Abstract
Objectives
Heart failure (HF) is characterized by perturbations in energy homeostasis and metabolism. The reversibility and prognostic value of circulating markers reporting on these changes remain unclear. We sought to describe the metabolomic profiles of patients along the spectrum of systolic HF, determine their association with adverse outcomes in a clinical trial of HF, and evaluate whether identified metabolites change with treatment for end-stage systolic HF.
Methods
To assess association of metabolites with clinical outcomes, a population of 453 chronic systolic HF patients who participated in the HF-ACTION trial, which randomized ambulatory, stable patients to exercise training versus usual care, were included. To assess change in metabolites with mechanical circulatory support, 41 patients with end-stage HF who underwent left ventricular assist device (LVAD) placement were studied. Targeted, quantitative profiling of 60 metabolites using tandem flow injection mass spectrometry was performed on frozen plasma samples obtained prior to randomization in the HF-ACTION group, as well as, prior to and ≥90 days post placement in the LVAD group. Principal components analysis (PCA) was used for data reduction; linear regression and Cox-proportional hazards regression modeling were used to assess the relation between the PCA-derived metabolite factor levels and clinical outcomes among patients from the HF-ACTION study. Differences between metabolite factors associated with outcomes in the HF-ACTION and LVAD groups were assessed using Wilcoxon rank sum tests.
Results
Five PCA-derived factors were significantly associated with peak VO2 levels at baseline in fully adjusted models. Of these, Factor 5 (composed of long-chain acylcarnitines) was associated with increased risk of all 3 pre-specified HF-ACTION clinical outcomes: all-cause mortality/all-cause hospitalization (HR: 1.24; 95% CI 1.09–1.42), all cause-hospitalization (HR: 1.42; 95% CI 1.16–1.74), and cardiovascular death or cardiovascular hospitalization (HR: 1.22; CI 1.06–1.39). Individual components of Factor 5 (C16, C18:1, and C18:2 acylcarnitine metabolites) were significantly higher in patients with end-stage HF prior to LVAD placement and decreased significantly after LVAD therapy.
Conclusions
In chronic HF patients, circulating long chain acylcarnitine metabolite levels were independently associated with clinical outcomes and decreased after long-term mechanical circulatory support. These metabolites, which report on mitochondrial fatty acid β-oxidation, highlight pathways that may serve as potential targets for new diagnostics or therapeutic interventions.
Keywords: Heart failure, biomarkers, mechanical circulatory support, prognosis, metabolomics
Introduction
Heart failure (HF) is a global health problem with an estimated prevalence of 38 million patients worldwide, a number that is increasing with ageing of the population. It is the most common diagnosis in patients aged 65 years or older admitted to hospital in high-income nations (1). The prognosis of HF remains worse than that of most cancers. Pharmaceutical treatments have primarily focused on neuro-hormonal blockade with β-blockers, angiotensin-converting enzyme inhibitors (ACE-I), angiotensin II receptor blockers (ARB), and aldosterone antagonists. However, HF is a complex syndrome and identification of novel molecular mechanisms might lead to new therapies.
The failing heart is characterized by structural, functional, inflammatory, and metabolic derangements that develop and worsen during progression of the disease state (2). Despite the multifactorial causes for HF, it has been suggested that as hearts begin to fail, altered energetics play an increasingly important role in pathogenesis: “an engine out of fuel” (3,4). The heart is among the most metabolically active organs in the body, utilizing an entire supply of ATP every 13 seconds; for this it primarily utilizes free fatty acids (FFAs) as energy substrates, and switches to favor glucose metabolism during states of stress (3). In HF, changes occur in myocardial mitochondrial functions that result in a shift towards the preferential use of glucose for metabolism rather than FFAs (3). Metabolomics, the study of small-molecule metabolites, aims to uncover the underlying pathophysiologic processes of the body with regards to energy homeostasis and metabolism; however, the prognostic or therapeutic implications of metabolic profile derangements in HF remain unclear (2,5–7). With the availability of novel compounds that stabilize or reverse mitochondrial dysfunction, human studies to delineate the circulating profile of this derangement, and establishing potential for reversibility of metabolic derangements in HF, could lead to a better understanding of whether they might be efficacious in this disease state (8,9).
We therefore sought to characterize circulating metabolites associated with poor outcomes in chronic systolic HF patients, and assess whether these prognostic profiles are modifiable with mechanical circulatory support for end-stage HF with long-term LVAD support.
