Abstract
Carotenoids and retinoids have several similar biological activities such as antioxidant properties, the inhibition of malignant tumour growth and the induction of apoptosis. Supplementation with carotenoids can affect cell growth and modulate gene expression and immune responses. Epidemiological studies have shown a correlation between a high carotenoid intake in the diet with a reduced risk of breast, cervical, ovarian, colorectal cancers, and cardiovascular and eye diseases. Cancer chemoprevention by dietary carotenoids involves several mechanisms, including effects on gap junctional intercellular communication, growth factor signalling, cell cycle progression, differentiation‐related proteins, retinoid‐like receptors, antioxidant response element, nuclear receptors, AP‐1 transcriptional complex, the Wnt/β‐catenin pathway and inflammatory cytokines. Moreover, carotenoids can stimulate the proliferation of B‐ and T‐lymphocytes, the activity of macrophages and cytotoxic T‐cells, effector T‐cell function and the production of cytokines. Recently, the beneficial effects of carotenoid‐rich vegetables and fruits in health and in decreasing the risk of certain diseases has been attributed to the major carotenoids, β‐carotene, lycopene, lutein, zeaxanthin, crocin (/crocetin) and curcumin, due to their antioxidant effects. It is thought that carotenoids act in a time‐ and dose‐dependent manner. In this review, we briefly describe the biological and immunological activities of the main carotenoids used for the treatment of various diseases and their possible mechanisms of action.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- ASTA
astaxanthin
- Aβ
amyloid‐β
- CAM
cell adhesion molecules
- H2O2
hydrogen peroxide
- iNOS
inducible NOS
- MCP‐1
monocyte chemoattractant protein‐1
- MHC II
major histocompatibility complex class II
- MMP
mitochondrial membrane potential
- PBMCs
peripheral blood mononuclear cells
- PP
Peyer's patch
- PTP
protein tyrosine phosphatase
- RA
retinoic acid
- TLR
toll‐like receptor
- TG
triglyceride
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Catalytic receptors c |
| Bcl‐2 | IFN‐α receptor |
| Bcl‐xL | IGF1R |
| IL‐1β | TLR4 |
| TNF‐α | Enzymes d |
| Nuclear hormone receptors b | AMPK |
| RARα | CDK2 |
| COX‐2 | |
| iNOS | |
| MMP9 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a,b,c,d).
Introduction
Carotenoids are colourful liposoluble pigments. They are found in plants, fungi, bacteria and algae and are present in many foods, for example, fruit, vegetables and fish (El‐Agamey et al., 2004; Tapiero et al., 2004). There are more than 600 carotenoids with natural structural variants which are divided into carotenes, xanthophylls and lycopene (Jomova and Valko, 2013; Rutz et al., 2016). Only ~40 carotenoids are present in a typical human diet and about 20 carotenoids have been identified in human blood and tissues. These carotenoids in the diet and human body include β‐carotene, α‐carotene, lycopene, lutein and cryptoxanthin (Rao and Rao, 2007). Carotenoids belong to the tetraterpenes family (C40‐based isoprenoid), responsible for the yellow, orange or red colour of fruits, leaves and flowers (Kaulmann and Bohn, 2014; Tapiero et al., 2004). For example, (a) the green vegetables contain high amounts of both hydrocarbon carotenes and xanthophylls; (b) lycopene is a lipophilic red pigment present in ripe tomatoes; (c) the orange colour of carrots is caused by β‐carotene; (d) capsanthine is responsible for the brilliant red pigment of peppers; and (e) the pink/red coloration of crustaceans is due to astaxanthin (ASTA) (Astorg, 1997). Most of the carotenoids are composed of a central carbon chain of alternating single and double bonds and carry various cyclic or acyclic end groups (Stahl and Sies, 2005; Figure 1). Epidemiological studies indicated that the use of diets rich in carotenoids is related to a lower incidence of cancer, cardiovascular diseases (CVDs), osteoporosis, diabetes, age‐related macular degeneration (AMD), cataract and also infectious diseases such as HIV infections (Rao and Rao, 2007; Pechinskii and Kuregyan, 2014; Saini et al., 2015). HIV patients usually have low plasma concentrations of carotenoids, because all carotenoids (e.g. lutein, cryptoxanthin, lycopene, β‐carotene and α‐carotene) are significantly destroyed in these patients and this is directly related to an increased risk of death. Both CD4+ and CD8+ lymphocytes can be increased significantly in HIV patients by the administration of 60 mg·day−1 of β‐carotene and the symptoms of the disease decrease over 24–36 months (Pechinskii and Kuregyan, 2014). In general, a number of biological actions of carotenoids have been demonstrated including antioxidant activity, immune enhancement, regression of malignant lesions and inhibition of mutagenesis (Rao and Rao, 2007). In this review, we briefly describe the biological activities of the main carotenoids used for the treatment of various diseases. Figure 2 shows the major functions and mechanisms of action of carotenoids.
Figure 1.
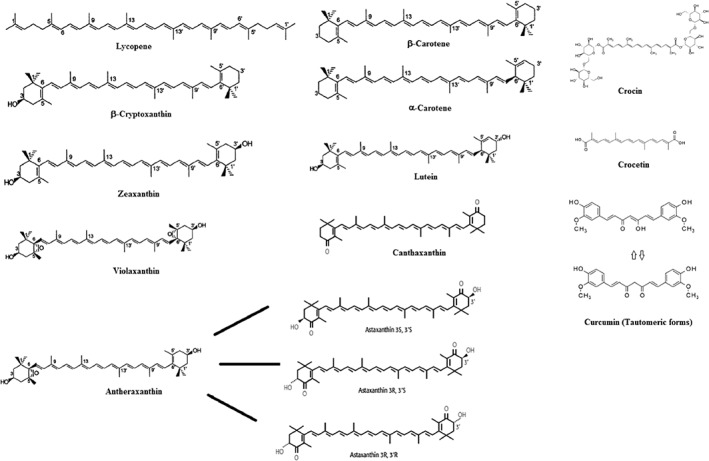
The structures of the major carotenoids.
Figure 2.
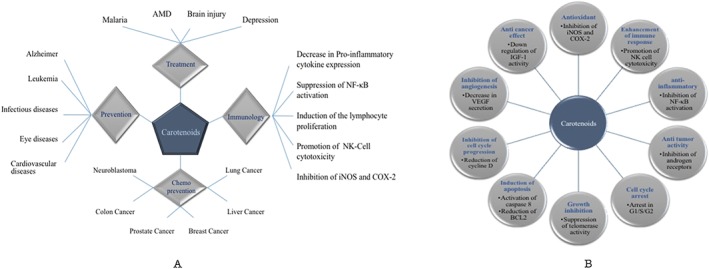
Biological activities of the main carotenoids used in the treatment of various diseases: (A) general effects; (B) their molecular mechanisms.
Chemistry of carotenoids
The main carotenoids include lycopene, β‐carotene, ASTA, lutein and zeaxanthin (Tapiero et al., 2004). Carotenoids are synthesized by the linkage of two C20 geranylgeranyldiphosphate molecules. All carotenoids contain a polyisoprenoid structure, a long conjugated chain of double bond and a near bilateral symmetry around the central double bond (Saini et al., 2015). Carotenoids can be divided into provitamin A (e.g. β‐carotene, α‐carotene and β‐cryptoxanthin) and non‐provitamin A compounds (Stahl and Sies, 2005). On the other hand, carotenoids can be classified based on their functional groups as follows: (a) xanthophylls (e.g. lutein, zeaxanthin) containing oxygen as functional group, and (b) carotenes (e.g. α‐carotene, β‐carotene and lycopene) containing only a parent hydrocarbon chain without any functional group (Saini et al., 2015). Other compounds such as apocarotenoids (e.g. retinoids, vitamin A, β‐ionone and α‐ionone aromatic volatile compounds) are also derived from carotenoids by oxidative cleavage using carotenoid cleavage dioxygenases (Saini et al., 2015). Generally, carotenoids are hydrophobic molecules with very low solubility in water that act in hydrophobic areas of the cell. Polar functional groups attached to the polyene chain can change the polarity of carotenoids, which may influence their localization within biological membranes and their interactions with different molecules (Jomova and Valko, 2013). Figure 1 shows the structures of the main carotenoids.
Antioxidant activities
Reactive oxygen and nitrogen species are produced during pathological processes and aerobic metabolism, and are involved in the pathobiochemistry of degenerative diseases (Stahl and Sies, 2005). The beneficial effects of carotenoids are mainly derived from their antioxidant properties as a main scavenger of the ROS such as single molecular oxygen (O2) and peroxyl radicals (Stahl and Sies, 2005; Rao and Rao, 2007). Carotenoids can scavenge radicals in three steps such as electron transfer (oxidation, reduction: CAR + ROO → CAR+ + ROO−), hydrogen abstraction (CAR + ROO → CAR + ROOH) and addition (CAR + ROO → ROOCAR) (El‐Agamey et al., 2004). The presence of conjugated double bonds enables these compounds to accept electrons from reactive species, and then neutralize free radicals (Rutz et al., 2016). A combination of two lipophilic antioxidants (e.g. vitamins E, C and β‐carotene) leads to synergistic effects as a result of scavenging reactive nitrogen species and inhibition of lipid peroxidation, which is significantly higher than that of a single effect (Stahl and Sies, 2005). β‐carotene functions as an effective chain‐breaking antioxidant. Studies have shown that β‐carotene can suppress the up‐regulation of haem oxygenase 1 gene expression in human dermal fibroblasts (FEK4) exposed to UV‐A, indicating a dose‐dependent pro‐oxidant effect (El‐Agamey et al., 2004; Rao and Rao, 2007). In addition, zeaxanthin can effectively scavenge both water‐ and lipid‐soluble peroxyl radicals, but β‐carotene is less effective at preventing lipid peroxidation (El‐Agamey et al., 2004). Lycopene is also known to be potent at decreasing the ROS generated by smoke and to modulate redox sensitive cell targets including protein kinases, protein tyrosine phosphatases (PTP), MAP kinase (MAPKs) and transcription factors (Kaulmann and Bohn, 2014).
Carotenoids and anti‐cancer properties
Carotenoids have been found to have beneficial effects on the treatment of various cancers. Table 1 shows biochemical activities of main carotenoids against different diseases.
Table 1.
Biological effects of the main carotenoids against different diseases
| Carotenoids | Source | Disease | In vitro/in vivo study | aDose of interest | Biochemical/clinical effects | Ref. |
|---|---|---|---|---|---|---|
| β‐carotene (derived from carrots, apricots, mangoes, red pepper, kale, spinach, broccoli) | Natural | Leukaemia | U937, HL‐60 cell lines (in vitro) | 20 μM | Cell cycle arrest in the G1 phase; induction of apoptosis, Antioxidant properties | Upadhyaya et al., 2007 |
| Natural | Colon cancer | COLO 320 HSR, WiDr, LS174 cell lines (in vitro) | 25 μM | Cell cycle arrest in the G2/M phase; reduction of the percentage of Bcl‐2 and Bcl‐xl positive cells | Palozza et al., 2002 | |
| Natural | Gastric cancer | AGS cell line (in vitro) | 100 μmol·L−1 | Induction of apoptosis | Jang et al., 2009 | |
| Natural | Adenocarcinoma | WiDr cell line (in vitro) | 50 μM | Induction of apoptosis by its pro‐oxidant properties | Palozza et al., 2001 | |
| Natural | Neuroblastoma | BE(2)C cell line, male BALB/c ν/ν mice (in vitro/ in vivo) | 20 μM | As a chemotherapeutic agent regulating the invasion and metastasis of neuroblastoma via hypoxia inducible factor‐ 1 α (HIF‐1α) | Kim et al., 2014 | |
| Natural | Hepatocarcinoma, Prostate cancer | SK‐Hep1, B16F10, PC‐3 cell lines (in vitro) | 20 μM | Inhibition of proliferation. Decrease in VEGF secretion | Chen et al., 2012 | |
| Natural | Cervical cancer | Chinese women (clinical) | Vitamin diet (8.22% for α‐carotene, 6.81% for β‐carotene) | Antioxidant compounds (e.g., α‐carotene, β‐carotene, and vitamins E and C) are beneficial in reducing the risk of cervical cancer | Guo et al., 2015 | |
| Natural | Prostate cancer | Finnish men (clinical) | High concentration of serum β‐carotene was associated with low risk of prostate cancer | Karppi et al., 2012 | ||
| Natural | HCV infection | HCV patient (clinical) | Decrease in serum retinol, β‐carotene, and retinol binding protein 4 (RBP4) was associated with early stages of HCV infection | Kataria et al., 2016 | ||
| Natural | Age‐related macular degeneration (AMD) | Patients (clinical) | Food intake | Low intake of n‐3 fatty acid, α‐tocopherol, zinc, vitamin D, vitamin C, β‐carotene and lutein was associated with neovascular AMD; but no change for retinol or cryptoxanthin | Aoki et al., 2016 | |
| Β‐carotene, canthaxanthin, phytoene | Natural | Skin tumours | Female SKH·h−1 mice (in vivo) | 3.3 mg (β‐carotene) 100 mg (canthaxanthin) 168 mg·kg−1·week−1 (phytoene) | β‐carotene decreased the number of skin tumours | Mathews‐Roth, 1982 |
| α‐carotene (AC) and β‐carotene (BC) [AC derived from carrots, bananas, pumpkins, peppers, avocados, apricots] | Natural | Hepatocarcinoma | SK‐Hep‐1 cell line (in vitro) | 2.5 μM | Inhibition of cell invasion. Anti‐tumour effects of AC were stronger than those of BC at the same concentration | Chen et al., 2013a,b |
| β‐carotene | Both forms [natural (derived from Dunaliellasalina) and synthetic] | Breast cancer | HaCat, MDA‐MB‐231 Cells (in vitro) | 10 μg·mL−1 | Induction of apoptosis using both natural and synthetic sources. Natural β‐carotene generated considerable higher rates of cell mortality as compared to synthetic form | Olmos et al., 2015 |
| Crocin (s), crocetin | Natural | Liver damage | Male Kunming mice (in vivo) | 400 mg·kg−1 | Low levels of serum alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP) | Chen et al., 2016 |
| Crocin | Natural | Breast cancer | MCF‐7 cells (in vitro) | 50 μg·mL−1 (IC50: 60 μg·mL−1 ) | Induction of apoptosis; activation of caspase‐8 | Lu et al., 2015 |
| Crocin, crocetin | Natural (derived from Crocus sativus) | Breast cancer | MCF‐7 and MDA‐MB‐231 cells (in vitro) | >200 μM (IC50 for MCF‐7: 350 μg·mL−1; IC50 for MDA‐MB‐231: 500 μg·mL−1) | Inhibition of the proliferation | Chryssanthi et al., 2007 |
| Crocin | Natural | Depression | Adult male Wistar Albino rats (in vivo) | 25 and 50 mg·kg−1 | Anti‐depressant like action by increasing CREB, BDNF and VGF levels in hippocampus | Vahdati Hassani et al., 2014 |
| Natural | Depression | Adult outpatients (clinical) | 30 mg·day−1 capsule of saffron | No side effects, treatment of mild to moderate depression (Anti‐depressant) | Noorbala et al., 2005 | |
| Crocetin | Natural | Brain injury | Adult Sprague–Dawley (SD) rats (in vivo) | 50 mg·kg−1 (once daily) | Decrease in BCL‐2 protein expression; inhibition of apoptosis; treatment of traumatic brain injury | Bie et al., 2011 |
| Natural | Gastric cancer | AGS, HFSF‐PI3 cell lines, Male Wistar albino rats (in vitro/ in vivo) | 200 μmol·L−1 | Induction of apoptosis. Reduction of the Bcl‐2/Bax mRNA ratio | Bathaie et al., 2013 | |
| Natural | Malignant cells | A‐549 (lung adenocarcinoma), VA‐13 (SV‐40 transformed fetal lung fibroblast), HeLa cell lines (in vitro) | Up to 200 μg·mL−1 (Non‐toxic); IC50 (for DNA: 35 or 42 μg·mL−1, for RNA: 57 or 65 μg·mL−1 , for protein: 45 or 55 μg·mL−1) | A dose dependent inhibitory effect on DNA/RNA synthesis in isolated nuclei and suppressed the activity of purified RNA polymerase II; Hela cells were less sensitive to the inhibition of intracellular protein synthesis than the A‐549 and VA‐13 cells | Abdullaev, 1994 | |
| Natural | Lung cancer | Male Swiss albino mice (in vivo) | 50 mg·kg−1 body weight (Non‐toxic) | Inhibition of cell proliferation | Magesh et al., 2009 | |
| Natural | Colorectal cancer | HCT‐116, HT‐29, SW‐480, NSCLC Cell lines (in vitro) | 3 mg·mL−1 | Anti‐proliferative effects | Aung et al., 2007 | |
| Natural | Cervical cancer caused by HPV infections | TC‐1 cell line, Female C57BL/6 mice (in vitro/ in vivo) | IC50 for crocin (2 mM) | Induction of a sub‐G1 peak. Apoptosis; anti‐tumour effect; prevention of cell growth; chemotherapeutic agent | Khavari et al., 2014 | |
| Curcumin | Natural | Breast cancer | MDA‐MB‐435 cell line, Female athymic nude mice (in vitro/ in vivo) | 50 μmol·L−1 | Enhanced apoptosis; decreased breast cancer metastasis to the lung; suppression of NF‐ĸB | Aggarwal et al., 2005 |
| Natural | Alzheimer | APPSw Tg+ and Tg− mice (in vivo) | 160 ppm | Reduction of oxidized proteins and interleukin‐1β pro‐inflammatory cytokine | Lim et al., 2001 | |
| Natural | Head and neck cancer | CCL23, CAL27, UM‐SCC1, UM‐SCC14A cell lines. Female athymic nude mice (in vitro/ in vivo) | 50 μmol·L−1 (toxic at 400 μmol·L−1) | Growth inhibition | LoTempio et al., 2005 | |
| Curcumin | Natural | Bladder cancer | KU‐7, 253JB‐V cell lines; athymic nude mice (in vitro/ in vivo) | 10 μmol·L−1 for cell line (toxic at 25 μmol·L−1); 50 mg·kg−1·day−1 for mice | Inhibition of tumour growth | Chadalapaka et al., 2008 |
| Natural | Skin tumour | Male Swiss ablino mice (in vivo) | 1% in regimen | Inhibition of the tumour number | Limtrakul et al., 1997 | |
| Natural | Helicobacter pylori | C57BL/6 mice (in vivo) | 50 μg·mL−1 | Growth inhibitor for Indian H. pylori strains; healing the overall damage caused by H. pylori | De et al., 2009 | |
| Natural | Liver and small intestine cancer | knockout mice Nrf2 (−/−) (in vivo) | 1000 mg·kg−1 | Chemoprevention | Shen et al., 2006 | |
| Natural | Ovarian cancer | CaOV3 cells (in vitro) | 50 μM | Curcumin induced AMPK activation | Pan et al., 2008 | |
| Natural | Pancreatic cancer | Patients (clinical) | 8 g | Loss of subcutaneous fat and muscle as compared to untreated subjects | Parsons et al., 2016 | |
| Natural | Prostate cancer | LNCaP, PC‐3 cell lines (in vitro) | 40 μM for LNCaP, 30 μM for PC3 | Therapeutic effect. Down‐regulation of transactivation and expression of androgen receptor (AR) and AR‐related cofactors including activator protein‐1 (AP‐1), NF‐ĸB, and CREB (cAMP response element‐binding protein)‐binding protein (CBP) | Nakamura et al., 2002 | |
| Natural | Leukaemia | HL60, Bel7402, SGC7901 cell lines; female BALB/c athymic (ν+/ν+) mice (in vitro/ in vivo) | 1 μM (IC50 for HL60: 3.11 μM, for Bel7402: 3.8 μM; for SGC7901: 8 μM) | Growth inhibition. Suppression of telomerase activity in the cancer cells, and regulation of telomere length | Cui et al., 2006 | |
| Curcumin | Natural | Malaria | Male Swiss mice (in vivo) | 100 mg·kg−1 | Malaria therapy. Reduction of blood parasitaemia by 80–90%, and significant enhancement of their survival | Reddy et al., 2005 |
| Natural | Melanoma | MMAN, MMRU, RPEP, PMWK, Sk‐mel‐2, Sk‐mel‐5, Sk‐mel‐28, MEWO cell lines (in vitro) | 100 μM | Induction of apoptosis | Bush et al., 2001 | |
| Curcuma biscuits | Natural | Cardiovascular disease | Healthy men (clinical) | Curcuma biscuits (daily up to 2 months) | Reduction of total cholesterol and LDL‐cholesterol, Prevention of cardiovascular disease | Madaric et al., 2013 |
| Thymoquinon, curcumin | Natural | Influenza | Turkey poults (in vivo) | 2.5 g·kg−1 | Synergistic anti‐influenza activity, High antibody titer against H9N2 AIV | Umar et al., 2016 |
| Curcumin, tetrahydrocurcumin (THC) | Natural | Colon cancer | Male B6C3F1 mice (in vivo) | 0.2% (THC) and 0.5% (curcumin) in diet | Chemopreventive agents THC is more active than the curcumin | Kim et al., 1998a,b |
| Hydrazinocurcumin | Synthetic | Endothelial liver cell | BAECs, HT29, NIH3T3 cells (in vitro) | Toxicity: 0.52 μM | New candidate for anti‐angiogenic agent | Sup Shim et al., 2002 |
| Hydrazinobenzoyl curcumin (HBC) | Synthetic | Lung cancer | A549 cells (in vitro) | Toxicity: 80 μM | Inhibition of the A549 cell proliferation via inducing autophagy | Zhou et al., 2014 |
| Curcumin analogues | Synthetic | Tumour (human fibrosarcoma cells) | HT‐1080, BAECs, HUVECs, JB6P+ cell lines (in vitro) | 5 μM (Non‐toxic) | Inhibition of endothelial cell migration, Inhibitory effect of AP‐1 transcription and anti‐angiogenic activity | Hahm et al., 2004 |
| Lutein/ zeaxanthin (derived from kale, spinach, broccoli, peas, cress, parsley, lettuce, maize, egg yolk) | Natural | Age‐related macular degeneration (AMD) | AMD patient (clinical) | Food intake | Strongly associated with a reduced risk for AMD | Seddon et al., 1994 |
