Abstract
OBJECTIVES
The aim of this study was to assess for a treatment interaction between statin use and ET response.
BACKGROUND
Recent data suggest that statins may attenuate exercise training (ET) response, but limited data exist in patients with heart failure (HF).
METHODS
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) was a randomized trial of 2,331 patients with chronic HF with ejection fraction ≤35% who were randomized to usual care with or without ET. We evaluated whether there was a treatment interaction between statins and ET response for the change in quality of life and aerobic capacity (peak oxygen consumption and 6-min walk distance) from baseline to 3 months. We also assessed for a treatment interaction among atorvastatin, simvastatin, and pravastatin and change in these endpoints with ET. Multiple linear regression analyses were performed for each endpoint, adjusting for baseline covariates.
RESULTS
Of 2,331 patients in the HF-ACTION trial, 1,353 (58%) were prescribed statins at baseline. Patients treated with statins were more likely to be older men with ischemic HF etiology but had similar use of renin angiotensin system blockers and beta-blockers. There was no evidence of a treatment interaction between statin use and ET on changes in quality of life or exercise capacity, nor was there evidence of differential association between statin type and ET response for these endpoints (all p values >0.05).
CONCLUSIONS
In a large chronic HF cohort, there was no evidence of a treatment interaction between statin use and short-term change in aerobic capacity and quality of life with ET. These findings contrast with recent reports of an attenuation in ET response with statins in a different population, highlighting the need for future prospective studies. (Exercise Training Program to Improve Clinical Outcomes in Individuals With Congestive Heart Failure; NCT00047437)
Keywords: chronic heart failure, exercise training, heart failure with reduced ejection fraction, statins
In recently updated lipid guidelines, statin use carries a Class I recommendation for secondary prevention in patients with atherosclerotic cardiovascular disease and for primary prevention in high-risk individuals (1,2). Statin use in heart failure (HF) patients is also common. Statin use has a Class III recommendation for patients with HF without other indications in the most recent U.S. and European HF guidelines (3–5). However, the most common etiology of HF in most developed countries is atherosclerotic cardiovascular disease.
Current guidelines recommend nonpharmacological interventions including aerobic exercise training (ET) and cardiac rehabilitation (CR) in patients with chronic HF with reduced ejection fraction (EF) (4). Although many patients with HF receive statins, and the use of ET interventions are increasingly incorporated into a patient-centered treatment approach, guidelines do not provide specific recommendations for a combined use strategy or appropriate timing for initiating ET and statin therapy (6,7).
Recent data, however, suggest that statins may attenuate the ET response in some populations (8). For example, in overweight or obese patients with metabolic risk factors, statins attenuated the improvement in muscle enzyme mitochondrial levels and cardiorespiratory fitness with ET (8). Consistent with this finding, several small studies have also shown statins to reduce skeletal muscle mitochondrial content and oxidative capacity (9–12). HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest clinical trial to examine the effect of ET in patients with chronic HF receiving contemporary optimal medical therapy (13). We performed a secondary analysis of HF-ACTION to assess for a treatment interaction between statin use and response to ET with respect to changes in aerobic capacity and health-related quality of life (HR-QoL) from baseline to 3 months.
METHODS
HF-ACTION was a multicenter, randomized, placebo-controlled trial of usual care with and without an ET regimen in 2,331 patients with stable chronic HF. The study design and results have been previously reported (13,14). The ET intervention included 36 supervised sessions of aerobic exercise over 3 months, followed by home training on a treadmill or stationary cycle for an additional 2 years. The usual care group received self-management educational materials with information on medications, fluid management, and sodium intake and recommendations for 30 min of moderate-intensity physical activity most days of the week as tolerated. Changes in baseline peak oxygen uptake (VO2) and 6-min walk distance (6MWD) were analyzed at 3months to characterize exercise capacity. HR-QoL was measured using the 23-item self-administered disease-specific Kansas City Cardiomyopathy Questionnaire (KCCQ) at 3 months. Each participating center’s ethics committee or institutional reviewboard approved the study, and all patients gave written informed consent. The present analysis focuses on patients enrolled in HF-ACTION who were on statin therapy at baseline compared with those not on a statin to evaluate for a treatment interaction of statin therapy on ET response.
