Abstract
Background
The reason why Cystic Fibrosis (CF) is the most common fatal genetic disease among Caucasians has been incompletely studied. We aimed at deepening the hypothesis that CF carriers have a relative protection against Mycobacterium tuberculosis (Mtb) infection.
Methods
Applying spatial epidemiology, we studied the link between CF carriership rate and tuberculosis (TB) incidence in Brazil. We corrected for 5 potential environmental and 2 immunological confounders in this relation: monthly income, sanitary provisions, literacy rates, racial composition and population density along with AIDS incidence rates and diabetes mellitus type 2. Smoking data were incomplete and not available for analysis.
Results
A significant, negative correlation between CF carriership rate and TB incidence, independent of any of the seven confounders was found.
Conclusion
We provide exploratory support for the hypothesis that carrying a single CFTR mutation arms against Mtb infections.
Keywords: Cystic fibrosis, Tuberculosis, Spatial epidemiology, Brazil, Resistance genetics
Background
In Europe, 1:20 to 1:80 people carry a mutation in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene [1], rendering Cystic Fibrosis (CF) the most common life-shortening autosomal recessive disorder among Caucasians. Eighty-seven percent of European patients with CF have at least one F508del allele [2], a deletion of the phenylalanine (F) codon at position 508 [3] and supposed to be a founder mutation in Northern Europe [4].
Thanks to a better understanding and treatment, the mean life expectancy of patients with CF has increased to over 30 years in developed countries nowadays. Until the 1970s however, most CF patients died before reaching reproductive age [5]. However, the reason why CF is still as dominantly present, despite having an expected high purifying index [6], remains unknown.
One hypothesis for this high CF carriership rate among the Caucasian population could be that carrying a single CF mutation has (had) an evolutionary selective advantage. It has been suggested that CF heterozygotes would be more resistant to cholera, typhoid fever or tuberculosis. Using estimates of mortality from TB in different regions, Poolman and Galvani [7] determined that only Tuberculosis (TB) could account for modern-day CF incidence rates in Euro-descendent populations. They suggest that reduced susceptibility to TB in CF heterozygotes could explain the modern gradient of CF in Europe and around the globe, following the White Plague.
We aimed at conducting an in-depth analysis of this putative link between CF mutations and TB and decided to do so in Brazil. The country meets four conditions vital to conduct meaningful research on this topic. First, 47•7% of the population are Brasileiros brancos, or ‘white Brazilians’ of European descent [8]. This results in significant CF incidence and carriership rate [9]. Second, Brazil is one of the 22 WHO-designated ‘high burden countries’ for TB [10]. Third, the dominant Brazilian Mycobacterium tuberculosis (Mtb) strain is the same in Europe and the Americas [11]. Finally, elaborate datasets for CF and TB are available.
Given this unique context in Brazil, we analyse whether support for the CF-TB hypothesis can be provided from a health geographical point of view. This is done through a multidisciplinary, multiscalar spatial epidemiological study, addressing the question: ‘Are Caucasian CF carriership rates in Brazil negatively associated with TB incidence rates when corrected for confounders?’.
Methods
We researched the link between CF and TB on two scales on the Brazilian territory: the state and municipality level. Demographic data were extracted from the 2010 national population census and 2014 population estimates [12].
The research was approved by the Institutional Ethics Committees and registered through Plataforma Brasil.
At the state level, accurate F508del CFTR carriership and TB data were available for six states: Bahia (BA), Minas Gerais (MG), Rio de Janeiro (RJ), São Paulo (SP), Paraná (PR) and Santa Catarina (SC) (Fig. 1). Together, these states cover 116 million (m) inhabitants (BA: 15•3 m, MG: 21•0 m, SP: 44•8 m, RJ: 16•7 m, PR: 11•3 m and SC: 6•9 m).
Fig. 1.
Overview of the 6 Brazilian states included in this research
The research of Raskin et al. [13] for MG, SP, PR and SC, Cabello et al. [14] for RJ, and Moura Costa et al. [15] for BA provide the data on F508del carriership. Information on the carriership frequency of other CFTR mutations is not systematically reported. TB data per state were obtained from the Health Ministry [16].
