Abstract
Anthocyanins are a class of water‐soluble flavonoids, which show a range of pharmacological effects, such as prevention of cardiovascular disease, obesity control and antitumour activity. Their potential antitumour effects are reported to be based on a wide variety of biological activities including antioxidant; anti‐inflammation; anti‐mutagenesis; induction of differentiation; inhibiting proliferation by modulating signal transduction pathways, inducing cell cycle arrest and stimulating apoptosis or autophagy of cancer cells; anti‐invasion; anti‐metastasis; reversing drug resistance of cancer cells and increasing their sensitivity to chemotherapy. In this review, the latest progress on the anticancer activities of anthocyanins and the underlying molecular mechanisms is summarized using data from basic research in vitro and in vivo, from clinical trials and taking into account theory and practice.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- ABC
ATP‐binding cassette
- AP‐1
activator protein‐1
- APC
adenomatous polyposis coli
- ARE
antioxidant response element
- Atg5
autophagy‐related gene 5
- BCRP
breast cancer resistance protein
- cAMP
cyclic AMP
- C‐3‐G
cyanidin‐3‐glucoside
- Cy‐g
cyanidin‐3‐O‐β‐glucopyranoside
- FDGP
freeze‐dried grape powder
- HCC
hepatocellular carcinoma cells
- iNOS
inducible NO synthase
- 3‐MA
3‐methyladenine
- MEK
MAPK/ERK kinase
- 8‐OHdG
8‐hydroxydeoxyguanosine
- P‐gp
P‐glycoprotein
- PMBE
Pinus massoniana bark extract
- RTK
receptor TK
- siRNA
small interfering RNA
- TG
Thapsigargin
- TPA
12‐O‐tetradecanoylphorbol‐13‐acetate
- YGM‐3
3‐(6,6′‐caffeylferulylsophoroside)‐5‐glucoside of cyanidin
- YGM‐6
3‐(6,6′‐caffeylferulylsophoroside)‐5‐glucoside of peonidin
Tables of Links
| TARGETS | ||
|---|---|---|
| Other protein targets a | Enzymes e | MMP2 |
| Bcl‐2 | Akt (PKB) | MMP9 |
| TNF‐α | Caspase family | MPO |
| Catalytic receptors b | CDK1 subfamily | PI3K |
| PDGFR | COX‐2 | PKA |
| VEGFR | EGFR | p38 MAPK |
| Nuclear hormone receptors c | ERK1 | Raf |
| AP‐1 | ERK2 | uPA |
| Transporters d | iNOS | |
| ABCB1, P‐gp | JNK1 | |
| ABCG2 | MEK1 |
| LIGANDS | |
|---|---|
| cAMP | Nitric oxide (NO) |
| EGF | PGE2 |
| IFN‐γ | Thapsigargin |
| Keap1 | TPA |
| LPS |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
Anthocyanins exist widely in plants and are the most abundant flavonoids. Anthocyanins are responsible for the varied colours of flowers and fruit that change with the change of seasons and are cell vacuole components. For example, strawberries, grapes, apples, purple cauliflower and corn can present a red, blue or purple colour respectively (Cooper‐Driver, 2001). To date, the number of reported types of anthocyanins exceeds 500, and these different anthocyanins can be detected in 27 families and 72 genera of plants (Sarma et al., 1997). Research since the 1980s has mainly focused on their extraction (Pace et al., 2014; Carbonaro et al., 2015), isolation (Correa‐Betanzo et al., 2014), purification and absorption (McGhie and Walton, 2007), metabolism (Jian and Giusti, 2010), bioavailability (Fernandes et al., 2014), pharmacokinetics (Kay, 2006; Semaming et al., 2015) and their analytical techniques (Welch et al., 2008). On the basis of studies in cell lines from the gastrointestinal tract, such as the intestines (Li et al., 2011), oesophageal, stomach, colorectal, liver, breast, cervical and prostate cancers (Bowen‐Forbes et al., 2010; Rugina et al., 2012; Hafidh et al., 2013; Jin et al., 2013; Li et al., 2014; Bishayee et al., 2016), animal models (Jiang et al., 2014) and human clinical trials (https://clinicaltrials.gov), anthocyanins have antioxidant (He and Giusti, 2010), bacteriostatic, anti‐inflammation, anti‐ageing and anticancer functions (Bagchi et al., 2004; Cui and Li, 2014; Chen et al., 2016b). They can be applied for the prevention of cardiovascular disease (Alvarez‐Suarez et al., 2014; Cerletti et al., 2016), obesity control (Wu et al., 2013), the alleviation of diabetes (Li et al., 2015a) and cancer therapy (Bobe et al., 2006). Here, we summarize briefly the possible antitumour or anticancer roles of anthocyanins in the different stages of tumourigenesis and carcinogenesis, highlighting their sources, structural characteristics and health effects, and focusing on their pharmacological aspects in the prevention and treatment of cancer in vitro and in vivo.
The chemical structure and characteristics of anthocyanins
Anthocyanin is the common name for a class of flavonoids that are easily dissolved in water. Their basic structural unit is 2‐phenylchromenylium (flavylium) (Figure 1) (Hou et al., 2004; Jing and Giusti, 2010). They exist in natural products, mainly in a form combined with glucose, galactose and rhamnose (Liu et al., 2010), and can be divided into at least six common types, such as pelargonidin, cyanidin, delphinidin, peonidin, petunidin and malvidin, according to the different substituent groups on the flavylium B‐ring (Holton and Cornish, 1995). The research has indicated that the ortho‐dihydroxyphenyl structure on the B‐ring is the active site that inhibits tumour growth and metastasis (Hou et al., 2004; Xu et al., 2010). In recent years, because of increased health consciousness (Pascual‐Teresa, 2014), people have paid close attention to anthocyanins' roles in tumour prevention and cancer therapy because of their extensive sources, low cytotoxicity and safe consumption. This review summarizes the related experimental and clinical data at the cellular and molecular levels in vitro and in clinic trails in vivo, analyses the existing critical questions and provides directions for further research.
Figure 1.
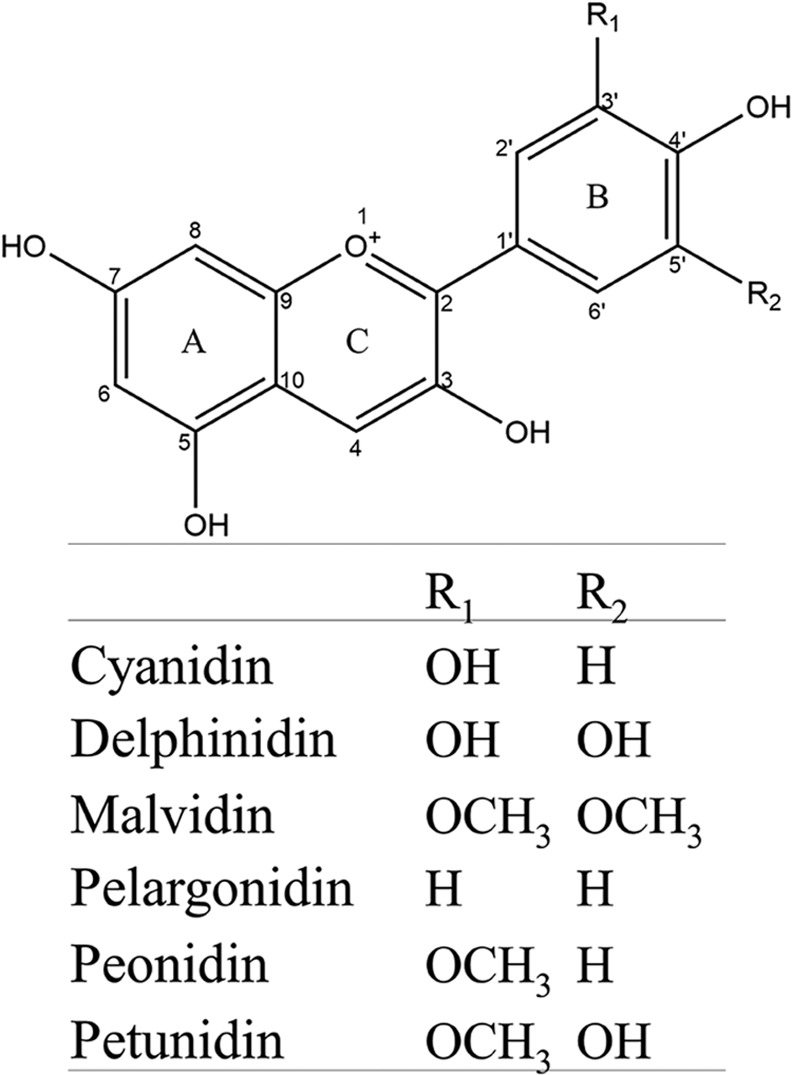
Chemical structure of the anthocyanidins most commonly found in foods.
The anti‐carcinogenic activities of anthocyanins in the initial stage of tumourigenesis
Antioxidant
Anthocyanins can act on the antioxidant system (Bowen‐Forbes et al., 2010; Li et al., 2015a), where they scavenge free radicals, thereby reducing damage to the genome of normal cells by oxidative stress and the subsequent malignant transformation by gene mutation, thus preventing the occurrence of tumours (Shih et al., 2007; Yi et al., 2010). Yi et al. (2010) found that the antioxidant effect of anthocyanins is determined by the 3′, 4′, 5′ hydroxyl on the B‐ring and the 3′ hydroxyl on the C‐ring. Shih et al. (2007) and Thoppil et al. (2012) found that anthocyanins (cyanidin, delphinidin and malvidin) could act on antioxidant response element (ARE) through the Keap1‐Nrf2 pathway and inhibit the activity of cysteinyl aspartate specific proteinase‐3 (caspase‐3) by regulating the expression of phase II antioxidases (glutathione reductase, glutathione peroxidase, glutathione transferase and quinone oxidoreductase), thus playing a role in antioxidant protection. In short, it is the promotion of the expressions of ARE‐regulated phase II enzymes by anthocyanins that defend normal cells against oxidative stress.
Anti‐inflammation
Chronic inflammation is often a harbinger of a tumour. The abnormal overexpression and secretion of inflammatory factors are critical to tumourigenesis. It is reported that anthocyanins can control the expression and secretion of inflammatory factors by inhibiting the transcription factor NF‐κB, through multiple pathways to exert their anti‐inflammatory function (Esposito et al., 2014; Peiffer et al., 2014). For example, cyanidin‐3‐glucoside (C‐3‐G), delphinidin‐3‐glucoside and petunidin‐3‐glucoside inhibit the activation of NF‐κB induced by external stimuli (e.g. LPS or IFN‐γ) by acting on the PI3K/PKB and MAPK pathways (Afaq et al., 2005; Limtrakul et al., 2015) and can inhibit the expression of COX‐2 and inducible NO synthase (iNOS), as well as the production of their products PGE2 and NO (Haseeb et al., 2013; Jeong et al., 2013; Peiffer et al., 2014). Miyake et al. (2012) and Burton et al. (2015) found that anthocyanins could also block the activation of STAT3 and inhibit the expression of NF‐κB.