| Lutein/ zeaxanthin | Natural | Age‐related macular degeneration | Women (clinical) | Food intake | Protection against intermediate AMD in healthy women younger than 75 years | Moeller et al., 2006 |
| Natural | Age‐related cataract | AMD patient (clinical) | Daily lutein/zeaxanthin (10 mg/2 mg) | No statistically significant overall effect on rates of cataract surgery or vision loss | Chew et al., 2013 | |
| Natural | Diabetic retinopathy | Type 2 diabetes pateints (clinical) | Lutein/ zeaxanthin (6 mg/ 0.5 mg) | Potent preventive and also therapeutic effects | Moshetova et al., 2015 | |
| Natural | Epidermal hyperproliferation and acute inflammation | Female SKH‐1 mice (in vivo) | 0.4% lutein plus 0.04% zeaxanthin enriched diet | Reduction of acute inflammatory responses and inhibition of UV‐induced rebound hyper proliferation | Gonzalez et al., 2003 | |
| Natural | Skin cancer | Adult patients (clinical) | Dietary foods intake | More than 50% reduction in the risk of squamous cell carcinoma (SCC) | Heinen et al., 2007 | |
| Natural | Atherosclerosis | Female apoE‐null mice (in vivo) | 0.2% (w w‐1) | Increased dietary intake of lutein is protective against the development of early atherosclerosis | Dwyer et al., 2001 | |
| Natural | Atherosclerosis | Male guinea pigs (in vivo) | 0.1 g 100 g‐1 of diet | Prevention of early atherosclerosis development by reducing cholesterol accumulation | Kim et al., 2011 | |
| Semi synthetic | Antioxidant activity | Male Swiss albino mice (in vivo) | 100 mg | Significant antioxidant activity | Firdous et al., 2010 | |
| Astaxanthin | Natural | Colon cancer | Male Crj:CD‐1 (ICR) mice (in vivo) | 200 ppm (No toxicity) | Suppression of colon carcinogenesis by effects on NF‐ĸB signalling pathway | Yasui et al., 2011 |
| Lycopene [Derived from Tomato, Red watermelon, Pink grapefruit, Papaya, Guava, Rose hip canned] | Natural | Prostate cancer | Men (clinical) | Dietary intake | Decrease in the risk of prostate cancer | Giovannucci et al., 1995 |
| Lycopene | Natural | Prostate cancer | Phase II randomized clinical trial before prostatectomy (clinical) | 15 mg (twice daily) (No adverse effects) | Reduction of IGF‐1 level; enhancement of IGFBP‐3 level; decrease in tumour growth | Kucuk et al., 2001 |
| Natural | Prostate cancer | DU145, PC‐3, LNCaP cell lines; Male BALB/c nude mice (in vitro/ in vivo) | 26.6 μmol·L−1, 40.3 μmol·L−1, 168.5 μmol·L−1; In mice:100 and 300 mg·kg−1 | Inhibition of tumour growth | Tang et al., 2005 | |
| Lycopene | Natural | Breast cancer | Female Wistar rats (in vivo) | 20 mg·kg−1 | Inhibition of tumour growth and expression of apoptosis associated proteins | Sahin et al., 2011 |
| Natural | Breast cancer | MCF‐10a, MCF‐7, HBL‐100, MDA‐MB‐231 Cell lines (in vitro) | 10 μM | Cell cycle arrest in G1/S phase, Increase in expression of BRCA1 and BRCA2 oncosuppressor genes | Chalabi et al., 2004 | |
| Natural | Breast cancer; endometrial cancer | MCF‐7,T‐47D; ECC‐1 cell lines (in vitro) | 10 μM | Inhibition of cell cycle progression via reduction of the cyclin D level and retention of p27 in the cyclin E‐cdk2 complexes | Nahum et al., 2001 | |
| Natural | Breast cancer | MCF‐7, MDA‐MB‐231 cell lines (in vitro) | Toxicity: 2.4 μM for MCF‐7; 3.0 μM for MDA‐MB‐231 | Enhancement of quinacrine activity synergistically and inhibition of Wnt‐TCF signalling through APC | Preet et al., 2013 | |
| Natural | Oral cavity and pharynx cancer | Smokers and non‐smokers (clinical) | Dietary intake | Low concentration of plasma lycopene is associated with increased mortality | Mayne et al., 2004 | |
| Natural | Digestive tract cancer | Human (clinical) | Raw tomato intake | Protection against digestive‐tract cancers | Franceschi et al., 1994 | |
| Natural | Lung cancer | Male adult ferrets (in vivo) | 1.1 and 4.3 mg·kg−1 body weight·day−1 | Protective effects against lung cancer by promotion of apoptosis and inhibition of cell proliferation | Liu et al., 2003 | |
| Natural | Lung cancer | Male B6C3F1 mice (in vivo) | 50 ppm | Inhibition of lung neoplasian development | Sup Shim et al., 2002 | |
| Natural | HPV infection | Women (clinical) | Dairy foods | Significant inverse association between HPV persistence and plasma cis‐lycopene concentrations (56% tumour reduction) | Sedjo et al., 2002 | |
| Natural | Atherosclerosis | Men (clinical) | Dietary intake | Low concentration of serum lycopene is associated with a high carotid atherosclerosis | Rissanen et al., 2003 | |
| Natural | Cardiovascular diseases (CVD) | CVD and healthy pateints (clinical) | 7 mg | Improvement of endothelial (vascular) function in CVD patients | Gajendragadkar et al., 2014 | |
| Lycopene | Synthetic crystalline | Embryo‐fetal toxicity/ teratogenicity | Rats; rabbits (in vivo) | For rat: 3000 mg·kg−1·day−1; For rabbit: 2000 mg·kg−1·day−1 | Developmental toxicity | Christian et al., 2003 |
| Synthetic and natural | – | Healthy participants (clinical) | 15 mg·day−1 | Synthetic and natural lycopene are equivalent sources of lycopene (identical bioavailability) | Hoppe et al., 2003 | |
| Lycopene, α‐ and β‐carotene | Natural | Breast cancer; lung cancer | MCF‐7; NCI‐H226 cell lines (in vitro) | 1–2 μM | Lycopene inhibited the IGF‐induced cell proliferation; lycopene is a more potent inhibitor than α‐ and β‐carotene | Levy et al., 1995 |
| Lycopene and β‐carotene | Natural | Breast cancer | MCF‐7, MDA‐MB‐231, MDA‐MB‐235 cell lines (in vitro) | 10 μM | Inhibition of cell proliferation; cell cycle arrest in different phases, Induction of apoptosis | Gloria et al., 2014 |
Dose of interest: the dose at which the sample was tested was non‐toxic.
Carotenes (lycopene, β‐carotene and β‐cryptoxanthin)
The carotenoids possessing pro‐vitamin A activity are α‐carotene, β‐carotene, γ‐carotene and β‐cryptoxanthin. The low bioavailability of β‐carotene in natural sources is due to the resistance of carotene‐protein complexes and the plant cell walls to digestion and degradation and subsequently its poor release (Donhowe and Kong, 2014). Thermal processing has been shown to enhance β‐carotene bioavailability and absorption as compared with mechanical processing (van het Hof et al., 2000; Donhowe and Kong, 2014). Studies have shown that higher circulating levels of these carotenoids in women may reduce the risk of breast cancer (Eliassen et al., 2012). The reports indicate that treatment of human breast cell lines (e.g. MCF‐7 cells) with lycopene and β‐carotene, for 48 and 96 h, potently inhibits cell proliferation, arrests the cell cycle in different phases and increases apoptosis (Gloria et al., 2014). The findings obtained after treating human chronic monocytic leukaemia (U937) and myeloid leukaemia with β‐carotene indicated that β‐carotene acts as an antioxidant at lower concentrations (up to 20 μM for 24 h), but shows pro‐oxidant properties at higher concentrations. Also, β‐carotene has been shown to arrest HL‐60 leukaemia cells in the G1 phase (~39.4%) and significantly reduce their viability at a concentration of 20 μM. In fact, the number of apoptotic bodies is enhanced with increasing concentrations of β‐carotene (Upadhyaya et al., 2007; Niranjana et al., 2015). Among the different human adenocarcinoma colon cancer cells, COLO 320 HSR, WiDr and LS174 cells are the most susceptible to β‐carotene treatment respectively. Treatment with β‐carotene significantly decreases the percentage of BCL‐2 and Bcl‐xL positive cells and induces cell cycle arrest in the G2/M phase by reducing the expression of cyclin A (a key regulator of the G2/M phase progression) in a dose‐dependent manner (Niranjana et al., 2015; Palozza et al., 2002). Other studies have shown that supplementation with β‐carotene increases the level of apoptotic p53 and decreases anti‐apoptotic BCL‐2 in a human gastric cancer cell line (AGS cells) after 24 h treatment (Jang et al., 2009; Niranjana et al., 2015). Also, β‐carotene reduces the expression of hypoxia‐inducible factor 1α, a well‐known tumour metastasis regulator, and attenuates the migratory and invasive ability of malignant neuroblastoma cells (i.e. anti‐metastasis effect) (Niranjana et al., 2015). Higher serum concentrations of α‐carotene and β‐carotene as well as some vitamins (e.g. vitamins E and C) are associated with a lower risk of cervical cancer in Chinese women (Guo et al., 2015). In the same way, a significant correlation was observed between high concentrations of serum β‐carotene and the risk of prostate cancer (PCa) (Karppi et al., 2012). In addition, natural β‐carotene derived from Dunaliella salina generated higher rates of cell mortality on MDA‐MB‐231 breast cancer cells as compared with synthetic β‐carotene (Olmos et al., 2015). The findings indicated that treatment with α‐carotene significantly suppresses metastasis of human hepatocarcinoma cells (SK‐Hep‐1), by prevention of invasion, migration and adhesion in a dose‐dependent manner (~2.5 μM). The anti‐apoptotic effects of α‐carotene were stronger than those of β‐carotene at the same concentration (Niranjana et al., 2015; Chen et al., 2013a,b). Lycopene, a potent single oxygen quenching agent present in tomatoes, shows different biological effects such as cardioprotective, antioxidant, anti‐inflammatory, anti‐mutagenic and anti‐carcinogenic activities (Bhuvaneswari and Nagini, 2005). A 24 h incubation of cells with lycopene showed that this carotenoid is more efficient than α‐ and β‐carotene in preventing the growth of human endometrial, lung and mammary cancer cells, through inhibition of the insulin‐like growth factor (IGF)‐induced cell proliferation. IGFs are potent autocrine mitogens for endometrial and breast cancer cells (Levy et al., 1995). Supplementation with lycopene may diminish the growth of PCa by up‐regulating Cx43 gap junction protein, decreasing the IGF‐1 level and/ or increasing IGF binding at protein‐3 level (Kucuk et al., 2001). The data showed that the cis form of lycopene found in tomato products was more bioavailable than the trans form found in fresh tomatoes (Boileau et al., 1999). Furthermore, the reduced risk of PCa was slightly stronger for high intakes of cooked tomato products than for high intakes of raw tomatoes (Giovannucci et al., 1995). Lycopene and genistein are known to be potent antioxidants and their combination provides maximum protection against 7, 12‐dimethylbenz [α] anthracene (DMBA)‐induced mammary carcinogenesis (Sahin et al., 2011). The results showed a late G1‐phase cell cycle arrest followed by an increase in the G1 phase cell number (Chalabi et al., 2004). Indeed, lycopene suppressed cell cycle progression through a reduction in the cyclin D level and retention of p27 in cyclinE‐cdk2, leading to inhibition of G1 CDK activities (Nahum et al., 2001). On the other hand, lycopene significantly enhanced BRCA1, D11‐BRCA1, BRCA2 and D12‐BRCA2 RNA expression in three breast tumour cells (e.g. MCF‐7, HBL‐100 and MDA‐MB‐231 cell lines) (Chalabi et al., 2004). It also decreased serum‐induced phosphorylation of the retinoblastoma protein and related pocket proteins (Nahum et al., 2001). Several reports have suggested that topoisomerase inhibitors and lycopene synergistically suppress Wnt‐TCF signalling in breast cancer cells without affecting the normal cells (Preet et al., 2013). Generally, the mechanisms involved in the inhibitory effects of lycopene on tumour growth or carcinogenesis include an up‐regulation of detoxification systems, ROS scavenging, interference with cell proliferation, inhibition of cell cycle progression, induction of gap‐junctional communication and modulation of signal transduction pathways. The anti‐inflammatory activity of lycopene is also considered to be an important mechanism involved in its suppressive effect on the promotion and progression of carcinogenesis. Moreover, lycopene can inhibit cell invasion, angiogenesis and metastasis. These activities were shown at physiological concentration in humans. Although the preclinical data strongly suggested lycopene has antitumour activity, several epidemiological studies indicate that its use for the prevention of cancers is controversial. However, because of its multiple tumour‐inhibitory activities, lycopene still remains a promising carotenoid for the prevention and treatment of human cancers (Bhuvaneswari and Nagini, 2005; Ono et al., 2015). Furthermore, a significant inverse association has been observed between HPV persistence and plasma cis‐lycopene concentrations; lycopene induced a ~56% reduction in viral load (Sedjo et al., 2002).
Crocin and crocetin
Saffron contains terpenes, terpene alcohol and their esters (Srivastava et al., 2010). The value of saffron is due to the presence of three main secondary metabolites: crocin, responsible for colour; picrocrocin, responsible for taste; and safranal, responsible for odour (Bolhasani et al., 2005; Bathaie et al., 2007; Alizadeh and Bolhassani, 2015). The stability of its carotenoids depends on storage conditions including light, temperature and humidity (Gutheil et al., 2012). Their hepatoprotective efficacy is mediated through the induction of an antioxidant pathway (Chen et al., 2016). Saffron extract significantly decreases the viability of hepatocellular carcinoma cells (HepG2) in a time‐ and dose‐dependent manner. It can reduce the expression of TNF receptor 1 protein, cell proliferation and oxidative stress, as well as increase the active form of caspase‐3, induce apoptosis and down‐regulate inflammatory markers, such as COX‐2, inducible NOS (iNOS) and NF‐κB‐p65 in vivo (Amin et al., 2011). Crocus sativus extract also shows potent anti‐proliferative effects on human colorectal cancer cells such as HCT‐116, SW‐480 and HT‐29 (Aung et al., 2007). Crocin significantly inhibits the proliferation of MCF‐7 cells and induces apoptosis via mitochondrial signalling pathways such as the activation of caspase‐8, up‐regulation of Bax, the disruption of mitochondrial membrane potential (MMP) and the release of cytochrome c (Lu et al., 2015). Crocin has also been shown to have efficacy as a treatment of mild to moderate depression by increasing cAMP response element binding protein, brain‐derived neurotrophic factor and VEGF levels in the hippocampus (Noorbala et al., 2005; Vahdati Hassani et al., 2014). Furthermore, crocin inhibited amyloid‐β (Aβ) fibrillogenesis in male Wistar rats at lower concentrations than dimethylcrocetin (a synthetic carotenoid of saffron), indicating the effective role of the sugars in structure (Khalili, 2010). Our data confirmed that the cytotoxic activity of saffron extract and its ingredients (e.g. crocin) is higher against a malignant TC‐1 cell line than non‐malignant cells (COS‐7) and this is mediated through the induction of apoptosis (Alizadeh and Bolhassani, 2015). In addition, therapeutic DNA vaccination accompanied by oral administration of crocin showed that 100% of mice treated with crocin were tumour‐free as compared with those receiving the DNA vaccine alone (~66.7% compared to DNA vaccine + crocin ~33.3%), suggesting the high efficacy of crocin as a chemotherapeutic agent (Khavari et al., 2014). Crocetin was also shown to be an effective treatment for traumatic brain injury; it inhibited apoptosis at the early stages of the injury and enhanced vessel angiogenesis at the sub‐acute stage of cerebral trauma (Bie et al., 2011). Crocetin has also been shown to protect neurons from the deleterious effects of 6‐hydroxydopamine and was helpful in the prevention of Parkinsonism (Ahmad et al., 2005). Crocetin treatment significantly reduced the Bcl‐2/Bax mRNA ratio and increased caspase activity in AGS cell lines. Crocetin can act as a chemopreventive agent against benzo (α) pyrene‐induced lung carcinogenesis by protecting the glycoprotein levels in serum and tissues (Magesh et al., 2009). Crocetin has been shown to significantly inhibit the proliferation of human pancreatic adenocarcinoma cell lines (e.g. MIAPaCa‐2, BxPC3, Capan‐1 and ASPC‐1) by suppressing EGF receptor activity and by increasing the Bax/Bcl‐2 ratio (Dhar et al., 2009). In addition, the administration of geniposide, crocin and crocetin significantly reduced serum alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase levels in carbon tetrachloride (CCl4)‐treated mice. The decreased levels of GSH and the activities of antioxidant enzymes [SOD and catalase (CAT)] were enhanced by these carotenoids (Chen et al., 2016).Furthermore, saffron extract containing carotenoids has been shown to have anti‐convulsant and anti‐alzheimer properties in animal and human trials. Administration of saffron extract and its ingredients augmented dopamine and glutamate levels in the brain in a concentration‐dependent manner. Moreover, these compounds can interact with the opioid system to decrease withdrawal syndrome (Khazdair et al., 2015).
Xanthophyll (lutein, zeaxanthin and astaxanthin)
Xanthophylls are different from other carotenoids because of their oxygenated substituents, that is free hydroxyl groups at each end of the molecule that allow their orientation within cell membranes and lipoproteins (Roberts et al., 2009). Zeaxanthin and lutein act as antioxidants protecting photoreceptor cells from the potential damage caused by free radicals (Krinsky et al., 2003). A high dietary intake of lutein and zeaxanthin has been associated with a reduced incidence of skin cancer (Heinen et al., 2007). Lutein, by lowering very‐low‐density lipoprotein (VLDL) and intermediate density lipoprotein (IDL) levels, reducing inflammation and oxidative stress in the artery wall and also decreasing IL‐10 concentrations, acts as a potent protective factor against the progression of atherosclerosis in animals and humans (Dwyer et al., 2001; Kim et al., 2011). ASTA is unique as it has more hydroxyl groups than other xanthophylls. ASTA contains two terminal rings linked by a polyene chain with both lipophilic and hydrophilic properties (Ambati et al., 2014). ASTA cannot be converted to vitamin A. Humans are not able to synthesize ASTA and need to take it from food. Due to the high lipophilicity of ASTA, it can cross the blood–brain barrier and reach the brain and eye structures (Schweigert et al., 1998). ASTA is a potent antioxidant. Low doses of ASTA have been shown to inhibit colon carcinogenesis in mice by modulating proliferation, G0/G1 phase arrest through down regulation of cyclin D and increasing the expression of p21, p53 and p27. ASTA markedly decreased the incidence of colon adenocarcinoma in mice in vivo and increased their survival rate by suppressing the expression of PCNA (Pashkow et al., 2008; Yasui et al., 2011; Niranjana et al., 2015). In vitro studies showed that natural ASTA from Haematococcus pluvialis microalgae can be more than 50 times stronger than synthetic ASTA in single oxygen quenching and nearly 20 times more effective in eliminating free radicals (antioxidant activity) (Capelli et al., 2013).
