (Lopresti et al., 2015).
Curcumin for skin diseases
Curcumin has also been shown to be very effective against various skin diseases such as psoriasis and vitiligo.
Psoriasis
Psoriasis is an autoimmune disorder characterized by patches of abnormal skin. In a clinical trial, curcumin was found to exhibit an antipsoriatic effect by altering PhK activity (Heng et al., 2000). Recently, in a randomized, double‐blind, placebo‐controlled clinical trial, patients treated with the curcumin formulation, Meriva, showed reduced disease conditions. It also increased the anti‐psoriatic effects of topical steroids in these patients when treated in combination. Thus, it was highly effective as an adjuvant therapy against psoriasis vulgaris and, notably, caused a reduction in serum levels of IL‐22 (Antiga et al., 2015). Moreover, the safety and efficacy of curcumin was documented in a phase II, open‐label, Simon's two‐stage trial where the plaque psoriasis patients received 4.5 g of oral curcuminoid C3 complex daily. The intention‐to‐treat analysis response rate was found to be 16.7%, and none of the participants had to withdraw from the study due to associated adverse events (Kurd et al., 2008).
Dermatitis
A randomized, double‐blind, placebo‐controlled clinical trial has been conducted to investigate curcumin's potential at reducing the severity of radiation‐associated dermatitis in 30 breast cancer patients. A decrease in the severity of radiation dermatitis was observed in those patients receiving 6 g·day−1 curcumin p.o. during their radiotherapy sessions (Ryan et al., 2013).
Vitiligo
Vitiligo is a chronic skin condition characterized by loss of pigmentation of the skin. The beneficial effect of curcumin on vitiligo has been demonstrated by Asawanonda and Klahan (2010); treatment with narrow band UVB plus topical tetrahydrocurcuminoid cream was found to be effective and well tolerated (Asawanonda and Klahan, 2010).
Curcumin for infectious diseases
Curcumin has also been shown to be effective in the treatment of various infectious diseases in humans.
Acquired immunodeficiency syndrome
Acquired immunodeficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV), which interferes with and weakens the immune system. A clinical trial from New England evaluated the effectiveness of curcumin as an antiviral agent in 40 AIDS patients. The patients were allotted to either a high dose group (2.5 g·day−1) or a low‐dose group in a random fashion for the treatment of 8 weeks. Though statistically insignificant, a mild increase in CD4 cells was observed in the high‐dose group and a consistent decrease in the low‐dose group. However, no evidence was obtained related to a decrease in viral load (James, 1996).
Curcumin for liver diseases
Curcumin exhibits effects against different liver diseases such as hepatitis B, hepatitis C, alcoholic liver disease, non‐alcoholic fatty liver disease, drug‐induced hepatotoxicity, liver cancer, biliary cirrhosis and primary sclerosing cholangitis. The antioxidant and inhibitory effects of curcumin on NF‐κB play a vital role in its effect against a diverse range of hepatic diseases (Nanji et al., 2003, Rivera‐Espinoza and Muriel, 2009, Nabavi et al., 2014). Curcumin was shown to reduce the liver damage in several animal models of liver injury (Bruck et al., 2007). The herbal formulation comprising of C. longa and Tinospora cordifolia was found to prevent anti‐tuberculosis treatment‐induced hepatotoxicity significantly without causing any toxic effects (Adhvaryu et al., 2008).
Other diseases
The multitargeting potential of curcumin is extended to many other diseases as well like arsenic carcinogenicity and dyspepsia. Curcumin with its intrinsic antioxidant properties could limit the toxic effects associated with arsenic (Biswas et al., 2010). It also inhibits exercise‐induced oxidative stress in humans and reduces the severity of premenstrual syndrome in women by modulating the levels of neurotransmitters and anti‐inflammatory biomolecules (Takahashi et al., 2014; Khayat et al., 2015). Administration of curcuminoids to β‐thalassemia/Hb E patients reduces oxidative damage (Kalpravidh et al., 2010). Furthermore, curcumin increased the quality of life in a 15‐year‐old Caucasian girl with Déjérine–Sottas (Burns et al., 2009). It also improves the post‐operative outcomes of patients who have undergone laparoscopic cholecystectomy (Agarwal et al., 2011). A randomized controlled trial demonstrated that curcumin, due to its anti‐inflammatory effects, can combat pruritus and improve the quality of life of these patients (Panahi et al., 2012a). Moreover, oral administration of curcumin suppresses the symptoms of lupus nephritis – inflammation of the kidney (Khajehdehi et al., 2012), and significantly reduces the paraprotein (a monoclonal protein) levels in the blood of patients with monoclonal gammopathy of undetermined significance (MGUS) (Golombick et al., 2009). In another study, curcumin slowed the disease progression of patients with MGUS and smouldering multiple myeloma (Golombick et al., 2012).
Synergy of curcumin with other nutraceuticals in the clinic
To attain an improved therapy with better efficacy and less toxicity, the effects of curcumin when used in combination with other safe agents have been investigated. Several clinical trials have attempted to explore the feasibility and tolerability of the combination of curcumin with various nutraceuticals. For example, oral curcumin with piperine can reverse lipid peroxidation efficiently in patients with tropical pancreatitis (Durgaprasad et al., 2005). Cruz‐Correa's group have evaluated the effect of a combination therapy of curcumin and quercetin to reduce adenomas in patients with familial adenomatous polyposis. The combined treatment caused a decrease in the number and size from baseline of polyps with negligible adverse reactions and no laboratory abnormalities (Cruz‐Correa et al., 2006). Rafailov and group conducted a phase I trial to evaluate the effect of a herbal preparation containing curcumin, known as ‘Zyflamend’, against prostatic intraepithelial neoplasia (PIN). The biopsy revealed benign prostatic hyperplasia alone at the end of 6 months, and after 18 months, the biopsy was negative for cancer and PIN (Rafailov et al., 2007; Sung et al., 2012). The application of Indian turmeric with honey is highly effective as a complementary therapy for oral mucositis (Francis and Williams, 2014). Moreover, Oxy‐Q bioflavonoid therapy with curcumin and quercetin improves the early graft function in dialysis‐dependent cadaveric kidney recipients (Shoskes et al., 2005). Likewise, in a cohort of 311 patients, Cinarepa, a mixture of various phytochemicals, including curcumin, chlorogenic acid, inulin and rosemary bud essential oil, was shown to suppress the symptoms of functional dyspepsia significantly (Sannia, 2010).
Curcumin has not only been combined with other natural compounds but also with different therapeutic drugs. In a prospective randomized study, the therapeutic effect of quercitin and curcumin (FlogMEV) in combination with prulifloxacin was assessed in chronic bacterial prostitis patients, and FlogMEV was found to improve the clinical efficacy of prulifloxacin (Cai et al., 2009). The efficacy of another 7‐day non‐antibiotic therapy, comprising curcumin, lactoferrin, N‐acetylcysteine and pantoprazole, at eradicating H. pylori infection and reducing gastric inflammation has also been determined. However, this therapy was not particularly effective (Di Mario et al., 2007). Nevertheless, curcumin has been found to have high potential against different diseases either alone or in combination with other agents. In addition, there are more than 100 ongoing clinical trials of curcumin (Table 3); the findings of these clinical trials can be anticipated to be of immense help in providing a better understanding of curcumin's potential and its future prospects in the clinicomanagement of various human diseases.