Methods
Study Populations
To assess the prognostic significance of metabolites we analyzed a subgroup of the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial of chronic systolic HF patients. Details of the participants have been described elsewhere (10). Specifically, 452 of 2331 enrolled in the who study had agreed to participate in a biomarker sub-study that required collection and banking of peripheral blood samples for purpose of research, and on whom metabolomics profiling was performed. Patients were age 18 or older, with left ventricular systolic dysfunction (LVEF <35%) and ambulatory HF; they were randomized to exercise training versus usual HF care. Outcomes of interest included changes in exercise capacity measured by distance walked during 6-minute walk test and peak oxygen consumption measured from cardiopulmonary exercise test; clinical endpoints included all-cause mortality, cardiovascular mortality, cardiovascular hospitalization, and heart failure hospitalization.
To assess modifiability of the prognostic metabolites with mechanical treatment for HF, we studied 41 consecutive patients, aged 18 and older, who were deemed to have end-stage HF and required mechanical circulatory support with continuous flow LVAD as bridge to transplantation or destination therapy at DUMC from January 1, 2011, to October 30, 2012. Details of the cohort and methodology are described elsewhere (11). These patients had agreed to collection and banking of peripheral blood samples for the purpose of research. Patients had blood samples collected prior to LVAD and had paired long-term samples available post-LVAD placement [median time>136 days (94–180)].
These studies were approved by the Duke University Medical Center Institutional Review Board, performed in accordance with the ethical guidelines of the Declaration of Helsinki; all patients provided written informed consent.
Metabolic Analysis
Using a targeted, quantative tandem flow injection mass-spectrometry based approach, we determined levels of 45 aclycarnitines and 15 amino acids in both study populations. Proteins were first removed by precipitation with methanol; aliquoted supernatants were dried and esterified with hot acidic methanol (acylcarnitines) and n-butanol (amino acids). For the analysis, we used tandem mass spectrometery with a Quattro Micro instrument (Waters Corp, Milford, MA).; addition of internal standards enabled quantitative assessment of metabolites. Testing for all of the assays was done in random batch order by the Metabolomics/Biomarker Core Laboratory of the Duke Molecular Physiology Institute, Duke University, Durham, NC; testing personnel were blinded to the clinical status of patients, and samples were randomly distributed without knowledge of event status.
Clinical Endpoints
In the HF-ACTION cohort, the association of metabolite component factors with peak oxygen consumption (VO2) and clinical outcomes were assessed. Paralleling outcomes from the main clinical trial, the primary clinical outcome was the composite variable of all-cause mortality or all-cause hospitalization; secondary clinical outcomes included (1) all-cause hospitalization, (2) cardiovascular death or cardiovascular hospitalization, (3) cardiovascular death or heart failure exacerbation.
Statistical Analysis
In the HF-ACTION cohort, principal components analysis (PCA) with varimax rotation was used to reduce the large number of correlated metabolites into uncorrelated factors as we have done previously (6,12). Multiple regressions were used to evaluate the association of baseline PCA-derived factor levels with baseline peak VO2, and Cox-proportional hazards regression modeling was used to assess the relation between factors and clinical outcomes. Models were conducted in three stages: (1) unadjusted; (2) adjusted for age, gender, and BMI; and (3) adjusted for all known predictors of each outcome, which had been previously identified in the full HF-ACTION cohort (13). In each model, all factors were considered simultaneously and stepwise selection used to select the set of significant factors. For the peak VO2 model, covariates were age, gender, race, region, BMI, diabetes, PVD, NYHA class, LVEF, ventricular conduction and test modality. Covariates included in clinical outcomes models were age, gender, race, geographic region, LVEF, BUN, presence of severe mitral regurgitation, medications, symptom scores, and measures from the baseline CPX test. Kaplan-Meier methods were used to generate time-to-event curves for significant metabolite factors. Individual metabolites were compared between the HF-ACTION and end-stage HF LVAD groups using Wilcoxon rank sum tests. The authors had full access to and take full responsibility for the integrity of the data. All analyses were performed with SAS 9.2 (SAS Institute Incorporated, Cary, NC) and R 2.15.3 (R Development Core Team). A P-value ≤ 0.05 was considered statistically significant for all analyses.