As it has been shown that big units of aggregation for TB can lead to associations not being present at smaller [17], a second, more detailed scale was defined: the municipality level. São Paulo state’s 645 municipalities were selected, as well-elaborated CF and TB registries exist for that state: the Brazilian Cystic Fibrosis Registry (REBRAFC) [18] and the national Information System for Notifiable Diseases – TB Registry (SINAN-TB) databases respectively. Eight out of nine main CF Centres of São Paulo State and the SINAN-TB registry approved the use of their anonymised data. For their 907 patients with CF registered in the REBRAFC, the municipality of residence could be identified. SINAN-TB comprises 155,317 registered TB cases in São Paulo state between 2007 and 2014. Again, the patients’ municipality of residence was retrieved.
Minimum CF mutation carriership for the municipalities was calculated multiplying CF prevalence by two, as it is certain both parents of a patient with CF are CFTR mutation carriers. The rareness of this disease might cause unrealistically high incidence and carriership rates if, for example, a single CF patient is registered in a relatively sparsely populated municipality. In order to exclude this ‘problem of the small numbers’, only municipalities with over 10,000 inhabitants were withheld.
We hypothesized that the diversity among municipalities would allow correction for several external determinants – potential confounders. Based on a study of the human ecology of both CF and TB, we gathered municipality data on five important environmental confounders: the per capita nominal monthly income, sanitary provisions, literacy rates, the racial composition of the population and the population density [8]. We also studied three comorbid risk factors that are known to impair the immunological response to Mtb: AIDS incidence rates, diabetes mellitus type 2 and smoking [19–21].
Subsequently, a cartographic analysis was performed using GIS software (ArcMap10•2 by ESRI®). Maps were produced for both levels, allowing visual interpretation of the data. Positive spatial autocorrelation might occur when rates of geographically close spatial areas are more likely to be highly related than those from distant areas. This was controlled for, guaranteeing a geographically unbiased analysis at the municipality level.
Next, the municipality level findings were analysed statistically using SPSS (IBM®) and SAS/STAT® software. As data were not normally distributed, we calculated Spearman correlation coefficients between incidence of TB and prevalence of CF (bivariate level). Partial Spearman rank correlations were adjusted for average income, population density, literacy rates, share of Caucasians, sanitary provisions, and AIDS incidence rates (multivariate level). We also applied parametric tests by use of linear regression models instead of Spearman rank correlations to assure consistency in the results. For the parametric tests, the TB incidence data were logarithmically transformed. A p-value ≤0•05 was considered statistically significant for all tests.
Results
Using this multidisciplinary, multiscalar spatial epidemiological approach, we analysed whether Caucasian CF carriership rates in Brazil negatively correlate with TB incidence rates.
At the state-level, we analysed F508del carriership rates versus TB incidence (Fig. 2). F508del carriership rates were higher in states with a large share of Caucasians. In the southernmost states for example, PR and SC, on average 80% of inhabitants is considered to be Caucasian [8]. The highest percentages of F508del CF mutation carriers are also noted in those states.
Fig. 2.
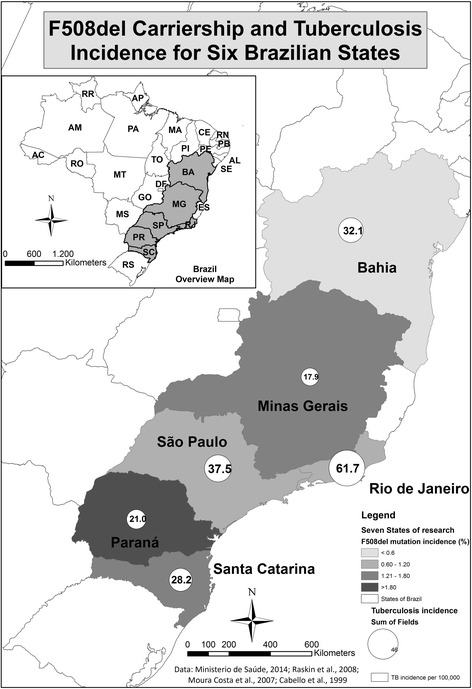
CF carriership prevalence and TB incidence for 6 Brazilian states. In states with a high carriership rate, TB incidence tends to be lower
Subsequently plotting F508del carriership against TB incidence, showed a spatial pattern suggesting that states with a higher share of F508del mutation carriers have lower TB incidence rates. PR, SC and MG, while having the highest percentage of carriers (2•38%, 1•79% and 1•37% respectively), are the states with the least TB cases (21•0, 28•2 and 17•9 per 100,000 inhabitants). For RJ, SP and BA, the opposite image is obtained. We conclude to a cartographic trend. Given there are only six observations at state level, carrying out a statistical analysis is not useful.
