Abstract
Background:
Discriminating latent tuberculosis infection (LTBI) from active TBI may be challenging. The objective of this study was to produce the recombinant L-alanine dehydrogenase (AlaDH) antigen and evaluate individuals with LTBI, those with active TBI, and uninfected individuals by enzyme-linked immunospot assay (ELISPOT) in order to distinguish LTBI from active TBI.
Methods:
This exploratory study was performed in the Iranian city of Shiraz from 2014 to 2015. The study population (N=99) was divided into 3 groups: individuals with newly diagnosed active TBI (n=33), their household contacts (n=33), and controls (n=33). AlaDH was produced through PCR and cloning methods. The diagnostic characteristics of AlaDH vs. ESAT-6/CFP-10 were evaluated in responses to interferon-γ (IFN-γ) and interleukin-2 (IL-2) with ELISPOT. Differences between the groups were assessed with the Kruskal–Wallis and Mann–Whitney tests for nonparametric data analysis. The statistical analyses were performed with SPSS, version 16.
Results:
IFN-γ responses to both ESAT-6/CFP-10 (P=0.81) and AlaDH (P=0.18) revealed that there were no significant differences between the individuals with LTBI and those with active TBI. The same results were determined for IL-2 responses to ESAT-6/CFP-10 between the 2 groups, while significantly higher IL-2 responses to AlaDH were observed in LTBI than in active TBI. According to the ROC curve analysis, a cutoff value of 275 SFC showed sensitivity of 75.8% and specificity of 78.8% for distinguishing LTBI from active TBI by IL-2 responses to AlaDH.
Conclusion:
The current study suggests that it may be possible to discriminate LTBI from active TBI by IL-2 responses to AlaDH.
Keywords: Enzyme-linked immunospot assay, Interferon-gamma, Interleukin-2, Mycobacterium tuberculosis
What’s Known
Distinguishing latent tuberculosis (LTBI) from active TB is important. Tuberculin skin test for several years was the only but inaccurate way to distinguish LTBI. Some studies have shown that IFN-γ and IL-2 responses to TB antigens such as AlaDH cannot discriminate the 2 kinds of infection.
What’s New
We are the 1st to demonstrate the diagnostic characteristics of the recombinant AlaDH antigen for the distinction between latent and active TB infection in adults.
ELISPOT responses of IL-2 to AlaDH between active and latent TB infections are significantly different, which in turn could detect individuals recently infected with LTBI.
Introduction
An estimated one-third of the world’s population is currently infected with Mycobacterium tuberculosis (M. tuberculosis). Iran is located in a tuberculosis (TB) endemic region in which there are 2 countries with a high prevalence of TB.1 The majority of people harbor the bacteria as a latent tuberculosis infection (LTBI), and even though they do not show the symptoms of active TBI, they are potentially vulnerable to progression to TB disease.2 The tuberculin skin test (TST) for several years was the only way to diagnose LTBI. Nonetheless, because the TST had low specificity and sensitivity and was difficult to perform, it was necessary that a more accurate method be devised.3
The M. tuberculosis genome was sequenced in the late 1990s and it later became clear that the section of the BCG genome was deleted. Two antigens of this part, namely early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10), are good candidates for stimulating T cells in persons who are infected with M. tuberculosis in order to stimulate the production of interferon-γ (IFN-γ) and other cytokines such as interleukin-2 (IL-2).4,5 IFN-γ is measured by methods such as enzyme-linked immunospot assay (ELISPOT) and is drawn upon to invent new methods for diagnosing TBI.6,7 Almost 30% of individuals in contact with patients suffering from active TBI exhibit some symptoms of this infection, and half of them will develop the disease in the first 2 years.8 Previous studies have shown that LTBI is more prevalent in persons in close contact with patients suffering from active TBI. Thus, the identification and treatment of individuals with LTBI can prevent the progression of the disease to active TBI.9-11 Since the distinction between LTBI and active TBI is impossible,12 a test that could distinguish between LTBI and active TBI will be very useful.13,14 Several antigens of M. tuberculosis in addition to ESAT-6 and CFP-10 are currently used for this purpose.15,16 L-alanine dehydrogenase (AlaDH) is one of these antigens, and it is involved in the metabolism of nitrogen and the adaptation of M. tuberculosis in anaerobic conditions.17 In a research performed in our laboratory, ESAT-6, CFP-10, and ESAT-6/CFP-10 fusion antigens were produced. It has been shown that ESAT-6/CFP-10 fusion antigens are valuable for the diagnosis of active TBI.18,19 Also, recent research has demonstrated that the analysis of IL-2 might help to distinguish active TBI from LTBI.20,21 Therefore, in the current study, we aimed to analyze IFN-γ and IL-2 responses to AlaDH and ESAT-6/CFP-10 fusion antigens in the diagnosis of LTBI vs. active TBI.