PATIENT POPULATION
Patients enrolled in HF-ACTION were required to have stable chronic HF with left ventricular EF ≤35% and New York Heart Association functional class II to IV symptoms while on optimal HF therapy for ≥6 weeks. Patients were excluded if they had major comorbidities or other limitations that would preclude ET, devices that would prevent achievement of target heart rates, or recent (≤6 weeks) or planned (≤6 months) major cardiovascular procedures. For the present analysis, statin use was defined as HMG-CoA reductase inhibitor use at baseline stratified by the specific statin marked on the case report form: atorvastatin, pravastatin, simvastatin, or other. Other lipid-lowering agents were also captured.
OUTCOMES OF INTEREST
The primary outcomes for the present analysis were change in peak VO2 and change in KCCQ from baseline to 3 months, as in previous HF-ACTION analyses, because of the increased missingness at later time points (15,16). The secondary outcome was change in 6MWD from baseline to 3 months.
STATISTICAL ANALYSIS
We performed post-hoc analyses of patients with and without statin treatment at baseline to assess their change in peak VO2, KCCQ, and 6MWD. The baseline characteristics, including demographics, physical and laboratory findings, medical history, and therapies, were summarized as frequencies and percentages for categorical variables and by medians and 25th and 75th percentiles for continuous variables in patients stratified by randomized treatment (ET vs. usual care) and statin use (no statin vs. statin) (see Table 1). Comparisons of baseline characteristics across statin use were conducted within each treatment group using Student t tests or Wilcoxon tests for continuous variables and chi-square tests or Fisher exact tests for categorical variables when appropriate.
TABLE 1.
Baseline Characteristics
| Usual Care | Exercise Training | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Nonstatin (n = 489) | Statin (n = 683) | p Value | Nonstatin (n = 489) | Statin (n = 670) | p Value | |
| Age, yrs | 55 (45–64) | 62 (55–70) | <0.001 | 57 (48–66) | 61 (53–69) | <0.001 |
|
| ||||||
| Female | 34.4 | 21.4 | <0.001 | 40.5 | 22.2 | <0.001 |
|
| ||||||
| African American | 41.1 | 25.0 | <0.001 | 40.7 | 26.6 | <0.001 |
|
| ||||||
| Ischemic etiology | 24.9 | 69.8 | <0.001 | 24.3 | 71.5 | <0.001 |
|
| ||||||
| PVD | 3.9 | 9.7 | <0.001 | 3.9 | 8.0 | <0.01 |
|
| ||||||
| Prior myocardial infarction | 19.8 | 58.9 | <0.001 | 17.6 | 58.8 | <0.001 |
|
| ||||||
| Hypertension | 50.7 | 63.3 | <0.001 | 53.3 | 68.0 | <0.001 |
|
| ||||||
| Atrial fibrillation/flutter | 19.9 | 21.1 | 0.61 | 20.2 | 22.1 | 0.45 |
|
| ||||||
| Never smoker | 46.1 | 31.6 | <0.001 | 45.7 | 30.6 | <0.001 |
|
| ||||||
| No CCS angina | 88.8 | 82.1 | <0.01 | 86.1 | 80.1 | 0.06 |
|
| ||||||
| NYHA functional classification | 0.30 | 0.90 | ||||
| II | 66.1 | 63.1 | 62.2 | 62.5 | ||
| III/IV | 33.9 | 36.9 | 37.8 | 37.5 | ||
|
| ||||||
| HF hospitalizations in previous 6 months | <0.01 | 0.78 | ||||
| None | 67.6 | 76.5 | 73.7 | 74.9 | ||
| 1 | 25.2 | 18.3 | 19.3 | 18.7 | ||