Curcumin
Curcumin, a hydrophobic polyphenol derived from the rhizome of the herb Curcuma longa, has a wide range of pharmacological activities (Anand et al., 2007). In a human breast cancer xenograft model, dietary administration of curcumin significantly reduced the incidence of breast cancer metastasis to the lung; this was mediated through the inhibition of the anti‐apoptotic transcription factor NF‐κB and NF‐κB‐regulated gene products in the tumour tissue (Aggarwal et al., 2005). This growth suppression was also observed in head and neck cancer (HNC) cell lines (LoTempio et al., 2005; Wilken et al., 2011), human bladder cancer cells (e.g. 253JB‐V and KU7) (Chadalapaka et al., 2008), melanoma (Bush et al., 2001) and skin tumours (Limtrakul et al., 1997). Curcumin has been shown to induce apoptosis in melanoma cells via a Fas receptor/caspase‐8 pathway independent of p53 (Bush et al., 2001). Both low and high doses of curcumin reduced the levels of IL‐1β in TG− mice and also significantly decreased the levels of oxidized proteins, insoluble and soluble amyloid and plaque burden in the brains of APPSw transgenic mice (Lim et al., 2001; Ringman et al., 2005). Curcumin was shown to have therapeutic potential against Helicobacter pylori infection irrespective of the disease status. It was highly effective in eradicating H. pylori from infected C57BL/6 mice as well as in repairing H. pylori‐induced gastric damage (De et al., 2009). The new curcumin derivative, tetrahydrocurcumin, was shown to be more active than curcumin in preventing the development of aberrant crypt foci and cell proliferation (Kim et al., 1998a,b; Chauhan, 2002). Furthermore, thymoquinone and curcumin have been shown to have a synergistic inhibitory effect on the influenza virus induced by increasing the efficiency of the immune response and this effect plays a significant role in diminishing the pathogenic effects of H9N2 in turkeys (Umar et al., 2016). Curcumin was found to induce the activation of AMPK in human ovarian cancer cells (CaOV3 cells) in a time‐ and concentration‐dependent manner (~25–50 μM of curcumin; time: 15–120 min), as curcumin activated p38 phosphorylation that is an important downstream signal of AMPK, and elicited CaOV3 cell death (Pan et al., 2008). In addition, the treatment of patients with advanced pancreatic cancer with curcumin showed significantly greater loss of subcutaneous fat and muscle than untreated controls (Parsons et al., 2016). Curcumin has been shown to suppress telomerase expression and activity in three cancer cells (e.g. HL60, Bel7402 and SGC7901 cell lines) by inducing apoptosis, which indicates it has an anti‐proliferating effect (Cui et al., 2006). There are data showing that a novel synthetic analogue of curcumin, hydrazinocurcumin, also exhibits anti‐angiogenic activity but is less potent (Sup Shim et al., 2002). In contrast, low concentrations of the synthetic analogue of curcumin, hydrazinobenzoylcurcumin, when applied for short time, can inhibit the proliferation of human lung adenocarcinoma A549 cells by inducing autophagy and has potential as a therapeutic anti‐cancer agent. This compound is different from other curcumin derivatives, which mostly stimulate cell apoptosis (Zhou et al., 2014). Also, other synthetic derivatives of curcumin, including compounds 4 [1, 7‐bis‐(3,4‐dimethoxyphenyl)‐1,6‐heptadiene‐ 3,5‐dione] and 8 [1, 7‐bis‐(4‐propargyl‐3‐methoxyphenyl)‐1,6‐ heptadiene‐3,5‐dione], have been found to have potent activity against Leishmania amazonensis (Gomes et al., 2002). Curcumin and its analogues have been demonstrated to have an inhibitory effect on AP‐1 transcription (responsible for cell proliferation and differentiation) and anti‐angiogenic activity on the developmental neovascularization of chick embryo. In fact, curcumin analogues can decrease the migration of bovine aortic endothelial cells (BAEC) and HUVEC invasion (Hahm et al., 2004).
Immunopharmacological role of carotenoids
The effects of different carotenoids on immune responses, such as lymphocyte proliferation, cytokine release, phagocytic and microbicidal capacities, natural killer cell cytotoxic activity and inflammation, has been studied in vitro and in vivo. In this section, we describe the various immunopharmacological roles of individual carotenoids. Table 2 shows the immunological properties of the major carotenoids used to treat different diseases.
Table 2.
Immunological properties of major carotenoids against different diseases
| Carotenoids | In vitro/in vivo | Stimulus | Dose | Immunological effects | Ref. |
|---|---|---|---|---|---|
| Astaxanthin | Human | Phytohaemmaglutinin (PHA), concanavalin A (Con A), pokeweed mitogen (PWM) | 2 and 8 mg | ↓ C‐reactive protein (CRP) at 2 mg; ↑Nkc activity and lymph proliferation at 8 mg; ↑IFN‐γ and IL‐6 at 8 mg; No change in IL‐2 and TNF‐α | Park et al., 2010 |
| Dog | Concanavalin A | 20 mg | ↑ lymph proliferation and NKc cytotoxicity; ↑ IgG, IgM; ↑ Beta cell population; no changes in the populations of CD4+, CD8+ and MHC class II | Chew et al., 2011 | |
| Cat | Con A, PHA, PWM | 10 mg | ↑ NKc cytotoxicity;↑cd5,cd4 population; ↓Beta cell population; no alteration in CD8 and MHCII; ↑IgG, IgM | Park et al., 2011 | |
| Human | –––––––––– | 4 mg | ↑ IgA secretion; no change in leukocyte count; reduction of pro‐oxidant‐antioxidant balance (PABC) | Baralic et al., 2015 | |
| Neutrophil from human peripheral blood | Lipopolysaccharide (LPS) | 5 μM | ↑ phagocytic (30%) and fungicide (28%) effects against Candida albicans; ↓ TNF‐α and IL‐6; ↑NO production | Macedo et al., 2010 | |
| U‐937 cell line | H2O2 | 10 μM | ↓TNF‐α, NF‐ĸB, IL‐6 and IL‐1β | Speranza et al., 2012 | |
| Astaxanthin stereoisomers | Mouse [K562 cell (target for NKc); lymphocytes and peritoneal macrophages] | –––––––– | 20 μmol·L−1 | ↑Lymph proliferation; ↑phagocytic activity; ↑NKc cytotoxicity; (3S, 3'S)‐trans‐astaxanthin was better than others | Sun et al., 2016 |
| Spleen and thymus from C57B/6 mice | PHA, ConA | 10−7‐10−9M | ↑ Ab production; ↑Thy‐1+ and Thy‐1− cell populations, No change in IL‐2 | Jyonouchi et al., 1991 | |
| Human PBMC (peripheral blood mononuclear cells) | PWM (for IgA); trinitrophenol‐modified keyhole limpet haemocyanin (TNP‐KLH); tetanus toxoid (for IgG) | 10−8mol·L−1 | ↑IgM; ↑IgG; ↑IgA | Jyonouchi et al., 1995 | |
| Peritoneal adherent cells of BALB/c mice | E.coli lipopolysaccharide (for Ab) | 2 × 10−7‐ 2 × 10−8M | ↑Thymocyte proliferation; ↑Ab production, ↑ TNF‐α and IL‐1α | Okai and Higashi‐Okai, 1996 | |
| Crocin | BV2 mouse microglial cells | LPS | 20 μM | ↓NO release in the cells stimulated with IFN‐γ and amyloid β (Aβ), ↓TNF‐α, NF‐ĸB, and IL‐1β | Nam et al., 2010 |
| Curcumin | Mice | E.G7/OT1 mouse lymphoma | 70 mg·kg−1·day−1 | ↑CD8 cytotoxicity , ↓ TGF‐β | Chang et al., 2012 |
| Mice | Mitogenic anti‐CD3/28 monoclonal antibodies (mAb) or antigenic stimulation by ovalbumin (OVA) | 1% of diet | ↓ NF‐ĸB activation; ↓ CD4 proliferation; ↓IL‐2 production (29.4%) in antigenic stimulation | Kim et al., 2009 | |
| Rat | Keyhole limpet haemocyanin (KLH) antigen | 40 mg·kg−1 | ↑IgG production | South et al., 1997 | |
| Curcumin + Cyclosporin‐A | Rat | PHA; ConA | 40 mg·kg−1·day−1 | ↑proliferation of lymph cells; No change in IFN‐γ and IL‐2 | Varalakshmi et al., 2008 |
| Curcumin | Human gastric epithelial cells (AGS) | H. pylori | 40 μM for IL‐8 80 μM for NF‐ĸB | ↓IKK activity, Suppression of IL‐8; no change in ERK1/2 and p38 | Foryst‐Ludwig et al., 2004 |
| Neutral unilamellar liposomes of curcumin | Mice | Sheep red blood cells (SRBC) | 200 μmol·Kg−1 | ↑Antibody titres; ↑phagocytic activity of macrophages; inhibition of delayed type hypersensitivity (DTH) reaction by about 39.75% | Antony et al., 1999 |
| Curcumin | Primary human CD4+ T cells | anti‐CD2/ CD3/CD28 antibody‐coated beads | 2 μg·mL−1 | ↓ T cell expansion; Down‐regulation of CD69 at early phase; up‐regulation of CCR7 and L‐selectin at late phase, ↓IL‐10, IL‐13, IL‐2, TNF‐α, and IFN‐γ | Kim et al., 2013 |
| RAW264.7 macrophages from mice | Lipopolysaccharide (LPS) | 5 μg·mL−1 | Down‐regulation of NF‐ĸB binding to the p40‐ĸB sequence; ↓kB binding activity; inhibition of IL‐12 secretion from macrophage | Kang et al., 1999a,b | |
| Splenic macrophages from mice | LPS; head‐killed Listeria monocytogenes (HKL) | 5 μg·mL−1 | Inhibition of IL‐12, ↑IL‐4; No change in IL‐10; ↓ IFN‐γ | Kang et al., 1999a,b | |
| Curcumin | DC from mice | LPS | 25 μM | Suppression of CD80,86, MHCII but not MHCI, ↓ TNF‐α, NF‐ĸB, IL‐6, and IL‐1β cytokines | Kim et al., 2005 |
| Human astrocyte cell line (U373‐MG) | LPS | 5 μM | ↓ IL‐6, ↓MMP‐9 enzyme activity, and MCP‐1 mRNA expression | Seyedzadeh et al., 2014 | |
| CD14+ monocytes, isolated from human peripheral blood | LPS; polycytidylic acid (polyI:C) | 30 μM | ↓DC‐induced CD4 proliferation; ↓dextran uptake by non‐stimulated cells; No significant increase in CD83,86; prevention of DC migration towards CCL19 and CCL21; ↓chemokines fractalkine (CX3CL1) and interferon producing factor (IP‐10), ↓ IL‐6 | Shirley et al., 2008 | |
| Human PBMCs; RAW253 | PHA (for IL‐2); IFN‐γ (for NKc); LPS | 0.01 and 0.05 μg·mL−1 | Inhibition of PHA‐induced T‐cell proliferation; ↑NK cell cytotoxicity; no significant decrease in TNF‐α, ↓IL‐2 and LPS‐induced NF‐ĸB; ↓NO product from macrophages | Yadav et al., 2005 | |
| Curcumin | RAW264.7 cells; Ba/F3 cells | LPS | 20 μM | ↓ NF‐ĸB, ↓Cox‐2 expression; inhibited dimerization of TLR4 in Ba/F3cells | Youn et al., 2006 |
| Curcumin and lutein | Chicks | LPS | 200 mg·kg−1 | Curcumin led to: ↑ beta cell proliferation (%5.6) and T cell proliferation (%30.4) as compared to lutein; | Rajput et al., 2013 |
| Lutein | Human (atherosclerosis patients) | ––––––––––– | 20 mg·day−1 | ↓monocyte chemoattractant protein‐1MCP‐1), ↓IL‐6 | Xu et al., 2013 |
| Silk lutein | Mice | LPS; ConA | 20 mg·kg−1 | ↑NKc activity; ↑Ab production; ↑CD3,CD4, lymph proliferation; ↑IFN‐γ and IL‐2 | Promphet et al., 2014 |
| FloraGloTM crystalline lutein | Cat | PHA,ConA,PWM | 10 mg·day−1 | ↑percentage of CD4,CD21 and IgG; no effect on CD8,MHCII; no change in IL‐2 | Kim et al., 2000a,b |
| FloraGloTM crystalline lutein | Dog | PHA,CnoA,PWM | 5‐20 mg | ↑CD4 (at 5 mg); ↑CD8,CD5,MHCII (at 20 mg), ↑IgG | Kim et al., 2000a,b |
| Lutein | Chickens | Salmonella LPS | 50 mg·kg−1 | ↓IL12, IL‐1β (in liver) ↑TLR‐4 mRNA (in spleen) | Moraes et al., 2016 |
| Lutein | Rat Muller cells (rMC‐1) | CoCl2 | 20 μM | ↓ COX‐2, No change in TNF‐α, ↓NF‐kB and IL‐1β | Li et al., 2012 |
| RAW264.7 and HaCaT | LPS, INF‐γ, TNF‐α (for COX‐2) | 30 μM | ↓COX‐2 mRNA; suppressed p38, JNK activation; ↓IL‐6 | Oh et al., 2013 | |
| SW‐1353 human | IL‐1β | 0.1 μmol·L−1 1 μmol·L−1 | ↑IL‐4; IL‐10, IL‐6 and TNF‐α not affected; ↑IFN‐γ (1 μmol L−1) and IL‐2 (0.1 μmol L−1); ↓NF‐kB (0.1 μmol L−1) | Di Filippo et al., 2012 | |
| Xanthophylls: lutein and zeaxanthin | Male finch | PHA | 7 μg·mL−1 | 21% difference in wing‐web‐swelling between carotenoid‐supplemented and control; Lutein and Zeaxanthin are different in cell‐mediated immune response by only 3% | McGraw and Ardia, 2004 |
| Meso‐zeaxanthin (MZ) | Balb/c mice | LPS | 25 μg·mL−1 | ↓iNOS and COX‐2 in macrophages; ↓TNF‐α, IL‐1β and IL‐6 | Firdous et al., 2015 |
| 40% of lutein and 60% of zeaxanthin | Hens/ chicks | LPS | 40 mg·kg−1 | ↓mRNA of IFN‐γ, IL‐6, IL‐1β; ↓LITAF; ↑IL‐10; IL‐4 not affected | Gao et al., 2012 |
| β‐carotene | Human | ––––––––––– | 60 mg·day−1 | ↑T cell CD4; ↑percentage of NK; ↑percentage of cells with markers for activation IL‐2 and transferrin | Watson et al., 1991 |
| Dog | ConA; PWM, PHA | 50 mg·day−1 | ↑IgG, not IgM; ↑CD4; CD8,CD21,MHC II not altered; No change in IL‐2 | Chew et al., 2000 | |
| Fish | A. hydrophila infection | 100 mg·kg−1 | ↑phagocytic activity | Anbazahan et al., 2014 | |
| Lutein, β‐carotene, astaxanthin | Spleen cells from mice (in vitro), In vivo | T‐dependent (TD) Antigen | 10–8 mol/1 | Lutein (↑Ab in vitro); all 3 carotenoids (↑Ab in vivo); astaxanthin (↑IgM more than others) | Jyonouchi et al., 1994 |
| β‐carotene | Cow | PHA, ConA, PWM | 300 mg for phagocyte; 600 mg for proliferation | ↑ Phagocyte effect of neutrophils; ↑lymph proliferation | Michal et al., 1994 |
| β‐carotene | Healthy women | PHA | 30 mg·day−1 | No effect on T lymphocyte proliferative response | Gossage et al., 2000 |
| Jejunum and ileum of mice after weaning | ––––––––––––––––– | 50 mg·kg−1 | ↑IgA concentrations in the jejunum; ↑IgA antibody‐secreting cells | Nishida et al., 2014 | |
| Aged humans | K562 (target for NKc) | 45 mg·day−1 | ↑34% NKc cytotoxicity; ↑31% total T cells | Wood et al., 2000 | |
| Mouse splenocytes; human peripheral blood lymphocytes | –––––––––––––––––– | 2.5, 5 μg·mL−1 | For human:↑ tumour cell lysis For murine Nkc: negative effect on NK cells; ↓lysis of YAC‐1 lymphoma cells | Ashfaq et al., 2000 | |
| RAW264 macrophage | LPS; INF‐γ | 10 μM | ↓IL‐12p40, IL‐6 and IL‐1β | Katsuura et al., 2009 | |
| β‐carotene | Peyer's patch (PP) cells were isolated from mice (ex vivo) | ConA | 5 mg·kg−1·day−1 | Weakly decreased the percentage of T cells; ↑IL‐2 | Yamaguchi et al., 2010 |
| Spleen and thymus from C57B/6 mice | PHA, ConA | 10–8 M | Ab and IL‐2 production didn't increase | Jyonouchi et al., 1991 | |
| PBMC | PWM (IgA); TNP‐KLH; tetanus toxoid (IgG) | 10–8 M | No increase in IgM and IgG; ↑IgA | Jyonouchi et al., 1995 | |
| Spleen,thymocytes from BALB/c mice | ConA; LPS (for Ab) | 2 × 10–8 to 2 × 10–7 M | ↑Thymocytes proliferation; ↑Ab production at 2 × 10‐7 M; ↑TNF‐α and IL‐1α | Okai and Higashi‐Okai, 1996 | |
| β‐carotene and Lycopene | Mice | –––––––––––––––––– | 300 mg·kg−1 | β‐carotene: ↑the percentages and total cell amounts of CD3+,CD4+,CD8+ Lycopene: ↑ the numbers of beta cells and T‐helper cells (CD4+ total cell numbers), ↑ IgG | Garcia et al., 2003 |
| PBMC from human | ConA | Tomato juice (330 mL·day−1): 40 mg lycopene and 1.5 mg β‐carotene | ↑IL‐2 and IL‐4 cytokines | Watzl et al., 1999 | |
| β‐cryptoxanthin | Rat | Myxomatosis vaccine | 5, 10 mg·kg−1 | ↑ CD4; No change in CD8; ↑IgM (all doses at 21 day); ↑IgG (10 mg at 14, 21 day; 5 mg at 21 day); ↑IL‐4 (5 mg at 21 day; 10 mg at 14, 21 day); No change in IFN‐γ | Ghodratizadeh et al., 2014 |
| RAW264 | LPS; IFN‐γ | 10 μM | mild suppression of IL‐12p40; ↓IL‐1β and IL‐6 | Katsuura et al., 2009 | |
| SW‐1353 human | IL‐1β | 1 μmol·L−1 | ↓IL‐10; IL‐4 not affected; inhibition of IL‐1α; ↓IFN‐γ, NF‐ĸB and IL‐2 | Di Filippo et al., 2012 | |
| Violaxanthin (isolated from C. ellipsoidea ) | Murine macrophage RAW 264.7 | LPS | 60 μM (below 100 μM) | ↓NO; ↓PGE2;↓INOS‐COX‐2 (mRNA); ↓binding to p65 DNA sequences (↓NF‐ĸB) | Soontornchaiboon et al., 2012 |
| Lycopene and lutein | Human | – | 500 mg full weight; (15 mg of carotenoid in corn oil) | Lycopene: ↑HLA‐DR, no change in other MHCII molecules. Lutein: no change | Hughes et al., 2000 |
| Carotenoid extract from Dunaliella salina algae (α‐carotene, β‐carotene, lutein and zeaxanthin) | Murine macrophage RAW264.7 | LPS | 5, 10, 25 μM | Inhibition of NO and PGE2; at 5 and 10 μM, the algae extract presented a significantly higher inhibitory activity for NO and IL‐1β than all‐trans‐β‐carotene; ↓IL‐1β , IL‐6, TNF‐α and NF‐kB | Yang et al., 2013 |
| Lycopene | Mice | Ovalbumin (OVA) | 4 mg 200·μL−1 of water | ↓ eosinophils; ↓IL‐4, IL‐5; IL‐13, and IFN‐γ not altered | Hazlewood et al., 2011 |
| Lycopene | Mice | The left anterior descending coronary artery (LAD) for post‐myocardial infarction (MI) model in mice | 10 mg·kg−1·day−1 | ↓TGF‐β1; ↓ caspase 3,8,9 (all in mRNA); ↓TNF‐α, NF‐kB and IL‐1β | He et al., 2015 |
| HUVECs | LPS | 20 μM | ↓CD14,TLR4, TNF‐α and NF‐ĸB | Bae and Bae, 2011 | |
| PBMC | K562 cell | 5 μM | ↑ NKc cytotoxicity and IFN‐γ | Li et al., 2014 | |
| PBMC | LPS; PHA | 4 μM | ↓IL‐10, IL‐2 and IFN‐γ; IL‐6, IL‐1ra not affected; ↑TNF‐α and IL‐1β | Bessler et al., 2008 | |
| Adipose tissue from mice; 3 T3‐L1 cells; human preadipocytes | TNF‐α | 2 μM | ↓MCP1in adipose tissue from mice and 3 T3‐L1; ↓IL‐1β, IL‐6 and NF‐ĸB (↓ phosphorylated IKKα/β in 3 T3‐L1) | Gouranton et al., 2011 | |
| Human THP‐1 macrophage | Cigarette smoke extract (CSE) | 2 μM | ↓NF‐ĸB; ↓IL‐8; ↓ROS production; ↓NOX‐4 expression | Simone et al., 2011 | |
| Lycopene | Pancreatic acinar cells from rat | Cerulein, a cholecystokinin (CCK) analogue | 5 μmol·l−1 | ↓ROS; IL‐6, NF‐kB activation | Kang et al., 2011 |
| RAW 264.7 macrophage | LPS | 0.5–2 μM | ↓JNK phosphorylation; no effect on p38 and ERK1/2 phosphorylation; ↓ TNF‐α (at 1 μM), IL‐1β (at 2 μM), NF‐kB (at 2 μM), and IL‐6 (at 0.5 μM) | Marcotorchino et al., 2012 | |
| PBMCs | Concanavalin A, anti‐CD3 (for T‐cell activation) | 1.18–2.93 μg·mL−1 | ↓T lymph proliferation; ↓CD69 in T‐cell subsets (at 1.18 μg·mL−1); ↓IL‐2 | Mills et al., 2012 | |
| RAW264.7 | Gliadin in association with IFN‐γ | 20 μM | ↓iNOS and NF‐kB; ↓signal transducer and activator of transcription‐1α (STAT‐1α); ↓COX‐2; ↓interferon regulatory factor‐1(IRF‐1) | De Stefano et al., 2007 | |
| PBMC | LPS; PMA (for INF‐γ, IL‐2) | 0.25 μM | ↓IL‐10; ↓IL‐1ra; no change in IL‐1β; ↓IL‐2, TNF‐α and IFN‐γ | Bergman et al., 2010 | |
| RAW 264.7 | LPS | 10 μM | ↓NO; ↓INOS (at both protein and mRNA levels); COX‐2 not affected | Rafi et al., 2007 |
Astaxanthin (ASTA)
ASTA (3, 3‐dihydroxy‐β, β‐carotene‐4, 4‐dione) significantly improve the phagocytic and microbicidal capacity of neutrophils and enhances the intracellular calcium concentration (Ca+2) and NO production. These activities were associated with a decrease in the levels of superoxide anion, hydrogen peroxide (H2O2), IL‐6 and TNF‐α cytokines. Indeed, oxidative damage in proteins and lipids is significantly reduced after ASTA treatment. Also, treatment with 5 μM ASTA has been shown to increase both the phagocytic (~30%) and fungicide (~28%) capacities of neutrophils (Macedo et al., 2010). In another study, ASTA treatment was found to decrease the secretion of pro‐inflammatory cytokines in stimulated U937 cells. These data indicate that ASTA suppresses the H2O2 mediated activation of NF‐κB and the secretion of cytokines by modulating the expression of SHP‐1. SHP‐1 is a PTP acting as a negative regulator of the cytokine signalling pathway (Speranza et al., 2012). The researchers also showed that all three stereoisomers of ASTA, (3S, 3'S)‐trans‐ASTA, (3R, 3'R)‐trans‐ASTA and meso‐trans‐ASTA, at a concentration of 20 μmol·L−1 significantly increased lymphocyte proliferation, the phagocytic capacity of peritoneal exudates cells and cytotoxic activity of natural killer (NK) cells. Moreover, the 3S, 3'S enantiomer demonstrated higher immunoregulatory activity than the two other enantiomers in vitro (Weihong et al., 2016). ASTA, a carotenoid without vitamin A activity, enhanced the production of human immunoglobulin (IgM, IgA and IgG) by peripheral blood mononuclear cells (PBMCs) in response to T‐cell‐dependent stimuli (polyclonal stimulants) (Jyonouchi et al., 1995). Dietary ASTA has been found to reduce a biomarker of DNA damage and acute phase protein (plasma C‐reactive protein) and enhance immune responses (e.g. NK cell cytotoxic activity and total T and beta cell subpopulations) in young healthy women (Park et al., 2010). In addition, dietary ASTA enhanced the levels of IgG, IgM and beta cell population as well as reducing plasma concentrations of C reactive protein and DNA damage in female Beagle dogs (Chew et al., 2011). ASTA was shown to have similar effects in cats, acting as a potent antioxidant and modulating the immune response (Park et al., 2011). ASTA supplementation also improved the immunological dysfunction, suppression of NK cell activity, induced in mice by stress (Kurihara et al., 2002). Furthermore, this carotenoid can increase the salivary IgA response and attenuate muscle damage in young soccer players, thus preventing inflammation induced by severe physical training or conditions of increased oxidative stress (Baralic et al., 2015).
