Abstract
Background:
Glioma is the most common primary brain tumor with poor prognosis. Temozolomide (TMZ) has been used with irradiation (IR) to treat gliomas. The aim of the present study was to evaluate the cytotoxic and radiosensitizing effect of TMZ when combined with high-dose and high-dose rate of gamma irradiation in vitro.
Methods:
Two ‘U87MG’ cell lines and skin fibroblast were cultured and assigned to five groups for 24, 48, and 72 hours. The groups were namely, TMZ group (2000 μM/L), IR group (5 Gy), TMZ plus IR group, control group, and control solvent group. MTT assay was applied to evaluate cell viability. Data were analyzed with SPSS 21.0 software using one-way ANOVA and Kruskal-Wallis test. P<0.05 were considered statistically significant.
Results:
The slope of growth curve U87MG cells in semi-logarithmic scale was equal to 27.36±0.89 hours. The viability of cells was determined for different TMZ and IR treatment groups. In terms of cell viability, there were no significant differences between the control and control solvent groups (P=0.35) and between treated group by IR (5 Gy) alone and TMZ (2000 µM/ml) alone (P=0.15). Data obtained for the cell viability of combined TMZ plus IR in both cell lines compared to TMZ or IR treated group alone showed a significant difference (P=0.002).
Conclusion:
The evaluation of cells viability showed that TMZ in combination with IR on glioma cells led to a significant radiosensitivity compared to IR alone.
Keywords: Glioblastoma, Radiation-sensitizing agents, Temozolomide, Radiotherapy
What’s Known
Glioblastoma is a tumor of the brain resistant to radiotherapy and chemotherapy. Although a combination of gamma-rays with TMZ is used for the treatment of such patients, but there is no universal agreement for using radiation and TMZ dose for achieving a significant therapeutic efficacy.
What’s New
A single dose of 5 Gy with higher dose rate, usually used in conventional radiotherapy in combination with various doses of TMZ on Glioblastoma and normal skin fibroblast cell lines, is shown to have significant killing effects. This regimen might increase therapeutic efficacy if used clinically.
Introduction
Glioblastoma multiforme (GBM) is the most aggressive and malignant brain tumors1 with an average survival time of 12–14 months.2 Despite various methods like surgery, radiotherapy, chemotherapy and even using a combination of different modalities,3 it is still a lethal disease with poor prognosis which does not offer completely effective and useful method for the treatment of glioma.4 Therefore, developing modern strategies for therapy is essential for such patients.
Temozolomide (TMZ)5,6 is currently the standard chemotherapeutic drug and radiosensitizing agent7 that is used for the treatment of glioblastoma. The development of TMZ drug dates back to early 1980s when the drug was first synthesized by scientists at Aston University. TMZ is orally available, imidazotetrazine-based, and DNA alkylating agent.8,9 TMZ is stable in acidic conditions, but the rate of degradation increases at high pH. The stability of TMZ in acidic condition allows it to be administered orally. TMZ is able to tolerate the high acidity condition of the stomach before entering the systemic circulation and passing blood-brain-barrier (BBB).10 Spontaneous hydrolysis of TMZ and change of pH convert its molecules into active metabolite form and consequently does not require hepatic metabolism for its activity.8,9 The ability of TMZ in delaying the growth of tumor cells and its easy prescription has turned it into a popular option for the treatment of glioblastoma.11 The anti-tumor effect of TMZ is methylation of tumor cell DNAs.12 Its cytotoxicity effect is apparently accomplished by adding methyl group to N7 and O6 position of guanine in DNA.13 This drug induces O6-MeG lesion and finally causes apoptosis death.14
The aim of the present study was to investigate cytotoxic and radiosensitizing effects of TMZ on U87MG cell line in vitro. We attempted to evaluate the extent of cell damage by TMZ in combination with gamma-rays of CO-60 in monolayer cells culture of glioma and primary fibroblast cells by MTT assay. The 3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay15 is a quantitative colorimetric method to measure and compare the metabolic viability of the cells in vitro under different treatment protocols.16 The results of this study could be considered as an effective step to determine the therapeutic strategy of applying radiosensitizing agents for the treatment of malignant tumors such as glioma.