| 2 | 4.9 | 2.8 | 4.3 | 4.5 | ||
| ≥3 | 2.3 | 2.4 | 2.7 | 1.8 | ||
|
| ||||||
| LVEF, % | 25 (20–30) | 25 (20–31) | 0.35 | 25 (20–30) | 25 (20–30) | 0.97 |
|
| ||||||
| BMI, kg/m2 | 30 (26–36) | 30 (26–34) | 0.01 | 30 (25–35) | 30 (26–35) | 0.20 |
|
| ||||||
| SBP, mm Hg | 110 (100–126) | 112 (100–126) | 0.73 | 112 (100–125) | 110 (100–126) | 0.65 |
|
| ||||||
| HR at rest, beats/min | 72 (64–79) | 68 (62–76) | 0.007 | 72 (64–80) | 68 (62–76) | <0.001 |
|
| ||||||
| Sodium, mmol/l | 139 (137–141) | 139 (137–141) | 0.55 | 139 (137–141) | 139 (137–141) | 0.61 |
|
| ||||||
| Creatinine, mg/dl | 1.1 (0.9–1.4) | 1.3 (1.0–1.6) | <0.001 | 1.1 (0.9–1.4) | 1.2 (1.0–1.5) | <0.001 |
|
| ||||||
| BUN, mg/dl | 18 (14–25) | 22 (16–30) | <0.001 | 19 (14–26) | 21 (16–29) | <0.001 |
|
| ||||||
| ACE inhibitor | 74.2 | 72.9 | 0.61 | 77.1 | 74.3 | 0.28 |
|
| ||||||
| Angiotensin-II receptor blocker | 21.7 | 22.5 | 0.72 | 22.9 | 25.7 | 0.28 |
|
| ||||||
| Beta-blocker | 94.7 | 95.0 | 0.80 | 93.0 | 94.9 | 0.18 |
|
| ||||||
| Aldosterone antagonist | 49.1 | 42.2 | 0.02 | 50.1 | 41.5 | <0.01 |
|
| ||||||
| Loop diuretic | 78.3 | 78.8 | 0.85 | 76.1 | 78.1 | 0.43 |
|
| ||||||
| ICD | 33.7 | 41.4 | <0.01 | 35.6 | 47.2 | <0.001 |
|
| ||||||
| Biventricular pacemaker | 15.1 | 18.9 | 0.09 | 18.8 | 18.5 | 0.90 |
Values are median (interquartile range) or %.
ACE = angiotensin converting enzyme; BMI = body mass index; BUN = blood urea nitrogen; CCS = Canadian Cardiovascular Society; HF = heart failure; HR = heart rate; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; PVD = peripheral vascular disease; SBP = systolic blood pressure.
Linear regression models were used in the analysis to examine the relationship between statin use and change in the individual endpoints from baseline to 3 months. To account for the missing data at 3 months for the outcomes of interest, inverse weighting for missingness was performed. Specifically, subjects in the regression model were weighted by the inverse of their probability of having a nonmissing response similar to recent analyses in HF-ACTION (15,16). This should reduce the bias that would have been present in a complete case analysis by assigning higher weight to observed data subjects who were similar to subjects who were more likely to have missing data (15). Inverse propensity weighting was also used to account for the nonrandom use of statin therapy at baseline (15). The final weight used in the analysis is the inverse of the product of the probability for missingness and the probability for statin use. To investigate for possible confounding variables, the propensity for statin treatment model was selected using backward stepwise variable selection methods (conservatively using p < 0.10 to enter and stay in the model) and further included all variables previously used in multivariable HF-ACTION models (see Table 2 footnote) (17). In the weighted linear models, we assessed interactions between randomized treatment and statin use for each outcome. From this model, we report the interaction p value and adjusted effect estimates for statin use within randomized subgroups.
TABLE 2.