Crocin and crocetin
Crocin and crocetin carotenoids found in saffron have a variety of pharmacological effects. Crocin derived from stigma of Crocus sativus has been confirmed as a powerful antioxidant, stronger than α‐tocopherol (Bathaie et al., 2014; Bolhassani et al., 2014; Bolhassani, 2015). Indeed, the neuroprotective effects of crocin and crocetin are mainly associated with their antioxidant properties (Ochiai et al., 2007). Crocin increases intracellular glutathione levels and consequently prevents cell death in hypoxic PC12 cells, a cell culture model for brain ischaemia (Ochiai et al., 2007). Crocetin also inhibits mRNA expression of TNF‐α, IL‐1β and iNOS in the liver and increased overall survival in a haemorrhagic shock model (Yang et al., 2006). Crocin and crocetin effectively inhibit LPS‐induced NO release from cultured BV2 mouse brain microglial cells as well as reducing NF‐κB activation, the levels of TNF‐α and IL‐1β cytokines and intracellular ROS. Crocin and crocetin have been shown to mediate neuroprotection by decreasing the production of different neurotoxic molecules from activated microglia induced by IFN‐γ and Aβ (Nam et al., 2010).
Curcumin
Curcumin is a safe and potent immunomodulator of the immune system. Curcumin can induce apoptosis specifically in tumour cells but not in primary cultures or non‐transformed cells under similar conditions (e.g. incubation with 25 μM curcumin for 24 h). Curcumin also modulates adaptive immunity by enhancing the proliferation of T‐cells (Varalakshmi et al., 2008). These observations were confirmed by a significant increase in macrophage phagocytic activity and the immuno‐stimulatory activity in curcumin‐treated Balb/c animals (Antony et al., 1999). Curcumin (diferuloylmethane) can inhibit the cellular mitogenic response of epithelial cells induced by H. pylori infection. Curcumin prevented IκBα degradation, the activity of IkB kinases a and b (IKKa and b) and NF‐κB DNA‐binding in a culture of the AGS infected by H. pylori. Indeed, a low dose of curcumin (~40 μM) was found to block H. pylori‐induced NF‐κB activation and IL‐8 synthesis (Foryst‐Ludwig et al., 2004). In another study, curcumin directly decreased the T‐cell‐dependent inflammatory stress in vitro by modulating the activation of CD4+ T‐cells at various levels, such as (a) inhibition of CD2/CD3/CD28‐initiated CD4+ T cell proliferation; (b) enhancement of CD69, CCR7, L‐selectin and TGFβ1 expression; and (c) suppression of IL‐12 production in a dose‐dependent manner (Kim et al., 2013). In fact, curcumin‐induced inhibition of IL‐12 production in mouse macrophages stimulated with LPS could mediate several of the biological effects of curcumin, for example, its anti‐inflammatory effects in chronic inflammatory diseases. Curcumin may directly modulate the NF‐κB‐DNA interaction by forming a complex with NF‐κB that is unable to bind kB sites (Kang et al., 1999a,b). A similar report also confirmed that pretreatment with curcumin significantly inhibits IL‐12 production by mouse macrophages stimulated with either LPS or head‐killed Listeria monocytogenes; this could have potential as a therapeutic effect of curcumin on Th1‐mediated immune diseases. Curcumin probably inhibits IL‐12 production in macrophages by down‐regulating the activity of NF‐κB on the IL‐12 p40 gene, known as the highly inducible component of IL‐12 (Kang et al., 1999a,b). The production of Th1 cytokines such as IL‐2 and IFN‐γ was suppressed in macrophages or splenic T lymphocytes pretreated with curcumin (Gao et al., 2004). Dietary curcumin and limonin have been found to suppress CD4+ T‐cell proliferation and IL‐2 production, and NF‐κB p65 nuclear translocation in activated CD4+ T‐cells in DO11.10 transgenic mice (Kim et al., 2009). Furthermore, dendritic cells (DCs) treated with curcumin are highly effective at antigen (Ag) capture through a mannose receptor‐mediated endocytosis, and this effect of curcumin is dose‐dependent manner. Curcumin inhibits LPS‐induced MAPK activation and the translocation of NF‐κBp65. Moreover, it significantly inhibited CD80, CD86 and major histocompatibility complex (MHC) class II expression, but not MHC class I expression in DCs. Curcumin suppressed the maturation of bone marrow‐derived murine DC at concentration of 25 μM (Kim et al., 2005) and other studies have shown that curcumin prevents DC migration and chemokine secretion. Curcumin suppressed migration towards CCL19 and CCL21 in a chemotaxis assay and also reduced the levels of chemokines fractalkine (CX3CL1) and interferon producing factor (IP‐10). The function of both fractalkine and IP‐10 is to attract inflammatory cells to sites of inflammation (Shirley et al., 2008). Activated astrocytes have been shown to play a dual functional role in CNS inflammatory disorders such as multiple sclerosis. Using a LPS‐induced inflammatory model in vitro, curcumin decreased the function of an astrocyte cell line (U373‐MG) by inhibiting the release of IL‐6 and also the activity of the enzyme MMP‐9. Indeed, this anti‐inflammatory of curcumin on neuroinflammation resulted in CNS repair (Seyedzadeh et al., 2014). Curcumin also inhibited the dimerization of toll‐like receptor (TLR) 4, the degradation of IRAK‐1 and NF‐κB activation induced by LPS in a dose‐dependent manner (~20 μM). Curcumin inhibited both MyD88‐ and TRIF‐dependent pathways in LPS‐induced TLR4 signalling (Youn et al., 2006). The immunomodulatory properties of curcumin were evaluated in several studies. Generally, curcumin inhibited phytohaemagglutinin‐induced T‐cell proliferation, IL‐2 production, NO generation, LPS‐induced NF‐κB and augmented NK‐cell cytotoxicity. These data suggest curcumin could have a therapeutic effect in Th1‐mediated autoimmune disorders, as it has been shown to have potent immunosuppressive properties in vitro. Curcumin is an effective scavenger of ROS such as hydroxyl radicals and superoxide anions (Yadav et al., 2005), and it can affect both endoplasmic reticulum (ER) stress and mitochondria functional pathways. Indeed, curcumin‐stimulated apoptosis in activated T‐cells was shown to be due to intense ER stress (Zheng et al., 2013). Curcumin may improve the therapeutic efficiency of adoptive T‐cell therapy (i.e. the ex vivo expansion and subsequent transfusion of tumour‐specific T lymphocytes) to eradicate tumours. Curcumin, when combined with adoptive therapy in male tumour‐bearing C57BL/6 mice, was shown to enhance the cytotoxicity of Ag‐specific CD8+ T‐cells against the tumours by modifying the tumour micro‐environment during treatment. T‐cell activity was enhanced by combined treatment due to the blockade of various immunosuppressors (e.g. regulatory T‐cells, indoleamine 2, 3‐dioxygenase, TGF‐β) (Chang et al., 2012). Numerous studies have demonstrated that curcumin modulates the proliferation and activation of T‐cells in a dose‐dependent manner. Indeed, low‐dose curcumin augments the proliferation of splenic lymphocytes, whereas high‐dose curcumin reduces this effect in mouse model. Curcumin can successfully restore populations of CD4+ and CD8+ cells in the tumour micro‐environment and inhibit the depletion of central and/or effector memory T‐cells. Curcumin significantly decreases the levels of IL10 and TGF‐β and Treg cell population; pretreatment of CD4+CD25+ Treg cells with curcumin diminished their immunosuppressive activity (Bose et al., 2015). Tumour‐derived exosomal proteins are known to suppress IL‐2‐induced NK‐cell activity in breast carcinoma and Zhang et al. showed that curcumin increased the proteasomal degradation of these proteins, partially restoring the NK‐cell activity against the tumour. Therefore, curcumin is able to target the immune escape strategies that are crucial for the immune responses (Zhang et al., 2007). Furthermore, oral administration of curcumin (50 mg·kg−1) suppressed the mast cell‐dependent IgE response in Ag‐induced local passive cuataneous anaphylaxis (Srivastava et al., 2011).
Lutein (Lu) and β‐cryptoxanthin (βCr)
Lutein (~30 μM) significantly decreases several skin inflammatory responses, such as the increased expression of IL‐6 from LPS‐treated macrophages, up‐regulation of COX‐2 from IFN‐𝛾/TNF‐𝛼‐treated aneuploid immortal keratinocyte cells (HaCaT cells) and the enhancement of MMP‐9 levels (a marker of acute inflammation) in UV‐irradiated keratinocytes (Oh et al., 2013). Lutein acts as a strong antioxidant; it reduces oxidative stress induced by benzo(a)pyrene (Vijayapadma et al., 2014), a hypercholestrolaemic diet (Kim et al., 2012), H2O2 (Gao et al., 2011) and D‐galactose (Mai et al., 2010). Lutein supplements (~20 mg·day−1) resulted in a significant decrease in serum IL‐6 and monocyte chemoattractant protein‐1 (MCP‐1), triglyceride (TG) and LDL in early atherosclerosis for 3 months (Xu et al., 2013). Cocoons from yellow silkworms have been found to be an abundant source of dietary lutein and this silk lutein extract can increase both innate and adaptive immune functions. The silk extract enhanced IL‐2 and IFN‐γ production, the populations of CD3+ and CD4+ CD3+ cells, antibody production and NK‐cell activity in female BALB/c (Promphet et al., 2014). Domestic cats fed 10 mg lutein on weeks 8 and 12 showed a high plasma IgG level and increased percentages of CD4 and CD21 lymphocytes, supporting the immunomodulatory action of lutein (Kim et al., 2000a,b). Dietary lutein also induced both cell‐mediated and humoral immune responses (IgG) and enhanced the percentages of cells expressing CD5, CD4, CD8 and MHC II molecules in domestic canines (Kim et al., 2000a,b). Lutein‐supplemented birds showed superior efficiency of pigmentation as compared with curcumin‐supplemented birds; however, both groups of birds had reduced lipid oxidation in the liver (Rajput et al., 2013). Lutein protected the retina from ischaemic/hypoxic damage by its anti‐oxidative, anti‐inflammatory and anti‐apoptotic activities (Li et al., 2012). Dietary lutein decreased mammary tumour growth and the expression of the anti‐apoptotic Bcl‐2 gene as well as increasing the mRNA expression of the pro‐apoptotic genes (e.g. p53 and Bax) and the Bax: Bcl‐2 ratio in tumours (Chew and Park, 2004). Lutein and β‐cryptoxanthin may down‐regulate factors involved in the inflammation associated with rheumatoid arthritis and osteoarthritis. βCr suppressed the generation of IFN‐γ, IL‐1α and IL‐2 cytokines while Lu increased their levels. Lu increased the levels of IL‐4 and IL‐10 while βCr decreased their concentrations. NF‐κB p50 production was suppressed by both Lu and βCr (Di Filippo et al., 2012). Some of the immunity‐related properties of β‐cryptoxanthin and lutein were studied on the murine macrophage cell line (RAW264). β‐cryptoxanthin (~10 μM) significantly decreased IL‐1β mRNA levels, but it did not reduce IL‐6 and IL‐12 p40 mRNA levels. Lutein did not significantly inhibit the transcription of these three cytokines (Katsuura et al., 2009). β‐cryptoxanthin has been shown to increase the blood CD4+ lymphocytes count and serum IgG, IgM and IgA levels (i.e. humoral immunity) in mammals (Ghodratizadeh et al., 2014). Nishi et al. reported βCr administration causes an increase in IL‐6 levels but does not affect IL‐4 and IFN‐γ levels, indicating the significant enhancement of immunoglobulin levels and CD4+ cells count (Nishi et al., 2012). Oxygenated carotenoids like βCr can be esterified with various fatty acid chains and the esterified form of this xanthophyll has a higher bioavailability than its free form (Fu et al., 2010). The esterified βCr with a fatty acid chain could be useful to further the immune stimulatory effect of βCr (Ghodratizadeh et al., 2014).
Lycopene
Lycopene suppresses LPS‐induced pro‐inflammatory responses by different methods: (a) enhancement of vascular barrier integrity, (b) inhibition of barrier permeability and expression of cell adhesion molecules (CAM) and (c) prevention of leukocyte adhesion and transendothelial migration. Indeed, the anti‐inflammatory properties of lycopene are mediated by the down‐regulation of NF‐κB expression and TNF‐α production (Bae and Bae, 2011). The expression of TNF‐α‐induced intercellular adhesion molecule‐1 (ICAM‐1) in HUVEC was inhibited by lycopene, whereas the expression of COX‐2 and platelet‐endothelial cell (EC) adhesion molecule was not influenced in HUVECs. Lycopene attenuated the TNF‐α‐induced IκB phosphorylation, NF‐κB expression and NF‐κB p65 translocation from cytosol to nucleus, suggesting its anti‐inflammatory effect could be used for the prevention of CVDs. Moreover, IFN‐γ‐induced ICAM‐1 expression was not affected by lycopene, indicating that lycopene firstly influences the TNF‐α‐induced signalling pathway (Hung et al., 2008). Pretreatment of human THP‐1 macrophages with lycopene resulted in a significant inhibition of cigarette smoke extract‐induced IL‐8 expression at both the RNA and protein levels; the molecular mechanism involved in this effect is mediated through NF‐κB inactivation. NF‐κB inactivation has been associated with an inhibition of redox signalling and activation of PPAR signalling (Simone et al., 2011). Lycopene has a positive effect on NK‐ cell viability and cytotoxicity at concentration of 5 μM. Its anti‐apoptosis effect on NK‐cells is associated with a down‐regulation of the expression of caspase 3 and 9 genes. In addition, lycopene did not affect the expression of functional receptors on NK cells including NKG2A, NKG2D, NKp30 and NKp44. Treatment with lycopene increased the expression of IFN‐γ at both the gene and protein levels after 7 days (Li et al., 2014). Lycopene has been shown to enhance the production of IL‐1β and TNF‐α in a dose‐dependent manner and decrease IL‐2, IL‐10 and IFN‐γ secretion in human PBMCs, whereas the levels of IL‐6 and the IL‐1 receptor antagonist were not affected. The increased generation of the pro‐inflammatory cytokines (IL‐1β and TNF‐α), as well as the decreased secretion of the anti‐inflammatory cytokine (IL‐10), indicate that lycopene can enhance inflammatory responses (Bessler et al., 2008). However, lycopene has been shown to reduce the expression of pro‐inflammatory cytokines and chemokines (e.g. IL‐6, IL‐1β and MCP‐1) at both the mRNA and protein levels in a variety of adipose tissues and adipocyte models (Gouranton et al., 2011). A high concentration of lycopene (10 μmol·L−1) showed a strong anti‐angiogenic effect that may be associated with the up‐regulation of IL‐12 (~163%) and IFN‐γ (~531%) as observed in HUVEC (Huang et al., 2013). Lycopene may be useful for the prevention or treatment of acute pancreatitis by suppressing the activation of NF‐κB and the expression of inflammatory cytokines (IL‐6), mediated by a decrease in the intracellular levels of ROS in pancreatic acinar cells (Kang et al., 2011). The anti‐inflammatory effects of lycopene on RAW 264.7 macrophages were associated with a decrease in LPS‐stimulated migration (Marcotorchino et al., 2012). The studies showed that pre‐incubation of mouse peritoneal macrophages with lycopene (~1 μM), lutein (~1 μM) and β‐carotene (~2 μM) before the addition of LPS caused a synergistic inhibition of NO, PGE2 and superoxide production, derived from the down‐regulation of iNOS, COX‐2 and NADPH oxidase at both the mRNA and protein expression levels and synergistic inhibition of TNF‐α secretion (Hadad and Levy, 2012). Indeed, lycopene treatment (~10 μM) decreased LPS‐induced iNOS protein and NO production (~40%) in RAW 264.7 in a dose‐dependent fashion (Rafi et al., 2007). However, supplementation with lycopene has also been shown to reduce allergic inflammation systemically, especially in the lungs, by diminishing the Th2 cytokine responses. Lycopene prevented the infiltration of inflammatory leukocytes (e.g. neutrophils, eosinophils, lymphocytes and macrophages) into bronchoalveolar lavage fluid and this was associated with the suppression of the Th2 transcription factor GATA‐3, the cytokine IL‐4 and the activity of eosinophil peroxidase and MMP‐9 (Lee et al., 2008; Hazlewood et al., 2011). Whereas lycopene has been shown to attenuate inflammation and apoptosis (e.g. reduction of caspase‐3, ‐8 and ‐9 expression) in post‐myocardial infarction remodeling, in a mouse model, by inhibiting the NF‐κB signalling pathway (e.g. NF‐κB p65 phosphorylation) (He et al., 2015). Lycopene has been found to inhibit LPS‐mediated release of high mobility group1 (HMGB1) and HMGB1‐mediated pro‐inflammatory signalling responses in both primary HUVEC and animals through down‐regulation of cell surface expression of CAMs, as well as HMGB1 receptors, TLR‐2 and ‐4, and receptors for advanced glycation end products, and to initiate pro‐inflammatory responses in ECs (Lee et al., 2012). Xanthophylls (containing 60% of zeaxanthin and 40% of lutein) have been shown to regulate anti‐inflammatory and pro‐inflammatory cytokine expression in various tissues of both chicks and hens. Dietary xanthophyll reduces the expression of pro‐inflammatory cytokines (e.g. IFN‐γ, IL‐6, IL‐1β and LITAF) in the liver, duodenum and jejunum of hens and increases anti‐inflammatory cytokine expression (e.g. IL‐4 and IL‐10) in the liver, jejunum and ileum (Gao et al., 2012). Furthermore, lycopene decreases peroxynitrite or oxidative stress‐induced DNA damage in Chinese hamster lung fibroblasts (Muzandu et al., 2006; Kim, 2011).