Patients and Methods
Specimen Collection
This exploratory study was performed in the Iranian city of Shiraz from 2014 to 2015. The study population (N=99) was divided into 3 groups. The 1st group (n=33) contained patients diagnosed with active TBI. They had a positive TST, confirmed by the examination of the sputum for acid-fast bacilli and/or culturing. Patients who had started treatment were excluded from the study. All the blood samples were obtained from the TB Center of Shiraz University of Medical Sciences. The mean age was 33 years (range=21–59 y), and there were 16 male individuals in this group. The 2nd group (n=33) comprised individuals with LTBI who were selected from the household contacts of the 1st group and had a positive TST with no clinical or radiographic evidence of active TBI. The mean age was 31 years (range=20–60 y), and there were 16 male individuals in this group. The 3rd group (n=33) was made up of healthy controls with a negative TST and without known contact with patients suffering from M. tuberculosis. The mean age was 34 years (range=19–56 y), and there were 17 male individuals in this group. HIV-positive persons were excluded from the analysis. Sex and age were matched in these groups. All the patients and normal subjects gave written informed consent ahead of participation in the study. The details of the 3 groups are given in table 1.
Table 1.
Demographic characteristics of the total study population
| n (%) | |||
|---|---|---|---|
| Control | LTBI | Active TBI | |
| Age (median) | 34 (19-56) | 31 (20-60) | 33 (21-59) |
| Emigrants | 5 (15.1) | 6 (18.2) | 5 (15.1) |
| Male | 17 (51.5) | 16 (48.5) | 16 (48.5) |
| Female | 16 (48.5) | 17 (51.5) | 17 (51.5) |
| BCG vaccinated | 29 | 27 | 28 |
| TST (mm) | |||
| <5 | 32 | 0 | 0 |
| 5-9 | 1 | 1 | 0 |
| 10-14< | 0 | 5 | 3 |
| ≥15 | 0 | 27 | 30 |
TBI: Tuberculosis infection; LTBI: Latent tuberculosis infection; TST: Tuberculin skin test
Cloning of the AlaDH Gene
The gene of the AlaDH protein was amplified by polymerase chain reaction (PCR) from M. tuberculosis H37Rv chromosomal DNA, which was obtained from Razi Vaccine Institute and Serum Research Institute in Tehran. The 2 primers used were upper: 5’ -CGG GGT ACC ATG CGC GTC GGT ATT C -3’ and down: 5’-CCC AAG CTT ACA GGC CAG CAC -3’. PCR amplification was performed with 35 cycles at 94 °C for 45 seconds, 66 °C for 45 seconds, 72 °C for 45 seconds, and 72 °C for 10 minutes. The expression and purification of the recombinant protein were performed under standard protocols. The PCR product was ligated to the PET32a vector, which is commercially available from Novagen. This vector permits the genes of interest to be fused to the thioredoxin fusion protein, trxA, for high levels of expression with good solubility and it also contains cleavable His-tag sequences for detection and purification. It was thereafter transferred into Escherichia coli (E. coli) strain DH5α, which was grown in a Luria–Bertani (LB) medium (Gibco Life Technologies PA, UK) containing ampicillin (Gibco Life Technologies PA, UK) (100 µg/mL). The recombinant plasmids were purified from the positive clones, and the PCR constructs were sequenced to verify their integrity. E. coli BL21 was transformed by PET32a and plated on an LB solid medium. An overnight culture (600 mL) of the resulting strain was used to inoculate 12 L of LB, and when it was in the mid-log phase (absorbance 600 reached 0.7), it was induced by isopropyl-D-thiogalacto-pyranoside (IPTG, 0.5 mM) (Fermentas AB, VL, Lithuania). The bacterial cells were harvested by centrifugation, and the cell pellets were frozen at -70 °C.22
SDS–PAGE and Western Blotting