Anti‐mutagenesis
During the transformation of normal cells towards cancer cells, somatic cell hypermutation can lead to instability of the genome and cause cancer (Martincorena and Campbell, 2015). Yoshimoto et al. (1999) used four different kinds of sweet potato root as experimental materials to investigate their anti‐mutation effect and found that Salmonella typhimurium TA98 presented reverse mutation under the action of a heterocyclic mutagen, while adding four different kinds of sweet potato root, whose main ingredients are 3‐(6,6′‐caffeylferulylsophoroside)‐5‐glucoside of cyanidin (YGM‐3) and 3‐(6,6′‐caffeylferulylsophoroside)‐5‐glucoside of peonidin (YGM‐6), could inhibit the reverse mutation of TA98 in a dose‐dependent manner. Thus, it was concluded that YGM‐3 and YGM‐6 could inhibit the reverse mutation of normal cells induced by a mutagen. Oxidative stress from free radical abnormalities can lead to DNA injury and mutation of related genes – oncogenes and anti‐oncogenes – resulting in carcinogenesis and finally causing cancer. Therefore, anthocyanins with antioxidant properties may protect human cells from malignant mutation from extreme levels of ROS and free radicals by inhibiting point mutations, thereby exerting their anti‐mutagenesis effects in human somatic cells.
The anti‐carcinogenic activities of anthocyanins in the cancer formation stage
Differentiation induction
Differentiation induction is a phenomenon whereby malignant cells differentiate towards normal and mature cells under the effect of differentiation inducers. A large number of malignant cells undergo mitosis, and these cells are poorly differentiated (Charepalli et al., 2015; Thwe et al., 2016). Anthocyanins can induce terminal differentiation of tumour cells and block tumourigenesis. Through detecting the markers and kinase inhibitors in the cell differentiation process, Fimognari et al. (2004) found that cyanidin‐3‐O‐β‐glucopyranoside (Cy‐g) could induce the differentiation of human acute promyelocytic leukaemia cell line HL‐60 in a dose‐dependent way by activating PI3K and PKC. Under treatment by Cy‐g (200 mg·mL−1), HL‐60 cells presented differentiation characteristics, such as increased adhesion and enhanced activity of esterase, and the expression of oncogene c‐Myc was decreased. However, following treatment by PI3K and PKC inhibitors, the effect of Cy‐g to induce the differentiation of HL‐60 was significantly reduced. Serafino et al. (2004) found that Cy‐g could induce the differentiation of melanoma cell line TVM‐A12 by up‐regulating cAMP levels, and the expressions of tyrosinase and the differentiation marker MART‐1. Liu‐Smith and Meyskens recently validated Cy‐g's effects on the induction of melanoma cell differentiation (Liu‐Smith and Meyskens, 2016). To some extent, the degree of differentiation determines the degree of tumour malignancy, and anthocyanins might play roles in the cancer formation stage by inducing differentiation, further determining the size of final tumour and its malignancy.
Inhibiting cellular transformation
Cellular transformation is one of the mechanisms underlying tumourigenesis. Some carcinogens, such as 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) and EGF, can induce the transformation of various cell lines through the transcription factors AP‐1 and NF‐κB in the Raf‐MEK‐ERK and PI3K/Akt pathways (Burton et al., 2015). Limtrakul et al. (2015) found that black rice whole grain extracts might suppress LPS‐induced inflammation via inhibition of the MAPK signalling pathway, leading to decreased NF‐κB and AP‐1 translocation. In addition, inflammation also has an important relationship with cellular transformation, and high expression of COX‐2 and PGE2 can enhance the tumorigenic effect (Hou et al., 2004). Anthocyanins can act on Ras‐ERK and PI3K/Akt pathways by decreasing the expression of AP‐1 and thus inhibit the cellular transformation. Hou et al. (2004, 2005) found that delphinidin, cyanidin and petunidin could inhibit the transformation of mouse skin cell line JB6P+ induced by TPA. Kang et al. (2008) found that delphinidin could bind with Raf1 or MEK1 in an ATP‐non‐competitive way to inhibit the expression of AP‐1 and NF‐κB in JB6P+ cells treated with TPA and further inhibit the expression of COX‐2 and the production of PGE2. In addition, delphinidin could also weaken the TPA‐induced cellular transformation through the Ras/Raf/MEK/ERK pathway by regulating the phosphorylation level of MEK, ERK, ribosomal protein S6 kinase and mitogen stress activator protein kinase. Early experiments demonstrated that this effect is associated with the antioxidant ability of anthocyanins to scavenge superoxide radicals (Hou et al., 2004). Recently, Song et al. (2012) found that the 1,1‐diphenyl‐2‐picrylhydrazyl radical 2,2‐diphenyl‐1‐(2,4,6‐trinitrophenyl) hydrazyl and 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid) diammonium salt of cyanidin and C‐3‐G showed no significant difference in their free radical scavenging abilities; however, their abilities to inhibit cellular transformation are significantly different: cyanidin has a strong ability to inhibit transformation, while C‐3‐G has hardly any ability to inhibit transformation, which suggested that the effect of cyanidin to inhibit transformation is not associated with its antioxidant ability. Cyanidin can directly combine with PI3K in an ATP‐competitive way to inhibit the expression of AP‐1 and NF‐κB through the PI3K/Akt/p70S6 pathway and inhibits the cellular transformation of JB6P+ cells by treatment with EGF.
Inhibiting cell proliferation
A significant characteristic of cancer cells is their uncontrolled cell cycle, which leads to continuous division and proliferation (Lee et al., 2010; Li et al., 2016a). Anthocyanins can selectively inhibit the proliferation of cancer cells but have little influence on the proliferation of normal cells (Malik et al., 2003). The main manifestations of anthocyanins' inhibition of the growth and proliferation of cancer cells are summarized below from three aspects.
Inhibition of signalling pathways to block signal transduction
Syed et al. (2008) found that delphinidin could inhibit the hepatocyte growth factor‐induced phosphorylation and activation of hepatocyte growth factor receptors on human normal mammary cell line MCF‐10 A and could block the Ras–ERK MAPK and PI3K/Akt pathways. Teller et al. (2009b) found that anthocyanins could also inhibit the autophosphorylation of receptor TKs (RTKs) extensively in cancer cells, and the inhibitory effect on oncogene ErbB3 was the most effective. Anthocyanins, especially malvidin, could inhibit the activity of PDE and the hydrolysis of cAMP effectively in human colon cancer HT29 cells and thus inhibit the MAPK signalling pathway (Marko et al., 2004). In short, anthocyanins can inhibit the growth and proliferation of cancer cells by inhibiting different kinase signalling pathways in vitro.
Regulating the expression of anti‐oncogenes and relevant proteins
Malik et al. (2003) and Ha et al. (2015) found that in addition to up‐regulating p53 in colon and prostate cancer cells to activate the DNA repair system, anthocyanins could also initiate the transcription of p21 and p27. p21, a broad‐spectrum inhibitor of cyclin‐dependent kinases (CDKs), can combine with CDKs and inhibit their activity to induce the cell cycle arrest of cancer cells. Anwar et al. (2016) found that a standardized berry anthocyanin rich extract inhibited the proliferation of Caco‐2 cells by up‐regulating the expression of p21Waf/Cif1, arresting their cell cycle, and further inducing them to undergo apoptosis by caspase‐3 activation. Meanwhile, anthocyanins can also down‐regulate the expressions of CDK‐1 and CDK‐2, inhibit the expressions of cyclin‐B, cyclin‐A and cyclin‐E, promote the expressions of CDK inhibitors (CDKIs) and induce cancer cells to arrest at the G0/G1 and G2/M stages (Chen et al., 2005). Thus, anthocyanins can inhibit cancer cell proliferation mainly by arresting the cell cycle at different division phases via up‐regulating the expressions of anti‐oncogenes and down‐regulating the expressions of oncogenes, accompanied by the expressions of different cyclins and their partners CDKs and/or CDKIs.
Other signalling pathways
Kausar et al. (2012) found that berry anthocyanidins could act on the β‐catenin, Wnt and Notch pathways, as well as their downstream target proteins, to inhibit the growth and proliferation of human non‐small‐cell lung cancer cells synergistically.
Overall, tumours and cancer are referred to as signal pathway diseases, and this concept helps researchers to broaden their research and develop safer, targeted chemopreventive and/or chemotherapeutic treatments, including anthocyanins and other natural products.
The anti‐carcinogenic activities of anthocyanins in the cancer development stage
Inducing apoptosis of tumour cells
Malignantly transformed cells display uncontrollable growth, and their excessive proliferation leads to the formation of a tumour (Četojević‐Simin et al., 2015). Apoptosis of tumour cells is inhibited; therefore, dying cells cannot be eliminated normally (Naomi et al., 2003). Anthocyanins can induce the apoptosis of cancer cells through the internal mitochondrial pathway and the external death receptor pathway (Figure 2).
Figure 2.
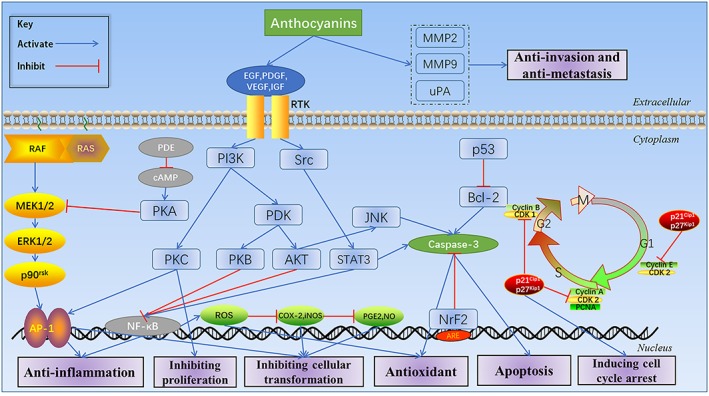
The main potential molecular mechanism of the antitumour effect of anthocyanins in vitro. Cancer cell growth might be inhibited by anthocyanidins through targeting RTKs (e.g. EGFR, PDGFR and VEGF/VEGFR) and acting on the Ras‐MAPK and PI3K/Akt signal cascade pathway. Inflammation might also be inhibited by anthocyanins through acting on the PI3K/Akt and NF‐κB pathway to suppress the expression of COX‐2 and iNOS and prevent cancer by regulating the expression of phase II antioxidant enzymes to achieve antioxidation through the Nrf2/ARE signalling system. During cancer initiation, anthocyanins might prevent malignant transformation by targeting the MAPK pathway and AP‐1 factor and by inhibiting RTK activity. Anthocyanins can initiate the expression of p21 and p27, whose products can combine with multiple cyclin‐CDKs to down‐regulate the expression of CDK‐1 and CDK‐2, further inhibiting the expression of cyclin‐B, cyclin−A and cyclin–E, which promote the expression of CDK inhibitors and induce cancer cells to arrest at the G0/G1 and G2/M stages. During cancer development, anthocyanins can induce apoptosis of cancer cells by activating caspases, mediated by ROS and JNK/p38‐MAPK. In addition, anthocyanins might exert their anti‐metastatic activities by targeting the VEGF signalling pathway and extracellular matrix degradation (via MMP2, MMP9, uPA).