Figure 3a shows the calculated municipal CFTR mutation carriership rates on the municipal level. Figure 3b shows the TB incidence rate for each respective municipality. Again, an inverted pattern between CF mutation carriership and TB incidence rates could be observed.
Fig. 3.
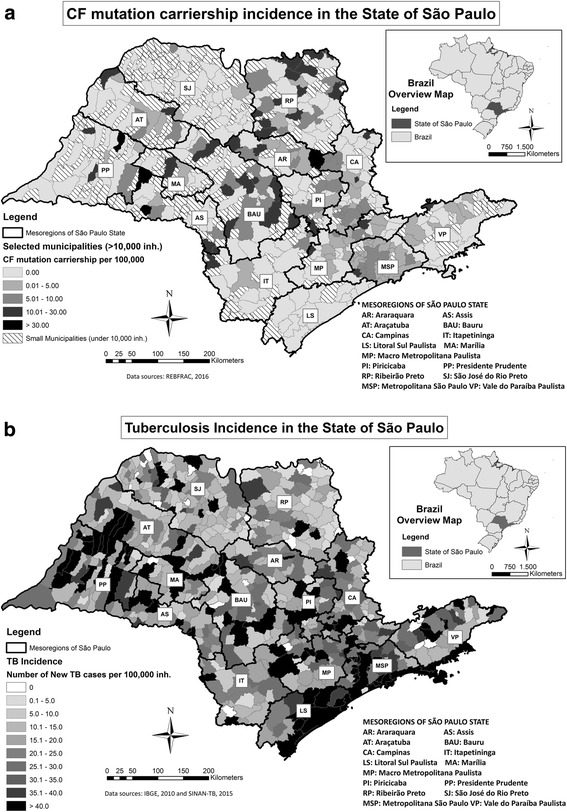
Map of the municipality-level data on the State of São Paulo for CF carriership prevalence (a) and TB incidence (b)
These cartographic observations were complemented by statistical analyses. TB incidence rates were spatially positively autocorrelated (Moran’s Index of 0•08, z-score of 5•44 and p < 0•001). A Spearman correlation coefficient (r) was therefore calculated to quantify the CF-TB relationship for the 171 municipalities with over 10,000 inhabitants in which at least one CF case has been registered (Fig. 4). In spite of the probable underestimation of the number of CF carriers in each municipality, CF carriership rate was significantly and inversely correlated with TB incidence (r = −0•48 and p < 0•001). With adjustments applied for monthly income, population density, literacy rates, the racial composition of the population, sanitary provisions and AIDS incidence rates as partial variables, the corresponding partial Spearman rank correlation was −0•39 (p < 0•001). Bivariate analysis of TB or CF with diabetes mellitus type 2 as a confounder was insignificant (p = 0.21 and p = 0.93 respectively). Data on smoking were not consistently registered and hence unavailable for statistical analysis.
Fig. 4.
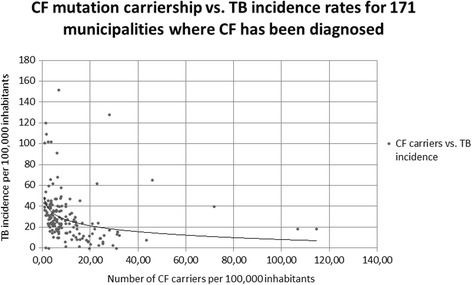
CF carriership versus TB incidence rates for the 171 Sao Paulo municipalities studied
Subsequently, a multivariate analysis was run (Table 1), showing that carriership rates correlate significantly and inversely with log-transformed TB incidence rates. This relation is independent of any of the five potential environmental confounders identified and AIDS incidence rates. Including diabetes mellitus data reduced the power of the overall model strongly so was not used as an explanatory variable.
Table 1.