The expression of the recombinant protein was investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and western blotting. The cell pellets were re-suspended with a lysis buffer containing phosphate-buffered saline, 1 mM of EDTA (pH=8.0), and 1% (v/v) Triton X-100. The cell lysates were sonicated (30-s pulses at 20-s intervals for 6 times), and the suspension was centrifuged. Next, 12.5% SDS–PAGE analysis was performed by utilizing Mini Format Vertical Electrophoresis (Bio-Rad, Hercules, CA, USA). Western blotting was done based on the recognition of His-tag. Accordingly, the SDS–PAGE-separated proteins were electrotransferred into nitrocellulose membranes (Millipore, Bedford, MA USA) using a Mini Trans-Blot Cell (Bio-Rad, Hercules, CA, USA). The membranes were incubated with horseradish peroxidase-conjugated anti-poly-His antibody (Santa Cruz, Dallas, TX, USA). The detection of the specific protein was facilitated using an enhanced chemiluminescence solution. Light emissions were captured by exposing the membranes to X-ray films.5
Purification of the AlaDH Recombinant Protein
PET32a supports the His-tag cloning system; therefore, the recombinant protein was purified by the Ni-NTA affinity column (Qiagen GmbH, Hilden, Germany) under denaturing and native conditions. After the sample was loaded on the column and extensive washing steps were performed with 8-M urea (Sigma, St. Louis, MO, USA) (pH=7.4), elution by using a linear 10–500 mM gradient of imidazole (Sigma, St. Louis, MO, USA) was performed. Then, the His-tagged protein was dialyzed against phosphate-buffered saline for 48 hours, while the buffer was changed every 12 hours to remove the urea and imidazole. The bicinchoninic acid method was used to assay the protein concentration (Pierce, Rockford, IL, USA).23
IFN-γ and IL-2 ELISPOT Assays
IFN-γ and IL-2 ELISPOT assays were performed for the quantification of the responses of the T cells to recombinant AlaDH and ESAT-6/CFP-10 antigens, which were produced in our lab previously,18 by utilizing a commercially available ELISPT kit (U-CyTech, Utrecht, Netherlands). A 6-mL blood sample from each participant was collected. Subsequently, the peripheral blood mononuclear cells (PBMCs) of the samples were separated with Ficoll (Sigma, St. Louis, MO, USA), and the number of the cells was quantified. MultiScreen 96-well plates (Millipore, Bedford, MA, USA) were individually pre-coated with anti-IFN-γ and anti-IL-2 antibodies (10 µg/mL) and incubated overnight at 4 °C. The mycobacterial antigens (1 µg/mL) and PBMCs (2×105 cells/well) were added to each well. The plain medium as a negative control and phytohemagglutinin (Sigma, St. Louis, MO, USA) as a positive control were applied and incubated for 24 hours for IFN-γ and 72 hours for IL-2 at 37 °C. The wells were washed, and biotinylated anti-IFN-γ, anti-IL-2, and φ-labeled goat anti-biotin antibodies were added separately. Finally, the spots were revealed by activators I and II, which display IFN-γ and IL-2 secreting cells, followed by the quantification of the spots using a stereomicroscope. The number of the spots from the unstimulated wells was subtracted from the number in each well. The mean of the duplicates was applied, and all the results were expressed as the number of spot-forming cells (SFC) per million PBMCs.24,25
Data Analysis
All the experiments were repeated independently twice. The results of the Shapiro–Wilk test for all the groups showed that the variables did not have a normal distribution. Accordingly, the differences between the groups were assessed with the Kruskal–Wallis and Mann–Whitney tests for nonparametric data analysis. A P≤0.05 was considered significant. The statistical analyses were performed using SPSS, version 16 (SPSS Inc., Chicago, IL, USA). The receiver operating characteristic (ROC) curve was plotted in order to find out the optimum cutoff for the ELISPOT assays of IL-2 and IFN-γ responses in discriminating individuals with active TBI from those with LTBI and also to determine the sensitivity and the specificity of these assays.
Ethical Approval
Ethical approval for the study was granted by the Research Ethics Committees of Shiraz University of Medical Sciences, and informed consent was obtained from all the participants.
