Abstract
Ageing, an unanswered question in the medical field, is a multifactorial process that results in a progressive functional decline in cells, tissues and organisms. Although it is impossible to prevent ageing, slowing down the rate of ageing is entirely possible to achieve. Traditional Chinese medicine (TCM) is characterized by the nourishing of life and its role in anti‐ageing is getting more and more attention. This article summarizes the work done on the natural products from TCM that are reported to have anti‐ageing effects, in the past two decades. The effective anti‐ageing ingredients identified can be generally divided into flavonoids, saponins, polysaccharides, alkaloids and others. Astragaloside, Cistanche tubulosa acteoside, icariin, tetrahydrocurcumin, quercetin, butein, berberine, catechin, curcumin, epigallocatechin gallate, gastrodin, 6‐Gingerol, glaucarubinone, ginsenoside Rg1, luteolin, icarisid II, naringenin, resveratrol, theaflavin, carnosic acid, catalpol, chrysophanol, cycloastragenol, emodin, galangin, echinacoside, ferulic acid, huperzine, honokiol, isoliensinine, phycocyanin, proanthocyanidins, rosmarinic acid, oxymatrine, piceid, puerarin and salvianolic acid B are specified in this review. Simultaneously, chemical structures of the monomers with anti‐ageing activities are listed, and their source, model, efficacy and mechanism are also described. The TCMs with anti‐ageing function are classified according to their action pathways, including the telomere and telomerase, the sirtuins, the mammalian target of rapamycin, AMP‐activated kinase and insulin/insulin‐like growth factor‐1 signalling pathway, free radicals scavenging and the resistance to DNA damage. Finally, Chinese compound prescription and extracts related to anti‐ageing are introduced, which provides the basis and the direction for the further development of novel and potential drugs.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- AMPK
AMP‐activated kinase
- AST
astragaloside
- CAG
cycloastragenol
- CCP
Chinese compound prescription
- CR
caloric restriction
- GSH‐Px
GSH peroxidase
- INS/IGF‐1
insulin/insulin‐like growth factor‐1
- JKSQ
Jinkui Shenqi
- LWDH
Liuwei Dihuang
- MDA
methane dicarboxylic aldehyde
- mTOR
mammalian target of rapamycin
- NRF2
nuclear factor erythroid 2‐related factor 2
- P. ginseng
Panax ginseng
- R. puerariae
Radix puerariae
- Sal B
salvia acid B
- SIRTs
sirtuins
- SIRT1
sirtuin1
- SIRT6
sirtuin6
- sMaf
small muscle aponeurotic fibrosarcoma
- S6 K
ribosomal protein S6 kinase
- TCM
traditional Chinese medicine
- TFE
total flavones from Epimedium brevicornu
- TOR
target of rapamycin
- VSMC
vascular smooth muscle cells
Tables of Links
| TARGETS | |
|---|---|
| Enzymes a | Transporters b |
| AMPK | GLUT4 |
| Mammalian target of rapamycin | |
| S6K | |
| Sirtuin 1 | |
| Sirtuin 2 | |
| Sirtuin 6 |
| LIGANDS | |
|---|---|
| AMP | Insulin‐like growth factor 1 |
| Angiotensin II | Luteolin |
| ATP | Quercetin |
| D‐galactose | Rapamycin |
| Epigallocatechin gallate | Resveratrol |
| 6‐Gingerol |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al. 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Ageing is the major risk factor for several life‐threatening diseases. Ageing, a complex molecular process driven by diverse molecular pathways and biochemical events that are influenced by interplay of multiple genetic and environmental factors, could lead to progressive and deleterious changes in the whole organism (Ideker et al. 2001; Argyropoulou et al. 2013; Wong et al. 2003). A myriad of theories including mitochondrial mutation, oxidative damage, carbonyl toxification and free radical theory, which is currently the most widely accepted one, have been used to explain the mechanisms underlying the phenomenon of senescence (Yin and Chen 2005).
Excessive amounts of free radicals can attack cell membrane, nucleic acids, proteins, enzymes and other biological macromolecules through peroxidation, causing lipid peroxidation of unsaturated fatty acids on the cell membrane, cross linking of nucleic acid and protein molecules, abnormality of DNA mutation or replication, together with decline of enzyme activity, which consequently leads to serious damage on cell function and eventually results in senility and even death (Huang 2007). There are a number of papers and reviews supporting or questioning this theory (Alexeyev 2009; Lapointe and Hekimi 2010; Ristow and Schmeisser 2011). Moreover, a variety of molecular pathways have been identified as the main molecular causes of ageing, such as cellular senescence, mitochondrial dysfunction and telomere attrition, which is considered one of the best known molecular mechanisms of ageing both in humans and mice (Harley et al. 1990; Flores et al. 2008; Lopez‐Otin et al. 2013). Telomere attrition could lead to age‐related pathologies by resulting in the exhaustion of tissue‐ and self‐renewal capacity of the stem cell compartments (Flores et al. 2005; Sharpless and Depinho 2007).
Mitochondrial DNA damage theory is also a research hotspot in recent years. Mitochondrial DNA is exposed to external environments thereby lacking protection from histones and DNA binding proteins and it is also vulnerable to oxygen free radical damage. What is worse, it is not easy to repair because of the lack of repair systems, after the injury (D'Aquila et al. 2012). Furthermore, the study found that the senescence of organisms is closely related to the regulation of genes including geronto genes, longevity genes and apoptosis genes. In support, Tom Johnson succeeded in positioning the first ‘longevity gene’, age‐1 (Friedman and Johnson 1988). Micro RNAs (miRNAs), post‐transcriptional regulators of gene expression, could lead to inhibition of protein translation by binding inexactly to the 3′‐untranslated regions of target mRNAs (Pan et al. 2015). In fact, ageing involves not only a myriad of genes and proteins but also changes in endogenous metabolites (Ryazanov and Nefsky 2002; Warner 2005; Panza et al. 2007; Yan et al. 2009). Metabolomics, the best analysis to fit the holistic concept of traditional Chinese medicine (TCM), has been widely used recently for the discovery of novel biological active compounds and targets, and a series of age‐related metabolites were proposed according to exploratory works on aged rats, dogs and humans (Williams et al. 2005; Williams et al. 2006; Berger et al. 2007; Schnackenberg et al. 2007; Wang et al. 2007; Lawton et al. 2008; Cao et al. 2015;) including metabolic syndromes, cardiovascular disease, neurodegeneration and diabetes (Li et al. 2013a). Therefore, tackling ageing and its vicious spiral would be an effective approach to combat age‐related diseases. In fact, research on age‐related diseases has become a hot topic recently in the field (Martin 2011).
Reportedly, the most effective intervention in extending longevity in model organisms is caloric restriction (CR), which can not only increases longevity but also reduces risk for most (if not all) age‐related diseases. However, CR requires a permanent diet, which makes it difficult for many people to accept, thus limiting its popularity. Although Western medicine with anti‐ageing effects has made some progress, side effects, specific targets and multiple drug resistance are worrying. For example, researchers in America found that rapamycin can prolong the lifespan of mice by about 14%; however, its immunosuppressive effect could lead to the invasion of infectious diseases. To the contrary, TCM can exert anti‐ageing functions with unique dialectical treatment systems, multi‐target mechanisms and few adverse reactions. For example, it was shown that the extracts obtained from Rhodiola rosea could increase longevity of worms and flies without negative effects on reproduction or metabolic rate (Jafari et al. 2008; Wiegant et al. 2009). Moreover, integration of TCM, as well as Chinese materia medica, into the national healthcare delivery system has become an essential national policy in China, indicating that considerable emphasis has been given to the TCM research and development (Dang et al. 2016; Gao et al. 2015). Additionally, the popular use of metabolomics in ageing indicated the possibility for reconciliation and integration of Chinese and Western medicine.
Mechanism of anti‐ageing by TCM
Although a number of theories on ageing mechanism have been put forward (Linda and David 2002), people know little about ageing compared with that of other areas in biology. Consequently, it is important as well as urgent to explore the mechanism of ageing and strategies of anti‐ageing. TCM represents an extraordinary inventory of high diversity structural scaffolds that can offer promising candidate chemical entities in the major healthcare challenge of increasing health span and/or delaying ageing. Referring to the relevant literatures published in the past two decades, the mechanisms of anti‐ageing/age‐related diseases of active ingredients from TCM are summarized below.
Regulation of telomeres and telomerase
Telomeres, composed of tandem repeats of the TTAGGG bound to an array of proteins, are specialized nucleotide sequences at the ends of chromosomes (Blackburn 2001; Chan and Blackburn 2004; Finkel et al. 2007). Telomere length is demonstrated to be related to the replicative lifespan of normal somatic cells. Indeed, the replication of normal somatic cells is limited by telomere shortening, which proceeds incrementally with each round of cell division, resulting in the loss of 50–200 terminal base pairs of the telomere in humans both in vitro and in vivo; thus, the telomeres become shorter (Watson 1972; Olovnikov 1973; Allsopp et al. 1992; Allsopp and Harley 1995). Telomere length mainly depends on telomerase, a ribonucleoprotein enzyme that can elongate telomeric repeats in the 5′‐to‐3′ direction, thus mitigating the end‐replication problem (Blackburn 1991; Chan and Blackburn 2004).
Recently, a growing number of results have demonstrated that some active ingredients and prescriptions of TCM could play distinct roles in anti‐ageing via improving telomerase activity or suppressing telomere shortening (Table 1). For example, astragaloside (AST) cycloastragenol (CAG) (Figure 1) could exert anti‐ageing effects in human embryonic lung fibroblasts by affecting activity of telomerase and expression of the klotho gene (Guo et al. 2010), a novel gene closely related to human ageing. AST, a macromolecular saponins, has poor bioavailability when taken orally. Specifically, Liu et al. studied the physicochemical property of AST and CAG and their metabolism in vivo and in vitro. The experimental data showed that AST was easily transformed by the intestinal flora into metabolites with strong pharmacological activity, especially CAG which was the potent component of AST, exerting most of its efficacy (Liu 2013). Moreover, telomerase activity in testicular tissues of mice, which were gavaged with Cynomorium songaricum (C. songaricum) polysaccharide at 40 or 80 mg·kg−1·d−1, was clearly higher than that of mice treated with D‐galactose, indicating that C. songaricum polysaccharide could exert anti‐ageing effect by improving telomerase activity (Ma et al. 2009). In addition, flavonoids of Epimedium brevicornu (E. brevicornu) could significantly extend the population doublings of human diploid fibroblast cells from 53 to 64 generations, decrease the expression of p16 mRNA, increase the content of phosphorated Rb protein and protect the telomere length without activating telomerase (Hu et al. 2004). Meanwhile, metabonomic studies using liquid chromatography coupled with MS‐investigated the anti‐ageing effects of total flavones from E. brevicornu (TFE) on 4, 10, 18 and 24‐month‐old rats. Clearly, the TFE‐treated group had smoother fur, more locomotor activities and better appetite compared with the untreated 24‐month‐old rats. The results indicated that the anti‐ageing effects exerted by TFE might be related to the intervention on lipid metabolism and its anti‐oxidation activity, as most of the age‐related metabolites, such as saturated fatty acids, unsaturated fatty acids, ergothioneine, carnosine and deoxycholic acid, were reset to a younger level (Yan et al. 2009).
Table 1.
Mechanisms of anti‐ageing/age‐related diseases via regulation of telomere and telomerase by TCM
| Active ingredients/source | Experimental model | Efficacy | Mechanism | Reference |
|---|---|---|---|---|
| Polysaccharide | ||||
| Cistanche deserticola Ma | D‐galactose‐induced subacute ageing model mice | Significantly decreases MDA content in heart and brain, enhances telomerase activity, lymphocyte proliferation, phagocytosis of peritoneal macrophages and peripheral blood IL‐2 content | Antagonizes free radical injury and enhances telomerase activity and the immunity of ageing mice | (Zhang et al., 2011a) |
| Cynomorium songaricum Rupr. | D‐galactose‐induced ageing of mice | Exerts the anti‐ ageing effect on the ageing mice | Significantly increases the activity of telomerase in testicle | (Ma et al., 2009) |
| Angelica sinensis Oliv. Diels | D‐galactose and sodium nitrite‐induced subacute senile dementia mice | Improves the ability of learning and memory, delays ageing | Might by increasing SOD and telomerase activity | (Li et al., 2013) |
| X‐ray irradiation‐induced ageing of murine haematopoietic stem cell | Significantly inhibits the cell ratio of in HSC G1 stage and the increase in the number of SA‐β‐Gal positive cells, down‐regulates the expression of p53 protein and increases the length of telomere and the vitality of telomerase in HSCs | May be related to the increase in the length of telomere and the activity of telomerase, as well as the down‐regulation of the expression of p53 protein | (Zhang et al., 2013a) | |
| Astragalus membranaeus Fisch. Bge. | Senile human embryonic lung diploid fibroblasts | Improves cell viability of HDF cells, reduces expression of SA‐β‐gal and shortening velocity of TRF | Modulates telomerase activity, regulates or changes telomere binding protein | (Zhu et al., 2012) |
| Flavonoid | ||||
| Euphorbia humifusa Willd. | D‐galactose‐induced ageing mice | Improves telomerase content and SOD activity in testes and brain tissues of aged mice, decreases MDA content | Antioxidant and regulation of telomerase activity | (Cao et al., 2011) |
| Epimedium brevicornu Maxim. | Senescent human diploid fibroblasts (2BS) | Significantly extends the population doublings of 2BS cells, decreases the expression of p16 mRNA, increases the content of phosphorylated Rb protein and improves the telomere length of 2BS cells rather than activates telomerase activity | Protects telomere length probably through inhibiting the p16 gene expression, promoting the production of phosphorylated Rb protein but not activating the telomerase | (Hu et al., 2004) |
| Acteoside | ||||
| Cistanche tubulosa Schenk Wight | D‐galactose‐induced ageing mice | Decreases MDA content, obviously enhances telomerase activity in heart and brain, lymphocyte proliferation, phagocytosis of peritoneal macrophages and peripheral blood IL‐2 content | Antagonizes free radical injury and enhances telomerase activity and the immunity of ageing mice | (Zhang et al., 2008b) |
| C21 steroidal glycoside | ||||
| Cynanchum bungei Decne. | D‐galactose‐induced ageing mice | Prompts the ability of anti‐ oxidation, anti‐fatigue and anti‐stress | Increases SOD activity and telomerase activity, decreases MDA level | (Zhang et al., 2007) |
| Astragaloside | ||||
| Astragalus membranaeus Fisch. Bge. | Aged human embryonic lung fibroblast | Reduces β‐ galactosidase activity, increases cell viability, telomerase activity and klotho mRNA expression | Regulates telomerase activity and klotho gene expression | (Guo et al., 2010) |
| Ginsenoside Rg1 | ||||
| Panax ginseng C. A. Meyer | Tert‐butylhydroperoxide‐induced Sca‐1+ haemopoietic stem cell ageing in mice | Reduces the percentage of positive cells expressed SA‐β‐Gal and the number of cells entered G1 phase, increases the number of colony of mixed haematopoietic progenitor, markedly decreases telomere shortening, reinforces telomerase activity | Activates telomerase activity, reduces the shortening of telomere length | (Zhou et al., 2011) |
| Tert‐butylhydroperoxide (t‐BHP)‐induced senescence in WI‐38 cells | Attenuates t‐BHP‐induced cell senescence, markedly reduces the RTF shortening, promotes telomerase expression | Probably by activating telomerase activity and preventing terminal restriction fragment shortening | (Zhao et al., 2005) | |
| Alkaloid | ||||
| Uncaria rhynchophylla Miq. Miq. ex Havil. | D‐galactose‐induced ageing model of rats aortic endothelial cells | Improves cell morphology and inhibits cell ageing | Reduces expression of β‐galactosidase and relative expression quantity of telomerase | (Jiang et al., 2011) |
| Allicin | ||||
| Allium sativum Linn. | t‐BHP‐induced senescence in fibroblast cells | Significantly attenuates t‐BHP‐induced sencscence, markedly decreases RTF shortening and results in telomerase activation | Activates telomerase activity and prolongs terminal restriction fragment length | (Ke et al., 2006) |
| Pine pollen | ||||
| Pinus massoniana Lamb. | Human embryonic lung fibroblasts of ageing | Increases cell population doubling level and enhances telomerase activity | By activating telomerase activity | (Zhao and Yu, 2004) |
Figure 1.