Results
Baseline Patient Characteristics
Table 1 displays the baseline characteristics of the chronic HF (HF-ACTION) and end-stage HF (LVAD) patient populations. The median age of the chronic HF group was 59 years, and 68 years in the end-stage HF group. The patients were similarly distributed with regards to sex, race, BMI, co-morbidities (including hypertension, hyperlipidemia, diabetes) as well as objective laboratory measures. The groups differed in objective measures of cardiovascular fitness: peak VO2 of 14.3 mL/kg·min in chronic HF compared to 12.5 mL/kg·min in end-stage HF; Ve-VCO2 slope of 32.3 in chronic HF compared to 42.1 in end-stage HF; and NT-proBNP of 823 ng/L in chronic HF compared to 3108 ng/L in end-stage HF. Left ventricular ejection fraction, as ascertained by echocardiogram, was not statistically significantly different: 25% in chronic HF compared to 20% in end-stage HF. Baseline characteristics of the subset of HF-ACTION patients used for this study were not substantially different from the overall cohort (Supplementary Table 1).
Table 1.
Baseline Characteristics
| Baseline Characteristics | Chronic HF (N=453) |
End-stage HF (N=41) |
|---|---|---|
| Age, years | 59 (51, 68) | 68 (54, 74) |
| Male, % | 71.5% (324) | 70.7% (29) |
| BMI, kg/m2 | 30.4 (26.3, 35.8) | 28.7 (24.3, 34.9) |
| Caucasian | 66.7% (302) | 70.7% (29) |
| Hypertension | 62.5% (282) | 68.3% (28) |
| Hyperlipidemia | 67.5% (306) | 61.0% (25) |
| Diabetes | 32.5% (147) | 61.0% (25) |
| Sodium, mmol/L | 139 (137, 141) | 135 (131, 138) |
| Blood Urea Nitrogen, mg/dL | 20 (15, 28) | 28 (17, 41) |
| Creatinine, mg/dL | 1.2 (1.0, 1.5) | 1.4 (1.2, 1.9) |
| Total Cholesterol, mg/dL | 162 (139, 192) | 119 (95, 138) |
| LDL, mg/dL | 89 (70, 117) | 61 (46, 76) |
| HDL, mg/dL | 38 (32, 48) | 31 (23, 40) |
| HgbA1C, % | 6.8 (6.0, 8.1) | 6.9 (5.8, 7.6) |
| LVEF, % | 25 (20, 30) | 20 (15, 20) |
| Peak VO2, ml/kg/min | 14.3 (11.3, 17.3) | 12.5 (9.2, 13.7) |
| Ve-VCO2 Slope | 32.3 (27.9, 38.1) | 42.1 (36.4, 47.6) |
| NT-proBNP, pg/mL | 823.3 (323.0, 2088.0) | 3108.0 (2160.5, 7417.0) |
Continuous variables are shown as median (25th, 75th percentile), and categorical variables as % (N).
Baseline Metabolomic Factors and Functional Status in Chronic HF
PCA identified thirteen metabolite factors grouping in biologically consistent pathways and similar to our previous studies, these are shown in Supplementary Table 2 (5,14). In the original HF-ACTION trial, baseline peak VO2 was the most significant predictor of mortality in this population (Χ2= 153) and we sought to determine if there was a similar association with metabolite profiles (15). As shown in Table 2, Factor 1 (medium-chain acylcarnitines), Factor 2 (long-chain dicarboxylacylcarnitines), Factor 4 (branched amino acids and related catabolites), Factor 5 (long-chain acylcarnitines), and Factor 8 (short chain dicarboxylacyclcarnitines) were all associated with peak VO2 in the fully adjusted model.
Table 2.
Association of Principal Component Factors with Baseline Peak VO2 in the Chronic HF Cohort (HF-ACTION)
| Outcome: Peak VO2 (mL/kg/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for age, gender, BMI | Adjusted for the full prediction model* | |||||||
| Factor | Estimate (Std Err) |
Wald χ2 | P | Estimate (Std Err) |
Wald χ2 | P | Estimate (Std Err) |
Wald χ2 | P |
| 1 | −1.28 (0.33) | 15.38 | <.0001 | −0.79 (0.30) | 6.90 | <0.01 | −0.69 (0.26) | 6.88 | <0.01 |
| 2 | −0.94 (0.32) | 8.33 | <0.01 | −0.94 (0.30) | 9.73 | <0.001 | −0.88 (0.27) | 10.56 | 0.001 |
| 3 | −0.57 (0.20) | 7.93 | 0.01 | −0.40 (0.19) | 4.65 | 0.03 | |||
| 4 | 0.42 (0.20) | 4.59 | 0.03 | 0.50 (0.18) | 7.42 | 0.006 | 0.58 (0.16) | 12.71 | <0.001 |
| 5 | −0.47 (0.23) | 4.23 | 0.04 | −0.68 (0.21) | 10.39 | 0.001 | −0.38 (0.19) | 4.10 | 0.04 |
| 7 | −0.71 (0.20) | 12.41 | <0.001 | −0.37 (0.19) | 4.01 | 0.05 | |||
Any factor not listed above was not significant at P≤0.05. Grey spaces indicate factors consistently associated with the outcome after all adjustments.
