Abstract
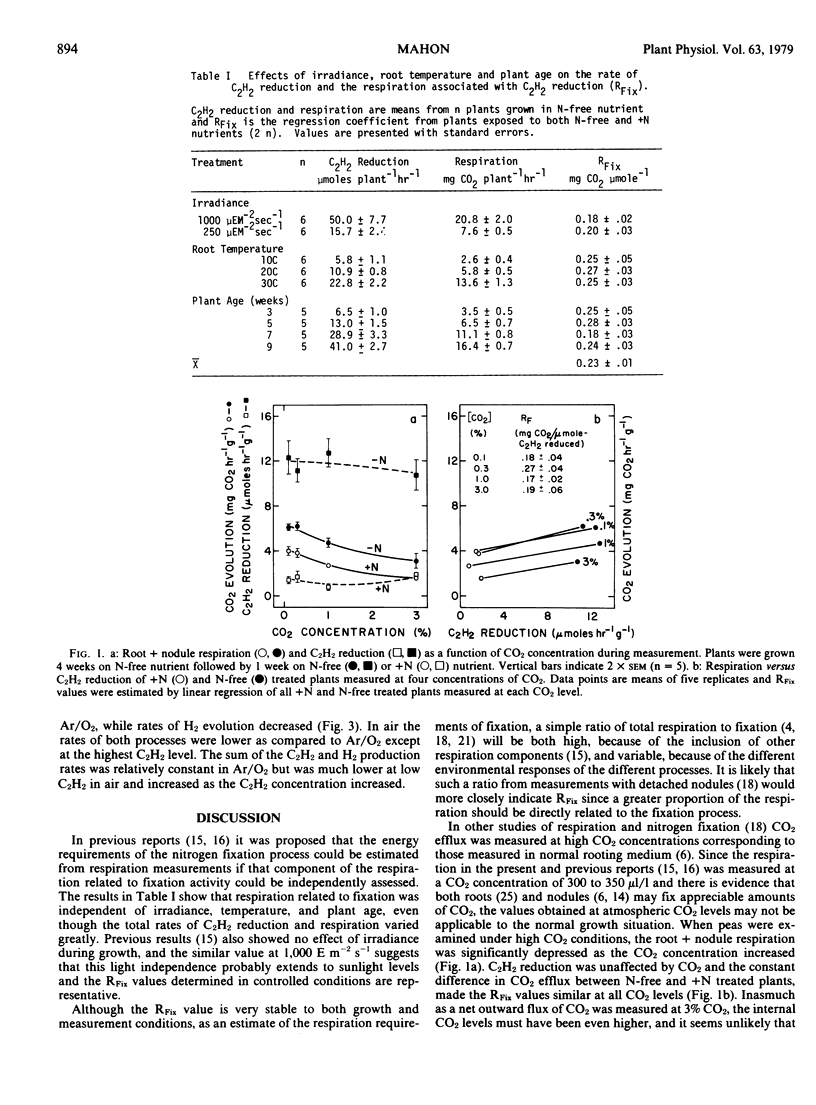
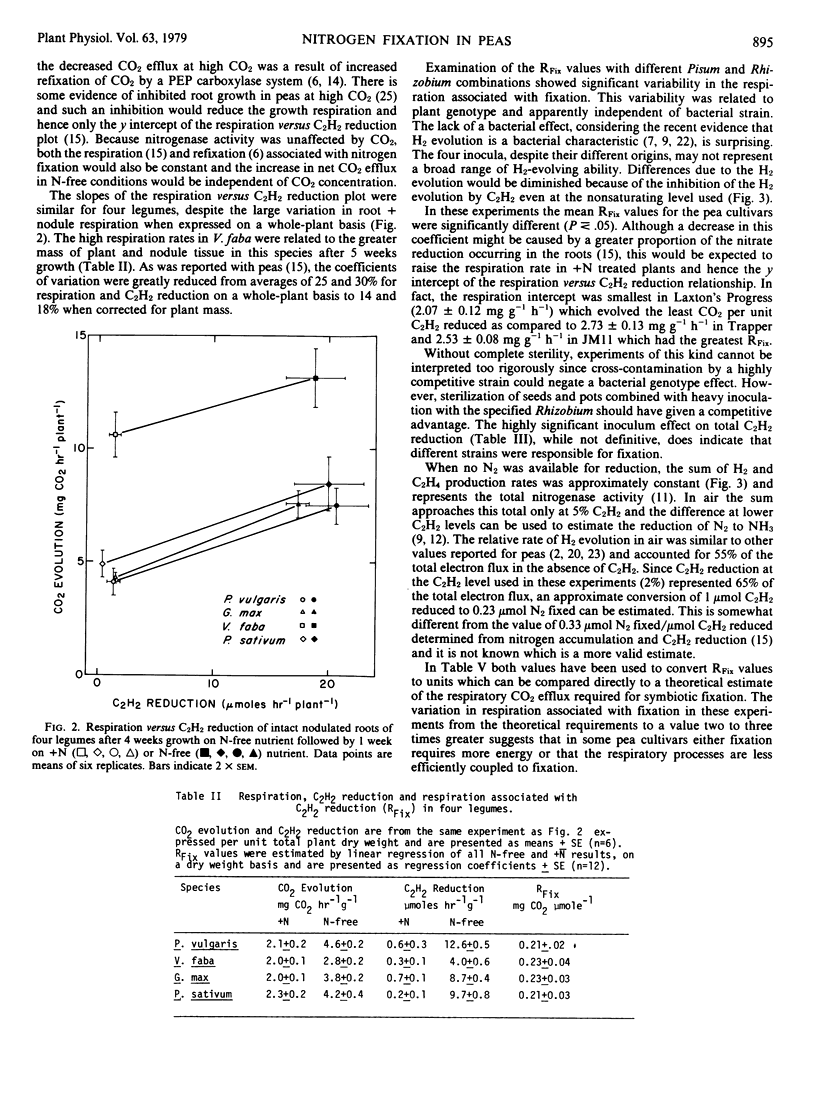
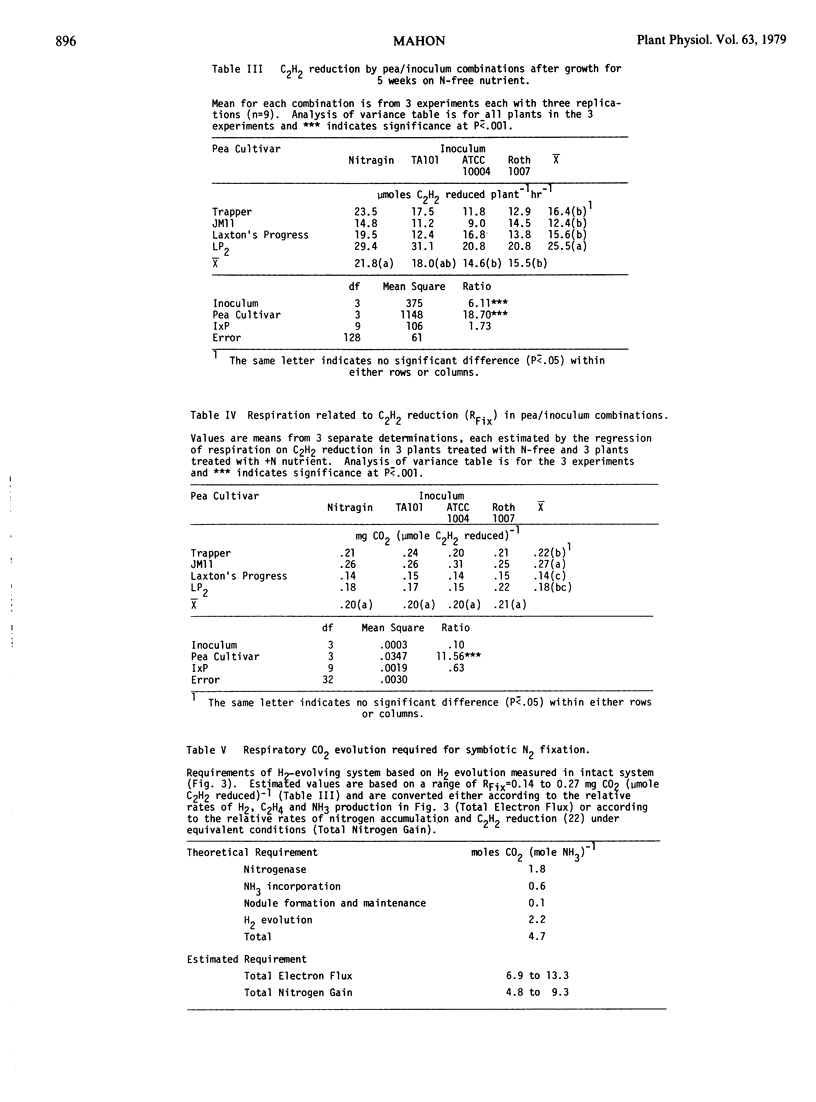
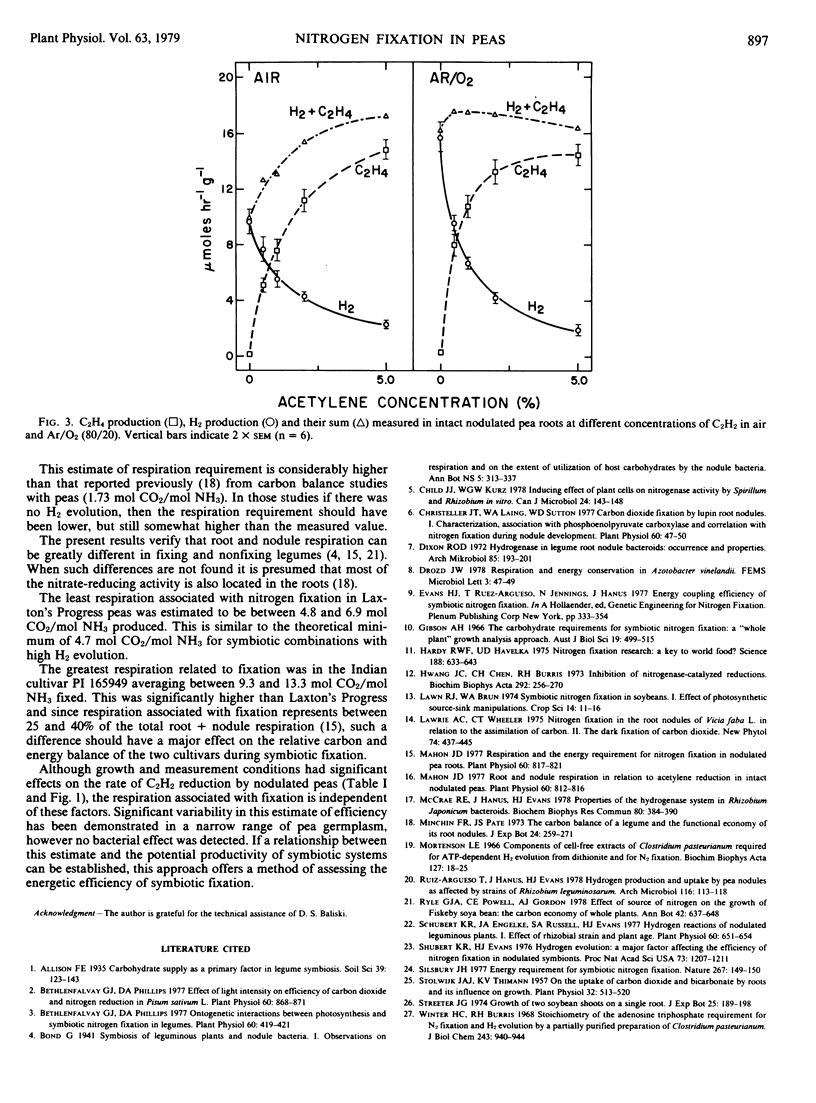
Estimated values for the respiration associated with symbiotic nitrogen fixation in Pisum sativum L. were independent of irradiance, temperature, plant age, and CO2 concentration, despite large variation in the total rates of C2H2 reduction and root + nodule respiration. Similar values were also found in Phaseolus vulgaris L., Vicia faba L. and Glycine max (L.) Merr. Among all combinations of four Pisum cultivars with four Rhizobium leguminosarum inoculants only the plant genotype significantly affected the fixation-linked respiration, although both plant and bacterial types significantly influenced the total rate of C2H2 reduction. On the basis of measured rates of H2 evolution and C2H2 reduction, or total nitrogen gain in the same system, the least respiration per unit of ammonia produced symbiotically was estimated as 4.8 to 6.9 moles CO2 (mole