Multivariate statistical analysis on the municipality level
| Variable [unit] | Estimate | Standard Error | Probability > |t| |
|---|---|---|---|
| Intercept | 2•3219 | 1•2420 | 0•06 |
| Log (TB incidence rates) [Annual number of new TB cases/100,000 inhabitants] | −0•2091 | 0•0843 | a 0•0141 |
| Average income [Per capita Brazilian Real/Month] |
−0•0002 | 0•0002 | 0•15 |
| Population density [Inhabitants/km2] | −0•0001 | 0•0000 | b < 0•0001 |
| Literacy rates [% of population] | −0•0156 | 0•0149 | 0•21 |
| Share of Caucasians [% of population] |
0•0050 | 0•0029 | 0•08 |
| No sanitary provisions [% of population] |
0•0767 | 0•0355 | a 0•0323 |
| AIDS incidence rates [Incidence/100,000 inhabitants] | 0•0023 | 0•0032 | 0•48 |
Results of the multivariate analysis of the correlation between CF carriership rates (number of CF carriers per 100,000 inhabitants) and TB incidence (annual number of new TB cases per 100,000 inhabitants) correcting for six potential confounders
aindicates significant findings at the 0•05 significance level
bindicates significant findings at the 0•001 significance level
Discussion
This multidisciplinary, multiscalar study in Brazil is, to our knowledge, the first to address the hitherto theoretical link between CF carriership and TB incidence on a contemporary patient dataset. We found a significant, inverse correlation between both, that remained valid after thorough correction for potential environmental and immunological confounders.
Our approach is innovative as it highlights 3 additional aspects to this hypothesis: 1. It extends the idea that human genetics are an important factor in infectious diseases; 2. It studies a possible evolutionary advantage in a representative contemporary environment; 3. It shows how health geography can contribute substantially to elucidating a medical hypothesis.
First, the human genetics of infectious diseases paradigm, the idea that a genetic defect can predispose to a specific infectious pathogen, has revolutionized modern medicine [22]. Increasingly, mutations and new genes are being identified that render the host susceptible to a specific pathogen [23]. Several genetic defects in the defence against TB have been identified [24]. This allows understanding why, whilst most TB-exposed individuals only have latent disease, particular patients develop fulminant TB disease (‘low disease burden’, ‘high susceptibility’). Our research explores the same idea of a genetic determinant in infectious disease. However, it changes the question to why, despite having a high chance of developing disease, some people have relative protection against the disease (‘high disease burden’, ‘low susceptibility’). Important examples of already elaborated ‘resistance genetics’ include sickle cell trait in survival advantage against malaria [25] and the CCR5-delta32 mutation in HIV resistance [26].
Yet, the field of ‘resistance genetics’ is still highly unexplored. Brazil figures among the WHO listed 22 ‘high burden’ countries for TB. At a municipality level, we showed that the group of CF carriers did not have a lower TB burden than other Brazilians [27]. Notwithstanding this high burden, we found an inverse correlation between CF carriership and TB incidence, suggesting a lower susceptibility of CF carriers to TB infection.
Second, in both malaria and HIV, the geographic spread of the ‘resistance allele’ led to the unravelling of the link with its respective infectious disease. Also for our research, the choice of an appropriate place has proven crucial. Brazil is the only country worldwide that combines high TB incidence with the European TB strain and a high CF carriership background, making statistical analyses possible. We were able to study this large cohort thanks to access to the detailed Brazilian registry for TB and CF. In this way evolutionary genetics can be studied in a representative contemporary environment.
Third, this study emphasizes the importance of a multidisciplinary approach to evolutionary genetics. For many years, the question why CF is so frequent amongst Caucasians has been posed in medical literature, yet no in-depth study has been undertaken to more definitively answer this question. We show that Brazil’s geographical, socio-economic and demographic diversity make it possible to approach the role and impact of CFTR mutations on TB infection, as well as of various external determinants. This complexity could only be unravelled by means of spatial analysis on medical registry data.
Now, the question goes back to the biomedical bench, as only by cell biological research it will be possible to elucidate and validate the molecular mechanisms behind the found correlation. This biomedical validation is essential to sustain this first preliminary evidence.