Statin Status and Changes in Exercise Capacity and Quality of Life From Baseline to 3 Months
| Test | Randomized Treatment Group | Statin Use | Baseline | 3 Months | Unweighted and Unadjusted Effect Estimate | Adjusted* Effect Estimate | Adjusted* Interaction p Value | Adjusted*† Interaction p Value | Unweighted and Unadjusted Interaction p Value |
|---|---|---|---|---|---|---|---|---|---|
| CPET peak VO2, ml/min/kg | Usual care | No statin | 15.5 ± 5.1 | 16.2 ± 5.1 | −0.22 (−0.6 to 0.1) | −0.13 (−1.0 to 0.7) | 0.54 | 0.57 | 0.68 |
| Statin | 14.7 ± 4.5 | 14.9 ± 4.8 | |||||||
| Exercise training | No statin | 15.1 ± 4.8 | 16.0 ± 5.0 | −0.11 (−0.4 to 0.2) | 0.34 (−1.7 to 2.4) | ||||
| Statin | 14.6 ± 4.5 | 15.6 ± 4.8 | |||||||
|
| |||||||||
| 6MWD, m | Usual care | No statin | 373.4 ± 109.6 | 386.9 ± 111.7 | −5.1 (−14.2 to 4.0) | −25.36 (−49.6 to −1.1) | 0.81 | 0.80 | 0.66 |
| Statin | 360.5 ± 104.0 | 363.1 ± 107.1 | |||||||
| Exercise training | No statin | 366.2 ± 99.1 | 396.1 ± 106.7 | −7.96 (−17.0 to 1.1) | −19.2 (−60.6 to 22.2) | ||||
| Statin | 361.0 ± 105.4 | 388.0 ± 103.2 | |||||||
|
| |||||||||
| KCCQ | Usual care | No statin | 64.5 ± 21.3 | 68.5 ± 21.5 | 0.3 (−1.5 to 2.1) | 0.9 (−4.4 to 2.6) | 0.96 | 0.97 | 0.16 |
| Statin | 68.0 ± 20.6 | 72.5 ± 19.8 | |||||||
| Exercise training | No Statin | 66.1 ± 20.7 | 70.8 ± 20.2 | −1.44 (−3.1 to 0.2) | −0.9 (−8.6 to 7) | ||||
| Statin | 65.6 ± 19.8 | 70.9 ± 20.1 | |||||||
Values are mean ± SD or effect estimate (95% confidence interval). Reference = no statin.
Adjusted (via inverse probability weighting) for the following variables: Peak VO2: age, sex, race, income, Hispanic ethnicity, history of diabetes, peripheral vascular disease, number of heart failure hospitalizations in prior 6 months, ischemic etiology, NYHA functional classification at baseline, aldosterone antagonist at baseline, pacemaker, resting heart rate (beats/min), KCCQ total symptom score at baseline, KCCQ physical limitation score at baseline, heart rate at peak exercise (beats/min), CPX test resting ECG rhythm, CPX test peak oxygen pulse, CPX test VeVCO2 slope, insulin use at baseline, BMI, LVEF, CPX test duration, CPX test peak respiratory exchange ratio, BUN, and angiotensin-II receptor blocker use at baseline. Model also adjusted via propensity weighting for statin use at baseline and missing exercise capacity measures at 3 months. 6MWD: age, sex, Hispanic ethnicity, prior revascularization, ischemic etiology, number of heart failure hospitalizations in prior 6 months, history of hypertension, baseline ACE inhibitor dose, nonloop diuretic or loop diuretic use at baseline, nonloop diuretic and loop diuretic use, aldosterone antagonist use at baseline, biventricular pacemaker, pacemaker, systolic blood pressure at baseline, resting heart rate (beats/min), BMI, KCCQ total summary score at baseline, CPX test heart rate reserve, CPX test heart rate (beats/min) at end of stage 2 of the CPX test, CPX test rest ECG rhythm, CPX test peak respiratory exchange ratio, CPX test VeVCO2 slope, peak VO2, KCCQ quality of life score at baseline, loop diuretic use at baseline, angiotensin II receptor blocker use at baseline, smoking status, KCCQ clinical summary score at baseline, LVEF, CPX test duration, and BUN. Model also adjusted via propensity weighting for statin use at baseline and missing exercise capacity measures at 3 months. KCCQ: age, sex, prior myocardial infarction, prior revascularization, history of diabetes, number of heart failure hospitalizations in prior 6 months, NYHA functional classification at baseline, aldosterone antagonist at baseline, nonloop diuretic and loop diuretic use at baseline, insulin use at baseline, AICD at baseline, systolic blood pressure at baseline, resting heart rate (beats/min), LVEF, KCCQ physical limitation score at baseline, KCCQ social limitation score at baseline, BDI score at baseline, CPX test heart rate reserve, CPX test heart rate at peak exercise (beats/min), CPX test resting ECG rhythm, CPX test peak oxygen pulse, ventricular conduction on CPX, peak VO2, loop diuretic use at baseline, angiotensin-II receptor blocker use at baseline, peripheral vascular disease, biventricular pacemaker, atrial fibrillation/flutter, BUN, and BMI. Model also adjusted via propensity weighting for statin use at baseline and missing exercise capacity measures at 3 months.
Random effect adjustment for site.