Table 3.
Ongoing clinical trials of curcumin for various diseases in humans
| Disease | Dose | Pts | Phase | Affiliation | Start date |
|---|---|---|---|---|---|
| Safety and tolerability | |||||
| Healthy individuals | 2, 4 gb | 12 | I | Gary N Asher; UNC‐CH USA | Mar 2011 |
| 500 mg | 23 | 0 | Jan Frank; UHOH, Germany | Oct 2011 | |
| 2790 mg·day−1; 18 months | 132 | II | Gary Small; UC, LA | Mar 2012 | |
| 80 mga | 23 | 0 | Jan Frank; UHOH, Germany | Nov 2012 | |
| NAa | 12 | I | Tetyana Pelipyagina; KGK Synergize | Apr 2015 | |
| Cancer | |||||
| ADH | 50 and 100 mg; 3 months | 30 | – | Lisa Yee; OSU, USA | June 2013 |
| Breast cancer | 500 mg | 2 | II | Andrew Mille; Emory University, USA | May 2015 |
| Curcumin gel; 4–6 ha | 180 | II | Gary Morrow, URCC NCORP | Oct 2015 | |
| Cancer | 200 mg·day−1; 28 days | 28 | I | David Hong; MDACC USA | Oct 2011 |
| 100–300 mg·m2−1; 8 weeksa | 28 | I/II | Richard Greil; Internistische Onkologie | Mar 2014 | |
| NAb | 40 | I | Aminah Jatoi; Mayo Clinic | Mar 2016 | |
| CIN | 1000 mg·day−1; 12 weeks | 14 | 0 | Carolyn Matthews; Texas Oncology | Mar 2016 |
| Colon cancer | NAb | 100 | III | Arie Figer; TASMC, Israel | Mar 2006 |
| 4 g·day−1; 30 daysa | 40 | I | Gary Asher; UNC‐CH, USA | Nov 2010 | |
| NA; 7 daysb | 35 | I | Donald Miller; JGBCC, USA | Jan 2011 | |
| 2–4 g·day−1; 6yearsb | 51 | I/II | William Steward; UHL, UK | Feb 2012 | |
| 1–4 g·day−1; 4 daysb | 20 | I | Gary Asher; UNC‐CH, USA | Jun 2013 | |
| 0.5,1 g·day−1; 28 daysa , b | 100 | II | Andrea DeCensi; Ente Ospedaliero Ospedali Galliera | Mar 2014 | |
| 100 mg·day−1b | 44 | II | Jeong‐Heum Baek; Gachon University | May 2015 | |
| 1000 mg·day−1; 2 weeksb | 14 | 0 | John Preskitt; Texas Oncology, PA | Mar 2016 | |
| EC | 2 g·day−1; 2 weeksa | 10 | II | Frederic Amant; UZ, Belgium | Oct 2013 |
| Glioblastoma | NA | 15 | 0 | Stephan Duetzmann; Goethe University Germany | Oct 2012 |
| H&NC | 8 g·day−1; 21–28 days | 33 | 0 | Cherie‐Ann Nathan; LSUHSC, USA | Jun 2010 |
| Lymphoma | NAb | 35 | II | Paolo Caimi Case; CCC, USA | Sep 2014 |
| Osteosarcoma | Curcumin powder | 24 | I/II | Manish Agarwal; TMH, India | May 2008 |
| NSCLC | 80 mg·day−1; 8 weeksa , b | 20 | I | Victor Cohen; LDI, Canada | Aug 2015 |
| Pancreatic cancer | NAb | – | III | Arber Nadir; TAU, Israel | Jun 2005 |
| Prostate cancer | NAb | 100 | II | Centre Jean Perrin | Mar 2014 |
| 120 mg·day−1; 3 daysa , b | 64 | II | Abolfazl Razzaghdoust; SBUMS, Iran | Mar 2016 | |
| Rectal cancer | 8 g·day−1 b | 45 | II | Sunil Krishnan; MDACC, USA | Aug 2008 |
| Cardiovascular disease | |||||
| CVD | NAa | 21 | – | Anwar Tandar; University of Utah,USA | Jun 2013 |
| MS | 240 mg·day−1; 6 weeks | 42 | II | Jan Frank; UHOH, Germany | Jul 2013 |
| Inflammatory diseases | |||||
| RA | 1–2 × 4 cap·day−1; 2 weeks | 40 | 0 | Dinesh Khanna;UC, USA | Jan 2010 |
| 2 and 4 g·day−1; 1 montha | 45 | I | Janet Funk; UA, USA | Nov 2015 | |
| Crohn's disease | 3 g·day−1; 6 monthsb | 122 | III | Gilles Bommelaer; UCF, France | Dec 2014 |
| FAP | 2 × 3 pills·day−1; 12 months | 50 | – | Cruz‐Correa; UPR, Puerto Rico | Nov 2007 |
| NA; 12 months | 50 | II | Francis Giardiello; NCI, USA | Oct 2010 | |
| Bowel syndrome | Coltect; 4 weeksa | 40 | II | Timna Naftali; TAU, Isreal | Apr 2011 |
| CP | 2 times; 4 weeks | 100 | IV | Agarwal; TKDC, India | Jan 2014 |
| Mucositis | 0.33–3 g·day−1; 4–6 weeks | 38 | I and II | Dhimant Patel; ABMC, USA | Feb 2015 |
| OSMF | Curcumin gel | 30 | II | SVSIDS; India | Dec 2013 |
| Orthodontis | Mouthwashb | 24 | I | Vitor H Panhóca; USP, Brazil | Jan 2014 |
| Osteoarthritis | 2 × 3 caps ·day−1; 3 monthsb | 22 | 0 | Caroline Castermans; CHL, Belgium | Mar 2012 |
| UC | 2 × 2 tab ·day−1; 2 months | 30 | – | Iris Dotan; TASMC, Israel | Nov 2008 |
| Curcumin capsules | 60 | III | Amit Assa; SCMCI, Israel | Sep 2016 | |
| 50–100 mg; 2 weeksb | 50 | III | Rupa Banerjee; AIG, India | Feb 2016 | |
| Metabolic disease | |||||
| Diabetes | 2 cap·day−1; 6 months | 70 | – | Alan Chous; Chous Eye Care Associates, USA | May 2012 |
| 500 mg | 50 | II/III | NNFTRI, Iran | Jul 2015 | |
| Neurological disease | |||||
| Alzheimer's disease | 2 or 3 g·day−1 | 26 | II | Fali Poncha ; JHRC, India | Oct 2009 |
| 800 mg·day−1 ; 6 monthsb | 80 | II | Sally Frautschy; VAORD, USA | Jan 2014 | |
| Schizophrenia | 720 mg·day−1 | 36 | I/II | Michael Davis; VAGLAHS, USA | Jul 2014 |
| 3 g·day−1; 6 months | 40 | IV | Vladimir Lerner; BMHC, Isreal | Jan 2015 | |
| 1200 mg·day−1; 8 weeks | 40 | II | Cenk Tek; Yale University, USA | Jan 2016 | |
| Skin disease | |||||
| Psoriasis | E2 per day; 28 daysa | 21 | I | Elorac, Inc. USA | Sep 2014 |