Results
Cloning, Expression, and Purification of the AlaDH Recombinant Antigen
The amplification of the AlaDH gene was performed by PCR. The PCR product (1100 bp) (figure 1) was successfully cloned into the PET32a expression vector. DNA sequencing confirmed the accurate integrity and orientation of the gene. The Ni–NTA column was used to purify the AlaDH protein, and SDS–PAGE analysis was performed to indicate the molecular weight of the desired protein around 40 kDa. In order to achieve a pure recombinant protein, we removed thioredoxin, trxA, by thrombin cleavage (figure 2). However, since this process reduces the yield of the protein, we utilized recombinant proteins with and without thrombin cleavage for ELISPOT to observe the differences. When similar results were obtained, recombinant protein without thrombin cleavage was applied. The AlaDH His-tag protein was recognized by the mouse anti-His-tag monoclonal antibody using western blotting, as is shown in figure 3.
Figure 1.
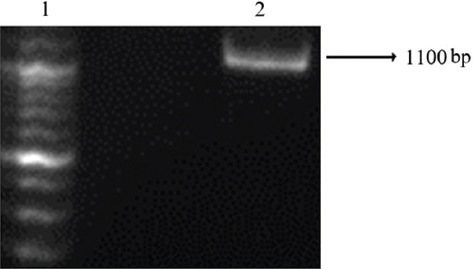
100-bp marker (lane 1), agarose gel analysis for the L-alanine dehydrogenase (AlaDH) gene (lane 2).
Figure 2.
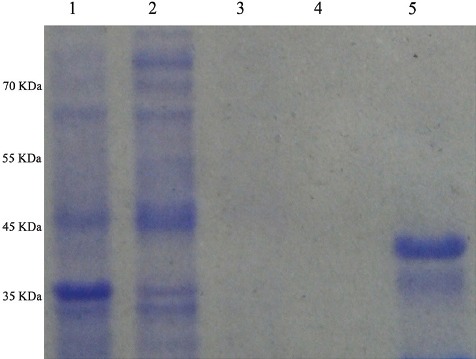
Expression and purification of L-alanine dehydrogenase (AlaDH) antigen samples, (lanes 1–5) resolved in a 12% (w/v) polyacrylamide gel, under reducing conditions followed by Coomassie blue staining. Escherichia coli (E. coli) extracts before isopropyl D-thiogalacto-pyranoside (IPTG) induction (lane 1). E. coli extracts 4 hours after induction with 0.1 mM of IPTG (lane 2). Washed Ni-NTA resin (lanes 3 and 4). Recombinant proteins purified by affinity chromatography using Ni-NTA resin with thrombin cleavage (lane 5). Molecular weight standards are shown to the left of lane 1.
Figure 3.

Western blot assay of the recombinant protein with His-tag monoclonal antibody.
IFN-γ and IL-2 Responses to AlaDH vs. ESAT-6/CFP-10 by ELISPOT
ELISPOT assays were performed in the study groups for IFN-γ and IL-2 responses to AlaDH and ESAT-6/CFP-10 fusion antigens. The data were presented as SFC and shown in table 2. The responses of IFN-γ and IL-2 to the aforementioned antigens were compared between the 3 groups. The ELISPOT assays of IFN-γ responses to AlaDH (P=0.18) and ESAT-6/CFP-10 (P=0.81) and IL-2 responses to ESAT-6/CFP-10 (P=0.99) were not significantly different between LTBI and active TBI, while IL-2 responses to AlaDH (P=0.001) were different significantly. The ROC curve analysis was performed, and the optimum cutoff for IL-2 responses to AlaDH was 275 SFC and LTBI was identified with sensitivity of 75.8% and specificity of 78.8%. As is shown in figure 4, the ROC curve analysis demonstrated that IL-2 responses to AlaDH were the most sensitive and specific indicator of LTBI (area under the curve=0.820), while the ROC curve analysis for IL-2 responses to ESAT-6/CFP-10, IFN-γ responses to ESAT-6/CFP-10, and IFN-γ responses to AlaDH showed much weaker responses.
Table 2.
