Abstract
Tannins are a heterogeneous group of high MW, water‐soluble, polyphenolic compounds, naturally present in cereals, leguminous seeds and, predominantly, in many fruits and vegetables, where they provide protection against a wide range of biotic and abiotic stressors. Tannins exert several pharmacological effects, including antioxidant and free radical scavenging activity as well as antimicrobial, anti‐cancer, anti‐nutritional and cardio‐protective properties. They also seem to exert beneficial effects on metabolic disorders and prevent the onset of several oxidative stress‐related diseases. Although the bioavailability and pharmacokinetic data for these phytochemicals are still sparse, gut absorption of these compounds seems to be inversely correlated with the degree of polymerization. Further studies are mandatory to better clarify how these molecules and their metabolites are able to cross the intestinal barrier in order to exert their biological properties. This review summarizes the current literature on tannins, focusing on the main, recently proposed mechanisms of action that underlie their pharmacological and disease‐prevention properties, as well as their bioavailability, safety and toxicology.
Linked Articles
This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc
Abbreviations
- GSE
grape seed extract
- HTs
hydrolyzable tannins
- NOAEL
no‐observed‐adverse effect level
- OPCs
oligomeric proanthocyanidins
Tables of Links
| TARGETS | ||
|---|---|---|
| Other protein targets a | Enzymes f | MMP‐9 |
| Bax/Bcl‐2 | Akt (PKB) | Glycosidase |
| TNF‐α | Amylase | HATs |
| GPCRs b | Caspase 1 | Lipase |
| CCR2 | Caspase 3 | Lipoxygenases |
| Nuclear hormone receptors c | CDKL | Proteases |
| PPARγ | ERK‐1 (p44 MAPK) | |
| Transporters d | ERK‐2 (p42 MAPK) | |
| GLUT4 | MAPKs | |
| Catalytic receptors e | MAPK 14 (p38 MAPK) | |
| NLRP3 | MMP‐2 | |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,e,fAlexander et al., 2015a,b,c,d,e,f).
Introduction
Tannins are a heterogeneous group of water‐soluble polyphenolic compounds of high molecular weight (500–3000 Daltons) – with as many as 20 hydroxyl groups – and are present in plants, foods and beverages (de Jesus et al., 2012). Being phenolic compounds, tannins are chemically reactive and form inter‐ and intra‐molecular hydrogen bonds that are able to interact with, and precipitate macromolecules, such as proteins and carbohydrates. They are also responsible for the astringent taste of many fruits and vegetables (de Jesus et al., 2012; Lamy et al., 2016). With one to five hydroxyl groups, astringency is increased, while from seven groups upwards, it decreases, given that steric hindrance starts to counterbalance the strength of the hydrogen bonds (Zou et al., 2015).
Tannins can be classified into two groups: hydrolysable tannins and condensed tannins (also named catechin tannins or proanthocyanidins). Hydrolysable tannins can be further divided into gallotannins, which provide sugar and gallic acid on hydrolysis, and ellagitannins, which on hydrolysis do not yield just sugar and gallic acid but also ellagic acid (see Lamy et al., 2016). These compounds, as the name suggests, are hydrolyzed by weak acids and decomposed by high temperatures to yield pyrogallol, a hepatotoxic and highly irritant compound (Jiménez et al., 2014).
The second class, condensed tannins, also referred to as proanthocyanidins, is the most abundant plant‐derived polyphenols. They are oligomers of flavan‐3‐ol (catechin monomers) and/or flavan‐3,4‐diol, usually linked by C‐C (4–8 or 6–8) and occasionally by C‐O‐C bonds with a wide structural diversity (de Jesus et al., 2012; Lamy et al., 2016) and are also called oligomeric proanthocyanidins (OPCs). These compounds are not readily hydrolyzed; they decompose in acidic alcoholic conditions giving red pigments called phlobaphenes. To date, though, the chemistry of proanthocyanidins is only partly known.
Despite their abundance in our diet (estimated daily intake 0.1–0.5 g), tannins have received little attention, probably due to their polymeric nature and high structural complexity (Serrano et al., 2009). In recent years, considerable attention has been paid to proanthocyanidins and their monomers due to the potential beneficial effects on human health, including immunomodulatory, anti‐inflammatory, anticancer, antioxidant, cardio‐protective and antithrombotic properties (Nile and Park, 2014; Smeriglio et al., 2014a; Sieniawska, 2015). However, to assess their precise role in human health, it is essential to gather further knowledge on their pharmacological and toxicological behaviour. In fact, although many plant‐derived proanthocyanidins are widely used now as nutritional supplements, evidence of their safety and potential for long‐term toxicity is still lacking (Berry et al., 2016).
Here we have reviewed the latest developments and knowledge on the occurrence, dietary intake and biological effects of tannins, with particular attention on the pharmacological and toxicological aspects.
Chemical features and sources
Tannins (proanthocyanidins and hydrolysable tannins) are one of the main secondary metabolites found in cacao beans, tea, wines (mainly red), fruits, juices, nuts, chocolate, legumes and cereal grains (Table 1). There are many ways to define tannins based on their properties, solubility and presence of substituents (Okuda and Ito, 2011). Taking into account, for instance, the structural features of hydrolysable tannins, they can be identified as follows: gallotannins, ellagitannins and complex tannins.
Table 1.
Main tannin families and related food sources
| Tannins (mg·100 g−1 or mg·100 mL−1) | ||||
|---|---|---|---|---|
| Species | Proanthocyanidins | Ellagitannins | Gallotannins | |
| Fruits | ||||
| Cranberries | Vaccinium oxycoccus L. | 194–496 | — | — |
| Chokeberries | Aronia melanocarpa (Michx.) Elliott | 553–2106 | — | — |
| Plums | Prunus domestica L. | 32–334 | — | — |
| Black diamond | Prunus spp | 210–267 | — | — |
| Blueberries | Vaccinium myrtillus L. | 87–274 | — | — |
| – | Vaccinium corymbosum L. | 311–335 | — | — |
| Black currants | Riges nigrum L. | 105–255 | 3–6 | — |
| Red currants | Ribes rubrum L. | 30–61 | — | — |
| Blackberries | Rubus fructicosus L. | 5–46 | 150–270 | — |
| Crowberries | Empetrum nigrum L. | 153–173 | — | — |
| Lingonberries | Vaccinium vitis‐idaea L. | 175–545 | — | — |
| Red Grapes | Vitis Labrusca L. | 8–75 | — | — |
| Grape seeds | Vitis Vinifera L. | 2180–6050 | — | — |
| Strawberries | Fragaria × ananassa Duchesne | 15–183 | 71–83 | — |
| Peaches | Prunus persica L. | 29–110 | — | — |
| Apricot | Prunus armeniaca L. | 8–73 | — | — |
| Raspberries | Rubus occidentalis L. | 3–74 | 160–326 | — |
| Pears | Pyrus communis L. | 5–81 | — | — |
| Apple | Malus domestica Borkh. | 46–278 | — | — |
| Pomegranate | Punica granatum L. | — | 58–177 | — |
| Guava | Pisidium spp. | — | 20–25 | — |
| Mango | Mangifera indica L. | — | — | 30–160 |
| Juices | ||||
| Cranberry | – | 20–21 | — | — |
| Apple | – | 52–69 | — | — |
| Grape | – | 45–47 | — | — |
| Nuts | ||||
| Almonds | Prunus dulcis (Mill.) D.A.Webb | 67–257 | — | 20–34 |
| Hazelnuts | Corylus spp. | 125–645 | — | — |
| Pecans | Carya illinoinensis (Wangenh.) K.Koch | 238–695 | 11–33 | — |
| Pistachio nuts | Pistacia vera L. | 113–271 | — | — |
| Walnuts | Juglans regia L. | 35–87 | 36–59 | — |
| Legumes | ||||
| Beans | Phaseolous vulgaris L. | 5–830 | — | — |
| Carob fibre | Ceratonia siliqua L. | 1–19 | — | — |
| Cowpeas | Vigna unguiculata (L.) Walp. | 17–319 | — | — |
| Lentils | Lens culinaris Medik. | 1–2 | — | — |
| Peanuts | Arachis hypogaea L. | 121–141 | — | — |
| Cereal grains | ||||
| Barley | Hordeum vulgare L. | 59–153 | — | — |
| Buckwheat | Fagopyrum esculentum Moench | 4–46 | — | — |
| Sorghum | Sorghum bicolor (L.) Moench | 413–5333 | — | — |
| Rice | Oryza sativa L. | 9–91 | — | — |
| Beverages | ||||
| Wine | – | 1–53 | 2–5 | — |
| Tea | – | 1–5 | — | — |
| Cacao beans | Theobroma cacao L. | 6100–8100 | — | — |
| Chocolate | – | 828–1332 | — | — |
Proanthocyanidins
Proanthocyanidins are oligomers or polymers of flavan‐3‐ols, where the monomeric units are linked mainly by C‐4 → C‐8 bonds, although less frequently also C‐4 → C‐6 linkages can be found. These types of linkages lead to the formation of the so‐called B‐type proanthocyanidins. A‐type proanthocyanidins, on the other hand, are characterized by an additional bond between C‐2 → C‐7 of the basic flavan‐3‐ol units (Figure 1). Proanthocyanidins are composed of different flavan‐3‐ol subunits, known as proanthocyanidin monomers or catechins. The most common monomeric units are the diastereomers of (epi)catechin, (epi)afzelechin and (epi)gallocatechin. In this class of flavonoids, two aromatic homocyclic rings (A and B) and a heterocyclic ring (C) are present, and they are joined by a three‐atom carbon bridge. Their C ring features a hydroxyl group at the C‐3 position, and it lacks a double bond between C‐2 and C‐3. The most abundant proanthocyanidins in vegetables are those composed of catechin and epicatechin units (procyanidins), while much less common are propelargonidin or prodelphinidin containing (epi)afzelechin or (epi)gallocatechin basic units (Figure 1), present in foods such as barley, broad beans, red kidney beans, redcurrants, pinto beans, black tea and cinnamon (Landete, 2011; de Jesus et al., 2012; Mateos‐Martín et al., 2012; Lamy et al., 2016). Procyanidins have been identified, in natural sources, as dimers (e.g. procyanidin B1‐B8, procyanidin A1‐A2), trimers (e.g. procyanidin C1 and C2, selligueain A and B), tetramers and oligomeric structures with a degree of polymerization ranging from 5 to 11. The most common acyl substituent (as in the case of tea‐ or wine‐derived species) is a galloyl group bound to the hydroxyl group in the C‐3 position. The carbohydrate moieties are usually linked to the C‐3 or the C‐5 position in glycosylated proanthocyanidin oligomers.
Figure 1.
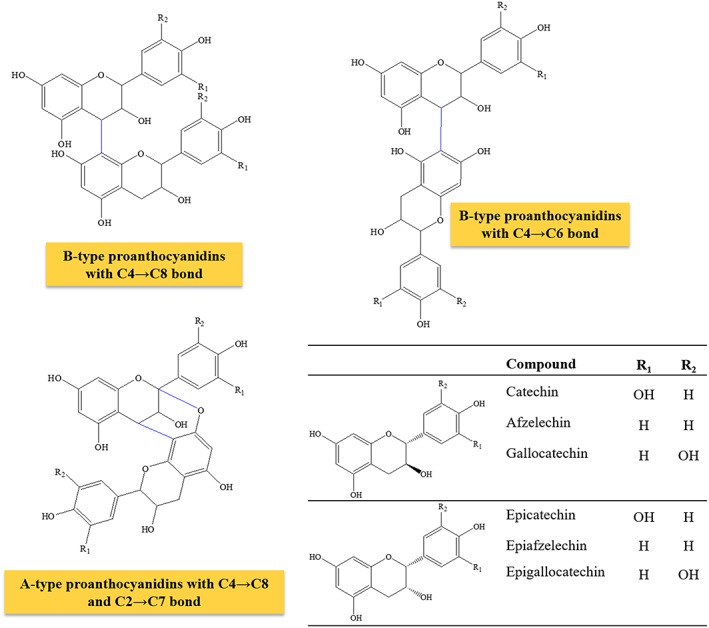
Basic chemical features of B‐ and A‐type proanthocyanidins and their monomeric units.