| Other | |||||
| AAA | 2 g·day−1; 2 days | 3500 | II | Amit Garg; LHRI, Canada | Nov 2011 |
| ADPKD | 25 mg·kg−1·day−1; 1 year | 68 | IV | Kristen Nowak; CU, USA | Nov 2015 |
| Bipolar disorder | 0.5–2 g·day−1; 3–8 weeks | 30 | II | Benjamin Goldstein; SHSC, Canada | Sep 2013 |
| Erectile dysfunction | 12 g·day−1; 8 weeksa | 44 | IV | Hyun Jun Park; PNUH, South Korea | Feb 2012 |
| ESRD | 3 cap·day−1; 8 weeks | 48 | I/II | SUMS, Iran | Apr 2011 |
| Fibromyalgia | 5 weeksa | 40 | – | Grégoire Cozon; HCL, France | Nov 2011 |
| H. Pylori infection | NAb | 150 | – | Gingold Rachel; RMC, Israel | Jan 2014 |
| Hyper prolactinoma | NA | 30 | I | Mashhad University of Medical Sciences | July 2011 |
| Inflammation | 2 capsulesb | 22 | – | Charles Couillard; LU, Canada | Oct 2013 |
| Kidney allografts | 2 mL of 12 mg·mL−1 a , b | 20 | I | Kaija Salmela; HUCH, Finland | Jan 2011 |
| Kidney disease | 90 mg·day−1; 6 months | 750 | III | Matthew Weir; LHRI, Canada | Sep 2015 |
| Migraine | 4 g·day−1; 2 months | 80 | IV | TUMS, Iran | Sep 2015 |
| Multiple sclerosis | 1 g·day−1 | 2780 | II | Merck KGaA; Germany | Apr 2012 |
| NAFLD | NAa | 150 | – | Giovanni de Gaetano; Neuromed IRCCS | May 2015 |
| Prostatectomy | 1 g·day−1; 6 months | 600 | II | Yair Lotan; UTSW, USA | May 2014 |
| Proteinuria | NA | 120 | III | Magdalena Madero; Inst Nacional de Cardiología | Feb 2013 |
| Vascular ageing | 500–2000 g·day−1 | 118 | – | Douglas Seals; CU, USA | Jun 2013 |
| Vascular reactivity | – | 21 | I/II | Jean‐René LUSSON; UCF, USA | Feb 2012 |
| Vascular stiffness | 200 mg·day−1 ; 7 days | 40 | I | Jamie Burr; UPEI, Canada | Nov 2014 |
Curcumin formulation.
Combination.
AAA, abdominal aortic aneurysm; ACF, aberrant crypt foci; ADH, atypical ductal hyperplasia; ADPKD, autosomal dominant polycystic kidney disease; CIN, cervical intraepithelial neoplasia; CP, chronic periodontitis; CVD, cardiovascular disease; EC, endometrial carcinoma; ESRD, end‐stage kidney disease; FAP, familial adenomatous polyposis; H&NC, head and neck cancer; MDS, myelodysplastic syndrome; MS, metabolic syndrome; NA, not available; NAFLD, non‐alcoholic fatty liver disease; NSCLC, non‐small cell lung cancer; OLP, oral lichen planus; OSMF, oral submucous fibrosis; RA, rheumatoid arthritis; T2D, type 2 diabetes; UC, ulcerative colitis.
Conclusions
There is an abundance of preclinical and clinical evidence indicating that curcumin has potential as a therapy for a wide variety of chronic diseases including cancer, cardiovascular, inflammatory, metabolic, neurological and skin diseases, and various infectious diseases. Unlike most pharmaceutical drugs, curcumin modulates multiple targets that affect different diseases. Safety, efficacy and affordability are some of the added advantages exhibited by this compound. There are also increasing lines of evidence suggesting it has a potent chemosensitizing effect in various cancers. Nevertheless, a few studies have reported that curcumin can function as an antagonistic as well. However, its therapeutic efficacy is hindered to a certain extent by its low bioavailability. Therefore, various strategies are being implemented, which include the development of curcumin analogues and formulations such as adjuvants, nanoparticles, liposomes, micelles and phospholipid complexes, to improve its bioavailability. In addition, several other approaches have been employed to enhance its bioavailability, which include altering the route of administering curcumin and obstructing the metabolic pathways via co‐treatment with other agents. Therefore, more detailed and well‐controlled clinical trials are inevitable to evaluate the efficacy of these new formulations as compared with the parental compound. Thus, the results of these further investigations are likely to increase the bioavailability, therapeutic importance and application of curcumin and make this agent a cutting edge therapeutic strategy for the prevention and treatment of a variety of chronic diseases.
Author contributions
B.B.A. and A.K. contributed to the study design and writing. D.B., G.P., J.M. and N.K.R. performed bibliographic search and artwork. S.P. contributed to proofreading and writing.
Conflict of interest
The authors declare no conflicts of interest.
Kunnumakkara, A. B. , Bordoloi, D. , Padmavathi, G. , Monisha, J. , Roy, N. K. , Prasad, S. , and Aggarwal, B. B. (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. British Journal of Pharmacology, 174: 1325–1348. doi: 10.1111/bph.13621.
Contributor Information
Ajaikumar B Kunnumakkara, Email: kunnumakkara@iitg.ernet.in.
Bharat B Aggarwal, Email: bbaggarwal@gmail.com.