Materials and Methods
Cell Lines and Monolayer Cell Culture
U87MG human glioma cell line (ATCC® HTB-14™) was provided by the cell bank of the Pasteur Institute of Iran and fibroblast cell line of primary culture from the eyelid skin biopsy of a 45-year-old woman. Cells were maintained in Dulbecco’s modified eagle’s medium (DMEM, GIBCO) containing 10% fetal bovine serum (FBS, GIBCO), 100 U/ml penicillin (Sigma), 100 U/ml streptomycin (Sigma), and 2 mM L-glutamine at 37°C in a humidified atmosphere of 5% CO2. For monolayer cells culture, both cell lines were cultured with the cell density of 104 cell/cm2 in T-25 flasks (Nunc).
Drug Treatment with Temozolomide (TMZ)
In order to evaluate the radiosensitizing effect of TMZ on glioma and primary fibroblast cells in the presence of irradiation, various concentrations of TMZ (1000 to 8000 µM) were added to plates. The plates contained monolayer cells with 2×103cell/cm2 density (noted concentrations acquired with a solution of 2.5 mg TMZ powder in 1 ml of 0.2% DMSO in ambient temperature and kept at -20 °C) and samples were maintained under drug treatment for a cell doubling time.
Irradiation Procedure
Cells were irradiated with gamma-rays generated from a CO-60 teletherapy unit (megavoltage telecobalt units) (Theratron 780, Canada) with a dose of 5 Gy and dose rate of 116.76 cGy/min at a distance of 80 cm from the source of CO-60, with a field size of 20×20 cm2 at ambient temperature.
MTT Cell Viability Assay
Untreated control cells, TMZ, and gamma irradiation cells were exposed to various concentrations of TMZ (1000, 2000, 4000, 6000, 8000 µM) for a cell doubling time (27 hours) and CO-60 gamma-rays with a dose of 5 Gy and a dose rate of 116.76 cGy/min. Cell viability at 24, 48, and 72 hours was determined with 300 µl MTT (Sigma) at a concentration of 0.5 mg/ml for 4 hours. Then, by adding 150 µl DMSO (Sigma), the optical density of the solution at 540 nm wavelength was measured using ELISA reader (Biotech-USA).17
Experimental Design
In order to evaluate the effects of IR and TMZ on U87MG and skin fibroblast cell lines, growth curve was drawn in semi-logarithmic scale with cell numbers versus time during 12 days. For the combined treatment based on IC50 values of TMZ, a concentration in which cells growth decreases to 50% was defined11 and approximately 4000 µM/ml was obtained. The concentration of TMZ in the last step was considered as 2000 µM in order to get the desirable effect with less TMZ dose in combination with irradiation. The groups after cells doubling time were treated with irradiation and TMZ were divided as follows:
Control group: Received neither drug nor irradiation
Control solvent group: Received neither drug nor irradiation, but received 0.2% DMSO
Treated group with dose 2000 µM of TMZ
Treated group with CO-60 gamma-rays (5 Gy)
Treated group with TMZ (2000 µM/ml) in combination with gamma-rays of CO-60 (5 Gy).
Statistical Analysis
Results from various experiments were analyzed using the statistical package for social science (SPSS version 21.0) and Excel 2010 software packages and data were obtained with triplicate experiments. The significance of the data was compared by one-way ANOVA and Kruskal-Wallis non-parametric tests. P values <0.05 were considered statistically significant.
Results
The growth curve was drawn in semi-logarithmic scale with cell numbers versus time during 12 days. The slope of the curve was calculated in a linear or logarithmic region of the curve, which was equal to 27.36±0.89 hours. Figure 1 shows U87MG cell growth curve in the monolayer cell culture model.
Figure 1.
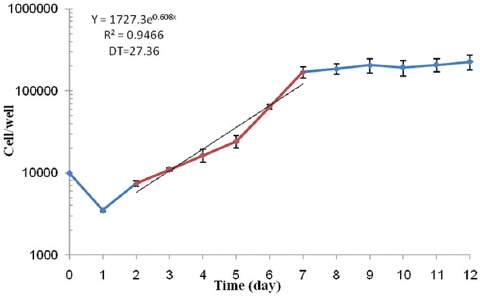
The growth curve of U87MG cell line (cell numbers versus time). All experiments were repeated three times. Error bars indicate the standard error of means (SEM).