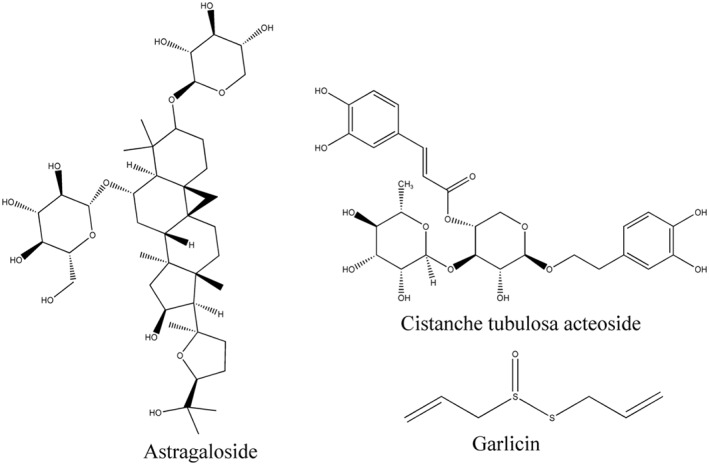
Structural formula of Cistanche tubulosa acteoside, garlicin and astragaloside with anti‐ageing/age‐related diseases effects from traditional Chinese medicine by regulating telomeres and telomerase.
Great attention has been paid to telomere and telomerase by the medical community in recent years. With the rapid development of molecular biology, more and more drugs with anti‐ageing features through controlling telomere length and telomerase activity will continue to be found and fully explored..
Regulation of sirtuins
Sirtuins (SIRTs), a group of NAD+‐dependent deacetylases belonging to a class of highly conserved proteins, are widely distributed across the range of organisms from bacteria to humans and play distinct roles in regulating some cellular functions, such as gene repair, cell cycle, metabolism and oxidative stress, via deacetylation of histones and non‐histones (Oberdoerffer and Sinclair 2007; Westphal et al. 2007). Notably, overexpression of SIRTs could extend lifespan in yeast, Drosophila and Caenorhabditis elegans (C. elegans) (Rogina and Helfand 2004; Viswanathan et al. 2005). Sirtuin1 (SIRT1) has been investigated most thoroughly and deeply among the SIRTs in mammals (Pillarisetti 2008). The possible mechanism involves two aspects. On one hand, SIRTs could increase stress resistance by activating negative regulation of pro‐apoptotic factors such as p53 and forkhead box‐O (FOXO) (Luo et al. 2001; Brunet et al. 2004). In fact, SIRT1 induced the deacetylation of p53 and subsequently reduced its binding capacity with cis‐DNA components, thereby preventing it from inducing DNA damage and apoptosis and suppressing cell proliferation. Meanwhile, SIRT1 could deacetylate FOXO1 and enhance nuclear ectopic transcriptional activity, thus increasing the expression of antioxidant enzymes such as SOD (Marfe et al. 2011). On the other hand, SIRTs might regulate body's energy metabolism to suppress fat accumulation and increase insulin secretion from islet beta cells via stimulating metabolism‐related genes such as PPARγ coactivator‐1α (Schilling et al. 2006), thus leading to an increase in stress resistance and extension in lifespan.
Various studies have demonstrated that TCM can exert anti‐ageing effects through the regulation of SIRTs (Table 2). One such example is resveratrol, which is a polyphenol particularly found in red wine, red grapes and tea and is the most potent regulatory factor of SIRT1 (Howitz et al. 2003; Li et al. 2016). Resveratrol can mimic the anti‐ageing effect of CR, thus being able to regulate the average lifespan of the organism (Baur et al. 2006; Mouchiroud et al. 2010). Accumulating data published have confirmed that resveratrol can prolong the lifespan of yeast, nematodes, fruit flies and fishes (Bass et al. 2007; Mouchiroud et al. 2010; Wood et al. 2004a). Moreover, icariin (Figure 2), a principal active ingredient of Epimedium in berberidaceae is another active compound that exerts anti‐ageing effects (Lee et al. 1995). Icariin could improve the expression of SIRT6 and reduce the expression of NF‐κB protein and the inflammatory response of old mice, indicating that the anti‐ageing mechanism of icariin was likely to be closely related to NF‐κB signalling pathway and SIRT6 histone deacetylase (Chen et al. 2012). It is likely that SIRT6 was up‐regulated after treatment with icariin, specifically combined with the RELA subunit of NF‐κB dimer, then attached to the downstream gene promoter of NF‐κB, leading to H3K9 histone deacetylation. As a result, the chromosome configuration was changed and coiled tightly, thereby silencing the downstream target genes of NF‐κB. Therefore, target gene transcription was reduced, and the cell senescence was diminished (Chen et al. 2012; Li et al. 2015). Additionally, Li et al. found that Cornus officinalis (C. officinalis) polysaccharide could slow the progression of age‐related cataracts by significantly increasing the activity of SOD, the expression of SIRT1 mRNA and FOXO1 mRNA and reducing the expression of p53 mRNA, indicating that C. officinalis polysaccharides probably regulated the expression of downstream genes p53 and FOXO1 through regulating SIRT1, eventually inhibiting or delaying apoptosis of epithelial cells in the lens (Li et al. 2014).
Table 2.
Molecular mechanisms of anti‐ageing/age‐related diseases via regulation of SIRTs by TCM
| Active ingredients | Source | Experimental model | Efficacy | Mechanism | References |
|---|---|---|---|---|---|
| Polysacchharides | Cornus officinalis Sieb. et Zucc. | Eye lens of D‐galactose‐induced ageing rats | Inhibits or delays apoptosis of epithelial cells in eye lens and slows the progression of age‐related cataract | May regulate the expression of downstream genes p53 and FOXO1 probably through regulation of SIRT1 | (Li et al., 2014) |
| Extracts | Ginkgo biloba Linn. | Natural ageing rats | Decreases the number of 8‐OHDG‐positive cells, delays relative telomere shortening, increases expression of SIRT1, declines expression of p21 without an obvious change of p53 in number | May be associated with the expression of SIRT1 and p21 protein | (Hao et al., 2013) |
| Icariin | Epimedium brevicornu Maxim. | Aged mice | Improves the expression of SIRT6 and reduces that of NF‐κB protein, as well as the inflammatory response of old mice | Closely related to NF‐κB signalling pathway and SIRT6 histone deacetylase | (Chen et al., 2012) |
| Butein | Butea monosperma Lam. Kuntze | Yeast Saccharomyces . cerevisiae (S. cerevisiae) | Increases lifespan by 31% at a concentration of 10 μM | Activates SIRT1 | (Howitz et al., 2003) |
| Resveratrol | Polygonum cuspidatum | Natural senescence of HUVECs | Reverses the senescence of HUVECs | Possibly increases the expression of SIRT1 thus decreasing the apoptosis levels of HUVECs | (Jiang et al., 2016) |
| Wild‐type adult worms | Increases lifespan | Dependent upon SIRT2.1 but not DAF‐16/FOXO activity | (Viswanathan et al., 2005) | ||
| Flies | Increases longevity by −20% at 200 μM | Dependent on SIRT2 | (Bauer et al., 2004; Wood et al., 2004b) | ||
| Budding yeast S. cerevisiae | Increases cell survival and extends lifespan by −70% at a concentration of 10 μM | Stimulates SIRT2 activity, increases DNA stability | (Howitz et al., 2003) | ||
| Caenorhabditis elegans | Extends lifespan | Through SIRT1‐dependent autophagy | (Morselli et al., 2010) | ||
| Drosophila and C. elegans | Extends lifespan | Through up‐regulation of SIRT2 and AMPK | (Bass et al., 2007) | ||
| Anoxic cardiocytes | Protects the cardiomyocytes from hypoxia‐induced apoptosis and promotes cell cycle arrest | Increases the level of SIRT1, which plays a role by the regulation of Foxo1 and its downstream genes such as Bin and p27 | (Wang et al., 2009b) | ||
| Silymarin | Silybum marianum Linn. Gaertn. | Isoproterenol‐induced injury in cultured rat neonatal cardiac myocytes | Protects isoproterenol‐treated rat cardiac myocytes from death, decreases production MDA, release of LDH and pro‐apoptotic cytochrome c from mitochondria, increases superoxide dismutase activity and mitochondrial membrane potential | Through resuming mitochondrial function and regulating the expression of SIRT1 and Bcl‐2 family members | (Zhou et al., 2006) |
| Quercetin | Herba hyperici | Male ICR mice 8 weeks of age | Increases mRNA expression of PPARγ coactivator‐1α (PGC‐1α) and SIRT1, mtDNA and cytochrome c concentration, maximal endurance capacity and voluntary wheel‐running activity | Increases mitochondrial biogenesis through up‐regulation of PGC‐1α, SIRT1 and mtDNA | (Davis et al., 2009) |
| Tetrahydrocurcumin | Curcuma aromatica Salisb. | Drosophila flies | Increases healthspan but not maximum lifespan, suppresses oxidative stress | Regulates sirtuins and FOXO‐responsive pathways | (Argyropoulou et al., 2013) |
Figure 2.
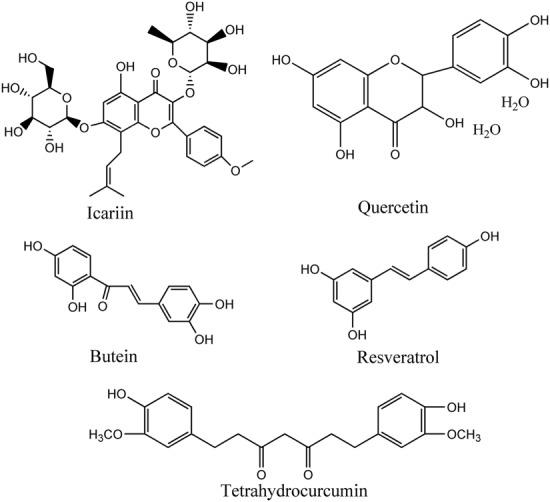
Structural formula of active ingredients with anti‐ageing/age‐related diseases effects from traditional Chinese medicine by regulating sirtuins.
Overall, many active ingredients of TCM can slow down ageing via the activation of SIRTs. So far, much attention has been paid to SIRT1, which is of great importance to anti‐ageing. With the in‐depth study on SIRT1 and molecular mechanisms of ageing, gene therapies targeted at SIRT1 will surely play a distinct role in extending human lifespan (Ling and Hu 2013).
Regulation of nutrient and energy sensing pathways
The lifespan of many species is controlled by the nutrient and energy sensing signal transduction pathways, including the target of rapamycin (TOR)/ribosomal protein S6 kinase (S6 K), the AMP‐activated kinase (AMPK) and the insulin/insulin‐like growth factor‐1 (INS/IGF‐1) signalling pathways (Kenyon 2010; Alic and Partridge 2011).
Regulation of mTOR
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that is evolutionarily highly conserved and can mediate the stress response. mTOR signalling, is emerging as a critical regulator of ageing (Rajapakse et al. 2011) and partial inhibition of its downstream targets, such as S6 K or protein synthesis, extends lifespan in yeast, worms, flies and mice (Kapahi et al. 2004; Kaeberlein et al. 2005; Hansen et al. 2007; Syntichaki et al. 2007).
It is clearly possible to cure age‐related diseases by rapamycin but the side effects (e.g. suppressing the immune system) are inevitable (Wu et al. 2015). Fortunately, TCM can function as rapamycin analogues, which are much safer, more effective with fewer side effects. Ginsenoside Rb1, a protopanaxdiol extracted from the roots of Panax ginseng (P. ginseng), which has been long used as a ‘precious tonic’ to support vitality and maintain homeostasis in China, was found to have preferable anti‐ageing activities (Helliwell et al. 2015). Specifically, the natural senile mouse models of 20 months old were prepared and injected with ginsenoside Rb1 (Figure 3) at first. During the experimental period, there was a remarkable reduction of MAO activity in Rb1 group, a decline of PAI‐1 protein expression in high‐dose Rb1 group and a decrease of mTOR protein phosphorylation levels in low‐dose Rb1 group as well as in high‐dose group, implying that the anti‐ageing effects of ginsenoside Rb1 on mice may be partially or completely related to the mTOR/p70s6k pathway (Peng et al., 2014) Similarly, 6‐gingerol (Figure 3) extracted from ginger could markedly decrease senescence in vascular smooth muscle cells (VSMCs) induced by angiotensin II, with cell cycle arrestin the G0/G1 phase and decreased protein leveld of mTOR and phosphorylated p70‐S6 K, suggesting that 6‐gingerol may attenuate VSMCs senescence through inhibition of the mTOR/P70‐S6 K pathway (Zhou et al. 2014).
Figure 3.
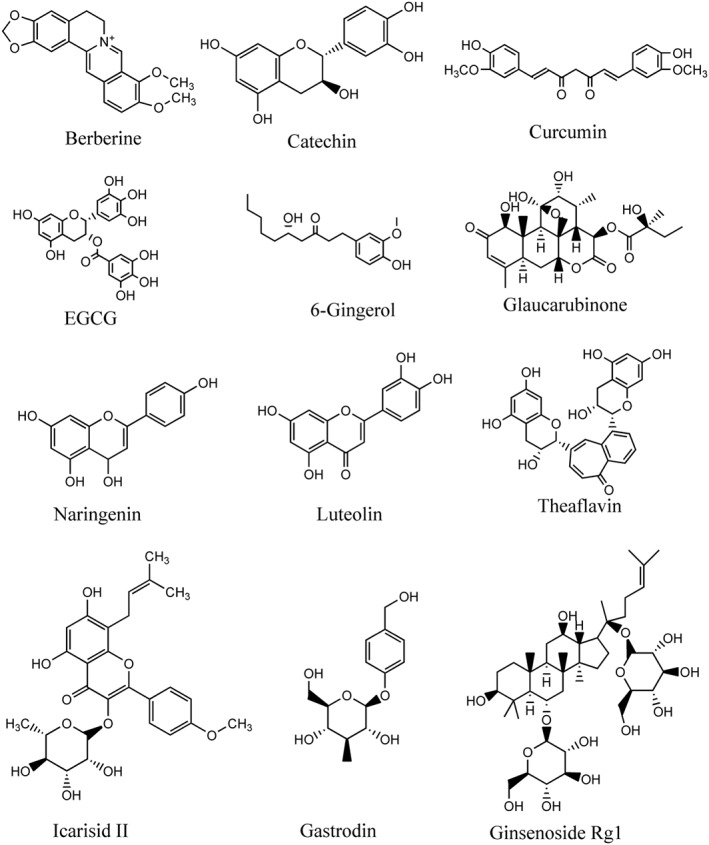
Structural formula of active ingredients with anti‐ageing/age‐related diseases effects from traditional Chinese medicine by regulating nutrient and energy sensing pathways.
Regulation of AMPK
AMPK has been defined as the ‘cellular energy regulator’, as it can sense the change in the AMP/ATP ratio and keep the balance between cellular carbon use efficiency and ATP yields (Geng et al. 2014; Zhang et al. 2014a). AMPK activity declines in ageing skeletal muscle of mammals, while overexpression of AMPK directly activates DAF‐16/FOXO by phosphorylation (Greer et al. 2007) and extends C. elegans lifespan even when CR starts in middle age animals (Apfeld et al. 2004).
In recent years, studies have found that TCM can fight against ageing and prevent age‐related diseases by modulating the activity of AMPK. For example, the total saponins of Panax notoginseng inhibited H9c2 apoptosis induced by serum, glucose and oxygen deprivation and prevented reduction of mitochondrial membrane potential, as well as reducing the positive rate of TdT‐mediated dUTP nick end labelling cells in myocardial tissue and increased levels of p‐AMPK protein, in a dose‐dependent manner, indicating that its anti‐ageing function may be related to AMPK activation (Yang et al. 2012). Reportedly, curcumin (Figure 3) activates signalling pathways downstream of the anti‐ageing modulators AMPK and the transcription factor Nrf2 and suppresses inflammatory processes mediated by NF‐kB signalling (Salminen et al. 2012; Surh et al. 2008). Because of these promising findings, curcumin was tested in humans as a possible treatment for Alzheimer's disease (Baum et al. 2008; Ringman et al. 2005).