References
- Abidi A, Gupta S, Agarwal M, Bhalla HL, Saluja M (2014). Evaluation of efficacy of curcumin as an add‐on therapy in patients of bronchial asthma. J Clin Diagn Res 8: HC19–HC24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhvaryu MR, Reddy N, Vakharia BC (2008). Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol 14: 4753–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal KA, Tripathi CD, Agarwal BB, Saluja S (2011). Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: a double‐blind, randomized placebo‐controlled study. Surg Endosc 25: 3805–3810. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sung B (2009). Pharmacological basis for the role of curcumin in chronic diseases: an age‐old spice with modern targets. Trends Pharmacol Sci 30: 85–94. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P (2008). Potential of spice‐derived phytochemicals for cancer prevention. Planta Med 74: 1560–1569. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Gupta SC, Sung B (2013a). Curcumin: an orally bioavailable blocker of TNF and other pro‐inflammatory biomarkers. Br J Pharmacol 169: 1672–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Yuan W, Li S, Gupta SC (2013b). Curcumin‐free turmeric exhibits anti‐inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res 57: 1529–1542. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Deb L, Prasad S (2015). Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 20: 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguas M, Hoyo JD, Faubel R, Nos P (2016). Use of telemedicine in inflammatory bowel disease: a real monitoring option? Expert Rev Gastroenterol Hepatol 10: 879–881. [DOI] [PubMed] [Google Scholar]
- Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R et al. (2012). Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res 32: 795–799. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Karawi D, Al Mamoori DA, Tayyar Y (2016). The role of curcumin administration in patients with major depressive disorder: mini meta‐analysis of clinical trials. Phytother Res 30: 175–183. [DOI] [PubMed] [Google Scholar]
- Allegri P, Mastromarino A, Neri P (2010). Management of chronic anterior uveitis relapses: efficacy of oral phospholipidic curcumin treatment. Long‐term follow‐up. Clin Ophthalmol 4: 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB et al. (2008). The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones 40: 201–210. [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007). Bioavailability of curcumin: problems and promises. Mol Pharm 4: 807–818. [DOI] [PubMed] [Google Scholar]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB (2008). Curcumin and cancer: an “old‐age” disease with an “age‐old” solution. Cancer Lett 267: 133–164. [DOI] [PubMed] [Google Scholar]
- Antiga E, Bonciolini V, Volpi W, Del Bianco E, Caproni M (2015). Oral curcumin (Meriva) is effective as an adjuvant treatment and is able to reduce IL‐22 serum levels in patients with psoriasis vulgaris. Biomed Res Int 2015: 283634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G, Belcaro G, Cornelli U, Luzzi R, Togni S, Dugall M et al. (2011). Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med 53: 43–49. [PubMed] [Google Scholar]
- Ara SA, Mudda JA, Lingappa A, Rao P (2016). Research on curcumin: a meta‐analysis of potentially malignant disorders. J Cancer Res Ther 12: 175–181. [DOI] [PubMed] [Google Scholar]
- Araujo NC, Fontana CR, Gerbi ME, Bagnato VS (2012). Overall‐mouth disinfection by photodynamic therapy using curcumin. Photomed Laser Surg 30: 96–101. [DOI] [PubMed] [Google Scholar]
- Asawanonda P, Klahan SO (2010). Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg 28: 679–684. [DOI] [PubMed] [Google Scholar]
- Basu P, Dutta S, Begum R, Mittal S, Dutta PD, Bharti AC et al. (2013). Clearance of cervical human papillomavirus infection by topical application of curcumin and curcumin containing polyherbal cream: a phase II randomized controlled study. Asian Pac J Cancer Prev 14: 5753–5759. [DOI] [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J et al. (2008). Six‐month randomized, placebo‐controlled, double‐blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28: 110–113. [DOI] [PubMed] [Google Scholar]
- Bayet‐Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C et al. (2010). Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 9: 8–14. [DOI] [PubMed] [Google Scholar]
- Behal R, Mali AM, Gilda SS, Paradkar AR (2011). Evaluation of local drug‐delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: a clinical and microbiological study. J Indian Soc Periodontol 15: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG et al. (2010a). Efficacy and safety of Meriva(R), a curcumin‐phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev 15: 337–344. [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG et al. (2010b). Product‐evaluation registry of Meriva(R), a curcumin‐phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med 52: 55–62. [PubMed] [Google Scholar]
- Belcaro G, Hosoi M, Pellegrini L, Appendino G, Ippolito E, Ricci A et al. (2014). A controlled study of a lecithinized delivery system of curcumin (Meriva(R)) to alleviate the adverse effects of cancer treatment. Phytother Res 28: 444–450. [DOI] [PubMed] [Google Scholar]
- Bergman J, Miodownik C, Bersudsky Y, Sokolik S, Lerner PP, Kreinin A et al. (2013). Curcumin as an add‐on to antidepressive treatment: a randomized, double‐blind, placebo‐controlled, pilot clinical study. Clin Neuropharmacol 36: 73–77. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Takada Y, Aggarwal BB (2004). Curcumin (diferuloylmethane) inhibits receptor activator of NF‐kappa B ligand‐induced NF‐kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol 172: 5940–5947. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Urolagin SS, Pentyala KB, Urolagin SB, K BM, Bhoi S (2014). Novel therapeutic approach for the treatment of periodontitis by curcumin. J Clin Diagn Res 8: ZC65–ZC69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas J, Sinha D, Mukherjee S, Roy S, Siddiqi M, Roy M (2010). Curcumin protects DNA damage in a chronically arsenic‐exposed population of West Bengal. Hum Exp Toxicol 29: 513–524. [DOI] [PubMed] [Google Scholar]
- Bordoloi D, Roy NK, Monisha J, Padmavathi G, Kunnumakkara AB (2016). Multi‐targeted agents in cancer cell chemosensitization: what we learnt from curcumin thus far. Recent Pat Anticancer Drug Discov 11: 67–97. [DOI] [PubMed] [Google Scholar]
- Boyanapalli SS, Tony Kong AN (2015). “Curcumin, the king of spices”: epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr Pharmacol Rep 1: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondino N, Re S, Boldrini A, Cuccomarino A, Lanati N, Barale F et al. (2014). Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. ScientificWorldJournal 2014: 174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck R, Ashkenazi M, Weiss S, Goldiner I, Shapiro H, Aeed H et al. (2007). Prevention of liver cirrhosis in rats by curcumin. Liver Int 27: 373–383. [DOI] [PubMed] [Google Scholar]
- Burns J, Joseph PD, Rose KJ, Ryan MM, Ouvrier RA (2009). Effect of oral curcumin on Dejerine–Sottas disease. Pediatr Neurol 41: 305–308. [DOI] [PubMed] [Google Scholar]
- Cai T, Mazzoli S, Bechi A, Addonisio P, Mondaini N, Pagliai RC et al. (2009). Serenoa repens associated with Urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicrob Agents 33: 549–553. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L et al. (2011). Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 4: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani‐Wu N, Silverman S Jr, Reingold A, Bostrom A, Mc Culloch C, Lozada‐Nur F et al. (2007). A randomized, placebo‐controlled, double‐blind clinical trial of curcuminoids in oral lichen planus. Phytomedicine 14: 437–446. [DOI] [PubMed] [Google Scholar]