Blank spaces indicate that the factor was not significant at P≤0.05 in that model.
Adjusting for age, gender, race, region, BMI, diabetes, PVD, NYHA class, LVEF, ventricular conduction and test modality.
Baseline Metabolomic Factors and Clinical Outcomes in Chronic HF
Table 3 shows the association of principle component factors with clinical outcomes. Factors 5 (long-chain acylcarnitines) and 7 (medium chain acylcarnitines) were associated with an increase in risk of the primary outcome of all-cause mortality or all-cause hospitalization (HR: 1.25, 95% CI 1.09–1.42 and HR: 1.16, 95% CI 1.02–1.31, respectively, for fully adjusted model). Factor 9 (amino acids) was associated with decreased risk of the primary outcome (HR: 0.88, 95% CI 0.78–0.99). With regards to secondary outcomes, Factor 5 (long-chain acylcarnitines) was associated with a greater risk of composite outcome of all-cause mortality or all-cause hospitalization (HR: 1.24; 95% CI 1.09–1.42), all cause-hospitalization (HR: 1.42; 95% CI 1.16–1.74), and cardiovascular death or cardiovascular hospitalization (HR: 1.22; CI 1.06–1.39). Of note, neither Factors 7 nor 9 has associations with risk of hospitalization or cardiovascular death. Figures 1 shows Kaplan Meier curves of the association between tertiles of Factor 5 for the primary end-point of the trial, with the highest tertile showing a greater rate of death or hospitalization compared to the middle and lowest tertile.
Table 3.
Association of Principal Component Factors with Clinical Outcomes in Chronic HF Cohort (HF-ACTION)
| Outcome: All-Cause Mortality or All-Cause Hospitalizations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for age, gender, BMI | Adjusted for full prediction model* | |||||||
| Factor | HR (CI) | Wald χ2 | P | HR (CI) | Wald χ2 | p-value | HR (CI) | Wald χ2 | P |
| 3 | 1.13 (1.01, 1.27) | 4.33 | 0.04 | ||||||
| 5 | 1.31 (1.16, 1.48) | 17.72 | <.0001 | 1.30 (1.14, 1.48) | 15.51 | <.0001 | 1.24 (1.09, 1.42) | 10.49 | 0.001 |
| 7 | 1.18 (1.06, 1.32) | 8.88 | <0.01 | 1.15 (1.02, 1.29) | 5.33 | 0.02 | 1.16 (1.02, 1.31) | 5.46 | 0.02 |
| 9 | 0.82 (0.73, 0.93) | 10.61 | <0.01 | 0.85 (0.75, 0.97) | 6.15 | 0.01 | 0.88 (0.78, 0.99) | 4.00 | <0.05 |
| 13 | 0.89 (0.79, 1.00) | 3.85 | <0.05 | ||||||
| Outcome: All-Cause Hospitalization | |||||||||
| Unadjusted | Adjusted for age, gender, BMI | Adjusted for full prediction model* | |||||||
| Factor | HR (CI) | Wald χ2 | P | HR (CI) | Wald χ2 | p-value | HR (CI) | Wald χ2 | P |
| 1 | 1.48 (1.08, 2.05) | 5.81 | 0.01 | ||||||
| 2 | 1.67 (1.25, 2.22) | 11.95 | <0.001 | 1.56 (1.15, 2.14) | 7.91 | <0.05 | |||
| 5 | 1.61 (1.30, 1.99) | 19.61 | <.0001 | 1.59 (1.28, 1.97) | 18.02 | <.0001 | 1.42 (1.16, 1.74) | 11.35 | <0.001 |
| 8 | 1.57 (1.21, 2.04) | 11.64 | <0.001 | 1.43 (1.08, 1.90) | 6.23 | <0.01 | |||
| Outcome: Cardiovascular Death or Cardiovascular Hospitalization | |||||||||
| Unadjusted | Adjusted for age, gender, BMI | Adjusted for full prediction model* | |||||||
| Factor | HR (CI) | Wald χ2 | P | HR (CI) | Wald χ2 | p-value | HR (CI) | Wald χ2 | P |
| 5 | 1.33 (1.16, 1.52) | 17.74 | <.0001 | 1.31 (1.15, 1.50) | 15.66 | <.0001 | 1.22 (1.06, 1.39) | 8.14 | <0.01 |
| 8 | 1.26 (1.06, 1.50) | 6.66 | <0.01 | 1.22 (1.01, 1.47) | 4.30 | 0.038 | |||
| 9 | 0.86 (0.76, 0.98) | 5.51 | 0.01 | ||||||
| Outcome: Cardiovascular Death or Heart Failure Hospitalization | |||||||||
| Unadjusted | Adjusted for age, gender, BMI | Adjusted for all known predictors* | |||||||
| Factor | HR (CI) | Wald χ2 | P | HR (CI) | Wald χ2 | p-value | HR (CI) | Wald χ2 | P |
| 2 | 1.37 (1.08, 1.73) | 6.97 | <0.01 | 1.35 (1.06, 1.73) | 5.87 | 0.02 | |||
| 5 | 1.41 (1.20, 1.67) | 16.82 | <.0001 | 1.41 (1.19, 1.67) | 15.95 | <.0001 | 1.28 (1.09, 1.51) | 8.61 | <0.01 |
| 8 | 1.55 (1.27, 1.91) | 17.88 | <.0001 | 1.51 (1.22, 1.88) | 14.13 | <0.001 | |||
Hazard ratios (CI) are for 1 unit increase in factor. Any factor not listed above was not significant at p≤0.05 in any model. Grey spaces indicate factors consistently associated with the outcome after all adjustments. Blank spaces indicate that the factor was not significant at p≤0.05 in that model.