NH3)−1 in Laxton's Progress and the greatest as 9.3 to 13.3 moles CO2 (mole NH3)−1 in an Indian cultivar, as compared to a theoretical minimum respiration requirement of 4.7 moles CO2 (mole NH3)−1 in peas.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethlenfalvay G. J., Phillips D. A. Effect of Light Intensity on Efficiency of Carbon Dioxide and Nitrogen Reduction in Pisum sativum L. Plant Physiol. 1977 Dec;60(6):868–871. doi: 10.1104/pp.60.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Ontogenetic Interactions between Photosynthesis and Symbiotic Nitrogen Fixation in Legumes. Plant Physiol. 1977 Sep;60(3):419–421. doi: 10.1104/pp.60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child J. J., Kurz W. G. Inducing effect of plant cells on nitrogenase activity by Spirillum and Rhizobium in vitro. Can J Microbiol. 1978 Feb;24(2):143–148. doi: 10.1139/m78-026. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Ruiz-Argüeso T., Jennings N., Hanus J. Energy coupling efficiency of symbiotic nitrogen fixation. Basic Life Sci. 1977;9:333–354. doi: 10.1007/978-1-4684-0880-5_21. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Havelka U. D. Nitrogen fixation research: a key to world food? Science. 1975 May 9;188(4188):633–643. doi: 10.1126/science.188.4188.633. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., Chen C. H., Burris R. H. Inhibition of nitrogenase-catalyzed reductions. Biochim Biophys Acta. 1973 Jan 18;292(1):256–270. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- Mahon J. D. Respiration and the energy requirement for nitrogen fixation in nodulated pea roots. Plant Physiol. 1977 Dec;60(6):817–821. doi: 10.1104/pp.60.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon J. D. Root and nodule respiration in relation to acetylene reduction in intact nodulated peas. Plant Physiol. 1977 Dec;60(6):812–816. doi: 10.1104/pp.60.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E. Components of cell-free extracts of Clostridium pasteurianum required for ATP-dependent H2 evolution from dithionite and for N2 fixation. Biochim Biophys Acta. 1966 Sep 26;127(1):18–25. doi: 10.1016/0304-4165(66)90470-3. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Engelke J. A., Russell S. A., Evans H. J. Hydrogen reactions of nodulated leguminous plants: I. Effect of rhizobial strain and plant age. Plant Physiol. 1977 Nov;60(5):651–654. doi: 10.1104/pp.60.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silsbury J. H. Energy requirement for symbiotic nitrogen fixation. Nature. 1977 May 12;267(5607):149–150. doi: 10.1038/267149a0. [DOI] [PubMed] [Google Scholar]
- Stolwijk J. A., Thimann K. V. On the Uptake of Carbon Dioxide and Bicarbonate by Roots, and Its Influence on Growth. Plant Physiol. 1957 Nov;32(6):513–520. doi: 10.1104/pp.32.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H. C., Burris R. H. Stoichiometry of the adenosine triphosphate requirement for N2 fixation and H2 evolution by a partially purified preparation of Clostridium pasteurianum. J Biol Chem. 1968 Mar 10;243(5):940–944. [PubMed] [Google Scholar]