Regulation of INS/IGF‐1
INS/IGF‐1 can affect the lifespan of a variety of organisms including yeast, worms, flies, mammals and humans, characterized by the weakening of insulin signalling, the enhancement of insulin sensitivity and the reduction of the plasma levels of insulin‐like growth factor‐1 (Bonafe et al.. 2003; Longo and Finch 2003; Cheng et al. 2004; Richardson et al. 2004). Roth et al. have reported that people with low insulin levels usually have a longer survival (Roth et al. 2002).
The INS/IGF‐1 signalling pathway can be used as a new target for developing drugs to prevent and treat age‐related diseases, thus delaying ageing and prolonging life. As a result, much attention has been paid to the correlation between INS/IGF1‐signalling pathway and senescence (Cheng et al. 2004). Cai et al. found that icariside II could increase thermo‐ and oxidative stress tolerance, decrease the rate of locomotion decline in late adulthood and extend lifespan by 20% in worms, and it was postulated that the lifespan extension caused by icariside II was dependent on the INS/IGF‐1 and DAF‐2/FOXO (and likely HSF1) signalling pathways (Cai et al. 2011). There is much work on TCM regulating nutrient and energy sensing pathways to delay ageing and prevent age‐related diseases and some specific examples are shown in Table 3.
Table 3.
Mechanisms of anti‐ageing/age‐related diseases via regulation of nutrient and energy sensing pathways by TCM
| Active ingredients | Source | Experimental model | Efficacy | Mechanism | References |
|---|---|---|---|---|---|
| Total saponins | Panax notoginseng Burk. F.H.Chen | Serum, glucose and oxygen deprivation (SGOD)‐induced apoptosis of H9c2 cells, ligation of the left anterior descending coronary artery‐induced apoptosis of cardiomyocytes in SD rats | Inhibits H9c2 apoptosis induced by SGOD, prevents reduction of mitochondrial membrane potential, reduces positive rate of TdT‐mediated dUTP nick end labelling cells in myocardial tissue and increases levels of p‐AMPK protein in a dose‐dependent manner | May be of great connact with activation of AMPK | (Zhang and Liu, 2011) |
| Berberine | Coptis chinensis Franch. | 6‐month‐old female db/db mice | Lowers body weight, fat pad weight and blood sugar levels, improves index of insulin sensitivity, activity of skeletal muscle mitochondrial COX and content of ATP, increases phosphorylation levels of AMPK, and enhances the transcriptional activity of PGC‐1α | Activates AMPK /PGC‐1α signalling pathway and improves mitochondrial energy metabolism | (Wang et al., 2014b) |
| Dietary obese rats | Improves metabolism | Activates AMPK by inhibiting the biosynthesis of ATP in mitochondria | (Yin et al., 2008) | ||
| Astragalus polysaccharide | Astragalus membranaeus Fisch. Bge. | Fat plus low‐dose streptozotocin (STZ)‐induced type 2 diabetic rats | Lowers content of blood glucose, serum triglycerides and glycosylated haemoglobin, enhances insulin sensitivity, increases phosphorylation levels of AMPK and acetyl‐CoA carboxylase (ACC) | May be associated with up‐regulation of AMPK activity | (Zhang et al., 2008b) |
| Fat plus STZ‐induced diabetic cardiomyopathy rats | Improves insulin resistance and that could be characterized by lowering blood sugar and elevating ISI index | May be related to up‐regulation of AMPK activity, uncoupling protein 2 expression and energy metabolism type 2 diabetes mellitus rats | (Wang et al., 2009b) | ||
| Gastrodin | Gastrodia elata Bl. | Oleic acid‐induced HL‐7702 cells | Inhibits oleic acid‐induced fat accumulation of HL‐7702 cells and lowers triglyceride content | Dependent on the activation of AMPK pathway in the cells | (Geng et al., 2015) |
| Ginsenoside Rb1 | Panax ginseng C. A. Meyer | Natural ageing mice | Decreases the activity of MAO, the expression of PAI 1 protein and the phosphorylation of mTOR protein | May be implemented by mTOR/p70s6k pathway partially or fully | (Peng et al., 2014) |
| 6‐Gingerol | Zingiber officinale Roscoe | Angiotensin II (Ang II)‐induced rat aortic VSMCs senescence | Markedly decreases Ang II‐induced VSMCs celluar senescence, cell cycle arresting in G0/G1 phase, the protein level of mTOR and phosphorylated p70‐S6 K | May attenuate VSMCs senescence through inhibition of mTOR/P70‐S6 K pathway | (Zhou et al., 2014) |
| Icariside II | Epimedium brevicornu Maxim. | C. elegans | Increases thermo and oxidative stress tolerance, decreases the rate of locomotion decline in late adulthood, extends lifespan by 20% | Dependent on the INS/IGF‐1 and DAF‐2/FOXO and likely HSF1 signalling pathways | (Cai et al., 2011) |
| Glaucarubinone | Simarouba glauca DC | C. elegans | Significantly extend −80% lifespan at 100 nM ; reduced the body fat content | May act through the nutrient sensing pathway | (Zarse et al., 2011) |
| Catechin | Camellia sinensis O. Ktze. | Hepa 1–6, L6, and 3 T3‐L1 cells and BALB/c mice | Up‐regulates the downstream target ACC | By the activation of LKB1/AMPK | (Murase et al., 2009) |
| Curcumin | Curcuma longa Linn. | Diabetic rats induced by high‐fat diet plus streptozotocin | Improves muscular insulin resistance by increasing oxidation of fatty acid and glucose | Mediated through LKB1‐AMPK pathway | (Na et al., 2011) |
| Alzheimer's disease trangenic mice model | Suppresses indices of inflammation and oxidative damage in the brain and decreases the overall amyloid content and plaque burden | Activates signalling pathways downstream of the anti‐ageing modulators AMPK and NRF2, suppress inflammatory processes mediated by NF‐kB signalling | (Lim et al., 2001; Salminen et al., 2012; Salvioli et al., 2007; Sikora et al., 2010; Surh et al., 2008) | ||
| Luteolin | Reseda odorata Linn. | A cell model of steatosis induced by palmitate | Reduces lipid accumulation | ActivateS AMPK, ACC‐1, CPT‐1, down‐regulates sterol regulatory element binding protein 1c and fatty acid synthase | (Liu et al., 2011b) |
| Naringenin | Citrus maxima Burm. Merr. | Skeletal muscle cells | Increases glucose uptake | Through activation of AMPK | (Zygmunt et al., 2010) |
| Quercetin | Herba hyperici | High‐cholesterol‐induced neurotoxicity in old mice | Reduces high‐cholesterol‐induced A β deposits and improves behavioural performance | Activates AMPK, increases HMGCR and ACC ,decreases elF2α phosphorylation | (Lu et al., 2010) |
| Theaflavin | Camellia sinensis O. Ktze. | HepG2 cells exposed to a long‐chain mixture of FAs and male Wistar rats 5 weeks old fed with high‐fat diet | Reduces lipid accumulation, suppresses fatty acid synthesis, stimulates fatty acid oxidation, inhibites acetyl‐coenzyme A carboxylase activities | By stimulating AMPK through the LKB1 and reactive oxygen species pathways | (Lin et al., 2007) |
| Epigallocatechin gallate | Gamellia sinensis O.Ktze | Rat pancreatic beta cells | Reduces glucotoxicity‐induced pancreatic beta cell death | Increases insulin sensitivity through activating AMPK signalling to inhibit the activities of lipogenic enzymes and ameliorating mitochondrial function | (Cai and Lin, 2009) |
| Rat L6 cells treated with dexamethasone | Improves insulin‐stimulated glucose uptake,improves insulin resistance | Increases GLUT4 translocation to plasma membrane, activates AMPK and PI3K/Akt | (Zhang et al., 2010) | ||
| Overnight‐fasted Wistar rats | Prevents free fatty acids‐induced peripheral insulin resistance, decreases plasma markers of oxidative stress, increases antioxidant enzymes and reverses IH‐induced | Through decreasing oxidative stress and PKCθ membrane translocation, activating the AMPK pathway and improving insulin signalling pathway in vivo | (Li et al., 2011a) | ||
| Ageing endothelial cells | Reduces endothelial cellular senescence and dysfunction | By inhibiting mTOR/S6 K signalling and ROS production | (Rajapakse et al., 2011) | ||
| Drosophila and C. elegans | Extends lifespan | Through up‐regulation of Sir2 and AMPK | (Bass et al., 2007) | ||
| Neuronal cells AD | Lowers Aβ accumulation | By activation of AMPK and induction of autophagy via inhibiting mTOR | (Vingtdeux et al., 2010) | ||
| HepG2 cells incubated with Arachidonic acid and iron | Inhibits apoptosis, ROS production and glutathione depletion, attenuates superoxide generation in mitochondria,... inhibits mitochondrial dysfunction | Through AMPK‐mediated inhibitory phosphorylation of GSK3β downstream of poly(ADP‐ribose)polymerase‐LKB1 pathway | (Shin et al., 2009) | ||
| Middle‐aged mice fed with a high‐calorie diet | Shifts the physiology of treated mice towards that of mice on a standard diet, significantly increases their survival | Restores normal insulin sensitivity, reduces IGF‐1 levels, increases AMPK activity, improves mitochondria number and function | (Baur et al., 2006; Lagouge et al., 2006) |
From the data shown above, we draw the conclusion that nutrient sensing signalling pathway could control lifespan in many species, and this possibility has received much support from a large number of experiments. What is more, INS/IGF, TOR and AMPK signalling pathways can systematically coordinate to modulate each other, thereby controlling cellular/organism homeostasis and function in response to adverse environmental conditions.
Free radicals scavenging
Generated from the mitochondria electron transport chain, ROS are closely related to ageing (Lee and Wei 2001). Although ROS are much needed (at low concentration) for the body to perform normal physiological functions, including transferring energy to maintain the vitality, killing cells, eliminating inflammation and decomposing poisons; abnormally high levels of ROS will lead to ageing and even death, as they can trigger free radical chain reactions because of its unpaired electrons and high reactive activities (Chen 2004; Jia et al. 2007).
TCM exerts free radical scavenging mainly through three ways. Firstly, TCM can achieve the purpose by enhancing function of the antioxidant system in the body through increasing the activity and content of various antioxidant enzymes such as SOD and GSH peroxidase (GSH‐Px). The stress‐induced synthesis of some of these enzymes is mainly triggered by Nrf2, which plays a central role in the protection of cells against oxidative and xenobiotic damage (Kensler and Wakabayashi 2010; Sykiotis and Bohmann 2010). Briefly, Nrf2 could activate transcription in response to oxidative stress mainly by translocating into the nucleus and recruiting the small muscle aponeurotic fibrosarcoma (sMaf) protein when stimulated (Espinosa et al. 2014). Then, the Nrf2‐sMaf heterodimer binds to the antioxidant response element, which is a cis‐acting DNA regulatory element that activates the promoter region of many genes encoding phase II detoxification enzymes and antioxidants, thereby contributing to the maintenance of cellular redox homeostasis (Lee et al. 2015). Reportedly, honokiol (Figure 4) could achieve desirable anti‐ageing effects by decreasing the content of methane dicarboxylic aldehyde (MDA) and increasing the activity of antioxidant enzymes, such as SOD and GSH‐Px in serums and tissues of mice injected with D‐galactose for six consecutive weeks to simulate natural‐aged mice (Hao et al. 2009). Secondly, TCM can scavenge free radicals directly. For example, C. songaricum extracts (20 mg·mL−1) enhanced cognitive behaviour, increased resistance to stress and extended female mean lifespan of flies, indicating that C. songaricum flavonoids acted as free radical scavengers (Yu et al. 2010; Liu et al. 2012). Thirdly, TCM can inhibit lipid peroxidation. Lipid peroxidation is a common way of damaging tissues by oxygen free radicals through following ways: oxygen free radicals + cell membrane lipid → lipid peroxidation reaction → lipid peroxidation → MDA + cell components → lipofuscin (Xu et al. 2006). In support, oxymatrine extracted from Sophora flavescens could improve the learning and memory ability of ageing mice induced by intraperitoneal injection of D (+)‐galactose, and the anti‐ageing effect was possibly related to its resistance to oxygen free radicals, as well as lipid peroxidation. Furthermore, a recent study demonstrated that oxymatrine could be in vivo converted to matrine that might be a novel drug used for curing type 2 diabetes and hepatic steatosis (Wang et al. 2005; Zeng et al. 2015). The majority of published studies are listed in Table 4.
Figure 4.
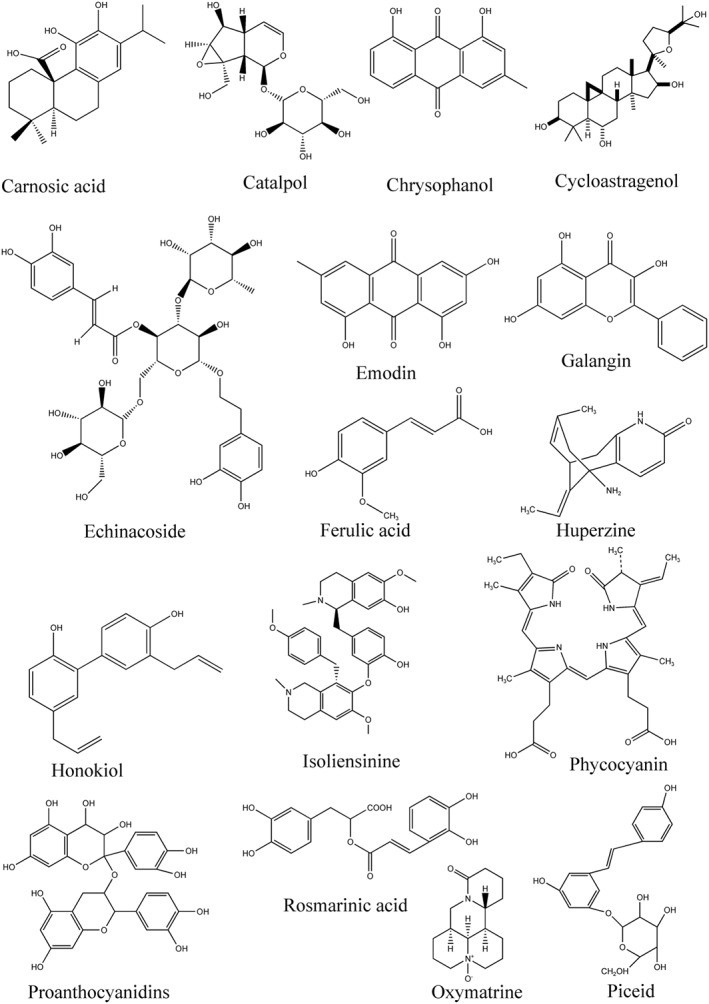
Structural formula of active ingredients with anti‐ageing/age‐related diseases effects from traditional Chinese medicine by free radical scavenging.
Table 4.