The death receptor pathway
Huang et al. (2012) determined the phosphorylation levels of p38, p53, protein Fas and FasL after treatment with the p38 inhibitor SB203580 and demonstrated that mulberry anthocyanin could induce the apoptosis of gastric cancer cells through the external receptor p38/Fas/FasL/Caspase‐8 pathway. Chang et al. (2005) found that delphinidin could activate p38‐FasL and the pro‐apoptosis protein Bid pathway, thereby inducing the apoptosis of HL‐60 cells in a time‐dependent and dose‐dependent manner.
The mitochondrial signalling pathway
Mitochondria‐mediated apoptosis include the caspase‐dependent and caspase‐independent pathways.
Caspase‐dependent pathway
Anthocyanins can act on proteins of the Bcl family and the inhibitor of apoptosis protein family to activate the apoptosis response through the caspase‐dependent cascade (Shih et al., 2007; Lee et al., 2009; Charepalli et al., 2016).
Caspase‐independent pathway
Anthocyanins extracted from potato could induce mitochondria to release endonuclease G and apoptosis‐inducing factor through the JNK pathway to trigger caspase‐independent apoptosis of prostate cancer LNCaP and PC‐3 cell lines (Reddivari et al., 2007; Liu et al., 2016).
The endoplasmic reticulum signalling pathway
Yu et al. (2011) investigated the mechanism underlying the anti‐apoptotic effects of freeze‐dried grape powder (FDGP) on human hepatocarcinoma Huh7 cells. Pretreatment with FDGP could inhibit the thapsigargin (TG)‐induced expression of binding immunoglobulin protein/glucose‐regulated protein 78 and cytochrome c and increased the TG‐reduced expression of PCNA and caspase‐12. Caspase‐7 can be transported to the endoplasmic reticulum (ER) to react with caspase‐12 to induce the apoptosis of cancer cells. By contrast, the protein Bax is also abundant in the ER, and research has confirmed that anthocyanins can cause down‐regulation or up‐regulation of pro‐apoptotic proteins, such as Bax and Bcl‐2 homologous antagonist/killer (Bak) (Pal et al., 2013; Liu et al., 2016). Therefore, anthocyanins might achieve their functions via the ER signalling pathway.
The lysosome signalling pathway – cooperation with an autophagy inhibitor
Autophagy is a process whereby intracellular substances are degraded by the lysosome, which has a dual function in carcinogenesis: on the one hand, autophagy deficiency can promote malignant transformation and carcinogenesis; on the other hand, autophagy can restrict the necrosis and inflammation of a tumour, thereby relieving chromosome injury and the metabolic stress response in tumour cells. Anthocyanins can induce autophagy of cells in an autophagy‐related protein 5 (encoded by Atg5)‐dependent manner (Tsuyuki et al., 2012; Schiavano et al., 2015). Longo et al. (2008) found that after human hepatocellular carcinoma (HCC) cell line PLC/PRF/5 was treated with anthocyanins, the expressions of downstream Bcl‐2 family and rapamycin target proteins were down‐regulated, while the expressions of eukaryotic initiation factor 2α and autophagy related gene LC3‐II were up‐regulated, which all suggested that anthocyanins can induce autophagy of human HCC cells. Using small interfering RNA (siRNA) to interfere to silence the Atg5 gene or using autophagy inhibitor 3‐methyladenine (3‐MA) to act on HCC cells treated with anthocyanins causes transfer of apoptin Bax into mitochondria, the release of cytochrome c and cleavage of caspase‐3, leading to the apoptosis cascade response. Feng et al. (2010) found that cyanidin‐3‐rutinoside and delphinidin could not induce the apoptosis of HCC cells and could only cause growth retardation of cells via vacuolisation, which could be inhibited by the type III PI3K inhibitor 3‐MA and proton pump inhibitors that blocked lysosome degradation. After using 3‐MA and siRNA to interfere with autophagy marker LC3 and using delphinidin to treat human HCC SMMC7721 cells, delphinidin could induce autophagy of SMMC7721 cells. In addition, 3‐MA and delphinidin acting together on SMMC7721 cells could lead to significant cell death.
Inhibiting angiogenesis of tumours
Tumour angiogenesis is a restrictive condition for the growth and metastasis of malignant tumours. The process of angiogenesis is controlled by many cytokines, of which the most important positive regulatory factor is VEGF. Therefore, inhibiting the receptor of angiogenesis VEGF receptor (VEGFR) could inhibit the metastasis of tumours effectively (Chen et al., 2015). Anthocyanins can inhibit RTK extensively, and the inhibitory effect on VEGFR‐3 is especially significant (Teller et al., 2009a). Delphinidin and cyanidin could strongly inhibit the expression of VEGF in vascular smooth muscle cells induced by PDGF by blocking the p38‐MAPK and JNK pathways (Oak et al., 2006). The inhibitory effect of delphinidin on angiogenesis is associated with not only VEGF inhibition but also inhibition of the PDGFR‐β (Lamy et al., 2008). Delphinidin could inhibit PDGF induced phosphorylation of PDGFR‐β in pulmonary artery smooth muscle cells in a time‐dependent and dose‐dependent manner, thus inhibiting VEGF‐induced microvessel formation in HUVECs. Hypoxia is a general pathophysiological characteristic of solid tumours. It is likely to induce angiogenesis of the tumour, and this process is mainly completed via the VEGF signalling pathway, mediated by hypoxia inducible factor‐1α (HIF‐1α). Inhibiting the protein level of HIF‐1α could lead to decreased transcription activity of HIF‐1α target genes, including VEGF (Huang et al., 2011). Freeze‐dried blackberry anthocyanins could reduce the expression of HIF‐1α and VEGF in oesophagus tumour cells of F344 rats induced by N‐nitrosomemethylbenzylamine and thus inhibit angiogenesis of oesophageal tumours (Wang et al., 2009).
Inhibiting the invasion and metastasis of tumours
Invasion and metastasis are the two main aspects of cancer cells that threaten patients' health and life. Cyanidin could inhibit effectively the invasion and metastasis of breast cancer cells (BT474, MDA‐MB231 and MCF‐7) with high expression of ErbB2 by blocking the ErbB2/cSrc/FAK pathway (Xu et al., 2010; Li et al., 2016a). Syed et al. (2008) found that delphinidin could reduce the membrane translocation of PKCα and the phosphorylation of STAT3 in MCF‐10 A cell lines mediated by hepatocyte growth factor, inhibit the nuclear translocation of NF‐κB/p65 and thus inhibit the invasion of cells. Invasion and metastasis involve three main processes: adhesion, degradation and movement. Anthocyanins can act on some adhesion molecules and proteolytic enzymes to inhibit the adhesion and degradation of cells (Zhu et al., 2008; Mauray et al., 2012).
Adhesion
Chen et al. (2011) found that delphinidin could reduce the expression of cell adhesion molecule‐1 and P‐selectin induced by oxidized LDL through the ROS/p38‐MAPK/NF‐κB pathway in a dose‐dependent manner, thus inhibiting the adhesion of monocytes to endothelial cells.
Degradation
Uridylyl phosphate adenosine (uPA) and MMPs are two key components for extracellular matrix degradation, which can promote the invasion of tumour cells and increase their migration ability. Anthocyanins can target uPA and MMPs and thus play a role in inhibiting tumour metastasis. Delphinidin could influence the expression of the uPA receptor and LDL receptor related protein, as well as the production of plasmin, by interfering with the clearance of uPA‐plasmin activator inhibitor complex in malignant glioma U‐87 cells, thus inhibiting the invasion of U‐87 cells (Lamy et al., 2007). In MDA‐MB‐453 cells, the capacity for migration, adhesion, motility and invasion was inhibited by black rice anthocyanins in a concentration‐dependent manner, accompanied by decreased activity of a transfer promoting factor, urokinase‐type plasminogen activator (Luo et al., 2014). Ho et al. (2010) found that peonidin could reduce the expression of uPA in lung cancer cells by inhibiting the phosphorylation of ERK1/2 and thus inhibited the invasion of cancer cells. Delphinidin could target reduced nicotinamide adenine dinucleotide phosphate oxidase to inhibit the phosphorylation of MAPK‐JNK1/2, MKK3/6‐p38 and MEK‐ERK1/2 and thus inhibit the expression of MMP‐1 in human epidermal fibroblasts induced by UV‐B radiation (Lim et al., 2013b). In addition, cyanidin and pelargonidin could inhibit the expression of MMP‐2 and MMP‐9 by inhibiting the PI3K/Akt pathway in cancer cells and thus reducing the invasion of cancer cells (Shin et al., 2011; Kuntz et al., 2015). A study also found that Vitis coignetiae anthocyanins could inhibit the expression of MMP‐2 and MMP‐9 through inhibiting the activation of NF‐κB in cancer cells (Yun et al., 2010). In addition, it is reported that anthocyanins (including delphinidin‐3‐glucoside, cyaniding‐3‐glucoside and malvidin‐3‐glucoside) could inhibit the expression of the tight junction proteins, claudin‐1 and claudin‐3, by activating the p38‐MAPK pathway in human colon cancer HCT‐116 cells, thus inhibiting their invasion (Shin et al., 2011).