Adjusted for age, gender, race, geographic region, LVEF, BUN, presence of severe mitral regurgitation, medications, symptom scores, and measures from the baseline CPX test
Figure 1. Kaplan-Meier Curves for the relationship between tertiles of the long-chain acylcarnitine factor (Factor 5) and time-to-event for the primary outcome of all-cause mortality and hospitalization.
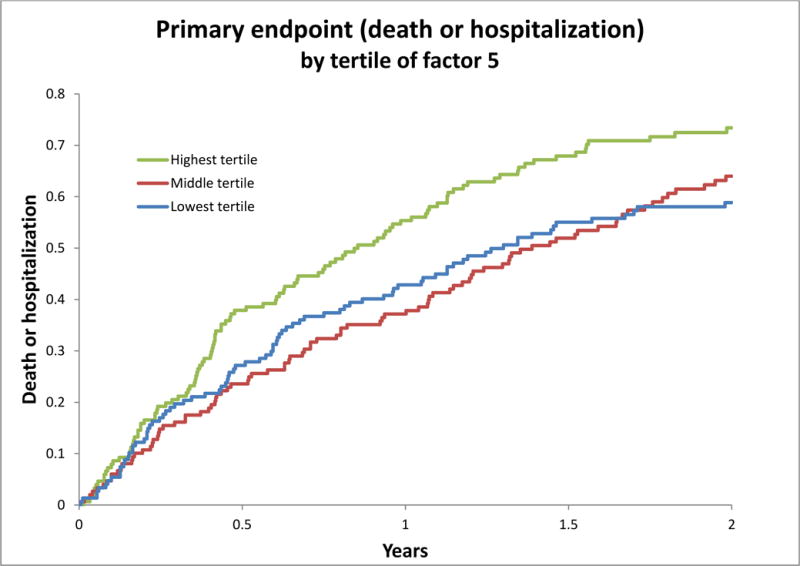
Adverse events showing adjusted Kaplan-Meier curve of relations between tertiles of metabolite factors 5 and risk of primary outcome.
Change in Metabolites with LVAD Support
Given the independent association between Factor 5 and peak VO2 as well as all clinical outcomes, we examined whether its constituent metabolites (i.e. metabolites with the highest factor load in the factor) changed significantly with LVAD support (Figure 2). As shown, C16, C18:1 and C18:2 were significantly higher at baseline in patients with end-stage HF prior to LVAD placement, and decreased after support. The other major components of Factor 5—Arginine, C18, and C20:4—did not change significantly with LVAD support.
Figure 2. Levels of Metabolites in the long-chain acylcarnitine factor (Factor 5) in Patients with Chronic Heart Failure and End-Stage Heart Failure before and after LVAD placement.