Mechanisms of anti‐ageing/age‐related diseases via free radicals scavenging by TCM
| Active ingredients | Source | Experimental model | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| Curcumin | Curcuma longa Linn. | Drosophila flies | Suppresses oxidative stress and lipid peroxidation, reduces accumulation of malondialdehyde (MDA), improves locomotor performance | Modulates a number of stress‐responsive genes, including the antioxidant enzyme superoxide dismutase | (Lee et al., 2010; Shen et al., 2013) |
| Echinacoside | Cistanche tubulosa Schenk Wight | D‐galactose‐induced aged mice | Repairs the damage induced by ROS, improves the memory ability, delays ageing process | Enhances the activities of GSH‐Px and SOD, reduces the content of MDA and the activity of MAO | (Muteliefu et al., 2004) |
| Isoliensinine | Nelumbo nucifera Gaertn | D‐galactose‐induced ageing mice | Markedly counteracts loss of body weight and liver index, significantly increases the antioxidative effect | Increases activities of SOD and GSH‐Px in surum and liver tissue, reduces content of MDA | (Liu et al., 2011a) |
| Huperzine a | Huperzia serrata Thunb. ex Murray Trev. | D‐galactose‐induced senile mice | Improves disorder of learning and memory and neuron protection | Significantly reduces the content of NO, the activity of NOS and the level of Ga2+ in brain cell plasma, increases the activities of GSH‐Px and SDH | (Lv et al., 2007) |
| Quercetin | Herba hyperici | C. elegans | Significantly improves the mean and maximum lifespan by 36 and 20% respectively with little effect on its reproductive capacity | Might improve the stress resistance | (Han et al., 2011) |
| Human RPE cells treated with oxidative stress mediated by H2O2 | Diminishes the decrease of mitochondrial function, reduces the activation of caspase‐3 from 1.9 to 1.4 fold, decreases the levels of caveolin‐1 mRNA and caveolin‐1 protein, attenuates the increase in β‐galactosidase–positive cells | Reduces mitochondrial dysfunction and cellular senescence | (Kook et al., 2008) | ||
| Polysaccharide | Arimillaria mellea Vahl ex Fr. Quel. | C. elegans | Significantly extends the lifespan without damage to the reproductive capacity, increases the expression of HSP‐16.2 and SOD‐3 | Maybe by increasing the capacity of stress resistance | (Chen et al., 2013) |
| Rosmarinic acid | Rosmarinus officinalis Linn. | D‐galactose‐induced ageing mice | Increases the activity of SOD and GSH‐Px in serum and brain, decreases the levels of MDA and triglyceride, extends hypoxia‐resistance time at normal pressure | Increases the activity of antioxidase, removes free radicals, reduces the production of lipid peroxidation | (Wang et al., 2009b) |
| Piceid | Polygonum cuspidatum | C. elegans model | Significantly increases the lifespan by 13% | Significantly enhances the swallowing rate, motility, intestinal lipofuscinosis and the reproductive capacity and remarkably decreases the lipofuscin | (Chen, 2012) |
| Green tea catechins | Gamellia sinensis O.Ktze | SAMP10 mice | Reduces carbonyl protein levels in the brain | Through decreasing carbonyl proteins and increasing GPx activity | (Kishido et al., 2007) |
| Mogroside | Siraitia grosvenorii Swingle C. Jeffrey ex Lu et Z. Y. Zhang | D‐galactose‐induced ageing mice and Drosophila melanogaster | Against ageing and prolongs the average life expectancy and maximum lifespan | Improves SOD activity and decreases MDA content | (Xiao et al., 2014) |
| Gypenosides | Gynostemma pentaphyllum Thunb. Makino | Human aged skin fibroblasts | Weakens oxidative stress, increases the ability of proliferation and therefore delays cells ageing | Increases the activity of SOD, CAT and GSH‐Px | (Cong et al., 2014) |
| Oxymatrine | Sophora flavescens Alt. | D‐galactose‐induced ageing mice | Improves the learning and memory ability | Defends against oxygen free radicals and reduces the lipid peroxidation | (Zi et al., 2012) |
| Total alkaloid | Corydalis yanhusuo W. T. Wang | D‐galactose‐induced ageing mice | Restores the ability of learning and memory and plays a role in anti‐ageing | Increases SOD, CAT and ChAT activity in the brain and reduces AChE activity | (Bai et al., 2008) |
| Resveratrol | Veratrum nigrum Linn. | D‐galactose‐induced ageing mice | Maintains the normal morphological structure of nerve cells, decreases oxidative stress responses and has protective effects on brain tissues | Significantly increases the number of nerve cells, the organ coefficients and activities of GSH‐Px, SOD and CAT, significantly decreases activity of MAO and content of MDA | (Cui et al., 2013) |
| D‐galactose‐induced myocardial cell senescence | Reduces the degree of D‐ galactose‐induced myocardial cell senescence | Reduces β‐ galactosidase and MDA levels, increases SOD activity and LC3II/LC3I level | (Guo et al., 2012) | ||
| Honokiol | Magnolia officinalis Rehd. et Wils. | D‐galactose‐induced ageing mice | Delays changes of quasi‐ageing | Enhances SOD, CAT and GSH‐Px activity, decreases MDA content | (Hao et al., 2009) |
| Chrysophanol | Rheum officinale Baill. | Scopolamine‐induced acquisition disturbance, sodium nitrite‐induced consolidation impairment, 30% ethanol‐induced retrieval deficit of memory and aluminium‐induced acute ageing in mice | Improves the impairments of memory acquisition and promotes the tolerance of rats | Increases plasma SOD activity | (Li et al., 2005) |
| Flavonoid | Oxytropis glabra Lam. DC. | D‐galactose‐induced ageing mice | Significantly prolongs the survival time under hypoxic condition and the swimming time at normal temperature and has obvious effects on anti‐senility | Significantly declines the content of MDA and LPO and increases the activity of SOD, GSH‐Px and CAT in serum and tissue | (Wang et al., 2013) |
| Galangin | Zingiber officinale Roscoe | D‐galactose‐induced senescent mice | Improves the cognitive function of aged mice | Attenuates the decreased activities of SOD, GPx and CAT, reduces MDA levels | (Fu et al., 2012) |
| Puerarin | Radix puerariae | D‐galactose‐induced ageing rats | Plays a part in anti‐ageing | Increases SOD and GSH‐Px activity in serum, decreases MDA and LPF levels | (Peng, 2009) |
| Sodium ferulate | Angelica sinensis Oliv. Diels/Ligusticum chuanxiong Hort. | D‐galactose‐induced ageing mice | Significantly promotes the activity of SOD in brain and serum, GSH‐Px in blood, remarkably inhibits the increase of MDA in serum and liver, MAO of brain, restrains the decrease of weight and the index of thymus and spleen | Increases the activity of antioxidase, removes the accumulation of metabolites in the body and increases the weight immune organ | (Zhu et al., 2004) |
| Carnosic acid | Rosmarinus officinalis Linn. | Human embryonic lung diploid fibroblasts 2BS cell line | Delays senescence of 2BS cells | Increases the cellular viability and the percentage of S distribution, dramatically reduces the SA‐β‐Gal positive rate, the percentage of G1/G0, the intracellular MDA level and p53 and p21 protein expression | (Tao et al., 2014) |
| Lotus seedpod procyanidins | Nelumbo nucifera Gaertn. | D‐galactose‐induced ageing mice | Has significant antioxidant effect | Significantly increases the activities of SOD and GSH‐Px in brain, remarkably decreases the content of MDA | (Chen et al., 2009) |
| Catalpol | Rehmannia glutinosa Gaert. Libosch. ex Fisch. et Mey. | D‐galactose‐induced sub‐acute senescent mice | Reverses the D‐galactose‐induced behavioural impairments | Increases the activities of SOD and GSH‐PX, decreases the MDA level | (Zhang and Liu, 2011) |
| Cycloastragenol | Astragalus propinquus Schischk. | D‐galactose‐induced ageing mice | Has a remarkable effect of anti‐decrepitude | May improve the activities of T‐SOD and T‐AOC, reduces the contents of MDA and HYP | (Cao et al., 2012) |
| Phycocyanin | Porphyra yezoensis veda | D‐Galactose‐induced mice models of subacute ageing | Has excellent anti‐ageing activity | Significantly increases SOD activity, thymus index and spleen index, as well as decreases MDA content | (Zhao and Tang, 2012) |
| Garlicin | Allium sativum Linn. | D‐galactose‐induced AD mice | Improves ability of spatial learning and memory | Reduces MDA content, increases SOD activity | (Hu et al., 2010) |
| Emodin | Rheum officinale Baill. | Hyperlipidaemia quail | Has significant lipid‐lowering effect and anti‐ageing effects | Lowers LPO content in serum and LF content in brain, increases SOD content and thymus weight as well as spleen weight | (Han et al., 2009) |
| Salvianolic acid B | Salvia miltiorrhiza Bunge. | Glucocorticoid‐induced ageing skin of rats | Significantly increases epidermal thickness and content of elastic fibres, alleviates ageing‐like changes | Inhibits lysophosphatidylcholine‐induced increase of matrix metalloproteinase‐2 activity, scavenges free radicals, improves immune status and has anti‐lipid peroxidation | (Zhang et al., 2008a) |
To sum up, a number of experiments have proved that ageing is closely related to free radicals, the theory of which has been widely accepted and becoming an active area. As stated above, TCM can exert anti‐ageing activities by free radicals scavenging, anti‐lipid peroxidation and up‐regulation of the antioxidative defence system.
Anti‐damage of DNA
DNA damage, the primary programme of ageing in the body, can be roughly divided into four types: base damage, glycosyl damage, bond rupture and DNA chain cross linking. Many studies have shown that DNA damage and self‐repair ability are closely related to ageing. Damage of DNA is the main reason leading to mutations, cancer, ageing and death, because it can directly affect DNA replication, transcription and protein synthesis, thereby affecting cells' growth, development, genetics, metabolism, reproduction and other vital biological activities (Jiang 2005).
TCM and its active ingredients could protect the integrity of DNA duplex and prevent gene mutation by resisting DNA damage (Jiang 2005). An experiment was carried out to study whether Radix puerariae (R. puerariae) and puerarin (Figure 5) have effects on delaying naturally senile mice of 18 months old and discovered that the rate of missing mtRNA in the elderly control group, the middle R. puerariae dose group and the middle puerarin dose group in all are 0.13, 0.11 and 0.11, respectively, indicating that puerarin could retard mitochondrial DNA damage (Wu et al. 2011). Furthermore, a metabolomics approach has been already used in a pharmacological study of Salvia miltiorrhiza (S. miltiorrhiza). For example, Jiang et al. identified 26 primary and secondary metabolites in S. miltiorrhiza from different regions and demonstrated that malonate and succinate possibly were the key markers for discriminating the geographical origin (Jiang et al. 2014). The application of metabolomics in S. miltiorrhiza provided novel insights into its essence. It was found that salvia acid B (Sal B) extracted from S. miltiorrhiza, exerted anti‐ageing effects on D‐galactose‐induced senile mice models presumably by promoting anti‐oxidation and affecting mitochondrial DNA levels, based on the findings that there was a better performance in Morris water maze test and stand‐jumping test, an increase of cerebral SOD activity, a reduction of MDA content and mitochondrial DNA level in Sal B treatment group (Gao et al. 2009). Additionally, Chen et al. showed that tea polyphenols have anti‐ageing effects and could significantly increase DNA methylating enzyme activity (Chen et al. 2001). Many other studies involved in the mechanism of anti‐damage of DNA by TCM active ingredients are shown in Table 5.
Figure 5.
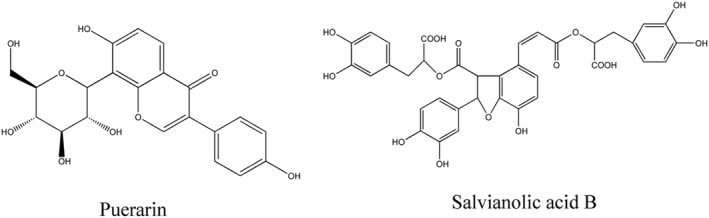
Structural formula of puerarin and salvianolic acid B with anti‐ageing/age‐related diseases effects from traditional Chinese medicine by anti‐damage of DNA.
Table 5.
Mechanisms of anti‐ageing/age‐related diseases via anti‐damage of DNA by TCM
| Active ingredients | Source | Experimental model | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| Tea polyphenols | Camellia sinensis O. Ktze. | 12–13‐month‐old mice | Significantly increases the DNA methylating enzyme vitality in the liver | Increases the DNA methylating enzyme vitality by altering conformation of DNA methylase and making it easier to transfer methyl to normal methylation sites | (Chen et al., 2001) |
| Resveratrol | Reynoutria japonica Houtt. | Natural ageing mice | Reduces the rate of mtRNA deletion and the percent of deletion on chief mtRNA | Avoids the mtDNA damage | (Zhang et al., 2011a) |
| Puerarin | Radix puerariae | Skin of natural ageing mice | Slows down the natural ageing of skin | Reduces deletion mutation of mtDNA, improves the vitality of GSH‐Px | (Li et al., 2011a; Wu et al., 2011) |
| Salvia acid B | Salvia miltiorrhiza Bunge | D‐galactose‐induced ageing mice | Exhibits better performance in Morris water maze test and stand‐jumping test, significantly reduces mitochondrial mtDNA and MDA levels, improves cerebral SOD activity | Promotes anti‐oxidation, reduces mitochondrial DNA level | (Gao et al., 2009) |
| Polysaccharide | Zostera marina L. | Reactive oxygen species‐induced lymphocyte DNA damage | Helps to maintain normal structure and function of cells | Scavenges free radical and reduces oxidative stress‐induced DNA damage | (Zhan et al., 1999) |
| Spirulina | UV‐induced DNA damage of human embryo lung diploid fibroblastic | Prevents the level of DNA damage induced by UV irradiation and promotes DNA repair capacity | Increases the activity of DNA endonuclease and DNA ligase in dose‐dependently | (Deng et al., 2001) |
Effects of TCM on DNA‐repair are being constantly revealed with the in‐depth understanding on the procedure of DNA damage and repair (Jiang 2004). However, the targets of drugs are rarely involved in current studies. Therefore, more researches are needed to explore the mechanisms of DNA repair by TCMs.
Chinese compound prescription and its extracts
Chinese compound prescription (CCP) is usually composed of several kinds of single herbs, each of which contains multiple effective constituents (Peng et al. 2015). In fact, the safety and effectiveness of CCP have been confirmed by the clinical practice for thousands of years. In the past decades, more and more attention in TCM has been focused on the effects of active ingredients from formulae (Yin et al. 2015). Of note, synergism, when the effects of the combination are greater than that of the individual drug, is a core principle of TCM and has played an essential role in improving its clinical efficacy (Zhang et al., 2014b).
Although the mechanisms of CCP are unclear owing to its complex composition, a large number of experimental studies have shown that several anti‐ageing mechanisms mentioned above are also applicable to CCP. Specifically, a traditional Chinese herbal formula, Zuoguiwan pill, composed of seven herbal constituents, was found to exert anti‐ageing effects by improving blood anti‐oxidative ability and decreasing DNA damage of lymphocytes (Xia et al. 2012). Similarly, it could significantly decrease the levels of 8‐hydroxy‐2'‐deoxyguanosine in the cerebral genome of 24‐month‐old rats, suggesting that the underlying mechanism relies on the protection and repair on DNA in cerebral genome (Zhao et al. 2002). Furthermore, there was a dose‐dependent increase in the expression of FOX, SIRT1 and c‐Myc in ovary cells of sub‐acutely ageing rats induced by D‐galactose after treatment with a decoction of Fallopia multiflora (F. multiflora), suggesting that this decoction could block ovary apoptosis probably by regulating the expression of FOX, SIRT1 and c‐Myc (Zhang et al., 2013a). Additionally, Zhang et al. applied ultra high performance liquid chromatography coupled with the quadrupole time‐of‐flight MS metabolomics to evaluate the therapeutic effect of Liuwei Dihuang (LWDH) pills, Jinkui Shenqi (JKSQ) pills and their combinations (administration of JKSQ pills in the morning and LWDH pills at night) on kidney deficiency in Sprague–Dawley rats induced by dexamethasone and D‐galactose (Zhang et al., 2014b). The results showed that the group treated with JKSQ pills in the morning and LWDH pills at night displayed the strongest rehabilitation for metabolic disorder, and it concluded that lipid metabolism and energy metabolism might be closely related to kidney deficiency and ageing as most of potential biomarkers identified of the kidney deficiency and ageing are related to fatty acids. Similarly, extracts of TCM are the other active ingredients possessing anti‐ageing functions under investigation. In support, aqueous extracts of Portulaca oleracea possibly exert anti‐ageing effects on senile mice by inhibiting expression of p53 gene and activating telomerase, so as to protect against telomere shortening in brain (Huang et al. 2007). Moreover, Guo et al. found that alcoholic extracts of C. officinalis can fight against senility by inhibiting the non‐enzymic glycosylation of proteins in vivo and reducing DNA damage of peripheral blood lymphocyte (Guo et al. 2005).