Reversal of multidrug resistance
Chemotherapy can inhibit proliferation or induce apoptosis of tumour cells by interfering with the DNA replication of tumour cells; however, multidrug resistance of tumour cells is a common reason for chemotherapy failure. A classical pathway for multidrug resistance of tumour cells is mediated by an ATP‐binding cassette (ABC) transmembrane protein superfamily, which mainly includes P‐glycoprotein (P‐gp), multidrug resistance associated protein and breast cancer resistance protein (BCRP). The ABC superfamily is highly expressed in the pharmacological barrier. Therefore, targeting these proteins to reverse multidrug resistance could be used to assist chemotherapy to treat cancer. The anthocyanins have MWs low enough to penetrate the blood–brain barrier (Andres‐Lacueva et al., 2005). A further study found that cyanidin could inhibit the expression of P‐gp in human epidermal carcinoma KB‐C2 cells that overexpressed P‐gp (Kitagawa, 2006). Anthocyanins have high affinity for BCRP, and among the anthocyanins determined, seven kinds (malvidin, petunidin, malvidin‐3‐galactoside, malvidin, cyaniding‐3‐galactoside, peonidin‐3‐glucoside and C‐3‐G) were potential substrates of BCRP, 12 types (cyanidin, peonidin, cyaniding‐3,5‐diglucoside, malvidin, pelargonidin, delphinidin, petunidin, delphinidin‐3‐glucoside, cyanidin‐3‐rutinoside, malvidin‐3‐glucoside, pelargonidin‐3,5‐diglucoside and malvidin‐3‐galactoside) were BCRP inhibitors and three kinds (malvidin, malvidin‐3‐galactoside and petunidin) demonstrated dual biological activity (Dreiseitel et al., 2009). In vitro, black rice anthocyanin could inhibit the activity of topoisomerase and the formation of topoisomerase‐DNA complex in colon cancer HT29 cells (Esselen et al., 2011). All these results suggested that anthocyanins might function to change pharmacokinetics and reverse multidrug resistance.
Experiments performed in different models
Animal models
In a BALB/c nude mice model that received a transplant of human breast cancer cell line MDA‐MB‐453, growth of cancer and the formation of its blood vessels were clearly inhibited by oral delivery of an anthocyanin‐rich extract from black rice (100 mg·kg−1 in the diet) (Chang et al., 2010). The study observed lower expressions of COX‐2, iNOS and c‐Jun, and angiogenic factors such as MMP‐9, MMP‐2 and uPA in tumour tissue (Chang et al., 2010). In a mouse model of adenomatous polyposis coli (APC) intestinal cancer, the number of mice that developed caecum cancer was reduced significantly (P < 0.05) (Lim et al., 2013a), compared with the control group, when they were fed with purple sweet potatoes rich in anthocyanins. Another mouse model of APC showed that mucosal expression of COX‐2 and cPLA2 was significantly decreased in the 0.5% anthocyanin‐rich extract group, by 32 and 62%, respectively, compared with the control group (Park et al., 2015). In a rat model of F344 colon cancer, the number of all tumours, which included glandular tumours and cancers, was reduced by 42, 45 and 71% compared with the control group, after a diet with 2.5, 5 and 10% freeze‐dried powders from black raspberries, and the 8‐hydroxydeoxyguanosine (8‐OHdG) level in urine was reduced by 73, 81 and 83% respectively (Kocic et al., 2011). Treatment with Hibiscus anthocyanins (HAs) caused reduction in the levels of aspartate transaminase, alanine transaminase, uric acid and myeloperoxidase. In addition, the results showed that oral administration of HAs (0.2%) × inhibited the progression of N‐nitrosomethylurea‐induced leukaemia by approximately 33.3% in rats (Tsai et al., 2014). Bishayee et al. (2011) tested the effects of black currant extract rich in anthocyanins on the prevention of liver cancer. Mice were fed with preventive anthocyanin for 4 weeks and fed for another 22 weeks after injecting diethylnitrosamine into the abdominal cavity to induce liver cancer. The result indicated that black currant anthocyanins could reduce the risk of abnormal proliferation before cancers formation, reduce the number of hyperplastic nodules and decrease in the sizes any nodules that did form. The effect was dose‐dependent, which was confirmed by later pathological examinations.
Human research
Epidemiological studies have demonstrated that consumption of fruit and vegetables rich in polyphenols is associated with reduced risk of colorectal cancer, while a recent study conducted by Nimptsch et al. (2016) did not support the hypothesis that a higher habitual intake of any flavonoid subclass decreases the risk of colorectal cancer. Although epidemiological studies do not show a lower risk of most human cancers after taking anthocyanins, anthocyanin intake might reduce oxidative damage (Wang and Stoner, 2008). DNA oxidative damage decreased in human subjects who had drunk a juice rich in anthocyanins and showed significant increases in reduced glutathione, compared with the control group (Weisel et al., 2006). Recently, Cerletti et al. (2016) summarized the results of two EU‐funded projects, which indicated that in breast cancer patients, moderate wine consumption may have a protective effect on skin toxicity induced by radiotherapy, attributing this effect to the possible antioxidant effect of the polyphenols contained in wine. In another study, the treatment group drank freeze‐dried powders from black raspberries every day, and after 6 months, 8‐iso‐PGF2 and 8‐OHdG levels were both decreased in their urine (Kresty et al., 2006). Urinary 8‐OHdG is considered a marker of total DNA damage in humans, while 8‐Iso‐PGF2, a prostaglandin‐like compound produced via COX‐independent enzymes, is used as a marker of lipid peroxidation. Both 8‐OHdG and 8‐Iso‐PGF2 are indicators of oxidative status in vivo (Kresty et al., 2006; Cocate et al., 2014). A randomized, placebo‐controlled, double blind, monocentric evaluation, including 300 patients, is reported in Table S1 of Cerletti et al.'s report in 2016 (ClinicalTrials.gov ID: NCT02195960). This trial is ongoing and will be completed in the coming months (Cerletti et al., 2016).
Pharmacokinetics
Yang et al. (2011) summarized the bioavailability, pharmacokinetics and metabolism of anthocyanins in their published review. De Ferrars et al. (2014) identified 17 13C‐labelled compounds in serum, which are metabolized to a structurally diverse range of metabolites that exhibit dynamic kinetic profiles. Research on the metabolites suggested that anthocyanins have longer enterohepatic recycling, which leads to prolonged residence time (Lila et al., 2016). Cerletti et al. (2016) also found three main metabolites in urine, namely, delphinidin‐3‐glucoside, C‐3‐G and cyanidin‐3‐(6‐malonylglucoside), reached a plateau level after the first week of ingestion of red orange juice. These compounds were considered markers of anthocyanin bioavailability in subsequent human studies. The peak plasma concentration of the four anthocyanins in black raspberries could be detected 2 h after taking oral black raspberries, whose elimination fits first‐order kinetics (Stoner et al., 2005). Four to eight hours after taking black raspberries, the anthocyanins are excreted through the urine in the form of complete anthocyanin or methyl derivatives. Experiments in vitro proved that anthocyanins in the range of 10− 6 to 10−4 M could inhibit the growth of malignant tumours, regulate cell signal transduction and induce apoptosis (Cooke et al., 2005; Wang and Stoner, 2008). When anthocyanins are taken, their concentration can reach a level of 10−8 M ~ 10−7 M or it can be far lower than that in vitro to have an anticancer effect (Yang et al., 2011). The bioavailability of anthocyanins is very low (<1% in plasma); however, their presence in their native form has been described recently in colonic tissues of patients, suggesting they can directly interact with colonic tissues (Núñez‐Sánchez et al., 2015). It remains unclear whether anthocyanins in experimental concentrations can play a role against cancer in the human body and whether they function as the original molecules or as metabolites.
In short, we have summarized the chemical structure of anthocyanins, their potential molecular mechanisms in tumour prevention and anticancer therapy, and the current research status of their pharmacological effects on both cell lines in vitro and animal model in vivo and in human clinic trails. Notably, their precursors, proanthocyanidins, share many of same functions with anthocyanins. Proanthocyanidins are molecules polymerized with flavan‐3‐ols (Chen et al., 2016a) and are extracted from flowers, fruit, leaves and barks of many plants. Proanthocyanidins, especially proanthocyanidin B from Pinus massoniana bark extract (PMBE), can scavenge free radicals and has antioxidant (Cui et al., 2005b), anti‐inflammation, antitumour and apoptosis induction and anti‐invasion effects at the cellular level in vitro (Cui et al., 2005a; Li et al., 2015b, 2016b) and in a mouse model in vivo (Li et al., 2007; Zhang et al., 2012). Animal experiments by Li et al. (2007) showed that Pinus koraiensis bark procyanidins extract has antitumour activity against U12 cervical cancer mice. Treatment with 100, 200 and 300 mg·kg−1 PMBE reduced the tumour weight and volume of S180‐bearing NIH mice by 9–67% and 13–68%, respectively (Zhang et al., 2012). In human trials, according to the No.NCT00100893 (https://clinicaltrials.gov), IH636 grape seed extract, which is rich in proanthocyanidins, has been used in a phase I clinical trial to study its side effects and the best dose to prevent breast cancer in postmenopausal women (40–75 years old) at risk of developing breast cancer.
Conclusions
The anticancer effect of anthocyanins differs according to the different substituents on their B‐rings. Anthocyanins with ortho‐dihydroxyphenyl on their B‐rings show the most obvious anticancer activity. Table 1 summarizes all the experimental data from cancer cell lines in vitro and animal models in vivo. Increasing evidence indicates that the main molecular mechanism of their antitumour effects lies in inhibiting the cancer cell growth and metastasis by targeting RTKs (EGFR, PDGFR and VEGF/VEGFR) by acting on Ras‐MAPK and PI3K/Akt signal cascade pathways. At the initial stage, anthocyanin inhibits inflammation by acting on the PI3K/Akt and NF‐κB pathway to suppress the expression of COX‐2 and iNOS, thus preventing normal cells from transformation by regulating the expression of phase II antioxidant enzymes to achieve antioxidation through Nrf2/ARE signal system. During the formation phase, anthocyanins prevent carcinogenesis by targeting the MAPK pathway and AP‐1 and by inhibiting RTK activity and its signal cascade pathway to regulate the expression of cancer‐related genes, which leads to cell cycle arrest and DNA repair. At the development stage, anthocyanins induce apoptosis of cancer cells by activating caspase, mediated by ROS and JNK/p38‐MAPK. In addition, anthocyanins inhibit cancer metastasis by targeting the VEGF signal pathway and extracellular matrix degradation. Furthermore, anthocyanins can reverse the multidrug resistance of cancer cells to improve their chemotherapy sensitivity. Figure 2 briefly summarizes the above related signal pathways.
Table 1.