AICD = Automatic implantable cardioverter-defibrillator; BDI = Beck Depression Inventory; CPET = cardiopulmonary exercise test; CPX = cardiopulmonary stress test; ECG = electrocardiogram; KCCQ = Kansas City Cardiomyopathy Questionnaire; VeVCO2 = minute ventilation/carbon dioxide production; VO2 = oxygen consumption; 6MWD = 6-min walk distance; other abbreviations as in Table 1.
Analysis of variance was used to examine the relationship between type of statin used and the change in exercise capacity and HR-QoL endpoints. Patients were classified into 5 groups: no statin, atorvastatin, simvastatin, pravastatin, and other. If a significant difference in outcomes by statin type was found, multiple comparison (Tukey) analysis was conducted to determine what those differences were.
Adherence to ET (defined as exercise >90 min/week for the first 3 months of the study) was assessed in the subset of patients randomized to ET comparing statin and nonstatin patients. This analysis evaluated for an interaction between statin use and exercise adherence in the ET group. These analyses were weighted for missingness and nonrandom statin use and adjusted for the same covariates as previously mentioned. Statistical significance was assessed using 2-sided p values, with values <0.05 considered statistically significant. All statistical computations were generated using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Statin therapy was used in 1,353 (58%) patients at baseline in HF-ACTION. The baseline characteristics of patients randomized to usual care or ET further dichotomized into those on statin therapy versus those not on statins are presented in Table 1. Patients treated with statins were more likely to be older men with hypertension, an ischemic etiology of HF, peripheral vascular disease, an elevated blood urea nitrogen, and a lower heart rate. Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use and beta-blocker use were similar between statin and nonstatin patients, whereas mineralocorticoid antagonist use was lower and implanted cardioverter-defibrillator use was higher in the statin-treated patients.
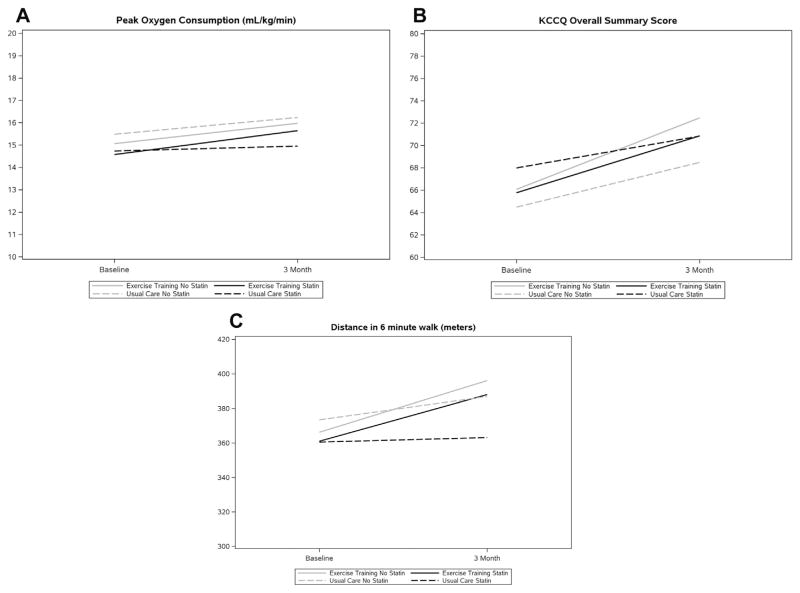
Patients randomized to usual care had higher rates of missing data compared with those randomized to ET at 3 months (Online Table 1). The changes in exercise capacity and HR-QoL from baseline to 3 months stratified by statin use and randomized treatment group are displayed in Tables 2 and 3 (statin type). There was no evidence for a treatment interaction between statin use and ET on changes in quality of life or exercise capacity, nor was there evidence of differential association between statin type and ET response for the endpoints (all p > 0.05). Figure 1 presents statin use and change in Peak VO2, 6MWD, and KCCQ from baseline to 3 months as a function of statin use and ET status. Online Figure 1 displays median and interquartile range of the changes in peak VO2, 6MWD, and KCCQ score on the basis of type of statin. There was also no interaction between statin use and exercise adherence with respect to exercise capacity or HR-QoL in patients randomized to ET (interaction p values for peak VO2, 6MWD, and KCCQ of 0.44, 0.80, and 0.60, respectively) (Table 4).