- Chainani‐Wu N, Collins K, Silverman S Jr (2012a). Use of curcuminoids in a cohort of patients with oral lichen planus, an autoimmune disease. Phytomedicine 19: 418–423. [DOI] [PubMed] [Google Scholar]
- Chainani‐Wu N, Madden E, Lozada‐Nur F, Silverman S Jr (2012b). High‐dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J Am Acad Dermatol 66: 752–760. [DOI] [PubMed] [Google Scholar]
- Chandran B, Goel A (2012). A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 26: 1719–1725. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS et al. (2001). Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high‐risk or pre‐malignant lesions. Anticancer Res 21: 2895–2900. [PubMed] [Google Scholar]
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S (2012). Curcumin extract for prevention of type 2 diabetes. Diabetes Care 35: 2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S (2014). Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem 25: 144–150. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Colletti A (2015). Combinations of phytomedicines with different lipid lowering activity for dyslipidemia management: the available clinical data. Phytomedicine 23: 1113–1118. [DOI] [PubMed] [Google Scholar]
- Cruz‐Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD et al. (2006). Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol 4: 1035–1038. [DOI] [PubMed] [Google Scholar]
- Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ et al. (2011). Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 74: 664–669. [DOI] [PubMed] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS et al. (2007). Curcumin effects on inflammation and performance recovery following eccentric exercise‐induced muscle damage. Am J Physiol Regul Integr Comp Physiol 292: R2168–R2173. [DOI] [PubMed] [Google Scholar]
- Deodhar SD, Sethi R, Srimal RC (1980). Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 71: 632–634. [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL et al. (2008). Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14: 4491–4499. [DOI] [PubMed] [Google Scholar]
- Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V et al. (2007). A curcumin‐based 1‐week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter 12: 238–243. [DOI] [PubMed] [Google Scholar]
- DiSilvestro RA, Joseph E, Zhao S, Bomser J (2012). Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiak K, McKinney J, Heilbrun LK, Sarkar FH (2010). Critical need for clinical trials: an example of a pilot human intervention trial of a mixture of natural agents protecting lymphocytes against TNF‐alpha induced activation of NF‐kappaB. Pharm Res 27: 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgaprasad S, Pai CG, Vasanthkumar AJF, Namitha S (2005). A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res 122: 315–318. [PubMed] [Google Scholar]
- Elad S, Meidan I, Sellam G, Simaan S, Zeevi I, Waldman E et al. (2013). Topical curcumin for the prevention of oral mucositis in pediatric patients: case series. Altern Ther Health Med 19: 21–24. [PubMed] [Google Scholar]
- Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar‐Sela G (2010). Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer 62: 1137–1141. [DOI] [PubMed] [Google Scholar]
- Esmaily H, Sahebkar A, Iranshahi M, Ganjali S, Mohammadi A, Ferns G et al. (2015). An investigation of the effects of curcumin on anxiety and depression in obese individuals: a randomized controlled trial. Chin J Integr Med 21: 332–338. [DOI] [PubMed] [Google Scholar]
- Farzaei MH, Abdollahi M, Rahimi R (2015). Role of dietary polyphenols in the management of peptic ulcer. World J Gastroenterol 21: 6499–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M, Williams S (2014). Effectiveness of indian turmeric powder with honey as complementary therapy on oral mucositis : a nursing perspective among cancer patients in Mysore. Nurs J India 105: 258–260. [PubMed] [Google Scholar]
- Gaffey A, Campbell J, Porritt K, Slater H (2015). The effects of curcumin on musculoskeletal pain: a systematic review protocol. JBI Database System Rev Implement Rep 13: 59–73. [DOI] [PubMed] [Google Scholar]
- Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S et al. (2014). Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. ScientificWorldJournal 2014: 898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB et al. (2005). Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 14: 120–125. [PubMed] [Google Scholar]
- Ghalaut VS, Sangwan L, Dahiya K, Ghalaut PS, Dhankhar R, Saharan R (2012). Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J Oncol Pharm Pract 18: 186–190. [DOI] [PubMed] [Google Scholar]
- Goel A, Aggarwal BB (2010). Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 62: 919–930. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB (2008). Curcumin as “curecumin": from kitchen to clinic. Biochem Pharmacol 75: 787–809. [DOI] [PubMed] [Google Scholar]
- Golombick T, Diamond TH, Badmaev V, Manoharan A, Ramakrishna R (2009). The potential role of curcumin in patients with monoclonal gammopathy of undefined significance – its effect on paraproteinemia and the urinary N‐telopeptide of type I collagen bone turnover marker. Clin Cancer Res 15: 5917–5922. [DOI] [PubMed] [Google Scholar]
- Golombick T, Diamond TH, Manoharan A, Ramakrishna R (2012). Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double‐blind placebo‐controlled cross‐over 4 g study and an open‐label 8 g extension study. Am J Hematol 87: 455–460. [DOI] [PubMed] [Google Scholar]
- Gottumukkala SN, Koneru S, Mannem S, Mandalapu N (2013). Effectiveness of sub gingival irrigation of an indigenous 1% curcumin solution on clinical and microbiological parameters in chronic periodontitis patients: a pilot randomized clinical trial. Contemp Clin Dent 4: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottumukkala SN, Sudarshan S, Mantena SR (2014). Comparative evaluation of the efficacy of two controlled release devices: chlorhexidine chips and indigenous curcumin based collagen as local drug delivery systems. Contemp Clin Dent 5: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010). Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29: 405–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Kannappan R, Reuter S, Dougherty PM, Aggarwal BB (2011a). Role of nuclear factor kappaB‐mediated inflammatory pathways in cancer‐related symptoms and their regulation by nutritional agents. Exp Biol Med (Maywood) 236: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK et al. (2011b). Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep 28: 1937–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Koh W, Aggarwal BB (2012). Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kismali G, Aggarwal BB (2013a). Curcumin, a component of turmeric: from farm to pharmacy. Biofactors 39: 2–13. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB (2013b). Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15: 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB (2013c). Multitargeting by turmeric, the golden spice: from kitchen to clinic. Mol Nutr Food Res 57: 1510–1528. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Tyagi AK, Deshmukh‐Taskar P, Hinojosa M, Prasad S, Aggarwal BB (2014). Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys 559: 91–99. [DOI] [PubMed] [Google Scholar]
- Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A et al. (2006). Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double‐blind, placebo‐controlled trial. Clin Gastroenterol Hepatol 4: 1502–1506. [DOI] [PubMed] [Google Scholar]
- Hasima N, Aggarwal BB (2012). Cancer‐linked targets modulated by curcumin. Int J Biochem Mol Biol 3: 328–351. [PMC free article] [PubMed] [Google Scholar]
- Hasima N, Aggarwal BB (2014). Targeting proteasomal pathways by dietary curcumin for cancer prevention and treatment. Curr Med Chem 21: 1583–1594. [DOI] [PubMed] [Google Scholar]