Summary of the experimental data on the health effects of anthocyanins from cancer cell lines in vitro and from animal models in vivo
| Anthocyanins | Source | Properties | Effects | Laboratory model | Reference |
|---|---|---|---|---|---|
| Cyanidin | Seeds of corn | Antioxidant, anti‐carcinogenesis | 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP) clearly exerts promoting effects on 1,2‐dimethylhydrazine‐induced colorectal carcinogenesis; these can be reduced by 5.0% potential of purple corn colour in the diet, under the present experimental conditions | F344 rats treated with PhIP | (Hagiwara et al., 2001) |
| Sweetpotato | Antioxidative, anti‐mutagenic, and anti‐proliferative | The multiplication medium extract, which exhibited the highest radical scavenging activities and anti‐proliferation activities, contained the highest level of anthocyanins | Human leukaemia HL‐60 cells | (Konczak‐Islam et al., 2003) | |
| Blackberry | Synergistically or additively in producing anticancer effects | Anthocyanins and non‐anthocyanin phenolics in anthocyanin‐containing blackberry extracts act synergistically or additively in producing anticancer effects | Human colon cancer HT‐29, breast cancer MCF‐7 and leukaemia HL‐60 cells | (Dai et al., 2009) | |
| Black currant | Up‐regulation of Bax and down‐regulation of Bcl‐2 expression | Mechanistic studies revealed that the anthocyanin‐rich black currant skin extract‐mediated proapototic signal during experimental hepatocarcinogenesis might be propagated via the up‐regulation of Bax and down‐regulation of Bcl‐2 expression at the translational level | Rats treated with diethylnitrosamine and human liver cancer HepG2 cells | (Bishayee et al., 2011) | |
| Chokeberry | Antioxidant, anti‐proliferative | Cyanidin glycosides inhibited HeLa human cervical tumour cell proliferation and increased the generation of reactive oxygen species after 48 h of treatment, suggesting that they could be responsible for the anti‐proliferative activity | Human colon cancer HT‐29 cells | (Rugina et al., 2012) | |
| Tart cherry | Anti‐inflammatory, anti‐carcinogenesis | Mice that were fed anthocyanin‐rich extract (at any dose) in combination with sulindac had fewer tumours and a smaller total tumour burden (total tumour area per mouse) in the small intestine compared with mice fed with sulindac alone | APC (Min) mice | (Bobe et al., 2006) | |
| Wild‐grown berries | Induction of autophagy | Anthocyanin‐induced autophagy switched to apoptosis, as shown by the activation of Bax, cytochrome c and caspase 3, terminal deoxynucleotide transferase‐mediated dUTP nick‐end labelling‐positive fragmented nuclei and cells with sub‐G(1) DNA content, which were prevented by z‐VAD | Human liver cancer PLC/PRF/5and HepG2 cells | (Longo et al., 2008) | |
| Black rice | Suppress metastasis | Black rice anthocyanins suppress metastasis in breast cancer cells by targeting the RAS/RAF/MAPK pathway | Human breast cancer cell lines MCF‐10A and MCF‐7 | (Chen et al., 2015) | |
| Black rice whole grain | Anti‐inflammation | Polar fraction of black rice whole grain extracts might suppress LPS‐induced inflammation via the inhibition of the MAPK signalling pathway, leading to decrease of NF‐kB and AP‐1 translocation | RAW 264.7 macrophage cells | (Limtrakul et al., 2015) | |
| Black currant | Antioxidant | Blackcurrant bioactive anthocyanins exert chemo‐preventive actions against diethylnitrosamine‐inflicted hepatocarcinogenesis by attenuating oxidative stress through activation of the Nrf2 signalling pathway | Rats | (Thoppil et al., 2012) | |
| Delphinidin | Grape | Block carcinogen‐DNA adduct formation | Concord grape extract and a component grape anthocyanin have breast cancer chemo‐preventive potential caused in part to their capacity to block carcinogen‐DNA adduct formation, modulate the activities of carcinogen‐metabolizing enzymes and suppress ROS in these noncancerous human breast cells. | Block carcinogen‐DNA human breast epithelial MCF‐10F cells treated with benzo[a]pyrene adduct formation | (Singletary et al., 2007) |
| Blueberry | Inhibit MMP‐2 and MMP‐9 proteolytic activity | Gallic acid inhibited MMP‐2 and MMP‐9 proteolytic activity with very similar potency. NMR and molecular modelling experiments confirmed the interaction of Gallic acid with MMP‐2 and suggested that it takes place within the catalytic centre | Human fibrosarcoma HT1080 cells | (Filipiak et al., 2014) | |
| Roselle | Antitumour | Treatment with HAs caused reduced levels of AST, ALT, uric acid and MPO. The results showed that oral administration of HAs (0.2%) remarkably inhibited progression of NMU‐induced leukaemia by approximately 33.3% in rats | N‐nitrosomethylurea‐induced leukaemia in rats | (Tsai et al., 2014) | |
| Muscadine grape skin | Mediated invasion, migration and osteoclastogenesis | Snail regulation of CatL might occur via STAT3 signalling and can be antagonized by Muscadine grape skin extract, leading to decreased cell invasion, migration and bone turnover | LNCaP prostate and MCF‐7 breast human cancer cells | (Burton et al., 2015) | |
| Malvidin | Blueberry | Antioxidant, anti‐proliferative, stimulate apoptosis | The ARF‐T was shown to have the highest anthocyanin content and antioxidant activity and inhibited B16‐F10 melanoma murine cells proliferation at concentrations higher than 500 μg·mL−1. In addition, ARF‐T stimulated apoptosis and increased total LDH activity in metastatic B16‐F10 melanoma murine cells | Mouse melanoma B16‐F10 cells | (Bunea et al., 2013) |
| Blueberry | Increase apoptosis through DNA fragmentation and caspase‐3 activity | Apoptosis was confirmed in HT‐29 cells when treated with anthocyanins from blueberry cultivars at 50–150 μg·mL−1 concentrations; however, these same concentrations decreased quinone reductase and glutathione‐S‐transferase activities rather than induced them | Human colon cancer HT‐29 cells | (Srivastava et al., 2007) | |
| Blackberry | Antioxidant, anti‐proliferative and anti‐inflammatory activities | The hydrolysed pulp and seed extracts showed significant antiproliferative activity. However, non‐hydrolysed extracts showed much less activity | Human lung cancer A549 cells | (Aqil et al., 2012) | |
| Bilberries and blueberries | Antioxidant | Anthocyanins had intracellular antioxidant activity if applied at very low concentrations (<1 μg·L−1; nM range), thereby providing a long‐sought rationale for their health protecting effects in spite of their unfavourable pharmacokinetic properties | Human colon cancer (Caco‐2), human hepatocarcinoma (HepG2), human endothelial (EA.hy926) and rat vascular smooth muscle (A7r5) | (Bornsek et al., 2012) | |
| Pelargonidin | Pomegranate | Antioxidant, antitumour and anti‐inflammatory | Pomegranate fruit extract possesses anti‐skin tumour‐promoting effects in CD‐1 mouse. Pomegranate fruit extract is capable of inhibiting conventional, as well as novel, biomarkers of TPA‐induced tumour promotion; therefore, it may possess chemopreventive activity in a wide range of tumour models | TPA‐induced skin tumour in CD‐1mice | (Afaq et al., 2005) |
| Peonidin | Sweet potato P40 | Antioxidant, anti‐proliferative, induce cell‐cycle arrest, apoptosis | Dietary P40 at 10–30% significantly suppressed azoxymethane‐induced formation of aberrant crypt foci in the colons of CF‐1 mice in conjunction with, at least in part, a lesser proliferative PCNA and a greater apoptotic caspase‐3 expression in the colon mucosal epithelial cells | Human colonic SW480 cancer cells | (Lim et al., 2013a) |
| Eugenia jambolana | Anti‐proliferative,pro‐apoptotic | Java plum fruit extract anthocyanins suppressed (P < 0.05) proliferation in HCT‐116 cells and elevated (P < 0.05) apoptosis in both HCT‐116 cells and colon CSCs | HCT‐116 colon cancer cell line and colon CSCs | (Charepalli et al., 2016) | |
| Petunidin | Jamun, Blackberry | Antioxidant and anti‐proliferative | The hydrolysed pulp and seed extracts showed significant anti‐proliferative activity. However, non‐hydrolysed extracts showed much less activity | Human lung cancer A549 cells. | (Aqil et al., 2012) |
| Blueberry | Antiproliferative, antioxidant, metastasis control | The ARF‐T had the highest anthocyanin content and antioxidant activity and inhibited B16‐F10 melanoma murine cells proliferation at concentrations higher than 500 μg·mL−1. In addition, ARF‐T stimulated apoptosis and increased total LDH activity in metastatic B16‐F10 melanoma murine cells | B16‐F10 metastatic melanoma murine cells | (Bunea et al., 2013) | |
| Black soybean | Suppress oxidative stresses, decreasing inflammatory responses | Mucosal expression of COX‐2 and cPLA2 were decreased significantly in the 0.5% AE group, by 32 and 62%, respectively, compared with the control group | APC (Min/+) mice | (Park et al., 2015) |
AE, anthocyanin‐rich extract; ALT, alanine transaminase; ARF‐T, anthocyanin rich‐fraction obtained from cultivar Torro; AST, aspartate transaminase; MPO, myeloperoxidase; NMU, N‐nitroso‐N‐methylurea.
Increasing in vitro experimental data have shown that anthocyanins can interfere with multiple signal pathways to exert their anticancer activities. However, it must be pointed out that most of these experiments were performed in vitro, and there is not necessarily an epidemiological connection between intake of anthocyanin and risk of carcinogenesis (Wang and Stoner, 2008). Data from animal models and in vitro and in vivo experiments indicated that anthocyanins have the potential to prevent and treat cancers; however, the metabolism of anthocyanins remains unclear. Considering that anthocyanins are rapidly metabolized into several active metabolites by the host and/or by gut microbiota and the evaluation of bioavailability of anthocyanins is complex (Núñez‐Sánchez et al., 2015), more sensitive instruments and efficient methods should be developed to further this research. More clinical trials are required to validate the potential anticancer activities of anthocyanins before their wide clinical use. In addition, experiments show that their availability in the human body is lower than that in vitro (Cooke et al., 2005, Núñez‐Sánchez et al., 2015). Therefore, improving the availability and the stability of anthocyanin in vivo represents another challenge to researchers. The latest review of preclinical and clinical evidence for the potential benefits of edible berries for human aerodigestive and gastrointestinal tract cancers indicated that the exact mechanisms of actions of phytochemicals (including anthocyanins) remain largely unexplained. These mechanisms include chronic inflammation and cancer; modulation of carcinogenesis; phytochemical interaction with vital proteins; signal pathways; and regulation of cancer cell proliferation, immortality and metastasis, and have attracted significant research attention (Bishayee et al., 2016). The mechanism of action of anthocyanins is summarized in Figure 2. Limited evidence from clinical data indicates that the use of anthocyanins can improve life quality (Bishayee et al., 2016) and might provide a survival advantage in terminal care for cancer patients, However, further rigorous scientific investigation and more clinical proof are required.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the University Students Innovation Training Program of Shanghai Municipal Government [grant number 201610247134 to HF Song] and the Yangfan Project of Tongji University School of Medicine [grant number 2012YF05 to YY Cui]. The authors thank the staff of the Key Laboratory of Arrhythmias, Ministry of Education (Tongji University) for their technical assistance. We also thank the editor and reviewers for their comments and suggestions, which have improved this review considerably.