The multidisciplinary population-based approach of our study covering 116 million Brazilian inhabitants is a major strength, yet this method also implies several limitations. First, the minimum CFTR mutation carriership rates for the municipalities are only a proxy of the real carriership frequency. As registry data were anonymized, it cannot be excluded that siblings are counted as individual patients, introducing an overestimation in the number of carriers. Most probably, however, our data are an underestimation of the CF carrier rate, as siblings with a CFTR mutation have not been taken into account, CF carriers not directly related to registered patients could not be identified, and not all CF cases are diagnosed correctly. Brazil has recently initiated systematic newborn screening for CF. As such, many CF cases that would go unnoticed in countries without screening, are now picked up. The frequency of CF disease has been shown to be linked with carriership rate [28].
Next, the structure of both SINAN-TB and REBRAFC registries limited the choice of aerial units that could be chosen for the analyses. The municipality level was the most detailed scale of research possible. Nevertheless, this scale remains susceptible to ecological fallacy. To address this limitation, a case-control study comparing the CF carrier rate of individual Brazilian patients with proven TB to age-, sex- and socio-economically matched healthy individuals from the same municipality could be envisaged. Future research could focus on the genetic background of individual patients with TB.
Lastly, we could not study the link between carrying CF and nontuberculous mycobacterial (NTMs) infection, as systematic municipality data on NTMs are lacking. Primary immune deficiency (PID) patients with Mendelian Susceptibility to Mycobacterial Disease develop severe Mtb and NTM infections [29], and patients with CF have a high susceptibility to infections with NTMs [30]. It can therefore feel counterintuitive that carrying one CFTR mutation would not result in an increased but rather a reduced Mtb infection rate. Yet Mtb and NTMs also differ importantly: they constitute a distinct group within the Mycobacteria family and have separate host traits, clinical features [31], and drug resistance patterns [32]. Recent findings in PID support that the immunological response to NTMs might be different from that to Mtb [33].
Notwithstanding these limitations, we found indications for the relative protective role of carrying a single CF mutation against infections with Mtb on two scales on the Brazilian territory.
The possibility that CF carriership has (had) an evolutionary advantage has been raised before. Candidate agents of selective pressure for CF include cholera [34], typhoid fever [35] and tuberculosis. Poolman and Galvani computed that only the European tuberculosis pandemic in the early 1600s can have provided sufficient selective pressure to explain the modern CF incidence [7]. On the other hand, it is also known that a mutation in CFTR cannot be fully protective against Mtb infection, as cases of patients with CF and TB have been reported [36].
Never before however, a study has addressed this theoretical hypothesis with patient data.
This observation is relevant, as a rationale for the high CF incidence in Euro-descendants could now be provided. Equally, once a potential human protection mechanism against Mtb infection can be uncovered, this opens up opportunities for the development of new treatment strategies against the disease and implies a vital step towards eradicating TB worldwide.
Conclusions
Using a multidisciplinary, multiscalar spatial epidemiological approach, we found exploratory support for the hypothesis that carrying a single CF mutation plays a relative protective role against Mtb infections. This could provide a rationale for the continued CF occurrence and encourages biomedical research into the human resistance genetics of tuberculosis and by extension other infectious diseases.
Acknowledgements
We would like to thank the patients and their families who agreed to the uptake of their data in SINAN-TB and REBRAFC.
We are grateful to the Centre Coordinators of São Paulo State who gave access to the anonymised data (genetics and municipality of birth) of the Brazilian CF Registry: Fabiola V. Adde, Sonia Chiba, Neiva Damaceno, Giesla F. Ferrari, Antonio Fernando Ribeiro, Joaquim C. Rodrigues, Rafael Stelmach and Lidia Alice Torres.
Funding
LB received an Erasmus Mundus Category B scholarship from the European Commission. BB is a Research Foundation Flanders (FWO) PhD fellow and receives grant 11V5316N. No other sources of funding were used for the current study.
Availability of data and materials
The study was made public through Plataforma Brasil. URL: http://aplicacao.saude.gov.br/plataformabrasil/login.jsf
The anonymized datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
The study was designed by LB, DV and CL from a geographical point of view and by BB, JC and LR from the medical side. The literature search providing data on state level was done by LB, BB and CL. Data collection on municipality level was performed in Brazil by LB under the supervision of JC and LR. LB also carried out the spatial analysis resulting in the cartography included in the manuscript. TN performed the statistical analysis. The initial draft of the manuscript based on data interpretation was prepared by LB, BB, KDB and IM. The final manuscript was reviewed and edited by all authors. BB is the corresponding author and guarantor of the paper. All authors read and approved the final manuscript.