A systematic review of the clinical and non‐clinical efficacy of anti‐ageing herbs was published based on six human and 61 animal studies, most of which showed significantly improved brain function, sexual disorder and skin wrinkling (Hasani‐Ranjbar et al. 2012). For example, Lee et al. investigated the clinical efficacy of P. ginseng in the cognitive performance of Alzheimer's disease patients and found that the mini‐mental state examination scores and Alzheimer's disease assessment scale scores both were significantly decreased after treatment with P. ginseng (Lee et al. 2008). Additionally, several other clinical trials were also performed with CPP or TCM extracts and demonstrated beneficial effects (Zhang et al. 1992; Zhang 1993; Wang 1994; Tian et al. 1997; Xu and Zhi 1998; Sugiyama 2006; Amagase and Nance 2008; Yonei et al. 2008; Gim et al. 2009). More related researches are listed in Table 6 as below.
Table 6.
Mechanisms of anti‐ageing/age‐related diseases by Chinese medicine compounds and extracts
| Active ingredient | Source | Experimental model | Efficacy mechanism | Mechanism references | References |
|---|---|---|---|---|---|
| Vigconic 28 | Panax ginseng/Cervus nippon/Cordyceps sinensis/Salvia miltiorrhiza/Allium tuberosum/Cnidium monnieri/Euodia rutaecarpa | C57BL/6 J mice | Retards ageing in mice | Likely by affecting mitochondria functionality | (Ko et al., 2010) |
| Liu Wei Dan Kun decoction | Rehmannia glutinosa Gaertn./Rhizoma dioscoreae/Cornus officinalis Sieb. et Zucc./Poria cocos Schw. Wolf./Alisma plantago‐aquatica Linn./Paeonia suffruticosa Andr./Salvia miltiorrhiza Bunge/Leonurus artemisia Lour. S. Y. Hu | Aged mice | Increases retention rate of double‐stranded DNA and restores partial damaged DNA | Reduces the damage of DNA in aged mice, increases resistance to radiation and improves the ability to repair DNA damage | (Yang et al., 1995) |
| Formula of tonifying kidney and spleen, nourishing blood and promoting blood flow | Lycium chinense Miller/Fallopia multiflora Thunb. Harald./Epimedium brevicornu Maxim./Fructus ligustri Lucidi/Angelica sinensis Oliv. Diels Cuscuta chinensis Lam. /Astragalus membranaeus Fisch. Bge./Polygonatum sibiricum Delar. ex Redoute | Senile Balb/c mice of 12–14‐month‐old | Decreases depletion of kidney mtDNA in senile Balb/c mouse significantly | Inhibits deletion mutation of mtDNA, reduces oxidative damage of mtDNA, protects mtDNA | (Wang et al., 2006) |
| Liuweidihuang Decoction | Rehmannia glutinosa Gaertn./Cornus officinalis Sieb. et Zucc./Rhizoma dioscoreae/Paeonia suffruticosa Andr./Alisma plantago‐aquatica Linn./Poria cocos Schw. Wolf. | Drosophila and D‐galactose‐induced ageing mice | Prolongs the surviving‐time, improves the anti‐oxidation ability, increases telomerase activity | Antioxidant and increases telomerase activity | (Wu and Dong, 2003) |
| Pill of kidney‐qi‐tonifying | Radix rehmanniae Exsiccata./Rhizoma dioscoreae/Cornus officinalis Sieb. et Zucc./Alisma plantago‐aquatica Linn./Poria cocos Schw. Wolf./Paeonia suffruticosa Andr./Cinnamomum cassia Presl/Aconitum carmichaeli Debx. | Cy‐clophosphamide‐induced damage of DNA in mice | Antagonizes DNA damage caused by cyclophosphamide | Enhances the body's ability to prevent DNA damage | (Zhou et al., 1998) |
| Decoction of four mild drugs | Panax ginseng C. A. Meyer/Atractylodes macrocephala Koidz./Poria cocos Schw. Wolf./Glycyrrhizae | D‐galactose‐induced ageing mice | Decreases the MDA content of heart, liver and brain, increases the telomerase activity in heart and brain tissues but with no effect on that in liver tissue | Antagonizes free radical injury, improves telomerase activity | (Yang et al., 2005) |
| D‐galactose‐induced ageing mice | Significantly improves the ability of learning and memory, increases the activities of GSH‐Px and SDH respectively in brain tissues, decreases the concentration of Ca2+ ions, prevents the damage of mtDNA in hippocampus | Enhances the antioxidative ability, regulates the homeostasis of Ga2+, inhibits the damage of mtDNA caused by oxidative stress for ageing brain | (Li et al., 2009) | ||
| Formula with effects of anti‐ageing and extending lifespan | Eucommia ulmoides Oliver/Lycium chinense Miller | Aged rats of 85 weeks old | Improves the biochemical changes | Decreases the DNA single chain break and increases their unscheduled DNA synthesis | (Guo et al., 1997) |
| Zuoguiwan pill | Rhizoma dioscoreae/Lycium chinense Miller/Cornus officinalis Sieb. et Zucc./Cyathula officinalis Kuan/Cuscuta chinensis Lam. Gelatinum cornu Cervi/Colla carapacis et Plastri Testudinis | D‐galactose‐induced ageing rats | Slows down the ageing progress | Through improving the blood anti‐oxidative ability and decreasing the DNA damage of lymphocytes | (Xia et al., 2012) |
| Natural ageing 24‐month‐old rats | Has effect of neural protection and repairment on brain | By down‐regulating levels of genomic DNA 8‐OH‐dG | (Zhao et al., 2002) | ||
| Five seeds combo | Lycium chinense Miller/Cuscuta chinensis Lam./Schisandra chinensis Turcz. Baill./Plantago asiatica Linn./Rubus idaeus Linn. | Thirty‐eight aged men with symptoms of senility and aged rats of 22 months old | Has protective effect on oxidative damage of mtDNA | Raises the activities of mitochondrial respiratory chain complexes I and IV, reduces the mitochondrial DNA deletions | (Wang et al., 2001) |
| Liquid of tonifying kidney and synergia | Rehmannia glutinosa Gaertn./Lycium chinense Miller/Scutellaria baicalensis Georgi/Angelica sinensis Oliv. Diels | 60Co γ irradiation‐induced damage on mice | Effectively prevents the apoptosis of lymphocyte, protects lymphocyte from injury | Reduces the cleavage rate of DNA | (Feng et al., 2005) |
| Capsule of tonifying qi and resolving turbidity | Astragalus membranaeus Fisch. Bge./Fructus ligustri Lucidi/Atractylodes lancea Thunb. DC./Bombyx mori L./Salvia miltiorrhiza Bunge/Euonymus alatus Thunb. Sieb. | The spontaneous type 2 diabetes KKAy mice fed with high fat diet | Improves insulin resistance | Probably related to raising expression levels of GLUT‐4 and AMPK protein in skeletal muscle and the activity of MLYCD in fat | (Guo et al., 2013) |
| Decoction of Fallopia multiflora | Fallopia multiflora Thunb. Harald./Cistanche deserticola Ma/Aerva sanguinolenta Linn. Blume/Epimedium brevicornu Maxim./Salvia miltiorrhiza Bunge/Poria cocos Schw. Wolf. | D‐galactose‐induced ageing rats | Reduces the incidence of ovary apoptosis | Maybe regulated by the expression of FOX, SIRT1 and c‐Myc and blocking apoptosis | (Zhang et al., 2013a) |
| Tablets of anti‐ageing; tablets of extending lifespan by Radix Polygoni Multiflori | Radix ginseng Rubra/Rehmannia glutinosa Gaert. Libosch. ex Fisch. et Mey./Asparagus cochin‐chinensis Lour. Merr./Ophiopogon japonicus Linn. f. Ker‐Gawl./Cortex Lycii/Poria cocos Schw. Wolf./Fallopia multiflora Thunb. Harald. | D‐galactose‐induced ageing rats | Improves the ageing symptom and the reduction of body weight, significantly increases the skin water content, sucrose consumption and bone narrow DNA content | May be related to enhance the capability of rapairing DNA damage | (Xiao et al., 2010) |
| Electuary of Li yongkang | Codonopsis pilosula Franch. Nannf./Dipsacus japonicus Miq./Cornus officinalis Sieb. et Zucc. | Ozone‐induced ageing mice | Enhances the ability of climbing rope, swimming, frost resistance, the total serum IgG concentration, blister of the sole, the thymus index and telomerase activity in thymus | Enhances telomerase activity, probably for the reason that the organs are rich in lymphocytes | (Yang et al., 2000) |
| Epimedii and Fructus Lycii | Epimedium brevicornu Maxim./Lycium chinense Miller | 22‐monthold rats | Protects aged rats from oxidative damage to mitochondria | Reduces the ratio of deleted/normal mtDNA, the activity of mitochondrial respiratory chain enzyme complexes and the rate of ATP synthesis | (Wang et al., 2002) |
| Erzhi pill | Ligustri Lucidi Ait./Eclipta prostratal | C. elegans | Increases the acute heat stress ability of C. elegans without affecting its fecundity | By regulating gene expression of IIS signalling pathway, neuroendocrine and biological clock | (Wang, 2010) |
| Extracts from TCM | Portulaca oleracea Linn. | D‐galactose‐induced ageing mice | Significantly increases the activity and length of telomerase in senile mouse brain, decreases the expression of p53 gene | Possibly through inhibiting the p53 gene expression and activating the telomerase | (Huang et al., 2007) |
| Rosa damascena | Drosophila flies | increased longevity by 27%, with no reduction in fecundity | Attributed to the antioxidant action | (Jafari et al., 2008) | |
| Hericium erinaceus Bull. Ex Fr. | D‐galactose‐induced ageing mice | Has significant antioxidative effect in apolexis brain tissues | Increases expression of SOD and GSH‐Px, decreases MDA content | (Liu et al., 2011b) | |
| Cornus officinalis Sieb. et Zucc. | D‐galactose‐induced ageing rats | Plays certain role in delaying ageing | Inhibits the in vivo protein non‐enzymatic glycosylation, decreases the DNA damage of peripheral blood lymphocyte | (Guo et al., 2005) | |
| Cynomorium songaricum Rupr. | Flies | Enhances cognitive behaviour and resistance to stress, extends female mean lifespan | The C. songaricum flavonoids act as free radical scavengers | (Liu et al., 2012; Yu et al., 2010) | |
| Vacciniumuliginosum Linn. | Drosophila flies | Significantly extends mean lifespan, enhances the locomotor performance | Via the up‐regulation of antioxidant enzymes (e.g. superoxide dismutase and catalase) | (Peng et al., 2012) | |
| Rhodiola rosea | Fruit fly and Drosophila melanogaster | Increases longevity without negative effects on reproduction or 2metabolic rate | strongly suggesting that Rhodiola is not a mere dietary restriction mimetic | (Jafari et al., 2007) | |
| Ginkgo biloba | nematode | Extends nematode lifespan by 10% | By enhancing resistance to thermal and oxidative stress | (Collins et al., 2006) | |
| Damnacanthus officinarum | C. elegans | Shows in vivo neuroprotective and lifespan extending activity by 10–30% | Mechanism unknown | (Yang et al., 2012) | |
| Ligusticum Chuanxiong Hort. | C. elegans | Increases the average life span of nematodes by 29.9% and the maximum life span by 9.4% | By antioxidant stress, regulating IIS signalling passway, inhibiting fat accumulation, improving mitochondrial activity and other genes related to energy metabolism | (Wang, 2010) | |
| Panax ginseng C. A. Meyer/Panax notoginseng Burkill F. H. Chen ex C. H. Chow/Ligusticum chuanxiong Hort. | Angiotensin II‐induced ageing of HUVECs | Delays the ageing of HUVECs induced by angiotensin II | Possibly by down‐regulating the expression of NADPH oxidase subunit p47phox through AT1R and further reducing superoxide anion production | (Yang et al., 2009) | |
| Coptis chinensis Franch. | High fat diet‐induced metabolic syndrome rats | Enhances the insulin sensitive index M‐value and protein level of p‐AMPK‐α, reduces wet weight of innards fat | Decreases the level of TC, TG in serum and improves insulin sensitivity by activation of AMPK in muscle tissues of metabolic syndrome rats | (Qiao et al., 2010) | |
| Decoction | Polygonatum sibiricum Delar. ex Redoute | D‐galactose‐induced ageing mice | Significantly increases the telomerase activity in gonad and brain without significant change of MDA levels | Significantly activates the telomerase activity | (Li et al., 2002) |
In summary, there is much evidence, as mentioned above, for the anti‐ageing effect of combinatorial intervention in TCM to achieve synergistic interactions that could produce sufficient effects at low doses. However, the regulation of compatibility, principles of composition and effective substance basis of CPP or TCM extracts are poorly understood, thus hampering the development and modernization of TCM (Sheridan et al. 2012). Therefore, chemical fingerprinting coupled with systems biology should be applied to TCM to scientifically and accurately explore the pharmacokinetics of multi‐ingredients in TCM (Zhang et al., 2014b).
Limitations and prospects
Above all, active ingredients from TCM without serious adverse reactions seem to provide an intriguing way forward to exert anti‐ageing effects. Unfortunately, it must be highlighted that there are still many limitations and problems unresolved at the current stage. Firstly, the efficacy and/or safety of many TCM products largely rely on poor‐quality researches that are probably limited by the inadequate or inconsistent methods being used and risk of bias of the included studies, thereby failing to draw firm conclusions of efficacy. Therefore, high quality research in the field of TCM is emphatically needed to firmly establish the efficacy and/or safety of many TCM products. Simultaneously, there are potentially serious adverse events (Chan 2015), although relatively infrequent, as well as the interactions (Izzo 2012; Milic et al. 2014) between herbal medicines and prescribed medicines, that have been described, implying that more meticulous attention should be paid to herbal research. In fact, it is advisable to make sure that the herbal remedies are chemically characterized, standardized if possible and of known quality when prescribing for population in specific conditions, such as during pregnancy and by breastfeeding women, in the paediatric and adolescent population, as well as in the geriatric population (Izzo et al. 2016). Furthermore, many factors, such as the herb–drug interactions, the methods used for processing, combining and decocting, as well as the clinical context and testing methods used, could affect the toxicity of TCM (Liu et al. 2014). Thus, predicting safety of TCM at an early stage is a great challenge for drug development and requires considerable effort (Wang et al. 2015).
As is known to us all, TCMs not only possess multiple bioactive components and various pharmacological activities but also might generate other bioactive or inactive metabolites when delivered in vivo. It is really difficult to figure out whether the anti‐ageing effect is attributed to a (n) single and exact mechanism or owing to the synergistic therapeutic efficacies. Probably, TCM cannot be expected to have the target as clearly defined as that of a single compound, leading to the paradox that the theory of the whole view of TCM is reflected in all its aspects, whereas new studies of TCM are becoming more and more detailed and molecular . On the other hand, multi‐component treatments might hit multiple targets and exert synergistic therapeutic efficacies, at least in some formulae, thereby controlling complex diseases (Fu et al. 2007; Grivicich et al. 2008; Panchabhai et al. 2008; Sharma et al. 2008). Meanwhile, the bioactive metabolites generated when delivered in vivo should also be considered for their multi‐targeting roles on complex diseases. Specifically, there were some published data in several clinical models of Alzheimer's disease confirming this (Qin et al. 2009; Ksiezak‐Reding et al. 2012; Wang et al., 2014b; Zhao et al. 2015; Ho et al. 2016). Moreover, the clinical implications of TCMs in ageing‐related diseases are still unclear for the reason that current studies on TCM with anti‐ageing effects mainly stayed in the very earliest stage, and clinical trials are infrequent or performed without sufficient rigour and recorded detail. Undoubtedly, more high quality clinical trials are much needed to confirm the promising activities of the ingredients from TCM, and the findings must be interpreted vigilantly and cautiously. Additionally, few studies at the current stage focus on the relationship between the anti‐ageing activities of TCM components and their chemical structures, which is potentially of great importance to the exploration of TCMs with anti‐ageing effects.