Lin, B.‐W. , Gong, C.‐C. , Song, H.‐F. , and Cui, Y.‐Y. (2017) Effects of anthocyanins on the prevention and treatment of cancer. British Journal of Pharmacology, 174: 1226–1243. doi: 10.1111/bph.13627.
References
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H (2005). Anthocyanin‐ and hydrolyzable tannin‐rich pomegranate fruit extract modulates MAPK and NF‐kappaB pathways and inhibits skin tumorigenesis in CD‐1 mice. Int J Cancer 113: 423–433. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Suarez JM, Giampieri F, Tulipani S, Casoli T, Stefano GD, González‐Paramás AM et al. (2014). One‐month strawberry‐rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem 25: 289–294. [DOI] [PubMed] [Google Scholar]
- Andres‐Lacueva C, Shukitt‐Hale B, Galli RL, Jauregui O, Lamuela‐Raventos RM, Joseph JA (2005). Anthocyanins in aged blueberry‐fed rats are found centrally and may enhance memory. Nutr Neurosci 8: 111–120. [DOI] [PubMed] [Google Scholar]
- Anwar S, Fratantonio D, Ferrari D, Saija A, Cimino F, Speciale A (2016). Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase‐3 activation and p21 upregulation. Mol Med Rep 14: 1397–1403. [DOI] [PubMed] [Google Scholar]
- Aqil F, Gupta A, Munagala R, Jeyabalan J, Kausar H, Sharma RJ et al. (2012). Antioxidant and antiproliferative activities of anthocyanin/ellagitannin‐enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry. Nutr Cancer 64: 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Sen CK, Bagchi M, Atalay M (2004). Anti‐angiogenic, antioxidant, and anti‐carcinogenic properties of a novel anthocyanin‐rich berry extract formula. Biochemistry (Mosc) 69: 75–80. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Haskell Y, Do C, Siveen KS, Mohandas N, Sethi G et al. (2016). Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: preclinical and clinical evidence. Crit Rev Food Sci Nutr 56: 1753–1775. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Mbimba T, Thoppil RJ, Haznagy‐Radnai E, Sipos P, Darvesh AS et al. (2011). Anthocyanin‐rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine‐induced hepatocellular carcinogenesis in rats. J Nutr Biochem 22: 1035–1046. [DOI] [PubMed] [Google Scholar]
- Bobe G, Wang B, Seeram NP, Nair MG, Bourquin LD (2006). Dietary anthocyanin‐rich tart cherry extract inhibits intestinal tumorigenesis in APC(Min) mice fed suboptimal levels of sulindac. J Agric Food Chem 54: 9322–9328. [DOI] [PubMed] [Google Scholar]
- Bornsek SM, Ziberna L, Polak T, Vanzo A, Ulrih NP, Abram V et al. (2012). Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem 134: 1878–1884. [DOI] [PubMed] [Google Scholar]
- Bowen‐Forbes CS, Zhang Y, Nair MG (2010). Anthocyanin content, antioxidant, anti‐inflammatory and anticancer properties of blackberry and raspberry fruits. J Food Compos Anal 23: 554–560. [Google Scholar]
- Bunea A, Rugina D, Sconta Z, Pop RM, Pintea A, Socaciu C et al. (2013). Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16‐F10 metastatic murine melanoma cells. Phytochemistry 95: 436–444. [DOI] [PubMed] [Google Scholar]
- Burton LJ, Smith BA, Smith BN, Loyd Q, Nagappan P, McKeithen D et al. (2015). Muscadine grape skin extract can antagonize Snail‐cathepsin L‐mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis 36: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro M, Nardini M, Maselli P, Nucara A (2015). Chemico‐physical and nutritional properties of traditional legumes (lentil, Lens culinaris L., and grass pea, Lathyrus sativus L.) from organic agriculture: an explorative study. Org Agri 5: 1–9. [Google Scholar]
- Cerletti C, de Curtis A, Bracone F, Digesu C, Morganti AG, Iacoviello L et al. (2016). Dietary anthocyanins and health: data from FLORA and ATHENA EU projects. Br J Clin Pharmacol . doi:10.1111/bcp.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Četojević‐Simin DD, Velićanski AS, Cvetković DD, Markov SL, Ćetković GS, Šaponjac VTT et al. (2015). Bioactivity of Meeker and Willamette raspberry (Rubus idaeus L.) pomace extracts. Food Chem 166: 407–413. [DOI] [PubMed] [Google Scholar]
- Chang H, Yu B, Yu X, Yi L, Chen C, Mi M et al. (2010). Anticancer activities of an anthocyanin‐rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr Cancer 62: 1128–1136. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang HP, Hsu JD, Yang SF, Wang CJ (2005). Hibiscus anthocyanins rich extract‐induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol Appl Pharmacol 205: 201–212. [DOI] [PubMed] [Google Scholar]
- Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, Agarwal R, Vanamala JK (2015). Anthocyanin‐containing purple‐fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J Nutr Biochem 26: 1641–1649. [DOI] [PubMed] [Google Scholar]
- Charepalli V, Reddivari L, Vadde R, Walia S, Radhakrishnan S, Vanamala JK (2016). Eugenia jambolana (Java Plum) fruit extract exhibits anti‐cancer activity against early stage human HCT‐116 colon cancer cells and colon cancer stem cells. Cancers (Basel) 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu JD et al. (2011). Inhibitory effect of delphinidin on monocyte‐endothelial cell adhesion induced by oxidized low‐density lipoprotein via ROS/p38MAPK/NF‐kappaB pathway. Cell Biochem Biophys 61: 337–348. [DOI] [PubMed] [Google Scholar]
- Chen MH, McClung AM, Bergman CJ (2016a). Bran data of total flavonoid and total phenolic contents, oxygen radical absorbance capacity, and profiles of proanthocyanidins and whole grain physical traits of 32 red and purple rice varieties. Data Brief 8: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PN, Chu SC, Chiou HL, Chiang CL, Yang SF, Hsieh YS (2005). Cyanidin 3‐glucoside and peonidin 3‐glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr Cancer 53: 232–243. [DOI] [PubMed] [Google Scholar]
- Chen WC, Liu HM, Liu JS (2016b). Progress in the research on anti‐carcinogenic activities of anthocyanins (Chinese). Food Res Dev 37: 211–215. [Google Scholar]
- Chen XY, Zhou J, Luo LP, Han B, Li F, Chen JY et al. (2015). Black rice anthocyanins suppress metastasis of breast cancer cells by targeting RAS/RAF/MAPK pathway. Biomed Res Int 2015: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocate PG, Natali AJ, Oliveira A, Longo GZ, Alfenas Rde C, Peluzio Mdo C et al. (2014). Fruit and vegetable intake and related nutrients are associated with oxidative stress markers in middle‐aged men. Nutrition 30: 660–665. [DOI] [PubMed] [Google Scholar]
- Cooke D, Steward WP, Gescher AJ, Marczylo T (2005). Anthocyans from fruits and vegetables‐‐does bright colour signal cancer chemopreventive activity? Eur J Cancer 41: 1931–1940. [DOI] [PubMed] [Google Scholar]
- Cooper‐Driver GA (2001). Contributions of Jeffrey Harborne and co‐workers to the study of anthocyanins. Phytochemistry 56: 229–236. [DOI] [PubMed] [Google Scholar]
- Correa‐Betanzo J, Allen‐Vercoe E, Mcdonald J, Schroeter K, Corredig M, Paliyath G (2014). Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem 165: 522–531. [DOI] [PubMed] [Google Scholar]
- Cui J, Li XY (2014). Progress on anti‐tumor mechanisms of anthocyanins(Chinese. Food Sci 35: 310–315. [Google Scholar]
- Cui YY, Xie H, Wang JF (2005b). Potential biomedical properties of Pinus massoniana bark extract. Phytother Res 19: 34–38. [DOI] [PubMed] [Google Scholar]
- Cui YY, Xie H, Qi KB, He YM, Wang JF (2005a). Effects of Pinus massoniana bark extract on cell proliferation and apoptosis of human hepatoma BEL‐7402 cells. World J Gastroenterol 11: 5277–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Gupte A, Gates L, Mumper RJ (2009). A comprehensive study of anthocyanin‐containing extracts from selected blackberry cultivars: extraction methods, stability, anticancer properties and mechanisms. Food Chem Toxicol 47: 837–847. [DOI] [PubMed] [Google Scholar]
- De Ferrars RM, Czank C, Zhang Q, Botting NP, Kroon PA, Cassidy A et al. (2014). The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol 171: 3268–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiseitel A, Oosterhuis B, Vukman KV, Schreier P, Oehme A, Locher S et al. (2009). Berry anthocyanins and anthocyanidins exhibit distinct affinities for the efflux transporters BCRP and MDR1. Br J Pharmacol 158: 1942–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D, Chen A, Grace MH, Komarnytsky S, Lila MA (2014). Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J Agric Food Chem 62: 7022–7028. [DOI] [PubMed] [Google Scholar]
- Esselen M, Boettler U, Teller N, Bachler S, Hutter M, Rufer CE et al. (2011). Anthocyanin‐rich blackberry extract suppresses the DNA‐damaging properties of topoisomerase I and II poisons in colon carcinoma cells. J Agric Food Chem 59: 6966–6973. [DOI] [PubMed] [Google Scholar]
- Feng R, Wang SY, Shi YH, Fan J, Yin XM (2010). Delphinidin induces necrosis in hepatocellular carcinoma cells in the presence of 3‐methyladenine, an autophagy inhibitor. J Agric Food Chem 58: 3957–3964. [DOI] [PubMed] [Google Scholar]
- Fernandes I, Faria A, Calhau C, Freitas VD, Mateus N (2014). Bioavailability of anthocyanins and derivatives. J Funct Foods 7: 54–66. [Google Scholar]
- Filipiak K, Hidalgo M, Silvan JM, Fabre B, Carbajo RJ, Pineda‐Lucena A et al. (2014). Dietary Gallic acid and anthocyanin cytotoxicity on human fibrosarcoma HT1080 cells. A study on the mode of action. Food Funct 5: 381–389. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Berti F, Nusse M, Cantelli‐Forti G, Hrelia P (2004). Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin‐3‐O‐beta‐glucopyranoside. Biochem Pharmacol 67: 2047–2056. [DOI] [PubMed] [Google Scholar]