TABLE 3.
Type of Statin and Changes in Exercise Capacity and Quality of Life: Baseline to 3 Months
FIGURE 1. Statin Use and Change in Peak VO2, 6MWD, and KCCQ From Baseline to 3 Months as a Function of Statin Use and Exercise Training Status.
KCCQ = Kansas City Cardiomyopathy Questionnaire; VO2 = oxygen consumption; 6MWD = 6-min walk distance.
TABLE 4.
Statin Use and Changes in Exercise Capacity and Quality of Life From Baseline to 3 Months on the Basis of Adherence to Exercise Training in the Exercise Training Treatment Arm
| Adjusted* Peak VO2 Effect Estimate | p Value | Interaction p Value | Adjusted* 6MWD Effect Estimate | p Value | Interaction p Value | Adjusted* KCCQ Effect Estimate | p Value | Interaction p Value | |
|---|---|---|---|---|---|---|---|---|---|
| ET adherent | 0.58 (−2.38 to 3.54) | 0.70 | 0.44 | 2.87 (−34.90 to 40.64) | 0.88 | 0.80 | −1.35 (−10.64 to 7.95) | 0.78 | 0.60 |
| ET nonadherent | 0.65 (−1.11 to 2.41) | 0.47 | −43.7 (−119.2 to 31.88) | 0.26 | 0.47 (−12.46 to 13.40) | 0.94 |
DISCUSSION
In the largest clinical trial of ET in patients with chronic HF, we evaluated for a treatment interaction between statin use and ET with respect to changes in exercise capacity and HR-QoL. We found that statin use was more prevalent in older men with hypertension, ischemic heart disease, and implanted cardioverter-defibrillators, but these patients had similar use of baseline guideline-directed medical therapy for HF compared with nonstatin patients. We found no evidence that statin treatment attenuated the response to ET in patients with chronic HF and reduced EF. Furthermore, various statins did not have a differential association with changes in quality of life or exercise capacity with ET. Our findings suggest that patients with HF on statin therapy who undergo ET do not have a blunted training response compared with nonstatin patients.
Previous studies suggest that statin use lowers mitochondrial content and oxidative capacity (9–12). Furthermore, atorvastatin impairs exercise-mediated mitochondrial adaptations in skeletal muscle and lowers running capacity in rodents (18,19). Many highly trained athletes are intolerant of statin treatment related to increased muscle enzyme release from skeletal muscle after significant endurance events such as marathons (20–23). However, limited data exist on changes in aerobic capacity in patients on statin therapy (24). A recent study that randomized 37 patients who were overweight or obese and at risk of metabolic syndrome to 12 weeks of aerobic ET with or without simvastatin demonstrated attenuated cardiorespiratory fitness responses and decreased skeletal muscle mitochondrial content in patients treated with simvastatin (8). However, 2 small studies evaluated the effects of 40 mg daily atorvastatin and 80 mg daily simvastatin in healthy subjects on maximal exercise capacity over 8 and 12 weeks of therapy, respectively, demonstrating no effect on maximal oxygen consumption (25,26). A prospective study that evaluated 1,201 patients with coronary artery disease undergoing 12 weeks of CR after an acute cardiac event found no effect of statin therapy on ET response (27). An age/sex-appropriate similar increase in peak oxygen consumption from CR in statin and nonstatin treatment was also found (+3.2 ± 3.7 ml/kg/min and +3.1 ± 3.7 ml/kg/min, respectively) (8,27). The STOMP (Statins on Skeletal Muscle Function and Performance) study randomized 420 healthy patients to evaluate symptoms, muscle strength, and exercise capacity in patients treated with atorvastatin or placebo over 6 months (28). STOMP found that atorvastatin therapy did not decrease endurance, aerobic capacity, or physical activity levels compared with placebo (change in peak VO2: −0.8 ml/kg/min (−1.3 to −0.3 ml/kg/min )(Post Minus Baseline) and −0.8 ml/kg/min (−1.4 to −0.2 ml/kg/min)(Post Minus Baseline), respectively) (28). Although these studies do not include patients with HF, they include patients who were on potent statin therapies for up to 6 months without demonstrating an impaired response to ET. These studies support our findings in the chronic HF population. Interesting, a study in rats already undergoing ET that then had statin therapy added also found no attenuated response to exercise (29).