Competing interests
None of the authors has a conflict of interest or competing interest regarding this publication.
Consent for publication
No individual patients participated in this study. All participating centres agreed to the use of their anonymised data for this study. All authors consented to the publication of the manuscript in its current form.
Ethics approval and consent to participate
Ethical approval for this observational study was obtained from all participating centres and registered through Plataforma Brasil - CAAE: 55,295,316.1.1001.5162. The study was given the certificate number: 032559/2016.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CF
Cystic Fibrosis
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- GIS
Geographical Information System
- IBGE
Instituto Brasileiro de Geografia e Estatística
- Mtb
Mycobacterium tuberculosis
- TB
Tuberculosis
- WHO
World Health Organization
Contributor Information
Lander Bosch, Email: lsmmb2@cam.ac.uk.
Barbara Bosch, Phone: +1 212-327-7335, Email: bbosch@rockefeller.edu.
Kris De Boeck, Email: christiane.deboeck@uzleuven.be.
Tim Nawrot, Email: tim.nawrot@uhasselt.be.
Isabelle Meyts, Email: isabelle.meyts@uzleuven.be.
Dominique Vanneste, Email: dominique.vanneste@kuleuven.be.
Cleonice Alexandre Le Bourlegat, Email: rf25@ucdb.br.
Julio Croda, Email: juliocroda@gmail.com.
Luiz Vicente Ribeiro Ferreira da Silva Filho, Email: vicres@usp.br.
References
- 1.Castellani C, Macek M, Jr, Cassiman J, et al. Benchmarks for cystic fibrosis carrier screening: a European consensus document. J Cyst Fibros. 2010;9(3):165–178. doi: 10.1016/j.jcf.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 2.De Boeck K, Zolin A, Cuppens H, Olesen HV, Viviani L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J Cyst Fibros. 2014;13(4):403–409. doi: 10.1016/j.jcf.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Kreindler JL. Cystic fibrosis: exploiting its genetic basis in the hunt for new therapies. Pharmacol Ther. 2010;125(2):219–229. doi: 10.1016/j.pharmthera.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucotte G, Hazout S. Geographic and ethnic distributions of the more frequent cystic fibrosis mutations in Europe show that a founder effect is apparent for several mutant alleles. Hum Biol. 1995;67(4):562–576. [PubMed] [Google Scholar]
- 5.Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 6.Pizzo L, Iriarte A, Alvarez-Valin F, Marín M. Conservation of CFTR codon frequency through primates suggests synonymous mutations could have a functional effect. Mutat Res. 2015;775:19–25. doi: 10.1016/j.mrfmmm.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Poolman EM, Galvani AP. Evaluating candidate agents of selective pressure for cystic fibrosis. J R Soc Interface. 2007;4(12):91–98. doi: 10.1098/rsif.2006.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IBGE. Instituto Brasileiro De Geografia E Estatistica . Sintese de Indicadores Sociais: Uma Analyse das Condições de Vida da População Brasileira. Rio de Janeiro: IBGE; 2009. [Google Scholar]
- 9.Faucz FR, Souza DAS, Olandoski M, Raskin S. CFTR allelic heterogeneity in Brazil: historical and geographical perspectives and implications for screening and counseling for cystic fibrosis in this country. J Hum Genet. 2010;55(2):71–76. doi: 10.1038/jhg.2009.123. [DOI] [PubMed] [Google Scholar]
- 10.WHO - World Health Organization. TB Country Profile – Brazil. Retrieved from URL: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=BR&LAN=EN&outtype=html. [Last accessed 20 Jan 2017].