Data analysis for lethal dose and IC50 value of TMZ treated cells showed that by increasing the dose of TMZ from 1,000 to 8,000 µM, the cell death rate was increased and up to 50% cytotoxicity was seen in up to 4000 µM doses (figure 2). In addition, figures 2-5 show that by increasing TMZ uptake time to 24 hours, cytotoxicity value of the TMZ and the cell death were increased (P=0.009) and by increasing the time to 72 hours after adding TMZ, less cytotoxicity was seen. With the combination of TMZ (1,000 to 8,000 µM) and IR, cytotoxicity was more pronounced at 72 hours post treatment (P=0.006).
Figure 2.
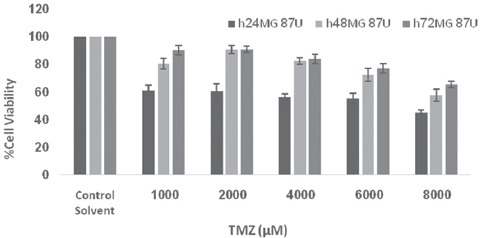
Viability of U87MG cells after treatment with various concentrations of TMZ assayed at values time intervals. Error bars represents the standard deviation of mean values obtained from triplicate experiments.
Figure 3.
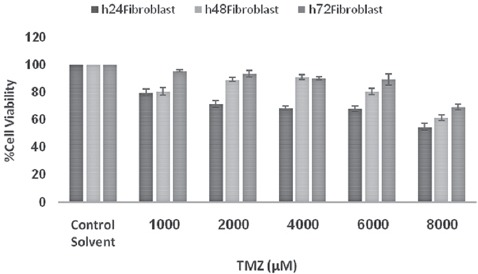
Viability of fibroblast cells after treatment with various concentrations of TMZ assayed at values time intervals. Error bars represents the standard deviation of mean values obtained from triplicate experiments.
Figure 4.

Viability of U87MG cells after treatment with various concentrations of TMZ in combination with irradiation assayed at values time intervals. Error bars represents the standard deviation of mean values obtained from triplicate experiments.
Figure 5.
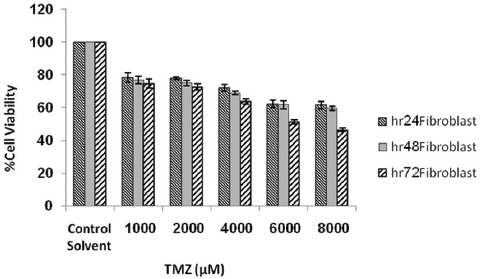
Viability of fibroblast cells after treatment with various concentrations of TMZ in combination with irradiation assayed at values time intervals. Error bars represents the standard deviation of mean values obtained from triplicate experiments.
Finally, after irradiation of samples by gamma-rays in combination with a dose of 2,000 µM/ml of TMZ, the percentage of viability and cytotoxicity of cells were assessed. Tables 1 and 2 show the percentage of cell viability acquired by different patterns under TMZ and IR treatment of cells. There were no statistical differences between data obtained for cell viability between the control and control solvent groups (P=0.35) and between IR alone and TMZ alone (P=0.15). Treated group with TMZ (2,000 µM/ml) in combination with IR (5 Gy) in both cell lines was compared to treated group with 2,000 µM dose of TMZ alone, showing a significant difference (P=0.02). Also, treated group with TMZ in combination with irradiation in both cell lines showed a significant difference compared to irradiation alone (P=0.02) (figure 6). There was no significance between the results obtained for U87MG cell lines and primary fibroblast (P=0.15) (figure 6).
Table 1.