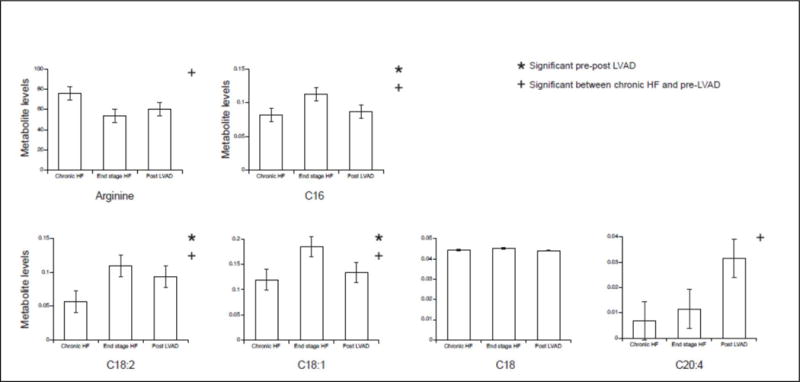
Metabolite levels that were significantly different before and after LVAD placement are marked by *, whereas those that were significantly different between chronic HF and pre-LVAD are marked by ┼. C16, C18:1 and C18:2 were significantly higher at baseline in patients with end-stage HF prior to LVAD placement, and decreased after support. The other major components of Factor 5—Arginine, C18, and C20:4—did not change significantly with LVAD support.
Discussion
This study examined the association of baseline metabolomic profiles with measures of cardiorespiratory fitness and clinical outcomes in 453 ambulatory patients with chronic systolic HF. We found a metabolite factor (Factor 5), composed mostly of long-chain acylcarnitines, that was independently associated with lower peak VO2 as well as both the primary and secondary clinical end-points of the parent trial (HF-ACTION). Of the major components of this factor, levels of C16, C18:1 and C18:2 acylcarnitines were significantly higher in patients with end-stage HF prior to LVAD implantation and decreased with circulatory support. This improvement was in a direction that would have predicted better outcomes in HF-ACTION. This pattern of metabolite abnormalities suggests impaired mitochondrial fatty acid oxidation, a finding previously described in HF, and also noted in rare mitochondrial disorders of lipid metabolism: Carnitine palmitoyltransferase 2 (CPT2) deficiency and Carnitine-acylcarnitine translocase (CACT) deficiency, both of which are associated with skeletal and cardiac myopathy (16). This further confirms that HF is characterized by dysfunction in a central pathway of energy utilization by the heart and peripheral musculature, that the measured abnormalities have prognostic importance, and that use of mitochondrial-based therapeutics might hold promise in the treatment of chronic systolic HF (4,17).
The mammalian heart has a unique ability to switch between fuel sources to adapt to changing physiological or dietary conditions— so-called metabolic flexibility. Healthy myocardium primarily meets its requirements for energy through the oxidation of long-chain fatty acids (LCFA), where carnitine plays a key role as a carrier (18). LCFAs are activated by esterification to CoA at the outer mitochondrial membrane. The inner mitochondrial membrane is impermeable to the acyl-CoA esters. The “carnitine shuttle” regulates the flux of acyl-CoA esters into the mitochondria. The shuttle requires the use of three major proteins: CPT I, CACT, and CPT II. Many clinical and experimental studies have demonstrated that the failing heart undergoes metabolic remodeling and develops a metabolic inflexibility, switching to glucose utilization at the expense of fatty acid oxidation (4). Although the molecular changes underlying the change in fuel utilization are complex and still incompletely understood, mitochondrial dysfunction remains a common pathological theme (19). In our study, we observed that elevated plasma levels of key long-chain acylcarnitines (C16 and C18) were independently associated with impaired cardiorespiratory capacity, and increased risk of all adverse clinical outcomes. Levels were significantly higher in patients with end-stage HF as compared with chronic systolic HF, and decreased with LVAD support. These findings are consistent with the notion that the syndrome of HF may be characterized by a general state of metabolic inflexibility and mitochondrial inefficiency that leads to accumulation of metabolic intermediates of fatty acid oxidation such as the long-chain acylcarnitines. Moreover, our findings indicate that these metabolic changes have distinct prognostic implications.
Our findings add further credence to the hypothesis that rare Mendelian disorders can provide key insights about mechanisms of common diseases and that the molecular targets in these conditions might serve as a target for more common conditions such as hypertension and dyslipidemias (20,21). In our study, we observed that elevated plasma levels of key long-chain acylcarnitines (C16 and C18) were independently associated with impaired cardiorespiratory capacity, and increased risk of all adverse clinical outcomes. Levels were significantly higher in patients with end-stage HF as compared with chronic systolic HF, and decreased with LVAD support. Abnormalities in plasma levels of these molecules are characteristic of disorders of the carnitine shuttle: Carnitine palmitoyltransferase II (CPT II) and Carnitine-acylcarnitine translocase (CACT) deficiencies, both of which are associated with skeletal and cardiac myopathy (16). CPT II deficiency can present with extreme phenotypic variability, with muscle weakness and cardiomyopathy being common associated findings. CACT is a far rarer and lethal disease that results in early infant demise from skeletal muscle damage and cardiomyopathy. Our findings suggest that chronic systolic HF recapitulates a milder form of these basic metabolic defects, that the degree of these defects worsen in patients with more advanced disease, and that they may be reversible with cardiac support.