Gallotannins
Gallotannins represent the simplest class of hydrolyzable tannins (HTs), containing gallic acid substituents esterified with a polyol residue (mainly D‐glucose). The β anomer of glucose is the dominant configuration, although rare examples of the α configuration can be found in natural derivatives. The glucose hydroxyl groups can be partly or totally substituted by phenol units to produce initially β‐glucogallin (1‐O‐galloyl‐β‐D‐glucopyranose). The biosynthetic pathway, starting from this molecule and after the galloylation reaction, yields di‐, tri‐, tetra, penta‐, hexa‐, hepta‐ and octagalloylglucoses. Of these, 1,2,3,4,6‐penta‐O‐galloyl‐β‐D‐glucopyranose is usually employed as the prototypical molecule of gallotannins (Figure 2). The formation of meta‐ or para‐depside bonds is characteristic of these molecules and involves esterification of the aromatic hydroxyl group rather than those present in aliphatic structures. However, many other derivatives can be found, for example gallotannins with 10 or more (up to 12) units of gallic acid esterified to a single glucose moiety, as in the case of tannins obtained from sumac (Rhus semialata) or oak galls (Quercus infectoria), utilized since ancient times to tan animal hides to produce leather. Although glucose is by far the most abundant polyol identified in tannins, glucitol, fructose, shikimic acid, xylose, hamamelose, saccharose, quercitol and quinic acid may constitute the core molecules for the subsequent galloylation processes. However, these derivatives are not very common and have only been isolated in maple, chestnut and witch hazel.
Figure 2.
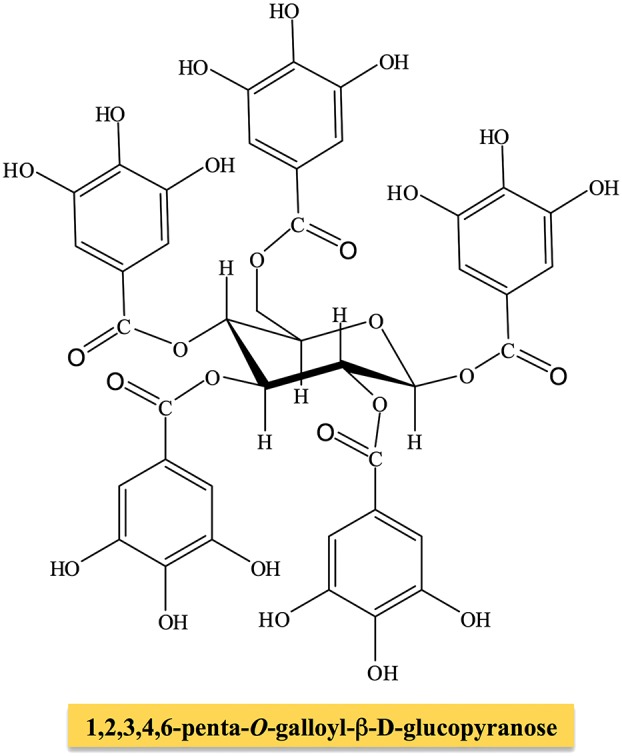
Chemical structure of pentagalloyl‐glucose.
Ellagitannins
Different from gallotannins (rarely found in nature), ellagitannins are present in many plant families, and almost 500 molecules have been isolated and identified to date (Okuda and Ito, 2011; Lamy et al., 2016). Although not all ellagitannins are hydrolyzable, they continue to be classified as hydrolyzable tannins for historical reasons. They can be found in numerous forms: monomeric (e.g. nupharin A, punicalagin, geraniin, eugeniin, davidiin, casuarictin and corilagin), dimeric (e.g. sanguiin), oligomeric (e.g. agrimoniin, nupharin E, nupharin C and hirtellin A) and C‐glycosidic (e.g. vescalagin, castalagin, casuarinin and stachyurin) molecules. Ellagitannins are formed from gallotannins by intermolecular carbon–carbon coupling between at least two galloyl units, yielding hexahydroxydiphenoyl acid, which in aqueous solution spontaneously lactonizes into ellagic acid (Figure 3). Ellagitannins and ellagic acid have been identified in both fruits as well as in nuts and seeds (Landete, 2011), see Table 1. The oxidative coupling commonly involves C‐4 and C‐6 (as in the case of eugeniin) and may extend to C‐2 and C‐3, leading to the formation of casuarictin (Figure 4). Other carbon atoms involved in this reaction are the C‐3/C‐6, C‐2/C‐4 or C‐1/C‐6 pairs. Hexahydroxydiphenoyl acid can undergo esterification not only with glucose but also with hamamelose and terpenoids. Ellagitannins can yield dimers following intermolecular oxidative coupling with other hydrolyzable tannins (as in the case of euphorbins) or high MW oligomers.
Figure 3.
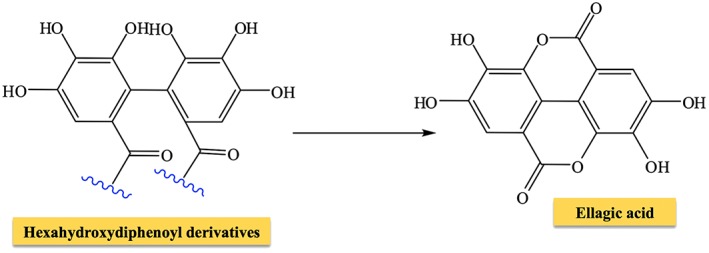
Formation of ellagic acid in aqueous solution.
Figure 4.
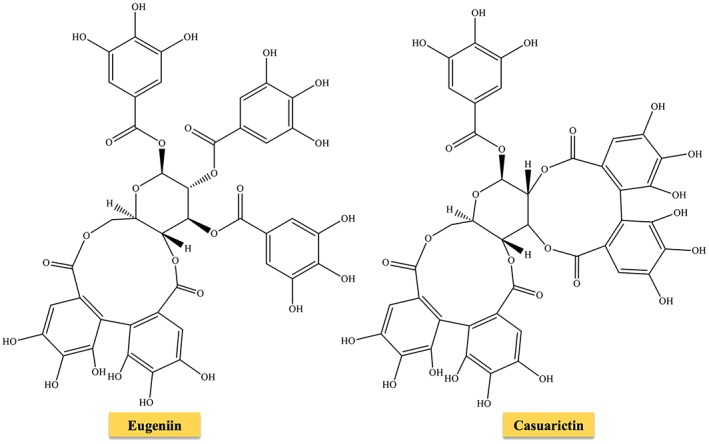
Chemical structure of eugeniin and casuarictin.
Complex tannins
The reaction of gallotannins or ellagitannins with catechin units yields complex tannins. Flavan‐3‐ols can link at C position of an open‐chain glucose. Typical examples of complex tannins are camelliatannins A and B, malabatrin A and acutissimin A (Okuda and Ito, 2011).
Distribution and content of proanthocyanidins and hydrolysable tannins in food
Tannins, in particular proanthocyanidins, are one of the most widespread compounds present in food ingested daily. They are commonly found in plants and have been identified in almost all their parts (fruits, leaves, roots, seeds, wood and bark). More than 1000 derivatives have been identified to date, and these compounds continue to be of interest to researchers due to the wide range of applications and the numerous biological processes influenced by their presence. These compounds are found in tea, wine, cereals, cacao and fruits in amounts of up to 184 mg·100 g−1, with epicatechin being the most abundant component, followed by catechin and type B‐procyanidin (de Jesus et al., 2012; Lamy et al., 2016). They are also the principal components found in ripe fresh nectarines, peaches and plums (Table 1). Recently, the presence of epicatechin and B‐type dimers and trimers of proanthocyanidins has been detected in the pulp of Annona cherimola Mill (Barreca et al., 2011). In red wine, the content of catechin, epicatechin, procyanidin B1, B2, B3 and B4 amounts to ~18 mg·100 g−1, more than double that found in white wine (Sanchez‐Moreno et al., 2003). Catechin and procyanidin B2 were the two principal components found in some cereals and fruits, as well as in tea and red wine. A complete list of proanthocyanidin content in foodstuffs can be found on the USDA database of Proanthocyanidin Content of Selected Foods (http:// www.nal.usda.gov/fnic/foodcomp/Data/PA/PA.html).
Gallotannins are present only in selected woody and herbaceous dicotyledons. Cereals are sources of hydrolyzable phenols due to phenolic compounds being linked to cell wall components. β‐Glucogallin has been reported to be one of the components of mango fruit pulp (Oliveira et al., 2016). Sumac and Chinese gallnuts (or nutgalls) are particularly rich in gallotannins. Sumac (Rhus coriaria L.) is a shrub grown in Spain, Italy, Turkey and some Mediterranean Arab countries from which a powder is obtained that has long been used as a tanning agent. The main compounds are D‐glucose derivatives esterified with gallic acid, such as penta‐, hexa‐, hepta‐, octa‐, nona‐, deca‐, undeca‐ and dodeca‐galloyl‐D‐glucose (Lu et al., 2013). Ellagitannins are present in only a few fruits and nuts (especially pecans and walnuts) consumed in the Western diet, and dietary intake (most probably far below 5 mg·day−1) is mainly resulting from the consumption of red fruits (strawberries, raspberries and blackberries or beverages and jams made from these) (Okuda and Ito, 2011), see Table 1. The principal derivatives identified in foods so far are as follows: sanguiin H‐6, punicalagin, sanguiin H‐5, vescalagin, castalagin, roburin E, casuarictin, pedunculagin, potentillin and lambertianin C. Estimates of the amount of ellagitannins present in food are based on detection of ellagic acid (after hydrolysis of these compounds) followed by HPLC detection, although care is needed during quantification to avoid underestimation deriving from solubility issues. Sanguiin H‐6, casuarictin, potentillin and pedunculagin are the predominantly ellagitannins identified in strawberries, raspberries and blackberries (Zorenc et al., 2016). These compounds are mainly contained in the peel and seed, while very low quantities or traces have been detected in the pulp. Pomegranates (fruits, juices and jams) are also a rich source of ellagitannins, mainly punicalagin and ellagic acid (Masci et al., 2016). Ellagitannins have been found in significant amount (Table 1) also in walnuts and pecan nuts (Landete, 2011), with pedunculagin being the main derivative present in walnuts.
Pharmacokinetic aspects
Aside from the pharmacological effects exerted locally in the gut, tannins have to be bioavailable in order to exert their health effects, and therefore, absorption, bioavailability and metabolism of these compounds must be carefully evaluated. Although the pharmacokinetics of simple phenols have been extensively investigated in animal and human studies (Velderrain‐Rodríguez et al., 2014), the same cannot be said for tannins, where results are still scarce and controversial. High MW tannins apparently are not absorbed intact, as demonstrated by Wiese et al. (2015), who reported that almost 90% of apple juice‐derived procyanidins consumed were retrieved in ileostomy effluent and were able to enter the colon (Wiese et al., 2015). The polymerization rate appears to play a major role in the fate of these compounds and seems to be indirectly related to the absorption of these molecules through the gut barrier and to their metabolism by gut microbiota to the monomers (i.e. catechin and epicathechin) (Zhang et al., 2016). Some in vivo and in vitro studies have investigated the hydrolysis mechanism of polymeric proanthocyanidins and whether it is possible for the derived oligomeric molecules to be absorbed by the small intestine; often the molecules that appear in biological fluids can be very different from those ingested (Zhang et al., 2016). Even though small quantities of proanthocyanidins monomers and dimers were found in the plasma compartment, methylated and glucuronidated derivatives seem to be the main metabolites. Furthermore, in the colon, the proanthocyanidins are catabolized by the gut microflora into a series of simpler metabolites such as phenyl valerolactone, phenylacetic and phenylpropionic acids (Zhang et al., 2016). However, these results are still controversial.
In vitro studies
Some in vitro experiments have suggested, for example that cocoa procyanidins are hydrolyzed into a mixture of epicatechin monomers and dimers under conditions similar to those present in the human stomach. Nevertheless, other studies have demonstrated that no significant depolymerization of cocoa procyanidins occurs in the stomach, thus suggesting a plausible buffer mechanism provided by the food bolus against the acid stomach environment (Ellam and Williamson, 2013; Kim et al., 2014). However, despite these conflicting observations, what is certain is that during digestion, high MW proanthocyanidins can form complexes with proteins, starches and digestive enzymes, resulting in the formation of less digestible complexes and this property is indirectly related to the degree of polymerization. Indeed, small molecules such as dimeric and trimeric proanthocyanidins can be more readily absorbed (Zhang et al., 2016).Absorption of OPCs has been reviewed recently by Ou and Gu, 2014. They reported that procyanidin A‐type dimers, trimers and tetramers were slowly transported across Caco‐2 cells monolayers (transport ratio of 0.6%, 0.4% and 0.2% respectively), suggesting their possible absorption after dietary intake. Furthermore, they reported similar permeability coefficients for (+)‐catechin and a proanthocyanidin dimer and trimer, while the permeability of proanthocyanidins with high polymerization was found to be approximately 10 times lower.