In HF-ACTION, ET reduced rehospitalizations and improved quality of life, offering significant benefits on top of guideline medical therapy for HF (13, 30, 31). With the recent Centers for Medicare and Medicaid expansion of coverage for CR to beneficiaries with HF, many patients are being referred for such care, including a sizeable proportion receiving statins. Our results suggest that there is no apparent blunting of the response to aerobic ET with concomitant statin use. Instead, the expanded CMS coverage for ET should be welcomed as a new therapeutic option to improve exercise capacity and quality of life and to potentially reduce hospitalizations.
STUDY LIMITATIONS
This was a retrospective analysis from a clinical trial of ET; thus, cause and effect relationships for statin therapy cannot be directly assessed. Although several small studies have suggested that statin use affects muscle strength, this patient-level data was unavailable for analysis in the HF-ACTION trial. Despite covariate adjustment, other measured and unmeasured variables may have influenced these results. Overadjustment bias may be present, as we do not know the timing of statin initiation relative to variables in the propensity model; thus, future prospective studies are needed to externally validate our findings. Prior statin use was not captured in the HF-ACTION trial. Thus, if a patient discontinued statin therapy due to statin intolerance, whether self-reported or clinically confirmed, prior to enrollment in HF-ACTION, they would be included in a nonstatin group for the current analysis; this would bias our findings toward the null hypothesis given that up to 15% of patients experience statin-related myalgias (32). It is plausible that these patients may be more likely to experience attenuated gains in exercise capacity or quality of life in response to ET when using statins. The inclusion and exclusion criteria of the HF-ACTION trial prevents generalization to the entire HF population, including those with preserved left ventricular EF and the very elderly. A final limitation is the modest increase in aerobic capacity with ET in HF-ACTION, due in part to low training adherence.
CONCLUSIONS
The results from this retrospective analysis of the large HF-ACTION trial suggest that statin therapy does not attenuate the improvement in aerobic capacity or quality of life response to ET in patients with chronic HF and reduced EF. Future prospective studies examining the effect of statin therapy on the responses to ET in the HF population are warranted to confirm our findings.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
ET and CR carry Class I and IIA recommendations, respectively, in patients with HF, but are uncommonly prescribed and completed in patients with HF related to various barriers. Our findings suggest that there does not appear to be an attenuated ET response in patients with HF on statin therapy.
TRANSLATIONAL OUTLOOK
Statin use does not blunt the ET response in patients with HF and reduced EF who engage in ET compared with nonstatin users. Future prospective studies are needed to confirm these findings and include patients with HF and preserved EF.
Acknowledgments
HF-ACTION was funded by the National Institutes of Health. The content of this paper is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health or the United States Government. Dr. Kelly is supported by grant 5T32 HL 7101-39 from the National Institute of Health. Dr. Kitzman has received a research grant from Novartis; has served as a consultant for Relypsa, GlaxoSmithKline, Abbvie, Corvia, Merck, and Bayer; and owns stock in Gilead and Relypsa. Dr. Mentz receives research support from the National Institutes of Health (U10HL110312), Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Novartis, Otsuka, and ResMed; has received honoraria from HeartWare, Janssen, Luitpold Pharmaceuticals, Novartis, ResMed, St. Jude and Thoratec; and has served on an advisory board for Luitpold Pharmaceuticals, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. John R. Teerlink, MD, served as Guest Editor for this paper.