A summary of data obtained for U87MG cells in treated samples under TMZ and gamma-rays (IR) alone or combined. Data shows mean values obtained from triplicate experiments. SD is the standard deviation of mean values
| Groups | Mean±SD | P value | ||||
|---|---|---|---|---|---|---|
| Control | Control solvent | TMZ (2,000 µM) | IR (5 Gy) | TMZ+IR | ||
| Times | ||||||
| 24 hrs | 1.15±0.15 | 1.26±0.35 | 0.78±0.03 | 0.69±0.09 | 0.55±0.16 | 0.009 |
| 48 hrs | 1.17±0.17 | 0.92±0.05 | 0.63±0.11 | 0.72±0.14 | 0.53±0.10 | 0.016 |
| 72 hrs | 1.23±0.19 | 1.20±0.29 | 0.73±0.08 | 0.64±0.10 | 0.40±0.05 | 0.015 |
Table 2.
A summary of data obtained for primary fibroblast cells in treated samples under TMZ and gamma-rays (IR) alone or combined. Data shows mean values obtained from triplicate experiments. SD is the standard deviation of mean values
| Groups | Mean±SD | P value | ||||
|---|---|---|---|---|---|---|
| Control | Control solvent | TMZ (2,000 µM) | IR (5 Gy) | TMZ+IR | ||
| Times | ||||||
| 24 hrs | 1.16±0.11 | 1.17±0.33 | 0.83±0.15 | 0.66±0.11 | 0.52±0.06 | 0.027 |
| 48 hrs | 1.22±0.28 | 1.16±0.35 | 0.79±0.08 | 0.60±0.14 | 0.40±0.07 | 0.013 |
| 72 hrs | 1.36±0.33 | 1.12±0.20 | 0.66±0.09 | 0.59±0.15 | 0.48±0.17 | 0.024 |
Figure 6.
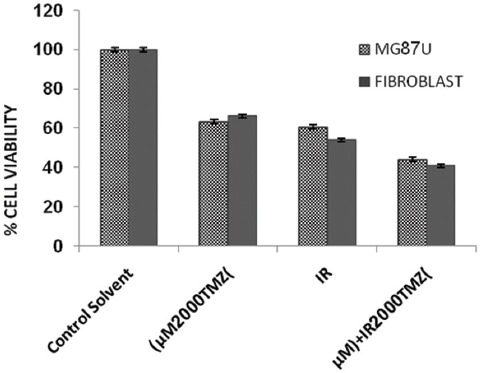
Viability of primary fibroblast and U87MG cells. Data represents the mean values obtained from triplicate experiments. Error bars indicate the standard deviation of mean values.
Discussion
Glioblastoma multiforme is a tumor in the central nervous system and accounts for 35-50% of intracranial tumors in adults. In addition, malignant glioma accounts for about 60% of glioma.18 Currently, surgery is the first-line treatment for glioma19 and it can be combined with radiotherapy or chemotherapy.20 External radiation therapy has a particular importance in the treatment of most cancers.21 The standard of care treatment for newly diagnosed glioblastoma changed in 200522 when radiation therapy plus chemotherapy replaced radiation therapy alone. Nowadays, for optimal use of external radiation therapy, combined techniques have been of interest to many investigators because of its potential in reducing radiation dose to normal tissues as well as increased damages to tumor cells. In a neurosurgery department, standard therapy in combination with chemotherapy and/or radiotherapy may achieve a mean survival time of 14.6 months.23,24 Adjuvant chemotherapy following initial surgery plays an important role in the management of patients with GBM and thus reduces the risk of cancer recurrence. Prior to 1999, nitrosourea-based combinations were the most commonly used chemotherapeutic agents in GBM, among which carmustine and lomustine were the most active agents. However, by adding these agents to combined surgery and postoperative radiotherapy, no significant improvements in terms of response rates and overall survival were observed. Since 1999 and by introducing TMZ, a modest improvement in median survival was observed. At present, concurrent chemoradiation followed by sequential adjuvant TMZ is recommended for patients with GBM. Radiosensitizers of TMZ can act like oxygen25 and thus intensify lesion due to irradiation.26 TMZ is an imidazotetrazine derivative and a novel oral alkylating agent27 with a molecular weight of 194.151 daltons.28,29 This drug is uptake by dividing cells and thus both cancer and normal cells are able to receive this drug. Its availability as an oral agent and efficacy support its potential in the treatment of glioma. It is also able to penetrate the blood-brain-barrier (BBB) and it does not require hepatic metabolism for activating the methylation of DNA. This is the principal mechanism for the cytotoxicity of TMZ against malignant cells, resulting in the fragmentation of DNA and disrupted DNA replication, and thus growth suppression and apoptotic cell death. TMZ remains stable in acidic conditions.30 For this reason, it is administered orally due to its resistant to high acidic conditions of the stomach. After leaving the systemic circulation and passing the BBB; under spontaneous hydrolysis and a series of chemical reactions, it leads to cell death.31 It is important to note that brain tumors tend to have higher pH rather than normal brain tissue and as mentioned earlier, the uptake of TMZ is more in alkaline environment. Thus, TMZ is used especially for brain cancer cells since its surrounding cells have less proliferation ability. Theoretically, glioma is the best option for this type of treatment and TMZ is evaluated in this study due to its widespread use in clinical studies.32