- He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J (2011). Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest 29: 208–213. [DOI] [PubMed] [Google Scholar]
- Hejazi J, Rastmanesh R, Taleban FA, Molana SH, Hejazi E, Ehtejab G et al. (2016). Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: a double blinded, randomized, placebo‐controlled study. Nutr Cancer 68: 77–85. [DOI] [PubMed] [Google Scholar]
- Heng MC, Song MK, Harker J, Heng MK (2000). Drug‐induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol 143: 937–949. [DOI] [PubMed] [Google Scholar]
- Henrotin Y, Gharbi M, Dierckxsens Y, Priem F, Marty M, Seidel L et al. (2014). Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2‐1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement Altern Med 14: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PR, Katz S, Kirshoff R (2005). Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci 50: 2191–2193. [DOI] [PubMed] [Google Scholar]
- Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, Muto S et al. (2010). Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate‐specific antigen. Prostate 70: 1127–1133. [DOI] [PubMed] [Google Scholar]
- Irving GR, Howells LM, Sale S, Kralj‐Hans I, Atkin WS, Clark SK et al. (2013). Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration – a clinical pilot study including assessment of patient acceptability. Cancer Prev Res (Phila) 6: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM (2014). Comparative absorption of curcumin formulations. Nutr J 13: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JS (1996). Curcumin: clinical trial finds no antiviral effect. AIDS Treat News : 1–2. [PubMed] [Google Scholar]
- Juan H, Terhaag B, Cong Z, Bi‐Kui Z, Rong‐Hua Z, Feng W et al. (2007). Unexpected effect of concomitantly administered curcumin on the pharmacokinetics of talinolol in healthy Chinese volunteers. Eur J Clin Pharmacol 63: 663–668. [DOI] [PubMed] [Google Scholar]
- Kalpravidh RW, Siritanaratkul N, Insain P, Charoensakdi R, Panichkul N, Hatairaktham S et al. (2010). Improvement in oxidative stress and antioxidant parameters in beta‐thalassemia/Hb E patients treated with curcuminoids. Clin Biochem 43: 424–429. [DOI] [PubMed] [Google Scholar]
- Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S et al. (2011). A phase I/II study of gemcitabine‐based chemotherapy plus curcumin for patients with gemcitabine‐resistant pancreatic cancer. Cancer Chemother Pharmacol 68: 157–164. [DOI] [PubMed] [Google Scholar]
- Kanai M, Otsuka Y, Otsuka K, Sato M, Nishimura T, Mori Y et al. (2013). A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol 71: 1521–1530. [DOI] [PubMed] [Google Scholar]
- Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH et al. (2011). Oral supplementation of turmeric attenuates proteinuria, transforming growth factor‐beta and interleukin‐8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double‐blind and placebo‐controlled study. Scand J Urol Nephrol 45: 365–370. [DOI] [PubMed] [Google Scholar]
- Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L et al. (2012). Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo‐controlled study. J Ren Nutr 22: 50–57. [DOI] [PubMed] [Google Scholar]
- Khayat S, Fanaei H, Kheirkhah M, Moghadam ZB, Kasaeian A, Javadimehr M (2015). Curcumin attenuates severity of premenstrual syndrome symptoms: a randomized, double‐blind, placebo‐controlled trial. Complement Ther Med 23: 318–324. [DOI] [PubMed] [Google Scholar]
- Kim SG, Veena MS, Basak SK, Han E, Tajima T, Gjertson DW et al. (2011). Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res 17: 5953–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickovic U, Doberer D, Gouya G, Aschauer S, Weisshaar S, Storka A et al. (2014). Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase‐1 expression in healthy male subjects. Biomed Res Int 2014: 458592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaadam B, Şanlier N (2015). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 3: 0. [DOI] [PubMed] [Google Scholar]
- Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J (2010). Investigation of the anti‐inflammatory effect of Curcuma longa in Helicobacter pylori‐infected patients. Int Immunopharmacol 10: 815–818. [DOI] [PubMed] [Google Scholar]
- Kumar A, Cannon CP (2009). Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 84: 917–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara AB, Anand P, Aggarwal BB (2008). Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269: 199–225. [DOI] [PubMed] [Google Scholar]
- Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C et al. (2014). Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging 9: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT et al. (2008). Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol 58: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuhara H, Furuie H, Inano A, Sunagawa A, Yamada S, Wu C et al. (2012). Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br J Pharmacol 166: 1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan R, Sudheeran PC, Josph CD (1987). Turmeric and curcumin as topical agents in cancer therapy. Tumori 73: 29–31. [DOI] [PubMed] [Google Scholar]
- Lahiff C, Moss AC (2011). Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm Bowel Dis 17: E66. [DOI] [PubMed] [Google Scholar]
- Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P et al. (1999). Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res 13: 318–322. [DOI] [PubMed] [Google Scholar]
- Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har‐Noy O et al. (2015). Curcumin in combination with mesalamine induces remission in patients with mild‐to‐moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol 13: 1444–1449 e1441. [DOI] [PubMed] [Google Scholar]
- Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ et al. (2015). Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis 9: 86–106. [DOI] [PubMed] [Google Scholar]
- Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM et al. (2006). Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro ML, Burch JM, Grimes CL (2016). The effect of NOD2 on the microbiota in Crohn's disease. Curr Opin Biotechnol 40: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda A, Belcaro G, Dugall M, Luzzi R, Scoccianti M, Togni S et al. (2012). Meriva(R), a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: a pilot, product evaluation registry study. Panminerva Med 54: 17–22. [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL (2004). Pancreatic cancer. Lancet 363: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD (2014). Curcumin for the treatment of major depression: a randomised, double‐blind, placebo controlled study. J Affect Disord 167: 368–375. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Maes M, Meddens MJ, Maker GL, Arnoldussen E, Drummond PD (2015). Curcumin and major depression: a randomised, double‐blind, placebo‐controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur Neuropsychopharmacol 25: 38–50. [DOI] [PubMed] [Google Scholar]
- Manuc TE, Manuc MM, Diculescu MM (2016). Recent insights into the molecular pathogenesis of Crohn's disease: a review of emerging therapeutic targets. Clin Exp Gastroenterol 9: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey PJ, Towler HM, Lightman S (2000). Management of chronic uveitis. BMJ 320: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi A, Sahebkar A, Iranshahi M, Amini M, Khojasteh R, Ghayour‐Mobarhan M et al. (2013). Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial. Phytother Res 27: 374–379. [DOI] [PubMed] [Google Scholar]
- Moreillon JJ, Bowden RG, Deike E, Griggs J, Wilson R, Shelmadine B et al. (2013). The use of an anti‐inflammatory supplement in patients with chronic kidney disease. J Complement Integr Med 10. pii: /j/jcim.2013.10.issue‐1/jcim‐2012‐0011/jcim‐2012‐0011.xml. [DOI] [PubMed] [Google Scholar]