As described above, most ingested tannins reach the colon. At this level, the gut microbiota play a key role in their metabolism leading to the formation of absorbable or unabsorbable metabolites which remain in the colonic lumen where they can counteract the effects of some pro‐oxidant agents (Marín et al., 2015). Proanthocyanidins seem to be extensively metabolized by gut microbiota producing mainly phenylacetic, phenylpropionic and phenylbutyric acids (Marín et al., 2015). The low concentration of 14C‐labelled metabolites detected indicates controversial results (Depréz et al., 2000). However, these underestimates may be due to the ability of some polyphenols to bind strongly to various molecules in gut cells and dietary fibres in the colonic lumen.
There is currently no data available regarding the intestinal bacteria responsible for the degradation of proanthocyanidins and their metabolites. Hydrolysable tannins are metabolized to gallic acid, pyrogallol, phloroglucinol and finally to acetate and butyrate via several bacterial enzymes (Marín et al., 2015).
In vivo studies
Despite some evidence to the contrary, the OPCs appear to be hydrolyzed into epicatechin in rat isolated small intestine (Zhang et al., 2016). Nevertheless, orally administered procyanidins in rat plasma and trace amounts of procyanidin dimers and trimers in urine (Li et al., 2013) were detected. These results suggest that proanthocyanidin trimers and dimers can be absorbed in vivo, also due to the similarity of the permeability coefficient to that of mannitol, and limited polymer bioavailability in the gut lumen (Ou and Gu, 2014).
Moreover, 16 metabolites, including phenylacetic, phenylpropionic and phenylbutyric acids, have been detected in rat urine after administration of proanthocyanidin isolated from willow tree catechins. The total yields significantly decreased in relation to the polymerization degree of the precursors: catechin monomer > dimer > trimer > polymer (Brenes et al., 2016).
Analysis of luminal content, after administration of grape seed extract (GSE) to Sprague–Dawley rats, showed distinct native and metabolite profiles for each region (caecum and proximal, mid and distal colon) allowing insight to be gained into the distribution and delivery of procyanidins and their microbial metabolites throughout the colon. In particular, procyanidins reached maximum concentrations approximately 3 h postgavage appearing earlier in the more proximal segments, while metabolites reached maximum concentrations from 3 to 18 h post‐gavage appearing later in more distal regions (Goodrich et al., 2015). Recently, the systemic absorption and metabolism of dietary procyanidin B4, after oral administration, were evaluated in pigs, demonstrating that this compound is absorbed as an intact molecule and in part excreted in urine. In addition, it was degraded to the monomeric subunits cathechin and epicathechin that were then further metabolized to methylated and glucuronidated conjugates (Bittner et al., 2014).
Human studies
Unlike condensed tannins, few studies on the bioavailability of hydrolysable tannins are currently available. However, the absorption rate of ellagic acid and gallic acid has been extensively documented. Some studies investigating the absorption of main compounds after oral administration of pomegranate juice detected low concentrations of ellagic acid in plasma after 0.5–3 h, while no intact forms of ellagitannins were detected (Nuñez‐Sánchez et al., 2014).
The low plasma concentration of free ellagic acid is probably due to its low water solubility and to its ability to complex calcium and magnesium ions in the intestine and thus compromise gut absorption (Serrano et al., 2009). The main ellagic acid derivatives have been found in human plasma and urine (Nuñez‐Sánchez et al., 2014). The intestinal absorption of gallic acid in humans is relatively slow (tmax, 87 min), as also found in rats (tmax, 60 min) (Serrano et al., 2009). Two different speeds of gastric absorption have been suggested for this simple phenol: rapid permeation for the unchanged molecule and slow permeation for the metabolites. The main metabolite of gallic acid observed in humans is 4‐O‐methylgallic acid (Serrano et al., 2009).
A plausible mechanism for ellagitannin degradation by human microbiota, via hydrolysis into ellagic acid and its microbial transformation into urolithin B, has been proposed. A study performed on human volunteers fed with a single dose of ellagitannin‐rich dietary sources demonstrated a large inter‐individual variation in the metabolite profiles among subjects within each group, suggesting the involvement of variable colonic microbiota (Espín et al., 2013). It is known that tannins are partially bioavailable for absorption in the gastrointestinal tract, small intestine for hydrolyzable tannins and large intestine for proanthocyanidins (Kamiloglu et al., 2016). These data correlate with some in vitro antioxidant properties ascribed to these tannins (Brenes et al., 2016).
It may be concluded that absorption through the gut barrier is probably limited to the absorption of tannins with a low degree of polymerization and of their metabolites formed in the colon. In view of this, it is critical to evaluate bioavailability studies in order to gain a better understanding of the effects of these phytochemicals on human health. In addition, the above findings highlight the necessity to choose appropriate biomarkers for the in depth study of the pharmacokinetics of these molecules. For some polyphenols, this has already been achieved (Espín et al., 2013; Stalmach et al., 2014). However, there is a great deal still to be done in this area.
Experimental and clinical pharmacology
The biological properties and health effects of tannins have been extensively reviewed (Coppo and Marchese, 2014; Katiyar, 2015; Salvadó et al., 2015; Verstraeten et al., 2015). Many of the in vitro and in vivo (human and animal) studies performed suggest that tannin intake may prevent the onset of several chronic diseases, as reported by recent reviews, systematic reviews and meta‐analysis (Holt et al., 2012; Blumberg et al., 2013; Wang et al., 2014; Salvadó et al., 2015; Turati et al., 2015). The recognized pharmacological activities have been ascribed to dietary monomeric flavan‐3‐ol derivatives and to various crude and purified tannins or proanthocyanidin fractions from the whole plant, fruits, leaves, rhizoma and bark of various medicinal plants (Table 2). Tannins exert their biological effects not only as an un‐absorbable (high MW) complex structure with binding properties that may produce local effects in the gastrointestinal tract but also as absorbable tannins (dimers and trimers) with their metabolites producing systemic effects (Jiménez et al., 2014; Nuñez‐Sánchez et al., 2014; Ou and Gu, 2014; Velderrain‐Rodríguez et al., 2014; Sieniawska, 2015). In addition to their well‐documented free radical scavenging and antioxidant activity, tannins seem able to exert antibacterial, antiviral, anticarcinogenic, anti‐inflammatory, anti‐allergic and vasodilatory effects (Holt et al., 2012; Blumberg et al., 2013; Nile and Park, 2014; Salvadó et al., 2015; Zhang et al., 2016). An overview of the main pharmacological activities ascribed to these compounds is provided in Table 3 and described in detail below.
Table 2.
Main herbal medicines containing tannins and their therapeutic indications. Source: www.ema.europa.eu
| Herbal drugs | Active constituents | Herbal preparations | Therapeutic applications |
|---|---|---|---|
| Agrimonia eupatoria L. (herba) | 3–11% tannins
(mainly proanthocyanidins and small amount of ellagitannins) |
Comminuted herbal substance;
Tincture (1:5), extraction solvent ethanol 45% (V/V); Liquid extract (DER 1:1), extraction solvent ethanol 25% (V/V). |
Mild diarrhoea;
Mild inflammation of the mouth and throat; Minor inflammation of the skin and small superficial wounds. |
| Hamamelis virginiana L. (dried or fresh leaves) | 3–10% tannins (gallotannins and proanthocyanidins) | Tincture prepared from fresh leaves (1:10), extraction solvent ethanol 45%;
Liquid extract prepared from fresh leaves (1:1) extraction solvent ethanol 45% V/V; Liquid extract from dried leaves (1:1) extraction solvent ethanol 30% m/m; Liquid extract from dried leaves (1:2) extraction solvent ethanol 60% V/V. |
Minor skin inflammation and dryness; Temporary relief of the symptoms associated with haemorrhoids, such as itching, burning sensation or pain; Minor inflammatory conditions of the oral mucosa. |
| Vaccinium myrtillus L. (ripe, dry fruits) | Proanthocyanidins | Comminuted herbal substance | Mild diarrhoea; Minor inflammations of the oral mucosa. |
| Quercus robur L. (cortex) | Hydrolyzable tannins (predominant) and proanthocyanidins | Comminuted herbal substance;
Powdered herbal substance; Dried extract (DER 5.0–6.5:1) extraction solvent ethanol 50% m/m. |
Mild diarrhoea;
Minor inflammatory conditions of the oral mucosa and skin; Relief of itching and burning associated with haemorrhoids; |
| Potentilla erecta (L.) Raeusch. (rhizoma) | 5–22% total tannins (15–20% condensed tannins, about 3.5% hydrolyzable tannins) | Comminuted herbal substance;
Tincture (1:5), extraction solvent ethanol 70% (V/V); Tincture (1:5), extraction solvent ethanol 45% (V/V); Liquid extract (DER 1:1), extraction solvent ethanol 25% (V/V); Dry extract (DER 3.5–4.5:1), extraction solvent ethanol 60% (V/V) |
Mild diarrhoea; Minor inflammations of the oral mucosa; |
| Rosa centifoglia L. (dried petals) | Proanthocyanidins | Comminuted herbal substance | Mild inflammations of the oral and pharyngeal mucosa; Minor skin inflammation. |
Table 3.
Main pharmacological activities ascribed to tannins
| Properties | Effects | References |
|---|---|---|
| Antioxidant and radical scavenging | Scavengers of hydroxyl, superoxide, and peroxyl radicals (Proanthocyanidins);
Inhibition of lipid peroxidation and lipoxygenases (Ellagitannins); Stronger antioxidant activity than ascorbic acid or α‐tocopherol (Procyanidins B1 and B3); Dose‐dependent radical scavenging action of galloylated condensed tannins. |
Nile and Park, 2014
Skrovankova et al.,
2015
Kancheva and Kasaikina, 2013 Sieniawska, 2015 |
| Anti‐cancer | Apoptosis p53‐dependent and Bax/Bcl‐2 proteins and caspase‐3 activation mediated in JB6 C141 cells (Grape seed proanthocyanidins);
Reduction of UV‐induced oxidative stress‐mediated phosphorylation and activation of NF‐κB in NHEK cells (Grape seed proanthocyanidins); Inhibition of cellular proliferation and expression of MMP‐2 and −9 in DU145 and LNCaP cells (Grape seed proanthocyanidins); Dose‐dependent inhibition of cell viability, proliferation and apoptosis in 4 T1, HT29 and LoVo cells (Grape seed proanthocyanidins); G0/G1 phase cell cycle arrest with increase in Cip1/p21 protein levels and decrease of cyclins and cyclin‐dependent kinases in MCF‐7 and A‐427 cells (Grape seed proanthocyanidins); Inhibition of KB, CAL‐27, MCF‐7, HT‐29, HCT116, DU145 and LNCaP cells growth and apoptosis induction (blackberry, black raspberry, blueberry, cranberry, red raspberry and strawberry proanthocyanidins);; Decrease in of UVB‐induced immune suppression in mice with induction of IL‐12 (Grape seed proanthocyanidins); Apoptosis induction and metastasis inhibition of 4 T1 murine mammary cancer cells (Grape seed proanthocyanidins); Inhibition of induced mammary tumours in Sprague–Dawley rats (Grape seed proanthocyanidins); In vitro and in vivo inhibition of HT29 cell growth (Grape seed proanthocyanidins); Inhibition of prostate tumour growth and progression in TRAMP mice (Grape seed proanthocyanidins);. |
Gollucke et al.,
2013
Nile and Park, 2014 Skrovankova et al., 2015 Ouédraogo et al., 2011 Sieniawska, 2015 Turati et al., 2015 |
| Antimicrobial | Inhibition of E. coli adherence to uroepithelium (Cranberry proanthocyanidins);
Activity against Staphylococcus aureus, Candida albicans and Campylobacter jejuni (Ellagitannins); Antiherpetic activity (hydrolyzable tannins); Inhibition of cytopathic effects of HIV (hydrolyzable tannins); Inhibition of HIV‐antigen expression in human lymphotropic virus type I‐positive MT‐4 cells (hydrolyzable tannins); Activity against Helicobacter pylori (hydrolyzable tannins). |
Marín et al.,
2015
Nile and Park, 2014
Sieniawska, 2015 de Jesus, 2012 |
| Anti‐nutrient | Inhibition of gastrointestinal enzymes (Proanthocyanidins);
Reducing capacity on Cr, Fe and Cu with alteration of their absorption (Proanthocyanidins); Modulation of chronic inflammatory bowl diseases (Procyanidin B3). |
Nile and Park, 2014
Sieniawska, 2015
Li et al., 2012 Dias et al., 2016 |
| Cardioprotective | Hypocholesterolemic effects (Proanthocyanidins);
Preventing myocardial ischaemic injury in adult rats (Proanthocyanidins); Cardioprotection in an experimental model of ischaemia–reperfusion damage (Proanthocyanidins); Anti‐atherosclerosi effect due to inhibition of differentiation of monocyte to macrophages (Oligomeric proanthocyanidins isolated from Crataegus oxyacantha L.); Protective effect on doxorubicin‐induced cardiac toxicity in rats (Grape‐seed proanthocyanidins); Suppression of doxorubicin‐induced electrocardiographic and biochemical changes (Grape‐seed proanthocyanidins); Protection against acute ischaemic brain damage in rats (Proanthocyanidins); Vascular protective effects (Procyanidin B2); Improved serum antioxidant status and decreased serum C‐reactive protein and plasma homocysteine concentrations (Proanthocyanidins); Decreased systolic and diastolic blood pressure (Grape‐wine proanthocyanidins). |
Blumberg et al.,
2013
Wang et al.,
2014
Holt et al., 2012 Guler et al., 2011 Mohana et al., 2015 Ammar et al., 2013 Yunoki et al., 2014 Yang et al., 2013 |
| Anti‐diabetic and anti‐obesity | Inhibition of salivary and pancreatic α‐amylase and α‐glucosidase activity (Hydrolyzable tannins and proanthocyanidins);
Stimulation of glucose uptake and glycogen and lipid synthesis (hydrolyzable tannins and proanthocyanidins); Reduced plasmatic insulin and improved homeostatic model assessment (HOMA) index (hydrolyzable tannins and proanthocyanidins); Modulation of the active glucagon‐like peptide‐1 (GLP‐1) levels (hydrolyzable tannins and proanthocyanidins); Hypocholesterolemic effect (tannin‐rich fibre); Acute effects on postprandial lipaemia, vascular function and blood pressure (Pomegranate ellagitannins); Insulinomimetic properties GLUT4 mediated (Grape seed proanthocyanidins); Insulin anabolic‐like and lipostabilizer properties (Grape seed proanthocyanidins); Inhibition of diabetes‐induced cataract formation in rats (Cacao proanthocyanidins); Increased antioxidant enzyme activities, induction of metal chelation activity, reduced resistin formation, and inhibition or activation of transcriptional factors such as NF‐kB and PPARɣ (Proanthocyanidins). |
Gonzalez‐Abuin et al.,
2015
Banihani et al.,
2013
Pinent et al., 2012 Gato et al., 2013 Mathew et al., 2012 Kooti et al., 2016 Cascaes et al., 2015 Cock, 2015 Stohs and Ray, 2015 Salvadó et al., 2015 |
Antioxidant and radical scavenging activity
Most activities of the proanthocyanidins and hydrolyzable tannins, including antioxidant and free radical‐scavenging capacity, largely depend on their structure; for example, an increase in anti‐radical effects was observed with an increase in the degree of polymerization (Sieniawska, 2015).