The patients were initially treated with 40 Gy whole brain conventional radiotherapy (daily fraction of 1.8-2 Gy and five fractions per week), which was continued to the primary tumor using reduced size fields up to 54 Gy. However, nowadays, a median dose of 54 (range 40–60) Gy is considered for all patients. Patients with a poor general condition were treated palliatively and only received 30 Gy in 10 fractions (i.e. 3 Gy per fraction).33 For this reason, in the present study, a single dose of 5 Gy with a high dose rate in combination with temozolomide was used. In a retrospective study, Ahmadloo et al. reported that the prognosis of adult patients with GBM remains poor; however, adjuvant treatments improve the overall survival.33 Wedge et al. in 2008 and 2011 have shown that the most damage due to radiosensitizing effect of TMZ drug is due to the presence of adenine and guanine in DNA. Methylation of DNA is the main mechanism of TMZ cytotoxicity against malignant cells that causes cell death with fragmentation and replication disorder of DNA.34,35 Based on this information and according to the repair process, researchers have found that such repair process can be disrupted and damage to tumor cells can be increased by ionizing radiation.34
Studies on the performance of TMZ in vitro and monolayer culture model of glioma cancer cells have been conducted.36 Results of these studies showed that TMZ has a synergistic effect in cancer cells’ radiosensitivity in combination with ionizing radiation.36 One of the main failures in radiation therapy of cancers is the presence of hypoxic cells. These cells are in the category of resistant cells to radiation. Consequently, radiosensitizers application of hypoxic cancer cells can help cancer cell death. In another study by Wei Shen et al. in 2006, they revealed that TMZ is inducing autophagy death.37 In addition, the treatment of cancer cells by irradiation in combination with TMZ causes autophagy death.38,39 Also, studies indicate that the number of cells after treatment with TMZ are less in S and G1 phases and are more in G2/M phase, which is a sign of cell arrest in G2/M phase.39 These findings and previous results show that TMZ causes cell cycle arrest, DNA methylation and inhibition of DNA DSB repair, and will lead to radiosensitivity of cancer cells. Additionally, Kil et al. revealed that radiosensitivity induced by TMZ do not cause an increased susceptibility to apoptosis and the main factor in evaluating cytotoxicity induced by radiation is by inducing and repair of DNA damage, particularly DSB.34
Tumors hypoxia increases by increasing the fraction number of the delivered dose. Therefore, the most radiosensitizers are more effective when used with a single dose of radiation.40,41 Since various doses of radiation is indicated for GBM in the literature, in this research a single dose of 5 Gy with a high dose rate was used to evaluate its efficacy when combined with TMZ. A decrease in cell viability following irradiation alone, TMZ treatment alone, or a combination of both modalities might be due to DNA DSB damage-induced leading to mitotic or apoptotic cell death.
Conclusion
A combination of high-dose with higher dose rate in a single fraction of irradiation with TMZ would lead to lower cell viability and eventually cell death. This observation may help to achieve a better therapeutic efficacy and clinical management of malignant glioma.
Acknowledgement
The authors are grateful to the Cancer Research Center of Cancer Institute of Iran and Ophthalmic Research Center. We also would like to thank Dr. Sahar Balagholi for her kind assistance.