Modern technology such as the in situ hybridization, immunohistochemistry and gene chip should be fully utilized to explore the ' regulatory effects of TCMs, which are characterized by multi‐tissues and multi‐indexes. Specifically, metabolomics, which has already been employed to identify and analyse the active ingredients of TCM, is inherently appropriate to provide novel insights into the essence and molecular basis of TCM (Shi et al. 2011; Cao et al. 2015). In conclusion, tackling ageing and age‐related diseases is a much needed task that, evidently, requires great commitment in both basic and clinical studies, aiming to identify and validate new drug targets and in new drug development.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This project was supported by the National Natural Foundation of China (31301453), Scientific Research Projects of the State Administration of traditional Chinese medicine (JDZX2015205), Guangdong Science and Technology Project (2013B090700015), Guangdong Provincial Science and Technology Department Project (2015B020211013).
Shen, C.‐Y. , Jiang, J.‐G. , Yang, L. , Wang, D.‐W. , and Zhu, W. (2017) Anti‐ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. British Journal of Pharmacology, 174: 1395–1425. doi: 10.1111/bph.13631.
Contributor Information
Jian‐Guo Jiang, Email: jgjiang@scut.edu.cn.
Wei Zhu, Email: zhuwei9201@163.com.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeyev MF (2009). Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J 276: 5768–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alic N, Partridge L (2011). Death and dessert: nutrient signalling pathways and ageing. Curr Opin Cell Biol 23: 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Harley CB (1995). Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res 219: 130–136. [DOI] [PubMed] [Google Scholar]
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB , et al. (1992). Telomere length predicts replicative capacity of human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America 89: 10114‐10118. [DOI] [PMC free article] [PubMed]
- Amagase H, Nance DM (2008). A randomized, double‐blind, placebo‐controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) juice, GoChi (TM. J Altern Complement Med 14: 403–412. [DOI] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R (2004). The AMP‐activated protein kinase AAK‐2 links energy levels and insulin‐like signals to lifespan in C‐elegans. Genes Dev 18: 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulou A, Aligiannis N, Trougakos IP, Skaltsounis A‐L (2013). Natural compounds with anti‐ageing activity. Nat Prod Rep 30: 1412–1437. [DOI] [PubMed] [Google Scholar]
- Bai X, Yang J, Liu CF, Song YL, Hao XY (2008). The anti‐aging effect of Corydalis yanhusuo W. T. Wang on aging mice induced by D‐galactose. Guizhou Med J 5: 399–401. [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L (2007). Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans . Mech Ageing Dev 128: 546–552. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL (2004). An accelerated assay for the identification of lifespan‐extending interventions in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America 101: 12980‐12985. [DOI] [PMC free article] [PubMed]
- Baum L, Lam CWK, Cheung SK‐K, Kwok T, Lui V, Tsoh J et al. (2008). Six‐month randomized, placebo‐controlled, double‐blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28: 110–113. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A et al. (2006). Resveratrol improves health and survival of mice on a high‐calorie diet. Nature 444: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Milgram E, Mitchell M, Lawton K, Hanson R, Kalhan S et al. (2007). The metabolomics of aging. FASEB J 21: A1040–A1040. [Google Scholar]
- Blackburn EH (1991). Structure and function of telomeres. Nature 350: 569–573. [DOI] [PubMed] [Google Scholar]
- Blackburn EH (2001). Switching and signaling at the telomere. Cell 106: 661–673. [DOI] [PubMed] [Google Scholar]
- Bonafe M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C et al. (2003). Polymorphic variants of insulin‐like growth factor I (IGF‐I) receptor and phosphoinositide 3‐kinase genes affect IGF‐I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. Journal of Clinical Endocrinology. Metabolism 88: 3299–3304. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin YX et al. (2004). Stress‐dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015. [DOI] [PubMed] [Google Scholar]
- Cai EP, Lin JK (2009). Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J Agric Food Chem 57: 9817–9827. [DOI] [PubMed] [Google Scholar]
- Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM et al. (2011). Icariin and its derivative icariside ii extend healthspan via insulin/IGF‐1 pathway in C. elegans . plos one 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao RZ, Wei YC, Zhang GW, Sun LJ (2011). Effect of total flavonoids of herba euphorbiae humifusae on the expression of telomerase activity in aged mice. West China. J Pharm Sci 2: 189–190. [Google Scholar]
- Cao YL, Li WL, Wei LY, Liu XY, Li ZH, He WG et al. (2012). Anti‐aging function of cycloastragenol in aging mice induced by D‐galactose. Chin J Exp Tradit Med Formulae 19: 208–211. [Google Scholar]
- Cao H, Zhang A, Zhang H, Sun H, Wang X (2015). The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother Res 29: 159–166. [DOI] [PubMed] [Google Scholar]
- Chan TYK (2015). Incidence and causes of aconitum alkaloid poisoning in Hong Kong from 1989 to 2010. Phytother Res 29: 1107–1111. [DOI] [PubMed] [Google Scholar]
- Chan S, Blackburn EH (2004). Telomeres and telomerase. Philos Trans R Soc Lond B‐Biol Sci 359: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX (2004). Research progress of the relationship between free radical and aging. J North Sichuan Med College 1: 207–209. [Google Scholar]
- Chen S (2012). The extraction, separation of piceid from cultivated Polygonum cuspidatum and preliminary study on its anti‐aging, Ji Shou University.
- Chen BT, Lin YP, Chen YC (2001). Study on the molecular biological mechanism of delaying aging by tea polyphenols‐the influence on the activity of DNA methylation enzyme in the liver of mouse. Food Sci 11: 56–57. [Google Scholar]
- Chen G, Tang Y, Yang L, Wang XK, Diao B, Zhu YL (2009). Antioxidative effects of LSPC in brain tissue of senile mice induced by D‐galactose. China Pharmacist 8: 1023–1025. [Google Scholar]
- Chen Y, Ping J, Zhang J, Huang JH, Xia SJ, Shen ZY (2012). Study of Icariin on raising SIRT6 activity and inhibiting NF‐kB inflammation signal pathway of mouse. Gerontol Health Care 18: 338–341. [Google Scholar]
- Chen LW, Zhang YX, Yu M, Wang SC (2013). Anti‐aging mechanism of polysaccharide from rhizomorph of Armillaria mellea in Caenorhabditis elegans. Chinese Traditional and Herbal Drugs 4: 449–453. [Google Scholar]
- Cheng CL, Gao TQ, Li DD (2004). Insulin /insulin‐like growth factor − 1 signaling pathway and aging. Foreign Med Sci Geriatr : 145–148. [Google Scholar]
- Collins JJ, Evason K, Kornfeld K (2006). Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans . Exp Gerontol 41: 1032–1039. [DOI] [PubMed] [Google Scholar]
- Cong J, Guo L, Ding XH, Wu JD, Gong Q (2014). Effect of gypenosides on antioxidative enzymes of human aged skin fibroblasts. Chin Med Herald 2: 32–34. [Google Scholar]
- Cui YF, Zhang BY, Zhang R, Li C, Zhao Y, Ren YH et al. (2013). Effects of resveratrol on morphology and oxidative stress of brain tissues in aging mice. Journal of Hygiene Research 6: 995–998 .+1003 [PubMed] [Google Scholar]
- Dang H, Wang Q, Wang H, Yan M, Liu X (2016). The integration of Chinese material medica into the Chinese health care delivery system, an update. Phytother Res 30: 292–297. [DOI] [PubMed] [Google Scholar]
- D'Aquila P, Rose G, Panno ML, Passarino G, Bellizzi D (2012). SIRT3 gene expression: a link between inherited mitochondrial DNA variants and oxidative stress. Gene 497: 323–329. [DOI] [PubMed] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD, Davis B (2009). Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol 296: R1071–R1077. [DOI] [PubMed] [Google Scholar]
- Deng YM, Zhang HQ, Xing SH, Sun Y (2001). The influence of polysaccharide from Spirulina platensis on human embryo lung diploid fibroblastic DNA damage and repair after UV irradiation. Chin J Marine Drugs 7: 27–31. [Google Scholar]
- Espinosa C, Perez‐Llamas F, Guardiola FA, Esteban MA, Arnao MB, Zamora S et al. (2014). Molecular mechanisms by which white tea prevents oxidative stress. J Physiol Biochem 70: 891–900. [DOI] [PubMed] [Google Scholar]
- Feng QS, Liu Y, Zhou Y, Liu JL, Huang GJ, Jin SR (2005). Effect of Bushen synergist on ~(60)Coγ radial‐induced mice splenic lymphocyte DNA damage. Sichuan J Tradit Chin Med 3: 25–26. [Google Scholar]
- Finkel T, Serrano M, Blasco MA (2007). The common biology of cancer and ageing. Nature 448: 767–774. [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML, Blasco MA (2005). Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA (2008). The longest telomeres: a general signature of adult stem cell compartments. Genes Dev 22: 654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE (1988). A mutation in the age‐1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S et al. (2007). Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res 21: 989–994. [DOI] [PubMed] [Google Scholar]
- Fu LQ, Li XY, Yang CX (2012). Galangin attenuates cognitive impairment in senescent mice. Herald Med 7: 863–866. [Google Scholar]
- Gao H, He L, Li CY, Lv JM (2009). Effects and mechanisms of salvianolic acid B on learning capability and memory in aging mice. Food Sci 19: 294–296. [Google Scholar]
- Gao F, Sun RJ, Ji Y, Yang BF (2015). Cardiovascular research is thriving in China. Br J Pharmacol 172: 5430–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng FH, Zhang P, Dong L, Gao F (2014). New findings in AMPK‐activated protein kinase. Chin Heart J 1: 97–100. [Google Scholar]
- Geng YN, Yu B, Kong WJ (2015). Gastrodin ameliorates oleic acid‐induced fat accumulation through activation of AMPK pathway in HL‐7702 cells. Chin Pharm Bull 1: 39–44. [Google Scholar]
- Gim GT, Kim H‐M, Kim J, Kim J, Whang W‐W, Cho S‐H (2009). Antioxidant effect of Tianwang Buxin pills, a traditional Chinese medicine formula: double‐blind, randomized controlled trial. Am J Chin Med 37: 227–239. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D et al. (2007). An AMPK‐FOXO pathway mediates longevity induced by a novel method of dietary restriction in C‐elegans. Curr Biol 17: 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivicich I, Ferraz A, Faria DH, Regner A, Schwartsmann G, Henriques AT et al. (2008). Synergistic effect of three benzopyrans isolated from hypericum polyanthemum in U‐373 MG glioblastoma cell line. Phytother Res 22: 1577–1580. [DOI] [PubMed] [Google Scholar]
- Guo YB, Zhang KC, Xu GX, Zeng YF, Liu QL, Yang LZ et al. (1997). Effect of Kangshuai Yanshou compound on DNA damage and repair capacity of old rats. Chin J Integr Tradit West Med S1: 68–70. [Google Scholar]
- Guo HY, Zhang PX, Ou Q, Wei XD, Xu H (2005). Effect of cornel on protein non‐enzymatic glycosylation and DNA damage in aging rats. Chin J Clin Rehab 19: 136–137. [Google Scholar]
- Guo L, Wei XD, Ou Q, Wang S, Zhu GM (2010). Effect of astragaloside on the expression of telomerase activity and klotho gene in aged HELF cells. Chin J Gerontol 13: 1819–1822. [Google Scholar]
- Guo HL, Liao LZ, Chen YL, Wu WK (2012). Resveratrol reverses D‐galactose‐induced senescence in cardiomyocytes. Chin J Pathophysiol 12: 2141–2146. [Google Scholar]
- Guo JJ, Pan YR, Zhao YL (2013). Experiment research about effects of Yiqi Huazhuo capsule on IR through AMPK signal channel. Chin Arch Tradit Chin Med 12: 2685–2688. [Google Scholar]
- Han W, Li XY, Zhou LL, Wu HP, Li SP, Lu Q (2009). Effect of emodin on lipid‐lowering and anti‐aging. Med Innov Chin 29: 14–16. [Google Scholar]
- Han HJ, Wang CL, Chen MH, Li FJ, Wang YR, Han XM et al. (2011). Study on the mechanisms of quercetin on extending lifespan of Caenorhabditis elegans . Amino Acids Biotic Resources 2: 35–38. [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee S‐J, Kenyon C (2007). Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans . Aging Cell 6: 95–110. [DOI] [PubMed] [Google Scholar]
- Hao QH, Chen GH, Feng YQ, Guo YX, Lei BS (2009). Antioxidative effect of honokiol in D‐galactose‐induced aging model mice. China J Chin Mat Med 6: 798–800. [Google Scholar]
- Hao L, Ren XH, Zhao Y, Xia CY, Guo CX, Wang YC et al. (2013). The effects of Ginkgo biloba extract on anti oxidantive DNA damage and delaying telomere shortening in prefrontal cortex of natural aging rats and its mechanisms. Pharmacol Clin Chin Mat Med 6: 38–42. [Google Scholar]
- Harley CB, Futcher AB, Greider CW (1990). Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460. [DOI] [PubMed] [Google Scholar]
- Hasani‐Ranjbar S, Khosravi S, Nayebi N, Larijani B, Abdollahi M (2012). A systematic review of the efficacy and safety of anti‐aging herbs in animals and human. Asian J Anim Vet Adv 7: 621–640. [Google Scholar]
- Helliwell RM, ShioukHuey CO, Dhuna K, Molero JC, Ye JM, Xue CC et al. (2015). Selected ginsenosides of the protopanaxdiol series are novel positive allosteric modulators of P2X7 receptors. Br J Pharmacol 172: 3326–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Bloom PA, Vega JG, Yemul S, Zhao W, Ward L et al. (2016). Biomarkers of resilience in stress reduction for caregivers of Alzheimer's patients. Neuromolecular Med 18: 177–189. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196. [DOI] [PubMed] [Google Scholar]
- Hu ZW, Shen ZY, Huang JH (2004). Experimental study on effect of Epimedium brevicornu flavonoids in protecting telomere length of senescence cells. Chin J Integr Tradit West Med 12: 1094–1097. [PubMed] [Google Scholar]
- Hu XQ, Li XH, Zheng YF, Long Y, Cao P, Zhang J (2010). Effect of allicin on learning and memory ability and antioxidative capability of brain tissue of Alzheimer's disease mice. Chin J Gerontol 7: 944–945. [Google Scholar]
- Huang YL (2007). Progress in studies of the anti‐aging traditional Chinese drugs. Lishizhen Med Mater Med Res 3: 691–693. [Google Scholar]
- Huang H, Yu NC, Liu Q, Yi YD, Ma W (2007). Effects of water extract of purslane herb on protecting telomere length of senile mouse. Chin J Clin Pharmacol Ther 7: 804–807. [Google Scholar]
- Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK et al. (2001). Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292: 929–934. [DOI] [PubMed] [Google Scholar]
- Izzo AA (2012). Interactions between herbs and conventional drugs: overview of the clinical data. Med Princ Pract 21: 404–428. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Hoon‐Kim S, Radhakrishnan R, Williamson EM (2016). A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother Res 30: 691–700. [DOI] [PubMed] [Google Scholar]
- Jafari M, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR et al. (2007). Rhodiola: a promising anti‐aging Chinese herb. Rejuvenation Res 10: 587–602. [DOI] [PubMed] [Google Scholar]
- Jafari M, Zarban A, Pham S, Wang T (2008). Rosa damascena decreased mortality in adult Drosophila. J Med Food 11: 9–13. [DOI] [PubMed] [Google Scholar]
- Jia XY, Gao YH, Zhao XL, Luan HY (2007). Research situation of free radical and anti‐aging. Heilongjiang Med Pharm 2: 75–76. [Google Scholar]
- Jiang LH (2004). Study on anti‐damage of DNA by traditional Chinese medicine. Herald Med 4: 250–252. [Google Scholar]
- Jiang LH (2005). Advance on the effect of DNA damage by traditional Chinese medicine (TCM) and its extractant. Pharm Biotechnol 1: 54–57. [Google Scholar]
- Jiang YH, Li YL, Zhao Q, Huo Q (2011). Uncaria alkaloids' intervention on the aged endothelial cell induced by D‐galactose. Chin J Arterioscler 6: 474–478. [Google Scholar]
- Jiang M, Wang C, Zhang Y, Feng Y, Wang Y, Zhu Y (2014). Sparse partial‐least‐squares discriminant analysis for different geographical origins of Salvia miltiorrhiza by H‐1‐NMR‐based metabolomics. Phytochem Anal 25: 50–58. [DOI] [PubMed] [Google Scholar]
- Jiang ZH, Ma S, Chen JW, Guo T, Li XJ, Fan MM et al. (2016). Resveratrol protects endothelial cells from senescence via inhibition of apoptosisi. Chin Heart J 6: 638–641. [Google Scholar]
- Kaeberlein M, Powers RW, Steffen KK, Westman EA, Hu D, Dang N et al. (2005). Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310: 1193–1196. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S (2004). Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke GA, You CH, Liu BL, Zhang H, Wang MM (2006). Study of mechanism of telomere and cell cycle in the proeess of allicin protection against senescence in fibroblast cells 1.