- 11.Gagneux S, Brennan MJ. Strain and antigenic variation in Mtb: implications for the development of new tools for TB. In: Nor NM, Acosta A, Sarmiento ME, editors. The art & Science of tuberculosis vaccine development. Second. Oxford: Oxford University Press; 2010. [Google Scholar]
- 12.IBGE. Instituto Brasileiro De Geografia E Estatistica . Estimativas de Populaçao 2014. Rio de Janeiro: IBGE; 2014. [Google Scholar]
- 13.Raskin S, Pereira-Ferrari L, Reis FC, et al. Incidence of cystic fibrosis in five different states of Brazil as determined by screening of p.F508del, mutation at the CFTR gene in newborns and patients. J Cyst Fibros. 2008;7(1):15–22. doi: 10.1016/j.jcf.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Cabello GM, Moreira AF, Horovitz D, et al. Cystic fibrosis: low frequency of DF508 mutation in 2 population samples from Rio de Janeiro, Brazil. Hum Biol. 1999;71(2):189–196. [PubMed] [Google Scholar]
- 15.Moura Costa FM, Santana MA, Moreira Lemos AC, Galvão-Castro B, Axosta AX. Low frequency of the ΔF508 mutation of the CFTR Gene in a highly admixed population in Bahia, Brazil. Hum Biol. 2007;79(3):293–297. doi: 10.1353/hub.2007.0040. [DOI] [PubMed] [Google Scholar]
- 16.Ministerio Da Saúde. Boletim Epidemiológico tuberculose 45. Retrieved from URL: http://bvsms.saude.gov.br/bvs/periodicos/boletim_epidemiologico_numero_2_2014.pdf [Last accessed 20 Jan 2017].
- 17.Harling G, Castro MC. A spatial analysis of social and economic determinants of tuberculosis in Brazil. Health Place. 2014;25:56–67. doi: 10.1016/j.healthplace.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 18.GBEFC – Grupo Brasileiro de Estudos de Fibrose Cística . Registro Brasileiro de Fibrose Cística. São Paulo: GBEFC; 2014. [Google Scholar]
- 19.Bates M, Marais BJ, Zumla A. Tuberculosis Comorbidity with Communicable and Noncommunicable Diseases. Cold Spring Harb Perspect Med. 2015 6;5(11). [DOI] [PMC free article] [PubMed]
- 20.SUS – Sistemo Único De Saúde. Indicadores e dados básicos do HIV/AIDS dos municípios brasileiros. Retrieved from URL: http://svs.aids.gov.br/aids/. [Last accessed 20 Jan 2017].
- 21.Sistema de informacoa de atencao basica. Ministry of Health Brazil. Retrieved from URL: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?siab/cnv/SIABSSP.def [Last accessed 20 Jan 2017].
- 22.Casanova JL. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc Natl Acad Sci U S A. 2015;112(51):E7128–E7137. doi: 10.1073/pnas.1521651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyts I, Bosch B, Bolze A, et al. Exome and genome sequencing for primary immunodeficiency inborn errors of immunity. J Allergy Clin Immunol. 2016;138(4):957–969. doi: 10.1016/j.jaci.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boisson-Dupuis S, Bustamante J, El-Baghdadi J, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev. 2015;264(1):103–120. doi: 10.1111/imr.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira A, Marguti I, Bechmann I, et al. Sickle hemoglobin confers tolerance to plasmodium infection. Cell. 2011;145(3):398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Novembre J, Galvani AP, Slatkin M. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 2005;3(11):e339. doi: 10.1371/journal.pbio.0030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Duguépéroux I, L'Hostis C, Audrézet MP, et al. Highlighting the impact of cascade carrier testing in cystic fibrosis families. J Cyst Fibros. 2016;15(4):452–459. doi: 10.1016/j.jcf.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin Immunol. 2014;26(6):454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36(2):217–224. doi: 10.1055/s-0035-1546751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendall BA, Varley CD, Choi D, et al. Distinguishing tuberculosis from nontuberculous mycobacteria lung disease, Oregon, USA. Emerg Infect Dis. 2011;17(3):506–509. doi: 10.3201/eid1703.101164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Scorpio A, Nikaido H, Sun Z. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol. 1999;181(7):2044–2049. doi: 10.1128/jb.181.7.2044-2049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Beaucoudrey L, Samarina A, Bustamante J, et al. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89(6):381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266(5182):107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- 35.Pier GB, Grout M, Zaidi T, et al. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature. 1998;393(6680):79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- 36.Patil N, Marco A, Montales MT, Bhaskar N, Mittadodla P, Mukasa LN. Pulmonary tuberculosis in a patient with cystic fibrosis. N Am J Med Sci. 2015;7(5):233–235. doi: 10.4103/1947-2714.157494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study was made public through Plataforma Brasil. URL: http://aplicacao.saude.gov.br/plataformabrasil/login.jsf
The anonymized datasets used and analysed during the current study are available from the corresponding author on reasonable request.