Conflict of Interest: None declared.
References
- 1.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Ushio Y, Kochi M, Hamada J, Kai Y, Nakamura H. Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol Med Chir (Tokyo) 2005;45:454–60. doi: 10.2176/nmc.45.454. [DOI] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Gao S. Temozolomide/PLGA microparticles and antitumor activity against glioma C6 cancer cells in vitro. Int J Pharm. 2007;329:122–8. doi: 10.1016/j.ijpharm.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Taphoorn MJ, Henriksson R, Bottomley A, Cloughesy T, Wick W, Mason WP, et al. Health-Related Quality of Life in a Randomized Phase III Study of Bevacizumab, Temozolomide, and Radiotherapy in Newly Diagnosed Glioblastoma. J Clin Oncol. 2015;33:2166–75. doi: 10.1200/JCO.2014.60.3217. [DOI] [PubMed] [Google Scholar]
- 7.Camphausen K, Tofilon PJ. Inhibition of histone deacetylation: A strategy for tumor radiosensitization. J Clin Oncol. 2007;25:4051–6. doi: 10.1200/JCO.2007.11.6202. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain MC. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev Neurother. 2010;10:1537–44. doi: 10.1586/ern.10.32. [DOI] [PubMed] [Google Scholar]
- 9.Omar AI, Mason WP. Temozolomide: The evidence for its therapeutic efficacy in malignant astrocytomas. Core Evid. 2010;4:93–111. doi: 10.2147/ce.s6010. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott AW, Tyler BM, Masi BC, Upadhyay UM, Patta YR, Grossman R, et al. Intracranial microcapsule drug delivery device for the treatment of an experimental gliosarcoma model. Biomaterials. 2011;32:2532–9. doi: 10.1016/j.biomaterials.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Osoba D, Brada M, Yung WK, Prados M. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol. 2000;18:1481–91. doi: 10.1200/jco.2000.18.7.1481. [DOI] [PubMed] [Google Scholar]
- 12.Tsuno T, Natsume A, Katsumata S, Mizuno M, Fujita M, Osawa H, et al. Inhibition of Aurora-B function increases formation of multinucleated cells in p53 gene deficient cells and enhances anti-tumor effect of temozolomide in human glioma cells. J Neurooncol. 2007;83:249–58. doi: 10.1007/s11060-007-9335-1. [DOI] [PubMed] [Google Scholar]
- 13.Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876. doi: 10.1038/cddis.2013.388. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weller M, Steinbach JP, Wick W. Temozolomide: A milestone in the pharmacotherapy of brain tumors. Future Oncol. 2005;1:747–54. doi: 10.2217/14796694.1.6.747. [DOI] [PubMed] [Google Scholar]
- 15.Buch K, Peters T, Nawroth T, Sanger M, Schmidberger H, Langguth P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay--a comparative study. Radiat Oncol. 2012;7:1. doi: 10.1186/1748-717X-71. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikzad S, Hashemi B, Hassan ZM, Mozdarani H. The Cell Survival of F10B16 Melanoma and 4T1 Breast Adenocarcinoma Irradiated to Gamma Radiation Using the MTT Assay Based on Two Different Calculation Methods. J Biomed Phys Eng. 2013;3:29–36. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 17.Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol. 2011;716:157–68. doi: 10.1007/978-1-61779-012-6959. [DOI] [PubMed] [Google Scholar]
- 18.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nava F, Tramacere I, Fittipaldo A, Bruzzone MG, Dimeco F, Fariselli L, et al. Survival effect of first- and second-line treatments for patients with primary glioblastoma: A cohort study from a prospective registry 1997-2010. Neuro Oncol. 2014;16:719–27. doi: 10.1093/neuonc/not316. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribba B, Kaloshi G, Peyre M, Ricard D, Calvez V, Tod M, et al. A tumor growth inhibition model for low-grade glioma treated with chemotherapy or radiotherapy. Clin Cancer Res. 2012;18:5071–80. doi: 10.1158/1078-0432.CCR-12-0084. [DOI] [PubMed] [Google Scholar]