Diagnostic performance of ELISPOT results in active TB patients, LTBI and healthy control groups
| Control median (IQR) | LTBI median (IQR) | Active TBI median (IQR) | P value control vs. active TB | P value LTBI vs. active TB | Area under the curve | Cutoff | Sensitivity% | Specificity% | |
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ ELISPOT result* | |||||||||
| ESAT-6/CFP-10 | 25 (15-40) | 265 (190-333) | 275 (190-368) | 0.001 | 0.65 | 0.532 | 65 | 33.3 | 84.8 |
| AlaDH | 20 (15-25) | 85 (65-130) | 105 (78-150) | 0.001 | 0.11 | 0.612 | 245 | 48.5 | 63.6 |
| IL-2 ELISPOT result* | |||||||||
| ESAT-6/CFP-10 | 45 (35-65) | 200 (130-310) | 180 (138-323) | 0.001 | 0.93 | 0.494 | 235 | 69.7 | 42.4 |
| AlaDH | 25 (15-38) | 380 (270-580) | 185 (103-270) | 0.001 | 0.0001 | 0.820 | 275 | 75.8 | 78.8 |
ELISPOT: Enzyme-linked immunospot assay; TBI: Tuberculosis infection; LTBI: Latent tuberculosis infection;
Results are illustrated as medians and interquartile ranges of spot forming colonies per million peripheral blood mononuclear cells (PBMCs)
Figure 4.
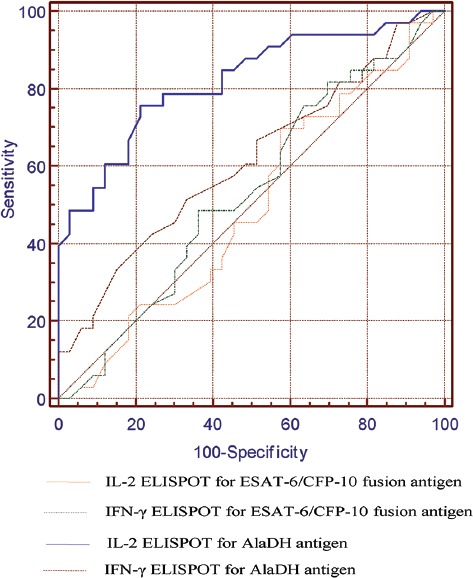
A receiver operating characteristic (ROC) plot is shown, illustrating sensitivity and specificity of the enzyme-linked immunospot (ELISPOT) assays of interferon-γ (IFN-γ) and interleukin-2 (IL-2) responses to AlaDH and ESAT-6/CFP-10 fusion antigens in discriminating latent tuberculosis infection (LTBI) from active TBI. Area under the ROC curve was 0.820 for AlaDH IL-2 ELISPOT, 0.612 for AlaDH IFN-γ ELISPOT, 0.494 for ESAT-6/CFP-10 IL-2 ELISPOT and 0.532 for ESAT-6/CFP-10 IFN-γ ELISPOT.
Discussion
In the present study, we demonstrated that AlaDH might be a promising marker for distinguishing LTBI from active TBI. The aims of the present study were cloning, expressing, and purifying the AlaDH recombinant antigen in order to evaluate the diagnostic characteristics of AlaDH and ESAT-6/CFP-10 in the distinction between adults with LTBI and adults with active TBI. It has been found that CFP-10 and ESAT-6 antigens distinguish individuals with active TBI from healthy individuals with high diagnostic sensitivity and specificity, whereas they are inadequate for the discrimination of active TBI from LTBI. In our previous study, ESAT-6/CFP-10 fusion was used to detect active TBI with 100% sensitivity and 100% specificity. In the current study, the ELISPOT assays of IFN-γ and IL-2 responses to ESAT-6/CFP-10 between individuals with active TBI and healthy controls were significantly different, which is consistent with previous reports.18,26,27 Nonetheless, the results were not significant considering the ELISPOT assays of IFN-γ and IL-2 responses to ESAT-6/CFP-10 and IFN-γ responses to AlaDH between the individuals with active TBI and those with LTBI. The SFC values of IL-2, induced by AlaDH, were significantly higher in LTBI than in active TBI, considering the number of individuals with LTBI-identified AlaDH compared with the patients with active TBI. Since this protein is involved in the adaptation of M. tuberculosis in anaerobic conditions, it is expected that antigens are exposed to more T cells and as a result more cytokine responses occur in persons with LTBI.17,28 AlaDH may play a role in cell wall synthesis as L-alanine is an important constituent of the peptidoglycan layer. Agren et al.17 (2008) demonstrated that AlaDH could alter the expression profile for adaptation to a state of latent infection. The authors also showed that the conformation and crystal structure of AlaDH was changed from open to closed ternary forms in the phase of latent infection, which indicated the different host immune responses of LTBI.