Although our primary hypothesis is that the elevations in long-chain acylcarnitine levels simply signal mitochrondrial dysfunction, there is a distinct possibility that they may also contribute to disease progression. Studies have demonstrated that cardiac myocytes exposed to hypoxia exhibit rapid accumulations in long-chain acylcarnitines, and these amphiphilic molecules have been shown to inhibit excitatory Na currents in vitro (22). Furthermore, long chain acylcarnitines increase calcium efflux in a concentration-dependent manner in isolated cardiac sarcoplasmic reticulum vesicles (22). This might predispose HF patients to malignant arrhythmias (23). Long-chain acylcarnitines are also associated with insulin resistance; these reside in cell membranes where they can potentially interfere with insulin signaling directly within the cell membrane (14). This might explain the noted state of insulin resistance seen in patients with HF (24). If true, this data will provide additional support for developing therapeutic strategies to improve these functions in the myocardium. Furthermore, there may be a role for testing the efficacy of currently available mitochondrial based therapies such as mitoprotective agents and L-carnitine supplementation in HF (25).
The improvement with LVAD support we noted indicates that these molecules may also play a role in the monitoring the efficacy of current and novel therapeutics in HF (26). Several strategies have been proposed for specific molecular targets for modifying mitochondrial function: micronutrient supplementation, increasing mitochondrial biogenesis, decreasing production of reactive oxygen species (ROS) and improvement of cellular iron homeostasis (25,27,28). Particularly interesting are a novel class of compounds that selectively target cardiolipin on the inner mitochondrial membrane to optimize efficiency of the electron transport chain and thereby restore cellular bioenergetics (9,29). A key next step might be to evaluate changes in cardiorespiratory capacity and metabolite profiles as biomarkers of response during trials of these and other agents in clinical trials.
Limitations
Several potential limitations of our study require consideration. Our patient population was a subset of the total HF-ACTION study; however, there were no meaningful differences in key baseline characteristics between these patients and the overall trial. The LVAD patient population was relatively small and from a single-center. These results should be confirmed in another similar population. Our current study should therefore be considered hypothesis-generating; further studies are required to confirm the findings. Lastly, we report on peripheral metabolite profiles and therefore cannot assume that they represent myocardial metabolism, but rather reflect global changes in metabolism.
Conclusions
In summary, we found that greater circulating levels of long-chain acylcarnitines were independently predictive of functional status and mortality in patients with chronic systolic HF. The abnormalities were modifiable with LVAD support in end-stage heart failure patients. These findings suggest a potentially novel way to prognosticate and manage HF patients in clinical practice while providing an impetus for pharmacological targeting of the mitochondria for treatment of HF.
Supplementary Material
Central Figure.
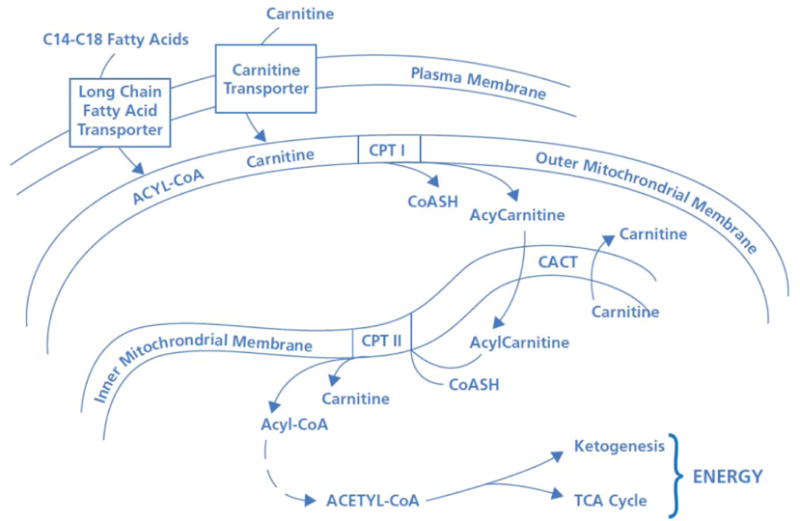
The primary energy source for the normally functioning human heart is free fatty acids, which are broken down by β-oxidation and entered into the Krebs cycle for eventual conversion into ATP. Long chain fatty acids are converted to their respective acyl-coenzyme A (CoA) ester by the ATP-dependent acyl-CoA synthetases. These acyl-CoA esters are then converted into acylcarnitine and free CoA by CPT-I at the outer mitochondrial membrane. The resulting acylcarnitine is then transported across the inner mitochondrial membrane by the carnitine:acylcarnitine translocase in exchange for free carnitine. Once inside the mitochondrial matrix, the acyl-CoA ester is reformed by CPT-II, and carnitine is released for further exchange by the carnitine:acylcarnitine translocase. In the failing heart, dysfunction in these key enzymes may lead to inadequate substrate utilization, which would be hypothesized to be reflected in serum elevations of long chain fatty acid intermediate metabolites such as long-chain acylcarnitines. Targeting these pathways might lead to novel therapeutics for heart failure.