- 21.Huang SH, O’Sullivan B. Oral cancer: Current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18:e233–40. doi: 10.4317/medoral.18772. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107:359–64. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Zhou C, Xu L, Xiao H. Hypoxia enhances stemness of cancer stem cells in glioblastoma: An in vitro study. Int J Med Sci. 2013;10:399–407. doi: 10.7150/ijms.5407. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today. 2015;20:899–905. doi: 10.1016/j.drudis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Sehedic D, Cikankowitz A, Hindre F, Davodeau F, Garcion E. Nanomedicine to overcome radioresistance in glioblastoma stem-like cells and surviving clones. Trends Pharmacol Sci. 2015;36:236–52. doi: 10.1016/j.tips.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Mouawad R, Sebert M, Michels J, Bloch J, Spano JP, Khayat D. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit Rev Oncol Hematol. 2010;74:27–39. doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Kitambi SS, Toledo EM, Usoskin D, Wee S, Harisankar A, Svensson R, et al. Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell. 2014;157:313–28. doi: 10.1016/j.cell.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Diez BD, Statkevich P, Zhu Y, Abutarif MA, Xuan F, Kantesaria B, et al. Evaluation of the exposure equivalence of oral versus intravenous temozolomide. Cancer Chemother Pharmacol. 2010;65:727–34. doi: 10.1007/s00280-009-1078-6. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5:144–51. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 30.Negreira N, Mastroianni N, Lopez de Alda M, Barcelo D. Multianalyte determination of 24 cytostatics and metabolites by liquid chromatography-electrospray-tandem mass spectrometry and study of their stability and optimum storage conditions in aqueous solution. Talanta. 2013;116:290–9. doi: 10.1016/j.talanta.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 31.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–97. [PubMed] [Google Scholar]
- 32.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013;15:4–27. doi: 10.1093/neuonc/nos273. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmadloo N, Kani AA, Mohammadianpanah M, Nasrolahi H, Omidvari S, Mosalaei A, et al. Treatment outcome and prognostic factors of adult glioblastoma multiforme. J Egypt Natl Canc Inst. 2013;25:21–30. doi: 10.1016/j.jnci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Kil WJ, Cerna D, Burgan WE, Beam K, Carter D, Steeg PS, et al. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14:931–8. doi: 10.1158/1078-0432.CCR-07-1856. [DOI] [PubMed] [Google Scholar]
- 35.Carmo A, Carvalheiro H, Crespo I, Nunes I, Lopes MC. Effect of temozolomide on the U-118 glioma cell line. Oncol Lett. 2011;2:1165–70. doi: 10.3892/ol.2011.406. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palumbo S, Pirtoli L, Tini P, Cevenini G, Calderaro F, Toscano M, et al. Different involvement of autophagy in human malignant glioma cell lines undergoing irradiation and temozolomide combined treatments. J Cell Biochem. 2012;113:2308–18. doi: 10.1002/jcb.24102. [DOI] [PubMed] [Google Scholar]
- 37.Turriziani M, Caporaso P, Bonmassar L, Buccisano F, Amadori S, Venditti A, et al. O6-(4-bromothenyl)guanine (PaTrin-2), a novel inhibitor of O6-alkylguanine DNA alkyl-transferase, increases the inhibitory activity of temozolomide against human acute leukaemia cells in vitro. Pharmacol Res. 2006;53:317–23. doi: 10.1016/j.phrs.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–57. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 39.Mhaidat NM, Zhang XD, Allen J, Avery-Kiejda KA, Scott RJ, Hersey P. Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer. 2007;97:1225–33. doi: 10.1038/sj.bjc.6604017. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merz F, Gaunitz F, Dehghani F, Renner C, Meixensberger J, Gutenberg A, et al. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol. 2013;15:670–81. doi: 10.1093/neuonc/not003. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JE, Khuntia D, Robins HI, Mehta MP. Radiotherapy and radiosensitizers in the treatment of glioblastoma multiforme. Clin Adv Hematol Oncol. 2007;5:894–902. 7, 15. [PubMed] [Google Scholar]