Violaxanthin
Violaxanthin of Chlorella ellipsoidea, a non‐synthetic natural product, has been proposed to be a safe and efficient anti‐inflammatory agent and can also function as an adjuvant. The anti‐inflammatory activity of violaxanthin may be based on inhibition of LPS‐mediated NF‐κB p65 subunit translocation into the nucleus and subsequently inactivation of the NF‐κB pathways. Violaxanthin (~60 μM) effectively inhibited the production NO in LPS‐treated RAW 264.7 macrophages, as well as the expression of iNOS and COX‐2, in a concentration‐dependent fashion (Soontornchaiboon et al., 2012).
β‐carotene
Treatment of human NK‐cells with β‐carotene at doses ranging from 2 to 200 ng·mL−1 induces a decrease in the tumorolytic effect in vitro; but treatment with β‐carotene at doses ranging from 0.1 to 10 μg·mL−1 induced a significant increase in tumorolytic function. However, other experiments have shown that α‐tocopherol increases the tumorolytic effect of mouse splenocytes, while β‐carotene suppresses this effect. Indeed, β‐carotene inhibits the positive effects of α‐tocopherol in mice, but the mechanism of this effect is unclear (Ashfaq et al., 2000). The accumulation of β‐carotene in murine macrophage cells (RAW264) was effectively related to cellular lipid peroxidation in a dose‐ and time‐dependent manner, indicating the pro‐oxidative activity of β‐carotene, and the synthesis of glutathione as an intracellular antioxidant. In addition, supplementation with β‐carotene suppressed the transcription of IL‐1β, IL‐6 and IL‐12 p40 cytokines (Katsuura et al., 2009). The number of lymphoid cells with surface markers for NK‐cells, IL‐2 and transferrin receptors (/or the number of Th and T‐inducer lymphocytes) has been shown to be increased in PBMCs from individuals supplemented with oral β‐carotene (Watson et al., 1991). Also Seifter et al. reported that β‐carotene stimulates the growth of the thymus gland and increases the number of thymic small lymphocytes (Seifter et al., 1981). Oral administration of three carotenoids, β‐carotene, β‐cryptoxanthin and lycopene resulted in modulated Th cytokine production in cultured Peyer's patch (PP) cells stimulated with the mitogen concanavalin A. In fact, oral administration of β‐carotene at 1 or 5 mg·kg−1·day−1 significantly increased IL‐2 production in PP cells after 72 h, while the level of IL‐4 was not changed. Also, oral administration of β‐carotene with capsaicin (both at 5 mg·kg−1) considerably improved the levels of IFN‐γ and IL‐5, three times higher than those induced by capsaicin alone (Yamaguchi et al., 2010). The immunomodulating activities of β‐carotene and its associated carotenoids, such as canthaxanthin and ASTA, were studied on the proliferation and functions of murine immunocompetent cells in vitro cell cultures. The results showed that canthaxanthin, β‐carotene and ASTA induce potent, but different, stimulatory effects on the proliferation of thymocytes and spleen cells from BALB/c mice. All three carotenoids significantly increased the release of IL‐1 and TNF‐α from murine peritoneal adherent cells at certain concentrations. However, the cytokine‐inducing activities were higher for ASTA than those for canthaxanthin and β‐carotene respectively (Okai and Higashi‐Okai, 1996). For the first time, Yang et al. showed that the carotenoid extract of Dunaliella salina composed of lutein, zeaxanthin α‐carotene and β‐carotene inhibits the production of NO, PGE2 and pro‐inflammatory cytokines (TNF‐α, IL‐1β and IL‐6) as well as the expression of iNOS and COX‐2 in LPS‐activated RAW264.7 cells. Indeed, the extract exhibited anti‐inflammatory activities through the inhibition of NF‐κB activation and JNK phosphorylation (Yang et al., 2013). Meso‐Zeaxanthin is also known as a xanthophyll carotenoid with profound antioxidant activity and has an anti‐inflammatory effect in the BALB/c mice model similar to the zeaxanthin supplement (Firdous et al., 2015). Other studies have indicated that lutein and β‐carotene enhance in vivo antibody production against T‐dependent Ags, not T‐independent Ags in old B6 mice, although at a lower level than that induced by ASTA. The in vivo or in vitro generation of antibody against TD Ags was notably lower in old than in young mice (Jyonouchi et al., 1994). Furthermore, supplementation with β‐carotene effectively increased the level of mucosal IgA (both the number and concentration of IgA antibody‐secreting cells) in the jejunum and ileum of weanling mice. These effects were mainly due to the mRNA expression of retinoid X receptorα, retinoic acid receptors [(RAR)α and (RAR)γ], and retinoic acid (RA)‐mediated immune response (Nishida et al., 2014). Selenium enhances the immune function (NK‐cell cytotoxicity) and phenotypic expression of T‐cell subsets, whereas β‐carotene affects only the immune function. Hence, supplemental selenium and β‐carotene could affect the immune function in aged subjects by enhancing the total percentage of T‐cells and increasing NK‐cell activity (Wood et al., 2000). Nevertheless, β‐carotene could be considered as a potential anti‐inflammatory agent for DNA‐virus infections and, especially, for the human herpes simplex virus, due to its ability to inhibit cytokine expression in Suid herpesvirus‐induced inflammation via NF‐κB inactivation (Lin et al., 2012; Guedes et al., 2014).
Epidemiological studies on carotenoids intake and randomized clinical trials
As carotenoids have been shown to act as antioxidants, the effects of their intake on the risk of developing certain cancer types (e.g. HNC, PCa and colon cancer) and other diseases (e.g. Parkinson's disease) have been extensively studied. Table 3 shows the relationship between intake of carotenoids and prevention of diseases.
Table 3.
The relationship between carotenoids intake and prevention of diseases
| Carotenoid | Disease | Clinical effect | Reference |
|---|---|---|---|
| Dietary carotenoids (combined) | Colorectal cancer | No significant effect | Panic et al., 2016 |
| β‐carotene | Prostate cancer | No significant effect | Wang et al., 2015 |
| α‐carotene, lycopene | Prostate cancer | Decrease in the risk of prostate cancer, but not at an advanced stage | Wang et al., 2015 |
| Tomatoes rich in carotenoids | Prostate cancer | Modest effect in the prevention of prostate cancer | Chen et al., 2013a,b |
| Lycopene | Benign prostatic hyperplasia (BPH) or prostate cancer | No significant effect | Ilic and Missob, 2012 |
| Lycopene | Cardiovascular disease | Improvement of endothelial function | Gajendragadkar et al., 2014 |
| Higher doses of lycopene | Prostate cancer | Reduced risk of prostate cancer | Chen et al., 2015 |
| β‐carotene | Oral cavity cancer and laryngeal cancer | 46 and 57% reduction in the risk of cancers respectively | Leoncini et al., 2015 |
| Lycopene and β‐cryptoxanthin | Laryngeal cancer | Reduction in the risk of cancer | Leoncini et al., 2015 |
| Combination of lycopene, α‐carotene and β‐cryptoxanthin | Oral and pharyngeal cancers | 26% reduction in the rate of cancers | Leoncini et al., 2015 |
| Micronutrients (α‐carotene, β‐carotene, β‐cryptoxanthin, lutein, lycopene, zeaxanthin and canthaxanthin) | Parkinson's disease | No significant effect | Takeda et al., 2014 |
| Vitamin A or β‐carotene supplementation | Maternal or infant mortality in pregnancy | No significant effect on birthweight indicators, preterm birth, stillbirth, miscarriage or fetal loss | Thorne‐Lyman and Fawzi, 2012 |
| Vitamin A or β‐carotene supplementation | Pregnancy in HIV‐positive women | Protective against low birthweight, no significant effects on preterm delivery or small‐for‐gestational age. Possible harmful effects through increased HIV transmission | Thorne‐Lyman and Fawzi, 2012 |
| β‐carotene | Non‐melanoma skin cancer | No significant effect | Greenberg et al., 1990 |
| β‐carotene | Breast cancer | Significant decrease in breast cancer risk | Aune et al., 2012 |
| β‐carotene and vitamin A | Cardiovascular disease | No effect and can even stimulate disease | Omenn et al., 1996 |
| Curcumin | Major depressive disorders | Anti‐depressant effect | Al‐Karawi et al., 2016 |
| Curcumin | Skin diseases | Therapeutic benefits for skin health | Vaughn et al., 2016 |
| Curcumin | Cardiovascular complications | Prevention of ventricular arrhythmias | Wongcharoen and Phrommintikul, 2009 |
| Astaxanthin | Cardiac diseases | Therapeutic role in the management of myocardial injury | Ciccone et al., 2013 |
Carotenoids and cancer
As is well‐known, carotenoid concentrations in blood are biomarkers of fruit and vegetable intake. However, among the six dietary carotenoids, only the intake of β‐carotene has been significantly related to a reduced risk of breast cancer (Aune et al., 2012). In contrast, Panic et al. showed that there is no significant correlation between the intake of specific carotenoids from dietary sources or combined carotenoids and the risk of colorectal cancer (Panic et al., 2016).In contrast, long‐term use of individual β‐carotene, retinol and lutein supplements did not show significant preventive effect for lung cancer, especially among smokers (Satia et al., 2009). Also in people with a previous non‐melanoma skin cancer, treatment with β‐carotene did not reduce the occurrence of novel skin cancers over a 5 year period of treatment with β‐carotene (Greenberg et al., 1990). Furthermore, Wang et al indicated that dietary β‐carotene intake and also its blood levels are not associated with reduced risk of PCa. In contrast, both dietary intake and blood levels of α‐carotene and lycopene could decrease the risk of PCa, but not the risk of advanced PCa. In general, α‐carotene and lycopene, but not β‐carotene, were inversely associated with the risk of PCa (Wang et al., 2015). Moreover, it was suggested that tomatoes can play a modest role in suppressing PCa (Chen et al., 2012). Meta‐analysis of four studies in men randomized to receive lycopene did not show any significant decrease in the incidence of benign prostatic hyperplasia (BPH) or PCa. However, two other studies indicated a decrease in prostate‐specific antigen levels in men diagnosed with PCa, who had received lycopene. Thus, it is not possible to support the use of lycopene for the prevention or treatment of BPH or PCa, due to the limited number of randomized controlled trials and various qualities of these studies (Ilic and Missob, 2012). Some studies showed that there is a reduced incidence of PCa with higher lycopene intake. Chen et al. demonstrated that higher lycopene consumption was linearly accompanied by a reduced risk of PCa with a threshold between 9 and 21 mg·day−1. In addition, higher levels of circulating lycopene (between 22 and 850 mg·L−1) significantly decreased the risk of PCa (Chen et al., 2015). Several other findings have supported the hypothesis that lycopene not only enhances the antioxidant responses in prostate cells but is also able to inhibit proliferation, induce apoptosis and decrease the metastatic capacity of PCa cells. However, there is no clear clinical evidence suggesting the use of lycopene in the prevention and/or treatment of PCa. Thus, further studies are required to determine its mechanisms of action in reducing the risk of PCa (Holzapfel et al., 2013). Recently, epidemiological studies were performed to investigate the correlation of the intake of specific carotenoids from dietary sources, with the risk of HNC. The risk reduction of oral cavity cancer and laryngeal cancer associated with β‐carotene intake was 46 and 57% respectively. Lycopene and β‐cryptoxanthin also decreased the risk of laryngeal cancer. The combination of lycopene, α‐carotene and β‐cryptoxanthin was associated with at least a 26% reduction in the rate of oral and pharyngeal cancer. These studies showed that dietary carotenoids especially as single nutrients have a protective role against HNC (Leoncini et al., 2015).
Carotenoids and cardiovascular disease
Several clinical trials have shown that carotenoids can reduce the risk of developing. The carotenoids could potentially decrease the risk of CVDs via several mechanisms such as: (a) lowering blood pressure, (b) reducing pro‐inflammatory cytokines, (c) decreasing markers of inflammation (e.g. C‐reactive protein) and (d) improving insulin sensitivity in liver, muscle and adipose tissues. Furthermore, the expression of specific genes involved in cell metabolism could be modulated by carotenoids (Gammone et al., 2015). There are several major reports about the clinical roles of carotenoids. For example, β‐carotene has been shown to have health‐enhancing properties including reducing the risk of developing certain diseases. However, it may stimulate disease through the activity of its derivatives that form in the presence of external agents, for example cigarette smoke or ROS generation in the body. An average of 4 years of carotenoids' supplementation showed that the combination of β‐carotene and vitamin A had no effect on the risk of death from lung cancer or CVD, and may even have an adverse effect on their incidence (Omenn et al., 1996). The dose‐dependent cardioprotective effects and also harmful properties of β‐carotene were studied in a rat model. The content of myocardial haem oxygenase 1 (HO1) was significantly elevated at a high dose of β‐carotene (150 mg·kg−1·day−1). It seems that Fe2 + generated as a metabolite of HO1 activity may determine the functions of β‐carotene as an antioxidant or pro‐oxidant agent. The high level of this enzyme in response to ischaemia/reperfusion‐induced oxidative stress may be a reason for the loss of cardioprotection at the high‐dose of β‐carotene (Csepanyi et al., 2015). In another study it was found that there was a significant correlation between the consumption of foods rich in β‐carotene and a reduced risk of coronary heart disease as compared with β‐carotene alone, because foods rich in β‐carotene usually possess other antioxidant vitamins and micronutrients effective in preventing diseases (van Poppel, 1996; Kritchevsky, 1999; Tavani and La Vecchia, 1999; Voutilainen et al., 2006). Some studies have suggested that lycopene may also prevent CVD in humans, likely due to significant antioxidant activity in vitro. However, lycopene levels are not lower in smokers than in non‐smokers, indicating that its possible preventive activity is not only due to antioxidant properties. In contrast, lycopene may inhibit cholesterol synthesis and enhance LDL degradation. The reports showed that risk of myocardial infarction was decreased in people with higher concentrations of lycopene in adipose tissue (Arab and Steck, 2000). Also lycopene supplementation was found to improve endothelial function in patients suffering from CVD on optimal secondary prevention (Gajendragadkar et al., 2014). It is well‐known that lycopene has a low rate of bioavailability. It is incorporated into chylomicrons and other apo‐B containing lipoproteins in the blood circulation. The recent studies showed that the accumulation of ROS and NO in oxidative stress may represent a major cause of lycopene depletion in CVD, type 2 diabetes mellitus and ageing; low levels of serum lycopene have been associated with CVD. However, there is a poor relationship between dietary and serum lycopene levels, which is due to the low bioavailability of lycopene from dietary sources (Petyaev, 2016). However, lycopene may improve blood flow, decrease inflammatory responses and induce protective effects against the progression of CVD (Muller et al., 2016). Several epidemiological studies have supported a dose–response relationship between lycopene and CVD, suggesting the role for lycopene in the prevention of CVD (Mordente et al., 2011; Böhm, 2012). Epidemiological reports indicate an inverse relationship between xanthophylls intake and both cataract and AMD suggesting they have a protective role in the eye. Several observational studies also showed that xanthophylls (lutein and zeaxanthin) may reduce the risk of cancer types especially breast and lung cancers, as well as suppressing heart disease and stroke (Ribaya‐Mercado and Blumberg, 2004). In contrast, dietary supplementation of long‐chain ω‐3 polyunsaturated fatty acids or macular xanthophylls as well as daily intake of minerals and vitamins did not decrease the risk of CVD in elderly subjects with AMD (Bonds et al., 2014). Whereas the antioxidant effects of curcumin were shown to attenuate adriamycin‐induced cardiotoxicity and also inhibit diabetic cardiovascular complications. The anti‐thrombotic, anti‐proliferative and anti‐inflammatory effects of curcumin as well as decreasing serum cholesterol levels could protect individuals against atherosclerosis. The p300‐histone acetyltransferase inhibitory effects of curcumin improve the development of cardiac hypertrophy and heart failure in animal models. In addition, the inflammatory effects of curcumin as well as its role in correcting the Ca2 + homeostasis may play a role in the prevention of ventricular arrhythmias (Wongcharoen and Phrommintikul, 2009). Among the carotenoids, ASTA exerts beneficial effects on heart health by different strategies such as: (a) decrease in inflammation associated with atherosclerosis, (b) modification of blood levels of LDL‐cholesterol (LDL‐C) and high density lipoprotein‐cholesterol (HDL‐C) and (c) reduction of apoptosis and macrophage infiltration in vascular lesions resulting in plaque stability by increasing adiponectin. Indeed, ASTA showed therapeutic effects in the management of myocardial injury, oxidized LDL‐C, rethrombosis after thrombolysis and other cardiac diseases (e.g. atrial fibrillation). Furthermore, ASTA could regulate macrophage atherogenesis‐related functions by suppressing the up‐regulation of scavenger receptors, activation of matrix metalloproteinases and expression of pro‐inflammatory cytokines (Ciccone et al., 2013).
Other diseases
Takeda et al. showed that there is no significant association between micronutrients (α‐carotene, β‐carotene, β‐cryptoxanthin, lutein, lycopene, zeaxanthin and canthaxanthin) and the risk of Parkinson's disease (Takeda et al., 2014). Other trials have indicated that vitamin A (VA) or β‐carotene supplementation during pregnancy did not have any significant effect on birthweight indicators, preterm birth, stillbirth, miscarriage or fetal loss. Among HIV‐positive women, supplementation was protective against low birthweight (<2.5 kg), without significant effects on preterm delivery or small‐for‐gestational age. VA supplementation could improve haemoglobin levels and decrease anaemia risk (<110 g·L−1) during pregnancy. Although, maternal supplementation with VA or β‐carotene may show benefits during pregnancy on maternal or infant mortality, it may also trigger harmful effects such as increasing HIV transmission in some individuals (Thorne‐Lyman and Fawzi, 2012).