Acknowledgments
Sources of Funding: This study was performed with a grant from the Daland Fellowship in Clinical Investigation. The HF-ACTION study was funded by grants from the NHLBI.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–24. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 2.Sutton BS, Crosslin DR, Shah SH, et al. Comprehensive genetic analysis of the platelet activating factor acetylhydrolase (PLA2G7) gene and cardiovascular disease in case-control and family datasets. Human molecular genetics. 2008;17:1318–28. doi: 10.1093/hmg/ddn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minear MA, Crosslin DR, Sutton BS, et al. Polymorphic variants in tenascin-C (TNC) are associated with atherosclerosis and coronary artery disease. Human genetics. 2011;129:641–54. doi: 10.1007/s00439-011-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neubauer S. The failing heart–an engine out of fuel. The New England journal of medicine. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 5.Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circulation Cardiovascular genetics. 2010;3:207–14. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 6.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126:1110–20. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng ML, Wang CH, Shiao MS, et al. Metabolic Disturbances Identified in Plasma Are Associated With Outcomes in Patients With Heart Failure: Diagnostic and Prognostic Value of Metabolomics. Journal of the American College of Cardiology. 2015;65:1509–20. doi: 10.1016/j.jacc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. Journal of cardiovascular pharmacology. 2014;64:543–53. doi: 10.1097/FJC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 9.Szeto HH, Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clinical pharmacology and therapeutics. 2014;96:672–83. doi: 10.1038/clpt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. Jama. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad T, Wang T, O’Brien EC, et al. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart failure. 2015;3:30–9. doi: 10.1016/j.jchf.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Shah SH, Newgard CB. Integrated metabolomics and genomics: systems approaches to biomarkers and mechanisms of cardiovascular disease. Circulation Cardiovascular genetics. 2015;8:410–9. doi: 10.1161/CIRCGENETICS.114.000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad T, Fiuzat M, Mark DB, et al. The effects of exercise on cardiovascular biomarkers in patients with chronic heart failure. American heart journal. 2014;167:193–202 e1. doi: 10.1016/j.ahj.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. Jama. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGarrah RW, Ahmad T, Koeberl DD, Patel CB. The heart is just a muscle. Circulation. 2015;131:914–22. doi: 10.1161/CIRCULATIONAHA.114.011647. [DOI] [PubMed] [Google Scholar]
- 18.Nickel A, Loffler J, Maack C. Myocardial energetics in heart failure. Basic Res Cardiol. 2013;108:358. doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- 19.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological reviews. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 20.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 21.Hall SS. Genetics: a gene of rare effect. Nature. 2013;496:152–5. doi: 10.1038/496152a. [DOI] [PubMed] [Google Scholar]
- 22.Kalim S, Clish CB, Wenger J, et al. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc. 2013;2:e000542. doi: 10.1161/JAHA.113.000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnet D, Martin D, Pascale De L, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–53. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 24.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–48. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 25.Soukoulis V, Dihu JB, Sole M, et al. Micronutrient deficiencies an unmet need in heart failure. Journal of the American College of Cardiology. 2009;54:1660–73. doi: 10.1016/j.jacc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Sanbe A, Tanonaka K, Kobayasi R, Takeo S. Effects of long-term therapy with ACE inhibitors, captopril, enalapril and trandolapril, on myocardial energy metabolism in rats with heart failure following myocardial infarction. J Mol Cell Cardiol. 1995;27:2209–22. doi: 10.1016/s0022-2828(95)91551-6. [DOI] [PubMed] [Google Scholar]
- 27.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. Journal of the American College of Cardiology. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai DF, Hsieh EJ, Chen T, et al. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circulation Heart failure. 2013;6:1067–76. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai DF, Chen T, Szeto H, et al. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. Journal of the American College of Cardiology. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