In a study conducted in Italy by Chiappini et al.29 (2012), the antibody responses of 6 antigens in children with LTBI and active TBI were compared, and their data revealed that IL-2 responses to the AlaDH antigen were significantly different between the children with active TBI and the healthy control group, whereas there was no difference between active TBI and LTBI in their study. The authors showed that AlaDH was not capable of discriminating active TBI from LTBI, which is in contrast to the results of our study, in which significant differences were observed between these 2 infections. The discrepancies between our findings may be due to actual differences in the immune responses between adults and children, which in turn indicates that the potency of AlaDH in the diagnosis of LTBI from active TBI is greater in our study.
We also showed that IL-2 responses to AlaDH were higher than IFN-γ responses to AlaDH in LTBI. It has been previously revealed that a higher response of IL-2 in LTBI was achieved after 72 hours’ incubation time, whereas 24 hours’ incubation time for IFN-γ had the same response. Thus, a prolonged incubation period seems to be essential to indicate an increased number of central memory T cells in adults with LTBI.30,31 Accordingly, we used the aforesaid experimental conditions. Given that IL-2-positive ELISPOT was related to ancient exposure, the increase in IL-2-secreting memory T cells may explain the high response of IL-2 compared with IFN-γ in our study.21
In the past few years, a large number of studies, systematic reviews, and meta-analyses have been conducted to evaluate ELISPOT in distinguishing individuals with LTBI.32-34 Some of these studies have used different antigens. For instance, Hougardy et al.35 (2007) showed that heparin-binding-hemagglutinin antigen had higher sensitivity than ESAT-6 for the detection of LTBI. Nevertheless, their study was carried out in a country with a low TB prevalence and as such cannot be generalized to other communities. In another study, Martinez et al.36 (2007) evaluated the cellular immune response of the Erp antigen and demonstrated that IFN-γ responses were higher in patients with LTBI than in those with active TBI. Be that as it may, Erp is present in the other species of mycobacteria and vaccine strain Mycobacterium bovis BCG. Chen and colleagues16 (2009) conducted a study that examined the 6 antigens and found that IFN-γ responses to the Rv1978 antigen were higher in individuals with LTBI than in those with active TBI, although this antigen was not sensitive enough.
The ROC curve analysis between the 2 antigens demonstrated that the ELISPOT assay for IL-2 responses to AlaDH had a greater discriminatory power. Our data revealed that IL-2 responses to the AlaDH antigen were significantly different between the individuals with LTBI and those who suffered from active TBI. As a result, the sequential usage of ESAT-6/CFP-10 and AlaDH ELISPOT assays could be useful for the detection of TBI and LTBI. For instance, when these 2 tests are positive for a person, the result could be considered as LTBI. If IL-2 responses to ESAT-6/CFP-10 are positive and the same responses to AlaDH are negative, the result may indicate a person with active TBI. When IL-2 responses to both ESAT-6/CFP-10 and AlaDH are negative, the result may indicate an uninfected person.
Our study has several limitations that should be mentioned. First, there is no gold standard for LTBI. Indeed, the only one currently available is for the detection of the later developmental stage of active TBI. The assumption of the diagnosis of LTBI in our study was based on a mixture of background exposure, which is an unavoidable limitation. Therefore, the definition of LTBI is probabilistic, based on the duration of exposures and history of contact with a patient suffering from active TBI. Second, the design of our study does not permit any valuation of conversion over time. In order to do that, we would need an additional assessment of the diagnostic value of ESAT-6/CFP-10 and AlaDH by ELISPOT in a large-scale study.
Conclusion
A diagnostic method that can specifically identify LTBI would allow more targeted therapy, which in turn could significantly reduce active TBI development. To that end, our study suggests that the ELISPOT assay of IL-2 responses to AlaDH can detect individuals with LTBI who have recently been infected. Accordingly, AlaDH has diagnostic value for individuals with active TBI. However, further prospective studies are needed to monitor the ELISPOT assay of IL-2 responses to AlaDH among patients exposed to TBI.