Experimental pharmacology
Proanthocyanidins are known to inhibit lipid peroxidation and lipoxygenases in vitro, and several studies have demonstrated their ability to scavenge hydroxyl, superoxide and peroxyl radicals, helping to restore the oxidative balance of the body (Georgiev et al., 2014). In some instances, some procyanidins such as procyanidin B1 and procyanidin B3 have been recognized to be stronger antioxidants than ascorbic acid or α‐tocopherol (Iglesias et al., 2012). These results observed in vitro have also been confirmed by several in vivo studies on animal models (Kancheva and Kasaikina, 2013; Nile and Park, 2014; Sieniawska, 2015; Skrovankova et al., 2015).
Fushimi et al. (2015) have investigated the antioxidant effects of unripe and mature persimmon in rats. Results showed that plasma phosphatidylcholine hydroperoxide levels, a biomarker of membrane lipid peroxidation, were significantly lower in the unripe persimmon group than in the control group, while no significant difference was observed between the mature persimmon group and the control group. The authors suggested that soluble tannins, found more in unripe fruits, could contribute to the difference in the antioxidant effect observed (Fushimi et al., 2015). A few years earlier, another study had shown that several proanthocyanidin‐type dimers, from peanut skins and persimmon pulp, possess high dose‐dependent antioxidant potency. B‐type dimers showed the highest radical scavenging activity in aqueous systems while A and B‐type dimers showed similar antioxidant potency in tissue or lipid systems highlighting the importance of the food matrix effect (Dong et al., 2013).
Protection against lipid peroxidation has also been observed in several tannin‐rich plants. For example, the ethanolic extract of Nigella sativa L. was found to counteract in vivo Fe(II)‐induced lipid peroxidation (Hassan et al., 2016). Also, Centella asiatica L. and its fractions were found to reduce lipid peroxidation induced by quinolinic acid and sodium nitroprusside in the rat brain (Marques et al., 2015).
Free radical‐scavenging activity of OPCs from Rhodiola rosea L. has been evaluated in mice serum, heart, liver and brain tissues. These compounds enhanced SOD and GSH peroxidase (GPx) activity by reducing malondialdehyde (MDA) content (Zhou et al., 2014). Another interesting and recent study has evaluated the protective effect of grape seed proanthocyanidins (GSPs) on cadmium‐induced renal toxicity (Nazima et al., 2015). Proanthocyanidins were also found able to improve lead‐induced cognitive impairments by blocking endoplasmic reticulum stress and NF ‐κB‐mediated inflammatory pathways in rats (Liu et al., 2014).
A meta‐analysis of experimental studies regarding the efficacy of proanthocyanidins against oxidative damage has recently been performed (Li et al., 2015a). Compared with the control group, proanthocyanidins significantly improved total antioxidative capacity, SOD, GSH, GPx and catalase (CAT) and reduced the MDA levels. Proanthocyanidins therefore effectively antagonize oxidative damage and enhance antioxidant capacity, but the antagonistic effect may be related to intervention time, intervention method and biological sample from which the indexes are estimated. The authors observed, in fact, significant differences in the effects of proanthocyanidins in relation to themode of administration (gavage vs. feeding) and dependent on the biological matrix analysed (Li et al., 2015a).
Clinical pharmacology
At present, there is only limited clinical evidence available about the antioxidant properties of hydrolyzable tannins and proanthocyanidins. Three pilot studies on human volunteers have recently been carried out using a French oak wood (Quercus robur L.) extract rich in roburin (a dimeric tannin), known with the brand name of Robuvit®. The first examined the pharmacokinetics and effects at transcriptome level of this formulation in healthy volunteers and showed that Robuvit® metabolites affected ribosomes, cell cycle and spliceosome pathways (Natella et al., 2014). The second study, which analysed the effect of 1 month intake of Robuvit® (300 mg·day−1) on oxidative stress markers in the plasma of healthy volunteers, demonstrated a decrease of the advanced oxidation protein products and lipid peroxides, an increased SOD and CAT activity and total antioxidant capacity (Horvathova et al., 2014). The third study, which was the most recent, showed Robuvit® to have no significant influence on GSH levels and paraoxonase (an antioxidant enzyme) activity, although homocysteine and cysteine levels decreased significantly (Deáková et al., 2015).
Finally, a double‐blind crossover trial was performed on 18 volunteers to evaluate the effects of procyanidin‐rich chocolate on fecal free radical production and antioxidant activity. Subjects were fed a low‐polyphenol diet and chocolate for two 4 week periods separated by a 4 week washout period. The authors observed a decreased free radical production in fecal water with respect to control group, suggesting that chocolate proanthocyanidins may be effective in rebalancing redox status (Sieniawska, 2015).
Anti‐cancer properties
Many studies, carried out to highlight the mechanisms of action of proanthocyanidins, have identified several molecular targets potentially useful for the prevention and treatment of cancer. It should be stated, however, that knowledge in this area is still at an embryonic stage, and further studies are needed to better clarify the anti‐cancer properties of these compounds.
Experimental pharmacology
Proanthocyanidins have been extensively studied on a wide range of cancer cells. GSPs induced p53‐dependent apoptosis on JB6 C141 mouse skin epidermal cells, through involvement of Bax/Bcl‐2 proteins and caspase‐3 activation (Gollucke et al., 2013). Furthermore, inhibition of the phosphorylation of proteins belonging to the MAPK family and activation of NF‐κB and its related genes were observed in normal human epidermal keratinocytes (Gollucke et al., 2013). The same molecular mechanisms were found to be involved in the inhibition of cell growth and in the down‐regulation of MMP‐2 and ‐9 in both androgen‐insensitive (DU145) and in androgen‐sensitive human prostate carcinoma cells (LNCaP) (Gollucke et al., 2013) on which GSPs also seem able to regulate androgen receptor‐mediated transcription through potent anti‐histone acetyltransferase activity (Park et al., 2011). The down‐regulation of these MMPs , involved in tumour development and metastasis, was also observed in pancreatic carcinoma cells (Chung et al., 2012). The inhibition of cell viability and proliferation and the pro‐apoptotic activity of GSPs were also observed on mouse mammary tumour 4T1 and colorectal cancer HT29 and LoVo cell lines (Sieniawska, 2015). GSPs interfered with normal regulation of cell‐cycle progression inducing an up‐regulation of Cip1/p21 and Kip1/p27 protein levels together with a down‐regulation of cyclins (D1, D2 and E) and cyclin‐dependent kinases (CDK2, CDK4 and CDK6) leading to Go/G1 phase arrest (Prasad and Katiyar, 2014). Concentration‐dependent effects of GSPs were observed on breast and lung cancer and gastric adenocarcinoma cells (Gollucke et al., 2013).
Besides GSPs, grapes and pine bark procyanidin‐rich fractions (with the highest percentage of galloylation and mean degree of polymerization) also efficiently inhibit cell proliferation, arresting the cell cycle in G2 phase and inducing apoptosis, as observed in HT29 human colorectal cancer cells (Ouédraogo et al., 2011). In vitro anti‐proliferative and pro‐apoptotic effects were also observed with several berry proanthocyanidin‐rich extracts on human oral (KB, CAL‐27), breast (MCF‐7), colon (HT‐29, HCT116) and prostate (LNCaP) cancer cells (Nile and Park, 2014; Skrovankova et al., 2015).
The in vitro anti‐cancer properties of proanthocyanidins have been confirmed by several animal studies. Dietary feeding of GSPs was found to inhibit UVB‐induced photocarcinogenesis in SKH‐1 hairless mice. This event seems to be correlated with the ability of these compounds to decrease UVB‐induced immune suppression in mice, by enhancing the production of IL‐12 and reducing the expression of IL‐10 (Gollucke et al., 2013; Sieniawska, 2015). A study performed in immunocompetent Balb/c mice fed with GSPs showed tumour‐growth inhibition with an increase in the survival period and inhibition of metastatic status (Gollucke et al., 2013). Similar results were observed in other in vivo cancer models (Gollucke et al., 2013). Moreover, it has been demonstrated that GSPs inhibit the spontaneous development of prostate cancer in male TRAMP mice (Sieniawska, 2015).
Clinical pharmacology
Although a number of experimental studies have been published, few clinical trials on the anti‐cancer properties of proanthocyanidins are available, to date.
A double‐blind, placebo‐controlled, randomized phase II trial was planned to elucidate the effects of supplementation with GSP extract (300 mg·day−1 for 6 months) in patients affected by radiation‐induced breast induration. At 12 months post‐randomization, ≥50% reduction in surface area (cm2) of breast induration in 29.5% of GSPs extract treated patients was recorded, while no significant difference between treatment and control groups in terms of external assessments of tissue hardness, breast appearance or patient self‐assessments of breast hardness, pain or tenderness was recorded (Sieniawska, 2015).
Proanthocyanidins have been also evaluated for their potential to attenuate the adverse effects of cancer radiotherapy. A randomized double blind, placebo‐controlled, pilot study was conducted using standardized cranberry capsules, containing 72 mg of proanthocyanidins, for the prevention and treatment of radiation cystitis in prostate cancer patients. The authors observed a statistically significant decrease in cystitis incidence in treated patients (65%) compared with control group (90%); severe cystitis was reported in 30% of treated patients and 45% in the control group. Moreover, the incidence of pain and burning was significantly lower in treated group. The results demonstrate that patients who receive radiation therapy for prostate cancer, particularly those on low hydration regimens or with baseline urinary symptoms, may benefit from cranberry supplementation (Hamilton et al., 2015).
Antimicrobial activity
The antimicrobial properties of proanthocyanidins and HTs present in many medicinal plants and foods are well documented. These phytochemicals seem to affect bacterial growth via several mechanisms such as inhibition of extracellular enzymes, deprivation of microbial essential substrates (for example by complexing metal ions), disintegration of bacterial outer membrane with cytoplasm leak or by direct action on microbial metabolism. Furthermore, these compounds can interfere with microbial cell wall polypeptides reacting with sulfhydryl groups, often leading to loss of function of the membrane proteins (Marín et al., 2015).