- Ha US, Bae WJ, Kim SJ, Yoon BI, Hong SH, Lee JY et al. (2015). Anthocyanin induces apoptosis of DU‐145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med J 56: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidh RR, Abdulamir AS, Bakar FA, Jalilian FA, Jahanshiri F, Abas F et al. (2013). Novel anticancer activity and anticancer mechanisms of Brassica oleracea L. var. capitata F. rubra . Eur J Intern Med 5: 450–464. [Google Scholar]
- Hagiwara A, Miyashita K, Nakanishi T, Sano M, Tamano S, Kadota T et al. (2001). Pronounced inhibition by a natural anthocyanin, purple corn color, of 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP)‐associated colorectal carcinogenesis in male F344 rats pretreated with 1,2‐dimethylhydrazine. Cancer Lett 171: 17–25. [DOI] [PubMed] [Google Scholar]
- Haseeb A, Chen D, Haqqi TM (2013). Delphinidin inhibits IL‐1beta‐induced activation of NF‐kappaB by modulating the phosphorylation of IRAK‐1(Ser376) in human articular chondrocytes. Rheumatology (Oxford) 52: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Giusti MM (2010). Anthocyanins: natural colorants with health‐promoting properties. Annu Rev Food Sci Technol 1: 163–187. [DOI] [PubMed] [Google Scholar]
- Ho ML, Chen PN, Chu SC, Kuo DY, Kuo WH, Chen JY et al. (2010). Peonidin 3‐glucoside inhibits lung cancer metastasis by downregulation of proteinases activities and MAPK pathway. Nutr Cancer 62: 505–516. [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC (1995). Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7: 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou DX, Kai K, Li JJ, Lin S, Terahara N, Wakamatsu M et al. (2004). Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure–activity relationship and molecular mechanisms. Carcinogenesis 25: 29–36. [DOI] [PubMed] [Google Scholar]
- Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M (2005). Anthocyanidins inhibit cyclooxygenase‐2 expression in LPS‐evoked macrophages: structure–activity relationship and molecular mechanisms involved. Biochem Pharmacol 70: 417–425. [DOI] [PubMed] [Google Scholar]
- Huang HP, Chang YC, Wu CH, Hung CN, Wang CJ (2012). Anthocyanin‐rich Mulberry extract inhibit the gastric cancer cell growth in vitro and xenograft mice by inducing signals of p38/p53 and c‐jun. Food Chem 129: 1703–1709. [Google Scholar]
- Huang L, Zhang Z, Zhang S, Ren J, Zhang R, Zeng H et al. (2011). Inhibitory action of Celastrol on hypoxia‐mediated angiogenesis and metastasis via the HIF‐1alpha pathway. Int J Mol Med 27: 407–415. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee WS, Shin SC, Kim GY, Choi BT, Choi YH (2013). Anthocyanins downregulate lipopolysaccharide‐induced inflammatory responses in BV2 microglial cells by suppressing the NF‐kappaB and Akt/MAPKs signaling pathways. Int J Mol Sci 14: 1502–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian H, Giusti MM (2010). Anthocyanins: natural colorants with health‐promoting properties. Annu Rev Food Sci Technol 1: 163–187. [DOI] [PubMed] [Google Scholar]
- Jiang X, Tang X, Zhang P, Liu G, Guo H (2014). Cyanidin‐3‐ O ‐β‐glucoside protects primary mouse hepatocytes against high glucose‐induced apoptosis by modulating mitochondrial dysfunction and the PI3K/Akt pathway. Biochem Pharmacol 90: 135–144. [DOI] [PubMed] [Google Scholar]
- Jin HL, Lim JD, Choung MG (2013). Studies on the anthocyanin profile and biological properties from the fruits of Acanthopanax senticosus (Siberian ginseng. J Funct Foods 5: 380–388. [Google Scholar]
- Jing P, Giusti MM (2010). Contribution of Berry Anthocyanins to Their Chemopreventive Properties. Springer: New York, pp. 3–40. [Google Scholar]
- Kang NJ, Lee KW, Kwon JY, Hwang MK, Rogozin EA, Heo YS et al. (2008). Delphinidin attenuates neoplastic transformation in JB6 Cl41 mouse epidermal cells by blocking Raf/mitogen‐activated protein kinase kinase/extracellular signal‐regulated kinase signaling. Cancer Prev Res (Phila) 1: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausar H, Jeyabalan J, Aqil F, Chabba D, Sidana J, Singh IP et al. (2012). Berry anthocyanidins synergistically suppress growth and invasive potential of human non‐small‐cell lung cancer cells. Cancer Lett 325: 54–62. [DOI] [PubMed] [Google Scholar]
- Kay CD (2006). Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr Res Rev 19: 137–146. [DOI] [PubMed] [Google Scholar]
- Kitagawa S (2006). Inhibitory effects of polyphenols on p‐glycoprotein‐mediated transport. Biol Pharm Bull 29: 1–6. [DOI] [PubMed] [Google Scholar]
- Kocic B, Filipovic S, Nikolic M, Petrovic B (2011). Effects of anthocyanins and anthocyanin‐rich extracts on the risk for cancers of the gastrointestinal tract. J BUON 16: 602–608. [PubMed] [Google Scholar]
- Konczak‐Islam I, Yoshimoto M, Hou DX, Terahara N, Yamakawa O (2003). Potential chemopreventive properties of anthocyanin‐rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L. J Agric Food Chem 51: 5916–5922. [DOI] [PubMed] [Google Scholar]
- Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD et al. (2006). Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer 54: 148–156. [DOI] [PubMed] [Google Scholar]
- Kuntz S, Kunz C, Rudloff S (2015). Inhibition of pancreatic cancer cell migration by plasma anthocyanins isolated from healthy volunteers receiving an anthocyanin‐rich berry juice. Eur J Nutr 22: 1–12. [DOI] [PubMed] [Google Scholar]
- Lamy S, Beaulieu E, Labbe D, Bedard V, Moghrabi A, Barrette S et al. (2008). Delphinidin, a dietary anthocyanidin, inhibits platelet‐derived growth factor ligand/receptor (PDGF/PDGFR) signaling. Carcinogenesis 29: 1033–1041. [DOI] [PubMed] [Google Scholar]
- Lamy S, Lafleur R, Bedard V, Moghrabi A, Barrette S, Gingras D et al. (2007). Anthocyanidins inhibit migration of glioblastoma cells: structure–activity relationship and involvement of the plasminolytic system. J Cell Biochem 100: 100–111. [DOI] [PubMed] [Google Scholar]
- Lee SH, Park SM, Park SM, Park JH, Shin DY, Kim GY et al. (2009). Induction of apoptosis in human leukemia U937 cells by anthocyanins through down‐regulation of Bcl‐2 and activation of caspases. Int J Oncol 34: 1077–1083. [DOI] [PubMed] [Google Scholar]
- Lee YK, Lee WS, Kim GS, Park OJ (2010). Anthocyanins are novel AMPKalpha1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol Rep 24: 1471–1477. [DOI] [PubMed] [Google Scholar]
- Li D, Zhang Y, Liu Y, Sun R, Xia M (2015a). Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr 145: 742–748. [DOI] [PubMed] [Google Scholar]
- Li K, Li Q, Li J, Zhang T, Han Z, Gao D et al. (2007). Antitumor activity of the procyanidins from Pinus koraiensis bark on mice bearing U14 cervical cancer. Yakugaku Zasshi 127: 1145–1151. [DOI] [PubMed] [Google Scholar]
- Li WX, Bao YH, Wang ZY (2011). Morphological observation of apoptotic human colon cancer cells ht29 induced by anthocyanins from Lonicera edulis (Chinese). Acta Nutrimenta Sinica 33: 575–579. [Google Scholar]
- Li X, Xu J, Tang X, Liu Y, Yu X, Wang Z et al. (2016a). Anthocyanins inhibit trastuzumab‐resistant breast cancer in vitro and in vivo. Mol Med Rep 13: 4007–4013. [DOI] [PubMed] [Google Scholar]
- Li YW, Wang D, Li XG, Jin Y (2014). Anthocyanins extracted from Chinese blueberry and its anticancer effects on HepG2 cells. Adv Mat Res : 887–888. [Google Scholar]
- Li YY, Feng J, Zhang XL, Cui YY (2015b). Pine bark extracts: nutraceutical, pharmacological, and toxicological evaluation. J Pharmacol Exp Ther 353: 9–16. [DOI] [PubMed] [Google Scholar]
- Li YY, Feng J, Zhang XL, Li MQ, Cui YY (2016b). Effects of Pinus massoniana bark extract on the invasion capability of HeLa cells. J Funct Foods 24: 520–526. [Google Scholar]
- Lila MA, Burton‐Freeman B, Grace M, Kalt W (2016). Unraveling anthocyanin bioavailability for human health. Annu Rev Food Sci Technol 7: 375–393. [DOI] [PubMed] [Google Scholar]
- Lim S, Xu J, Kim J, Chen TY, Su X, Standard J et al. (2013a). Role of anthocyanin‐enriched purple‐fleshed sweet potato p40 in colorectal cancer prevention. Mol Nutr Food Res 57: 1908–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TG, Jung SK, Kim JE, Kim Y, Lee HJ, Jang TS et al. (2013b). NADPH oxidase is a novel target of delphinidin for the inhibition of UVB‐induced MMP‐1 expression in human dermal fibroblasts. Exp Dermatol 22: 428–430. [DOI] [PubMed] [Google Scholar]
- Limtrakul P, Yodkeeree S, Pitchakarn P, Punfa W (2015). Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF‐kB and AP‐1 signaling pathways. Asian Pac J Cancer Prev 16: 4277–4283. [DOI] [PubMed] [Google Scholar]
- Liu HL, Jiang WB, Xie MX (2010). Flavonoids: recent advances as anticancer drugs. Recent Pat Anticancer Drug Discov 5: 152–164. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou J, Wu Z, Wang X, Liu L, Yao C (2016). Cyanidin 3‐O‐beta‐glucoside ameliorates ethanol‐induced acute liver injury by attenuating oxidative stress and apoptosis: the role of SIRT1/FOXO1 signaling. Alcohol Clin Exp Res 40: 457–466. [DOI] [PubMed] [Google Scholar]
- Liu‐Smith F, Meyskens FL (2016). Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res 60: 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo L, Platini F, Scardino A, Alabiso O, Vasapollo G, Tessitore L (2008). Autophagy inhibition enhances anthocyanin‐induced apoptosis in hepatocellular carcinoma. Mol Cancer Ther 7: 2476–2485. [DOI] [PubMed] [Google Scholar]
- Luo LP, Han B, Yu XP, Chen XY, Zhou J, Chen W et al. (2014). Anti‐metastasis activity of black rice anthocyanins against breast cancer: analyses using an ErbB2 positive breast cancer cell line and tumoral xenograft model. Asian Pac J Cancer Prev 15: 6219–6225. [DOI] [PubMed] [Google Scholar]
- Malik M, Zhao C, Schoene N, Guisti MM, Moyer MP, Magnuson BA (2003). Anthocyanin‐rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr Cancer 46: 186–196. [DOI] [PubMed] [Google Scholar]
- Marko D, Puppel N, Tjaden Z, Jakobs S, Pahlke G (2004). The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol Nutr Food Res 48: 318–325. [DOI] [PubMed] [Google Scholar]