- Muglikar S, Patil KC, Shivswami S, Hegde R (2013). Efficacy of curcumin in the treatment of chronic gingivitis: a pilot study. Oral Health Prev Dent 11: 81–86. [DOI] [PubMed] [Google Scholar]
- Na LX, Yan BL, Jiang S, Cui HL, Li Y, Sun CH (2014). Curcuminoids target decreasing serum adipocyte‐fatty acid binding protein levels in their glucose‐lowering effect in patients with type 2 diabetes. Biomed Environ Sci 27: 902–906. [DOI] [PubMed] [Google Scholar]
- Nabavi SF, Daglia M, Moghaddam AH, Habtemariam S, Nabavi SM (2014). Curcumin and liver disease: from chemistry to medicine. Compr Rev Food Sci Food Saf 13: 62–77. [DOI] [PubMed] [Google Scholar]
- Nair HB, Sung B, Yadav VR, Kannappan R, Chaturvedi MM, Aggarwal BB (2010). Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol 80: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Mukai S, Yamada S, Matsuoka M, Tarumi E, Hashimoto T et al. (2014). Short‐term effects of highly‐bioavailable curcumin for treating knee osteoarthritis: a randomized, double‐blind, placebo‐controlled prospective study. J Orthop Sci 19: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Tsuge N, Sawada H, Masamura N, Yamada S, Satomi S et al. (2014). A single consumption of curry improved postprandial endothelial function in healthy male subjects: a randomized, controlled crossover trial. Nutr J 13: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ (2003). Curcumin prevents alcohol‐induced liver disease in rats by inhibiting the expression of NF‐kappa B‐dependent genes. Am J Physiol Gastrointest Liver Physiol 284: G321–G327. [DOI] [PubMed] [Google Scholar]
- Nautiyal J, Banerjee S, Kanwar SS, Yu Y, Patel BB, Sarkar FH et al. (2011). Curcumin enhances dasatinib‐induced inhibition of growth and transformation of colon cancer cells. Int J Cancer 128: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C, Gopfert E (1999). The effect of chelidonium‐ and turmeric root extract on upper abdominal pain due to functional disorders of the biliary system. Results from a placebo‐controlled double‐blind study. Med Klin (Munich) 94: 425–430. [DOI] [PubMed] [Google Scholar]
- Ono T, Takada S, Kinugawa S, Tsutsui H (2015). Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp Physiol 100: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Oppenheimer A (1937). Turmeric (curcumin) in biliary diseases. Lancet 229: 619–621. [Google Scholar]
- Panahi Y, Sahebkar A, Amiri M, Davoudi SM, Beiraghdar F, Hoseininejad SL et al. (2012a). Improvement of sulphur mustard‐induced chronic pruritus, quality of life and antioxidant status by curcumin: results of a randomised, double‐blind, placebo‐controlled trial. Br J Nutr 108: 1272–1279. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Sahebkar A, Parvin S, Saadat A (2012b). A randomized controlled trial on the anti‐inflammatory effects of curcumin in patients with chronic sulphur mustard‐induced cutaneous complications. Ann Clin Biochem 49: 580–588. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A (2014a). Lipid‐modifying effects of adjunctive therapy with curcuminoids‐piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med 22: 851–857. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A (2014b). Curcuminoid treatment for knee osteoarthritis: a randomized double‐blind placebo‐controlled trial. Phytother Res 28: 1625–1631. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Saadat A, Beiraghdar F, Sahebkar A (2014c). Adjuvant therapy with bioavailability‐boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double‐blind placebo‐controlled trial. Phytother Res 28: 1461–1467. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Badeli R, Karami GR, Sahebkar A (2015a). Investigation of the efficacy of adjunctive therapy with bioavailability‐boosted curcuminoids in major depressive disorder. Phytother Res 29: 17–21. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Ghanei M, Bashiri S, Hajihashemi A, Sahebkar A (2015b). Short‐term curcuminoid supplementation for chronic pulmonary complications due to sulfur mustard intoxication: positive results of a randomized double‐blind placebo‐controlled trial. Drug Res (Stuttg) 65: 567–573. [DOI] [PubMed] [Google Scholar]
- Pinsornsak P, Niempoog S (2012). The efficacy of Curcuma longa L. extract as an adjuvant therapy in primary knee osteoarthritis: a randomized control trial. J Med Assoc Thai 95 (Suppl 1): S51–S58. [PubMed] [Google Scholar]
- Plummer SM, Hill KA, Festing MF, Steward WP, Gescher AJ, Sharma RA (2001). Clinical development of leukocyte cyclooxygenase 2 activity as a systemic biomarker for cancer chemopreventive agents. Cancer Epidemiol Biomarkers Prev 10: 1295–1299. [PubMed] [Google Scholar]
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB (2014a). Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv 32: 1053–1064. [DOI] [PubMed] [Google Scholar]
- Prasad S, Tyagi AK, Aggarwal BB (2014b). Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K (2001). Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health 32: 208–215. [PubMed] [Google Scholar]
- Pulikkotil SJ, Nath S (2015). Effects of curcumin on crevicular levels of IL‐1beta and CCL28 in experimental gingivitis. Aust Dent J 60: 317–327. [DOI] [PubMed] [Google Scholar]
- Pungcharoenkul K, Thongnopnua P (2011). Effect of different curcuminoid supplement dosages on total in vivo antioxidant capacity and cholesterol levels of healthy human subjects. Phytother Res 25: 1721–1726. [DOI] [PubMed] [Google Scholar]
- Rafailov S, Cammack S, Stone BA, Katz AE (2007). The role of Zyflamend, an herbal anti‐inflammatory, as a potential chemopreventive agent against prostate cancer: a case report. Integr Cancer Ther 6: 74–76. [DOI] [PubMed] [Google Scholar]
- Rainey N, Motte L, Aggarwal BB, Petit PX (2015). Curcumin hormesis mediates a cross‐talk between autophagy and cell death. Cell Death Dis 6: e2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasyid A, Lelo A (1999). The effect of curcumin and placebo on human gall‐bladder function: an ultrasound study. Aliment Pharmacol Ther 13: 245–249. [DOI] [PubMed] [Google Scholar]
- Rasyid A, Rahman AR, Jaalam K, Lelo A (2002). Effect of different curcumin dosages on human gall bladder. Asia Pac J Clin Nutr 11: 314–318. [DOI] [PubMed] [Google Scholar]
- Ravindran J, Prasad S, Aggarwal BB (2009). Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J 11: 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Aggarwal BB (1994). Curcumin is a non‐competitive and selective inhibitor of phosphorylase kinase. FEBS Lett 341: 19–22. [DOI] [PubMed] [Google Scholar]
- Remely M, Lovrecic L, de la Garza AL, Migliore L, Peterlin B, Milagro FI et al. (2015). Therapeutic perspectives of epigenetically active nutrients. Br J Pharmacol 172: 2756–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB (2011). Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr 6: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL (2005). A Potential role of the curry spice curcumin in Alzheimer's disease. Current Alzheimer Research 2: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera‐Espinoza Y, Muriel P (2009). Pharmacological actions of curcumin in liver diseases or damage. Liver Int 29: 1457–1466. [DOI] [PubMed] [Google Scholar]
- Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP et al. (2013). Curcumin for radiation dermatitis: a randomized, double‐blind, placebo‐controlled clinical trial of thirty breast cancer patients. Radiat Res 180: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebkar A (2014a). A systematic review and meta‐analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin Nutr 33: 406–414. [DOI] [PubMed] [Google Scholar]