Experimental pharmacology
The anti‐adhesion properties of berry fruits and derived products have long been known. Cranberry juice, for example, inhibits E. coli adherence to uroepithelium. However, the molecular structure of proanthocyanidins seems to play a major role in conferring bacterial anti‐adhesion activity to each matrix. In fact, cranberry proanthocyanidins, characterized by a series of catechin oligomers with one or more A‐type linkages – and therefore structurally quite different with respect to proanthocyanidins from other sources like grape and apple– have been observed to be the only ones able to elicit bacterial anti‐adhesion activity in humans (Micali et al., 2014). These results have been confirmed by several animal studies, which suggest that cranberry proanthocyanidins and/or their metabolites are able to inhibit bacterial adhesion to uroepithelium (Micali et al., 2014).
Cloudberry, raspberry and strawberry extracts, rich in ellagitannins, were found to be the best inhibitors against Campylobacter jejuni and Candida albicans (Lipińska et al., 2014). Bilberry and blueberry extracts showed bacteriostatic activity on Gram‐positive bacteria (Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis and Enterococcus fecalis) and Gram‐negative bacteria (Citrobacterfreundii, E. coli, Pseudomonas aeruginosa and Salmonella enteric ser. Typhimurium) (Nile and Park, 2014). The bacteria most sensitive to berry extracts were Helicobacter pylori and Bacillus cereus, while yeasts were found to be resistant to the extracts (Lipińska et al., 2014).
Several ellagitannin‐rich plants have shown antibacterial properties. For example, Pteleopsis hylodendron Mildbr. extracts, containing mainly ellagic acid, were active against Klebsiella pneumoniae, B. cereus, E. coli and Salmonella typhi (Marín et al., 2015). Pomegranate peel extract has also been observed to be active against S. aureus, S. typhi, L. monocytogenes and E. coli. Ellagic acid was found able to inhibit biofilm formation by S. aureus, including themethicillin‐resistant strains, and E. coli, as well as by the fungal pathogen C. albicans (Bakkiyaraj et al., 2013). Antifungal properties against C. albicans, C. neoformans and Aspergillus fumigates have been shown by this compound as well as by punicalagin, punicalin and gallagic acids (Marín et al., 2015).
Antimalarial activity against chloroquine and mefloquine‐resistant Plasmodium falciparum has been reported for ellagic acid, which showed synergy when combined with conventional antimalarial drugs (Marín et al., 2015).
Antiviral activity has also been described and seems to be strictly related to the tannin structure. The most active tannins appear to be the most cytotoxic. Several studies have suggested that tannins may interfere with Herpes simplex virus absorption as well as the absorption of HIV and its cytopathic effects (Lipińska et al., 2014; Ekambaram et al., 2016). This mechanism seems to be related to the capacity of tannins to bind the viral envelope components. Furthermore, ellagitannins and several proanthocyanidins have been demonstrated to be potent reverse transcriptase inhibitors (Ekambaram et al., 2016). One hydrolyzable tannin, ellagic acid, also shows specific antiviral activity against hepatitis B virus (HBV) by inhibiting HBeAg secretion in HBV‐infected cells (Marín et al., 2015).
Clinical pharmacology
There are several clinical studies on dietary supplements containing ellagitannins or proanthocyanidins and their antimicrobial properties and the most recent are discussed below.
A randomized, double‐blind, placebo‐controlled, acute study was performed to evaluate the E. coli anti‐adhesion activity following administration of several cranberry extracts, containing a standardized level of 36 mg proanthocyanidins, in healthy subjects. The authors observed, for all formulations studied, a significantly anti‐adhesion effect after intake of a single dose of cranberry extracts compared with the control group (Howell et al., 2015).
Another randomized, placebo‐controlled study was carried out in order to clarify how cranberry fruit powder (500 mg·per day for 6 months), with a standardized 0.56% proanthocyanidin content, could prevent recurrent urinary tract infection (UTI) in 182 women with two or more UTI episodes in the previous year. Results showed significantly fewer UTIs in the cranberry group with respect to the control group (10.8% vs. 25.8% respectively). The treated group also showed a much longer latency period before developing a new UTI episode than the placebo group (Vostalova et al., 2015). It should be mentioned, however, that the ability of cranberry to prevent UTIs has been recently questioned (Jepson et al., 2013; Izzo et al., 2016).
The health benefits of pomegranate consumption are attributed, as reported above, to ellagitannins and their metabolite content. In a recent study, performed on 20 healthy volunteers consuming 1000 mg·day−1 of pomegranate extract for 4 weeks, three different behaviours – based on urinary and fecal content of the pomegranate metabolite urolithin A – were observed: 1) in nine subjects no baseline urolithin A was present but its formation was induced by pomegranate extract consumption; 2) in five subjects baseline urolithin A formation was present and enhanced by pomegranate extract consumption; and 3) in six subjects there was no baseline urolithin A production and none was induced. Results showed an increase of Actinobacteria and Verrucomicrobia phyla and a decrease of Firmicutes in urolithin A producers with respect to baseline values. After 4 weeks, Enterobacter, Escherichia, Lactobacillus, Prevotella, Serratia and Veillonella genders also increased, in urolithin A producers compared with baseline values, while Collinsella decreased significantly. On this basis, the authors suggested that health benefits may be induced by pomegranate extract consumption due to changes in the microbiota (Li et al., 2015b).
Anti‐nutrient activity
Tannins, known to be astringent compounds due to their ability to complex and precipitate proteins, particularly proline‐rich proteins, are present in the human saliva through hydrophilic and hydrophobic interactions. Recently, a study was performed to elucidate the interaction between procyanidin B3, a common food tannin, and different wheat‐derived proline‐rich peptide fractions, responsible for the onset of celiac disease (CD). This study identified several soluble B3‐peptide complexes containing immunoreactive peptides, of different sizes and diversity in CD epitopes, demonstrating the potential beneficial effects of proanthocyanidins as a nutritional approach in the modulation of gut diseases (Dias et al., 2016).
The complexing ability of proanthocyanidins can also inhibit enzymes (pectinase, amylase, lipase, protease and β‐galactosidase) and may interfere with absorption of other compounds like proteins and carbohydrates. Several in vitro studies have supported this claim. However, the in vivo studies have not confirmed it, except at high doses, probably due to adaptation mechanisms such as induction of bile acids secretion in the gut and increase of proline‐rich salivary proteins (Brandão et al., 2014).
The limited absorption through the gut barrier of proanthocyanidins with a high degree of polymerization and the relatively weak affinity of those with a low polymerization degree, for proteins, have led to the hypothesis that non‐specific binding of proanthocyanidins to proteins is involved in the biological properties exerted by these compounds in inner tissues (Sieniawska, 2015). On the contrary, greater affinity to the shortest human saliva peptides has been observed (procyanidins C2 > B1 > B3) (Cala et al., 2012). Tannins have also been found to possess reductive capacity for some metals, for example Cr, Fe and Cu, thereby reducing their absorption (Sieniawska, 2015; Li et al., 2012).
No data are available from human studies about tannins and their anti‐nutritional effects.
Cardioprotective properties
The links between proanthocyanidin intake and cardioprotective properties have been extensively reviewed (Kay et al., 2012; Wang et al., 2014; Sieniawska, 2015; Bladé et al., 2016). A number of in vitro and animal studies have recently been performed to elucidate the role of proanthocyanidins as promising molecules that could prevent the development of several coronary syndromes by inhibiting the atherogenic process and balancing blood pressure and lipid homeostasis (Hort et al., 2012; Pons et al., 2014; Quifer‐Rada et al., 2016).
Recently, other mechanisms of action have been proposed. For example, an in vitro and in vivo study showed inhibition of monocyte to macrophages differentiation in atherosclerosis, by OPC isolated from Crataegus oxyacantha L. In summary, the OPCs decreased vascular cell adhesion protein 1 andchemokine CCL2 levels, down‐regulating the inflammatory pathway and macrophage markers, MMP 2 and 9 and PPARγ. These results highlight the potential role of these phytochemicals in the initial stages of atherosclerosis development and in overt disease. dependent on macrophage function (Mohana et al., 2015). Another animal study evaluated the cardio‐protective effect of GSPs on doxorubicin‐induced cardiac toxicity in rats. Administration of proanthocyanidins significantly suppressed doxorubicin‐induced electrocardiographic changes and normalized the aconitine dose producing ventricular tachycardia. Furthermore, proanthocyanidins significantly suppressed the biochemical changes induced by doxorubicin on creatine kinase‐myocardial band, LDH, MDA, SOD, CAT and reduced GSH (Ammar et al., 2013).
GSPs have also been found to possess antioxidant, anti‐inflammatory and antiapoptotic effects through which they are able to protect the liver against ischaemia/reperfusion injury by attenuating endoplasmic reticulum stress (Xu et al., 2015). In addition, there is evidence that a proanthocyanidin‐rich diet protects against acute ischaemic brain damage in rats, improving motor function, reducing cerebral infarction volume and decreasing both peroxidative markers such as 4‐hydroxynonenal, advanced glycation end products, 8‐hydroxy‐2‐deoxyguanosine as well as inflammatory markers such as CCL2, ionized calcium‐binding adapter molecule‐1 and TNF‐α (Yunoki et al., 2014).
Finally, another recent study investigating whether procyanidin B2 inhibits NLRP3 inflammasome activation in endothelial cells showed a significant down‐regulation of NLRP3 inflammasome in HUVECs followed by an inhibition of caspase‐1 activation and IL‐1β secretion in response to LPS. In addition, procyanidin B2 reduced LPS‐induced formation of ROS and down‐regulated the transcriptional activity of activator protein‐1 (Yang et al., 2014).
Clinical pharmacology
A cross‐sectional study has recently demonstrated the potential role of dietary antioxidants, including proanthocyanidins, in the prevention of cardiovascular diseases because they are able to improve serum antioxidant status and decrease serum C‐reactive protein and plasma homocysteine concentrations (Yang et al., 2013).
In a double‐blind, placebo‐controlled crossover study, the effect of two grape extracts (grape‐red wine and grape alone) on blood pressure and vascular function was assessed in mildly hypertensive subjects. Results showed that only the grape wine extract, rich in catechins and proanthocyanidins, was effective in decreasing the 24‐hour ambulatory systolic and diastolic blood pressure and the plasma concentration of the vasoconstrictor endothelin‐1, demonstrating that catechins and procyanidin‐rich food products may help to maintain physiological blood pressure and contribute to preventing the onset of heart disease (Draijer et al., 2015).
Also, a randomized, double‐blind, placebo‐controlled study performed in women affected by menopausal symptoms supplemented with GSPs tablets showed an improvement of physical and psychological symptoms (Terauchi et al., 2014).
In conclusion, while several in vitro and animal studies are available, clinical trials are sparse and have yielded controversial results. Further human studies are needed to confirm the preliminary findings obtained so far.
Metabolic disorders
A number of reviews and systematic reviews have recently been published about the in vitro and animal studies available, elucidating the role of proanthocyanidin‐rich plant extract in the treatment of diabetes, diabetic complications and other metabolic disorders like obesity (Bertoia et al., 2015; Gonzalez‐Abuin et al., 2015; Akaberi and Hosseinzadeh, 2016; Bladé et al., 2016). They highlight the main pharmacological mechanisms by which tannins may act on metabolic disorders.
Experimental pharmacology
Several molecular mechanisms and molecular targets have been proposed to explain the role of proanthocyanidins and hydrolyzable tannins in metabolic disorders. These phytochemicals have been found able to: (i) stimulate glucose uptake and glycogen and lipid synthesis by activation of insulin receptors, PKB, p44/42 and p38 MAPKs signalling pathways and translocation of glucose transporter type 4 (GLUT4) to the plasma membrane; (ii) exert insulin‐like effect on insulin‐sensitive tissues; (iii) delay the onset of insulin‐dependent diabetes mellitus by regulating the antioxidant status of pancreatic beta cells; (iv) reduce plasmatic insulin and improve the homeostatic model assessment (HOMA) index by down‐regulation of PPARɣ2, GLUT4 and insulin receptor substrate 1 in mesenteric white adipose tissue (WAT); (v) modulate active glucagon‐like peptide‐1 levels; (vi) inhibit intestinal enzymes like α‐amylase (proanthocyanidins) and α‐glucosidase (hydrolyzable tannins); (vii) inhibit both salivary and pancreatic amylase; and (viii) induce triglyceride turnover (Banihani et al., 2013; Cascaes et al., 2015; Cock, 2015; Gonzalez‐Abuin et al., 2015; Salvadó et al., 2015; Stohs and Ray, 2015; Kooti et al., 2016).