- Martincorena I, Campbell PJ (2015). Somatic mutation in cancer and normal cells. Science 349: 1483–1489. [DOI] [PubMed] [Google Scholar]
- Mauray A, Felgines C, Morand C, Mazur A, Scalbert A, Milenkovic D (2012). Bilberry anthocyanin‐rich extract alters expression of genes related to atherosclerosis development in aorta of apo E‐deficient mice. Nutr Metab Cardiovas 22: 72–80. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Walton MC (2007). The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res 51: 702–713. [DOI] [PubMed] [Google Scholar]
- Miyake S, Takahashi N, Sasaki M, Kobayashi S, Tsubota K, Ozawa Y (2012). Vision preservation during retinal inflammation by anthocyanin‐rich bilberry extract: cellular and molecular mechanism. Lab Invest 92 (1): 102–109. [DOI] [PubMed] [Google Scholar]
- Naomi K, Keiko I, Tojiro T, Koji Y, Masuko K (2003). Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins. J Agric Food Chem 51 (1): 68–75. [DOI] [PubMed] [Google Scholar]
- Nimptsch K, Zhang X, Cassidy A, Song M, O'Reilly EJ, Lin JH et al. (2016). Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am J Clin Nutr 103: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez‐Sánchez MA, Gonzalez‐Sarrias A, Romo‐Vaquero M, Garcia‐Villalba R, Selma MV, Tomas‐Barberan FA et al. (2015). Dietary phenolics against colorectal cancer‐‐From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol Nutr Food Res 59: 1274–1291. [DOI] [PubMed] [Google Scholar]
- Oak MH, Bedoui JE, Madeira SV, Chalupsky K, Schini‐Kerth VB (2006). Delphinidin and cyanidin inhibit PDGF(AB)‐induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br J Pharmacol 149: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C, Giacosa S, Torchio F, Segade SR, Cagnasso E, Rolle L (2014). Extraction kinetics of anthocyanins from skin to pulp during carbonic maceration of winegrape berries with different ripeness levels. Food Chem 165: 77–84. [DOI] [PubMed] [Google Scholar]
- Pal HC, Sharma S, Strickland LR, Agarwal J, Athar M, Elmets CA et al. (2013). Delphinidin reduces cell proliferation and induces apoptosis of non‐small‐cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One 8: 108–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MY, Kim JM, Kim JS, Choung MG, Sung MK (2015). Chemopreventive Action of anthocyanin‐rich black soybean fraction in APC (Min/+) intestinal polyposis model. J Cancer Prev 20: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Teresa SD (2014). Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Arch Biochem Biophys 559: 68–74. [DOI] [PubMed] [Google Scholar]
- Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, Carmella SG, Kuo CT et al. (2014). Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res (Phila) 7: 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddivari L, Vanamala J, Chintharlapalli S, Safe SH, Miller JC Jr (2007). Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase‐dependent and caspase‐independent pathways. Carcinogenesis 28: 2227–2235. [DOI] [PubMed] [Google Scholar]
- Rugina D, Sconta Z, Leopold L, Pintea A, Bunea A, Socaciu C (2012). Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J Med Food 15: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma AD, Sreelakshmi Y, Sharma R (1997). Antioxidant ability of anthocyanins against ascorbic acid oxidation. Phytochemistry 45: 671–674. [Google Scholar]
- Schiavano GF, De Santi M, Brandi G, Fanelli M, Bucchini A, Giamperi L et al. (2015). Inhibition of breast cancer cell proliferation and in vitro tumorigenesis by a new red apple cultivar. PLoS One 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaming Y, Pannengpetch P, Chattipakorn SC, Chattipakorn N (2015). Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid Based Complement Alternativ Med 2015: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafino A, Sinibaldi‐Vallebona P, Lazzarino G, Tavazzi B, Rasi G, Pierimarchi P et al. (2004). Differentiation of human melanoma cells induced by cyanidin‐3‐O‐beta‐glucopyranoside. FASEB J 18: 1940–1942. [DOI] [PubMed] [Google Scholar]
- Shih PH, Yeh CT, Yen GC (2007). Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress‐induced apoptosis. J Agric Food Chem 55: 9427–9435. [DOI] [PubMed] [Google Scholar]
- Shin DY, Lu JN, Kim GY, Jung JM, Kang HS, Lee WS et al. (2011). Anti‐invasive activities of anthocyanins through modulation of tight junctions and suppression of matrix metalloproteinase activities in HCT‐116 human colon carcinoma cells. Oncol Rep 25: 567–572. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Jung KJ, Giusti M (2007). Anthocyanin‐rich grape extract blocks breast cell DNA damage. J Med Food 10: 244–251. [DOI] [PubMed] [Google Scholar]
- Song NR, Yang H, Park J, Kwon JY, Kang NJ, Yong SH et al. (2012). Cyanidin suppresses neoplastic cell transformation by directly targeting phosphatidylinositol 3‐kinase. Food Chem 133: 658–664. [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). NC‐IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Akoh CC, Fischer J, Krewer G (2007). Effect of anthocyanin fractions from selected cultivars of Georgia‐grown blueberries on apoptosis and phase II enzymes. J Agric Food Chem 55: 3180–3185. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V et al. (2005). Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze‐dried black raspberries daily for 7 days. J Clin Pharmacol 45: 1153–1164. [DOI] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V et al. (2008). Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol 231: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller N, Thiele W, Boettler U, Sleeman J, Marko D (2009a). Delphinidin inhibits a broad spectrum of receptor tyrosine kinases of the ErbB and VEGFR family. Mol Nutr Food Res 53: 1075–1083. [DOI] [PubMed] [Google Scholar]
- Teller N, Thiele W, Marczylo TH, Gescher AJ, Boettler U, Sleeman J et al. (2009b). Suppression of the kinase activity of receptor tyrosine kinases by anthocyanin‐rich mixtures extracted from bilberries and grapes. J Agric Food Chem 57: 3094–3101. [DOI] [PubMed] [Google Scholar]
- Thoppil RJ, Bhatia D, Barnes KF, Haznagy‐Radnai E, Hohmann J, Darvesh AS et al. (2012). Black currant anthocyanins abrogate oxidative stress through Nrf2‐ mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr Cancer Drug Targets 12: 1244–1257. [DOI] [PubMed] [Google Scholar]
- Thwe A, Valan Arasu M, Li X, Park CH, Kim SJ, Al‐Dhabi NA et al. (2016). Effect of different agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (Fagopyrum tataricum Gaertn). Front Microbiol 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TC, Huang HP, Chang YC, Wang CJ (2014). An anthocyanin‐rich extract from Hibiscus sabdariffa linnaeus inhibits N‐nitrosomethylurea‐induced leukemia in rats. J Agric Food Chem 62: 1572–1580. [DOI] [PubMed] [Google Scholar]
- Tsuyuki S, Fukui S, Watanabe A, Akune S, Tanabe M, Yoshida K (2012). Delphinidin induces autolysosome as well as autophagosome formation and delphinidin‐induced autophagy exerts a cell protective role. J Biochem Mol Toxicol 26: 445–453. [DOI] [PubMed] [Google Scholar]
- Wang LS, Stoner GD (2008). Anthocyanins and their role in cancer prevention. Cancer Lett 269: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C et al. (2009). Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila) 2: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel T, Baum M, Eisenbrand G, Dietrich H, Will F, Stockis JP et al. (2006). An anthocyanin/polyphenolic‐rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol J 1: 388–397. [DOI] [PubMed] [Google Scholar]
- Welch CR, Wu Q, Simon JE (2008). Recent advances in anthocyanin analysis and characterization. Curr Anal Chem 4: 75–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Yu Z, Tang Q, Song H, Gao Z, Chen W et al. (2013). Honeysuckle anthocyanin supplementation prevents diet‐induced obesity in C57BL/6 mice. Food Funct 4: 1654–1661. [DOI] [PubMed] [Google Scholar]
- Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M et al. (2010). Cyanidin‐3‐glucoside inhibits ethanol‐induced invasion of breast cancer cells overexpressing ErbB2. Mol Cancer 9: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Koo SI, Song WO, Chun OK (2011). Food matrix affecting anthocyanin bioavailability: review. Curr Med Chem 18: 291–300. [DOI] [PubMed] [Google Scholar]
- Yi L, Chen CY, Jin X, Mi MT, Yu B, Chang H et al. (2010). Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low‐density lipoprotein and correlation with radical scavenging activity. FEBS Lett 584: 583–590. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Okuno S, Yoshinaga M, Yamakawa O, Yamaguchi M, Yamada J (1999). Antimutagenicity of sweetpotato (Ipomoea batatas) roots. Biosci Biotechnol Biochem 63: 537–541. [DOI] [PubMed] [Google Scholar]
- Yu J, Xu Y, Khaoustov V, Yoffe B (2011). Identification of components of grape powder with anti‐apoptotic effects. Toxicol Ind Health 27: 19–28. [DOI] [PubMed] [Google Scholar]
- Yun JW, Lee WS, Min JK, Jing NL, Kang MH, Kim HG et al. (2010). Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti‐invasive activity on HT‐29 human colon cancer cells. Food Chem Toxicol 48: 903–909. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Feng DR, Ma HL, Liu B, Wang HB, Xie H et al. (2012). Antitumor effects of Pinus massoniana bark extract in murine sarcoma S180 both in vitro and in vivo. Am J Chin Med 40: 861–875. [DOI] [PubMed] [Google Scholar]
- Zhu H, Ma J, Wang Q, Hou M, Tang Z, Guo H (2008). Anthocyanin attenuates CD40‐mediated endothelial cell activation and apoptosis by inhibiting CD40‐induced MAPK activation. Atherosclerosis 202: 41–47. [DOI] [PubMed] [Google Scholar]