Al‐Karawi et al. showed that curcumin has anti‐depressant effects in patients with major depressive disorders (MDDs). The administration of curcumin induced a significant reduction in the symptoms of depression. Indeed, it had the highest effect in middle‐aged patients at higher doses and for a longer duration of administration. Moreover, the administration of novel formulation of curcumin (BCM‐95) had no significant effect on depression as compared with the conventional curcumin‐piperine formula (Al‐Karawi et al., 2016). Turmeric/curcumin products and supplements, both oral and topical, can also provide therapeutic benefits for skin health and improve certain skin diseases. Thus, the development of effective curcumin delivery methods with high bioavailability and solubility would be useful for treatment of skin problems (Vaughn et al., 2016).
Pharmacokinetics, tolerable dose and safety of carotenoids
Carotenoids are absorbed in the body similar to lipids and transported through the lymphatic system into the liver. The absorption of carotenoids depends on dietary ingredients. For instance, a high cholesterol diet increases the absorption of carotenoids, whereas a low‐fat diet reduces their absorption (Ambati et al., 2014). All‐trans‐carotenoids have a better bioavailability than the 9‐cis‐forms. Elimination of carotenoids takes 5–7 and 2–3 days for β‐carotene and lycopene respectively. The bioconversion of β‐carotene to retinal is dose‐dependent and ranges between 27 and 2% for a 6 and 126 mg dose respectively (Schwedhelm et al., 2003). Pharmacokinetic parameters for trans‐ and cis‐lycopene isomers showed that the formulation was well tolerated with minimal side effects (Gustin et al., 2004). Although, high tissue concentrations of carotenoids showed pro‐oxidant activity under certain conditions, there are no known adverse effects at intake levels for dietary or formulated lycopene in healthy population. Therefore, synthetic lycopene, tomato lycopene extracts and crystallized lycopene extract are recognized as safe for use as an ingredient in a variety of foods (Trumbo, 2005). Lycopene supplementation in men with biochemically relapsed PCa is safe and well tolerated. The plasma levels of lycopene were similar for a wide dose range (15 to 90 mg·day−1) and had plateaued by 3 months (Clark et al., 2006). A physiological pharmacokinetic model was developed to describe the disposition of lycopene, delivered as a tomato beverage formulation in five doses (10, 30, 60, 90 or 120 mg), for a phase I study in healthy male subjects. The absorption rate at the 10 mg dose was significantly higher than at the higher doses; but the amount of lycopene absorbed was not statistically different between doses, suggesting a possible saturation of absorptive mechanisms. Independent of dose, 80% of the subjects absorbed less than 6 mg of lycopene. This may be useful for clinical trials with pharmacological doses of lycopene in cancer prevention, if absorption saturation occurs in the population (Diwadkar‐Navsariwala et al., 2003). In clinical trials, the β‐carotene supplements were given in the range of 15–50 mg·day−1 with good safety in intake level. β‐carotene has also been successfully used to treat inherited photosensitivity diseases at a dose of 180 mg·day−1 or more, without any adverse effects except for hypercarotenaemia. Toxicity studies in animals showed that β‐carotene is not mutagenic, carcinogenic, embryotoxic or teratogenic and did not cause hypervitaminosis A (Bendich, 1988). In several clinical studies, a high incidence of lung cancer was observed after administration of β‐carotene in high doses to smokers. As known, carotenoid breakdown products (CBP) including reactive aldehydes, and epoxides are formed during oxidative stress. The lung might be a critical organ in CBP formation. Indeed, the inhibition of mitochondrial respiration in state 3 by CBP was associated with a low content of protein sulfhydryl groups reducing glutathione levels and redox state, and also an elevated accumulation of malondialdehyde. The findings showed possible side effects of β‐carotene supplementation in intense oxidative stress induced by CBP indicating a class of lipid oxidation products and unwanted pro‐oxidative reactions (Siems et al., 2005).
Curcumin has been shown to protect against hepatic conditions, chronic arsenic exposure and alcohol intoxication. In clinical trials, curcumin has been used either alone or in combination with other agents. Curcumin's pleiotropic activities were derived from its ability to modulate different signalling molecules such as pro‐inflammatory cytokines, apoptotic proteins, NF‐κB, COX‐2, 5‐LOX, STAT3, C‐reactive protein, PGE2, prostate‐specific Ag, adhesion molecules, phosphorylase kinase, transforming growth factor‐β, TG, Endothelin 1 (ET‐1), creatinine, HO‐1, AST and ALT in human (Gupta et al., 2013). Phase I clinical trials showed that curcumin was safe even at high doses (12 g·day−1) over 3 months but indicated poor bioavailability. Major reasons for the low levels of curcumin in plasma and tissue may be due to poor absorption, rapid metabolism or systemic elimination. Different approaches have been used to improve the bioavailability of curcumin including: (a) the use of adjuvant like piperine interfering with glucuronidation, (b) the use of liposomal curcumin or curcumin nanoparticles, (c) the use of curcumin/phospholipid complex and (d) the use of structural analogues of curcumin (e.g. EF‐24). The EF‐24 showed a rapid absorption with a high plasma half‐life. Despite the lower bioavailability of curcumin, its therapeutic efficiency has been proven in different human diseases including CVDs, diabetes, cancer, arthritis, Crohn's disease and neurological diseases (Anand et al., 2007; Gupta et al., 2013). Crocin could be an effective treatment of patients with MDD without side effects (Talaei et al., 2015). Furthermore, crocin tablets (20 mg) were evaluated for short‐term safety and tolerability in healthy volunteers. No major adverse effects were reported during the clinical evaluation (Mohamadpour et al., 2013).
Among several natural carotenoids, ASTA was considered one of the best carotenoids for protection of cells, lipids and membrane lipoproteins against oxidative damage. Its intake includes the following sequential steps: (a) the mixture of ASTA with bile acid after ingestion and generation of micelles in the intestinum tenue; (b) partial absorption of the micelles with ASTA by intestinal mucosal cells; (c) incorporation of ASTA into chylomicra by intestinal mucosal cells; (d) digestion of chylomicra with ASTA by lipoprotein lipase after releasing into the lymph within the systemic circulation; and (e) deletion of chylomicron remnants rapidly by the liver and other tissues. Indeed, ASTA is associated with lipoproteins and is transported into the tissues (Ambati et al., 2014). ASTA was safe, with no side effects when it was used with food. It accumulated in rat tissues, but no toxic effects were observed. After oral administration of ASTA, the levels of antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidase were significantly increased in animals. However, excessive consumption of ASTA led to yellow to reddish pigmentation of the skin in animal models. On the other hand, the use of 50 mg·kg−1 ASTA decreased blood pressure in stroke prone rats and also in hypertensive rats for 5 weeks and 14 days respectively. ASTA also significantly protected animals against naproxen‐induced gastric, antral ulcer and suppressed lipid peroxidation levels in gastric mucosa. The higher therapeutic concentrations of ASTA had no adverse effects on platelet, coagulation and fibrinolytic function. The researchers recommended the administration of ASTA (~2–4 mg·day−1) with ω‐3 rich seed oils (e.g. walnuts and almonds). In these clinical studies, no side effects were found with higher doses of ASTA (~6 mg·day−1) in adult human subjects (Ambati et al., 2014).
Future directions
Different carotenoids have shown various biochemical and immunological functions. For example, astaxanthin and canthaxanthin carotenoids are known as a neuroprotective agents for neurodegenerative disorders (e.g. Parkinson) due to their anti‐oxidative and anti‐inflammatory activities, which can enhance the activity of glutathione peroxidase and catalase and reduce the production of IL‐1, IL‐6 and TNF‐α cytokines. Violaxanthin is also an anti‐inflammatory agent for therapeutic purposes, due to its ability to inhibit LPS‐mediated NF‐κB pathways. Lutein can also scavenge ROS generated during the inflammatory process and inhibit TNF‐α induction in cultured ECs. In addition, β‐carotene blocks nuclear translocation of the NF‐κB p65 subunit that is correlated with its inhibitory effect on phosphorylation and degradation of the NF‐κB inhibitor. Generally, all carotenoids show antioxidant activities such as quenching free radicals, reducing damage from reactive oxidant species and inhibiting lipid peroxidation. Furthermore, carotenoids can play important roles in immuno‐regulation and immuno‐stimulation in vertebrates. However, there are several major issues for the use of dietary carotenoids that should be considered to prevent or treat various disorders including: (a) the efficiency of individual carotenoids may depend on concentrations of other carotenoids. Indeed, carotenoids may interact synergistically and supplementation with a single carotenoid may be ineffective; (b) these compounds are highly sensitive to oxidation, chemical or enzymatic pathways, and consequently, they can be converted to other products, the effects of which are not fully known; (c) different genetic susceptibility can lead to variations in the response to treatment with carotenoids among individuals with similar dietary intakes; and (d) the efficiency of carotenoids is determined in a time‐ and dose‐dependent manner. Finally, the safety, metabolism and molecular biological properties of carotenoids should be elucidated through further studies before they are used to prevent carcinogenesis.
Author contributions
A.M., M.B. and S.S. contributed to the drafting and writing the manuscript. A.B. contributed to the drafting, writing, conception and final approval of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Milani, A. , Basirnejad, M. , Shahbazi, S. , and Bolhassani, A. (2017) Carotenoids: biochemistry, pharmacology and treatment. British Journal of Pharmacology, 174: 1290–1324. doi: 10.1111/bph.13625.
References
- Abdullaev FI (1994). Inhibitory effect of crocetin on intracellular nucleic acid and protein synthesis in malignant cells. Toxicol Lett 70: 243–251. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso‐Ramos CE et al. (2005). Curcumin suppresses the paclitaxel‐induced NF‐ĸB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res 11: 7490–7498. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN et al. (2005). Neuroprotection by crocetin in a hemi‐parkinsonian rat model. Pharmacol Biochem Behav 81: 805–813. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh F, Bolhassani A (2015). In vitro cytotoxicity of Iranian saffron and two main components as a potential anti‐cancer drug. SM J Pharmac Ther 1: 1–5. [Google Scholar]
- Al‐Karawi D, Al Mamoori DA, Tayyar Y (2016). The role of curcumin administration in patients with major depressive disorder: mini meta‐analysis of clinical trials. Phytother Res 30: 175–183. [DOI] [PubMed] [Google Scholar]
- Ambati RR, Phang SM, Ravi S, Aswathanarayana RG (2014). Astaxanthin: sources, extraction, stability, biological activities and its commercial applications: a review. Mar Drugs 12: 128–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S (2011). Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology 54: 857–867. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007). Bioavailability of curcumin: problems and promises. Mol Pharm 4: 807–818. [DOI] [PubMed] [Google Scholar]
- Anbazahan SM, Mari LS, Yogeshwari G, Jagruthi C, Thirumurugan R, Arockiaraj J et al. (2014). Immune response and disease resistance of carotenoids supplementation diet in Cyprinus carpio against Aeromonas hydrophila . Fish Shellfish Immunol 40: 9–13. [DOI] [PubMed] [Google Scholar]
- Antony S, Kuttan R, Kuttan G (1999). Immunomodulatory activity of curcumin. Immunol Invest 28: 291–303. [DOI] [PubMed] [Google Scholar]
- Aoki A, Inoue M, Nguyen E, Obata R, Kadonosono K, Shinkai S et al. (2016). Dietary n‐3 fatty acid, α‐tocopherol, zinc, vitamin D, vitamin C, and β‐carotene are associated with age‐related macular degeneration in Japan. Sci Rep 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab L, Steck S (2000). Lycopene and cardiovascular disease. Am J Clin Nutr 71: 1691S–1695S. [DOI] [PubMed] [Google Scholar]
- Ashfaq MK, Zuberi HS, Waqar MA (2000). Vitamin E and β‐carotene affect natural killer cell function. Int J Food Sci Nutr 51: S13–S20. [PubMed] [Google Scholar]
- Astorg P (1997). Food carotenoids and cancer prevention: an overview of current research. Trends Food Sci Technol 8: 406–413. [Google Scholar]
- Aune D, Chan DSM, Vieira AR, Rosenblatt DAN, Vieira R, Greenwood DC et al. (2012). Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta‐analysis of prospective studies. Am J Clin Nutr 96: 356–373. [DOI] [PubMed] [Google Scholar]
- Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT et al. (2007). Crocin from Crocus sativus possesses significant anti‐proliferation effects on human colorectal cancer cells. Exp Oncol 29: 175–180. [PMC free article] [PubMed] [Google Scholar]
- Bae JW, Bae JS (2011). Barrier protective effects of lycopene in human endothelial cells. Inflamm Res 60: 751–758. [DOI] [PubMed] [Google Scholar]
- Baralic I, Andjelkovic M, Djordjevic B, Dikic N, Radivojevic N, Suzin‐Zivkovic V et al. (2015). Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Hindawi Publishing Corporation: 1–9. [DOI] [PMC free article] [PubMed]
- Bathaie SZ, Bolhasani A, Hoshyar R, Ranjbar B, Sabouni F, Moosavi‐Movahedi AA (2007). Interaction of saffron carotenoids as anticancer compounds with ctDNA, Oligo (dG.dC)15, and Oligo (dA.dT)15. DNA Cell Biol 26: 533–540. [DOI] [PubMed] [Google Scholar]
- Bathaie SZ, Hoshyar R, Miri H, Sadeghizadeh M (2013). Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem Cell Biol 91: 397–403. [DOI] [PubMed] [Google Scholar]
- Bathaie SZ, Bolhassani A, Tamanoi F (2014). Anticancer effect and molecular targets of saffron carotenoids. Enzymes 36: 57–86. [DOI] [PubMed] [Google Scholar]
- Bendich A (1988). The safety of beta‐carotene. Nutr Cancer 11: 207–214. [DOI] [PubMed] [Google Scholar]
- Bergman M, Djaldetti M, Salman H, Bessler H (2010). On the combined effect of statins and lycopene on cytokine production by human peripheral blood cells. Heart Vessels 25: 426–431. [DOI] [PubMed] [Google Scholar]
- Bessler H, Salman H, Bergman M, Alcalay Y, Djaldetti M (2008). In vitro effect of lycopene on cytokine production by human peripheral blood mononuclear cells. Immunol Invest 37: 183–190. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari V, Nagini S (2005). Lycopene: a review of its potential as an anticancer agent. Curr Med Chem Anticancer Agents 5: 627–635. [DOI] [PubMed] [Google Scholar]
- Bie X, Chen Y, Zheng X, Dai H (2011). The role of crocetin in protection following cerebral contusion and in the enhancement of angiogenesis in rats. Fitoterapia 82: 997–1002. [DOI] [PubMed] [Google Scholar]
- Böhm V (2012). Lycopene and heart health. Mol Nutr Food Res 56: 296–303. [DOI] [PubMed] [Google Scholar]
- Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW (1999). Cis‐lycopene is more bioavailable than trans‐lycopene in vitro and in vivo in lymph‐cannulated ferrets. J Nutr 129: 1176–1181. [DOI] [PubMed] [Google Scholar]
- Bolhasani A, Bathaie SZ, Yavari I, Moosavi‐Movahedi AA, Ghaffari M (2005). Separation and purification of some components of iranian saffron. Asian J Chem 17: 725–729. [Google Scholar]
- Bolhassani A (2015). Cancer chemoprevention by natural carotenoids as an efficient strategy. Anticancer Agents Med Chem 15: 1026–1031. [DOI] [PubMed] [Google Scholar]
- Bolhassani A, Khavari A, Bathaie SZ (2014). Saffron and natural carotenoids: biochemical activities and anti‐tumor effects. Biochim et Biophys Acta 1845: 20–30. [DOI] [PubMed] [Google Scholar]
- Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J et al. (2014). Effect of long‐chain ω‐3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the age‐related eye disease study 2 (AREDS2) randomized clinical trial. JAMA Intern Med 174: 763–771. [DOI] [PubMed] [Google Scholar]
- Bose S, Panda AK, Mukherjee S, Sa G (2015). Curcumin and tumor immune‐editing: resurrecting the immune system (RW). Cell Div 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JA, Cheung KJ, Li G (2001). Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase‐8 pathway independent of p53. Exp Cell Res 271: 305–314. [DOI] [PubMed] [Google Scholar]
- Capelli B, Bagchi D, Cysewski GR (2013). Synthetic astaxanthin is significantly inferior to algal‐based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 12: 145–152. [Google Scholar]
- Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, Li X et al. (2008). Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res 68: 5345–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalabi N, Le Corre L, Maurizis JC, Bignon YJ, Bernard‐Gallon DJ (2004). The effects of lycopene on the proliferation of human breast cells and BRCA1 and BRCA2 gene expression. Eur J Cancer 40: 1768–1775. [DOI] [PubMed] [Google Scholar]
- Chang YF, Chuang HY, Hsu CH, Liu RS, Gambhir SS, Hwang JJ (2012). Immunomodulation of curcumin on adoptive therapy with T cell functional imaging in mice. Cancer Prev Res 5: 444–452. [DOI] [PubMed] [Google Scholar]
- Chauhan DP (2002). Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des 8: 1695–1706. [DOI] [PubMed] [Google Scholar]
- Chen HY, Huang SM, Yang CM, Hu ML (2012). Diverse effects of β‐carotene on secretion and expression of VEGF in human hepatocarcinoma and prostate tumor cells. Molecules 17: 3981–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Yueh TC, Chen YC, Huang CH, Yang CM, Hu ML (2013a). Antimetastatic effects of α‐carotene and possible mechanisms of action in human hepatocarcinoma SK‐Hep‐1 cells. J Agric Food Chem 61: 10368–10376. [DOI] [PubMed] [Google Scholar]
- Chen J, Song Y, Zhang L (2013b). Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta‐analysis of prospective studies. J Nutr Sci Vitaminol 59: 213–223. [DOI] [PubMed] [Google Scholar]
- Chen P, Zhang W, Wang X, Zhao K, Negi DS, Zhuo L et al. (2015). Lycopene and risk of prostate cancer: a systematic review and meta‐analysis. Medicine 94: e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chen Y, Wang Y, Cai S, Deng L, Liu J et al. (2016). Comparative evaluation of hepatoprotective activities of geniposide, crocins and crocetin by CCl4‐induced liver injury in mice. Biomol Ther 24: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew BP, Park JS (2004). Carotenoid action on the immune response. J Nutr 134: 257S–261S. [DOI] [PubMed] [Google Scholar]
- Chew BP, Park JS, Wong TS, Kim HW, Weng BB, Byrne KM et al. (2000). Dietary beta‐carotene stimulates cell‐mediated and humoral immune response in dogs. J Nutr 130: 1910–1913. [DOI] [PubMed] [Google Scholar]
- Chew BP, Mathison BD, Hayek MG, Massimino S, Reinhart GA, Parka JS (2011). Dietary astaxanthin enhances immune response in dogs. Vet Immunol Immunopathol 140: 199–206. [DOI] [PubMed] [Google Scholar]
- Chew EY, SanGiovanni JP, Ferris FL, Wong WT, Agron E, Clemons TE et al. (2013). Lutein/zeaxanthin for the treatment of age‐related cataract: AREDS2 randomized trial report. JAMA Ophthalmol 131: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian MS, Schulte S, Hellwig J (2003). Developmental (embryo‐fetal toxicity/teratogenicity) toxicity studies of synthetic crystalline lycopene in rats and rabbits. Food Chem Toxicol 41: 773–783. [DOI] [PubMed] [Google Scholar]
- Chryssanthi DG, Lamari FN, Iatrou G, Pylara A, Karamanos NK, Cordopatis P (2007). Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res 27: 357–362. [PubMed] [Google Scholar]
- Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G et al. (2013). Dietary intake of carotenoids and their antioxidant and anti‐inflammatory effects in cardiovascular care. Mediators Inflamm 2013: 782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PE, Hall MC, Borden LS Jr, Miller AA, Hu JJ, Lee WR et al. (2006). Phase I‐II prospective dose‐escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 67: 1257–1261. [DOI] [PubMed] [Google Scholar]
- Csepanyi E, Czompa A, Haines D, Lekli I, Bakondi E, Balla G et al. (2015). Cardiovascular effects of low versus high‐dose β‐carotene in a rat model. Pharmacol Res 100: 148–156. [DOI] [PubMed] [Google Scholar]
- Cui SX, Qu XJ, Xie YY, Zhou L, Nakata M, Makuuchi M et al. (2006). Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med 18: 227–231. [PubMed] [Google Scholar]
- De Stefano D, Maiuri MC, Simeon V, Grassia G, Soscia A, Cinelli MP et al. (2007). Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN‐γ. Eur J Pharmacol 566: 192–199. [DOI] [PubMed] [Google Scholar]
- De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB et al. (2009). Antimicrobial activity of curcumin against Helicobacter pylori isolates from india and during infections in mice. Antimicrob Agents Chemother 53: 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A, Mehta S, Dhar G, Dhar K, Banerjee S, Van Veldhuizen P et al. (2009). Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther 8: 315–323. [DOI] [PubMed] [Google Scholar]
- Di Filippo MM, Mathison BD, Park JS, Chew BP (2012). Lutein and β‐cryptoxanthin inhibit inflammatory mediators in human chondrosarcoma cells induced with IL‐1β. Open Nutr J 6: 41–47. [Google Scholar]
- Diwadkar‐Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA et al. (2003). A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res 44: 1927–1939. [DOI] [PubMed] [Google Scholar]
- Donhowe EG, Kong F (2014). Beta‐carotene: digestion, microencapsulation, and in vitro bioavailability. Food Bioproc Tech 7: 338–354. [Google Scholar]
- Dwyer JH, Navab M, Dwyer KM, Hassan K, Sun P, Shircore A et al. (2001). Oxygenated carotenoid lutein and progression of early atherosclerosis: the Los Angeles atherosclerosis study. Circulation 103: 2922–2927. [DOI] [PubMed] [Google Scholar]
- El‐Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG et al. (2004). Carotenoid radical chemistry and antioxidant/pro‐oxidant properties. Arch Biochem Biophys 430: 37–48. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q et al. (2012). Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst 104: 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdous AP, Preethi KC, Kuttan R (2010). Antioxidant potential of meso‐zeaxanthin a semi‐synthetic carotenoid. Food Chem 119: 1096–1101. [Google Scholar]
- Firdous AP, Kuttan G, Kuttan R (2015). Anti‐inflammatory potential of carotenoid meso‐zeaxanthin and its mode of action. Pharm Biol 53: 961–967. [DOI] [PubMed] [Google Scholar]
- Foryst‐Ludwig A, Neumann M, Schneider‐Brachert W, Naumanna M (2004). Curcumin blocks NF‐ĸB and the motogenic response in Helicobacter pylori‐infected epithelial cells. Biochem Biophys Res Commun 316: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Franceschi S, Bidoli E, La Vecchia C, Talamini R, D'Avanzo B, Negri E (1994). Tomatoes and risk of digestive‐tract cancers. Int J Cancer 59: 181–184. [DOI] [PubMed] [Google Scholar]
- Fu H, Xie B, Fan G, Ma S, Zhu X, Pan S (2010). Effect of esterification with fatty acid of β‐cryptoxanthin on its thermal stability and antioxidant activity by chemiluminescence method. Food Chem 122: 602–609. [Google Scholar]
- Gajendragadkar PR, Hubsch A, Maki‐Petaja KM, Serg M, Wilkinson IB, Cheriyan J (2014). Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS One 9: e99070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammone MA, Riccioni G, D'Orazio N (2015). Carotenoids: potential allies of cardiovascular health? Food Nutr Res 59: 26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Kuo J, Jiang H, Deeb D, Liu Y, Divine G et al. (2004). Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell‐mediated cytotoxicity, and cytokine production in vitro . Biochem Pharmacol 68: 51–61. [DOI] [PubMed] [Google Scholar]
- Gao S, Qin T, Liuetal Z (2011). Lutein and zeaxanthin supplementation reduces H2O2‐induced oxidative damage in human lens epithelial cells. Mol Vis 17: 3180–3190. [PMC free article] [PubMed] [Google Scholar]
- Gao YY, Xie QM, Jin L, Sun BL, Ji J, Chen F et al. (2012). Supplementation of xanthophylls decreased pro‐inflammatory and increased anti‐inflammatory cytokines in hens and chicks. Br J Nutr 108: 1746–1755. [DOI] [PubMed] [Google Scholar]
- Garcia AL, Ruhl R, Herz U, Koebnick C, Schweigert FJ, Worm M (2003). Retinoid‐ and carotenoid‐enriched diets influence the ontogenesis of the immune system in mice. Immunology 110: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodratizadeh S, Kanbak G, Beyramzadeh M, Dikmen ZG, Memarzadeh S, Habibian R (2014). Effect of carotenoid β‐cryptoxanthin on cellular and humoral immune response in rabbit. Vet Res Commun 38: 59–62. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1995). Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 87: 1767–1776. [DOI] [PubMed] [Google Scholar]
- Gloria NF, Soares N, Brand C, Oliveira FL, Borojevic R, Teodoro AJ (2014). Lycopene and beta‐carotene induce cell‐cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res 34: 1377–1386. [PubMed] [Google Scholar]
- Gomes DC, Alegrio LV, de Lima ME, Leon LL, Araujo CA (2002). Synthetic derivatives of curcumin and their activity against Leishmania Amazonensis . Arzneimittelforschung 52: 120–124. [PubMed] [Google Scholar]
- Gonzalez S, Astner S, An W, Goukassian D, Pathak MA (2003). Dietary lutein/zeaxanthin decreases ultraviolet B‐induced epidermal hyperproliferation and acute inflammation in hairless mice. J Invest Dermatol 121: 399–405. [DOI] [PubMed] [Google Scholar]
- Gossage C, Deyhim M, Moser‐Veillon PB, Douglas LW, Kramer TR (2000). Effect of beta‐carotene supplementation and lactation on carotenoid metabolism and mitogenic T lymphocyte proliferation. Am J Clin Nutr 71: 950–955. [DOI] [PubMed] [Google Scholar]
- Gouranton E, Thabuis C, Riollet C, Malezet‐Desmoulins C, Yazidi CE, Amiot MJ et al. (2011). Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem 22: 642–648. [DOI] [PubMed] [Google Scholar]
- Greenberg ER, Baron JA, Stukel TA, Stevens MM, Mandei JS, Spencer SK et al. (1990). A clinical trial of β‐carotene to prevent basal‐cell and squamous‐cell cancers of the skin. N Engl J Med 323: 789–795. [DOI] [PubMed] [Google Scholar]
- Guedes AC, Amaro HM, Sousa‐Pinto I, Malcata FX (2014). Bioactive carotenoids from microalgae. Bioactive Compounds from Marine Foods: Plant and Animal Sources: 130–151.
- Guo L, Zhu H, Lin C, Che J, Tian X, Han S et al. (2015). Associations between antioxidant vitamins and the risk of invasive cervical cancer in chinese women: A case–control study. Sci Rep 5: 13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB (2013). Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15: 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin DM, Rodvold KA, Sosman JA, Diwadkar‐Navsariwala V, Stacewicz‐Sapuntzakis M, Viana M et al. (2004). Single‐dose pharmacokinetic study of lycopene delivered in a well‐defined food‐based lycopene delivery system (tomato paste‐oil mixture) in healthy adult male subjects. Cancer Epidemiol Biomarkers Prev 13: 850–860. [PubMed] [Google Scholar]
- Gutheil WG, Reed G, Ray A, Dhar A (2012). Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol 13: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad N, Levy R (2012). The synergistic anti‐inflammatory effects of lycopene, lutein, β‐carotene, and carnosic acid combinations via redox‐based inhibition of NF‐ĸB signaling. Free Radic Biol Med 53: 1381–1391. [DOI] [PubMed] [Google Scholar]
- Hahm ER, Gho YS, Park S, Park C, Kim KW, Yang CH (2004). Synthetic curcumin analogs inhibit activator protein‐1 transcription and tumor‐induced angiogenesis. Biochem Biophys Res Commun 321: 337–344. [DOI] [PubMed] [Google Scholar]
- Hazlewood LC, Wood LG, Hansbro PM, Foster PS (2011). Dietary lycopene supplementation suppresses Th2 responses and lung eosinophilia in a mouse model of allergic asthma. J Nutr Biochem 22: 95–100. [DOI] [PubMed] [Google Scholar]
- He Q, Zhou W, Xiong C, Tan G, Chen M (2015). Lycopene attenuates inflammation and apoptosis in post‐myocardial infarction remodeling by inhibiting the nuclear factor‐κB signaling pathway. Mol Med Rep 11: 374–378. [DOI] [PubMed] [Google Scholar]
- Heinen MM, Hughes MC, Ibiebele TI, Marks GC, Green AC, van der Pols JC (2007). Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer 43: 2707–2716. [DOI] [PubMed] [Google Scholar]
- Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW (2013). The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int J Mol Sci 14: 14620–14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe PP, Kramer K, van den Berg H, Steenge G, van Vliet T (2003). Synthetic and tomato‐based lycopene have identical bioavailability in humans. Eur J Nutr 42: 272–278. [DOI] [PubMed] [Google Scholar]
- Huang CS, Chuang CH, Loa TF, Huc ML (2013). Anti‐angiogenic effects of lycopene through immunomodualtion of cytokine secretion in human peripheral blood mononuclear cells. J Nutr Biochem 24: 428–434. [DOI] [PubMed] [Google Scholar]
- Hughes DA, Wright AJ, Finglas PM, Polley AC, Bailey AL, Astley SB et al. (2000). Effects of lycopene and lutein supplementation on the expression of functionally associated surface molecules on blood monocytes from healthy male nonsmokers. J Infect Dis 182: S11–S15. [DOI] [PubMed] [Google Scholar]
- Hung CF, Huang TF, Chen BH, Shieh JM, Wu PH, Wu WB (2008). IFN‐γ‐induced ICAM‐1 expression at 8 and 16 h were not affected by lycopene, suggesting that lycopene primarily affects TNF‐α‐induced signaling pathway. Eur J Pharmacol 586: 275–282. [DOI] [PubMed] [Google Scholar]
- Ilic D, Missob M (2012). Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas 72: 269–276. [DOI] [PubMed] [Google Scholar]
- Jang SH, Lim JW, Kim H (2009). Mechanism of β‐carotene‐induced apoptosis of gastric cancer cells: Involvement of ataxia‐telangiectasia‐mutated. Ann N Y Acad Sci 1171: 156–162. [DOI] [PubMed] [Google Scholar]
- Jomova K, Valko M (2013). Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem 70: 102–110. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Hill RJ, Tomita Y, Good RA (1991). Studies of immunomodulating actions of carotenoids: Effects of beta‐carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutr Cancer 16: 93–105. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Zhang L, Gross M, Tomita Y (1994). Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T‐dependent antigens. Nutr Cancer 21: 47–58. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Gross M (1995). Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T‐dependent stimulant and antigen. Nutr Cancer 23: 171–183. [DOI] [PubMed] [Google Scholar]
- Kang BY, Chung SW, Chung WJ, Im SY, Hwang SY, Kim TS (1999a). Inhibition of interleukin‐12 production in lipopolysaccharide‐activated macrophages by curcumin. Eur J Pharmacol 384: 191–195. [DOI] [PubMed] [Google Scholar]
- Kang BY, Song YJ, Kim KM, Cho YK, Hwang SY, Kim TS (1999b). Curcumin inhibits Th1 cytokine profile in CD4+ T cells by suppressing interleukin‐12 production in macrophages. Br J Pharmacol 128: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Park KS, Seo JY, Kim H (2011). Lycopene inhibits IL‐6 expression in cerulein‐stimulated pancreatic acinar cells. Genes Nutr 6: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karppi J, Kurl S, Laukkanen JA, Kauhanen J (2012). Serum beta‐carotene in relation to risk of prostate cancer: The kuopio ischaemic heart disease risk factor study. Nutr Cancer 64: 361–367. [DOI] [PubMed] [Google Scholar]
- Kataria Y, Deaton RJ, Enk E, Jin M, Petrauskaite M, Dong L et al. (2016). Retinoid and carotenoid status in serum and liver among patients at high‐risk for liver cancer. BMC Gastroenterol 16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuura S, Imamura T, Bando N, Yamanishi R (2009). β‐carotene and β‐cryptoxanthin but not lutein evoke redox and immune changes in RAW264 murine macrophages. Mol Nutr Food Res 53: 1396–1405. [DOI] [PubMed] [Google Scholar]
- Kaulmann A, Bohn T (2014). Carotenoids, inflammation, and oxidative stress‐implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 34: 907–929. [DOI] [PubMed] [Google Scholar]
- Khalili M (2010). Behavioral and histological analysis of Crocus sativus effect in intracerebroventricular streptozotocin model of alzheimer disease in rats. Iranian J Pathol 5: 27–33. [Google Scholar]
- Khavari A, Bolhassani A, Alizadeh F, Bathaie SZ, Balaram P, Agi E et al. (2014). Chemo‐immunotherapy using saffron and its ingredients followed by E7‐NT (gp96) DNA vaccine generates different anti‐tumor effects against tumors expressing the E7 protein of human papillomavirus. Arch Virol 160: 499–508. [DOI] [PubMed] [Google Scholar]
- Khazdair MR, Boskabady MH, Hosseini M, Rezaee R, Tsatsakis AM (2015). The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J Phytomed 5: 376–391. [PMC free article] [PubMed] [Google Scholar]
- Kim H (2011). Inhibitory mechanism of lycopene on cytokine expression in experimental pancreatitis. Ann N Y Acad Sci 1229: 99–102. [DOI] [PubMed] [Google Scholar]
- Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba‐Toriyama H et al. (1998a). Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2‐dimethylhydrazine initiation. Carcinogenesis 19: 81–85. [DOI] [PubMed] [Google Scholar]
- Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba‐Toriyama H et al. (1998b). Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2‐dimethylhydrazine initiation. Carcinogenesis 19: 81–85. [DOI] [PubMed] [Google Scholar]
- Kim HW, Chew BP, Wong TS, Park JS, Weng BBC, Byrne KM et al. (2000a). Modulation of humoral and cell‐mediated immune responses by dietary lutein in cats. Vet Immunol Immunopathol 73: 331–341. [DOI] [PubMed] [Google Scholar]
- Kim HW, Chew BP, Wong TS, Park JS, Weng BBC, Byrne KM et al. (2000b). Dietary lutein stimulates immune response in the canine. Vet Immunol Immunopathol 74: 315–327. [DOI] [PubMed] [Google Scholar]
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO et al. (2005). Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF‐ĸB as potential targets. J Immunol 174: 8116–8124. [DOI] [PubMed] [Google Scholar]
- Kim W, Fan YY, Smith R, Patil B, Jayaprakasha GK, McMurray DN et al. (2009). Dietary curcumin and limonin suppress CD4+ T‐cell proliferation and interleukin‐2 production in mice. J Nutr 139: 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Leite JO, DeOgburn R, Smyth JA, Clark RM, Fernandez ML (2011). A lutein‐enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J Nutr 141: 1458–1463. [DOI] [PubMed] [Google Scholar]
- Kim JE, Clark RM, Park Y, Lee J, Fernandez ML (2012). Lutein decreases oxidative stress and inflammation in liver and eyes of guinea Pigs fed a hypercholesterolemic diet. Nutr Res Pract 6: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Jang MS, Son YM, Seo MJ, Ji SY, Han SH et al. (2013). Curcumin inhibits CD4+ T cell activation, but augments CD69 expression and TGF‐β1‐mediated generation of regulatory T cells at late phase. PLoS One 8: e62300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Lee HA, Lim JY, Kim Y, Jung CH, Yoo SH et al. (2014). β‐Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia‐inducible factor‐1α. J Nutr Biochem 25: 655–664. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA (2003). Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23: 171–201. [DOI] [PubMed] [Google Scholar]
- Kritchevsky SB (1999). β‐carotene, carotenoids and the prevention of coronary heart disease. J Nutr 129: 5–8. [DOI] [PubMed] [Google Scholar]
- Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F et al. (2001). Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev 10: 861–868. [PubMed] [Google Scholar]
- Kurihara H, Koda H, Asami S, Kiso Y, Tanaka T (2002). Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sci 70: 2509–2520. [DOI] [PubMed] [Google Scholar]
- Lee CM, Chang JH, Moon DO, Hyun Choi Y, Choi IW, Park YM et al. (2008). Lycopene suppresses ovalbumin‐induced airway inflammation in a murine model of asthma. Biochem Biophys Res Commun 374: 248–252. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Bae JW, Bae JS (2012). Inhibitory effects of lycopene on HMGB1‐mediated pro‐inflammatory responses in both cellular and animal models. Food Chem Toxicol 50: 1826–1833. [DOI] [PubMed] [Google Scholar]
- Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S (2015). Carotenoid intake from natural sources and head and neck cancer: A systematic review and meta‐analysis of epidemiological studies. Cancer Epidemiol Biomarkers Prev 24: 1003–1011. [DOI] [PubMed] [Google Scholar]
- Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M et al. (1995). Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha‐carotene or beta‐carotene. Nutr Cancer 24: 257–266. [DOI] [PubMed] [Google Scholar]
- Li SY, Fung FKC, Fu ZJ, Wong D, Chan HHL, Lo ACY (2012). Anti‐inflammatory effects of lutein in retinal ischemic/ hypoxic injury: In vivo and In vitro studies. Invest Ophthalmol Vis Sci 53: 5976–5984. [DOI] [PubMed] [Google Scholar]
- Li Q, Huyan T, Ye LJ, Ren HL, Shi JL, Huang QS (2014). Lycopene prolongs the lifespan and enhances the cytotoxicity of NK cells after Ex vivo expansion. Bioeng Biosci 2: 23–30. [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM (2001). The curry spice curcumin reduces oxidative damage and amyloid pathology in an alzheimer transgenic mouse. J Neurosci 21: 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW (1997). Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett 116: 197–203. [DOI] [PubMed] [Google Scholar]
- Lin HW, Chang TJ, Yang DJ, Chen YC, Wang M, Chang YY (2012). Regulation of virus induced inflammatory response by β‐carotene in RAW264.7 cells. Food Chem 134: 2169–2175. [DOI] [PubMed] [Google Scholar]
- Liu C, Lian F, Smith DE, Russell RM, Wang XD (2003). Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up‐regulating insulin‐like growth factor‐binding protein3 in cigarette smoke‐exposed ferrets. Cancer Res 63: 3138–3144. [PubMed] [Google Scholar]
- LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN et al. (2005). Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 11: 6994–7002. [DOI] [PubMed] [Google Scholar]
- Lu P, Lin H, Gu Y, Li L, Guo H, Wang F et al. (2015). Antitumor effects of crocin on human breast cancer cells. Int J Clin Exp Med 8: 20316–20322. [PMC free article] [PubMed] [Google Scholar]
- Macedo RC, Bolin AP, Marin DP, Otton R (2010). Astaxanthin addition improves human neutrophils function: In vitro study. Eur J Nutr 49: 447–457. [DOI] [PubMed] [Google Scholar]
- Madaric A, Kadrabova J, Krajcovicova‐Kudlackova M, Valachovicova M, Spustova V, Mislanova C et al. (2013). The effect of bioactive complex of quercetin, selenium, catechins and curcumin on cardiovascular risk markers in healthy population after a two month consumption. Bratisl Lek Listy 114: 84–87. [DOI] [PubMed] [Google Scholar]
- Magesh V, DurgaBhavani K, Senthilnathan P, Rajendran P, Sakthisekaran D (2009). In vivo protective effect of crocetin on benzo(α) pyrene‐induced lung cancer in Swiss albino mice. Phytother Res 23: 533–539. [DOI] [PubMed] [Google Scholar]
- Mai J, Shen X, Shi D, Wei Y, Shen H, Wu M (2010). Effect of lutein on relieving oxidative stress in mice induced by D‐galatose. Wei Sheng Yan Jiu 39: 430–432. [PubMed] [Google Scholar]
- Marcotorchino J, Romier B, Gouranton E, Riollet C, Gleize B, Malezet‐Desmoulins C et al. (2012). Lycopene attenuates LPS‐induced TNF‐secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage‐conditioned media. Mol Nutr Food Res 56: 725–732. [DOI] [PubMed] [Google Scholar]
- Mathews‐Roth MM (1982). Antitumor activity of beta‐carotene, canthaxanthin and phytoene. Oncology 39: 33–37. [DOI] [PubMed] [Google Scholar]