Clinical pharmacology
Despite the wealth of experimental evidence available regarding tannins and metabolic disorders, human studies are still scarce, and findings to date are controversial.
A randomized, double‐blind, placebo‐controlled trial, which investigated the hypocholesterolemic effects of persimmon fruit tannin‐rich fibre, showed a significant decrease of total plasma cholesterol levels as well as of LDL cholesterol levels with no changes in plasma HDL cholesterol or plasma triglyceride levels were observed (Gato et al., 2013).
Another randomized, controlled crossover trial was conducted on 19 young, healthy men with an energy drink (containing ellagitannin‐rich pomegranate extract). Results showed no significant differences in terms of capillary plasma triacylglycerol (TAG) between treated groups and control group while systolic blood pressure was found to be higher in treated groups than the control group. No appreciable differences were observed for reflection and stiffness indices or diastolic blood pressure (Mathew et al., 2012).
Safety and toxicity
When discussing t safety, it is important to make a distinction between biofunctional constituents in foods and the same molecules as purified or semi‐purified compounds, as found in dietary supplements. The latter, in fact, contain high amounts of bioactive compounds, and their contribution to overall dietary intake of tannins needs to be taken into account. The dangers of diets containing very high levels of these compounds together with food‐based polyphenol enrichment and supplements with purified agents or mixtures have not been studied in detail (Margină et al., 2015). This fact makes polyphenol consumption potentially problematic in terms of alterations in bioavailability and the consequent possible occurrence of side effects due to pharmacokinetic interactions. In theory, the risk related to dietary ingestion of these substances is very low due to their poor bioavailability. Moreover, there are encouraging results from some epidemiological studies reporting a dietary intake of more than 1 g·day−1 (i.e. related to eating habits) (Kumari and Jain, 2012) to be associated with a reduced onset of many chronic diseases (Sieniawska, 2015). However, further studies on the potential adverse events that might be associated with high intakes of these constituents are needed.
Animal studies
Tannin toxicity is based essentially on three mechanisms: (i) microbial enzyme inhibition (i.e. cellulases, pectinases, xylanases, GPx, laccase and glycosyltransferase) and substrates for decreased microbial growth (polysaccharides such as pectins, hemicelulose or polygalacturonic acid and less frequently peptides or aminoacids probably due to the lower affinity of tannins for these small molecules with respect to polymers), closely related to tannin‐protein interactions; (ii) action on membranes (inactivation of membrane‐bound proteins; inhibition of oxidative phosphorylation acting as ionophores; inhibition of electron transport system); and (iii) chelation of metal ions (e.g. iron and zinc) (Guil‐Guerrero et al., 2016).
Toxicology studies on tannic acid have so far been limited to the evaluation of genotoxicity, short‐term repeat‐dose toxicity and carcinogenicity. For example, a subchronic toxicity study, in female rats, demonstrated that a dietary intake of 2.5% grape seed or grape skin extract produced a no‐observed‐adverse effect level (NOAEL) (Serrano et al., 2016). Subchronic oral toxicity of GSE was also examined in another study on rats; the NOAEL of dietary GSE was found equal to 1.4 g·kg−1 body weight·day−1 in males and 1.5 g·kg−1 body weight·day−1 in females (Fiume et al., 2014). Furthermore, the LD50, after grape seed and skin extract ingestion in rats, was found to be higher than 5 g·kg−1 body weight.
Although numerous studies on the mutagenicity/genotoxicity are available (Kaur et al., 2000; Lehmann et al., 2000; Fuentes et al., 2006; de Rezende et al., 2009; Lluís et al., 2011), many of them are not validated for evaluating genotoxic risk or do not conform with modern protocols as outlined in the current OECD methodological guidelines, so further investigations in this field are highly recommended. Furthermore, no studies on reproductive toxicology are available. What can be said is that hydrolysable tannins are poorly absorbed per se by the gut lumen because they are extensively degraded in the gastrointestinal tract. Furthermore, their metabolites in target species, experimental animals and humans are very similar and appear to be efficiently excreted, so the expected lack of residues in foods of animal origin suggests that it is unlikely that consumers will receive an appreciable amount of the parent compound or its metabolites such as to exert a potentially toxic effect (EFSA, 2014).
Human studies
Although many in vitro and in vivo studies on tannins, and in particular proanthocyanidins, have provided important information with respect to the potential health effects of dietary intake of these compounds, to date, only 35 clinical trials are available, of which 27 are complete, five ongoing and three with unknown status (www.clinicaltrials.gov) (Table 4). Very few of these studies have been performed on potentially more susceptible subjects such as postmenopausal women, and there are even fewer or no studies at all relating to other vulnerable groups, including pregnant and lactating women (Smeriglio et al., 2014b), children and the elderly. Information regarding potential adverse events cannot be more comprehensively assessed until this data becomes available.
Table 4.
Current available list of completed clinical trials provided by U.S. National Institutes of Health (www.clinicaltrials.gov)
| Number ID | Title | Subjects enrolled |
|---|---|---|
| NCT01688154 | Ability of Grape Seed Proanthocyanidins to Reduce Postprandial Triglycerides in Humans | Both (20–40 years) |
| NCT00742287 | Cardiovascular Effects of Oligomeric Procyanidins (OPCs) in Smokers | Male (30–60 years) |
| NCT01483508 | Absorption and Metabolism of Dietary Cocoa Procyanidins in Humans | Both (18–70 years) |
| NCT01687114 | Urinary Proanthocyanidin‐A2 as a Biomarker of Compliance to Intake of Cranberry Products | Both (20–40 years) |
| NCT02515929 | Prospective Double‐Blind Randomized Controlled Clinical Trial in the Gingivitis Prevention With OPCs | Both (18–50 years) |
| NCT01707615 | Beneficial Effects of Grape Seed Proanthocyanidin Extrat on Progression of Atherosclerotic Plaques in Clinical Use | Both (43–75 years) |
| NCT02408289 | The Randomized Controlled Cocoa‐Appetite Trial | Male (18–35 years) |
| NCT02039648 | The Influence of Rumex Acetosa L on the Intraoral Colonization With Porphyromonas Gingivalis | Both (>18 years) |
| NCT01219595 | Cranberry Proanthocyanidins for Modification of Intestinal E. Coli Flora and Prevention of Urinary Tract Infections in UTI‐Susceptible Women | Female (18–65 years) |
| NCT01669317 | Mechanisms Underlying the Sleep Promoting Effect of Cherry Juice Standardized to Its Proanthocyanidin Content | Both (>65 years) |
| NCT01099150 | Dark Chocolate and Platelet Function in Humans | Both (18–70 years) |
| NCT00100893 | IH636 Grape Seed Extract in Preventing Breast Cancer in Postmenopausal Women at Risk of Developing Breast Cancer | Female (40–75 years) |
| NCT02087735 | Measurement of Urinary Catabolites of PACs as Biomarkers of Consumption of Cranberry Extracts | Female (18–40 years) |
| NCT01969994 | Absorption, Metabolism, and Excretion of (−)‐[2‐14C]Epicatechin in Humans | Male (18–50 years) |
| NCT01847053 | Bioavailability Study of Cinnamon in Healthy Subjects | Male (18–40 years) |
| NCT01010841 | Trial of Two Dietary Programs on Cardiometabolic Risk Factors in Subjects With Metabolic Syndrome | Female (20–75 years) |
| NCT01289860 | Investigating the Acute Effects of Flavonoids in Blueberries on Cognitive Function. | Both (18–75 years) |
| NCT00568152 | Effect of Apple Flavanols on Risk of Cardiovascular Disease | Both (19–64 years) |
| NCT02063477 | Effect of Oligopin® on Blood Pressure. | Both (>18 years) |
| NCT00713167 | The Efficacy of Red Grape Seed Extract on Lipid Profile and Oxidized Low‐Density Lipoprotein (OX‐LDL) | Both (21–64 years) |
| NCT01398150 | Cranberry Enhances Human Immune Function and Reduces Illness | Both (18–50 years) |
| NCT00740077 | Bioavailability of Flavonoids and Phenolic Acids From Cranberry Juice Cocktail in Healthy Older Adults | Both (50–70 years) |
| NCT01691430 | A Trial of Cranberry Capsules for Urinary Tract Infection Prevention in Nursing Home Residents | Female (>65 years) |
| NCT00318019 | Effect of OPC Factor on Energy Levels | Both (45–65 years) |
| NCT02333461 | Evaluation of Botanicals for Mechanisms Related to Appetite and Fat Metabolism | Both (18–70 years) |
| NCT01346774 | Preventing Urinary Tract Infection Post‐Surgery | Female (>18 years) |
| NCT01681394 | Effect of the Administration of a Polyphenol‐rich Cocoa Extract on Peripheral Blood Mononuclear Cells Gene Expression | Male (18–40 years) |
Conclusion
Tannins are bioactive compounds that are widely found in nature, and commonly present in edible and medicinal plants. Nowadays, most of the interest in this field derives from the possible implications of tannin intake for disease prevention, and, to this end, significant advances in understanding the biological properties of these bioactive compounds have been made. Current epidemiological and clinical data have shown a very preliminary correlation between tannin intake and health benefits in humans, although further studies are necessary to better investigate the effect of tannins in particular categories of the population. However, there are still numerous issues to be addressed. One of these is the lack of scientific rigour, especially as far as experimental design is concerned. The common use of non‐standardized food or plant extracts generates results that are often controversial and difficult to interpret. Furthermore, studies on the pharmacological targets and the mechanisms of action by which tannins exert their biological effects in vivo are still scarce. Further studies on the pharmacokinetic, safety and toxicological features should be performed to gain a better understanding of bioavailability, metabolism, tissue distribution and behaviour of these complex molecules, also in relation to other bioactive compounds in order to identify possible interactions and adverse effects that may arise from the co‐administration or by use of dietary supplements. In some cases, polyphenol intake can easily reach very high levels normally not encountered in the typical diet. Given the peculiar pharmacokinetics of tannins, there are other aspects that should not be overlooked, that is the food matrix constituents which could exert their effects in the intestinal lumen. Also, the genetic aspects of individuals that could affect gut cell uptake. Finally, individual variations in microbiota can affect the metabolism and, thus, the ultimate health effect in some cases. In our opinion, all these aspects should be taken into account, and an appropriate risk–benefit assessment should be made before making claims about the health effects in humans.
Conflict of interest
The authors declare no conflicts of interest.
Smeriglio, A. , Barreca, D. , Bellocco, E. , and Trombetta, D. (2017) Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. British Journal of Pharmacology, 174: 1244–1262. doi: 10.1111/bph.13630.