Acknowledgement
This study was done as a part of a PhD thesis by B. Movahedi and was supported by a grant (# 92-6714) from the office of the Vice Chancellor for Research and the Committee for Advanced Biomedical Sciences, Shiraz University of Medical Sciences. We would like to specially thank Dr. Moghadami (Vice Chancellor for Health) and Dr. Honarvar (Associate Principal for Research of the Health Policy Research Center), who provided the possibility of collecting samples from the TB Center of Shiraz University of Medical Sciences.
Conflict of Interest: None declared.
References
- 1.Rafiee S, Besharat S, Jabbari A, Golalipour F, Nasermoaadeli A. Epidemiology of tuberculosis in northeast of Iran: a population-based study. Iran J Med Sci. 2009;34:193–7. [Google Scholar]
- 2.Lalvani A, Pareek M. A 100 year update on diagnosis of tuberculosis infection. Br Med Bull. 2010;93:69–84. doi: 10.1093/bmb/ldp039. [DOI] [PubMed] [Google Scholar]
- 3.Shiratori B, Nakajima C, Chagan-Yasutan H, Saitoh H, Zhao J, Usuzawa M, et al. Immunological Diagnosis of Active and Latent TB. New Jersey: INTECH Open Access Publisher; 2012. [Google Scholar]
- 4.Mustafa AS. Mycobacterial gene cloning and expression, comparative genomics, bioinformatics and proteomics in relation to the development of new vaccines and diagnostic reagents. Med Princ Pract. 2005;14(Suppl 1):27–34. doi: 10.1159/000086182. [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn. 2006;6:423–32. doi: 10.1586/14737159.6.3.423. [DOI] [PubMed] [Google Scholar]
- 6.Amicosante M, Ciccozzi M, Markova R. Rational use of immunodiagnostic tools for tuberculosis infection: guidelines and cost effectiveness studies. New Microbiol. 2010;33:93–107. [PubMed] [Google Scholar]
- 7.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States. MMWR Recomm Rep 2010. 2010;59:1–25. [PubMed] [Google Scholar]
- 8.Moosazadeh M, Khanjani N, Parsaee M. The prevalence of latent tuberculosis infection and smear positive pulmonary tuberculosis in people with household close contact with tuberculosis in north of iran. Iran J Med Sci. 2015;40:161–5. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 9.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–8. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 10.Richeldi L. An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2006;174:736–42. doi: 10.1164/rccm.200509-1516PP. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad S. New approaches in the diagnosis and treatment of latent tuberculosis infection. Respir Res. 2010;11:169. doi: 10.1186/1465-9921-11-169. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37:100–11. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 13.Kunst H. Diagnosis of latent tuberculosis infection: the potential role of new technologies. Respir Med. 2006;100:2098–106. doi: 10.1016/j.rmed.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 15.Bai XJ, Liang Y, Yang YR, Li N, Zhang XY, An HR, et al. Immune responses to latent tuberculosis antigen Rv2659c in Chinese populations. J Microbiol Immunol Infect. 2015;48:381–9. doi: 10.1016/j.jmii.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Su X, Zhang Y, Wang S, Shao L, Wu J, et al. Novel recombinant RD2- and RD11-encoded Mycobacterium tuberculosis antigens are potential candidates for diagnosis of tuberculosis infections in BCG-vaccinated individuals. Microbes Infect. 2009;11:876–85. doi: 10.1016/j.micinf.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Agren D, Stehr M, Berthold CL, Kapoor S, Oehlmann W, Singh M, et al. Three-dimensional structures of apo- and holo-L-alanine dehydrogenase from Mycobacterium tuberculosis reveal conformational changes upon coenzyme binding. J Mol Biol. 2008;377:1161–73. doi: 10.1016/j.jmb.2008.01.091. [DOI] [PubMed] [Google Scholar]