- Kensler TW, Wakabayashi N (2010). Nrf2: friend or foe for chemoprevention? Carcinogenesis 31: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ (2010). The genetics of ageing. Nature 464: 504–512. [DOI] [PubMed] [Google Scholar]
- Kishido T, Unno K, Yoshida H, Choba D, Fukutomi R, Asahina S et al. (2007). Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology 8: 423–430. [DOI] [PubMed] [Google Scholar]
- Ko KM, Chiu PY, Leung HY, Siu AHL, Chen N, Leong EPK et al. (2010). Long‐term dietary supplementation with a Yang‐invigorating Chinese herbal formula increases lifespan and mitigates age‐associated declines in mitochondrial antioxidant status and functional ability of various tissues in male and female C57BL/6 J mice. Rejuvenation Res 13: 168–171. [DOI] [PubMed] [Google Scholar]
- Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A et al. (2008). The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci 49: 1712–1720. [DOI] [PubMed] [Google Scholar]
- Ksiezak‐Reding H, Ho L, Santa‐Maria I, Diaz‐Ruiz C, Wang J, Pasinetti GM (2012). Ultrastructural alterations of Alzheimer's disease paired helical filaments by grape seed‐derived polyphenols. Neurobiol Aging 33: 1427–1439. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart‐Hines Z, Meziane H, Lerin C, Daussin F et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1 alpha. Cell 127: 1109–1122. [DOI] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S (2010). When a theory of aging ages badly. Cell Mol Life Sci 67: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L et al. (2008). Analysis of the adult human plasma metabolome. Pharmacogenomics 9: 383–397. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH (2001). Mitochondrial alterations, cellular response to oxidative stress and defective degradation of proteins in aging. Biogerontology 2: 231–244. [DOI] [PubMed] [Google Scholar]
- Lee MK, Choi YJ, Sung SH, Shin DI, Kim JW, Kim YC (1995). Antihepatotoxic activity of icariin, a major constituent of Epimedium koreanum. Planta Med 61: 523–526. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Sim JY, Heo JH, Kim M (2008). Ponax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord 22: 222–226. [DOI] [PubMed] [Google Scholar]
- Lee KS, Lee BS, Semnani S, Avanesian A, Um C‐Y, Jeon H‐J et al. (2010). Curcumin extends life span, improves health span, and modulates the expression of age‐associated aging genes in Drosophila melanogaster . Rejuvenation Res 13: 561–570. [DOI] [PubMed] [Google Scholar]
- Lee YH, Lee SJ, Jung JE, Kim JS, Lee NH, Yi HK (2015). Terrein reduces age‐related inflammation induced by oxidative stress through Nrf2/ERK1/2/HO‐1 signalling in aged HDF cells. Cell Biochem Funct 33: 479–486. [DOI] [PubMed] [Google Scholar]
- Li YY, Yang Y, Deng HB, Hu XQ, Chen LL (2002). Effect of ploygonatum sibiricum on telomerase activity in aging mice tissue. Cent China Med J 4: 225–226 .+230 [Google Scholar]
- Li SJ, Zhang L, Zhang DS, Shen LX, Dong XH, Wu HX et al. (2005). Experimental study on antisenility effect of Chrysophanol. Chin J Gerontol 11: 78–80. [Google Scholar]
- Li B, Fang WJ, Meng H (2009). Influences of Sijunzi decoction on the mtDNA damage and antioxidative ability in brain of aging mice induced by D‐galactose. J Beihua Univ (Natural Science) 3: 218–222. [Google Scholar]
- Li DZ, Wu JD, Wang P (2011a). Effect of Radices puerarire and puerarin on activities of GSH‐Px of naturally sensile mice. Chinese Aesthet Med 12: 1921–1922. [Google Scholar]
- Li CS, Deng HB, Li DD, Li ZH (2013a). Advances and challenges in screening traditional Chinese anti‐aging materia medica. Chin J Integr Med 19: 243–252. [DOI] [PubMed] [Google Scholar]
- Li J, Chen LJ, Hu SS, Wang YF (2014). Effect of dogwood polysacchharide on the expression of SIRT1 gene in eye lens of the aging rats. Eval Anal Drug‐Use Hosp China 10: 875–878. [Google Scholar]
- Li Y, Yan H, Zhang Z, Zhang G, Sun Y, Yu P et al. (2015). Andrographolide derivative AL‐1 improves insulin resistance through down‐regulation of NF‐B signalling pathway. Br J Pharmacol 172: 3151–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Wang L, Li A, Qiu Z, Qi LW et al. (2016). The role of metformin and resveratrol in the prevention of hypoxia‐inducible factor 1 alpha accumulation and fibrosis in hypoxic adipose tissue. Br J Pharmacol 173: 2001–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang FS, Beech W, Frautschy SA, Cole GM (2001). The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21: 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Huang HC, Lin JK (2007). Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J Lipid Res 48: 2334–2343. [DOI] [PubMed] [Google Scholar]
- Linda P, David G (2002). Mechanisms of ageing: public or private? Nat Rev Genet 3. [DOI] [PubMed] [Google Scholar]
- Ling LJ, Hu QL (2013). Progress in the study of molecular mechanism of SIRT1and aging. J Green Sci Technol 5: 292–295. [Google Scholar]
- Liu XY (2013). Metabolism study in vivo and in vitro of astragaloside and cycloastragenol Beijing University of Chinese Medicine.
- Liu H, Li MG, Han L (2011a). Effects of Hericium extract on antioxidative system in apolexis brain tissues in mice induced by D‐galactose. Clin Misdiag Misther 7: 11–12. [Google Scholar]
- Liu JF, Ma Y, Wang Y, Du ZY, Shen JK, Peng HL (2011b). Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother Res 25: 588–596. [DOI] [PubMed] [Google Scholar]
- Liu HP, Chang RF, Wu YS, Lin WY, Tsai FJ (2012). The Yang‐tonifying herbal medicine cynomorium songaricum extends lifespan and delays aging in Drosophila. Evidence‐Based Complementary and Alternative Medicine. [DOI] [PMC free article] [PubMed]
- Liu XM, Wang Q, Song GQ, Zhang GP, Ye ZG, Williamson EM (2014). The classification and application of toxic Chinese materia medica. Phytother Res 28 (3): 334–347. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE (2003). Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299: 1342–1346. [DOI] [PubMed] [Google Scholar]
- Lopez‐Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell 153: 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Shan Q et al. (2010). Quercetin activates AMP‐activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol‐induced neurotoxicity. J Pathol 222: 199–212. [DOI] [PubMed] [Google Scholar]
- Luo JY, Nikolaev AY, Imai S, Chen DL, Su F, Shiloh A et al. (2001). Negative control of p53 by Sir2 alpha promotes cell survival under stress. Cell 107: 137–148. [DOI] [PubMed] [Google Scholar]
- Lv JH, Tang DL, Fang WJ, Zhang SP (2007). Influences of huperzine A on the antioxidative ability and Ga2+ concentration in brain of senile mice induced by D‐galactose. Chin J Hosp Pharm 10: 1403–1406. [Google Scholar]
- Ma LJ, Chen GL, Jia HY, Xie J (2009). Anti‐senescence effect of Cynomorium songaricum polysaccharide on D‐galactose‐induced aging mice. Chin J Hosp Pharm 14: 1186–1189. [Google Scholar]
- Marfe G, Tafani M, Fiorito F, Pagnini U, Iovane G, De Martino L (2011). Involvement of FOXO transcription factors, TRAIL‐FasL/Fas, and sirtuin proteins family in canine coronavirus type II‐induced apoptosis. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martin GM (2011). The biology of aging: 1985–2010 and beyond. FASEB J 25: 3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic N, Milosevic N, Kon SG, Bozic T, Abenavoli L, Borrelli F (2014). Warfarin interactions with medicinal herbs. Nat Prod Commun 9: 1211–1216. [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K et al. (2010). Caloric restriction and resveratrol promote longevity through the sirtuin‐1‐dependent induction of autophagy. Cell Death Dis 1, e10; DOI: 10.1038/cddis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Molin L, Dalliere N, Solari F (2010). Life span extension by resveratrol, rapamycin and metformin: the promise of dietary restriction mimetics for an healthy aging. Biofactors 36: 377–382. [DOI] [PubMed] [Google Scholar]
- Murase T, Misawa K, Haramizu S, Hase T (2009). Catechin‐induced activation of the LKB1/AMP‐activated protein kinase pathway. Biochem Pharmacol 78: 78–84. [DOI] [PubMed] [Google Scholar]
- Muteliefu G, Lei L, Tu PF, Guo DA, Lu JF (2004). Study on molecular mechanism of echinacoside for against aging. Acta Biophys Sin 3: 183–187. [Google Scholar]
- Na LX, Zhang YL, Li Y, Liu LY, Li R, Kong T et al. (2011). Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis 21: 526–533. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Sinclair DA (2007). The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol 8: 692–702. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM (1973). A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41: 181–190. [DOI] [PubMed] [Google Scholar]
- Pan ZW, Lu YJ, Yang BF (2015). Advances in exploring the role of microRNAs in the pathogenesis, diagnosis and therapy of cardiac diseases in China. Br J Pharmacol 172: 5435–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchabhai TS, Ambarkhane SV, Joshi AS, Samant BD, Rege NN (2008). Protective effect of Tinospora cordifolia, Phyllanthus emblica and their combination against antitubercular drugs induced hepatic damage: an experimental study. Phytother Res 22: 646–650. [DOI] [PubMed] [Google Scholar]
- Panza F, D'Introno A, Capurso C, Colacicco AM, Seripa D, Pilotto A et al. (2007). Lipoproteins, vascular‐related genetic factors, and human longevity. Rejuvenation Res 10: 441–458. [DOI] [PubMed] [Google Scholar]
- Peng SJ (2009). Experimental study on anti‐aging effect of puerarin. Shandong Med J 20: 45–46. [Google Scholar]
- Peng C, Zuo Y, Kwan KM, Liang Y, Ma KY, Chan HYE et al. (2012). Blueberry extract prolongs lifespan of Drosophila melanogaster . Exp Gerontol 47: 170–178. [DOI] [PubMed] [Google Scholar]
- Peng CC, Jin HZ, Liu RH (2015). Summary of the pharmacokinetic methods of traditional Chinese medicine prescription. J Pharm Pract 1: 5–8. [Google Scholar]
- Peng P, Song ZM, Yu SJ, Liu Y, Liu DH, Huang SY , et al. (2015). Ginsenoside Rb1 against natural aging of brain in mice and its influence on mTOR/p70s6k signaling pathway 1. Monograph of the sixteenth international congress of cardiology south China. 1.
- Pillarisetti S (2008). A review of SIRT1 and SIRT1 modulators in cardiovascular and metabolic diseases. Recent Pat Cardiovasc Drug Discov 3: 156–164. [DOI] [PubMed] [Google Scholar]
- Qiao LL, Huang F, Yan XG, Gong H, Li Y (2010). Effect of Rhizoma Coptidis apozem on expression of AMP‐activated protein kinase in skeletal muscle of metabolic syndrome rats. Chin J Tradit Chin Med Pharm 1: 145–148. [Google Scholar]
- Qin W, Ho L, Wang J, Peskind E, Pasinetti GM (2009). s100 a7, a novel alzheimer's disease biomarker with non‐amyloidogenic alpha‐secretase activity acts via selective promotion of ADAM‐10. PLoS One 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I et al. (2011). Hyperactive S6 K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF (2004). The role of insulin and insulin‐like growth factor‐1 in mammalian ageing. Best practice research clinical endocrinology. Metabolism 18: 393–406. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL (2005). A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res 2: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S (2011). Extending life span by increasing oxidative stress. Free Radic Biol Med 51: 327–336. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL (2004). Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proceedings of the National Academy of Sciences of the United States of America 101: 15998–16003. [DOI] [PMC free article] [PubMed]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD et al. (2002). Biomarkers of caloric restriction may predict longevity in humans. Science 297: 811–811. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Nefsky BS (2002). Protein turnover plays a key role in aging. Mech Ageing Dev 123: 207–213. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Kaarniranta K (2012). Phytochemicals suppress nuclear factor‐kappa B signaling: impact on health span and the aging process. Curr Opin Clin Nutr Metab Care 15: 23–28. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Sikora E, Cooper EL, Franceschi C (2007). Curcumin in cell death processes: a challenge for CAM of age‐related pathologies. Evid Based Complementary Altern Med 4: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling MM, Oeser JK, Boustead JN, Flemming BP, O'Brien RM (2006). Re‐evaluating the FOXO1‐PGC‐1 alpha connection. Nature 443: E10–E11. [DOI] [PubMed] [Google Scholar]
- Schnackenberg LK, Sun J, Espandiari P, Holland RD, Hanig J, Beger RD (2007). Metabonomics evaluations of age‐related changes in the urinary compositions of male Sprague Dawley rats and effects of data normalization methods on statistical and quantitative analysis. BMC Bioinformatics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PR, Shanmugavel M, Saxena AK, Qazi GN (2008). Induction of apoptosis by a synergistic lignan composition from Cedrus deodara in human cancer cells. Phytother Res 22: 1587–1594. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Depinho RA (2007). How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8: 703–713. [DOI] [PubMed] [Google Scholar]
- Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD et al. (2013). Curcumin‐supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age 35: 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan H, Krenn L, Jiang R, Sutherland I, Ignatova S, Marmann A et al. (2012). The potential of metabolic fingerprinting as a tool for the modernisation of TCM preparations. J Ethnopharmacol 140: 482–491. [DOI] [PubMed] [Google Scholar]
- Shi X, Lu XG, Zhan LB, Qi X, Liang LN, Hu SY et al. (2011). The effects of the Chinese medicine ZiBu PiYin recipe on the hippocampus in a rat model of diabetes‐associated cognitive decline: a proteomic analysis. Diabetologia 54: 1888–1899. [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG (2009). Resveratrol protects mitochondria against oxidative stress through AMP‐activated protein kinase‐mediated glycogen synthase kinase‐3 beta inhibition downstream of poly(ADP‐ribose)polymerase‐LKB1 pathway. Mol Pharmacol 76: 884–895. [DOI] [PubMed] [Google Scholar]
- Sikora E, Scapagnini G, Barbagallo M (2010). Curcumin, inflammation, ageing and age‐related diseases. Immun ageing: I A 7: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S (2006). The health benefits of gambir. Yakushigaku Zasshi 41: 47–49. [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK (2008). Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74: 1526–1539. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D (2010). Stress‐activated Cap'n'collar transcription factors in aging and human disease. Sci Signal 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki P, Troulinaki K, Tavernarakis N (2007). eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans . Nature 445: 922–926. [DOI] [PubMed] [Google Scholar]
- Tao PH, Wen XL, Wang YZ, Mao GX (2014). The experimental study on effect of carnosic acid on delaying the aging process of human diploid 2BS fibroblasts. Acta Nutri Sin 3: 273–277. [Google Scholar]
- Tian J, Du H, Yang H, Liu X, Li Z (1997). A clinical study on compound Da Huang (Radix et Rhizoma Rhei) preparations for improvement of senile persons' memory ability. J Tradit Chin Med = Chung i tsa chih ying wen pan /sponsored by All‐China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine 17: 168–173. [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE et al. (2010). AMP‐activated protein kinase signaling activation by resveratrol modulates amyloid‐beta peptide metabolism. J Biol Chem 285: 9100–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L (2005). A role for SIR‐2.1 regulation of ER stress response genes in determining C‐elegans life span. Dev Cell 9: 605–615. [DOI] [PubMed] [Google Scholar]
- Wang HJ (1994). Clinical application of anti‐aging capsule on 95 cases of middle aged and old peopleJiangsu. J Tradit Chin Med : 45–46.8196418 [Google Scholar]
- Wang XY (2010). Study of the anti‐aging effects of tcms that could promote Qi and activate blood or nourish kidney and lver on C.elegans and the underlying molecular mechanisms. China Academy of Chinese Medical Sciences.