References
- Akaberi M, Hosseinzadeh H (2016). Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother Res 30: 540–556. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G Protein‐Coupled Receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015f). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar el‐SM, Said SA, El‐Damarawy SL, Suddek GM (2013). Cardioprotective effect of grape-seed proanthocyanidins on doxorubicin-induced cardiac toxicity in rats. Pharm Biol 51: 339–344. [DOI] [PubMed] [Google Scholar]
- Bakkiyaraj D, Nandhini JR, Malathy B, Pandian SK (2013). The anti‐biofilm potential of pomegranate (Punicagranatum L.) extract against human bacterial and fungal pathogens. Biofouling 29: 929–937. [DOI] [PubMed] [Google Scholar]
- Banihani S, Swedan S, Alguraan Z (2013). Pomegranate and type 2 diabetes. Nutr Res 33: 341–348. [DOI] [PubMed] [Google Scholar]
- Barreca D, Laganà G, Ficarra S, Tellone E, Leuzzi U, Galtieri A et al. (2011). Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Annona cherimola Mill. (Annonaceae. Food Res Int 44: 2302–2310. [Google Scholar]
- Berry AC, Nakshabendi R, Abidali H, Atchaneeyasakul K, Dholaria K, Johnson C et al. (2016). Adverse Effects of Grape Seed Extract Supplement: A Clinical Case and Long‐Term Follow‐Up. J Diet Suppl 13: 232–235. [DOI] [PubMed] [Google Scholar]
- Bertoia ML, Rimm EB, Mukamal KJ, Hu FB, Willett WC, Cassidy A (2015). Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124,086 US men and women followed for up to 24 years. Br Med J 352: i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner K, Kemme T, Peters K, Kersten S, Dänicke S, Humpf HU (2014). Systemic absorption and metabolism of dietary procyanidin B4 in pigs. Molecular Nutrition and. Food Res 58: 2261–2273. [DOI] [PubMed] [Google Scholar]
- Bladé C, Aragonès G, Arola‐Arnal A, Muguerza B, Bravo FI, Salvadó MJ et al. (2016). Proanthocyanidins in health and disease. Biofactors 42: 5–12. [DOI] [PubMed] [Google Scholar]
- Blumberg JB, Camesano TA, Cassidy A, Kris‐Etherton P, Howell A, Manach C et al. (2013). Cranberries and their bioactive constituents in human health. Advance in. Nutrition 4: 618–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão E, Soares S, Mateus N, de Freitas V (2014). In vivo interactions between procyanidins and human saliva proteins: effect of repeated exposures to procyanidins solution. J Agric Food Chem 62: 9562–9568. [DOI] [PubMed] [Google Scholar]
- Brenes A, Viveros A, Chamorro S, Arija I (2016). Use of polyphenol‐rich grape by‐products in monogastric nutrition. A review. Anim Feed Sci Technol 211: 1–17. [Google Scholar]
- Cala O, Dufourc EJ, Fouquet E, Manigand C, Laguerre M, Pianet I (2012). The colloidal state of tannins impacts the nature of their interaction with proteins: the case of salivary proline‐rich protein/procyanidins binding. Langmuir 28: 17410–17418. [DOI] [PubMed] [Google Scholar]
- Cascaes MM, Guilhon GM, Andrade EH, Zoghbi M, Santos LS (2015). Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int J Mol Sci 16: 23881–23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Huang CC, Chen CH, Chiang HC, Chen KB, Chen YJ et al. (2012). Grape‐seed procyanidins inhibit the in vitro growth and invasion of pancreatic carcinoma cells. Pancreas 41: 447–454. [DOI] [PubMed] [Google Scholar]
- Cock IE (2015). The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae. Inflammopharmacology 23: 203–229. [DOI] [PubMed] [Google Scholar]
- Coppo E, Marchese A (2014). Antibacterial activity of polyphenols. Curr Pharm Biotechnol 15: 380–390. [DOI] [PubMed] [Google Scholar]
- de Jesus NZ, de Souza Falcão H, Gomes IF, de Almeida Leite TJ, de Morais Lima GR, Barbosa‐Filho JM et al. (2012). Tannins, peptic ulcers and related mechanisms. Int J Mol Sci 13: 3203–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déprez S, Brézillon C, Rabot S, Philippe C, Mila I, Lapierre C et al. (2000). Polymeric proanthocyanidins are catabolized by a human colonic microflora into low molecular weight phenolic acids. J Nutr 130: 2733–2738. [DOI] [PubMed] [Google Scholar]
- de Rezende AA, Graf U, Guterres Zda R, Kerr WE, Spanó MA (2009). Protective effects of proanthocyanidins of grape (Vitis vinifera L.) seeds on DNA damage induced by Doxorubicin in somatic cells of Drosophila melanogaster. Food Chem Toxicol 47: 1466–1472. [DOI] [PubMed] [Google Scholar]
- Deáková Z, Országhová Z, Andrezálová L, Slezák P, Lehotay J, Muchová J (2015). Influence of oak wood polyphenols on cysteine, homocysteine and glutathione total levels and PON1 activities in human adult volunteers ‐ a pilot study. Gen Physiol Biophys 34: 73–80. [DOI] [PubMed] [Google Scholar]
- Dias R, Perez‐Gregorio MR, Mateus N, De Freitas V (2016). Interaction study between wheat‐derived peptides and procyanidin B3 by mass spectrometry. Food Chem 194: 1304–1312. [DOI] [PubMed] [Google Scholar]
- Dong XQ, Zou B, Zhang Y, Ge ZZ, Du J, Li CM (2013). Preparation of A‐type proanthocyanidin dimers from peanut skins and persimmon pulp and comparison of the antioxidant activity of A‐type and B‐type dimers. Fitoterapia 91: 128–139. [DOI] [PubMed] [Google Scholar]
- Draijer R, de Graaf Y, Slettenaar M, de Groot E, Wright CI (2015). Consumption of a polyphenol‐rich grape‐wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients 7: 3138–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2014). Scientific Opinion on the safety and efficacy of tannic acid when used as feed flavouring for all animal species. EFSA J 12: 3828. [Google Scholar]
- Ekambaram SP, Perumal SS, Balakrishnan A (2016). Scope of Hydrolysable Tannins as Possible Antimicrobial Agent. Phytother Res 30: 1035–1045. [DOI] [PubMed] [Google Scholar]
- Ellam S, Williamson G (2013). Cocoa and human health. Annu Rev Nutr 33: 105–128. [DOI] [PubMed] [Google Scholar]
- Espín JC, Larrosa M, García‐Conesa MT, Tomás‐Barberán F (2013). Biological Significance of Urolithins, the Gut Microbial Ellagic Acid‐Derived Metabolites: The Evidence So Far. Evid Based Complement Alternat Med 2013: 270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC (2014). Safety assessment of Vitis vinifera (grape)‐derived ingredients as used in cosmetics. Int J Toxicol 33: 48S–83S. [DOI] [PubMed] [Google Scholar]
- Fuentes JL, Vernhe M, Cuetara EB, Sánchez‐Lamar A, Santana JL, Llagostera M (2006). Tannins from barks of Pinus caribaea protect Escherichia coli cells against DNA damage induced by gamma‐rays. Fitoterapia 77: 116–120. [DOI] [PubMed] [Google Scholar]
- Fushimi S, Myazawa F, Nakagawa K, Burdeos GC, Miyazawa T (2015). Young persimmon ingestion suppresses lipid oxidation in rats. J Nutr Sci Vitaminol (Tokyo) 61: 90–95. [DOI] [PubMed] [Google Scholar]
- Gato N, Kadowaki A, Hashimoto N, Yokoyama S, Matsumoto K (2013). Persimmon fruit tannin‐rich fiber reduces cholesterol levels in humans. Ann Nutr Metab 62: 1–6. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Ananga A, Tsolova V (2014). Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 6: 391–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollucke AP, Aguiar O Jr, Barbisan LF, Ribeiro DA (2013). Use of grape polyphenols against carcinogenesis: putative molecular mechanisms of action using in vitro and in vivo test systems. J Med Food 16: 199–205. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Abuin N, Pinent M, Casanova‐Marti A, Arola L, Blay M, Ardevol A (2015). Procyanidins and their healthy protective effects against type 2 diabetes. Curr Med Chem 22: 39–50. [DOI] [PubMed] [Google Scholar]
- Goodrich KM, Smithson AT, Ickes AK, Neilson AP (2015). Pan‐colonic pharmacokinetics of catechins and procyanidins in male Sprague–Dawley rats. J Nutr Biochem 26: 1007–1014. [DOI] [PubMed] [Google Scholar]
- Guil‐Guerrero JL, Ramos L, Moreno C, Zúñiga‐Paredes JC, Carlosama‐Yepez M, Ruales P (2016). Antimicrobialactivityofplant‐foodby‐products:Areviewfocusing on thetropics. Livest Sci 189: 32–49. [Google Scholar]
- Guler A, Sahin MA, Yucel O, Yokusoglu M, Gamsizkan M, Ozal E et al. (2011). Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit 17: BR326–BR331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K, Bennett NC, Purdie G, Herst PM (2015). Standardized cranberry capsules for radiation cystitis in prostate cancer patients in New Zealand: a randomized double blinded, placebo controlled pilot study. Support Care Cancer 23: 95–102. [DOI] [PubMed] [Google Scholar]
- Hassan W, Noreen H, Khalil S, Hussain A, Rehman S, Sajjad S et al. (2016). Ethanolic extract of Nigella sativa protects Fe(II) induced lipid peroxidation in rat's brain, kidney and liver homogenates. Pak J Pharm Sci 29: 231–237. [PubMed] [Google Scholar]
- Holt RR, Heiss C, Kelm M, Keen CL (2012). The potential of flavanol and procyanidin intake to influence age‐related vascular disease. J Nutr Gerontol Geriatr 31: 290–323. [DOI] [PubMed] [Google Scholar]
- Hort MA, Straliotto MR, Duz MS, Netto PM, Souza CB, Schulz T (2012). Cardioprotective effects of a proanthocyanidin‐rich fraction from Croton celtidifolius Baill: focus on atherosclerosis. Food Chem Toxicol 50: 3769–3775. [DOI] [PubMed] [Google Scholar]
- Horvathova M, Orszaghova Z, Laubertova L, Vavakova M, Sabaka P, Rohdewald P et al. (2014). Effect of the French oak wood extract Robuvit on markers of oxidative stress and activity of antioxidant enzymes in healthy volunteers: a pilot study. Oxid Med Cell Longev 2014: 639868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A, Souza D, Roller M, Fromentin E (2015). Comparison of the Anti‐Adhesion Activity of Three Different Cranberry Extracts on Uropathogenic P‐fimbriated Escherichia coli: a Randomized, Double‐blind, Placebo Controlled, Ex Vivo, Acute Study. Nat Prod Commun 10: 1215–1218. [PubMed] [Google Scholar]
- Iglesias J, Pazos M, Torres JL, Medina I (2012). Antioxidant mechanism of grape procyanidins in muscle tissues: redox interactions with endogenous ascorbic acid and α‐tocopherol. Food Chem 134: 1767–1774. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Hoon‐Kim S, Radhakrishnan R, Williamson EM (2016). A critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phytother Res 30: 691–700. [DOI] [PubMed] [Google Scholar]
- Jepson R, Craig J, Williams G (2013). Cranberry products and prevention of urinary tract infections. JAMA 310: 1395–1396. [DOI] [PubMed] [Google Scholar]
- Jiménez N, Esteban‐Torres M, Mancheño JM, de las Rivas B, Muñoz R (2014). Tannin Degradation by a Novel Tannase Enzyme Present in Some Lactobacillus plantarum Strains. Appl Environ Microbiol 80: 2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiloglu S, Capanoglu E, Bilen FD, Gonzales GB, Grootaert C, Van de Wiele T et al. (2016). Journal of Agricoltural and. Food Chem 64: 2450–2458. [DOI] [PubMed] [Google Scholar]
- Kancheva VD, Kasaikina OT (2013). Bio‐antioxidants a chemical base of their antioxidant activity and beneficial effect on human health. Curr Med Chem 20: 4784–4805. [DOI] [PubMed] [Google Scholar]
- Katiyar SK (2015). Proanthocyanidins from grape seeds inhibit UV‐radiation‐induced immune suppression in mice: detection and analysis of molecular and cellular targets. Photochem Photobiol 91: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur SJ, Grover IS, Kumar S (2000). Modulatory effects of a tannin fraction isolated from Terminalia arjuna on the genotoxicity of mutagens in Salmonella typhimurium. Food Chem Toxicol 38: 1113–1119. [DOI] [PubMed] [Google Scholar]
- Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A (2012). Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid‐rich food products. Mol Nutr Food Res 56: 1605–1616. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim J, Shim J, Lee CY, Lee KW, Lee HJ (2014). Cocoa phytochemicals: recent advances in molecular mechanisms on health. Critical Review Food Scince. Nutrition 54: 1458–1472. [DOI] [PubMed] [Google Scholar]
- Kooti W, Farokhipour M, Asadzadeh Z, Ashtary‐Larky D, Asadi‐Samani M (2016). The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician J 8: 1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Jain S (2012). Tannins: An Antinutrient with Positive Effect to Manage Diabetes. Research Journal of Recent. Sciences 1: 70–73. [Google Scholar]