- Sahebkar A (2014b). Are curcuminoids effective C‐reactive protein‐lowering agents in clinical practice? Evidence from a meta‐analysis. Phytother Res 28: 633–642. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Henrotin Y (2016). Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta‐analysis of randomized controlled trials. Pain Med 17: 1192–1202. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Mohammadi A, Atabati A, Rahiman S, Tavallaie S, Iranshahi M et al. (2013). Curcuminoids modulate pro‐oxidant‐antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother Res 27: 1883–1888. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Cicero AF, Simental‐Mendía LE, Aggarwal BB, Gupta SC (2016). Curcumin downregulates human tumor necrosis factor‐α levels: a systematic review and meta‐analysis ofrandomized controlled trials. Pharmacol Res 107: 234–242. [DOI] [PubMed] [Google Scholar]
- Sahu PK, Sahu PK, Sahu PL, Agarwal DD (2016). Structure activity relationship, cytotoxicity and evaluation of antioxidant activity of curcumin derivatives. Bioorg Med Chem Lett 26: 1342–1347. [DOI] [PubMed] [Google Scholar]
- Sanmukhani J, Satodia V, Trivedi J, Patel T, Tiwari D, Panchal B et al. (2014). Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother Res 28: 579–585. [DOI] [PubMed] [Google Scholar]
- Sannia A (2010). Phytotherapy with a mixture of dry extracts with hepato‐protective effects containing artichoke leaves in the management of functional dyspepsia symptoms. Minerva Gastroenterol Dietol 56: 93–99. [PubMed] [Google Scholar]
- Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T et al. (2011). Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 34: 660–665. [DOI] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM et al. (2001). Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7: 1894–1900. [PubMed] [Google Scholar]
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR et al. (2004). Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10: 6847–6854. [DOI] [PubMed] [Google Scholar]
- Shimouchi A, Nose K, Takaoka M, Hayashi H, Kondo T (2009). Effect of dietary turmeric on breath hydrogen. Dig Dis Sci 54: 1725–1729. [DOI] [PubMed] [Google Scholar]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64: 353–356. [DOI] [PubMed] [Google Scholar]
- Shoskes D, Lapierre C, Cruz‐Correa M, Muruve N, Rosario R, Fromkin B et al. (2005). Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation 80: 1556–1559. [DOI] [PubMed] [Google Scholar]
- Simian D, Fluxá D, Flores L, Lubascher J, Ibáñez P, Figueroa C et al. (2016). Inflammatory bowel disease: a descriptive study of 716 local Chilean patients. World J Gastroenterol 22: 5267–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P et al. (2014). Induction with NCB‐02 (curcumin) enema for mild‐to‐moderate distal ulcerative colitis – a randomized, placebo‐controlled, pilot study. J Crohns Colitis 8: 208–214. [DOI] [PubMed] [Google Scholar]
- Soare A, Weiss EP, Holloszy JO, Fontana L (2014). Multiple dietary supplements do not affect metabolic and cardio‐vascular health. Aging (Albany NY) 6: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M (1972). Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci 26: 269–270. [PubMed] [Google Scholar]
- Steigerwalt R, Nebbioso M, Appendino G, Belcaro G, Ciammaichella G, Cornelli U et al. (2012). Meriva(R), a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med 54: 11–16. [PubMed] [Google Scholar]
- Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T et al. (2012). Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens 25: 651–656. [DOI] [PubMed] [Google Scholar]
- Suhag A, Dixit J, Dhan P (2007). Role of curcumin as a subgingival irrigant: a pilot study. PERIO: Periodontal Pract Today 2: 115–121. [Google Scholar]
- Sung B, Prasad S, Yadav VR, Aggarwal BB (2012). Cancer cell signaling pathways targeted by spice‐derived nutraceuticals. Nutr Cancer 64: 173–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W (2013). Tolerability of curcumin in pediatric inflammatory bowel disease: a forced‐dose titration study. J Pediatr Gastroenterol Nutr 56: 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Suzuki K, Kim HK, Otsuka Y, Imaizumi A, Miyashita M et al. (2014). Effects of curcumin supplementation on exercise‐induced oxidative stress in humans. Int J Sports Med 35: 469–475. [DOI] [PubMed] [Google Scholar]
- Tuntipopipat S, Judprasong K, Zeder C, Wasantwisut E, Winichagoon P, Charoenkiatkul S et al. (2006). Chili, but not turmeric, inhibits iron absorption in young women from an iron‐fortified composite meal. J Nutr 136: 2970–2974. [DOI] [PubMed] [Google Scholar]
- Tuorkey MJ (2014). Curcumin a potent cancer preventive agent: mechanisms of cancer cell killing. Interv Med Appl Sci 6: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi AK, Prasad S, Yuan W, Li S, Aggarwal BB (2015). Identification of a novel compound (beta‐sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: comparison with curcumin. Invest New Drugs 33: 1175–1186. [DOI] [PubMed] [Google Scholar]
- Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N (2008). Effect of NCB‐02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel‐group, placebo‐controlled, 8‐week study. Drugs R D 9: 243–250. [DOI] [PubMed] [Google Scholar]
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z et al. (2008). Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 17: 1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res 2016. doi: 10.1002/ptr.5640. [Epub ahead of print] Review. PubMed PMID: 27213821. [DOI] [PubMed] [Google Scholar]
- Vitaglione P, Barone Lumaga R, Ferracane R, Radetsky I, Mennella I, Schettino R et al. (2012). Curcumin bioavailability from enriched bread: the effect of microencapsulated ingredients. J Agric Food Chem 60: 3357–3366. [DOI] [PubMed] [Google Scholar]
- Volak LP, Hanley MJ, Masse G, Hazarika S, Harmatz JS, Badmaev V et al. (2013). Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br J Clin Pharmacol 75: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS (2011). Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study. J Contemp Dent Pract 12: 221–224. [DOI] [PubMed] [Google Scholar]
- Wheat CL, Clark‐Snustad K, Devine B, Grembowski D, Thornton TA, Ko CW (2016). Worldwide incidence of colorectal cancer, leukemia, and lymphoma in inflammatory bowel disease: an updated systematic review and meta‐analysis. Gastroenterol Res Pract 2016 .1632439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JE, Brown RE, Buryanek J, Pfister S, Vats TS, Rytting ME (2012). Preliminary experience with personalized and targeted therapy for pediatric brain tumors. Pediatr Blood Cancer 59: 27–33. [DOI] [PubMed] [Google Scholar]
- Wongcharoen W, Jai‐Aue S, Phrommintikul A, Nawarawong W, Woragidpoonpol S, Tepsuwan T et al. (2012). Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am J Cardiol 110: 40–44. [DOI] [PubMed] [Google Scholar]
- Yadav VR, Aggarwal BB (2011). Curcumin: a component of the golden spice, targets multiple angiogenic pathways. Cancer Biol Ther 11: 236–241. [DOI] [PubMed] [Google Scholar]
- Yang YS, Su YF, Yang HW, Lee YH, Chou JI, Ueng KC (2014). Lipid‐lowering effects of curcumin in patients with metabolic syndrome: a randomized, double‐blind, placebo‐controlled trial. Phytother Res 28: 1770–1777. [DOI] [PubMed] [Google Scholar]
- Yang H, Xu W, Zhou Z, Liu J, Li X, Chen L et al. (2015). Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid‐derived 2‐like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp Clin Endocrinol Diabetes 123: 360–367. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL (2015). Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: a randomized, double‐blind, placebo‐controlled pilot study. J Clin Psychopharmacol 35: 406–410. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Fu M, Gao SH, Liu JL (2013). Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med 2013: 636053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McClain SA, Lee HM, Elburki MS, Yu H, Gu Y et al. (2016). A novel chemically modified curcumin “normalizes” wound‐healing in rats with experimentally induced type I diabetes: initial studies. J Diabetes Res 2016: 5782904. [DOI] [PMC free article] [PubMed] [Google Scholar]