- Mayne ST, Cartmel B, Lin H, Zheng T, Goodwin WJ (2004). Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J Am Coll Nutr 23: 34–42. [DOI] [PubMed] [Google Scholar]
- McGraw JK, Ardia RD (2004). Immunoregulatory activity of different dietary carotenoids in male zebra finches. Chemoecology 14: 25–29. [Google Scholar]
- Michal JJ, Heirman LR, Wong TS, Chew BP, Frigg M, Volker L (1994). Modulatory effects of dietary beta‐carotene on blood and mammary leukocyte function in periparturient dairy cows. J Dairy Sci 77: 1408–1421. [DOI] [PubMed] [Google Scholar]
- Mills LM, Wilson H, Thies F (2012). Lycopene inhibits lymphocyte proliferation through mechanisms dependent on early cell activation. Mol Nutr Food Res 56: 1034–1042. [DOI] [PubMed] [Google Scholar]
- Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB et al. (2006). Associations between intermediate age‐related macular degeneration and lutein and zeaxanthin in the carotenoids in age‐related eye disease study (careds): ancillary study of the Women's health initiative. Arch Ophthalmol 124: 1151–1162. [DOI] [PubMed] [Google Scholar]
- Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H (2013). Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Med Sci 16: 39–46. [PMC free article] [PubMed] [Google Scholar]
- Moraes ML, Ribeiro AM, Santin E, Klasing KC (2016). Effects of conjugated linoleic acid and lutein on the growth performance and immune response of broiler chickens. Poult Sci 95: 237–246. [DOI] [PubMed] [Google Scholar]
- Mordente A, Guantario B, Meucci E, Silvestrini A, Lombardi E, Martorana GE et al. (2011). Lycopene and cardiovascular diseases: An update. Curr Med Chem 18: 1146–1163. [DOI] [PubMed] [Google Scholar]
- Moshetova LK, Vorob'eva IV, Alekseev IB, Mikhaleva LG (2015). Results of the use of antioxidant and angioprotective agents in type 2 diabetes patients with diabetic retinopathy and age‐rrelated macular degeneration. Vestn Oftalmol 131: 34–40. [DOI] [PubMed] [Google Scholar]
- Muller L, Caris‐Veyrat C, Lowe G, Bohm V (2016). Lycopene and its antioxidant role in the prevention of cardiovascular diseases‐ A critical review. Crit Rev Food Sci Nutr 56: 1868–1879. [DOI] [PubMed] [Google Scholar]
- Muzandu K, Ishizuka M, Sakamoto KQ, Shaban Z, El Bohi K, Kazusaka A et al. (2006). Effect of lycopene and beta‐carotene on peroxynitrite‐mediated cellular modifications. Toxicol Appl Pharmacol 215: 330–340. [DOI] [PubMed] [Google Scholar]
- Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J et al. (2001). Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E‐cdk2 complexes. Oncogene 20: 3428–3436. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S et al. (2002). Curcumin down‐regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol 21: 825–830. [PubMed] [Google Scholar]
- Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU et al. (2010). Anti‐inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 648: 110–116. [DOI] [PubMed] [Google Scholar]
- Niranjana R, Gayathri R, Nimish Mol S, Sugawara T, Hirata T, Miyashita K et al. (2015). Carotenoids modulate the hallmarks of cancer cells. J Funct Foods 18: 968–985. [Google Scholar]
- Nishi K, Muranaka A, Nishimoto S, Kadotac A, Sugahara T (2012). Immunostimulatory effect of β‐cryptoxanthin in vitro and in vivo . J Funct Foods 4: 618–625. [Google Scholar]
- Nishida K, Sugimoto M, Ikeda S, Kume S (2014). Effects of supplemental β‐carotene on mucosal IgA induction in the Jejunum and Ileum of mice after weaning. Br J Nutr 111: 247–253. [DOI] [PubMed] [Google Scholar]
- Noorbala AA, Akhondzadeh S, Tahmacebi‐Pour N, Jamshidi AH (2005). Hydro‐alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double‐blind, randomized pilot trial. J Ethnopharmacol 97: 281–284. [DOI] [PubMed] [Google Scholar]
- Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H et al. (2007). Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo . Biochim Biophys Acta 1770: 578–584. [DOI] [PubMed] [Google Scholar]
- Oh J, Kim JH, Park JG, Yi YS, Park KW, Rho HS et al. (2013). Radical scavenging activity‐based and AP‐1‐targeted anti‐inflammatory effects of lutein in macrophage‐like and skin keratinocytic cells. Hindawi Publishing Corporation, Mediators of Inflammation: 1–8. [DOI] [PMC free article] [PubMed]
- Okai Y, Higashi‐Okai K (1996). Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. Int J Immunogenet 18: 753–758. [DOI] [PubMed] [Google Scholar]
- Olmos J, Gómez R, Rubio V (2015). Apoptosis comparison effects between synthetic and natural β‐Carotene from Dunaliella salina on MDA‐MB‐231 breast cancer cells. J Microb Biochem Technol 7: 051–056. [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A et al. (1996). Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334: 1150–1155. [DOI] [PubMed] [Google Scholar]
- Ono M, Takeshima M, Nakano S (2015). Mechanism of the anticancer effect of lycopene (Tetraterpenoids). Enzymes 37: 139–166. [DOI] [PubMed] [Google Scholar]
- Palozza P, Calviello G, Serini S, Maggiano N, Lanza P, Ranelletti FO et al. (2001). β‐carotene at high concentrations induces apoptosis by enhancing oxy‐radical production in human adenocarcinoma cells. Free Radic Biol Med 30: 1000–1007. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Maggiano N, Angelini M, Boninsegna A, Di Nicuolo F et al. (2002). Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by β‐Carotene through down‐regulation of cyclin A and Bcl‐2 family proteins. Carcinogenesis 23: 11–18. [DOI] [PubMed] [Google Scholar]
- Pan W, Yang H, Cao C, Song X, Wallin B, Kivlin R et al. (2008). AMPK mediates curcumin‐induced cell death in CaOV3 ovarian cancer cells. Oncol Rep 20: 1553–1559. [PubMed] [Google Scholar]
- Panic N, Nedovic D, Pastorino R, Boccia S, Leoncini E (2016). Carotenoid intake from natural sources and colorectal cancer: a systematic review and meta‐analysis of epidemiological studies. Eur J Cancer Prev. (In press) [DOI] [PubMed] [Google Scholar]
- Park JS, Chyun JH, Kim YK, Line LL, Chew BP (2010). Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Mathison BD, Hayek MG, Massimino S, Reinhart GA, Chew BP et al. (2011). Astaxanthin stimulates cell‐mediated and humoral immune responses in cats. Vet Immunol Immunopathol 144: 455–461. [DOI] [PubMed] [Google Scholar]
- Parsons HA, Baracos VE, Hong DS, Abbruzzese J, Bruera E, Kurzrock R (2016). The effects of curcumin (Diferuloylmethane) on body composition of patients with advanced pancreatic cancer. Oncotarget. doi:10.18632/oncotarget.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkow FJ, Watumull DG, Campbell CL (2008). Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol 101: S58–S68. [DOI] [PubMed] [Google Scholar]
- Pechinskii SV, Kuregyan AG (2014). Molecular‐biological problems of drug design and mechanism of drug action. Pharmaceut Chem J 47: 509–513. [Google Scholar]
- Petyaev IM (2016). Lycopene deficiency in aging and cardiovascular disease. Hindawi publishing corporation Oxidative medicine and cellular longevity: 1–6. [DOI] [PMC free article] [PubMed]
- Preet R, Mohapatra P, Das D, Satapathy SR, Choudhuri T, Wyatt MD et al. (2013). Lycopene synergistically enhances quinacrine action to inhibit wnt‐TCF signaling in breast cancer cells through APC. Carcinogenesis 34: 277–286. [DOI] [PubMed] [Google Scholar]
- Promphet P, Bunarsa S, Sutheerawattananonda M, Kunthalert D (2014). Immune enhancement activities of silk lutein extract from Bombyx mori cocoons. Biol Res 47: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi MM, Yadav PN, Reyes M (2007). Lycopene inhibits LPS‐induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J Food Sci 72: S69–S74. [DOI] [PubMed] [Google Scholar]
- Rajput N, Naeem M, Ali S, Zhang JF, Zhang L, Wang T (2013). The effect of dietary supplementation with the natural carotenoids curcumin and lutein on broiler pigmentation and immunity. Poult Sci 92: 1177–1185. [DOI] [PubMed] [Google Scholar]
- Rao AV, Rao LG (2007). Carotenoids and human health. Pharmacol Res 55: 207–216. [DOI] [PubMed] [Google Scholar]
- Reddy RC, Vatsala PG, Keshamouni VG, Padmanaban G, Rangarajan PN (2005). Curcumin for malaria therapy. Biochem Biophys Res Commun 326: 472–474. [DOI] [PubMed] [Google Scholar]
- Ribaya‐Mercado JD, Blumberg JB (2004). Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr 23: 567S–587S. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL (2005). A potential role of the curry spice curcumin in alzheimer's disease. Curr Alzheimer Res 2: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen TH, Voutilainen S, Nyyssonen K, Salonen R, Kaplan GA, Salonen JT (2003). Serum lycopene concentrations and carotid atherosclerosis: The kuopio ischaemic heart disease risk factor study. Am J Clin Nutr 77: 133–138. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Green J, Lewis B (2009). Lutein and zeaxanthin in eye and skin health. Clin Dermatol 27: 195–201. [DOI] [PubMed] [Google Scholar]
- Rutz JK, Borges CD, Zambiazi RC, da Rosa CG, da Silva MM (2016). Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem 202: 324–333. [DOI] [PubMed] [Google Scholar]
- Sahin K, Tuzcu M, Sahin N, Akdemir F, Ozercan I, Bayraktar S et al. (2011). Inhibitory effects of combination of lycopene and genistein on 7,12‐dimethyl Benz(a)anthracene‐induced breast cancer in rats. Nutr Cancer 63: 1279–1286. [DOI] [PubMed] [Google Scholar]
- Saini RK, Nile SH, Park W (2015). Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int 76: 735–750. [DOI] [PubMed] [Google Scholar]
- Satia JA, Littman A, Slatore CG, Galanko JA, White E (2009). Long‐term use of beta‐carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the vitamins and lifestyle (vital) study. Am J Epidemiol 169: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedhelm E, Maas R, Troost R, Böger RH (2003). Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin Pharmacokinet 42: 437–459. [DOI] [PubMed] [Google Scholar]
- Schweigert F, Britton G, Liaaen‐Jensen S, Pfander H (1998). Metabolism of carotenoids in mammals. Carotenoids Biosynthesis Metabol 3: 249–284. [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC et al. (1994). Dietary carotenoids, vitamins A, C, and E, and advanced age‐related macular degeneration. Eye Disease Case–Control Study Group Jama 272: 1413–1420. [PubMed] [Google Scholar]
- Sedjo RL, Roe DJ, Abrahamsen M, Harris RB, Craft N, Baldwin S et al. (2002). Vitamin A, carotenoids, and risk of persistent oncogenic human papillomavirus infection. Cancer Epidemiol Biomarkers Prev 11: 876–884. [PubMed] [Google Scholar]
- Seifter E, Rettura G, Levenson SM (1981). Carotenoids and cell mediated immune responses In: The Quality of Foods and Beverages, Chemistry and Technology, Vol. 2 Academic Press: New York, pp. 335–347. [Google Scholar]
- Seyedzadeh MH, Safari Z, Zare A, Gholizadeh Navashenaq J, Razavi SA, Kardar GA et al. (2014). Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int Immunopharmacol 22: 230–235. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S et al. (2006). Modulation of nuclear factor E2‐related factor 2‐mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther 5: 39–51. [DOI] [PubMed] [Google Scholar]
- Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS (2008). Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun 374: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siems W, Wiswedel I, Salerno C, Crifo C, Augustin W, Schild L et al. (2005). β‐Carotene breakdown products may impair mitochondrial functions‐potential side effects of high‐dose β‐carotene supplementation. J Nutr Biochem 16: 385–397. [DOI] [PubMed] [Google Scholar]
- Simone RE, Russo M, Catalano A, Monego G, Froehlich K, Boehm V et al. (2011). Lycopene inhibits NF‐kB‐mediated IL‐8 expression and changes redox and PPARc signalling in cigarette smoke‐stimulated macrophages. PLoS One 6: e19652. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Soontornchaiboon W, Joo SS, Kim SM (2012). Anti‐inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol Pharm Bull 35: 1137–1144. [DOI] [PubMed] [Google Scholar]
- South EH, Exon JH, Hendrix K (1997). Dietary curcumin enhances antibody response in rats. Immunopharmacol Immunotoxicol 19: 105–119. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza L, Pesce M, Patruno A, Franceschelli S, de Lutiis MA, Grilli A et al. (2012). Astaxanthin treatment reduced oxidative induced pro‐inflammatory cytokines secretion in U937: SHP‐1 as a novel biological target. Mar Drugs 10: 890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Ahmed H, Dixit RK, Dharamveer , Saraf SA (2010). Crocus sativus L.: A comprehensive review. Pharmacol Rev 4: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RM, Singh S, Dubey SK, Misra K, Khar A (2011). Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol 11: 331–341. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H (2005). Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta 1740: 101–107. [DOI] [PubMed] [Google Scholar]
- Sun W, Xing L, Lin H, Leng K, Zhai Y, Liu X (2016). Assessment and comparison of in vitro immunoregulatory activity of three astaxanthin stereoisomers. J Ocean Univ China 15: 283–287. [Google Scholar]
- Sup Shim J, Hoon Kim D, Jung HJ, Hee Kim J, Lim D, Lee SK et al. (2002). Hydrazinocurcumin, a novel synthetic curcumin derivative, is a potent inhibitor of endothelial cell proliferation. Bioorg Med Chem 10: 2439–2444. [DOI] [PubMed] [Google Scholar]
- Takeda A, Nyssen OP, Syed A, Jansen E, Bueno‐de‐Mesquita B, Gallo V (2014). Vitamin A and carotenoids and the risk of Parkinson's disease: a systematic review and meta‐analysis. Neuroepidemiology 42: 25–38. [DOI] [PubMed] [Google Scholar]
- Talaei A, Hassanpour Moghadam M, Sajadi Tabassi SA, Mohajeri SA (2015). Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double‐blind, placebo‐controlled, pilot clinical trial. J Affect Disord 174: 51–56. [DOI] [PubMed] [Google Scholar]
- Tang L, Jin T, Zeng X, Wang JS (2005). Lycopene inhibits the growth of human androgen‐independent prostate cancer cells in vitro and in BALB/c nude mice. J Nutr 135: 287–290. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Townsend DM, Tew KD (2004). The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother 58: 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavani A, La Vecchia C (1999). β‐carotene and risk of coronary heart disease: A review of observational and intervention studies. Biomed Pharmacother 53: 409–416. [DOI] [PubMed] [Google Scholar]
- Thorne‐Lyman AL, Fawzi WW (2012). Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta‐analysis. Paediatr Perinat Epidemiol 26: 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo PR (2005). Are there adverse effects of lycopene exposure? J Nutr 135: 2060S–2061S. [DOI] [PubMed] [Google Scholar]
- Umar S, Shah MA, Munir MT, Yaqoob M, Fiaz M, Anjum S et al. (2016). Synergistic effects of thymoquinone and curcumin on immune response and anti‐viral activity against avian influenza virus (H9N2) in turkeys. Poult Sci pii: pew069. [DOI] [PubMed] [Google Scholar]
- Upadhyaya KR, Radha KS, Madhyastha HK (2007). Cell cycle regulation and induction of apoptosis by β‐carotene in U937 and HL‐60 leukemia cells. J Biochem Mol Biol 40: 1009–1015. [DOI] [PubMed] [Google Scholar]
- Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H (2014). Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. DARU J Pharmaceut Sci 22: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van het Hof KH, de Boer BC, Tijburg LB, Lucius BR, Zijp I, West CE et al. (2000). Carotenoid bioavailability in humans from tomatoes processed in different ways determined from the carotenoid response in the triglyceride‐rich lipoprotein fraction of plasma after a single consumption and in plasma after four days of consumption. J Nutr 130: 1189–1196. [DOI] [PubMed] [Google Scholar]
- van Poppel G (1996). Epidemiological evidence for β‐carotene in prevention of cancer and cardiovascular disease. Eur J Clin Nutr 50: S57–S61. [PubMed] [Google Scholar]
- Varalakshmi C, Mubarak Ali A, Pardhasaradhi BVV, Srivastava RM, Singh S, Khar A (2008). Immunomodulatory effects of curcumin: In vivo . Int Immunopharmacol 8: 688–700. [DOI] [PubMed] [Google Scholar]
- Vaughn AR, Branum A, Sivamani RK (2016). Effects of turmeric (Curcuma longa) on skin health: A systematic review of the clinical evidence. Phytother Res 30: 1243–1264. [DOI] [PubMed] [Google Scholar]
- Vijayapadma V, Ramyaa P, Pavithra D, Krishnaswamy R (2014). Protective effect of lutein against benzo(a)pyrene‐induced oxidative stress in human erythrocytes. Toxicol Ind Health 30: 284–293. [DOI] [PubMed] [Google Scholar]
- Voutilainen S, Nurmi T, Mursu J, Rissanen TH (2006). Carotenoids and cardiovascular health. Am J Clin Nutr 83: 1265–1271. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui R, Xiao Y, Fang J, Xu Q (2015). Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose–response meta‐analysis of observational studies. PLoS One 10: e0137427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RR, Prabhala RH, Plzia PM, Alberts DS (1991). Effects of β‐carotene on lymphocyte subpopulations in elderly humans: Evidence for a dose–response relationship. Am J Clin Nutr 53: 90–94. [DOI] [PubMed] [Google Scholar]
- Watzl B, Bub A, Brandstetter BR, Rechkemmer G (1999). Modulation of human T‐lymphocyte functions by the consumption of carotenoid‐rich vegetables. Br J Nutr 82: 383–389. [DOI] [PubMed] [Google Scholar]
- Weihong S, Lihong X, Hong L, Kailiang L, Yuxiu Z, Xiaofang L (2016). Assessment and comparison of in vitro immunoregulatory activity of three astaxanthin stereoisomers. J Ocean Univ China (Oceanic and Coastal Sea Research) 15: 283–287. [Google Scholar]
- Wilken R, Veena MS, Wang MB, Srivatsan ES (2011). Curcumin: a review of anti‐cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 10: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongcharoen W, Phrommintikul A (2009). The protective role of curcumin in cardiovascular diseases. Int J Cardiol 133: 145–151. [DOI] [PubMed] [Google Scholar]
- Wood SM, Beckham C, Yosioka A, Darban H, Watson RR (2000). Carotene and selenium supplementation enhances immune responses in aged humans. Intern Med 2: 85–92. [DOI] [PubMed] [Google Scholar]
- Xu XR, Zou ZY, Xiao X, Huang YM, Wang X, Lin XM (2013). Effects of lutein supplement on serum inflammatory cytokines, apoE and lipid profiles in early atherosclerosis population. J Atheroscler Thromb 20: 170–177. [DOI] [PubMed] [Google Scholar]
- Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK (2005). Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol 27: 485–497. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hasegawa I, Yahagi N, Ishigaki Y, Akano F, Ohta T (2010). Carotenoids modulate cytokine production in peyer's patch cells Ex vivo . J Agric Food Chem 58: 8566–8572. [DOI] [PubMed] [Google Scholar]
- Yang R, Tan X, Thomas AM, Shen J, Qureshi N, Morrison DC et al. (2006). Crocetin inhibits mRNA expression for tumor necrosis factor‐alpha, interleukin‐1beta, and inducible nitric oxide synthase in hemorrhagic shock. J Parenter Enteral Nutr 30: 297–301. [DOI] [PubMed] [Google Scholar]
- Yang DJ, Lin JT, Chen YC, Liu SC, Lu FJ, Chang TJ et al. (2013). Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS‐stimulated pro‐inflammatory mediators in RAW264.7 cells via NF‐ĸB and JNK inactivation. J Funct Foods 5: 607–615. [Google Scholar]
- Yasui Y, Hosokawa M, Mikami N, Miyashita K, Tanaka T (2011). Dietary astaxanthin inhibits colitis and colitis‐associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem Biol Interact 193: 79–87. [DOI] [PubMed] [Google Scholar]
- Youn HS, Saitoh SI, Miyake K, Hwang DH (2006). Inhibition of homodimerization of Toll‐like receptor 4 by curcumin. Biochem Pharmacol 72: 62–69. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE et al. (2007). Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta 1773: 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Zhang Q, Joe Y, Lee BH, Ryu DG, Kwon KB et al. (2013). Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. Int Immunopharmacol 15: 517–523. [DOI] [PubMed] [Google Scholar]
- Zhou GZ, Zhang SN, Zhang L, Sun GC, Chen XB (2014). A synthetic curcumin derivative hydrazinobenzoylcurcumin induces autophagy in A549 lung cancer cells. Pharm Biol 52: 111–116. [DOI] [PubMed] [Google Scholar]