- Wang XM, Fu H, Liu GX (2001). Clinical and experimental study on effect of Wuzi Yanzong pill on oxidative damage of mitochondrial DNA in aging. Integr Tradit Chin West Med Pract Crit Care Med 6: 331–334. [Google Scholar]
- Wang XM, Fu H, Liu GX (2002). Effect of Herba Epimedii and Fructus Lycii on mitochondrial DNA deletion, activity of respiratory chain en‐zyme complexes and ATP synthesis in aged rats. J Beijing Med Univ 1: 68–71. [Google Scholar]
- Wang SJ, Wang GJ, Li XT, Sun JG, Ma RL, Sheng LS (2005). Simultaneous determination of oxymatrine and its active metabolite matrine in dog plasma by liquid chromatography‐mass spectrometry and its application to pharmacokinetic studies. Journal of Chromatography B‐Analytical Technologies in the Biomedical and Life Sciences 817: 319–325. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Zhang XR, Lan FH (2006). Effect of Bushenjianpi Yangxuehuoxue formula on deletion mutation of kidney mitochondria DNA in senile mouse. Chin J Tradit Chin Med Pharm 11: 652–654. [Google Scholar]
- Wang Y, Lawler D, Larson B, Ramadan Z, Kochhar S, Holmes E et al. (2007). Metabonomic investigations of aging and caloric restriction in a life‐long dog study. J Proteome Res 6: 1846–1854. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu M, Gu JX, Gu JY (2009b). Study of the antioxidant effect of Rosmarinic acid on aging mice induced by D‐galactose. Chin J Gerontol 5: 549–551. [Google Scholar]
- Wang S, Jiang H, Xi LQ, Chen GY, Zhang L, Ma CH (2013). Study on the anti‐aging effect of flavonoid from Oxytropis glabra DC on mice. Chin Agric Sci Bull 5: 37–41. [Google Scholar]
- Wang J, Bi WN, Cheng A, Freire D, Vempati P, Zhao W et al. (2014b). Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer's disease‐experimental approach and therapeutic implications. Front Aging Neurosci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ji Y, Yang B (2015). Chinese innovation in cardiovascular drug discovery. Br J Pharmacol 172: 5425–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner HR (2005). Longevity genes: from primitive organisms to humans. Mech Ageing Dev 126: 235–242. [DOI] [PubMed] [Google Scholar]
- Watson JD (1972). Origin of concatemeric T7 DNA. Nat New Biol 239: 197–201. [DOI] [PubMed] [Google Scholar]
- Westphal CH, Dipp MA, Guarente L (2007). A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci 32: 555–560. [DOI] [PubMed] [Google Scholar]
- Wiegant FAC, Surinova S, Ytsma E, Langelaar‐Makkinje M, Wikman G, Post JA (2009). Plant adaptogens increase lifespan and stress resistance in C‐elegans. Biogerontology 10: 27–42. [DOI] [PubMed] [Google Scholar]
- Williams RE, Lenz EM, Lowden JS, Rantalainen M, Wilson ID (2005). The metabonomics of aging and development in the rat: an investigation into the effect of age on the profile of endogenous metabolites in the urine of male rats using H‐1 NMR and HPLC‐TOF MS. Mol Biosyst 1: 166–175. [DOI] [PubMed] [Google Scholar]
- Williams R, Lenz EM, Wilson AJ, Granger J, Wilson ID, Major H et al. (2006). A multi‐analytical platform approach to the metabonomic analysis of plasma from normal and zucker (fa/fa) obese rats. Mol Biosyst 2: 174–183. [DOI] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu YS et al. (2003). Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421: 643–648. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M et al. (2004a). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M et al. (2004b). Sirtuin activators mimic caloric restriction and delay ageing in metazoans (vol 430, pg 686, 2004. Nature 431: 107–107. [DOI] [PubMed] [Google Scholar]
- Wu Q, Dong C (2003). The anti‐aging acion of Liuweidihuang decoction and its mechanism. Pharmacol Clin Chin Mater Med 3: 6–7 .+49 [Google Scholar]
- Wu JD, Wang P, Li DZ (2011). Effect of Radices puerarire and reducing mtRNA of naturally sensile mice. Liaoning J Tradit Chin Med 11: 2119–2120. [Google Scholar]
- Wu BY, Liu XG, Chen WC (2015). Effects of rapamycin induced cellular autophagy in aging‐related diseases. Chinese Pharmacological Bulletin 1: 11–14. [Google Scholar]
- Xia HY, Zhang JH, Xie YX, Yao LA, Li ZP, Xu YW (2012). Effects of Zuogui pill on blood anti‐oxidative abilities and DNA damage of lymphocytes in aging rats induced by D‐galactose. Chin J Tradit Chin Med Pharm 11: 2937–2939. [Google Scholar]
- Xiao H, Tao T, Chen ML, Leng XX, Pan YM, Zhu KY et al. (2010). Changes of general physical signs and bone marrow DNA content of ageing model rat induced by D‐galactose and interventional effect of traditional Chinese medicine. Chin J Comparat Med 5: 37–40 .+49 [Google Scholar]
- Xiao G, Chen Z, Li WN (2014). The anti‐aging effects of mogroside. Chin J Gerontol 15: 4263–4265. [Google Scholar]
- Xu CZ, Zhang BS (1998). Clinical observation on anti‐aging effects of Huatanquyu Chinese herbal medicine. Hebei Med 8: 38–39. [Google Scholar]
- Xu AX, Zhang ZM, Ge B, Pu JF (2006). Study effect and its mechanism on resisting senility of PCPN. Chin J Mod Appl Pharm S2: 729–731. [Google Scholar]
- Yan S, Wu B, Lin Z, Jin H, Huang J, Yang Y et al. (2009). Metabonomic characterization of aging and investigation on the anti‐aging effects of total flavones of Epimedium. Mol Biosyst 5: 1204–1213. [DOI] [PubMed] [Google Scholar]
- Yang XH, Lu J, Yang BF, Rao YQ, Li SC, Wang CS (1995). DNA damage and repair in old mouses pleen lymphoeytes modified by Liu Wei Dan Kun decoction. Pharmacol Clin Chin Mater Med 3: 4–6. [Google Scholar]
- Yang SM, Yan JH, Zang SQ, Chen F, Zhang CG, Wang TJ et al. (2000). Influence of Li Yongkang Gaozi on mouse thymus telomerase activity and other immune function. J Tradit Chin Med 9: 556–558. [Google Scholar]
- Yang J, Zhan XH, Sun Y, Li XC, Li HG (2005). Effect of Sijunzi decoction on malondialdehyde content and telomerase activity in heart, liver and brain tissues of D‐galactose induced aging model mice. Chin J Integr Tradit West Med 6: 531–533. [PubMed] [Google Scholar]
- Yang J, Lei Y, Fang SP, Cui W, Chen KY (2009). Study on acting mechanism of extracts from Ginseng, Notoginseng and Chuanxiong for delaying the aging of endothelial cells induced by angiotensin II. Chin J Integr Tradit West Med 6: 524–528. [PubMed] [Google Scholar]
- Yang X, Zhang P, Wu J, Xiong S, Jin N, Huang Z (2012). The neuroprotective and lifespan‐extension activities of Damnacanthus officinarum extracts in Caenorhabditis elegans . J Ethnopharmacol 141: 41–47. [DOI] [PubMed] [Google Scholar]
- Yin DZ, Chen KJ (2005). The essential mechanisms of aging: irreparable damage accumulation of biochemical side‐reactions. Exp Gerontol 40: 455–465. [DOI] [PubMed] [Google Scholar]
- Yin J, Gao Z, Liu D, Liu Z, Ye J (2008). Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab 294: E148–E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q, Lu H, Bai Y, Tian A, Yang Q, Wu J et al. (2015). A metabolite of Danshen formulae attenuates cardiac fibrosis induced by isoprenaline, via a NOX2/ROS/p38 pathway. Br J Pharmacol 172: 5573–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonei Y, Takahashi Y, Hibino S, Watanabe M, Yoshioka T (2008). Effects on the human body of a dietary supplement containing L‐carnitine and Garcinia cambogia extract: a study using double‐blind tests. J Clin Biochem Nutr 42: 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FR, Liu Y, Cui YZ, Chan EQ, Xie MR, McGuire PP et al. (2010). Effects of a flavonoid extract from Cynomorium songaricum on the swimming endurance of rats. Am J Chin Med 38: 65–73. [DOI] [PubMed] [Google Scholar]
- Zarse K, Bossecker A, Mueller‐Kuhrt L, Siems K, Hernandez MA, Berendsohn WG et al. (2011). The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans . Horm Metab Res 43: 241–243. [DOI] [PubMed] [Google Scholar]
- Zeng XY, Wang H, Bai F, Zhou X, Li SP, Ren LP et al. (2015). Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br J Pharmacol 172: 4303–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan LS, Zhang XS, Wu XH, Wang YL, Wang ZX (1999). The protective effect of the sulfated polysaccharides from brown seaweed on lymphocyte DNA damage caused by oxygenradial. Chin J Mar Drugs : 4–7. [Google Scholar]
- Zhang J (1993). Research situation on anti‐aging effects of traditional Chinese medicine compound prescription “Huan Jing Jian”. J Gansu Coll Tradit Chin Med 4: 49–51. [Google Scholar]
- Zhang XL, Liu XZ (2011). Effects of catalpol on memory and antioxidative enzyme activity in D‐galactose induced sub‐acute senescent mice. Chin J Biochem Pharm 2: 103–106. [Google Scholar]
- Zhang XR, Du J, Lin YZ, Lin JP, Xu SF (1992). Clinical study on the treatment of aging with strong anti aging liquid – a report of 30 cases. J Fujian Coll Tradit Chin Med 3: 141–144. [Google Scholar]
- Zhang SX, Li X, Yin JL, Chen LL, Zhang HQ (2007). Study on anti‐aging effect of C21 steroidal glycoside from the root of Cynanchum auriculatum planted in Jiangsu. Pract Geriatr 2: 104–107. [Google Scholar]
- Zhang HQ, Weng XJ, Chen LL, Li X (2008a). Effect of Cistanche tubulosa (Scheuk) Whight acteoside on telomerase activity and mimunity of aging mice. Chin J Pharmacol Toxicol 4: 270–273. [Google Scholar]
- Zhang YJ, Wu T, Zhou XJ, Liu LM (2008b). Glucocorticoid‐induced skin aging and the effects of salvia miltiorrhiza and salvianolic acid B in vivo. Chin J Hosp Pharm 20: 1767–1770. [Google Scholar]
- Zhang ZF, Li Q, Liang J, Dai XQ, Ding Y, Wang JB et al. (2010). Epigallocatechin‐3‐O‐gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine 17: 14–18. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Li Y, Song YY (2011a). Effect of polysaccharides of Cistanche deserticola on immune cells and telomerase activity in aging mice. Chin Pharm J 14: 1081–1083. [Google Scholar]
- Zhang MM, Jia XM, Wang C, Gao W, Peng KN, Zhao M et al. (2013a). Effects of Heshouwuyin on the expression of apoptosis‐associated genes FOX, SIRT1 and c‐Myc protein in the ovary of the aging rats. J Med Pest Control 12: 1368–1370. [Google Scholar]
- Zhang A, Sun H, Wang X (2014a). Potentiating therapeutic effects by enhancing synergism based on active constituents from traditional medicine. Phytother Res 28: 526–533. [DOI] [PubMed] [Google Scholar]
- Zhang L, Han X, Li Z, Liu R, Xu W, Tang C et al. (2014b). Metabolomics research on time‐selected combination of Liuwei Dihuang and Jinkui Shenqi pills in treating kidney deficiency and aging by chemometric methods. Chemom Intel Lab Syst 130: 50–57. [Google Scholar]
- Zhao YJ, Tang YC (2012). Separation, purification and anti‐aging activity of phycocyanin from Porphyra yezoensis . Food Sci 17: 94–97. [Google Scholar]
- Zhao LX, Yu L (2004). Pine pollen delays cell senescence and its effects on telomerase activity. Sichuan J Tradit Chin Med 4: 11–13. [Google Scholar]
- Zhao G, Cai DF, Fan J, Yuan ZJ (2002). Effect of Zuogui Wan on 8‐hydroxy‐2c‐deoxyguanosine of neocortex of aging rats. J Shanghai Med (University) 3: 208–210. [Google Scholar]
- Zhao CH, Chen XC, Zhu YG, Huang C, Shi GB, Ceng YQ et al. (2005). Roles of telomere and telomerase in the process of ginseno‐side Rg1 protection against tert‐butylhydroperoxide‐induced senescence in W I‐38 cells. Chin Pharm Bull 1: 61–66. [Google Scholar]
- Zhao W, Wang J, Bi W, Ferruzzi M, Yemul S, Freire D et al. (2015). Novel application of brain‐targeting polyphenol compounds in sleep deprivation‐induced cognitive dysfunction. Neurochem Int 89: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KF, Wang MY, Chen QL, Wu HT, Zhao MF, Zhang X (1998). Mechanism of Jinkui Shenqi pill in preventing aging. J Nanjing Univ Tradit Chin Med 6: 33–34 .+68 [Google Scholar]
- Zhou B, Wu LJ, Li LH, Tashiro S, Onodera S, Uchiumi F et al. (2006). Silibinin protects against isoproterenol‐induced rat cardiac myocyte injury through mitochondrial pathway after up‐regulation of SIRT1. J Pharmacol Sci (Print Edition) 102: 387–395. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang R, Yang B, Yao X, Wang P, Liu DF et al. (2011). Changes of telomere and telomerase in effect of ginsenoside Rg1 to delay hematopoietic stem cell senescence. China J Chin Mater Med 22: 3172–3175. [PubMed] [Google Scholar]
- Zhou YF, Zhang GH, Wang H (2014). A study on 6‐gingerol attenuate vascular smooth muscle cells senescence through inhibition of mTOR pathway molecular. Chongqing Med 14: 1687–1689. [Google Scholar]
- Zhu Z, Hu YZ, Qian ZG (2004). Studies of sodium terulate on anti‐oxidation of ageing mice inducal by D‐galactose. J Yunnan Coll Chin Med 1: 16–19. [Google Scholar]
- Zhu GM, Jiang XD, Wang DD, Ou Q, Zhang L, Sun J et al. (2012). Effect of Astragalus membranaeus polysaccharide on chromosome terminal restriction fragment length of the aged HDF cell. Chin J Gerontol 8: 1635–1637. [Google Scholar]
- Zi XH, Zhou W, Chen Q, Li M, Gu SL (2012). The effect of oxymatrine on aging mice caused by D(+)—galactose. J Chin Med Mater 9: 1455–1459. [PubMed] [Google Scholar]
- Zygmunt K, Faubert B, MacNeil J, Tsiani E (2010). Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun 398: 178–183. [DOI] [PubMed] [Google Scholar]