- 18.Hemmati M, Seghatoleslam A, Rasti M, Ebadat S, Mosavari N, Habibagahi M, et al. Expression and Purification of Recombinant Mycobacterium Tuberculosis (TB) Antigens, ESAT-6, CFP-10 and ESAT- 6/CFP-10 and Their Diagnosis Potential for Detection of TB Patients. Iran Red Crescent Med J. 2011;13:556–63. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmati M, Seghatoleslam A, Rasti M, Ebadat S, Naghibalhossaini F, Mostafavi-Pour Z. Additive effect of recombinant Mycobacterium tuberculosis ESAT-6 protein and ESAT-6/CFP-10 fusion protein in adhesion of macrophages through fibronectin receptors. J Microbiol Immunol Infect. 2016;49:249–56. doi: 10.1016/j.jmii.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Biselli R, Mariotti S, Sargentini V, Sauzullo I, Lastilla M, Mengoni F, et al. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin Microbiol Infect. 2010;16:1282–4. doi: 10.1111/j.1469-0691.2009.03104.x. [DOI] [PubMed] [Google Scholar]
- 21.Krummel B, Strassburg A, Ernst M, Reiling N, Eker B, Rath H, et al. Potential role for IL-2 ELISpot in differentiating recent and remote infection in tuberculosis contact tracing. PLoS One. 2010;5:e11670. doi: 10.1371/journal.pone.0011670. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poletto SS, da Fonseca IO, de Carvalho LP, Basso LA, Santos DS. Selection of an Escherichia coli host that expresses mutant forms of Mycobacterium tuberculosis 2-trans enoyl-ACP(CoA) reductase and 3-ketoacyl-ACP(CoA) reductase enzymes. Protein Expr Purif. 2004;34:118–25. doi: 10.1016/j.pep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Saini DK, Pant N, Das TK, Tyagi JS. Cloning, overexpression, purification, and matrix-assisted refolding of DevS (Rv.3132c) histidine protein kinase of Mycobacterium tuberculosis. Protein Expr Purif. 2002;25:203–8. doi: 10.1006/prep.2002.1628. [DOI] [PubMed] [Google Scholar]
- 24.Saini DK, Pant N, Das TK, Tyagi JS. Cloning, overexpression, purification, and matrix-assisted refolding of DevS (Rv 3132c) histidine protein kinase of Mycobacterium tuberculosis. Protein Expr Purif. 2002;25:203–8. doi: 10.1006/prep.2002.1628. [DOI] [PubMed] [Google Scholar]
- 25.Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–8. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 26.Adetifa IM, Lugos MD, Hammond A, Jeffries D, Donkor S, Adegbola RA, et al. Comparison of two interferon gamma release assays in the diagnosis of Mycobacterium tuberculosis infection and disease in The Gambia. BMC Infect Dis. 2007;7:122. doi: 10..1186/1471-2334-7-122. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Li Q, Liang Y, Yang Y, Zhang J, Liang J, et al. Clinical evaluation of a homemade enzyme-linked immunospot assay for the diagnosis of active tuberculosis in China. Mol Biotechnol. 2011;47:18–25. doi: 10.1007/s12033-010-9307-0. [DOI] [PubMed] [Google Scholar]
- 28.Amanatidou V, Syridou G, Mavrikou M, Tsolia MN. Latent tuberculosis infection in children: diagnostic approaches. Eur J Clin Microbiol Infect Dis. 2012;31:1285–94. doi: 10.1007/s10096-011-1524-3. [DOI] [PubMed] [Google Scholar]
- 29.Chiappini E, Della Bella C, Bonsignori F, Sollai S, Amedei A, Galli L, et al. Potential role of M. tuberculosis specific IFN-gamma and IL-2 ELISPOT assays in discriminating children with active or latent tuberculosis. PLoS One. 2012;7:e46041. doi: 10.1371/journal.pone.0046041. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 31.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–26. doi: 10.4049/jimmunol.178.8.5217. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang KC, Leung CC. Systematic review of interferon-gamma release assays in tuberculosis: focus on likelihood ratios. Thorax. 2010;65:271–6. doi: 10.1136/thx.2009.126771. [DOI] [PubMed] [Google Scholar]
- 33.Lange C, Pai M, Drobniewski F, Migliori GB. Interferon-gamma release assays for the diagnosis of active tuberculosis: sensible or silly? Eur Respir J. 2009;33:1250–3. doi: 10.1183/09031936.00019709. [DOI] [PubMed] [Google Scholar]
- 34.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 35.Hougardy JM, Schepers K, Place S, Drowart A, Lechevin V, Verscheure V, et al. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007;2:e926. doi: 10.1371/journal.pone.0000926. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez V, Carcelain G, Badell E, Jouan M, Mauger I, Sellier P, et al. T-cell and serological responses to Erp, an exported Mycobacterium tuberculosis protein, in tuberculosis patients and healthy individuals. BMC Infect Dis. 2007;7:83. doi: 10..1186/1471-2334-7-83. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