- Lamy E, Pinheiro C, Rodrigues L, Capela e Silva F, Lopes OS, Tavares S et al. (2016). Determinants of Tannin‐Rich Food and Beverage Consumption: Oral Perception vs. Psychosocial Aspects In: Combs CA. (ed). Tannins: Biochemistry, Food Sources and Nutritional Properties. Nova Science Publishers Inc: New York, USA, pp. 29–58. [Google Scholar]
- Landete JM (2011). Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res Int 44: 1150–1160. [Google Scholar]
- Lehmann M, Graf U, Reguly ML, Rodrigues De Andrade HH (2000). Interference of tannic acid on the genotoxicity of mitomycin C, methylmethanesulfonate, and nitrogen mustard in somatic cells of Drosophila melanogaster. Environ Mol Mutagen 36: 195–200. [DOI] [PubMed] [Google Scholar]
- Li M, Jia X, Yang J, Deng J, Zhao G (2012). Effect of tannic acid on properties of soybean (Glycine max) seed ferritin: a model for interaction between naturally occurring components in foodstuffs. Food Chem 133: 410–415. [DOI] [PubMed] [Google Scholar]
- Li S, Xu M, Niu Q, Xu S, Ding Y, Yan Y et al. (2015a). Efficacy of Procyanidins against In Vivo Cellular Oxidative Damage: A Systematic Review and Meta‐Analysis. PLoS One 10: e0139455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sui Y, Xiao J, Wu Q, Hu B, Xie B et al. (2013). Absorption and urinary excretion of A‐type procyanidin oligomers from Litchi chinensis pericarp in rats by selected ion monitoring liquid chromatography‐mass spectrometry. Food Chem 138: 1536–1542. [DOI] [PubMed] [Google Scholar]
- Li Z, Henning SM, Lee RP, Lu QY, Summanen PH, Thames G et al. (2015b). Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct 6: 2487–2495. [DOI] [PubMed] [Google Scholar]
- Lipińska L, Klewicka E, Sójka M (2014). The structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment 13: 289–299. [DOI] [PubMed] [Google Scholar]
- Liu CM, Ma JQ, Liu SS, Zheng GH, Feng ZJ, Sun JM (2014). Proanthocyanidins improves lead‐induced cognitive impairments by blocking endoplasmic reticulum stress and nuclear factor‐κB‐mediated inflammatory pathways in rats. Food Chem Toxicol 72: 295–302. [DOI] [PubMed] [Google Scholar]
- Lluís L, Muñoz M, Nogués MR, Sánchez‐Martos V, Romeu M, Giralt M et al. (2011). Toxicology evaluation of a procyanidin‐rich extract from grape skins and seeds. Food Chem Toxicol 49: 1450–1454. [DOI] [PubMed] [Google Scholar]
- Lu C, Luo X, Lu L, Li H, Chen X, Ji Y (2013). Preliminary extraction of tannins by 1‐butyl‐3‐methylimidazole bromide and its subsequent removal from Galla chinensis extract using macroporous resins. J Sep Sci 36: 959–964. [DOI] [PubMed] [Google Scholar]
- Margină D, Ilie M, Grădinaru D, Androutsopoulos VP, Kouretas D, Tsatsakis AM (2015). Natural products‐friends or foes? Toxicol Lett 236: 154–167. [DOI] [PubMed] [Google Scholar]
- Marín L, Miguélez EM, Villar CJ, Lombó F (2015). Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int 2015: 905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques NF, Stefanello ST, Froeder AL, Busanello A, Boligon AA, Athayde ML et al. (2015). Centella asiatica and Its Fractions Reduces Lipid Peroxidation Induced by Quinolinic Acid and Sodium Nitroprusside in Rat Brain Regions. Neurochem Res 40: 1197–1210. [DOI] [PubMed] [Google Scholar]
- Masci A, Coccia A, Lendaro E, Mosca L, Paolicelli P, Cesa S (2016). Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem 202: 59–69. [DOI] [PubMed] [Google Scholar]
- Mateos‐Martín ML, Fuguet E, Quero C, Pérez‐Jiménez J, Torres JL (2012). New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI‐TOF/TOF mass spectrometry. Anal Bioanal Chem 402: 1327–1336. [DOI] [PubMed] [Google Scholar]
- Mathew AS, Capel‐Williams GM, Berry SE, Hall WL (2012). Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods Hum Nutr 67: 351–357. [DOI] [PubMed] [Google Scholar]
- Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M (2014). Cranberry and recurrent cystitis: more than marketing? Crit Rev Food Sci Nutr 54: 1063–1075. [DOI] [PubMed] [Google Scholar]
- Mohana T, Navin AV, Jamuna S, Sakeena Sadullah MS, Niranjali Devaraj S (2015). Inhibition of differentiation of monocyte to macrophages in atherosclerosis by oligomeric proanthocyanidins ‐In‐vivo and in‐vitro study. Food Chem Toxicol 82: 96–105. [DOI] [PubMed] [Google Scholar]
- Natella F, Leoni G, Maldini M, Natarelli L, Comitato R, Schonlau F et al. (2014). Absorption, metabolism, and effects at transcriptome level of a standardized French oak wood extract, Robuvit, in healthy volunteers: pilot study. J Agric Food Chem 62: 443–453. [DOI] [PubMed] [Google Scholar]
- Nazima B, Manoharan V, Miltonprabu S (2015). Grape seed proanthocyanidins ameliorates cadmium‐induced renal injury and oxidative stress in experimental rats through the up‐regulation of nuclear related factor 2 and antioxidant responsive elements. Biochem Cell Biol 93: 210–226. [DOI] [PubMed] [Google Scholar]
- Nile SH, Park SW (2014). Edible berries: bioactive components and their effect on human health. Nutrition 30: 134–144. [DOI] [PubMed] [Google Scholar]
- Nuñez‐Sánchez MA, García‐Villalba R, Monedero‐Saiz T, García‐Talavera NV, Gómez‐Sánchez MB, Sánchez‐Álvarez C et al. (2014). Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res 58: 1199–1211. [DOI] [PubMed] [Google Scholar]
- Okuda T, Ito H (2011). Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 16: 2191–2217. [Google Scholar]
- Oliveira BG, Costa HB, Ventura JA, Kondratyuk TP, Barroso ME, Correia RM et al. (2016). Chemical profile of mango (Mangifera indica L.) using electrospray ionisation mass spectrometry (ESI‐MS. Food Chem 204: 37–45. [DOI] [PubMed] [Google Scholar]
- Ou K, Gu L (2014). Absorption and metabolism of proanthocyanidins. J Funct Foods 7: 43–53. [Google Scholar]
- Ouédraogo M, Charles C, Ouédraogo M, Guissou IP, Stévigny C, Duez P (2011). An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr Cancer 63: 1163–1173. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee YH, Choi KC, Seong AR, Choi HK, Lee OH et al. (2011). Hwang HJ, Yoon HG (2011). Grape seed extract regulates androgen receptor‐mediated transcription in prostate cancer cells through potent anti‐histone acetyltransferase activity. J Med Food 14: 9–16. [DOI] [PubMed] [Google Scholar]
- Pinent M, Cedó L, Montagut G, Blay M, Ardévol A (2012). Procyanidins improve some disrupted glucose homoeostatic situations: an analysis of doses and treatments according to different animal models. Crit Rev Food Sci Nutr 52: 569–584. [DOI] [PubMed] [Google Scholar]
- Pons Z, Guerrero L, Margalef M, Arola L, Arola‐Arnal A, Muguerza B (2014). Effect of low molecular grape seed proanthocyanidins on blood pressure and lipid homeostasis in cafeteria diet‐fed rats. J Physiol Biochem 70: 629–637. [DOI] [PubMed] [Google Scholar]
- Prasad R, Katiyar SK (2014). Down‐regulation of miRNA‐106b inhibits growth of melanoma cells by promoting G1‐phase cell cycle arrest and reactivation of p21/WAF1/Cip1 protein. Oncotarget 5: 10636–10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quifer‐Rada P, Choy YY, Calvert CC, Waterhouse AL, Lamuela‐Raventos RM (2016). Use of metabolomics and lipidomics to evaluate the hypocholestreolemic effect of Proanthocyanidins from grape seed in a pig model. Mol Nutr Food Res 60: 2219–2227. [DOI] [PubMed] [Google Scholar]
- Salvadó MJ, Casanova E, Fernández‐Iglesias A, Arola L, Bladé C (2015). Roles of proanthocyanidin rich extracts in obesity. Food Funct 6: 1053–1071. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Moreno C, Cao G, Ou B, Prior RL (2003). Anthocyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J Agric Food Chem 51: 4889–4896. [DOI] [PubMed] [Google Scholar]
- Serrano J, Casanova‐Martí À, Gil‐Cardoso K, Blay MT, Terra X, Pinent M et al. (2016). Acutely administered grape‐seed proanthocyanidin extract acts as a satiating agent. Food Funct 7: 483–490. [DOI] [PubMed] [Google Scholar]
- Serrano J, Puupponen‐Pimiä R, Dauer A, Aura AM, Saura‐Calixto F (2009). Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 2: S310–S329. [DOI] [PubMed] [Google Scholar]
- Sieniawska E (2015). Activities of Tannins‐‐From In Vitro Studies to Clinical Trials. Nat Prod Commun 10: 1877–1884. [PubMed] [Google Scholar]
- Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J (2015). Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int J Mol Sci 16: 24673–24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeriglio A, Monteleone D, Trombetta D (2014a). Health effects of Vaccinium myrtillus L.: evaluation of efficacy and technological strategies for preservation of active ingredients. Mini Rev Med Chem 14: 567–584. [DOI] [PubMed] [Google Scholar]
- Smeriglio A, Tomaino A, Trombetta D (2014b). Herbal products in pregnancy: experimental studies and clinical reports. Phytother Res 28: 1107–1116. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson E, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmach A, Williamson G, Crozier A (2014). Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct 5: 1727–1737. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Ray S (2015). Anti‐diabetic and Anti‐hyperlipidemic Effects and Safety of Salacia reticulata and Related Species. Phytother Res 29: 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi M, Horiguchi N, Kajiyama A, Akiyoshi M, Owa Y, Kato K et al. (2014). Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle‐aged women: a randomized, double‐blind, placebo‐controlled pilot study. Menopause 21: 990–996. [DOI] [PubMed] [Google Scholar]
- Turati F, Rossi M, Pelucchi C, Levi F, La Vecchia C (2015). Fruit and vegetables and cancer risk: a review of southern European studies. Br J Nutr 113: S102–S110. [DOI] [PubMed] [Google Scholar]
- Velderrain‐Rodríguez GR, Palafox‐Carlos H, Wall‐Medrano A, Ayala‐Zavala JF, Chen CY, Robles‐Sánchez M et al. (2014). Phenolic compounds: their journey after intake. Food Funct 5: 189–197. [DOI] [PubMed] [Google Scholar]
- Verstraeten SV, Fraga CG, Oteiza PI (2015). Interactions of flavan‐3‐ols and procyanidins with membranes: mechanisms and the physiological relevance. Food Funct 6: 32–41. [DOI] [PubMed] [Google Scholar]
- Vostalova J, Vidlar A, Simanek V, Galandakova A, Kosina P, Vacek J et al. (2015). Are High Proanthocyanidins Key to Cranberry Efficacy in the Prevention of Recurrent Urinary Tract Infection? Phytother Res 29: 1559–1567. [DOI] [PubMed] [Google Scholar]
- Wang X, Jun YY, Zhao G (2014). Flavonoid intake and risk of CVD: a systematic review and meta‐analysis of prospective cohort studies. Br J Nutr 111: 1–11. [DOI] [PubMed] [Google Scholar]
- Wiese S, Esatbeyoglu T, Winterhalter P, Kruse HP, Winkler S, Bub A et al. (2015). Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan‐3‐ols: a randomized cross‐over study in humans. Mol Nutr Food Res 59: 610–621. [DOI] [PubMed] [Google Scholar]
- Xu ZC, Yin J, Zhou B, Liu YT, Yu Y, Li GQ (2015). Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J Gastroenterol 21: 7468–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xiao L, Yuan Y, Luo X, Jiang M, Ni J et al. (2014). Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem Pharmacol 92: 599–606. [DOI] [PubMed] [Google Scholar]
- Yang M, Chung SJ, Floegel A, Song WO, Koo SI, Chun OK (2013). Dietary antioxidant capacity is associated with improved serum antioxidant status and decreased serum C‐reactive protein and plasma homocysteine concentrations. Eur J Nutr 52: 1901–1911. [DOI] [PubMed] [Google Scholar]
- Yunoki T, Deguchi K, Omote Y, Liu N, Liu W, Hishikawa N (2014). Anti‐oxidative nutrient‐rich diet protects against acute ischemic brain damage in rats. Brain Res 1587: 33–39. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Li D, Ho CT, Li J, Wan X (2016). The absorption, distribution, metabolism and excretion of procyanidins. Food Funct 7: 1273–1281. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Yin ZP, Ma L, Zhao W, Hao HW, Li HL (2014). Free radical‐scavenging activities of oligomeric proanthocyanidin from Rhodiolarosea L. and its antioxidant effects in vivo. Nat Prod Res 28: 2301–2303. [DOI] [PubMed] [Google Scholar]
- Zorenc Z, Veberic R, Stampar F, Koron D, Mikulic‐Petkovsek M (2016). White versus blue: Does the wild ‘albino’ bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem 211: 876–882. [DOI] [PubMed] [Google Scholar]
- Zou Y, Guo J, Yin SW, Wang JM, Yang XQ (2015). Pickering Emulsion Gels Prepared by Hydrogen‐Bonded Zein/Tannic Acid Complex Colloidal Particles. J Agric Food Chem 63: 7405–7414. [DOI] [PubMed] [Google Scholar]