ABBREVIAT IONS AND ACRONYMS
- CR
cardiac rehabilitation
- ET
exercise training
- HF
heart failure
- HR-QoL
health-related quality of life
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- VO2
oxygen uptake
- 6MWD
6-min walk distance
APPENDIX
For a supplemental table and figure, please see the online version of this article.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–31. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 3.Levy WC. Observational studies of statins in systolic heart failure. Heart Fail Clin. 2008;4:201–8. doi: 10.1016/j.hfc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 6.Opie LH. Exercise-induced myalgia may limit the cardiovascular benefits of statins. Cardiovasc Drugs Ther. 2013;27:569–72. doi: 10.1007/s10557-013-6483-8. [DOI] [PubMed] [Google Scholar]
- 7.Murlasits Z, Radak Z. The effects of statin medications on aerobic exercise capacity and training adaptations. Sports Med. 2014;44:1519–30. doi: 10.1007/s40279-014-0224-4. [DOI] [PubMed] [Google Scholar]
- 8.Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62:709–14. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem Biophys Res Comm. 2005;329:1067–75. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 10.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006;291:C1208–12. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 11.Sirvent P, Bordenave S, Vermaelen M, et al. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem Biophys Res Comm. 2005;338:1426–34. doi: 10.1016/j.bbrc.2005.10.108. [DOI] [PubMed] [Google Scholar]
- 12.Wu JS, Buettner C, Smithline H, Ngo LH, Greenman RL. Evaluation of skeletal muscle during calf exercise by 31-phosphorus magnetic resonance spectroscopy in patients on statin medications. Muscle Nerve. 2011;43:76–81. doi: 10.1002/mus.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whellan DJ, O’Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Mentz RJ, Bittner V, Schulte PJ, et al. Race, exercise training, and outcomes in chronic heart failure: findings from Heart Failure–a Controlled Trial Investigating Outcomes in Exercise TraiNing (HF-ACTION) Am Heart J. 2013;166:488–95. doi: 10.1016/j.ahj.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mentz RJ, Schulte PJ, Fleg JL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Am Heart J. 2013;165:193–9. doi: 10.1016/j.ahj.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraki A, Miyashita K, Mitsuishi M, Tamaki M, Tanaka K, Itoh H. Coenzyme Q10 reverses mitochondrial dysfunction in atorvastatin-treated mice and increases exercise endurance. J App Physiol (1985) 2012;113:479–86. doi: 10.1152/japplphysiol.01362.2011. [DOI] [PubMed] [Google Scholar]
- 19.Bouitbir J, Charles AL, Rasseneur L, et al. Atorvastatin treatment reduces exercise capacities in rats: involvement of mitochondrial impairments and oxidative stress. J App Physiol (1985) 2011;111:1477–83. doi: 10.1152/japplphysiol.00107.2011. [DOI] [PubMed] [Google Scholar]
- 20.Parker BA, Augeri AL, Capizzi JA, et al. Effect of statins on creatine kinase levels before and after a marathon run. Am J Cardiol. 2012;109:282–7. doi: 10.1016/j.amjcard.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 21.Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol. 2004;57:525–8. doi: 10.1111/j.1365-2125.2004.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearns AK, Bilbie CL, Clarkson PM, et al. The creatine kinase response to eccentric exercise with atorvastatin 10 mg or 80 mg. Atheroscler. 2008;200:121–5. doi: 10.1016/j.atherosclerosis.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Thompson PD, Parker B. Statins, exercise, and exercise training. J Am Coll Cardiol. 2013;62:715–6. doi: 10.1016/j.jacc.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan GM, Thompson PD. The effects of statins on skeletal muscle strength and exercise performance. Curr Opin Lipidol. 2010;21:324–8. doi: 10.1097/MOL.0b013e32833c1edf. [DOI] [PubMed] [Google Scholar]
- 25.Chung J, Brass EP, Ulrich RG, Hiatt WR. Effect of atorvastatin on energy expenditure and skeletal muscle oxidative metabolism at rest and during exercise. Clin Pharmacol Ther. 2008;83:243–50. doi: 10.1038/sj.clpt.6100264. [DOI] [PubMed] [Google Scholar]
- 26.Traustadottir T, Stock AA, Harman SM. High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults. Age (Dordr) 2008;30:283–91. doi: 10.1007/s11357-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rengo JL, Savage PD, Toth MJ, Ades PA. Statin therapy does not attenuate exercise training response in cardiac rehabilitation. J Am Coll Cardiol. 2014;63:2050–1. doi: 10.1016/j.jacc.2014.02.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouitbir J, Daussin F, Charles AL, et al. Mitochondria of trained skeletal muscle are protected from deleterious effects of statins. Muscle Nerve. 2012;46:367–73. doi: 10.1002/mus.23309. [DOI] [PubMed] [Google Scholar]
- 30.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308:465–74. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: findings from the HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol. 2013;29:1553–68. doi: 10.1016/j.cjca.2013.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.